Abstract
Cardiovascular collapse is the major factor contributing to the mortality of trauma-hemorrhage (T-H) patients. Toll-like receptors (TLRs) play a critical role in T-H-induced cardiac dysfunction. This study evaluated the role of TLR9 agonist, CpG-ODN 1826, in cardiac functional recovery after T-H. Trauma-hemorrhage was induced in a murine model by soft tissue injury and blood withdrawals from the jugular vein to a mean arterial pressure of 35±5 mm Hg. Mice were treated with CpG-ODN 1826 (10 µg/30g body weight) by intra-peritoneal injection one hour prior to T-H (N=5–8/group). Hemodynamic parameters were measured before, during hemorrhage, and at 60 min after T-H. Trauma-hemorrhage significantly decreased the mean arterial pressure and left ventricular pressure compared with sham controls. In contrast, CpG-ODN administration significantly attenuated the decrease in arterial pressure and left ventricular pressure due to T-H. Trauma-hemorrhage markedly decreased myocardial levels of phosphorylated Akt by 57.9%. However, CpG-ODN treatment significantly blunted the decrement in phospho-Akt by activating the PI3K/Akt signaling pathway. The PI3K inhibitor LY294002 partially abolished CpG-induced cardioprotection, indicating that additional signaling pathways are involved in the protective effect of CpG-ODN after T-H. We observed that CpG-ODN treatment also significantly attenuated the decrease in myocardial phospho-ERK levels after T-H. Inhibition of ERK by U0126 also partially abolished the cardioprotective effect of CpG-ODN after T-H. Our data suggests that CpG-ODN significantly attenuates T-H-induced cardiac dysfunction. The mechanisms involve activation of both PI3K/Akt and ERK signaling pathways. The TLR9 agonist, CpG-ODN 1826, may provide a novel treatment strategy for preventing or managing cardiac dysfunction and enhancing recovery in T-H patients.
Keywords: Shock, cardiac function, inflammation, innate immune, PI3K/Akt, MEK/ERK, NF-κB
Introduction
Traumatic injury, often accompanied by hemorrhage, is the leading cause of death in people between the age of one to forty-four in the United States (1). Cardiovascular collapse is one of the major factors contributing to the mortality of these patients (1). However, the cellular mechanisms by which trauma-hemorrhage (T-H) induces cardiac dysfunction have not been elucidated completely.
Trauma and hemorrhage cause a systemic inflammatory response syndrome (SIRS) that is clinically much like sepsis (2, 3, 4). Microbial pathogen-associated molecular patterns (PAMPs) activate immunocytes through pattern recognition receptors (PRRs). Similarly, injured/damaged tissues in T-H release endogenous ‘damage’-associated molecular patterns (DAMPs) that activate innate immunity and inflammatory responses through their PRRs. Toll-like receptors (TLRs) are a family of pattern recognition receptors that recognize both microbial products and endogenous molecules released by degraded tissue matrix or necrotic cells (4). Recent studies have shown that activation of TLR-mediated signaling pathways contribute to organ injury in sepsis and ischemic models (5).
TLR9 recognizes bacterial DNA and synthesized unmethylated CpG oligodeoxynucleotide (CpG-ODN) (6). Preclinical studies have shown that CpG-ODN could be a new immunomodulator which blunts harmful inflammatory responses, such as asthma and other allergic diseases (7). CpG-ODN has also been reported to accelerate wound healing (8). However, it is unknown whether CpG-ODN treatment will have beneficial effects on cardiac function following T-H.
Activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway has been shown to protect the myocardium from ischemic injury (9, 10), to attenuate cardiac dysfunction in sepsis/septic shock (11), and to increase survival in polymicrobial sepsis (12). Recent studies have identified cross talk between TLR signaling and the PI3K/Akt pathway (13, 14). Activation of the PI3K/Akt signaling pathway may serve as a negative feedback regulator for TLR-mediated innate immune and inflammatory responses (10, 12, 13). However, whether the CpG-ODN modulates PI3K/Akt signaling pathways in T-H has not been studied.
Activation of extracellular signal-regulated kinases (ERK) plays an important role in cell growth, proliferation, and survival (15, 16). Ras, a small G-protein, is an important signaling mediator which leads to activation of mitogen-activated protein kinase kinases 1,2 (MEK1,2) which activate ERKs(17). Recent evidence indicates that CpG-ODN administration activates the ERK signaling pathway (18). However, the role of the ERK signaling pathway in CpG-ODN treated T-H has not been investigated.
This study evaluated whether modulation of TLR9 with CpG-ODN 1826 will attenuate cardiac depression during T-H shock. We observed that CpG-ODN administration significantly ameliorated cardiac dysfunction in T-H and the mechanism involves activation of the PI3K/Akt and MEK/ERK signaling pathways.
Materials and Methods
Reagents
Unmethylated CpG oligodeoxynucleotide (CpG-ODN) 1826 (5’-TCCATGACGTTCCTGACGTT-3’), was synthesized by Integrated DNA Technologies (Coralville, IA, USA), and dissolved in sterile, pyrogen-free saline. LY294002 is considered as a specifically competitive inhibitor for the ATP binding site of phosphatidylinositol 3-kinase (PI3K) and was purchased from Alomone labs Ltd, Israel. U0126, a MEK inhibitor, was purchased from Promega (Madison, WI, USA) and dissolved following the manufacturers’ instructions.
Experimental Animals
Male C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). Experiments were performed when body weights were about 30 grams. The mice were maintained in the Division of Laboratory Animal Resources at East Tennessee State University. The experiments outlined in this article conformed to the Guide for the Care and use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85–23, Revised 1996). All aspects of the animal care and experimental protocols were approved by the East Tennessee State University Committee on Animal Care.
Animal Model of Trauma-Hemorrhage
Mice were anesthetized by isoflurane inhalation (induction, 5.0%; maintenance 1.5%) driven by 100% oxygen flow. After the left carotid artery was exposed, a Millar microtip pressure catheter (Millar Instruments Inc., Houston, TX) was inserted for blood pressure monitoring and the right jugular vein was cannulated with polyurethane tubing (BPU-T20, Instech Solomon, Plymouth Meeting, PA) for blood withdrawal at 0.82 ml/10 min/30 g body weight using a Harvard syringe pump (Harvard Apparatus, Holliston, MA). A thoracotomy was performed at the left fifth intercostal space with a 1.5 cm incision. A micro-conductance pressure catheter was positioned in the left ventricle (LV) via the apex of the heart for continuous registration of LV pressure-volume loops using the PowerLab system (AD Instruments, Inc., Colorado Springs, CO). Blood was withdrawn from the jugular vein until the mean arterial pressure (AP) reached 35±5 mm Hg and was maintained at this level during the first 10 min before the animal was observed for another 50 min (total time after hemorrhage = 60 min). Hemodynamic parameters were measured at three time points, i.e. “Before” (before hemorrhage), “During” (during the first 10 minutes of hemorrhage the mean AP was maintained at 35±5 mmHg) and “After” (at 60 minutes after the initiation of hemorrhage). The sham control group was subjected to the same procedure as the T-H group, but without bleeding. Following the final hemodynamic measurement, the mice were euthanized and tissue samples were collected and stored at −80°C for further analysis.
