Abstract
The WNT/β-CATENIN signaling pathway is a critical regulator of chondrocyte and osteoblast differentiation during multiple phases of cartilage and bone development. While the importance of β-CATENIN signaling during the process of endochondral bone development has been previously appreciated using a variety of genetic models that manipulate β-CATENIN in skeletal progenitors and osteoblasts, genetic evidence demonstrating a specific role for β-CATENIN in committed growth plate chondrocytes has been less robust. To identify the specific role of cartilage-derived β-CATENIN in regulating cartilage and bone development, we studied chondrocyte-specific gain- and loss-of-function genetic mouse models using the tamoxifen-inducible Col2CreERT2 transgene in combination with β-cateninfx(exon3)/wt or β-cateninfx/fx floxed alleles, respectively. From these genetic models and biochemical data, three significant and novel findings were uncovered. First, cartilage-specific β-CATENIN signaling promotes chondrocyte maturation, possibly involving a BMP2 mediated mechanism. Second, cartilage-specific β–CATENIN facilitates primary and secondary ossification center formation via the induction of chondrocyte hypertrophy, possibly through enhanced MMP expression at sites of cartilage degradation, and potentially by enhancing IHH signaling activity to recruit vascular tissues. Finally, cartilage-specific β-CATENIN signaling promotes perichondrial bone formation possibly via a mechanism in which BMP2 and IHH paracrine signals synergize to accelerate perichondrial osteoblastic differentiation. The work presented here supports the concept that the cartilage-derived β-CATENIN signal is a central mediator for major events during endochondral bone formation, including chondrocyte maturation, primary and secondary ossification center development, vascularization, and perichondrial bone formation.
Keywords: β-CATENIN, chondrocyte, cartilage, perichondrium, skeletal development
Introduction
Endochondral bone development, the process by which long bones in the skeleton are formed, occurs through a progression of well-ordered steps (1). First, mesenchymal progenitor cells condense and begin the process of chondrogenesis and osteogenesis, transitioning through an intermediate phase known as the chondro-osteo progenitor. Chondro-osteo progenitors express Col2a1 and are capable of differentiating into either the chondrocyte or osteoblast lineages (2). Chondrogenic and osteogenic differentiation processes are coupled, and each cell type influences the other during proliferation and maturation phases (3, 4). In the cartilage of endochondral elements, chondrocytes near the ends of the element proliferate rapidly while those nearest the center of the element exit the cell cycle and begin the process of hypertrophy and maturation. At the onset of chondrocyte hypertrophy, surrounding perichondrial cells undergo osteoblast differentiation and maturation beginning the process of perichondrial bone formation. As perichondrial osteoblasts mature near the diaphyseal region, a population of these cells migrates into the emerging primary ossification center (POC), which is created by the removal of terminally hypertrophic chondrocytes, the resorption of cartilage matrix, the invasion by both vascular and hematopoietic cells, and the synthesis of osteoid by migrating osteoblasts. A similar process occurs again during postnatal development in the epiphyseal regions to give rise to the secondary ossification center (SOC), which separates growth plate cartilage and articular cartilage of the joints. While the events leading to POC formation have been well studied, those leading to SOC formation are not as clearly understood. SOC formation is believed to occur in a process distinct from POC formation, although each has been suggested to be heavily dependent on MMP activity that can be regulated by multiple signaling pathways (5–8).
During endochondral bone development, WNT/β-CATENIN signaling is required for maintaining the immature phenotype of mesenchymal progenitor cells (9–11), determining osteoblast versus chondrocyte cell fate in chondro-osteo progenitors (9), and promoting chondrocyte proliferation and maturation (12, 13). While in vivo mouse genetic studies where β-CATENIN signaling has been manipulated in skeletal progenitors and osteoblasts have been instrumental in establishing the importance of this signaling pathway during cartilage and bone development (14–18), these studies have not determined the specific importance of cartilage-derived β-CATENIN in regulating these processes. To address this question directly, we employed chondrocyte-specific gain- and loss-of-function genetic mouse models using the tamoxifen-inducible Col2CreERT2 transgene in combination with β-cateninfx(exon3)/wt or β-cateninfx/fx floxed alleles, respectively. Cellular and molecular analyses of mutant embryos at multiple stages of cartilage and bone development, as well as biochemical analyses of primary chondrocyte culture models revealed multiple novel findings by which cartilage-derived β-CATENIN signals regulate chondrocyte maturation, primary and secondary ossification center development, vascularization, and perichondrial bone formation.
Materials and Methods
Mouse strains
Col2CreERT2 transgenic animals were bred from previously generated animals (19, 20). Both β–cateninfx(exon3)/fx(exon3) and β–cateninfx/fx animals were generous gifts from Dr. Di Chen’s laboratory and were described previously (21, 22). In the gain-of-function (GOF) model, cleavage of exon 3 of the β-catenin gene, which codes for phosphorylation sites, renders β-catenin resistant to degradation and, therefore, constitutively active. In the loss-of-function (LOF) model, introns 1 and 6 of the β-catenin gene are floxed and cleavage of these sites results in the transcription/translation of inactive β-catenin. In both GOF and LOF models, when an animal is treated with Tamoxifen (TM), the estrogen-receptor-conjugated Cre-recombinase is activated in Col2a1 expressing cells and gene recombination occurs. Col2CreERT2; β–cateninfx(exon3)/wt GOF and Col2CreERT2; β–catenin fx/fx LOF embryos were bred at Mendelian ratios until embryonic day 18.5 (E18.5). Since Col2a1 is expressed exclusively in chondrocytes only after E12.5, cartilage-specific recombination of floxed alleles was achieved via injections of tamoxifen into pregnant female mice at E13.5 (0.1mg/gram body weight of tamoxifen in corn oil) as described previously (2). All animal studies were performed in accordance with the guidelines set forth by the University Committee on Animal Resources at the University of Rochester Medical Center.
Tissue preparation, histology, immunohistochemistry (IHC), and in situ hybridization (ISH)
Embryos were harvested at E14.5, E15.5, E16.5 or E18.5 using the dissection and processing techniques described previously (23, 24). Upon harvest, tissue was dissected free of skin and fixed in 10% neutral buffered formalin. Embryos at or older than E15.5 were decalcified in formic acid (Decal Chemical Corporation, Tallman, NY) or 14% EDTA. Forelimbs and hind limbs were paraffin-processed, embedded, and sectioned at 5μM. For frozen sections, embryos were fixed in 4% PFA for 30 minutes, washed in PBS, decalcified in 14% EDTA overnight, rinsed in PBS and processed through 15% and 30% sucrose gradients overnight at 4°C. Limbs were embedded in Tissue-Tek OTC (Sakura Fine Technical Co., Ltd., Torrance, CA) and sectioned at 5μM. Hematoxylin and Eosin (H&E), Beta-galactosidase (LacZ), and Tartrate Resistant Acid Phosphatase (TRAP) staining were performed using standard protocols as described on our Histology, Biochemistry, and Molecular Imaging (HBMI) Core facility website (http://www.urmc.rochester.edu/musculoskeletal-research/core-services/histology/protocols.cfm). Measurements of cartilage lengths were performed on at least three sections from at least three mutant and control specimens. Where appropriate, data were averaged and a Student’s t-test was performed to determine significance.
Immunohistochemistry (IHC) staining was performed according to standard protocols. Briefly, sections were baked at 60°C for one hour, treated with xylene, and then progressively rehydrated through graded alcohols. Sections were quenched in 3% hydrogen peroxide and antigen retrieval was achieved by incubating sections in 0.01M Sodium Citrate Buffer at 75°C or 95°C for 60–90 minutes. Primary antibodies and dilutions used are as follows: Rabbit α-Phospho-Smad 1/5/8 (1:100, Cell Signaling, Danvers, MA), Rabbit α-β-catenin (1:30, Cell Signaling, Boston, MA), and Rabbit α-BMP2 (1:50, Abcam, Cambridge, MA).
In situ hybridization (ISH) was performed using 35S radiolabeled probes. Briefly, probes were generated from DNA plasmids for genes of interest using T7, T3, and SP6 RNA polymerases (Roche, Branchburg, NJ). The following cDNAs were used to generate antisense probes: Ihh (cut with XbaI; transcribe with T7), Col10a1 (XbaI; T7), Col1a1 (HindIII; T7), Bsp (NotI; SP6), Oc (XbaI; T3), Mmp13 (HindIII; T7), Mmp9 (BamHI; SP6), Mmp14 (BamHI; T7). Individual probes are available upon request. Briefly, de-waxed and rehydrated slides were postfixed with 4% PFA for 15 minutes, washed with PBS, treated with Proteinase K for 15 minutes, then fixed again in 4% PFA. Slides were treated with 0.2N HCl for 10 minutes, washed in PBS, and then treated with 0.1M Triethanolamine and acetic anhydride. Following dehydration, hybridization solution containing formamide, Tris-HCL, tRNA, Denhardt’s Solution, Dextran Sulfate, NaCl, SDS, water, DTT, and radiolabeled probe was applied to slides. Slides were cover-slipped then incubated at 57°C for 18–24 hours. Slides were then washed at 50°C with 5X SSC, followed by 2X SSC/50% Formamide. At 37°C, sections were incubated for 10 minutes in TNE, followed by 90 minutes in TNE containing RNAse A (Invitrogen, Carlsbad, CA). Sections were washed in TNE, 2X SSC, and twice in 0.2X SSC before being dehydrated in graded alcohols and dried. Slides were dipped in a silver-based emulsion (Kodak, Rochester, NY) and exposed for variable amounts of time depending on the strength of the signal as determined by pre-exposure to film. Slides were developed with developer and fixer (Kodak), counterstained with 0.5% Teledyne Blue, dehydrated and cover-slipped. For a more detailed protocol please refer to the website above.
