Summary
Accumulation of DNA damage is implicated in aging. This is supported by the fact that inherited defects in DNA repair can cause accelerated aging of tissues. However, clear-cut evidence for DNA damage accumulation in old age is lacking. Numerous studies report measurement of DNA damage in nuclear and mitochondrial DNA from tissues of young and old organisms, with variable outcomes. Variability results from genetic differences between specimens or the instability of some DNA lesions. To control these variables and test the hypothesis that elderly organisms have more oxidative DNA damage than young organisms, we measured 8,5′-cyclopurine-2′-deoxynucleosides (cPu), which are relatively stable, in tissues of young and old wild-type and congenic progeroid mice. We found that cPu accumulate spontaneously in the nuclear DNA of wild-type mice with age and to a greater extent in DNA repair-deficient progeroid mice, with a similar tissue-specific pattern (liver>kidney>brain). These data, generated under conditions where genetic and environmental variables are controlled, provide strong evidence that DNA repair mechanisms are inadequate to clear endogenous lesions over the lifespan of mammals. The similar, although exaggerated, results obtained from progeroid, DNA repair-deficient mice and old normal mice support the conclusion that DNA damage accumulates with, and likely contributes to aging.
Keywords: ageing, progeria, DNA damage, nucleotide excision repair, oxidative DNA lesion
DNA damage is implicated in contributing to the aging process. This is largely supported by the fact that inherited defects in DNA repair lead to accelerated aging of tissues (Hasty et al. 2003). For example, XFE progeroid syndrome is caused by mutations in XPF, one subunit of the XPF-ERCC1 endonuclease, required for several DNA repair pathways including nucleotide excision repair (NER) (Sijbers et al. 1996). XFE patients display premature aging of several organ systems, which is recapitulated in mice with reduced expression of XPF-ERCC1 (Ercc1−/Δ mice) (Niedernhofer et al. 2006; Gregg et al. 2011). However, it is not known which spontaneous endogenous DNA lesions drive accelerated aging when repair is attenuated and whether the same lesions accumulate in normal organisms with age.
Numerous studies have investigated the accumulation of DNA damage with age in nuclear and mitochondrial genomes from mammalian tissues with varying results (Bohr 2002; Loft et al. 2012). This variability can be attributed to the inherent difficulties in measuring endogenous oxidative DNA lesions (Collins et al. 2004). 8,5′-cyclopurine-2′-deoxynucleosides (cPu, Fig. S1) can be induced by endogenous reactive oxygen species (Jaruga & Dizdaroglu 2008), and they are relatively stable. Generation of cPu during sample preparation is greatly inhibited by O2 (Jaruga & Dizdaroglu 2008), rendering these lesions reliable biomarkers of oxidative stress and DNA damage.
cPu are substrates for NER (Brooks et al. 2000; Kuraoka et al. 2000); therefore, we predict that cPu lesions should accumulate in tissues of NER-deficient organisms. In addition, cPu strongly block DNA replication and transcription, causing replication and transcriptional mutagenesis (Kuraoka et al. 2000; Marietta & Brooks 2007; Yuan et al. 2011). Thus, upon accumulation, cPu lesions are anticipated to induce deleterious consequences to living cells thereby potentially contributing to aging.
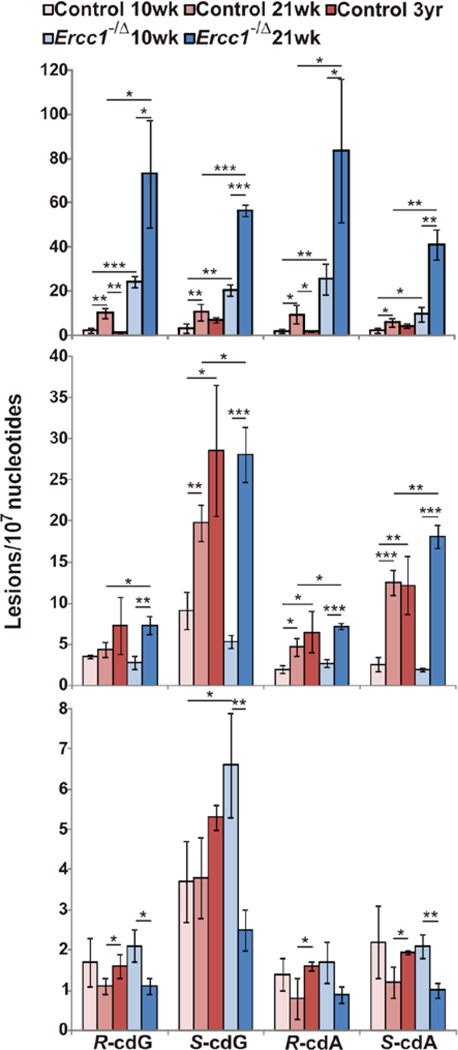
We employed LC-MS/MS/MS (Wang et al. 2011) and measured both 5′R and 5′S diastereomers of 8,5′-cyclo-2′-deoxyadenosine (cdA) and 8,5′-cyclo-2′-deoxyguanosine (cdG) (Fig. 1, Table S1, and Fig. S2) in nuclear DNA from liver, kidney and brain from congenic wild-type and Ercc1−/Δ mice. Adduct levels were measured in young adult mice (10 weeks of age), at 21 weeks of age, by which time Ercc1−/Δ mice have profound progeroid symptoms (Gregg et al. 2011), and in old wild-type mice (3 years old). In the liver, there were significantly more cPu in Ercc1−/Δ mice than normal littermates at both 10 and 21 weeks of age, providing further evidence that NER is critical for clearing cPu in vivo. In both normal and progeroid animals, there were significant increases in the levels of all four lesions with aging (at 21 weeks of age compared to 10 weeks), indicating that, even with a normal complement of DNA repair, NER is unable to completely clear cPu from the nuclear genome and the adduct levels increase over time. Surprisingly, there were significantly lower levels of the R diastereomers of cdG and cdA in the liver of old wild-type mice (3 years) compared to 21 week-old mice, possibly reflecting the fact that the liver can undergo regeneration under stress (Michalopoulos 2007).