Experimental Design
To test the hypothesis that TLR9 ligand CpG-ODN 1826 may have a protective effect against cardiac dysfunction following T-H, mice were treated with CpG-ODN (10 µg/30g body weight) by intraperitoneal injection one hour prior to T-H (N=5–8/group). CpG-ODN 1826 at 10 µg/30g body weight was the optimal dose based on our other experimental study.
To evaluate the role of the PI3K/Akt and MEK/ERK signaling pathways in TLR9 ligand-induced cardioprotection, the PI3K-specific inhibitor, LY 294002 (1 mg/30g body weight) or the MEK inhibitor (U0126, 300 µg/30g body weight) was administered intraperitoneally at 15 min prior to CpG-ODN administration (N=5/group). Hemodynamic parameters were measured at three time points, “Before” (before hemorrhage), “During” (during the first 10 minutes of hemorrhage) and “After” (at 60 minutes after the initiation of hemorrhage). The sham control group was subjected to the same procedure as T-H group, but without bleeding. Following the final hemodynamic measurement, the mice were euthanized and heart samples were collected for examination of phosphorylation of Akt and ERK using the cytoplasmic protein preparations.
Western Blot
Western blot was performed as described in our previous publications (10). Briefly, the cellular proteins from hearts were separated by SDS-polyacrylamide gel electrophoresis and transferred onto Hybond enhanced chemiluminescence (ECL) membranes (Amersham Pharmacia, Piscataway, NJ). The ECL membranes were incubated with appropriate primary antibody [anti-phospho-Akt, anti-Akt, anti-phospho-ERK and anti-ERK (Cell Signaling Technology, Beverly, Ma)], followed by an incubation with peroxidase-conjugated second antibodies (Cell Signaling Technology). The membranes were analyzed by the ECL system (Amersham Pharmacia). The signals were quantified by scanning densitometry and computer-assisted image analysis.
NF-κB Binding Activity Assay
Nuclear proteins were isolated using a method described previously (19, 10). NF-κB binding activities were analyzed using the LightShift Chemiluminescent EMSA Kit (Thermo Scientific, Rockford, IL) according to the suggested protocol. Briefly, binding reaction mixtures contain 2 µl binding buffer, 15 µg nuclear proteins and 2 µl (100 fmols) 3’-Biotin labeled double-stranded NF-κB consensus oligonucleotide in a 15 µl mixture. The reaction mixture was separated on 6% non-denaturing polyacrylamide gels and the density of the binding bands was detected by G: Box and the picture analyzed by the Gene Tools of Syngene (Frederick, MD).
Statistics
All data were expressed as mean ± SEM. Comparisons of data between groups were made using one-way analysis of variance (ANOVA) and Tukey’s procedure for multiple range tests was performed. When comparing functional changes “Before”, “During” or “After” T-H of the same group of animals; paired t test was applied. P< 0.05 was considered to be statistical significance.
Results
Trauma-Hemorrhage Decreased Cardiac Function
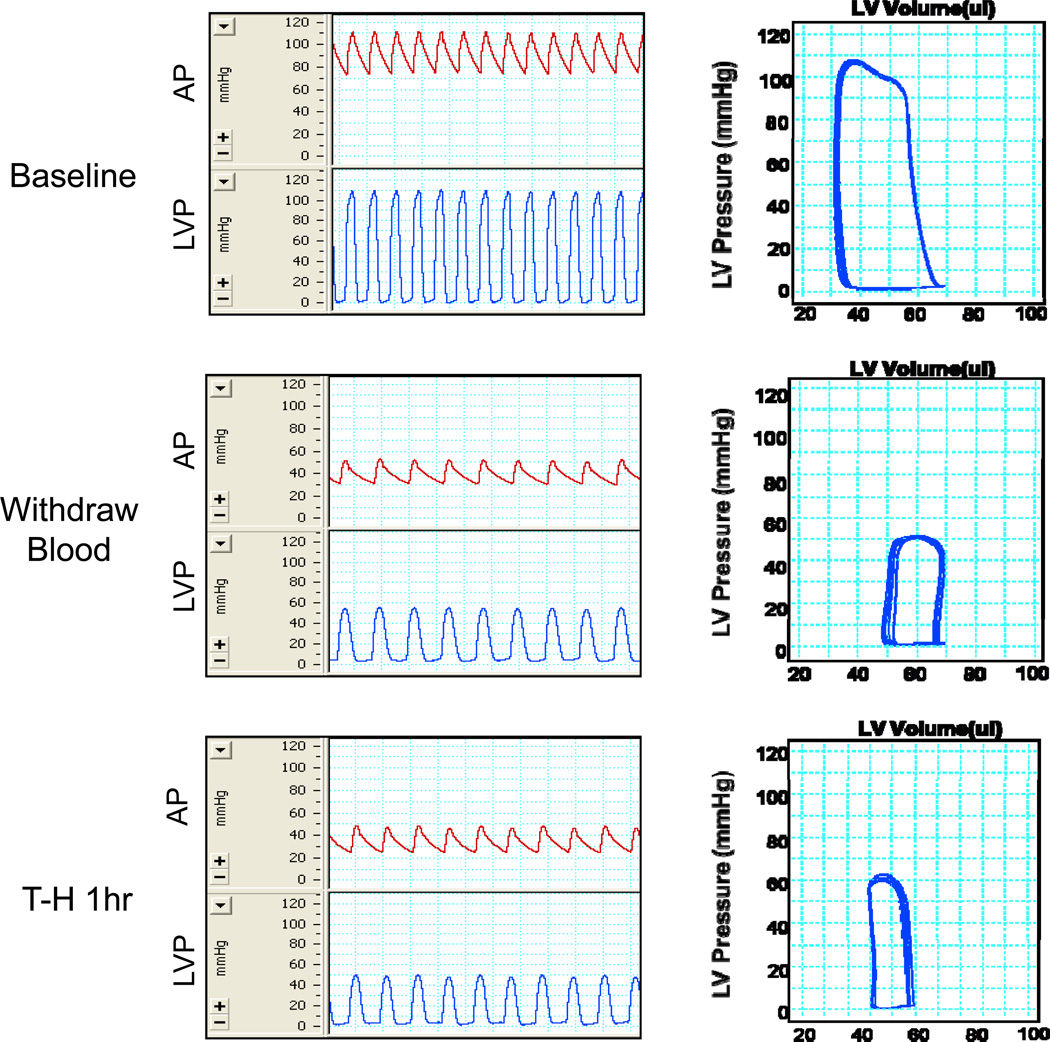
Blood was withdrawn from the jugular vein until mean arterial pressure reached 35±5 mm Hg. Sixty min after T-H, left ventricular pressure-volume loops were examined. As shown in Figure 1, T-H significantly decreased cardiac function as evidence by a characteristic right shift and a decrease of width in the pressure-volume loops and a decline in amplitude of the pressure signal. Cardiac function indices, including stroke volume (SV, ↓ 38.2%), cardiac output (CO, ↓ 55.7%), stroke work (SW, ↓ 65.8%), and dP/dtmax (↓ 58.2%), were dramatically decreased after T-H compared with the sham group (Table 1).