Primary chondrocyte cultures
Primary chondrocytes from the sterna and ribs of P3 GOF or LOF mice were isolated as previously described (25, 26). Cells were seeded at 7.5 × 104 cells per well in 24-well plates. Cells were treated immediately after plating with 4-hydroxytamoxifen (4OHT) to induce gene recombination or were treated with vehicle control. Alternatively, cells were infected with Ad5-CMV-GFP or Ad5-CMV-Cre adenoviruses. Adenoviral vectors (Ad5-CMV-GFP and Ad5-CMV-Cre) were purchased from Baylor College of Medicine and used at a 1:1000 dilution of the supplied stock. Cells were infected one day post-plating in complete media, and media was changed as needed. At the end of five days, when cells were 80–90% confluent, whole cell lysates, mRNA, and media were collected for further analyses (see below).
Protein was extracted using lysis buffer containing NaCl, EDTA, 10%TritonX-100, Tris-Cl, DTT, PMSF, proteinase inhibitor, and sodium orthovanadate. Western Blot analyses were completed using standard procedures with the following antibodies: Rabbit α-Phospho-SMAD1/5/8 (1:1000, Cell Signaling, Boston, MA); Mouse α-β-CATENIN (1:500, BD Transduction Laboratories, San Jose, California); Rabbit α-SMAD/5/8 (1:200, Santa Cruz, Santa Cruz, CA); Rabbit α-BMP2 (1:1000, Abcam, Cambridge, MA); Mouse α-β-ACTIN (1:5000, Sigma-Aldrich, St. Louis, MO);α-Alpha-TUBULIN (1:1000, Cell Signaling).
RNA was extracted using the Invitrogen Purlink RNA Mini Kit (#12183-018A), then reverse-transcribed using the i-Script cDNA synthesis kit (Biorad, Hercules, CA) per manufacturer’s instructions. Real-time RT-PCR was performed with the following primer pairs purchased from Integrated DNA Technologies (Coralville, IA): Bmp2 (Forward (F): 5′-TGG AAG TGG CCC ATT TAG AG-3′, Reverse (R): 5′-GCT TTT CTC GTT TGT GGA GC-3′), Ibsp (F: 5′-AAA TGG AGA CGG CGA TAG TTC CGA-3′, R: 5′-TGG AAA GTG TGG AGT TCT CTG CCT-3′), Osteocalcin (F: 5′-TGC TTG TGA CGA GCT ATC AG-3′, R: 5′-GAG GAC AGG GAG GAT CAA GT-3′), Runx2 (F: 5′-TGA TGA CAC TGC CAC CTC TGA CTT-3′, R: 5′-ATG AAA TGC TTG GGA ACT GCC TGG-3′), Alkaline phosphatase (F: 5′-TGA CCT TCT CTC CTC CAT CC-3′, R: 5′CTT CCT GGG AGT CTC ATC CT-3′), and β-actin (F: 5′-TGT TAC CAA CTG GGA CGA CA-3′, R: 5′-CTG GGT CAT CTT TTC ACG GT-3′). The PCR reaction was conducted in the RotorGene real-time DNA amplification system (Corbett Research, Sydney, Australia). cDNA samples were combined in a 20μl final volume with 1mM primers and SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA).
An assay for secreted BMP2 protein was performed using a sandwich-ELISA kit for BMP2 according to manufacturer instructions (R&D Systems, Minneapolis, Minnesota). Briefly, 50μl of each media sample was added to a microplate well and incubated at room temperature on an orbital shaker. After incubation, BMP2 protein was captured by the coated antibody. Following extensive washing, a BMP2 monoclonal antibody was added to detect the captured BMP2 protein. Hydrogen peroxide and tetramethylbenzidine solution was added and allowed to incubate at RT for 30 minutes, protected from light. Sulfuric acid stop solution was added, then optical density proportional to the amount of protein present was measured (absorbance of each well was measured at λ = 450nm). All samples were measured in duplicate and each condition was represented by three samples.
For co-culture experiments, primary sternal chondrocytes from P4 β-cateninfx(exon3)/wt mice were isolated and plated at high density on Transwell polycarbonate membrane inserts with a 0.4μm pore size (Corning, Lowell, MA). Twenty-four hours later, cells were infected with either Ad5-CMV-GFP or Ad5-CMV-Cre adenoviruses at equivalent MOIs. Forty-eight hours later, virus was removed and chondrocyte inserts were immediately transferred to plates containing confluent MC3T3 E1 or ST2 osteoprogenitor cells. Using this co-culturing system, the chondrocytes were suspended above the osteoprogenitor cells. Cell-to-cell contact was prohibited, but the high pore density of the Transwell membrane allowed for paracrine signaling between the two cells types to occur. Cells were co-cultured in osteogenic differentiation medium (alpha-MEM containing 10% FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml ascorbic acid, and 10 mM β-glycerophosphate) with media changes every two days until harvest. MC3T3 E1 cells were harvested after five days in co-culture and ST2 cells after four days in co-culture. mRNA and protein were isolated using the PARIS kit (Life Technologies, Grand Island, NY). For alkaline phosphatase staining experiments, cells were first fixed in 4% paraformaldehyde and then stained with a NBT/BCIP solution (Thermo Scientific, Rockford, IL).
Results
Cartilage-derived β-CATENIN promotes chondrocyte maturation and POC formation
To determine whether cartilage derived WNT/β-CATENIN signaling directly regulates chondrocyte maturation, we performed in vivo studies analyzing cartilage-specific βcat GOF and βcat LOF mutant embryos at E14.5, E15.5, E16.5, and E18.5 for defects in cartilage and endochondral bone development (Fig. 1; Supp. Fig. 1). H&E stained longitudinal sections of the humerus from E14.5 βcat GOF mouse embryos displayed lengthening of the hypertrophic region of cartilage as compared to wild-type (WT) littermate controls, suggesting an earlier onset or a more accelerated progression of chondrocytes towards hypertrophy (Fig. 1A). By E16.5 and E18.5, differences suggestive of advanced chondrocyte hypertrophy and maturation are observed (Fig. 1B,C). The distance from the epiphysis to the edge of mineralized bone is consistently shorter in βcat GOF mutant sections than in control sections (Fig. 1B). In E18.5 WT and βcat GOF humerus sections, the average hypertrophic zone to epiphysis distances were 1.2673 mm and 1.1641 mm (p<0.05), respectively, as measured by taking the greatest perpendicular distance from the pre-hypertrophic/hypertrophic chondrocyte boundary to the articular region of the epiphysis. Such a finding suggests advanced chondrocyte maturation since as chondrocyte maturation occurs, the hypertrophic front progresses towards the articular region of the epiphysis and the mineralized bone front follows. At E16.5 and E18.5 when pre-hypertrophic and hypertrophic cells are normally organized into columns, cells of βcat GOF mutant mice are highly disorganized and exist in clusters such that columnar organization of cartilage cells appears to be lost (Fig. 1B,C).
Figure 1. H&E histology of humerus from βcat GOF, LOF, and WT embryos.
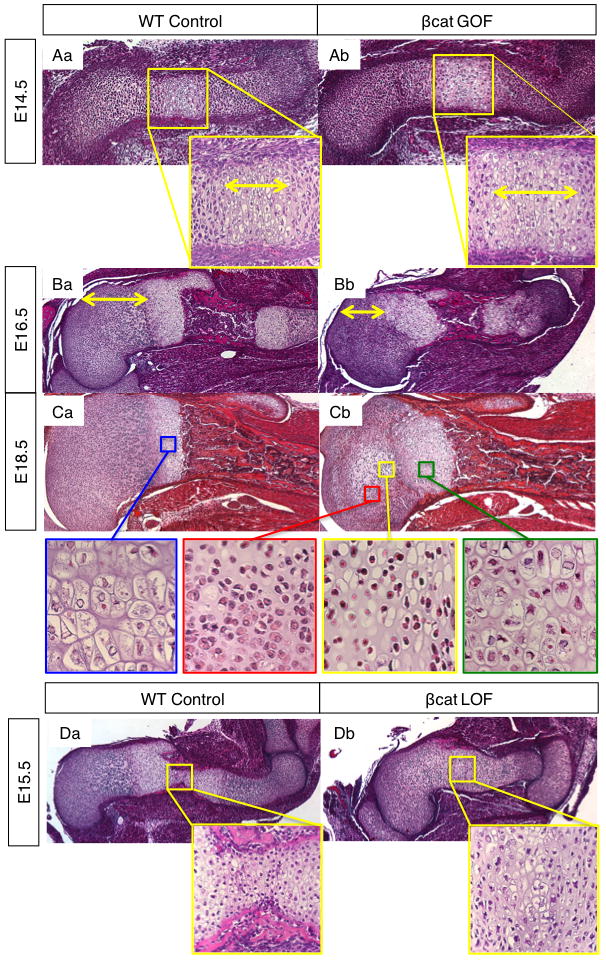
(A) Humerus sections from E14.5 WT (Aa) and β-cat GOF (Ab) littermate embryos (10X) with hypertrophic zone highlighted by double-headed arrows at high power (40X). (B) WT (Ba) and β-cat GOF (Bb) humerus at E16.5 with epiphysis to growth plate highlighted by yellow arrows (5X). (C) WT (Ca) and β-cat GOF (Cb) humerus at E18.5 (5X) with high power (40X) images of WT growth plate hypertrophic chondrocytes (blue box), and of GOF resting (red box), SOC (yellow box), and growth plate chondrocytes (green box). (D) Humerus from WT (Da) and βcat LOF (Db) littermates at E15.5 (5X) with high power (40x) magnification of mid-diaphysis.