Fig. 1. Levels of 8,5′-cyclopurine-2′-deoxynucleosides in nuclear DNA of mouse tissues.
Liver (top), kidney (middle), and brain (bottom) of Ercc1−/Δ mice and age-matched littermates were examined. ‘*’, p < 0.05; ‘**’, p < 0.01; ‘***’, p < 0.001. The p values were calculated using unpaired two-tailed t-test. The values represent the mean and standard error of results obtained from tissues of three different animals per group.
In kidney, like the liver, cPu levels increased significantly with age in both normal and Ercc1−/Δ mice (from 10 to 21 weeks of age). However, not until 21 weeks of age was there a significant difference in cPu levels between normal and ERCC1-deficient mice, suggesting that accumulation of cPu lesions begins later in the kidney than in the liver. Between the ages of 10 to 21 weeks, the levels of S-cdG increased 2–3-fold in the liver and kidney of normal mice as well as the liver from Ercc1−/Δ mice, but >5-fold in Ercc1−/Δ kidney. Similarly, the increase in S-cdA in Ercc1−/Δ kidney from 10 to 21 weeks dramatically exceeded that of the liver and kidney of normal mice, perhaps suggesting an acute aging-related degenerative process in the kidney of Ercc1−/Δ mice that causes oxidative stress. Overall, the levels of all of the cPu adducts were at least 2-fold greater in the liver than the kidney in Ercc1−/Δ mice, at any age.
In the brains of normal mice, a significant increase in cPu levels was detected in the 3 year-old mice, illustrating a time-dependent accumulation of cPu with age. In contrast, Ercc1−/Δ mice had significantly more S-cdG than normal mice at 10 weeks of age, but the levels of cPu decreased as the animals aged and were significantly lower at 21 weeks than 10 weeks. This could be because neurons harboring DNA damage are particularly vulnerable to cell death. Indeed, there is evidence of neuron attrition with aging in rodents (O'Callaghan & Miller 1991), which is accelerated in progeroid Ercc1−/Δ mice (Gregg et al. 2011). Interestingly, the 5’S diastereomers of cPu were present at higher levels than the corresponding 5′R diastereomers in kidney and brain of both normal and Ercc1−/Δ mice, likely reflecting the more efficient repair of the 5′R isomers (Kuraoka et al. 2000).
These data, generated from samples in which genetic and environmental variables were optimally controlled, establish that cPu lesions spontaneously accumulate in nuclear DNA in vivo with aging. DNA repair-deficient mice with accelerated aging generally have more lesions than normal mice. Remarkably, the levels of cPu lesions increase significantly as wild-type mice age, indicating that DNA repair mechanisms are inadequate to cope with endogenous DNA damage over a lifetime. Moreover, the pattern of cPu accumulation was different among the three tissues studied, with liver accumulating the most damage. The levels of cPu lesions are ~2 orders of magnitude lower than 8-oxoG under aerobic conditions (Chatgilialoglu et al. 2011). However, since cPu block transcription and replication, and are mutagenic, there is a strong likelihood that cPu could contribute to the degenerative aging-related changes seen in progeroid Ercc1−/Δ mice (Gregg et al. 2011) and with normal aging.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (NIH) (R01CA101864, R01ES016114, P30AG024827, P30CA047904). C.L.C. is supported by a Biomedical Research Fellowship from the Hartwell Foundation.
Footnotes
Author Contributions
J. W., C. L. C., P. D. R., L. J. N. and Y. W. designed research; J. W., C. L. C., L. J. N. and Y. W. wrote the paper; and J. W. conducted experiments.