Figure 1. Cardiac performance before, during and after trauma-hemorrhage.
Blood was withdrawn from jugular vein till mean arterial pressure reached 35±5 mmHg. Arterial pressure (AP, mmHg), left ventricle pressure (LVP, mmHg) and left ventricular pressure-volume loops were measured at the time points “Before” (before hemorrhage), “During” (during the first 10 minutes of hemorrhage, the mean AP was maintained at 35±5 mmHg) and “After” (at 60 minutes after the initiation of hemorrhage) in the trauma-hemorrhage model. Representative graphs of untreated mice are shown above.
Table1.
Hemodynamic parameters measured before, during and after trauma-hemorrhage.
| Group | Time | HR(bmp) | LVDP(mmHg) | SV(bmp*µl) | CO(ml/min) | SW(mmHg*µl) | dP/dtmax (mmHg/s) |
Mean AP (mmHg) |
|---|---|---|---|---|---|---|---|---|
| Vehicle Sham | Before | 445±11 | 107.0±3.0 | 23.5±2.4 | 10.7±1.2 | 2120±191 | 12359±932 | 90.4±2.9 |
| During | 458±12 | 105.1±2.2 | 23.7±3.1 | 10.9±1.5 | 2107±256 | 12567±1011 | 89.1±2.7 | |
| After | 550±42 | 106.4±2.4 | 23.7±2.9 | 13.0±1.8 | 2185±254 | 12342±1064 | 86.0±3.6 | |
| CpG Sham | Before | 425±24 | 104.5±1.4 | 30.9±2.4 | 13.2±1.3 | 2594±240 | 9551±958 | 88.2±0.6 |
| During | 429±23 | 108.6±1.3 | 32.2±2.8 | 13.8±1.4 | 2745±264 | 10170±1072 | 91.4±1.0 | |
| After | 571±22‡ | 109.0±2.0 | 26.4±1.8 | 15.2±1.6 | 2454±156 | 10367±688 | 92.0±2.2 | |
| Vehicle T-H | Before | 461±18 | 104.6±3.9 | 23.1±1.1 | 10.6±0.7 | 2047±150 | 7819±652 | 88.4±5.0 |
| During | 433±30 | 52.1±1.5‡* | 14.1±2.1‡* | 6.3±1.2‡* | 536±96‡* | 3113±453‡* | 35.8±0.8‡* | |
| After | 396±24‡* | 60.5±4.3‡* | 14.4±1.5‡* | 5.7±0.7‡* | 722±133‡* | 3263±457‡* | 42.9±4.2‡* | |
| CpG T-H | Before | 424±9 | 103.3±4.0 | 28.8±1.6 | 12.2±0.5 | 2363±147 | 9179±356 | 80.5±2.4 |
| During | 413±20 | 51.9±1.9‡ | 18.9±2.9‡ | 7.6±0.9‡ | 712±124‡ | 2591±124‡ | 35.5±1.1‡ | |
| After | 556±15‡# | 101.6±1.6# | 21.4±0.9‡# | 11.9±0.7# | 1561±147‡# | 6437±436‡# | 81.9±2.2# |
CpG-ODN was intraperitoneally administrated 1 hour before trauma-hemorrhage (T-H). Hemodynamic parameters were measured “Before” (before hemorrhage), “During” (during the first 10 minutes of hemorrhage, the mean AP was maintained at 35±5 mmHg) and “After” (at 60 minutes after the initiation of hemorrhage). Sham control was subjected to the same procedures as T-H group, but without bleeding. HR: heart rate; LVDP: left ventricular developed pressure (left ventricular systolic pressure- left ventricular diastolic pressure); SV: stroke volume; CO: cardiac output; SW: stroke work; dP/dtmax: maximum value of dP/dt; Mean AP: mean arterial pressure. Results are presented as mean ± SEM (N = 5–8/group). ,
P < 0.05 compared to corresponding “Before”;
P < 0.05 Vehicle T-H vs. Vehicle sham;
P < 0.05 CpG T-H vs. Vehicle T-H.
CpG-ODN Administration Attenuated Cardiac Dysfunction Following Trauma-Hemorrhage
We examined whether TLR9 ligand, CpG-ODN 1826, attenuated T-H-induced cardiac dysfunction. Table1 shows that the mean AP and left ventricular developed pressure (LVDP) were significantly decreased to the same levels in vehicle and CpG-ODN groups during T-H. However, after T-H the mean AP and LVDP were significantly higher in the CpG-ODN group when compared with the T-H vehicle group (mean AP 42.9±4.2 vs. 81.9±2.2; LVDP 60.5±4.3 vs. 101.6±1.6, mm Hg). CpG-ODN administration also significantly improved SV by 19.2%, CO by 81.4%, SW by 87.3%, and dP/dtmax by 68%, respectively, following T-H compared with the vehicle T-H group (Table1) at the end of study. There was no significant difference in CpG-ODN treated sham group and vehicle-treated sham group.
