SUMMARY
Understanding how bone growth is regulated by hormonal and mechanical factors during early growth periods is important for optimizing the attainment of peak bone mass to prevent or postpone the occurance of fragility fractures later in life. Using genetic mouse models that are deficient in thyroid hormone (TH) (Tshr−/− and Duox2−/−), growth hormone (GH) (Ghrhrlit/lit) or both (Tshr−/− ;Ghrhrlit/lit), we demonstrate that there is an important period prior to puberty when the effects of GH are surprisingly small and TH plays a critical role in the regulation of skeletal growth. Daily administration of T3/T4 during days 5 to 14, the time when serum levels of T3 increase rapidly in mice, rescued the skeletal deficit in TH-deficient mice but not in mice lacking both TH and GH. However, treatment of double-mutant mice with both GH and T3/T4 rescued the bone density deficit. Increased body fat in the TH-deficient as well as TH/GH double mutant mice was rescued by T3/T4 treatment during days 5–14. In vitro studies in osteoblasts revealed that T3 in the presence of TH receptor (TR) α1 bound to a TH response element in intron 1 of the IGF-I gene to stimulate transcription. In vivo studies using TRα and TRβ knockout mice revealed evidence for differential regulation of IGF-I expression by the two receptors. Furthermore, blockade of IGF-I action partially inhibited the biological effects of TH, thus suggesting that both IGF-I-dependent and independent mechanisms contribute to TH effects on prepubertal bone acquisition.
Keywords: Growth Hormone; Osteoblasts; Thyroid hormone receptor alpha; Thyroid hormone receptor beta; Dual oxidase-2, Hypothyroidism; Acid labile subunit; IGF binding protein
INTRODUCTION
Childhood and adolescence is a particularly important time to maximize bone acquistion since the skeleton undergoes rapid changes owing to the processes of growth, modeling and remodeling (1,2). Peak bone mass is attained in young adulthood, remains relatively constant in early adulthood, and then gradually declines in aging men and women (3). The achievement of an optimal bone mass is recognized as a primary health goal during early growth periods since it is widely understood that the risk of fragility fractures in old age has its origin during growth (4). For example, it has been reported that 60% of the risk of osteoporosis can be explained by the amount of bone mineral acquired by early adulthood (5). Therefore, information learned from studies on the mechanisms regulating bone acquisition during postnatal growth periods is of considerable importance in developing strategies to postpone or reduce the risk of osteoporotic fractures. With regard to the potential signaling molecules that contribute to skeletal growth, the findings from a variety of conditional knockout (KO) models and clinical studies of patients with mutations in genes that regulate insulin-like growth factor (IGF)-I action have illustrated a critical role for IGF-I in bone acquisition during postnatal growth (2,5–9). In terms of the mechanisms regulating IGF-I action, it is known since the discovery of IGF-I as a sulfation factor and somatomedin, that a much of IGF-I action is regulated by growth hormone (GH)-dependent mechanisms (10,11). This conclusion is based on findings that disruption of production and/or actions of GH results in impaired skeletal growth and markedly reduced serum IGF-I levels as revealed in both animal and human clinical studies (7,12–14).
Twin and family studies have demonstrated a strong genetic component in the determination of peak bone mass (15,16). Consistent with the human data, animal studies have revealed there is considerable variation in peak bone mass between inbred strains of both mice and rats, and that these differences are largely attributable to small differences in their genetic background (15–17). Studies have shown that bone mineral density (BMD) has a relatively high heritability, which ranges from 0.5 to 0.9 in human and mouse (15–17). In order to identify the critical developmental period when the differences in bone mass accrual occur, we have compared the relative gain in various parameters at several time points from day 7 to day 56 in C57BL/6J and C3H/HeJ inbred strains of mice, and have demonstrated a 70% difference in femoral BMD (18). These studies revealed that days 7 to 14 of the prepubertal period and days 23 to 31 of the pubertal period represent critical time points for skeletal growth as well as increases in serum IGF-I levels in mice (18). In subsequent studies using mice with inactivation of the IGF-I gene, GH deficiency and deletion of the GH receptor, it was determined that there is an important critical period during pre-pubertal growth when the effects of GH are small and IGF-I remains an important regulator (7). Accordingly, poor growth responses to GH treatment during the prepubertal growth period are frequently seen in clinical studies using recombinant human GH therapy in children with certain growth disorders (19,20). While these and other data have provided irrefutable evidence for the involvement of other factors besides GH in prepubertal bone acquisition, GH-independent mechanisms that regulate IGF-I expression and bone acquisition remain to be elucidated.
In this study, we evaluated the role of thyroid hormone (TH) in regulating prepubertal bone acquisition, since childhood hypothyroidism causes growth failure and since targeted disruption of TH receptor in mice influences skeletal growth (21–24). Furthermore, studies by Bassett et al. (25) have shown using 3,5,3’-L-triiodothyronine (T3) receptor alpha (TRα) knock-in mutant mice that there is a discrete period of time during post-natal growth in which TRα is essential for the actions of TH. In addition to the skeletal phenotype, we also evaluated the consequence of TH deficiency on the fat phenotype because of growing awareness of a bone-fat connection (26) and because TH effects on fat metabolism is poorly understood despite the well known effect of TH on the metabolic rate and heat production (27). Our data using mutant mouse models with TH and/or GH deficiency provide direct evidence that TH deficiency produces a greater skeletal deficit than GH deficiency during the prepubertal growth period. While T3/T4 treatment rescues the skeletal deficit in TH deficient mice, administration of both GH and T3 are required during the prepubertal growth period to rescue the skeletal deficit in the GH/TH deficient double-mutant mice. By contrast, increased body fat in the TH-deficient as well as TH/GH double mutant mice is rescued by T3/T4 treatment.
MATERIALS AND METHODS
Chemicals, recombinant proteins and biological reagents
T3 and T4 were purchased from Sigma (Saint Louis, MO). Recombinant human insulin-like growth factor binding protein-4 (IGFBP4) was purified from E. Coli as previously reported (28). Plasmid pTAL-luc-IGF-I was generated by inserting a DNA fragment from intron 1 of IGF-I gene (2113 to 2152 downstream of the transcription start site) containing a putative TH response element (TRE) in front of the TK minimal promoter of pTAL (Clontech, Mountain View, CA). Small interference RNA (siRNA) specifically against both TRα1 and TRα2, and non-specific control siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Adenoviruses expressing TRα1, TRα2 or β-Gal were generated as described (29).
Mouse models
Ghrhrlit/+ heterozygous mice with a point mutation in GH releasing hormone receptor and Tshr−/+ heterozygous mice with a point mutation in the coding region of the thyroid stimulating hormone receptor gene were purchased from the Jackson laboratory (Bar Harbor, Maine). Dual oxidase-2 mutant mice (Duox2−/−) with hypothyroidisim due to a missense mutation in Duox2 were maintained at Jackson Laboratory as described (30). Duox2 is an enzyme that is involved in the biosynthesis of THs by generating H2O2 required for organification of iodine. Homozygous Duox2 mutant mice are dwarf, hypothyroid (serum T4 about one-tenth of the controls) and hearing impaired (30). Colonies of TRα0/0 mutant mice harboring a targeted deletion of all transcripts from the TRα locus (31) and TRβ mutant mice harboring targeted deletion of both TRβ1 and TRβ2 mRNAs (32) were maintained at Imperial College, London, U.K. Mice with a conditional IGF-I KO in cells of osteoblastic lineage were reported previously (6). DNA extracted from tail snips were used for PCR-based genotyping. Newborn C57BL/6J mice with hypothyroidism were generated by treating pregnant mice with 0.05% methimazole in drinking water, as described previously (33). Both male and female mice in approximately equal number were used and the data from both genders were pooled for analyses. All mice were housed at the Jerry L. Pettis Memorial VA Medical Center Veterinary Medical Unit (Loma Linda, CA), Jackson Laboratory (Bar Harbor, ME) or Imperial College (London, U.K.) under standard approved laboratory conditions with controlled illumination (14 hours light, 10 hours dark), temperature (22°C) and unrestricted food and water. All of the procedures were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of the Jerry L Pettis Memorial VA Medical Center, Jackson Laboratory or Imperial College.