In H&E sections from βcat LOF embryos, an opposing phenotype is observed such that there is a delayed onset of chondrocyte hypertrophy, as well as a stunted progression towards chondrocyte maturation. While E15.5 WT humerus sections reveal the cellular appearance of regularly organized growth plates and newly forming primary ossification centers (POC) with wedges of mineralized bone entering the diaphysis, sections from βcat LOF littermates show only a small hypertrophic zone, disorganized pre-hypertrophic cells, and no primary ossification center (Fig. 1D). In contrast, sections from βcat GOF mice at E15.5 show advanced chondrocyte maturation and POC development (Supp. Fig. 1A). At E18.5, βcat LOF embryos appear to catch up in terms of developing a POC; however the POC is shorter (Supp. Fig. 1B) and the growth plate cartilage remains disorganized with fewer hypertrophic chondrocytes as compared to that of WT littermate controls (Supp. Fig. 1B). These findings suggest that β-catenin expressed in chondrocytes is critical in promoting chondrocyte maturation, organization, and POC formation.
In situ hybridization gene expression studies further elucidate the phenotypes revealed by H&E staining. At E14.5, Ihh expression domains were further apart from one another and more dispersed into regions of columnar chondrocytes in βcat GOF humerus sections than in WT controls (Fig. 2Aa–b). Correspondingly, the Col10a1 expression domain that marks hypertrophic chondrocytes is longer in βcat GOF humerus sections than in WT controls (Fig. 2Ac–d). These findings support that activation of β-catenin in immature chondrocytes induces an early hypertrophic differentiation program. TUNEL staining of E18.5 cartilage sections reveal no differences in cell death, suggesting that programmed cell death is not activated prematurely throughout the growth plate region. Complementing these findings, at E15.5, βcat LOF (Col2a1-CreERT2;β-cateninfx/wt) specimens show diminished markers of chondrocyte maturation; Ihh as well as Col10a1 domains are smaller (Fig. 2B). Taken together, the findings presented here distinguish cartilage-specific β-CATENIN as an integral factor that promotes the initiation of the POC by supporting chondrocyte organization and maturation.
Figure 2. Cartilage-specific β-CATENIN signaling regulates chondrocyte maturation.
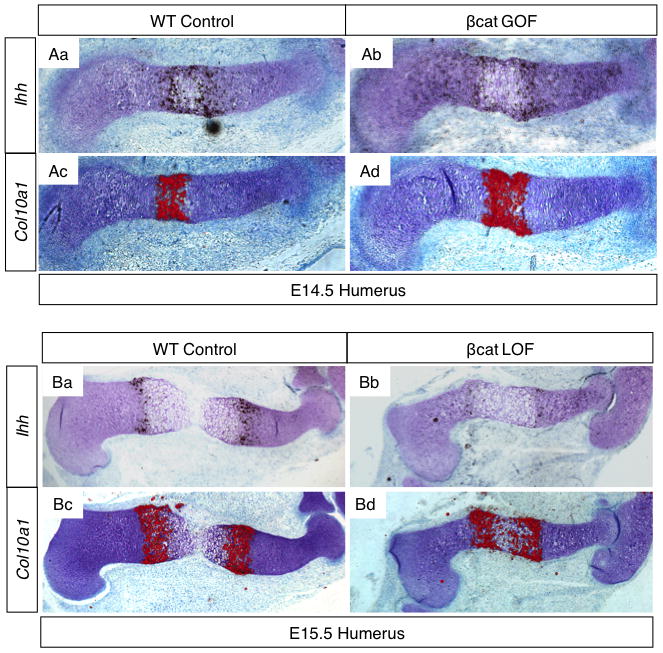
(A) In situ hybridization for Ihh (Aa–b) and Col10a1 (Ac–d) on WT and βcat GOF humerus sections at E14.5 (signal in red). (B) Ihh (Ba–b) and Col10a1 (Bc–d) gene expression in WT and βcat LOF (Col2a1-CreERT2; β-cateninfx/wt) humerus sections at E15.5.
Cartilage-derived β-CATENIN induces perichondrial bone formation
Further examination of histological sections from embryos of βcat GOF and LOF mice reveal not only changes in cartilage phenotypes but also in perichondrial bone formation. H&E humerus sections of E18.5 βcat GOF forelimbs (Fig. 3Ab) display a robustly thickened perichondrial bone collar with numerous cuboid, osteoblastic cells within the cambial layer of the perichondrium. Alternatively, WT littermate control sections displayed a very thin perichondrial bone matrix with primarily flattened osteoblastic cells lining the matrix (Fig. 3Aa). The enhanced perichondrial bone formation can be observed in the long bones of both fore- and hind-limbs from βcat GOF mice, suggesting that cartilage-specific over-expression of β-CATENIN results in a cell non-autonomous bone phenotype in addition to the effects observed on chondrocyte maturation. To verify that the enhanced perichondrial bone formation phenotype was derived specifically from cartilage in a cell non-autonomous manner and not due to a cell autonomous β-catenin mediated trans-differentiation of chondrocytes to the osteoblast lineage, we analyzed lineage specific recombination in E18.5 Col2CreERT2; R26R fx/+ and Col2CreERT2; β-catenin fx(ex3)/fx(ex3); R26R fx/+ mice following tamoxifen induction at E13.5. Staining for β-galactosidase activity was performed on frozen sections of the humerus (Supp. Fig. 2). Lineage tracing analyses of the β-galactosidase stained sections revealed that recombination caused by the inducible Col2CreERT2 transgene, either in the absence or presence of activated βcat GOF alleles, remains primarily chondrocyte specific and cannot be observed in the developing perichondrium. Therefore, these data support that β-CATENIN accumulation in chondrocytes has cell non-autonomous effects resulting in enhanced perichondrial bone formation.
Figure 3. Cartilage-specific β-CATENIN signaling regulates perichondrial bone formation.
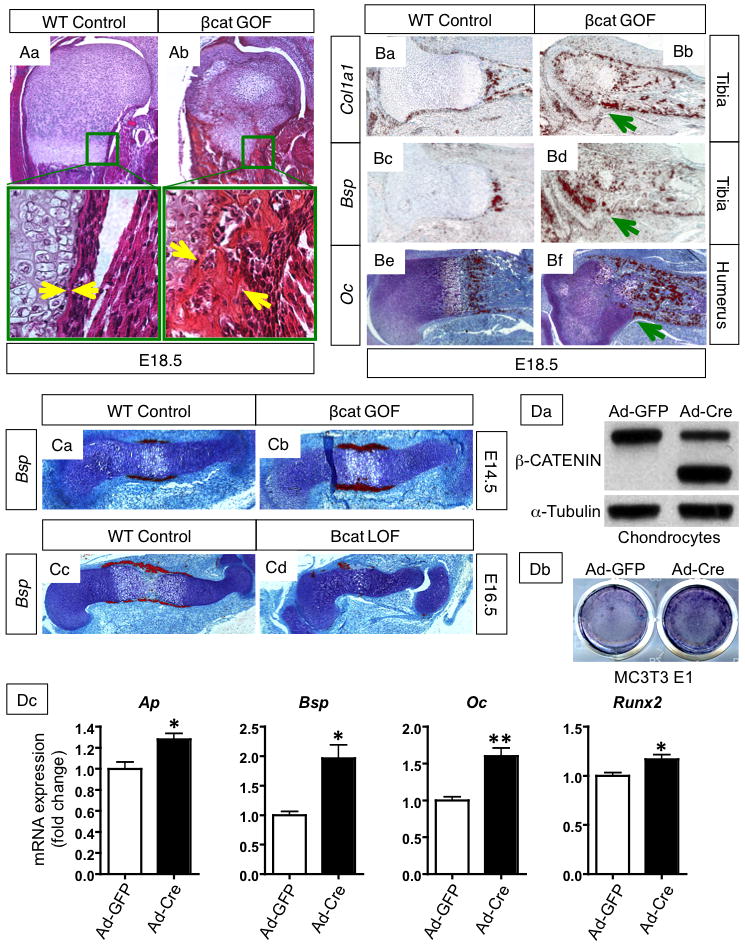
(A) H&E stained sections of the proximal humerus from E18.5 WT (Aa) and βcat GOF (Ab) littermates (10X), with corresponding areas around the growth-plate and bone collar boxed in green and shown at higher magnification (20X). Yellow arrows mark the width of the perichondrial bone collar. (B) In situ hybridization for osteoblast differentiation markers Col1a1 in the tibia (Ba–b), Bsp in the tibia (Bc–d), and Oc (Be–f) in the humerus of E18.5 WT and GOF embryos (10X). Green arrows denote advanced osteoblastic gene expression in the perichondrium. (C) In situ hybridization for osteoblast differentiation marker, Bsp, at E14.5 in WT and βcat GOF humerus sections (10X) (Ca–b) and at E16.5 in WT and βcat LOF littermates (5X) (Cc–d). (D) Data from co-culture of MC3T3 E1 osteoprogenitors with β-cat GOF chondrocytes. (Da) Alkaline phosphatase activity in osteoprogenitors co-cultured with β-CATENIN overexpressing chondrocytes and control. (Db) Overexpression of β-CATENIN in Ad-Cre infected chondrocytes is shown by Western blot with alpha-tubulin control. (Dc) mRNA expression of osteoblast differentiation markers (Alkaline phosphatase, Bone sialoprotein, Osteocalcin) and the transcription factor, Runx2, in MC3T3 E1 cells after co-culture with β-cat GOF chondrocytes.