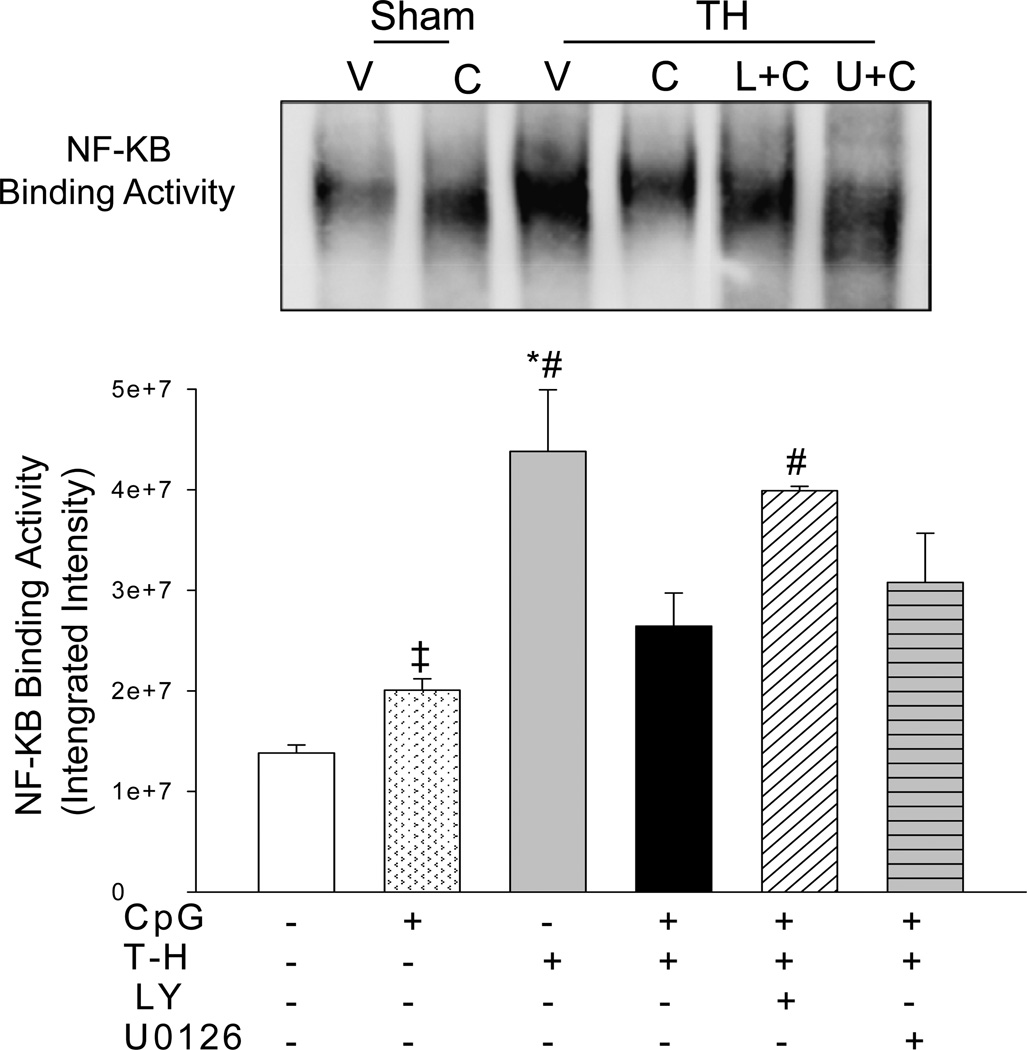
CpG-ODN Administration Decreased NF-κB Binding Activities Following Trauma-Hemorrhage
Activation of the NF-κB pathway contributes to cardiac dysfunction in ischemia and sepsis (20, 21). We examined the effect of CpG-ODN on NF-κB activation following T-H. Since TLR9 signaling is reported to mediate through PI3K/Akt and MEK/ERK pathways, we employed specific inhibitors to evaluate their effects on NF-κB activation. As shown in Figure 2, NF-κB binding activity markedly increased following T-H when compared with sham control. In contrast, CpG-ODN administration significantly attenuated T-H-increased NF-κB binding activity by 39.7%. Interestingly, PI3K inhibition effectively eliminated the beneficial effect of CpG-ODN on NF-κB binding activity. However, MEK/ERK inhibition by U0126 did not significantly affect the levels of NF-κB binding activity in CpG-ODN-treated mice.
Figure 2. CpG-ODN attenuated the increase in myocardial NF-κB binding activity associated with trauma-hemorrhage.
CpG-ODN (10ug/30g body weight) was intraperitoneally administered one hour before trauma-hemorrhage (T-H). The PI3K inhibitor, LY 294002 (LY; 1mg/30g body weight) and ERK inhibitor, U0126 (312 ug/30 g body weight) was administered by intraperitoneal injection, 15 min prior to CpD-ODN administration. Sham surgical operation severed as sham control. Sixty min after T-H, hearts were harvested and the nuclear proteins were isolated. NF-κB binding activity was measured by EMSA. V: vehicle; C: CpG-ODN; L+C: LY+CpG-ODN; U+C: U0126+CpG-ODN. N=3–5 mice/group.
‡ P < 0.05 compared to Vehicle sham; *P < 0.05 vs. corresponding sham; #P < 0.05 vs. CpG-ODN treated T-H.
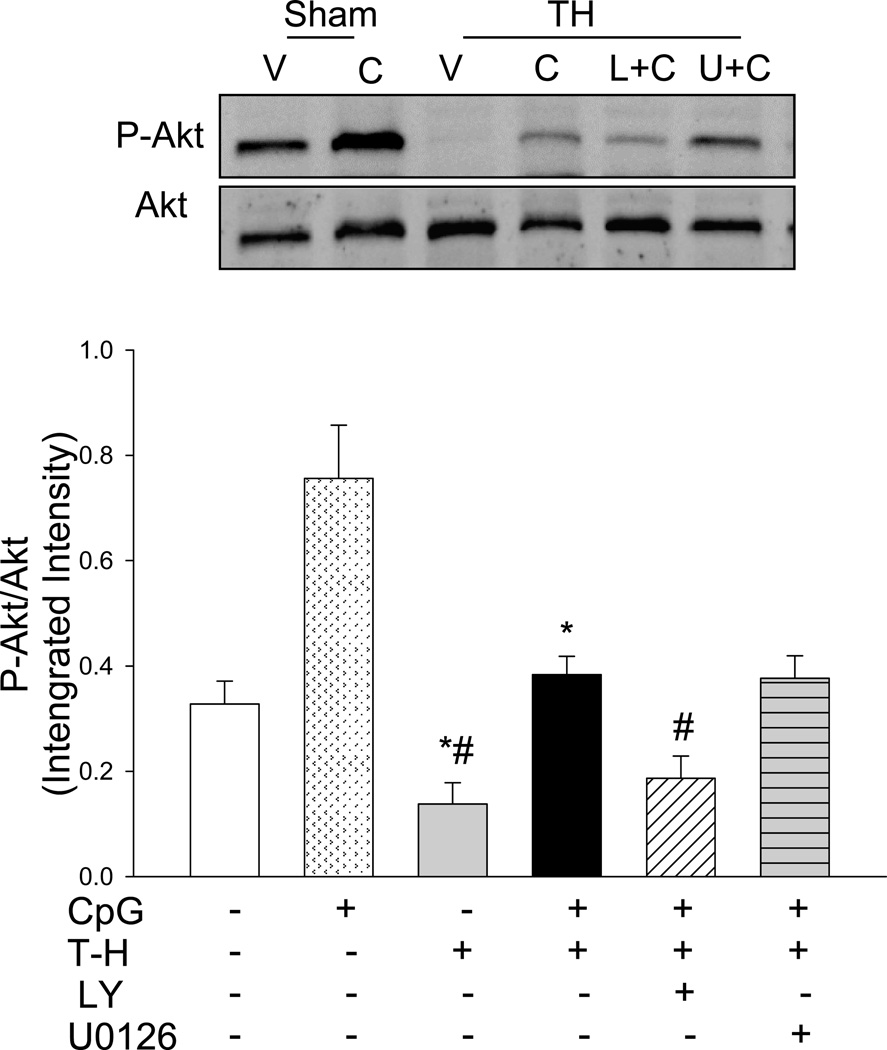
CpG-ODN Administration Increased the Levels of Phospho-Akt in the Myocardium
To investigate the mechanism by which CpG-ODN improved cardiac function following T-H, we examined the levels of phospho-Akt (P-Akt) in the myocardium. As shown in Figure 3, CpG-ODN treatment significantly increased the levels of P-Akt by 130.7% in sham control group compared with sham vehicle control, suggesting that CpG-ODN activated the PI3K/Akt signaling pathway. T-H significantly decreased the ratio of P-Akt/Akt by 57.9% when compared to the sham control. In contrast, CpG-ODN treatment prevented T-H induced decrease of P-Akt/Akt ratio. PI3K inhibition with LY294002 abolished the beneficial effect of CpG-ODN. MEK/ERK inhibition did not alter Akt phosphorylation in the presence of T-H.