Evaluation of bone phenotypes
Total bone mineral content (BMC) and BMD were measured by dual-energy X-ray absorptiometry with a PIXImus instrument (LunarCorp., Madison, WI) as described previously (7,12). The volumetric BMD and geometric parameters at the mid-diaphysis of the femur isolated from 3- and 21-day age of mice were determined by Peripheral Quantitative Computed Tomography (pQCT; Norland Stratec XCT 960M, Stratec Medzizintechnik GmbH, Madison, WI) as reported (7,12). Trabecular bone microarchitecture of the femur isolated from 21 day old mice were assessed by µ-CT (VIVA CT40, Scanco). The femurs were scanned by X-ray (55–70 kVp volts). The voxel size was 10.5 microns. Reconstruction analysis was performed with SCANCO software (SCANCO Medical, Bruttisellen, Switzerland). A fixed section of 1.8 mm starting at 0.36 mm proximal to the growth plate was analyzed for trabecular measurements using SCANCO software.
Serum measurements
IGF-I was measured by RIA after removing IGF binding proteins using an acid gel filtration protocol involving a Bio Spin method (34). T3 levels were measured by a solid phase RIA using a Coat-A-Count Total T3 kit from Diagnostic Products Corporation (Los Angeles, CA).
Cell culture
Primary osteoblasts were isolated from the calvariae of 2 to 4 day old C57BL/6J mice using a modified sequential digestion protocol described previously (35,36). Primary calvarial osteoblasts and MC3T3-E1 cells were plated at 1.5 × 104/cm2 (1.5 × 105/well) in 6-well culture plates in α-minimal essential medium (α-MEM) containing 10% FBS, penicillin (100 units/ml), and streptomycin (100 µg/ml). Bone marrow stromal cells were isolated from 4-week old mice as described (37). The cells were cultured to 80–90% confluence prior to use in experiments. Twenty-four hours prior to treatment, cells were incubated in the presence of serum-free αMEM medium containing 0.1% bovine serum albumin (BSA) and antibiotics. THs (T3 and T4) stocks were prepared in 5mM NaOH and diluted in αMEM prior to adding to cell cultures.
Transfection and transduction in osteoblasts
For knockdown studies, MC3T3-E1 cells were transiently transfected by electroporation as reported previously (36,38). Briefly, MC3T3-E1 cells (1.5 × 106) were resuspended in 100 µl of fibroblast nucleofector buffer (Amaxa, Gaithersburg, MD) containing 1.25 µg TRα1/2 siRNA or 1.25 µg negative control siRNA (Santa Cruz). The cells were then transferred into a 2-mm gap width electroporation cuvette, and electroporated at 165 V for 15 milliseconds, using a Gene Pulser (Bio-Rad, Hercules, CA). After electroporation, the cells were plated at high density (150,000 cells/cm2) in prewarmed, αMEM growth medium in a 6-well plate, and cultured in a humidified 37 °C incubator with 5% CO2, 95% air until further treatment. For overexpression studies, MC3T3-E1 cells were transduced with adenoviral particles expressing TRα1, TRα2 or β-Gal control as described (29).
RNA extraction and real-time quantitative polymerase chain reaction
RNA was extracted from MC3T3-E1 cells, primary calvarial osteoblasts, primary bone marrow stromal (BMS) cells or the mouse tissues as described previously (36,39). An aliquot of RNA (2 µg) was reverse-transcribed into cDNA in 20 µl volume of reaction by oligo(dT)12–18 primer. Real-time PCR contained 0.5 µl template cDNA, 1× SYBR GREEN master mix (Qiagen), and 100 nM of specific forward and reverse primers in a 25 µl reaction volume. Primers used for real-time PCR are listed in Table 1.
Table 1.
Primer sequences for RT real-time PCR
| GENE | FORWARD | REVERSE |
|---|---|---|
| ALS | 5’- GCTCTGTGGCTTGACGGAAAC | 5’- CCAGGAGGTTGTTGCCCAAA |
| DLX5 | 5`- AGAAGAGTCCCAAGCATCCGA | 5`- GCCATAAGAAGCAGAGGTAGG |
| IGFBP-3 | 5’- CCAGAACTTCTCCTCCGAGTCTAAG | 5`- CTCAGCACATTGAGGAACTTCAGAT |
| IGFBP-5 | 5`- ATACAACCCAGAACGCCAGCT | 5`- ACCTGGGCTATGCACTTGATG |
| IGF-1 | 5’- GCTCTTCAGTTCGTGTGTGGAC | 5’- CATCTCCAGTCTCCTCAGATC |
| OSTEOCALCIN | 5`- CTCTCTCTGCTCACTCTGCT | 5`- TTTGTAGGCGGTCTTCAAGC |
| OSTERIX | 5`- AGAGGTTCACTCGCTCTGACGA | 5`- TTGCTCAAGTGGTCGCTTCTG |
| PPIA | 5’- CCATGGCAAATGCTGGACCA | 5’- TCCTGGACCCAAAACGCTCC |
| RUNX2 | 5`- AAAGCCAGAGTGGACCCTTCCA | 5`- ATAGCGTGCTGCCATTCGAGGT |
| TRAP | 5`- CACTCAGCTGTCCTGGCTCAA | 5`- CTGCAGGTTGTGGTCATGTCC |
| TRα1 | 5’- CTGCCTTGCGAAGACCAGATC | 5’- CAGCCTGCAGCAGAGCCACTTCCGT |
| TRα2 | 5’- AATGGTGGCTTGGGTGTGGT | 5’- CCTGAACAACATGCATTCCGA |
| TR β* | 5’- GGTGCTGGATGACAGCAAGA | 5’- GCATTCACGATGGGTGCTTGT |
| TRΔα1 | 5’- CTCTGTGATCCTGCTGTTCCACAG | 5’- TTTCATGTGGAGGAAGCGGC |
| GC TRΔα2 | 5’- CTCTGTGATCCTGCTGTTCCACAG | 5’- CCTGAACAACATGCATTCCGA |
Recognizes both TRβ1 and TRβ2
Luciferase reporter and ALP activity assays
The pTAL-luc-IGF-I and control reporter constructs were transfected into MC3T3-E1 cells with lipofectamine (Invitrogen). After a 48-hour treatment with TH (10 ng/ml T3) or vehicle, the transfected cells were lysed for luciferase assay as described (36). Alkaline phosphatase (ALP) activity in MC3T3-E1 cells treated with TH or vehicle in serum-free medium for 3 days was measured as previously reported (38).