To determine whether cartilage-specific β-CATENIN signaling promotes perichondrial osteoblast differentiation, we performed in situ hybridization gene expression studies analyzing markers of osteoblast differentiation. At E14.5, Bone sialoprotein (Bsp) is expressed in a thicker and broader domain within the developing perichondrium of βcat GOF embryos as compared to WT controls (Fig. 3Ca–b). Alternatively, even as late as E16.5, βcat LOF humerus sections have minimal Bsp expression within the perichondrium, while WT controls show significant Bsp expression throughout the perichondrium surrounding the pre-hypertrophic and hypertrophic chondrocytes (Fig. 3Cc–d). At E18.5, the type I collagen (Col1a1), Bsp, and Osteocalcin (Oc) expression domains, which mark progressively more differentiated osteoblasts, are more robust and extend further within the perichondrium towards the groove of Ranvier in βcat GOF tibia and humerus sections as compared to WT littermate controls (Fig. 3B).
To determine specifically whether βcat GOF chondrocytes were capable of inducing osteogenic differentiation in a cell-non-autonomous manner, we performed co-culture experiments in which osteoprogenitors (MC3T3 E1 and ST2 cells) were physically separated from chondrocytes overexpressing β-CATENIN via a transwell membrane, which allows cells to be exposed to the same media. Western blot analyses indicated that β-CATENIN was overexpressed in Ad5-CMV-Cre infected βcat GOF chondrocyte cultures (Fig. 3Da; Supp. Fig. 4). Co-cultured MC3T3 E1 and ST2 osteoprogenitors demonstrated increased alkaline phosphatase activity in Ad5-CMV-Cre infected βcat GOF chondrocyte co-cultures as compared to Ad5-CMV-GFP infected controls (Fig 3Db; Supp. Fig. 4). Consistent with enhanced osteogenic differentiation, MC3T3 E1 cells co-cultured with β-CATENIN overexpressing chondrocytes displayed elevated levels of the osteogenic markers, Alkaline phosphatase, Bone sialoprotein, and Osteocalcin, as well as, the osteoblast transcription factor, Runx2, as compared to controls (Fig. 3Dc).
TRAP (Tartrate Resistant Acid Phosphatase) staining of frozen sections of E18.5 βcat GOF and WT forelimbs showed no detectable difference in the number of TRAP-positive stained cells when comparing βcat GOF mutant and control sections (Supp. Fig. 3). This suggests the enhanced perichondrial bone collar was caused solely by osteoblastic formation and not as a result of impaired bone resorption. Taken together, these findings demonstrate that β-CATENIN regulates differentiation and cellular communication not only within populations of chondrocytes, but also between chondrocytes and osteoblasts.
Cartilage-derived β-CATENIN promotes SOC formation
While POCs of endochondral bones form at E15.5 during mouse embryonic development, SOCs normally begin to form after birth around post-natal day 5–7. However, cartilage-specific βcat GOF induces formation of the SOC during embryonic development while cartilage-specific βcat LOF delays SOC formation during post-natal development (Fig. 4 and Supp. Fig. 5B). H&E staining of E18.5 fore and hind limbs from βcat GOF embryos reveals enlarged cells within the epiphyseal cartilage regions, reminiscent of hypertrophic cells found in the epiphyses during SOC formation in post-natal mice (Fig. 1C, 3Ab, and 4Ab). At high power, the group of cells in the center of the epiphyses (Fig. 1Cb; yellow box) are distinct from the round, resting chondrocytes surrounding them (Fig. 1Cb; red box) in that they have more cytoplasmic area. They also appear to be distinct from growth plate hypertrophic cells (Fig. 1Cb; green box) in that their nuclei are more condensed and total cell volume appears to be less; at high magnification, they resemble the hypertrophic chondrocytes in secondary ossification centers as shown by others in the literature (6, 27). Using in situ hybridization gene expression analyses for cartilage differentiation markers, we determined that the enlarged cells in the E18.5 βcat GOF epiphyses are indeed hypertrophic chondrocytes as they express the hypertrophic marker, Col10a1 (Figure 4Ac–d) and the pre-hypertrophic marker, Ihh (Fig. 6Ba–b). In mice with the most robust SOC phenotype at E18.5, the hypertrophic chondrocytes of the SOC and the hypertrophic chondrocytes localized to the end of the growth plate appear to nearly merge (Fig. 1F, 3Ab, 4Ad, 4Cb).
Figure 4. Cartilage-specific β-CATENIN signaling regulates SOC formation.
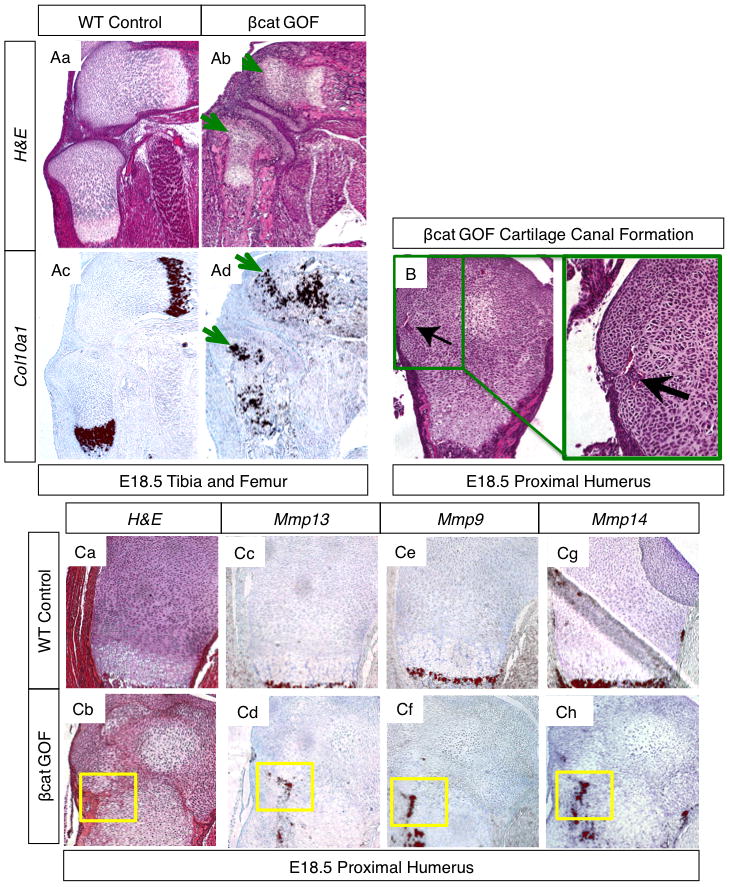
(A) H&E stained sections of E18.5 tibia and femur from WT (Aa) and βcat GOF (Ab) littermates, green arrows depict hypertrophic chondrocytes in the epiphyses of βcat GOF specimens (5x). In situ hybridization for Col10a1, a hypertrophic chondrocyte marker, in adjacent WT (Ac) and GOF (Ad) sections. Green arrows highlight hypertrophic marker expression in the epiphyses of βcat GOF hind limb sections. (B) H&E stained sections at 10X and 20X of the proximal humerus from an E18.5 βcat GOF embryo. Green box and black arrows highlight a forming cartilage canal. (C) H&E stained sections (Ca–b) of WT and βcat GOF proximal humerus with corresponding in situ hybridization of adjacent sections for Mmp13 (Cc–d), Mmp9 (Ce–f), and Mmp14 (Cg–h). Yellow boxes highlight an area of cartilage canal formation (10X).
Figure 6. β-CATENIN regulates IHH signaling in cartilage.
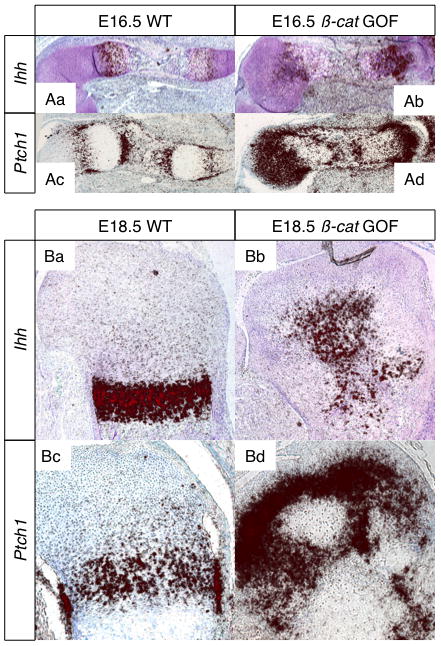
(A) In situ hybridization for Ihh (Aa–b) and Ptch-1 (Ac–d) expression (red signal) in the humerus of E16.5 WT and βcat GOF littermates (5X). (B) In situ hybridization for Ihh (Ba–b) and Ptch-1 (Bc–d) expression in the proximal humerus of E18.5 WT and βcat GOF littermates (5X).
When Cre-recombination in βcat GOF mice was induced at post-natal day 1 (P1) by subcutaneous tamoxifen injection into the dorsal cervical fat pad, the proximal humerus from P4 GOF pups displayed early SOC formation and vascular invasion when compared to littermate controls (Supp. Fig. 5A). Additionally, induction of Cre-recombination at P1 in βcat LOF mutants resulted in delayed progression of SOC formation. Careful inspection of serial sections of the proximal humerus in mutant and WT animals showed that even at P5, when the WT proximal humerus has started to display hypertrophic chondrocytes in the epiphysis, that from the LOF mutant has not (Supp. Fig. 5B). These data indicate that cartilage-specific β-CATENIN signals are important mediators of epiphyseal chondrocyte hypertrophy, which precedes normal SOC formation.