Figure 3. CpG-ODN prevents the decrease in myocardial Akt phosphorylation that occurs after trauma-hemorrhage.
CpG-ODN (10ug/30g body weight) was intraperitoneally administered one hour before T-H. The PI3K inhibitor, LY 294002 (LY; 1mg/30g body weight) or the ERK inhibitor, U0126 (312 ug/30 g body weight) was administered by intraperitoneal injection, 15 min prior to CpD-ODN administration. Sham surgical operation served as sham control. Sixty min after T-H, hearts were harvested and the cytosolic proteins were isolated. The levels of phospho-Akt/Akt (P-Akt/Akt) were examined by Western blot with specific antibodies. V: vehicle; C: CpG-ODN; L+C: LY+CpG-ODN; U+C: U0126+CpG-ODN. N=4–5 mice/group. *P < 0.05 vs. corresponding sham; #P < 0.05 vs. CpG-ODN treated T-H.
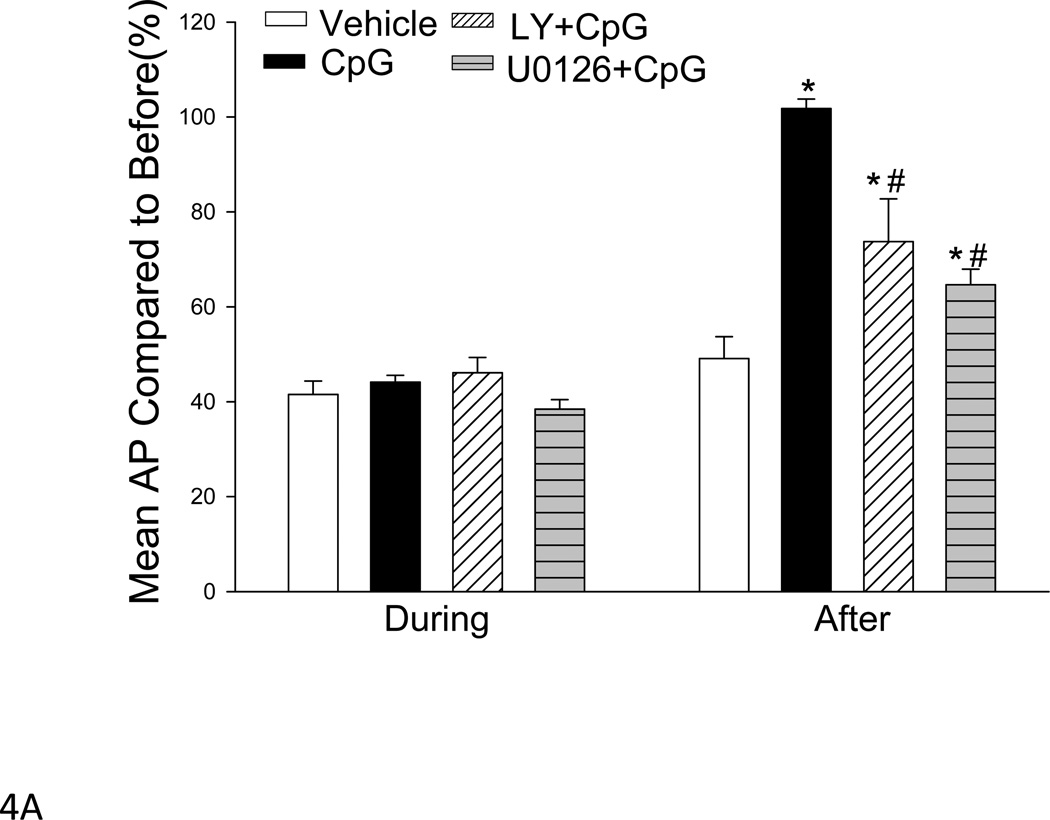
PI3K Inhibition Partially Abolished CpG-ODN-Induced Cardioprotection Following Trauma-Hemorrhage
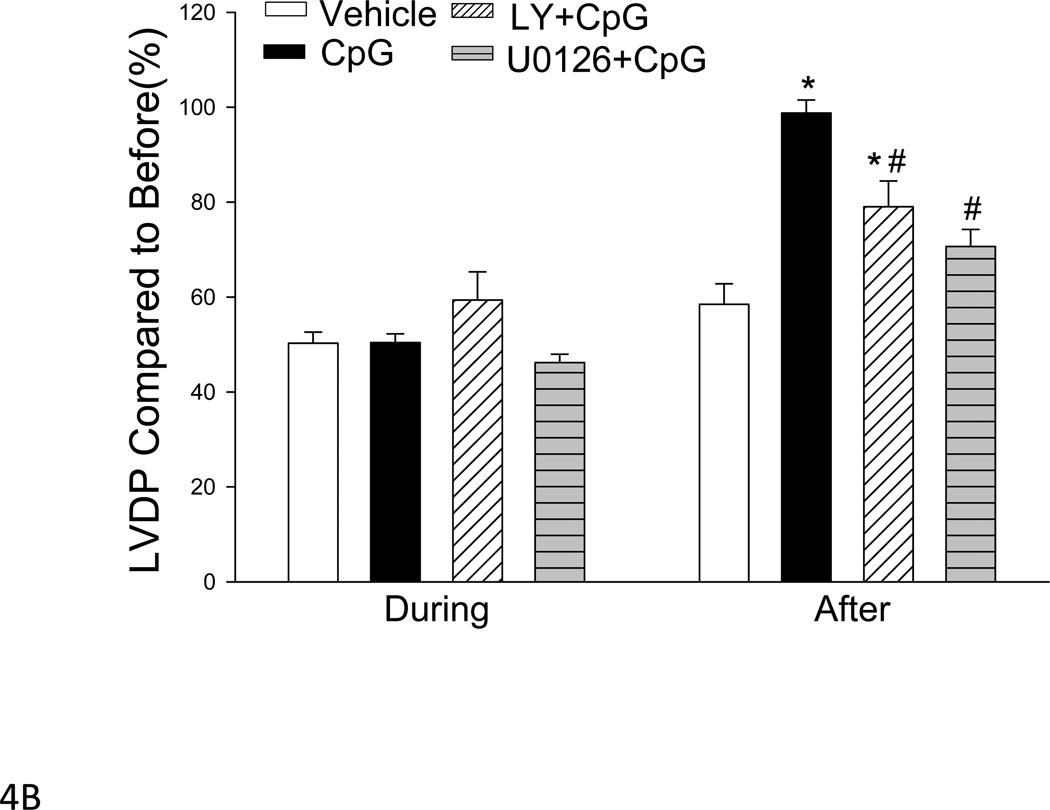
To determine whether activation of the PI3K/Akt signaling pathways contribute to the cardioprotective effect of CpG-ODN in T-H, we treated mice with a PI3K specific inhibitor, LY294002, 15 min prior to CpG-ODN administration. As shown in Figure 4, the mean AP (A) and LVDP (B) values in LY294002 treated mice that received CpG-ODN treatment were significantly lower following T-H compared with CpG-ODN-treated group (mean AP 57.6±5.1 vs. 81.9±2.2; LVDP 73.6±3.0 vs. 101.6±1.6, mm Hg). However, the levels of mean AP and LVDP in LY294002 treated mice were still greater than in vehicle control T-H mice, suggesting that activation of the PI3K signaling pathway partially contributed to CpG-ODN-induced cardioprotection following T-H.