Electrophoretic mobility shift assay
Nuclear extracts from MC3T3-E1 cells were prepared as described previously (36,39). Double-stranded oligonucleotides were labeled as described previously (36). Nuclear extracts (4 µg) from MC3T3-E1 cells were incubated in a binding buffer containing 10 mM Tris-HCl (pH 7. 9), 50 mM NaCl, 3 mM DTT, 10 % glycerol, 0.05% NP-40, 0.1 mM ZnCl2, 50 µg/ml poly dI-dC, and 20 fmole of labeled DNA probe. Excess unlabeled DNA competitors were added into the reaction 5 minutes before addition of the radiolabeled probe. The reactions were incubated at room temperature for 20 minutes, and then antibody against TRα or control IgG was added to the reactions. The reactions were incubated at room temperature for another 20 minutes, and analyzed on 5% non-denaturing polyacrylamide gels in 1 × TBE buffer (50 mM Tris-borate-EDTA, pH 8.0). Gels were dried and visualized by autoradiography.
Mouse Metatarsal Bone Culture
Metatarsal bones were surgically isolated from 2-day old C57BL/6 mice and were incubated in serum-free αMEM containing 0.5% BSA, 50 µg/ml ascorbic acid, 1 mM β-glycerol phosphate, 100 units/ml penicillin and 100 µg/ml streptomycin at 37 °C in humidified air with 5% CO2 as reported (40). T3 (10 ng/ml) or the same volume of vehicle control was added to medium 1 day later, and metatarsals were cultured for 10 days. Imaging was performed at day 10 under a microscope attached to a Nikon camera. The length of metatarsals was measured using a computer with Amira software (Mercury Computer Systems, Inc., Chelmsford, MA).
Statistical Analysis
Data were analyzed by Student s t-test or ANOVA as appropriate. One- and two-way ANOVA tests were performed using STATISTICA software (Statsoft, Tulsa, OK).
RESULTS
TH is an important regulator of IGF-I expression and bone acquisition during the prepubertal growth period
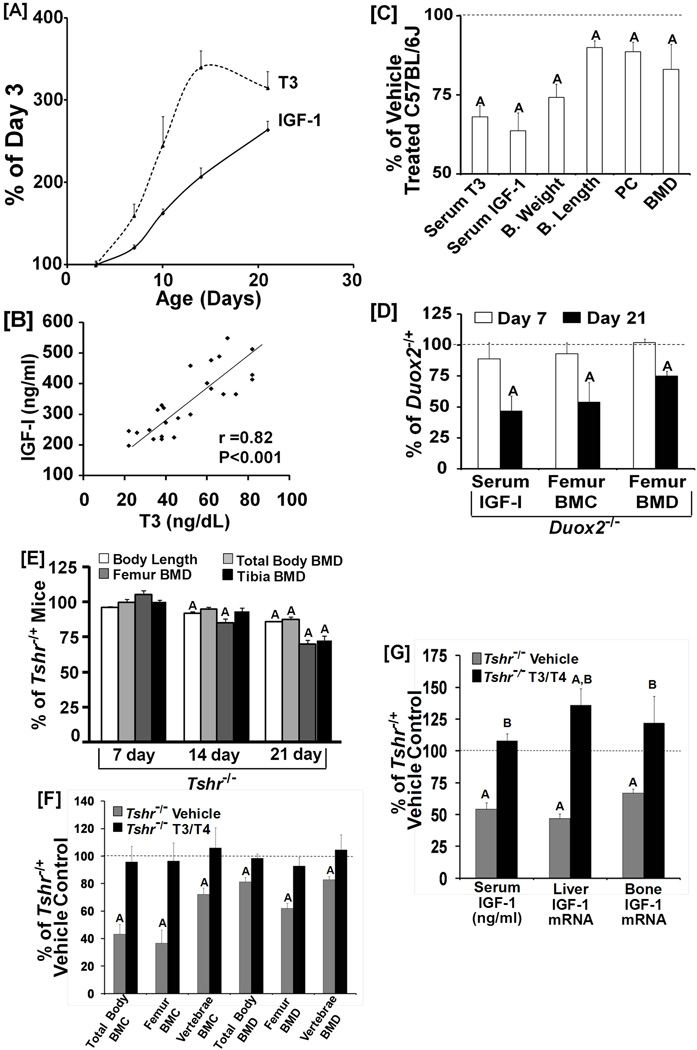
To determine if TH is a major regulator of IGF-I production during the prepubetal growth period, we first measured the postnatal changes in serum levels of total T3 and IGF-I in C57BL/6J mice. As seen in Figure 1A, serum levels of IGF-I increased almost three-fold in mice during the prepubertal growth period (i.e. first three weeks after birth). This increase in IGF-I was preceded by changes in serum T3 levels, which increased two-fold between days 7 and 14. The serum levels of T3 correlated with serum IGF-I levels (r = 0.82; Figure 1B).
Figure 1. TH is essential for the prepubertal increase in serum IGF-I level and bone mass.
[A]: Postnatal changes in serum levels of total T3 and IGF-I in C57BL/6J mice. Values are percentage of day 3 and are mean ± SEM (n = 7). IGF-I and T3 levels at day 3 were 88 ng/ml and 20 ng/dl, respectively. A = P < 0.05 as compared to day 3. [B]: Correlation between serum levels of total T3 and IGF-I during prepubertal growth in C57BL/6J mice. [C]: Serum and skeletal parameters in pups from methimazole treated experimental mice at day 14 of age. Values are percentage of control mice and are mean ± SEM (N = 7 to 16). A = P < 0.05 vs. control. B = Body; PC = periosteal circumference. [D]: Serum levels of IGF-I, femur BMC and femur BMD in Duox2 mutant and control mice at 7 and 21 days of age. Values are % of day 7 and 21 control mice (Mean ± SEM, n = 5 to12). A = P < 0.01 vs. control mice. [E]: Body length and BMD in TH-deficient Tshr−/− and Tshr−/+ control mice at 7, 14 and 21 days of age. Values are percentage of day 7 of control mice (Mean ± SEM, n = 8). A = P<0.01 vs corresponding control mice. [F-G]: Effects of T3/T4 treatment (days 5–14) on skeletal (F) and IGF-I (G) phenotype in TH-deficient Tshr−/− mice. At day 21, mice were euthanized and used for end point analyses. Values are % of H/+ vehicle (5 mM NaOH) treated control mice (Mean ± SEM, n = 8. A = P < 0.01 vs. control mice, B = P < 0.01 vs. corresponding vehicle treated mice.
To determine a cause and effect relationship between changes in serum T3 and IGF-I levels, hypothyroidism was induced in newborn mice by treating pregnant mice with 0.05% methimazole in drinking water. The serum level of total T3 in pups from mothers treated with methimazole was significantly reduced by 32% at 14 days of age compared to untreated control mice (Figure 1C). Consistent with a role for TH in regulating IGF-I production, serum levels of IGF-I were reduced by 36% in the TH-deficient mice compared to control mice, and correlated with serum levels of T3 (r = 0.76, p < 0.05). Body weight and length were reduced by 25% and 10%, respectively, in TH-deficient mice compared to control mice. Furthermore, femur periosteal circumference (PC) and BMD were reduced by 12% and 20%, respectively. IGF-I mRNA levels, determined by real-time RT-PCR, was reduced by 33% (n = 7, P < 0.01) in the femurs in the hypothyroid mice as compared to control mice.
To obtain further evidence that the increase in IGF-I production during prepubertal growth is dependent on TH, we used two additional mouse models, Duox2−/− and Tshr−/− which exhibit hypothyroidism (30,41). A missense mutation in the Duox2 gene results in a 90% reduction in serum T4 (30). Serum levels of IGF-I, femur BMC and BMD were normal in Duox2−/− at 7 days of age (Figure 1D). However, at day 21, IGF-I was reduced by 53% in Duox2−/− mice. Femur BMC and BMD were decreased by 46% and 25%, respectively, in Duox2−/− mice at day 21. A similar phenotype was identified in Tshr−/− mice (Figure 1E).