An important event in SOC formation is the appearance of cartilage canals, which bring vascularization to the epiphyseal area and, eventually, invading osteogenic cells. H&E stained humerus sections from multiple cartilage elements at E18.5 in both WT and βcat LOF embryos showed no evidence of cartilage canal invasion and vascularization within the epiphyseal cartilage, while E18.5 βcat GOF embryos displayed robust cartilage canals containing early vascular tissue complete with red blood cells (Fig. 4B). These data suggest that cartilage-specific β-CATENIN signals induce vascular cues necessary for SOC formation. This finding is further supported by the early expression of Mmp9, Mmp13, and Mmp14 present in the epiphyses of βcat GOF, but not WT, endochondral bones (Fig. 4C). MMP signals have been shown to be important mediators of cartilage canal and SOC formation during normal development (6), and these data suggest that β–CATENIN signals may drive the expression of several Mmp genes during this process. Furthermore, the βcat GOF embryos that display the most advanced SOC formation at E18.5 also show significant osteoblastic gene expression, Col1a1 and Bsp, in regions closely associated with the cartilage canals, vascular invasion, and SOC formation (Fig. 3Bb, Bd). These data likely reflect the presence of invading osteoblasts in the primitive SOC that have migrated from the perichondrium via cartilage canals or have been brought in by vascular invasion.
Cartilage-derived β-CATENIN regulates BMP2 and IHH signaling during endochondral bone development
During endochondral bone development, multiple signals, including WNT/β-CATENIN, BMP, and IHH, influence chondrogenesis and osteogenesis, chondrocyte proliferation and hypertrophy, and vascular invasion. BMP2, in particular, is known to play a critical role in both bone development (28–30) and bone repair (31, 32), while several BMP ligands (BMP2, 4, 6, 7, and GDF5) have been identified as targets of WNT/β-CATENIN signaling in various skeletal lineages (11, 33–35). IHH has also been identified as an important regulator of chondrocyte hypertrophy, osteoblast differentiation, and perichondrial bone formation (16, 24, 36–38), as well as, a direct target of the BMP pathway in chondrocytes (39). We therefore set out to examine whether chondrocyte-derived β-CATENIN signaling regulates chondrocyte maturation, POC and SOC formation, and perichondrial bone formation via modulations of the BMP and IHH pathways.
To determine whether cartilage-specific β-CATENIN signals regulate the BMP pathway, we first performed IHC analyses for β-CATENIN, BMP2, and pSMAD 1/5/8 on E18.5 humerus sections from WT and βcat GOF embryos (Fig. 5). These data show a marked increase in β-CATENIN throughout most of the immature cartilage with a specific concentration in pre-hypertrophic cells surrounding the hypertrophic chondrocytes of the growth plate and SOC of βcat GOF (Fig. 5Ab) sections as compared to WT sections (Fig. 5Aa). We also observed enhanced BMP2 immunostaining in the same cartilage cell populations (Fig. 5Ad). Corresponding to the elevated levels of BMP2 in βcat GOF cartilage sections, we observed significantly higher levels of phosphorylated SMAD 1/5/8 immunostaining that was primarily restricted to the same cell populations as those expressing β-CATENIN and BMP2 (Fig. 5Af).
Figure 5. β-CATENIN regulates BMP/SMAD signaling in cartilage in vivo and in βcat GOF and LOF sternal chondrocyte cultures.
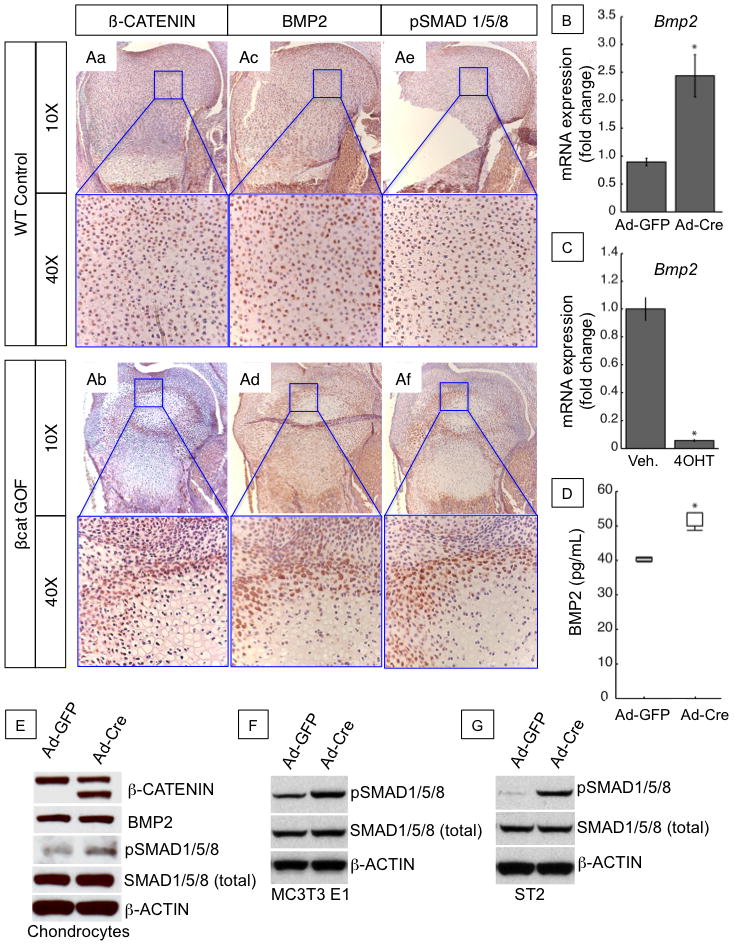
(A) Immunohistochemistry for β-CATENIN (Aa–b), BMP2 (Ac–d) and phosphorylated SMAD 1/5/8 (Ae–f) proteins in sections of the proximal humerus of E18.5 WT and βcat GOF littermates at low power (10x) and high power (40X). (B) Bmp2 mRNA expression in cultured sternal chondrocytes from 3-day old β-catenin(exon3)/(exon3) (βcat GOF) mice treated with adenoviral infection of Cre-recombinase (Ad-Cre) or GFP (Ad-GFP) control. (C) Bmp2 mRNA expression in cultured sternal chondrocytes from 3-day old Col2a1-ERT2Cre+;β-cateninfx/fx (βcat LOF) mice treated with tamoxifen (4OHT) or vehicle (veh.) control. (D) Secreted BMP2 protein expression in culture media as detected by ELISA from βcat GOF cells infected with Ad-GFP control or Ad-Cre. (E) Western Blot for β-CATENIN, BMP2, phosphorylated SMAD 1/5/8, total SMAD 1/5/8, and β-ACTIN proteins in βcat GOF and control sternal chondrocyte culture as described in (B). (F–G) Western Blot for phosphorylated SMAD 1/5/8, total SMAD 1/5/8, and β-ACTIN in MC3T3 E1 cells (G) and ST2 cells (H) co-cultured with β-cat GOF cells.
To examine whether chondrocyte-specific β-CATENIN activation can regulate BMP/SMAD signaling in both chondrocytes and osteoblasts, we performed several in vitro GOF and LOF studies in which primary chondrocytes were isolated from β–catenin fx(exon3)/wt or Col2CreERT2; β–catenin fx/fx mice and treated with adenoviral-Cre-recombinase (ad-Cre) or 4-hydroxytamoxifen (4OHT) to induce gene recombination. We found that activation of β-CATENIN signals in primary chondrocyte cultures led to a 2.5 fold increase in Bmp2 expression when compared to untreated or adenoviral-GFP (Ad-GFP) infected controls (Fig. 5B), while other Bmp genes (Bmp4, Bmp6) showed no significant changes in gene expression as assessed by real-time RT-PCR. Conversely, treatment of Col2CreERT2; β-catenin fx/fx chondrocyte cultures with 4OHT to induce recombination and deletion of β-catenin floxed alleles resulted in a greater than 80% decrease in Bmp2 expression (Fig. 5C). To assess β-CATENIN mediated effects on BMP2 production, secretion, and signaling, we performed BMP2 ELISA assays and Western blot analyses on protein collected from cultured chondrocyte media and total cell lysates, respectively. These data demonstrated that secreted BMP2 protein levels, as measured by ELISA, were increased by 30% in Ad-Cre infected GOF cultures over Ad-GFP controls (Fig. 5D). Western blot analyses of Ad-Cre infected GOF cultures revealed a significant accumulation of the truncated, degradation-resistant β-CATENIN protein (lower band), which equaled or exceeded endogenous levels (upper band) (Fig. 5E). While cellular BMP2 levels in Ad-Cre infected cultures did not appear to be significantly higher than Ad-GFP controls, we clearly observed enhanced phosphorylated SMAD 1/5/8 (pSMAD 1/5/8) with no change in total SMAD 1/5/8 levels (Fig. 5E). Taken together, we interpret these data as a chondrocyte-derived β-CATENIN mediated enhancement of BMP2 secretion and signaling, leading to activation of the SMAD pathway in chondrocytes. MC3T3 E1 and ST2 cells co-cultured with β-CATENIN overexpressing chondrocytes also displayed elevated phosphorylated SMAD 1/5/8 (pSMAD 1/5/8) and no change in total SMAD 1/5/8 levels (Fig. 5F,G). These data support a potential role for chondrocyte-specific β-CATENIN induced BMP2 secretion and paracrine activation of BMP/SMAD signaling in osteoblasts.