Figure 4. Inhibiting PI3K or MEK/ERK activity abolished the cardioprotective effect of CpG-ODN in trauma-hemorrhage.
CpG-ODN (10ug/30g body weight) was intraperitoneally administered one hour before T-H. The PI3K inhibitor, LY 294002 (LY; 1mg/30g body weight) or ERK inhibitor, U0126 (312 µg/30 g body weight) were administered by intraperitoneal injection, 15 min prior to CpG-ODN administration. Hemodynamic parameters were measured before, during and 60 minutes after the initiation of hemorrhage. Mean AP: mean arterial pressure; LVDP: left ventricular developed pressure. Data were displayed as of percentage of before. Results were presented as mean ± SEM (N = 5–8/group). *P < 0.05 T-H vs. vehicle T-H; #P < 0.05 vs. CpG-ODN
CpG-ODN Administration Enhanced Myocardial ERK Phosphorylation
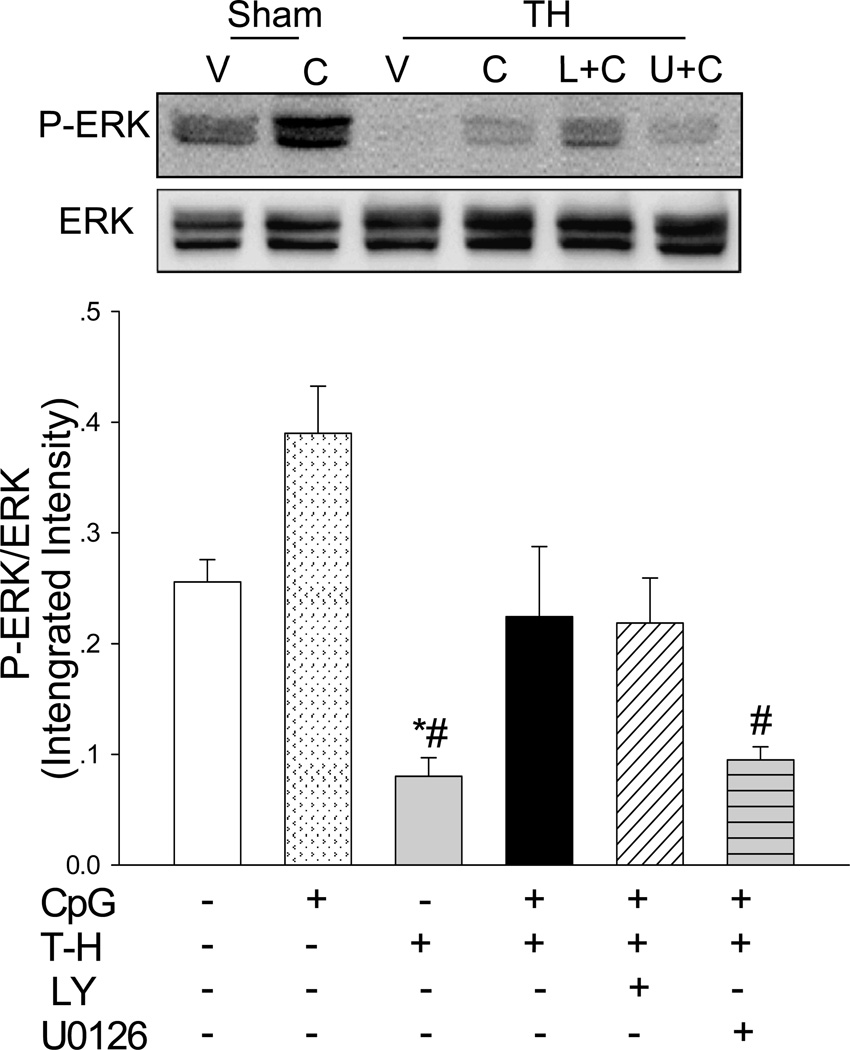
We examined the effect of CpG-ODN administration on ERK phosphorylation in the myocardium following T-H. Figure 5 shows that the levels of phospho-ERK (P-ERK) were significantly greater (↑ 52.5%) in CpG-ODN treated sham control than in vehicle sham mice. The levels of P-ERK in the myocardium were significantly decreased by 68.7% in T-H mice compared with sham control. However, CpG-ODN administration markedly attenuated deleterious effect of T-H on ERK phosphorylation. The levels of P-ERK/ERK in CpG-ODN treated mice were significantly greater (↑ 180.3%) than in the T-H control group. Inhibition of MEK/ERK by U0126 abolished the effect of CpG-ODN on ERK phosphorylation after T-H.
Figure 5. CpG-ODN prevented the decrement in myocardial ERK phosphorylation that is associated with trauma-hemorrhage.
CpG-ODN (10ug/30g body weight) was intraperitoneally administered one hour before T-H. The PI3K inhibitor, LY 294002 (LY; 1mg/30g body weight) or ERK inhibitor, U0126 (312 ug/30 g body weight) was administered by intraperitoneal injection, 15 min prior to CpG-ODN administration. Sham surgical operation served as sham control. Sixty min after T-H, hearts were harvested and the cytosolic proteins were isolated. The levels of phospho-ERK/ERK (P-ERK/ERK) ratios were examined by Western blot with specific antibodies. V: vehicle; C: CpG-ODN; L+C: LY+CpG-ODN; U+C: U0126+CpG-ODN. N=4–5 mice/group. *P < 0.05 vs. corresponding sham; #P < 0.05 vs. CpG-ODN treated T-H.