If the reductions in serum IGF-I and bone growth were a direct consequence of a lack of increase in TH production during the prepubertal growth period, it should be possible to rescue these deficiencies by giving TH during the time when TH levels increase. Thus, Tshr−/− mice were treated with a combination of 1 µg T3 and 10 µg of T4 per day (42) from days 5 to 14 after birth or with the same volume of 5 mM NaOH vehicle. Tshr−/+ heterozygous mice that are euthyroid (43) were treated with NaOH vehicle and used as controls. As expected, Tshr−/− mice treated with vehicle exhibited significant reductions in both BMC and BMD of total body, femur and vertebra (Figure 1F). T3/T4 treatment rescued these deficits in bone acquisition (Figure 1F). In vehicle treated Tshr−/− mice, the serum IGF-I level was reduced by 50% and associated with reduced IGF-I expression in the liver and bone (Figure 1G). TH treatment during prepubertal growth increased IGF-I expression in both liver and bone and normalized the serum IGF-I level.
TH regulates bone and fat phenotypes independent of GH during the prepubetal growth period
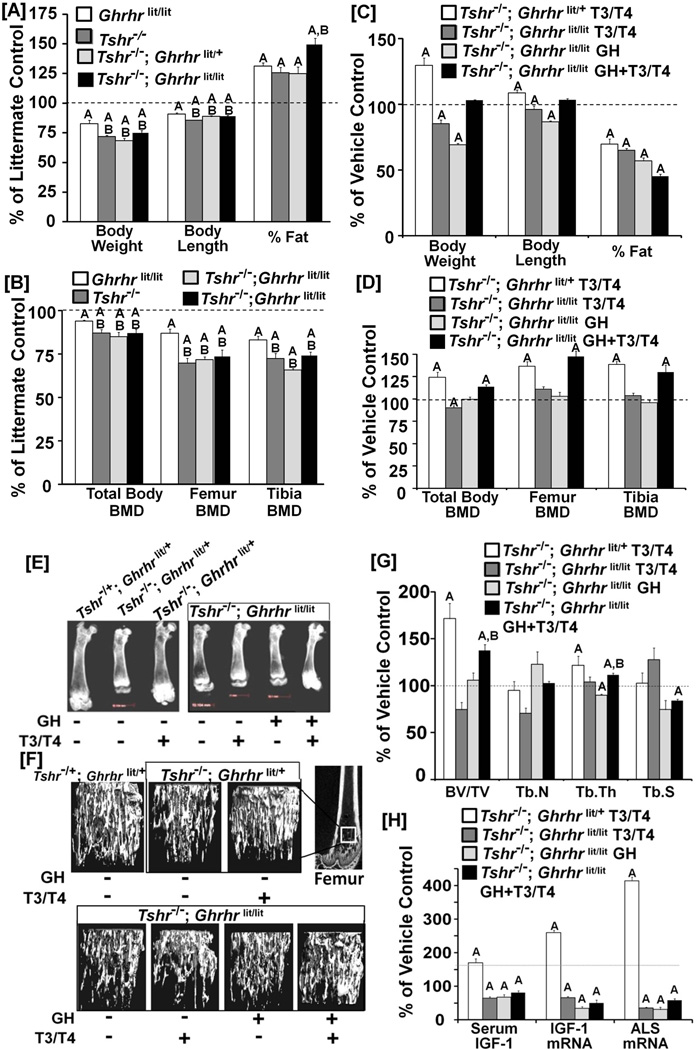
If TH is a major regulator of the prepubertal rise in IGF-I expression and bone acquisition, we then should expect a greater deficit in bone mass in TH deficient mice compared to GH deficient mice. We, therefore, examined the skeletal phenotypes of mice deficient in GH, TH or both. Figure 2A shows that deficits in body weight and length were greater in TH deficient mice compared to GH deficient mice. TH or GH deficiency increased the percentage of fat which was further increased by the combined deficiency of GH and TH. Although increased body fat in the hypothyroid animals is anticipated based on the well established effects of TH to accelerate resting energy expenditure, published data from individuals transitioning from hypothyroid to hyperthyroidism and vice versa, as well as from hypothyroid rats placed on a high fat diet failed to establish such a connection (27). The deficits in total body, femur and tibia BMD were significantly greater in the TH deficient mice compared to GH deficient mice (Figure 2B). Surprisingly, Tshr−/−; Ghrhrlit/lit double-mutant mice that are deficient in both TH and GH did not exhibit a greater BMD deficit than the mice with TH deficiency alone at day 21. We next addressed the question of whether the skeletal deficit in double-mutant mice can be rescued by TH and/or GH treatment between days 5 to14. Treatment with T3/T4 or GH alone for 10 days significantly decreased body weight as well as length in the double mutant mice (Figure 2C). Combined treatment with GH and T3/T4 did not significantly influence either the body weight or length in double mutant mice. However, treatment of TH deficient Tshr−/−; Ghrhrlit/+ mice with T3/T4 increased both body weight and length. TH treatment rescued the body fat phenotype in the TH deficient mice, and treatment of Tshr−/−; Ghrhrlit/lit mice with either TH or GH alone, or both also rescued the body fat phenotype. Surprisingly, neither TH nor GH treatment rescued skeletal deficits in the double-mutant mice (Figures 2D and E). TH treatment of double mutant mice decreased total body BMD but had no effect on femur or tibia BMD. GH treatment alone did not influence total body, femur or tibia BMD in the double mutant mice either. However, combined GH and TH treatment during days 5–14 increased total body, femur and tibia BMD in double-mutant mice. In addition, TH administration alone rescued deficits in total body, femur and tibia BMD in TH deficient Tshr−/−; Ghrhrlit/+ mice.
Figure 2. TH regulates bone and fat phenotypes independent of GH during the prepubetal growth period.
[A-B]: Body weight, length and percentage of fat (A) and total body, femur and tibia BMD (B) in Ghrhrlit/lit, Tshr−/− and Tshr−/−; Ghrhrlit/+, or Tshr−/−; Ghrhrlit/lit mice. Values are percentage of littermate control (Ghrhrlit/+ ; Tshr−/+ ). A = P < 0.01 vs. control littermate mice (n = 10 to 16/per group). A = P<0.05 vs WT littermate control mice. B = <0.05 vs Ghrhrlit/lit mice. [C-D]: Treatment effects of T3/T4, GH and GH + T3/T4 on body weight, length and percentage of fat (C) and total body, femur and tibia BMD (D) in Tshr−/−; Ghrhrlit/lit mice. Tshr−/−; Ghrhrlit/+ mice treated with T3/T4 were used as positive control. Values are percentage of vehicle treated mice of the same genotype. A = P < 0.01 vs. vehicle treated mice of the same genotype (n = 7 to10 per group). Tshr−/−; Ghrhrlit/lit and Tshr−/−; Ghrhrlit/+ mice were treated with T3/T4 or vehicle from day 5 to day 14 after birth. For GH treatment, 4mg/kg body weight dose of GH was equally divided into two doses and administered once in the morning and once in the evening daily. [E]: Representative X-ray images of the femurs isolated from the mice in C. [F] Representative µ-CT images of distal metaphysis of femurs from the mice in C. [G] Effect of TH and/or GH treatment on bone volume (BV)/total volume (TV), trabecular number (Tb.N), trabecular thickness (Tb.Th) and trabecular spacing (Tb.S) of distal metaphysic of femur in mice in C, measured by µ-CT. A = P < 0.01 vs vehicle treated control mice of the same genotype, B = P < 0.01 vs. GH treated Tshr−/−; Ghrhrlit/lit mice. [H]: Effects of TH and/or GH on serum level of IGF-I, IGF-I mRNA and ALS mRNA in the livers isolated from the mice in C. A = P < 0.01 vs. vehicle treated mice of the same genotype (n = 7 to 10 per group).