Since Indian Hedgehog (IHH) is a critical mediator of chondrocyte maturation, vascularization, and perichondrial bone formation during endochondral bone development as well as it is a target of BMP/SMAD signaling, we assessed Ihh expression and IHH signaling activity in WT and βcat GOF humerus sections. At E16.5, Ihh is expressed in an expanded domain of pre-hypertrophic and hypertrophic chondrocytes in βcat GOF humerus sections (Fig. 6Aa,b), while Ptc1, a downstream target of IHH signaling, is highly up-regulated throughout the immature chondrocytes of the epiphyses and the surrounding perichondrial osteoblasts (Fig. 6Ac,d) as compared to WT control sections. By E18.5, βcat GOF humerus sections display diffuse Ihh expression throughout the pre-hypertrophic and hypertrophic chondrocytes of the developing SOC and the growth plate (Fig. 6Ba,b). Additionally, in E18.5 βcat GOF sections, Ptc1 expression continues to be elevated in the immature chondrocytes of the epiphyses and in the surrounding perichondrial osteoblast population when compared to WT controls (Fig. 6Bc,d).
We also performed in vitro co-culture assays to determine whether chondrocyte-specific β-CATENIN overexpression activates the IHH pathway in both chondrocytes and osteoblasts. Following activation of β-CATENIN in chondrocytes, we were able to detect a modest upregulation of IHH protein expression, although expression of the IHH target genes, Ptch1 and Gli1, was not enhanced in either the chondrocytes or co-cultured osteoprogenitors (MC3T3 E1 and ST2 cells).
Discussion
Utilizing in vivo and in vitro cartilage-specific β-catenin GOF and LOF models, we have provided data to support that β-CATENIN from chondrocytes promotes chondrocyte maturation, enhances perichondrial bone formation, initiates cartilage vascularization, and drives the formation of primary and secondary ossification centers via direct signaling from chondrocytes. Furthermore, we provide molecular data supporting that cartilage-specific β-CATENIN regulation of these processes are potentially mediated via modulations of the paracrine signaling factors, BMP2 and IHH.
WNT/β-CATENIN signaling has previously been implicated in regulating chondrocyte hypertrophy and maturation in vivo, although whether this process is regulated directly by β-CATENIN signals in committed chondrocytes or surrounding perichondrial tissue has not previously been resolved. Several studies have incorporated gain- or loss-of-function floxed alleles of β-catenin using the Dermo1Cre (16), Prx1Cre (10, 11), and Col2Cre (9, 12, 13) transgenes that target recombination to mesenchymal progenitors and chondro-osteoprogenitors, which give rise to both committed chondrocytes and osteoblasts. These studies demonstrate the importance of WNT/β-CATENIN signaling in regulating the onset and progression of chondrocyte hypertrophic differentiation, establish WNT/β-CATENIN as a vital regulator of the perichondrial osteoblast lineage, and show that the perichondrium is critically important in controlling chondrocyte proliferation and differentiation. The current study supports previous reports of β-CATENIN’s role in endochondral bone formation, while providing further detail as to the specific role of chondrocyte-derived β-CATENIN.
To our knowledge, this is the first work to identify cartilage-derived β-CATENIN signals as an initiating factor in both POC and SOC formation. These are complicated processes that require precise timing of chondrocyte maturation, cartilage matrix turnover, vascular invasion, and migration of osteoblastic and hematopoietic cell populations. While processes that initiate SOC formation are largely unknown, one well accepted model is that cartilage canal formation, followed by vasculogenesis, precedes SOC formation, and that members of the family of matrix metalloproteinases (namely MMP9, MMP13 and MMP14 (MT1-MMP)) are critical in cartilage canal formation (5, 6, 27, 40). As suggested by our in situ hybridization findings, β-CATENIN from chondrocytes possibly regulates these signals to promote cartilage canal formation and SOC initiation. IHH/GLI2 signaling is another critical regulator of the earliest stages of cartilage vascularization, as well as, a player in maintaining the persistence of vascular structures in the developing cartilage (41, 42). Further, in other cell types, IHH has been shown to increase MMP14 expression and localization to promote cellular migration (43). Our in vivo βcat GOF data suggests a role for β-CATENIN in mediating both MMP and IHH activity in cartilage, which ultimately promote clearance of cartilage matrix and vascular invasion at sites of both POC and SOC formation. Another striking finding uncovered by the chondrocyte-specific genetic manipulation of β-CATENIN signaling is the cell non-autonomous regulation of perichondrial bone formation.
Since gene recombination in both our βcat GOF and LOF mouse models is induced at a developmental time by which chondrocyte and osteoblast cell fates have been determined, and since gene recombination is specific to chondrocytes, the resulting bone phenotypes are not likely due to changes in cell fate via cell autonomous mechanisms. Instead, the alterations to perichondrial bone formation are highly suggestive of a paracrine effect elicited by cartilage-specific modulations in β-CATENIN signaling. In fact, our in vitro co-culture experiments demonstrated that β-CATENIN activation in chondrocytes promoted both osteogenic differentiation and BMP/SMAD signaling within osteoprogenitors via a cell non-autonomous paracrine effect. While the β-CATENIN mediated BMP/SMAD signaling effects were recapitulated both in vivo and in vitro, we were only able to identify β-CATENIN mediated effects on IHH signaling to chondrocytes and osteogenitors in vivo. Since IHH induces the expression of one of its own co-receptors, PTCH1, it is possible that the abundance of PTCH1 on the chondrocyte cell surface would sequester IHH and limit its availability to co-cultured osteoprogenitors. More intricate in vitro studies would need to be designed in the future to confirm a β-CATENIN mediated IHH signaling effect on co-cultured cells as seen in vivo. Taken together, β-CATENIN mediated induction of these secreted molecules potentially leads to the early onset of osteoblast differentiation and enhanced perichondrial bone formation.
β-CATENIN and BMP/IHH signaling have been shown to interact in other contexts. In tissues of varied origin, BMP and WNT signaling appear to interact with one another to regulate processes involving differentiation and proliferation, and both inhibitory and facilitative relationships have been described between these pathways (44–46). During limb development, the relationship between these two pathways is varied and shown to be important during multiple processes (10, 17, 28, 30, 34, 47–61). Our cartilage-specific genetic manipulations of β-CATENIN have uncovered more examples in which the β-CATENIN and BMP/IHH signaling pathways synergistically regulate multiple aspects of cartilage and bone development. Our finding regarding β-CATENIN’s influence on BMP2 signaling in vivo and in vitro resonates with the recent literature in which BMP2 was identified as the critical BMP ligand regulating chondrocyte maturation (62). Interestingly, we found that βcat GOF enhanced IHH signaling in vivo, conventionally thought to inhibit chondrocyte maturation. However, this signal was not enough to inhibit the β-CATENIN/BMP2 mediated chondrocyte maturation. It has been demonstrated that IHH can promote chondrocyte maturation independent of normal IHH/PTHrP signaling, which delays chondrocyte hypertrophy (38), and it may be that in the context of β-CATENIN mediated IHH signaling, IHH signals in a manner to promote chondrocyte maturation.
To summarize, our findings support that cartilage-derived β-CATENIN is a key player in regulating chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. Our findings lead us to suggest the model depicted in Figure 7. In this model, chondrocyte-specific β-CATENIN is a prominent inducer of chondrocyte hypertrophy and maturation, as well as, osteoblast differentiation and perichondrial bone formation, via BMP2 signaling. Additionally, chondrocyte-derived β-CATENIN potentially mediates the induction of MMPs and IHH during POC and SOC formation to initiate cartilage matrix resorption and vascularization in vivo.
Figure 7. Model for chondrocyte-derived β-CATENIN regulation of chondrocyte maturation, ossification center formation, and perichondrial bone development.
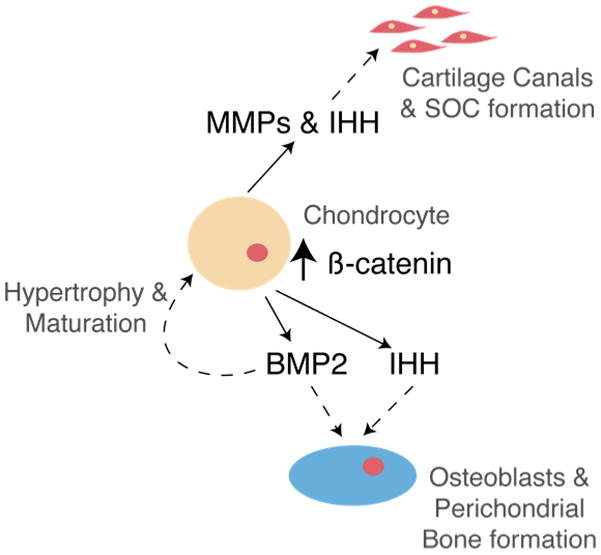
The findings presented here fit a model in which chondrocyte-derived β-CATENIN not only influences chondrocyte maturation but also results in the increased expression of MMPs and IHH in vivo, which potentially contribute to cartilage canal formation, vascularization, and POC and SOC development. Additionally, β-CATENIN activation in chondrocytes results in enhanced BMP2, and possibly IHH, signaling that promotes osteoblast differentiation and perichondrial bone formation in a cell non-autonomous manner. Finally, the β-CATENIN mediated enhancement of BMP2 signaling in chondrocytes results in accelerated chondrocyte maturation and hypertrophy.