Inhibition of ERK Partially Abolished CpG-ODN-Attenuation of T-H-Induced Cardiac Dysfunction following Trauma-Hemorrhage
We examined the role of the MEK/ERK signaling pathway in CpG-ODN-induced cardioprotection after T-H. A MEK/ERK specific inhibitor, U0126, was administered to mice 15 min prior to CpG-ODN administration. After T-H, cardiac function was measured. We observed that the levels of mean AP (Figure 4A) and LVDP (Figure 4B) were significantly decreased by 36.4% and 28.3% in U0126 treated mice that received CpG-ODN, compared with the CpG-ODN group. However, the level of mean AP in U0126 treated mice was significantly greater than the T-H control group (Figure 4A).
Discussion
The important finding in the present study was that administration of the TLR9 ligand, CpG-ODN 1826, significantly attenuated cardiac dysfunction following T-H. The mechanisms involve activation of both PI3K/Akt and MEK/ERK signaling pathways. Inhibition of PI3K or MEK/ERK partially abolished CpG-ODN-attenuation of T-H-decreased cardiac function. Our data suggest that modulation of TLR9 by its ligand, CpG-ODN might offer potential benefit for T-H patients.
Trauma causes tissue and cell damage or even necrosis. When accompanying hemorrhage, trauma reduces cardiac output and induces organ ischemia (5). T-H injured tissues release endogenous “damage signals” or “alarmins” which are recognized by TLRs to initiate innate immune and inflammatory responses (4). It is well known that uncontrolled inflammatory responses intensify the tissue injury and result in multiple organ dysfunction (3, 22). Previous reports have shown that TLR4 contributes to T-H-induced organ dysfunction (5, 23). Indeed, a TLR4 antagonist has been shown to exert a beneficial effect in ischemic organ dysfunction (23). Interestingly, administration a low dose of TLR agonists has been reported to be a potentially new and novel approach for the treatment and management of several diseases, including sepsis/septic shock (14). In this study, we observed that administration of CpG-ODN significantly attenuated T-H-induced cardiac dysfunction. CpG-ODN 1826 is a synthetic type B TLR9 agonist, which mainly induces the innate immune and inflammatory responses through TLR9-dependent mechanism. Recent evidence suggests that CpG-ODN may be a new clinical approach for immunotherapy (24, 25) via modification of TLR-mediated inflammatory responses.
Recent studies have shown that T-H activates NF-κB activity (20, 21), a key transcriptional factor which regulates inflammatory gene expression. In the present study, we observed that CpG-ODN administration significantly attenuated T-H induced myocardial NF-κB activation which was positively correlated with improved cardiac function after T-H. In the present study, we also observed that CpG-ODN administration significantly attenuated T-H-decreased Akt phosphorylation in the myocardium following T-H. The data suggests that CpG-ODN treatment can activate the PI3K/Akt signaling pathway. Activation of the PI3K/Akt signaling pathway has been reported to be an endogenous negative-feedback mechanism to prevent over-responses by TLR-mediated signaling pathways to endotoxin challenge (13). Previous studies have reported that activation of the PI3K/Akt signaling pathway improved cardiac functional recovery and cardiomyocyte contractility in T-H animals (26). In addition, numerous studies have reported that CpG-ODN stimulated Akt phosphorylation in multiple cell types (27). Collectively, activation of the PI3K/Akt signaling pathway may be one of the mechanism by which CpG-ODN attenuates T-H-induced cardiac dysfunction. To determine the role of activation of the PI3K/Akt signaling induced by CpG-ODN, we treated mice with a specific PI3K inhibitor, LY294002, 15 min before CpG-ODN administration. We observed that LY294002 administration significantly abolished CpG-ODN-attenuation of T-H-decreased Akt phosphorylation in the myocardium. More significantly, PI3K inhibition only partially abolished CpG-ODN-improved cardiac function after T-H. Our data demonstrated that CpG-ODN attenuated T-H-induced cardiac dysfunction is partially mediated via activation of the PI3K/Atk signaling pathway. The data also indicated that additional mechanisms could be involved in CpG-ODN-induced cardioprotection in T-H.
Activation of MEK/ERK has been implicated in cellular survival and confers protection against T-H induced injury (28). We determined whether CpG-ODN-induced cardioprotection involves activation of MEK/ERK signaling. We observed that CpG-ODN treatment significantly increased the levels of ERK phosphorylation in sham mice, suggesting that CpG-ODN activated MEK/ERK signaling pathway. A recent study reported that CpG-ODN induced protection against allergic inflammation is via an ERK dependent mechanism (28). We also observed that CpG-ODN treatment prevented the decrement in ERK phosphorylation associated with T-H. It is a great significance that administration of U0126, a specific MEK/ERK inhibitor, to mice 15 mice prior to CpG-ODN administration partially abolished the cardioprotective effect of CpG-ODN in T-H. Our observation suggests that CpG-ODN-induced cardioprotection in T-H is partially mediated via an ERK signaling pathway.
In summary, we observed that TLR9 ligand CpG-ODN 1826 attenuates cardiac dysfunction in T-H. The mechanisms involve activation of both PI3K/Akt and MEK/ERK signaling pathways. Inhibition of either PI3K/Akt or MEK/ERK with their specific inhibitors partially abolished the cardioprotective effect of CpG-ODN in T-H. Thus, our data demonstrate that CpG-ODN-induced cardioprotection is mediated, in part, through activation of both PI3K/Akt and MEK/ERK dependent mechanisms. Our results also suggest that TLR9 ligand CpG-ODN could be a potential treatment of T-H patients.. However, future studies are needed to evaluate the beneficial effect of post-treatment with CpG-ODN on cardiac functional recovery during T-H. In addition, when isoflurane is used for inhalation anesthesia of experimental animals, room air will be employed at the onset of trauma hemorrhage model because full oxygen flow during anesthesia may affect the experimental results.