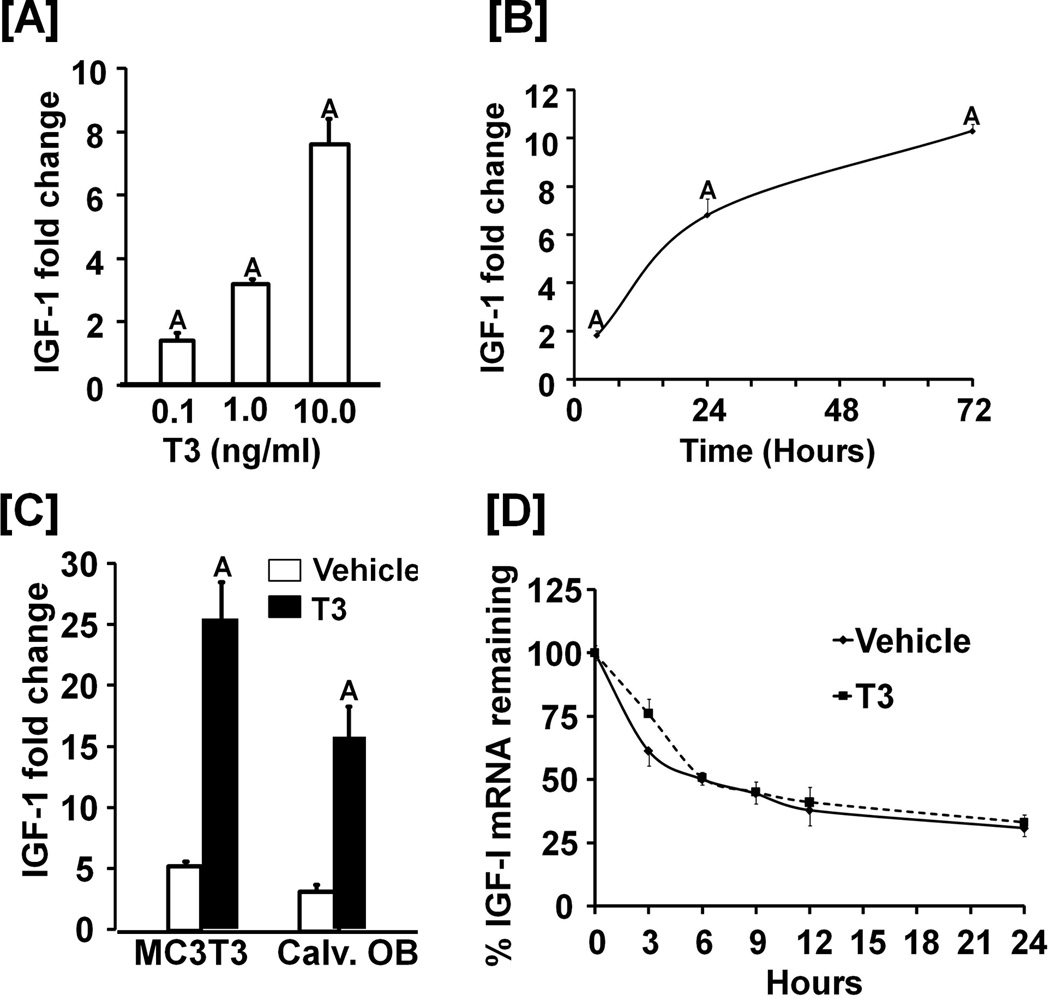
Figure 3. T3 stimulates IGF-1 expression in osteoblasts.
[A-B]: Dose (A) and time (B) –dependent effects of T3 on IGF-I expression in MC3T3-E1 cells. For the dose response experiment, cells were treated with 0.1–10 ng/ml of T3 for 24 hours. For time course studies, cells were treated with 10 ng/ml of T3 for 24 hours. Values are fold-change over the expression level of vehicle treated cells and expressed as the mean ± SEM (n = 3). A = P < 0.01 vs. vehicle control. [C]: T3 stimulates IGF-I protein expression. MC3T3-E1 cells or primary osteoblasts (Calv. OB) were treated with 10 ng/ml of T3 for 72 hours. Conditioned medium was collected for measurement of secreted IGF-I by RIA. Values are the mean ± SEM (n = 3). A = P < 0.01 vs. vehicle control. [D] Effect of T3 treatment on IGF-I mRNA stability. MC3T3-E1 cells were treated with 10 ng/ml T3 or vehicle for 24 hrs prior to the addition of actinomycin (10 µg/ml). Cells were harvested for RNA extraction and real time RT-PCR at the time points indicated. Data are expressed as mean ± SEM from 3 replicates.
Micro-CT analysis demonstrated a decrease in trabecular bone volume in TH deficient mice, which was rescued by TH replacement (Figures 2F and G). Neither TH nor GH treatment alone was sufficient to rescue the decreased trabecular bone volume in Tshr−/−; Ghrhrlit/lit double mutant mice. However, treatment of double-mutant mice with both GH and TH restored trabecular bone volume. TH replacement increased trabecular thickness in TH deficient mice while the combined treatment of TH and GH increased trabecular thickness and decreased trabecular separation in Tshr−/−; Ghrhrlit/lit double mutant mice.
Consistent with the bone phenotypic changes, TH treatment increased serum IGF-I levels in the TH deficient mice which was accompanied by increases in expression of both IGF-I and acid-labile subunit (ALS) in the liver (Figure 2H). Serum IGF-I level was decreased by 84% (P<0.001) in Tshr−/−; Ghrhrlit/lit mice compared to Tshr−/+; Ghrhrlit/+ control mice. Treatment of double mutant mice with T3/T4, GH or T3/T4 + GH resulted in a 20–40% decrease in serum IGF-I compared to vehicle treatment. Accordingly, IGF-I and ALS mRNA levels were reduced in the double mutant mice treated with T3/T4, GH or T3/T4 + GH compared to vehicle treatment.
TH directly regulates IGF-I transcription predominantly via TRα in osteoblasts
To determine whether TH regulates IGF-I expression directly, we treated MC3T3-E1 cells in serum-free conditions with T3 and demonstrated a dose- and time-dependent effect of T3 on IGF-I mRNA levels (Figures 3A and 3B). IGF-I expression increased by 8-fold after 24 hrs of treatment with 10 ng/ml of T3. IGF-I protein in serum-free conditioned medium was increased by 5-fold in both MC3T3-E1 and primary osteoblasts after T3 treatment (Figure 3C). Furthermore, the half-life of IGF-I mRNA was similar between T3 and vehicle control (Figure 3D).