Supplementary Material
(A) WT (Aa) and β-cat GOF (Ab) humerus at E15.5 (5x). (B) Humerus sections from E18.5 WT (Ba) and LOF (Bb) embryos with POC lengths highlighted by yellow arrows and high power magnification (20X) of growth plate area as highlighted by blue boxes.
β-galactosidase stained frozen humerus sections from a Col2CreERT2; R26R fx/+ control (A) and a Col2CreERT2; β-cateninfx(ex3)/wt; R26R fx/+ mutant animal (B). Note the high efficiency of recombination specifically in chondrocytes and the lack of perichondrial β-galactosidase staining in each of the mouse models.
(A) TRAP staining of frozen sections from the humerus from a WT control littermate (Aa) and a CreERT2;β-cateninfx(ex3)/wt E18.5 embryo (Ab). (B) The number of osteoclasts in the humeral shaft per histological section was counted and there was no difference between the two samples (n=3). Images presented were taken at 10X magnification and are from the mid-shaft trabecular bone.
(A) Alkaline phosphatase activity in osteoprogenitors (ST2 cells) co-cultured with β-CATENIN overexpressing chondrocytes (Ad-Cre) and control (Ad-GFP). (B) Overexpression of β-CATENIN in Ad-Cre infected chondrocytes is shown by Western blot with α-TUBULIN control.
(A) Proximal humerus of WT (Aa) and β-cat GOF (Ab) P4 animals; green arrow highlights area of vascular invasion and SOC formation in the GOF sample. (B) Proximal humerus of WT (Ba) and LOF (Bb) P5 animals; green arrow in highlights hypertrophic chondrocytes of the SOC in the WT animal. There is no initiated SOC in serial sections of the sample from the LOF animal as represented in (Bb).
Acknowledgments
We would like to gratefully acknowledge the technical expertise and assistance of Ryan Tierney and Sarah Mack within the Center for Musculoskeletal Research (CMSR) Histology, Biochemistry, and Molecular Imaging (HBMI) Core for the processing and preparation of tissue samples. We would also like to thank Anthony J. Mirando for his expertise in the propagation of mice, dissections, and isolation of primary chondrocytes. Authors’ roles: Study design: DYD, MJH, and RJO. Data collection: DYD, JHJ, YZ. Data analysis: DYD, JHJ, MJH, and RJO. Data interpretation: DYD, JHJ, DC, WH, MJH, and RJO. Drafting manuscript: DYD and MJH. Revising manuscript content: DYD, MJH, JHJ, and RJO. Approving final version of manuscript: DYD, JHJ, WH, DC, MJH, and RJO. DYD takes responsibility for the integrity of the data analyses. This work was supported by a NIH/NIAMS R01 grants AR38945 to RJO and AR057022 to MJH. DYD was supported by a T32 NIH training grant (AR053459) to RJO. DYD is also a trainee in the University of Rochester School of Medicine and Dentistry Medical Scientist Training Program, which is funded by an NIH T32 training grant (GM07356).
Footnotes
Disclosures
All authors state that they have no conflict of interests.
References
- 1.Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 2.Hilton MJ, Tu X, Long F. Tamoxifen-inducible gene deletion reveals a distinct cell type associated with trabecular bone, and direct regulation of PTHrP expression and chondrocyte morphology by Ihh in growth region cartilage. Dev Biol. 2007 Aug 1;308(1):93–105. doi: 10.1016/j.ydbio.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long F, Linsenmayer TF. Tissue-specific regulation of the type X collagen gene. Analyses by in vivo footprinting and transfection with a proximal promoter region. J Biol Chem. 1995 Dec 29;270(52):31310–4. doi: 10.1074/jbc.270.52.31310. [DOI] [PubMed] [Google Scholar]
- 4.Colnot C, Lu C, Hu D, Helms JA. Distinguishingthe contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol. 2004 May 1;269(1):55–69. doi: 10.1016/j.ydbio.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez J, Costales L, Lopez-Muniz A, Lopez JM. Chondrocytes are released as viable cells during cartilage resorption associated with the formation of intrachondral canals in the rat tibial epiphysis. Cell Tissue Res. 2005 Jun;320(3):501–7. doi: 10.1007/s00441-004-1034-z. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez J, Costales L, Serra R, Balbin M, Lopez JM. Expression patterns of matrix metalloproteinases and vascular endothelial growth factor during epiphyseal ossification. J Bone Miner Res. 2005 Jun;20(6):1011–21. doi: 10.1359/JBMR.050204. [DOI] [PubMed] [Google Scholar]
- 7.Blumer MJ, Longato S, Fritsch H. Structure, formation and role of cartilage canals in the developing bone. Ann Anat. 2008;190(4):305–15. doi: 10.1016/j.aanat.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Burkus JK, Ganey TM, Ogden JA. Development of the cartilage canals and the secondary center of ossification in the distal chondroepiphysis of the prenatal human femur. Yale J Biol Med. 1993 May-Jun;66(3):193–202. [PMC free article] [PubMed] [Google Scholar]
- 9.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005 May;8(5):739–50. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005 May;8(5):727–38. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Hill TP, Taketo MM, Birchmeier W, Hartmann C. Multiple roles of mesenchymal beta-catenin during murine limb patterning. Development. 2006 Apr;133(7):1219–29. doi: 10.1242/dev.02298. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004 May 1;18(9):1072–87. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo X, Mak KK, Taketo MM, Yang Y. The Wnt/beta-catenin pathway interacts differentially with PTHrP signaling to control chondrocyte hypertrophy and final maturation. PLoS One. 2009;4(6):e6067. doi: 10.1371/journal.pone.0006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004 May 1;18(9):1072–87. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000 Jul;127(14):3141–59. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 16.Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005 Jan;132(1):49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 17.Enomoto-Iwamoto M, Kitagaki J, Koyama E, Tamamura Y, Wu C, Kanatani N, Koike T, Okada H, Komori T, Yoneda T, Church V, Francis-West PH, Kurisu K, Nohno T, Pacifici M, Iwamoto M. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol. 2002 Nov 1;251(1):142–56. doi: 10.1006/dbio.2002.0802. [DOI] [PubMed] [Google Scholar]
- 18.Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005 May 13;280(19):19185–95. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 19.Zhu M, Chen M, Lichtler AC, O’Keefe RJ, Chen D. Tamoxifen-inducible Cre-recombination in articular chondrocytesof adult Col2a1-CreER(T2) transgenic mice. Osteoarthritis Cartilage. 2008 Jan;16(1):129–30. doi: 10.1016/j.joca.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O’Keefe RJ, Chen D. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007 Jan;45(1):44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brainmalformation and failure of craniofacial development. Development. 2001 Apr;128(8):1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 22.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999 Nov 1;18(21):5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual. 3. Woodbury: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
- 24.Hilton MJ, Tu X, Cook J, Hu H, Long F. Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development. 2005 Oct;132(19):4339–51. doi: 10.1242/dev.02025. [DOI] [PubMed] [Google Scholar]
- 25.Li TF, Darowish M, Zuscik MJ, Chen D, Schwarz EM, Rosier RN, Drissi H, O’Keefe RJ. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Miner Res. 2006 Jan;21(1):4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefebvre V, Garofalo S, Zhou G, Metsaranta M, Vuorio E, De Crombrugghe B. Characterization of primary cultures of chondrocytes from type II collagen/beta-galactosidase transgenic mice. Matrix Biol. 1994 Aug;14(4):329–35. doi: 10.1016/0945-053x(94)90199-6. [DOI] [PubMed] [Google Scholar]
- 27.Lee ER, Lamplugh L, Davoli MA, Beauchemin A, Chan K, Mort JS, Leblond CP. Enzymes active in the areas undergoing cartilage resorption during the development of the secondary ossification center in the tibiae of rats ages 0–21 days: I. Two groups of proteinases cleave the core protein of aggrecan. Dev Dyn. 2001 Sep;222(1):52–70. doi: 10.1002/dvdy.1168. [DOI] [PubMed] [Google Scholar]
- 28.Rosen V. BMP2 signaling in bone development and repair. Cytokine GrowthFactor Rev. 2009 Oct-Dec;20(5–6):475–80. doi: 10.1016/j.cytogfr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006 Dec;38(12):1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 30.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 31.Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007 Aug;77(8):626–31. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- 32.Katayama Y, Matsuyama Y, Yoshihara H, Sakai Y, Nakamura H, Imagama S, Ito Z, Wakao N, Kamiya M, Yukawa Y, Kanemura T, Sato K, Iwata H, Ishiguro N. Clinical and radiographic outcomes of posterolateral lumbar spine fusion in humans using recombinant human bone morphogenetic protein-2: an average five-year follow-up study. Int Orthop. 2009 Aug;33(4):1061–7. doi: 10.1007/s00264-008-0600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan Y, Tang D, Chen M, Huang J, Xie R, Jonason JH, Tan X, Hou W, Reynolds D, Hsu W, Harris SE, Puzas JE, Awad H, O’Keefe RJ, Boyce BF, Chen D. Axin2 controls bone remodeling through the beta-catenin-BMP signaling pathway in adult mice. J Cell Sci. 2009 Oct 1;122(Pt 19):3566–78. doi: 10.1242/jcs.051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Whetstone HC, Youn A, Nadesan P, Chow EC, Lin AC, Alman BA. Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J Biol Chem. 2007 Jan 5;282(1):526–33. doi: 10.1074/jbc.M602700200. [DOI] [PubMed] [Google Scholar]