Acknowledgments
This work was supported, in part, by AHA 09GRNT2020111, NIH GM093878 to R.L.K.; NIH HL071837 to C.L.; NIH GM083016 to C.L. and D.L.W.; and NIH GM53522 to D.L.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angele MK, Schneider CP, Chaudry IH. Bench-to-bedside review: latest results in hemorrhagic shock. Crit Care. 2008;12:218. doi: 10.1186/cc6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mi Q, Constantine G, Ziraldo C, Solovyev A, Torres A, Namas R, Bentley T, Billiar TR, Zamora R, Puyana JC, Vodovotz Y. A dynamic view of trauma/hemorrhage-induced inflammation in mice: principal drivers and networks. PLoS ONE. 2011;6:e19424. doi: 10.1371/journal.pone.0019424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38:1336–1345. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursai T, Junger W, Brohl K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:41–42. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng X, Ao L, Song Y, Raeburn CD, Fullerton DA, Harken AH. Signaling for myocardial depression in hemorrhagic shock: roles of Toll-like receptor 4 and p55 TNF-alpha receptor. Am J Physiol Regul Integr Comp Physiol. 2005;288:R600–R606. doi: 10.1152/ajpregu.00182.2004. [DOI] [PubMed] [Google Scholar]
- 6.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nature Immunology. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 7.Gupta GK, Agrawal DK. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic application in allergy and asthma. BioDrugs. 2010;24:225–235. doi: 10.2165/11536140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto M, Sato T, Beren J, Verthelyi D, Klinman DM. The acceleration of wound healing in primates by the local administration of immunostimulatory CpG oligonucleotides. Biomaterials. 2011;32:4238–4242. doi: 10.1016/j.biomaterials.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt Promotes Survival of Cardiomyocytes In Vitro and Protects Against Ischemia-Reperfusion Injury in Mouse Heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao RL, Browder W, Williams DL. Modulating Toll-like receptor mediated signaling by (1-->3)-b-D-glucan rapidly induces cardioprotection. Cardiovascular Research. 2003;61:538–547. doi: 10.1016/j.cardiores.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Ha T, Lu C, Liu L, Hua F, Hu Y, Kelley J, Singh K, Kao RL, Kalbfleisch J, Williams DL, Gao X, Li C. TLR2 ligands attenuate cardiac dysfunction in polymicrobial sepsis via a phosphoinositide-3-kinase dependent mechanism. Am J Physiol Heart Circ Physiol. 2010;298:H984–H991. doi: 10.1152/ajpheart.01109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, Brooks L, Breuel K, Schweitzer JB. Modulation of the phosphoinositide 3-Kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 13.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends in Immunology. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 14.Ha T, Hu Y, Liu L, Lu C, McMullen JR, Shioi T, Isumo S, Kelley J, Kao RL, Williams DL, Gao X, Li C. TLR2 ligands induce cardioprotection against ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Cardiovascular Research. 2010;87:694–703. doi: 10.1093/cvr/cvq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steelman LS, Franklin RA, Abrams SL, Chappell W, Kempf CR, Baseke J, Stivala F, Donia M, Fagone P, Nicoletti F, Libra M, Ruvolo P, Ruvolo V, Evangelisti C, Martelli AM, McCubrey JA. Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy. Leukemia. 2011;25:1080–1094. doi: 10.1038/leu.2011.66. [DOI] [PubMed] [Google Scholar]
- 16.Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F, Mazzarino MC, Donia M, Fagone P, Malaponte G, Nicoletti F, Libra M, Milella M, Tafuri A, Bonati A, Baseke J, Cocco L, Evangelisti C, Martelli AM, Montalto G, Cervello M, McCubrey JA. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011;3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashton-Beaucage D, Therrien M. The greater RTK/RAS/ERK signalling pathway: how genetics has helped piece together a signalling network. Med Sci (Paris) 2010;26:1067–1073. doi: 10.1051/medsci/201026121067. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, An H, Yu Y, Zhang M, Qi R, Cao X. Ras participates in CpG oligodeoxynucleotide signaling through association with toll-like receptor 9 and promotion of interleukin-1 receptor-associated kinase/tumor necrosis factor receptor-associated factor 6 complex formation in macrophages. J Biol Chem. 2003;278:36334–36340. doi: 10.1074/jbc.M305698200. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Browder W, Kao RL. Early activation of transcription factor NF-kB during ischemia in perfused rat heart. Am J Physiol. 1999;276:H543–H552. doi: 10.1152/ajpheart.1999.276.2.H543. [DOI] [PubMed] [Google Scholar]
- 20.Carlson DL, White DJ, Maass DL, Nguyen RC, Giroir B, Horton JW. I kappa B overexpression in cardiomyocytes prevents NF-kappa B translocation and provides cardioprotection in trauma. Am J Physiol Heart Circ Physiol. 2003;284:H804–H814. doi: 10.1152/ajpheart.00394.2001. [DOI] [PubMed] [Google Scholar]
- 21.Shahani R, Klein LV, Marshall JG, Nicholson S, Rubin BB, Walker PM, Linksay TF. Hemorrhage-induced alpha-adrenergic signaling results in myocardial TNF-alpha expression and contractile dysfunction. Am J Physiol Heart Circ Physiol. 2001;281:H84–H92. doi: 10.1152/ajpheart.2001.281.1.H84. [DOI] [PubMed] [Google Scholar]
- 22.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 23.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of Toll-like Receptor 4 with Eritoran Attenuates Myocardial Ischemia-Reperfusion Injury. Circulation. 2006;114:I-270–I-274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 24.Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Advanced Drug Delivery Reviews. 2008;60:795–804. doi: 10.1016/j.addr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nature Reviews Drug Discovery. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 26.Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. The PI3K/Akt Pathway Mediates the Nongenomic Cardioprotective Effects of Estrogen Following Trauma-hemorrhage. Ann Surg. 2007;245:971–977. doi: 10.1097/01.sla.0000254417.15591.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoarau C, Gerard B, Lescanne E, Henry D, Francois S, Lacapere J, Benna JE, Dang PM, Grandchamp B, Lebranchu Y, Gougerot-Pocidalo M-A, Elbim C. TLR9 Activation Induces Normal Neutrophil Responses in a Child with IRAK-4 Deficiency: Involvement of the Direct PI3K Pathway. J Immunol. 2007;179:4754–4765. doi: 10.4049/jimmunol.179.7.4754. [DOI] [PubMed] [Google Scholar]
- 28.Choudhury BK, Wild JS, Alam R, Klinman DM, Boldogh I, Dharajiva N, Mileski WJ, Sur S. In vivo role of p38 mitogen-activated protein kinase in mediating the anti-inflammatory effects of CpG oligodeoxynucleotide in murine asthma. J Immunol. 2002;169:5955–5961. doi: 10.4049/jimmunol.169.10.5955. [DOI] [PubMed] [Google Scholar]
- 29.Meier F, Schittek B, Busch S, Garbe C, Smalley K, Satyamoorthy K, Li G, Herlyn M. The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front Biosci. 2005;10:2986–3001. doi: 10.2741/1755. [DOI] [PubMed] [Google Scholar]
- 30.Brzezianska E, Pastuszak-Lewandoska D. A minireview: the role of MAPK/ERK and PI3K/Akt pathways in thyroid follicular cell-derived neoplasm. Front Biosci. 2011;16:422–439. doi: 10.2741/3696. [DOI] [PubMed] [Google Scholar]