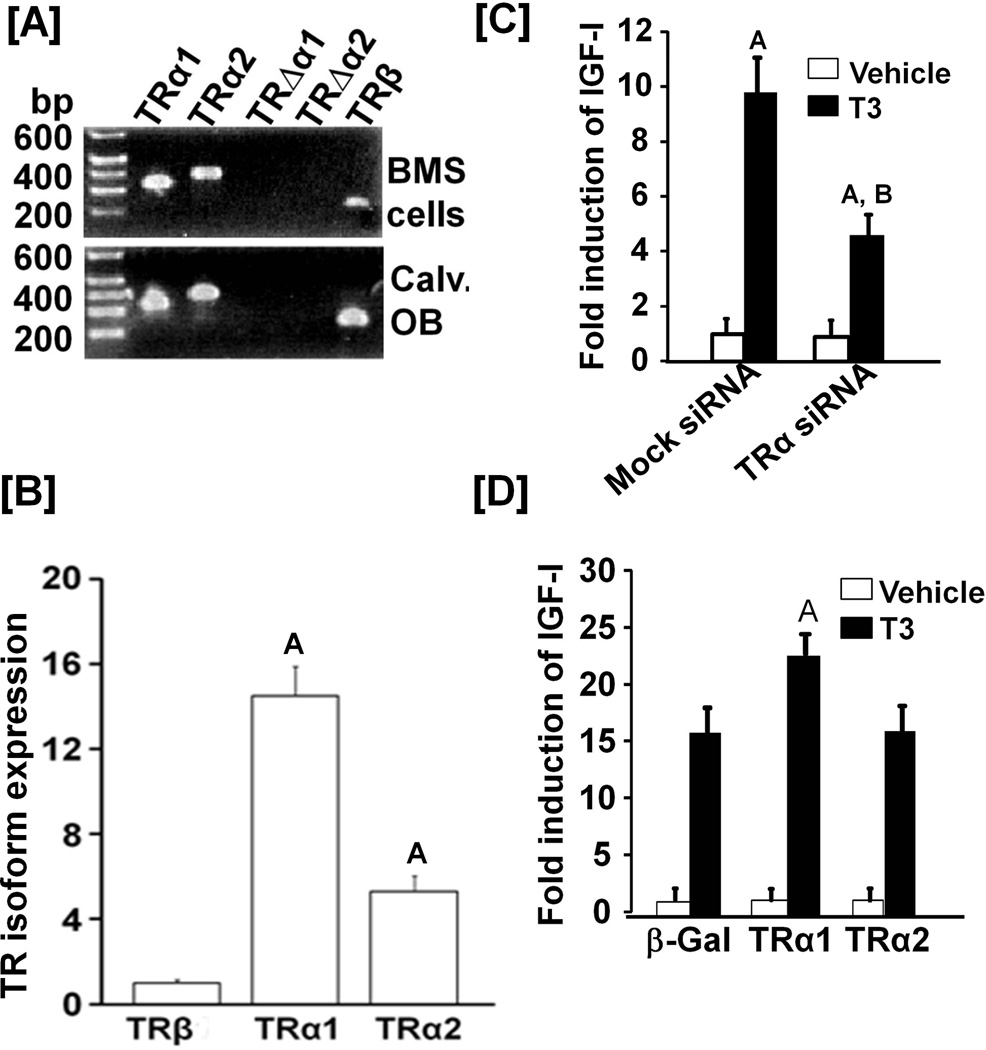
To determine which TR isoform is predominantly expressed in primary BMS cells, calvarial osteoblasts and MC3T3-E1 cells, transcript levels of TRα1, TRα2, TRΔα1, TRΔα2 and TRβ1 were determined by RT-PCR. TRα1, TRα2 and TRβ1 were expressed in all 3 cell types but expression of TRΔα1, TRΔα2 and TRβ2 mRNAs was undetectable (Figures 4A & B). TRα1 expression was 32-fold and 6-fold greater than TRβ1 and TRα2, respectively in MC3T3-E1 cells. To evaluate the role of TRα in mediating TH effects on IGF-I expression, we transfected MC3T3-E1 cells with a siRNA that targets both TRα1 and TRα2 or control siRNA. MC3T3 cells transfected with TRα siRNA led to a 70% and 90% reduction in expression of TRα1 and TRα2, respectively (data not shown). By contrast, expression of TRβ was unaffected in TRα siRNA treated cells (1.05 ± 0.2, n = 3, P = 0.78). TRα siRNA treatment led to a greater than 50% inhibition of the TH-induced increase in IGF-I expression in MC3T3-E1 osteoblasts (Figure 4C). Overexpression of TRα1 but not TRα2 resulted in upregulation of IGF-I expression by T3 stimulation (Figure 4D). Osteocalcin mRNA expression was increased in TRα1 overexpressed cells as compared to the control cells that expressed β-gal (54.6 ± 3.4 vs 40.2 ± 3.7 fold, P < 0.05). There was no significant change in osteoccalcin expression in the MC3T3-E1 cells overexpressing TRα2 (42.1 ±5.2 vs 40.2 ± 3.7 fold, P = 0.64). While neither TRα1 nor TRα2 overexpression decreased basal IGF-I expression, TRα2 overexpression resulted in a 40% decrease (P < 0.01) in basal osteocalcin expression.
Figure 4. TRα1 is predominantly expressed in bone cells and mediates T3 induction of IGF-I expression.
[A]: Expression levels of various isoforms of TH receptors in osteoblasts. RNA extracted from bone marrow stromal (BMS) cells and calvarial osteoblasts (Calv. OB) were reverse transcribed for RT-PCR. PCR products were visualized on an agarose gel. [B]: Quantitative analyses of TH receptor (TR) expression in primary osteoblasts by real time RT-PCR. Values are expressed as percentage of TRβ1 and mean ± SEM (n = 3). A = P < 0.01 vs. TRα1. [C]: Effect of knockdown of TRα expression by siRNA on the TH-induced increase in IGF-I expression. Twenty-four hours after transfection of MC3T3-E1 cells with siRNA specific to TRα1/2 or control siRNA, the cells were treated with 10 ng/ml T3 or vehicle for another 24 hrs prior to RNA extraction and real-time PCR. Values are the mean ± SEM (n = 3). A = P < 0.01 vs. vehicle; B = P<0.01 vs corresponding control siRNA treatment. [D]: Effect of overexpression of TRα1, TRα2 and β-gal on the TH-induced increase in IGF-I expression. Twenty-four hours after transduction of MC3T3-E1 cells with adenoviral particles expressing β-gal, TRα1 or TRα2, cells were treated with T3 (10 ng/ml) or vehicle. After 24 hrs of treatment, RNA was extracted for real time RT-PCR. Values are the mean ± SEM (n = 3).