- 35.Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O’Keefe RJ, Zuscik M, Chen D. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009 Jan;24(1):12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999 Aug 15;13(16):2072–86. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long F, Chung UI, Ohba S, McMahon J, Kronenberg HM, McMahon AP. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 2004 Mar;131(6):1309–18. doi: 10.1242/dev.01006. [DOI] [PubMed] [Google Scholar]
- 38.Mak KK, Kronenberg HM, Chuang PT, Mackem S, Yang Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development. 2008 Jun;135(11):1947–56. doi: 10.1242/dev.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Retting KN, Song B, Yoon BS, Lyons KM. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009 Apr;136(7):1093–104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davoli MA, Lamplugh L, Beauchemin A, Chan K, Mordier S, Mort JS, Murphy G, Docherty AJ, Leblond CP, Lee ER. Enzymes active in the areas undergoing cartilage resorption during the development of the secondary ossification center in the tibiae of rats aged 0–21 days: II. Two proteinases, gelatinase B and collagenase-3, are implicated in the lysis of collagen fibrils. Dev Dyn. 2001 Sep;222(1):71–88. doi: 10.1002/dvdy.1160. [DOI] [PubMed] [Google Scholar]
- 41.Joeng KS, Long F. The Gli2 transcriptional activator is a crucial effector for Ihh signaling in osteoblast development and cartilagevascularization. Development. 2009 Dec;136(24):4177–85. doi: 10.1242/dev.041624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colnot C, de la Fuente L, Huang S, Hu D, Lu C, St-Jacques B, Helms JA. Indian hedgehog synchronizes skeletal angiogenesis and perichondrial maturation with cartilage development. Development. 2005 Mar;132(5):1057–67. doi: 10.1242/dev.01649. [DOI] [PubMed] [Google Scholar]
- 43.Shinozaki S, Ohnishi H, Hama K, Kita H, Yamamoto H, Osawa H, Sato K, Tamada K, Mashima H, Sugano K. Indian hedgehog promotes the migration of rat activated pancreatic stellate cells by increasing membrane type-1 matrix metalloproteinase on the plasma membrane. J Cell Physiol. 2008 Jul;216(1):38–46. doi: 10.1002/jcp.21372. [DOI] [PubMed] [Google Scholar]
- 44.Ille F, Atanasoski S, Falk S, Ittner LM, Marki D, Buchmann-Moller S, Wurdak H, Suter U, Taketo MM, Sommer L. Wnt/BMP signal integration regulates the balance between proliferationand differentiation of neuroepithelial cells in the dorsal spinal cord. Dev Biol. 2007 Apr 1;304(1):394–408. doi: 10.1016/j.ydbio.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 45.Yang L, Yamasaki K, Shirakata Y, Dai X, Tokumaru S, Yahata Y, Tohyama M, Hanakawa Y, Sayama K, Hashimoto K. Bone morphogenetic protein-2 modulates Wnt and frizzled expression and enhances the canonical pathway of Wnt signaling in normal keratinocytes. J Dermatol Sci. 2006 May;42(2):111–9. doi: 10.1016/j.jdermsci.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Tian Q, He XC, Hood L, Li L. Bridging the BMP and Wnt pathways by PI3 kinase/Akt and 14-3-3zeta. Cell Cycle. 2005 Feb;4(2):215–6. [PubMed] [Google Scholar]
- 47.Villacorte M, Suzuki K, Hayashi K, de Sousa Lopes SC, Haraguchi R, Taketo MM, Nakagata N, Yamada G. Antagonistic crosstalk of Wnt/beta-catenin/Bmp signaling within the Apical Ectodermal Ridge (AER) regulates interdigit formation. Biochem Biophys Res Commun. 2010 Jan 22;391(4):1653–7. doi: 10.1016/j.bbrc.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, Mishina Y. Wnt Inhibitors Dkk1 and Sost are Downstream Targets of BMP Signaling Through the Type IA Receptor (BMPRIA) in Osteoblasts. J Bone Miner Res. 2009 Oct 29; doi: 10.1359/jbmr.090806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakou T, Onishi T, Yamamoto T, Nagamine T, Sampath T, Ten Dijke P. Localization of Smads, the TGF-beta family intracellular signaling components during endochondral ossification. J Bone Miner Res. 1999 Jul;14(7):1145–52. doi: 10.1359/jbmr.1999.14.7.1145. [DOI] [PubMed] [Google Scholar]
- 50.Ferguson CM, Schwarz EM, Reynolds PR, Puzas JE, Rosier RN, O’Keefe RJ. Smad2 and 3 mediate transforming growth factor-beta1-induced inhibition of chondrocyte maturation. Endocrinology. 2000 Dec;141(12):4728–35. doi: 10.1210/endo.141.12.7848. [DOI] [PubMed] [Google Scholar]
- 51.Hanada K, Solchaga LA, Caplan AI, Hering TM, Goldberg VM, Yoo JU, Johnstone B. BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J Cell Biochem. 2001 Mar 26;81(2):284–94. doi: 10.1002/1097-4644(20010501)81:2<284::aid-jcb1043>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi T, Lyons KM, McMahon AP, Kronenberg HM. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc Natl Acad Sci U S A. 2005 Dec 13;102(50):18023–7. doi: 10.1073/pnas.0503617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mbalaviele G, Sheikh S, Stains JP, Salazar VS, Cheng SL, Chen D, Civitelli R. Beta-catenin and BMP-2 synergize to promote osteoblast differentiation and new bone formation. J Cell Biochem. 2005 Feb 1;94(2):403–18. doi: 10.1002/jcb.20253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006 Dec;2(12):e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adams SL, Cohen AJ, Lassova L. Integration of signaling pathways regulating chondrocyte differentiation during endochondral bone formation. J Cell Physiol. 2007 Dec;213(3):635–41. doi: 10.1002/jcp.21262. [DOI] [PubMed] [Google Scholar]
- 56.Wu X, Shi W, Cao X. Multiplicity of BMP signaling in skeletal development. Ann N Y Acad Sci. 2007 Nov;1116:29–49. doi: 10.1196/annals.1402.053. [DOI] [PubMed] [Google Scholar]
- 57.Andrade AC, Nilsson O, Barnes KM, Baron J. Wnt gene expression in the post-natal growth plate: regulation with chondrocyte differentiation. Bone. 2007 May;40(5):1361–9. doi: 10.1016/j.bone.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong Y, Drissi H, Chen M, Chen D, Zuscik MJ, Schwarz EM, O’Keefe RJ. Wnt-mediated regulation of chondrocyte maturation: modulation by TGF-beta. J Cell Biochem. 2005 Aug 1;95(5):1057–68. doi: 10.1002/jcb.20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong YF, Soung do Y, Chang Y, Enomoto-Iwamoto M, Paris M, O’Keefe RJ, Schwarz EM, Drissi H. Transforming growth factor-beta and Wnt signals regulate chondrocyte differentiation through Twist1 in a stage-specific manner. Mol Endocrinol. 2007 Nov;21(11):2805–20. doi: 10.1210/me.2007-0199. [DOI] [PubMed] [Google Scholar]
- 60.Hartmann C. Skeletal development--Wnts are in control. Mol Cells. 2007 Oct 31;24(2):177–84. [PubMed] [Google Scholar]
- 61.Macsai CE, Foster BK, Xian CJ. Roles of Wnt signalling in bone growth, remodelling, skeletal disorders and fracture repair. J Cell Physiol. 2008 Jun;215(3):578–87. doi: 10.1002/jcp.21342. [DOI] [PubMed] [Google Scholar]
- 62.Shu B, Zhang M, Xie R, Wang M, Jin H, Hou W, Tang D, Harris SE, Mishina Y, O’Keefe RJ, Hilton MJ, Wang Y, Chen D. BMP2, but not BMP4, is critical for chondrocyte proliferation and maturation during endochondral bone development. Journal of Cell Science. 2011 doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) WT (Aa) and β-cat GOF (Ab) humerus at E15.5 (5x). (B) Humerus sections from E18.5 WT (Ba) and LOF (Bb) embryos with POC lengths highlighted by yellow arrows and high power magnification (20X) of growth plate area as highlighted by blue boxes.
β-galactosidase stained frozen humerus sections from a Col2CreERT2; R26R fx/+ control (A) and a Col2CreERT2; β-cateninfx(ex3)/wt; R26R fx/+ mutant animal (B). Note the high efficiency of recombination specifically in chondrocytes and the lack of perichondrial β-galactosidase staining in each of the mouse models.
(A) TRAP staining of frozen sections from the humerus from a WT control littermate (Aa) and a CreERT2;β-cateninfx(ex3)/wt E18.5 embryo (Ab). (B) The number of osteoclasts in the humeral shaft per histological section was counted and there was no difference between the two samples (n=3). Images presented were taken at 10X magnification and are from the mid-shaft trabecular bone.
(A) Alkaline phosphatase activity in osteoprogenitors (ST2 cells) co-cultured with β-CATENIN overexpressing chondrocytes (Ad-Cre) and control (Ad-GFP). (B) Overexpression of β-CATENIN in Ad-Cre infected chondrocytes is shown by Western blot with α-TUBULIN control.
(A) Proximal humerus of WT (Aa) and β-cat GOF (Ab) P4 animals; green arrow highlights area of vascular invasion and SOC formation in the GOF sample. (B) Proximal humerus of WT (Ba) and LOF (Bb) P5 animals; green arrow in highlights hypertrophic chondrocytes of the SOC in the WT animal. There is no initiated SOC in serial sections of the sample from the LOF animal as represented in (Bb).