Supporting Information
Additional supporting information may be found in the online version of this article.
Fig. S1 Structures of cdA and cdG.
Fig. S2 Representative LC-MS/MS/MS data.
Table S1 Levels of 8,5'-cyclo-2'-deoxyguanosine (cdG) and 8,5'-cyclo-2'-deoxyadenosine (cdA) in nuclear DNA of tissues of Ercc1−/Δ mice and age-matched littermates (n = 3).
Contributor Information
Jin Wang, Email: jinwang@ucr.edu.
Cheryl L. Clauson, Email: clausoncl@upmc.edu.
Paul D. Robbins, Email: probb@pitt.edu.
Laura J. Niedernhofer, Email: niedlx@upmc.edu.
Yinsheng Wang, Email: yinsheng.wang@ucr.edu.
References
- Bohr VA. Repair of oxidative DNA damage in nuclear and mitochondrial DNA, some changes with aging in mammalian cells. Free Radic. Biol. Med. 2002;32:804–812. doi: 10.1016/s0891-5849(02)00787-6. [DOI] [PubMed] [Google Scholar]
- Brooks PJ, Wise DS, Berry DA, Kosmoski JV, Smerdon MJ, Somers RL, Mackie H, Spoonde AY, Ackerman EJ, Coleman K, Tarone RE, Robbins JH. The oxidative DNA lesion 8,5'-(S)-cyclo-2'-deoxyadenosine is repaired by the nucleotide excision repair pathway and blocks gene expression in mammalian cells. J. Biol. Chem. 2000;275:22355–22362. doi: 10.1074/jbc.M002259200. [DOI] [PubMed] [Google Scholar]
- Chatgilialoglu C, Ferreri C, Terzidis MA. Purine 5',8-cyclonucleoside lesions: chemistry and biology. Chem. Soc. Rev. 2011;40:1368–1382. doi: 10.1039/c0cs00061b. [DOI] [PubMed] [Google Scholar]
- Collins AR, Cadet J, Moller L, Poulsen HE, Vina J. Are we sure we know how to measure 8-oxo-7,8-dihydroguanine in DNA from human cells? Arch. Biochem. Biophys. 2004;423:57–65. doi: 10.1016/j.abb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Gregg SQ, Robinson AR, Niedernhofer LJ. Physiological consequences of defects in ERCC1-XPF DNA repair endonuclease. DNA Repair. 2011;10:781–791. doi: 10.1016/j.dnarep.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–1359. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- Jaruga P, Dizdaroglu M. 8,5'-Cyclopurine-2'-deoxynucleosides in DNA: Mechanisms of formation, measurement, repair and biological effects. DNA Repair. 2008;7:1413–1425. doi: 10.1016/j.dnarep.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Kuraoka I, Bender C, Romieu A, Cadet J, Wood RD, Lindahl T. Removal of oxygen free-radical-induced 5',8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc. Natl. Acad. Sci. U S A. 2000;97:3832–3837. doi: 10.1073/pnas.070471597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loft S, Danielsen P, Lohr M, Jantzen K, Hemmingsen JG, Roursgaard M, Karotki DG, Moller P. Urinary excretion of 8-oxo-7,8-dihydroguanine as biomarker of oxidative damage to DNA. Arch. Biochem. Biophys. 2012;518:142–150. doi: 10.1016/j.abb.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Marietta C, Brooks PJ. Transcriptional bypass of bulky DNA lesions causes new mutant RNA transcripts in human cells. EMBO Rep. 2007;8:388–393. doi: 10.1038/sj.embor.7400932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK. Liver regeneration. J. Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. The concentration of glial fibrillary acidic protein increases with age in the mouse and rat brain. Neurobiol. Aging. 1991;12:171–174. doi: 10.1016/0197-4580(91)90057-q. [DOI] [PubMed] [Google Scholar]
- Sijbers AM, de Laat WL, Ariza RR, Biggerstaff M, Wei YF, Moggs JG, Carter KC, Shell BK, Evans E, de Jong MC, Rademakers S, de Rooij J, Jaspers NG, Hoeijmakers JH, Wood RD. Xeroderma pigmentosum group F caused by a defect in a structure-specific DNA repair endonuclease. Cell. 1996;86:811–822. doi: 10.1016/s0092-8674(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Wang J, Yuan B, Guerrero C, Bahde R, Gupta S, Wang Y. Quantification of oxidative DNA lesions in tissues of Long-Evans Cinnamon rats by capillary high-performance liquid chromatography-tandem mass spectrometry coupled with stable isotope-dilution method. Anal. Chem. 2011;83:2201–2209. doi: 10.1021/ac103099s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Wang J, Cao H, Sun R, Wang Y. High-throughput analysis of the mutagenic and cytotoxic properties of DNA lesions by next-generation sequencing. Nucleic Acids Res. 2011;39:5945–5954. doi: 10.1093/nar/gkr159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.