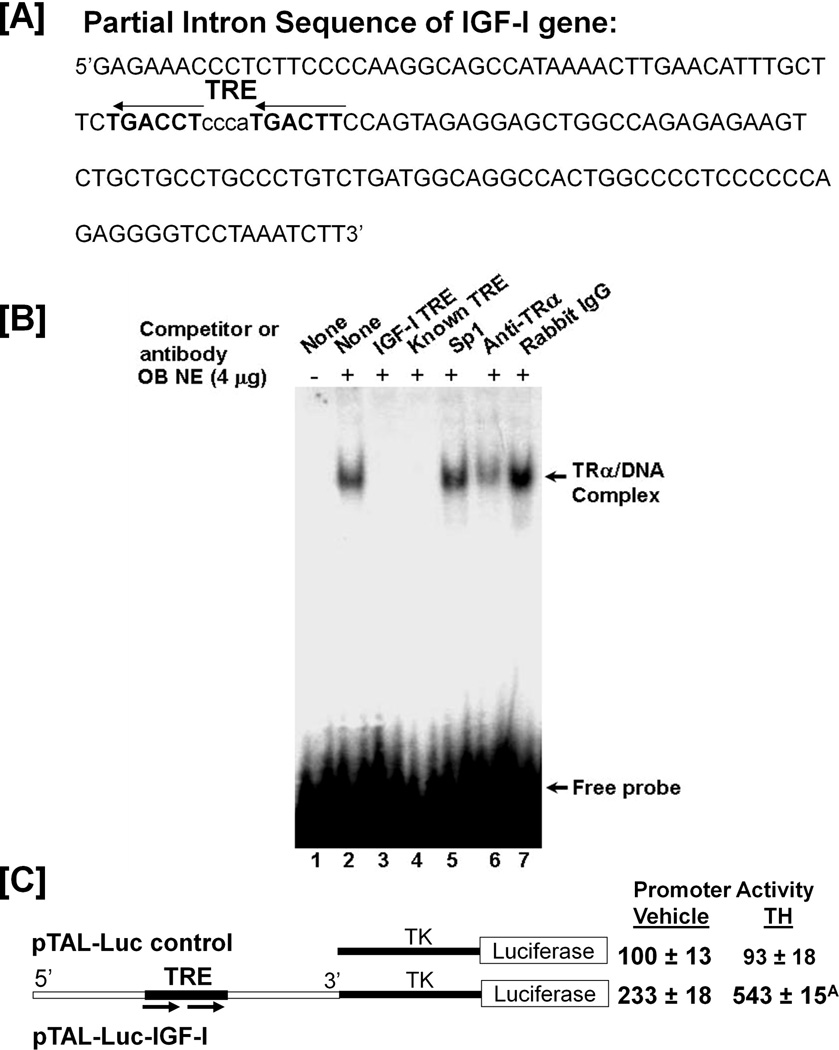
Because of the rapid effect of T3 on IGF-I expression, we predicted a direct effect of TH on IGF-I gene transcription. We identified a putative TRE in intron 1 of the mouse IGF-I gene located between nucleotides 2113 to 2152 downstream of the transcription start site (Figure 5A). To determine whether TRα present in mouse osteoblasts binds to this putative TRE, EMSA was carried out. As shown in figure 5B, nuclear extracts from MC3T3-E1 cells bound specifically to the putative TRE. The formation of DNA-protein complex was sequence specific since the presence of a 200-fold excess of unlabeled IGF-I TRE or a consensus TRE (44), but not an unlabeled SP1 binding site was able to compete for the binding of the nuclear extract to the labeled IGF-I TRE. Furthermore, the protein-DNA complex was disrupted by a TRα antibody but not by control IgG (Figures 5B, lane 6 & 7). To determine whether the IGF-1 TRE mediates TH effects on IGF-I transcription, a 155 bp IGF-I intron 1 fragment containing the putative TRE was inserted upstream of the TK minimal promoter in pTAL-Luc to generate a pTAL-Luc-IGF-I reporter construct. MC3T3-E1 cells were transiently transfected with reporter plasmid, and treated with 10 ng/ml T3 or vehicle for 48 hours prior to luciferase assay. As shown in 5C, T3 treatment increased luciferase activity by 2.3-fold.
Figure 5. T3 directly regulates IGF-I transcription.
[A]: DNA sequence of intron 1 of the mouse IGF-I gene contains putative TRE. [B]: TRα binds to the TRE in the mouse IGF-I gene. EMSA was conducted using 4 µg of nuclear extract (NE) with a 32P-labeled TRE probe. The sequences of the oligonucleotides are as follows: IGF-I intron TRE: 5’TACTGGAAGTCATTGGGAGGTCAGA; Consensus TRE: 5’AGCTTCAGGTCACAGGAGGTCAGA; Sp1: 5’ATTCGATCGGGGCGGGGCGAGC. [C]: TH stimulates luciferase activity of pTAL-IGF-I reporter. A portion of the IGF-I intron containing TRE (top panel) was inserted in front of TK minimal promoter of the pTAL-Luc vector to generate the pTAL-IGF-I reporter. MC3T3-E1 cells were transfected with the reporter constructs, and then treated with 10 ng/ml T3 or vehicle. Luciferase activity was analyzed 48 hrs after treatment and expressed as percentage of the luciferase activity from the cells tranfected with pTAL-Luc and treated with vehicle. A = P < 0.01 vs. vehicle treated pTAL-IGF-I.
TRα and TRβ differentially regulate expression of IGF system components in mice
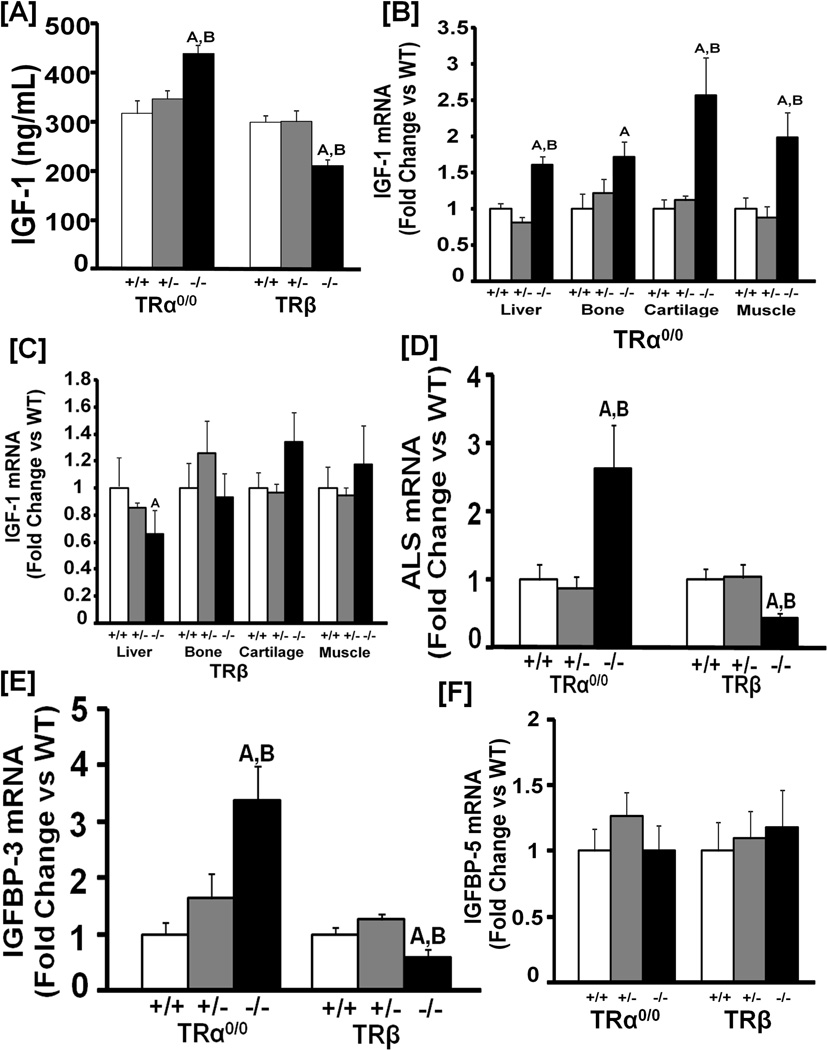
To determine the role of TRs in regulating IGF-I expression in vivo, we measured serum levels of IGF-I in homozygous and heterozygous mice lacking functional TRα or TRβ and their corresponding WT littermate controls. Serum IGF-I was increased in homozygous TRα KO mice compared to both WT and heterozygous littermates (Figure 6A). By contrast, homozygous mutant mice lacking both TRβ1 and β2 showed a 29% decrease (P<0.05) in serum IGF-I compared to heterozygous and WT mice. Accordingly, IGF-I mRNA expression was elevated in TH target tissues including the bone and the cartilage in homozygous TRα KO mice (Figure 6B). By contrast, IGF-I expression was decreased in the liver but not in other tissues of TRβ KO mice (Figure 6C). Furthermore, ALS and IGFBP-3 expressions were increased in the liver in homozygous TRα KO mice but decreased in TRβ KO mice (Figures 6D and E). IGFBP-5 mRNA expression was unaffected by deletion of either TRα or TRβ (Figure 6F).
Figure 6. Targeted disruption of TRα and TRβ differentially modulates IGF-I expression in mice.
[A]: Serum IGF-I levels in homozygous and heterozygous TRα0/0 and TRβ mutant mice and corresponding WT littermates at 14 days of age. A = P<0.01 vs WT mice of the same genotype, B = P<0.01 vs heterozygous mice of the same genotype. N = 8–12 mice per genotype. [B-C]: IGF-I mRNA levels in the liver, bone, cartilage and muscle of homozygous and heterozygous TRα0/0 (B) and TRβ (C) mutant mice and corresponding WT littermate control mice at 14 days of age. Values are fold-change of WT control mice. A = P<0.01 vs WT mice of the same genotype, B = P<0.01 vs heterozygous mice of the same genotype. N = 8 mice per group. [D-F]: ALS (D), IGFBP-3 (E) and IGFBP-5 (F) mRNA levels in the liver of homozygous and heterozygous TRα0/0 and TRβ mutant mice and corresponding WT littermates at 14 days of age. A = P<0.01 vs WT mice of the same genotype, B = P<0.01 vs heterozygous mice of the same genotype. N = 8–12 mice per genotype.
TH biological effects on bone cells is in part dependent on local IGF-I
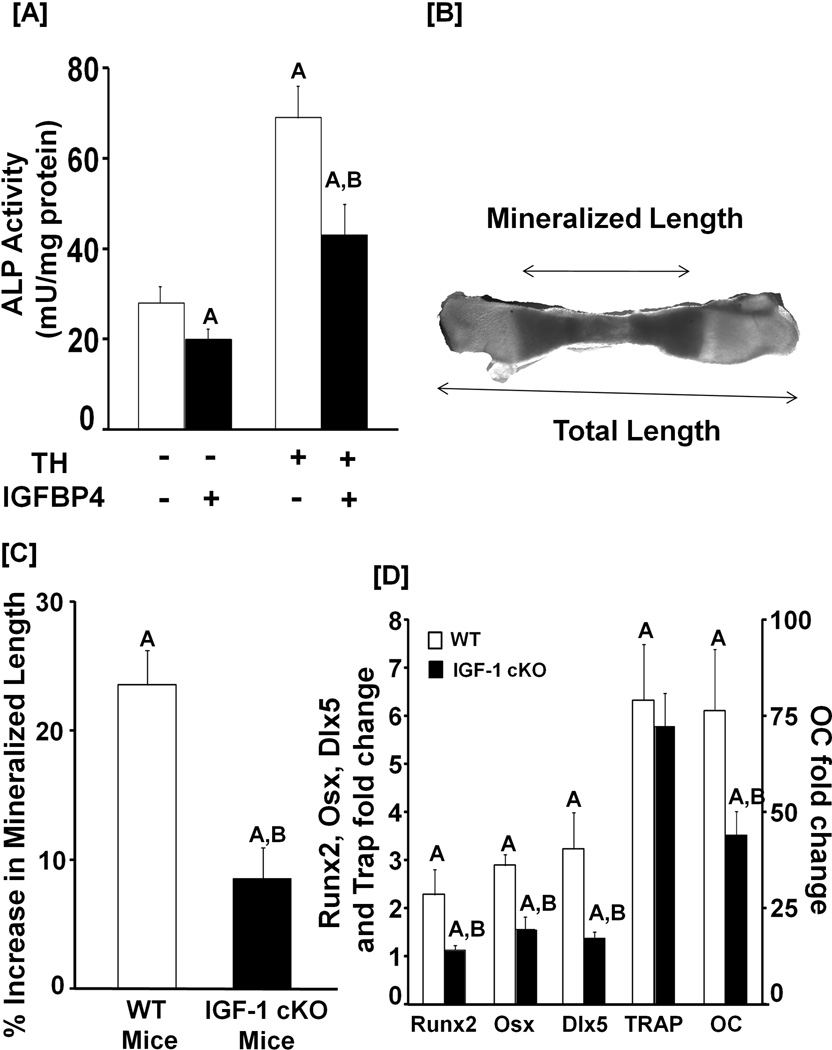
We next addressed if TH effects on bone depend on local IGF-I production. We evaluated the effects of T3 on ALP activity in MC3T3-E1 cells after neutralizing IGF activity with an excess of inhibitory IGFBP4. TH-induction of ALP activity was inhibited by IGFBP4 (Figure 7A). To examine whether local IGF-I mediates TH effects on bone growth, metatarsals were isolated from 3-day old IGF-I conditional KO mice, and incubated in serum-free conditions in the absence or presence of TH for 10 days. Total and mineralized lengths were measured from digital images of the metatarsals (Figure 7B). Ten days of TH treatment resulted in a 25% increase in mineralized length adjusted for total length whereas only a 10% increase was observed in the mice lacking IGF-I in osteoblasts (Figure 7C). Consistent with these data, mRNA levels of transcription factors (Runx2, Osx and Dlx5) and osteocalcin were increased to a greater extent by 10 days of TH treatment compared to vehicle in the metatarsals from WT mice compared to the metatarsals from IGF-I conditional KO mice (Figure 7D). By contrast, TH treatment caused a similar increase in the mRNA levels of TRAP, a marker of bone resorption, in the metatarsals-derived from WT and IGF-I conditional KO mice.
Figure 7. TH biological effects in bone cells is in part mediated via local IGF-I.
[A]: Neutralization of IGF-I with inhibitory IGFBP-4 blocks the TH-induced increase in ALP activity. MC3T3-E1 cells were incubated with or without T3 or IGFBP-4. Three days later, cells were lysed for ALP activity determinations and total protein measurements. Values are the Mean ± SEM (n = 8). A = P < 0.01 vs. vehicle control. B = P < 0.01 vs. T3. [B]: A microscope image of a mineralized metatarsal bone-derived from a 3-day old WT mouse after a 10 day incubation in DMEM containing 0.5% BSA, 50 µg/ml ascorbic acid and 1 mM β-glycerol phosphate. [C]: Quantitative data of cultured metatarsals-derived from WT and IGF-I conditional knockout (cKO) mice. Metatarsals from 3-day old IGF-I conditional KO and WT control mice were incubated in serum-free medium containing 0.5% BSA 50 µg/ml ascorbic acid and 1 mM β-glycerol phosphate for 10 days in the presence (10 ng/ml) or absence of T3. The mineralized length of the bone was determined using microscopy. The TH-induced increase in the length of mineralized metatarsals-derived from IGF-I cKO was compared to the metatarsals from the corresponding control WT mice. Mineralized length was adjusted for total length to account for differences in the length of metatarsals for the two genotypes. A = P < 0.01 vs. vehicle treated metatarsals. B = P < 0.01 vs. T3 treated metatarsals from WT mice. [D]: TH-increases the expression of bone specific transcription factors and bone formation markers. RNA was isolated from the cultured metatarsals in C, and used for real time RT-PCR for measurement of expression of transcription factors and bone formation markers as described in the methods. A = P < 0.01 vs. vehicle treated metatarsals. B = P < 0.01 vs. T3 treated metatarsals from WT mice.
DISCUSSION
The importance of IGF-I in skeletal development is well established since peak bone mass is considerably reduced as a consequence of deficiency in IGF-I action in both humans and experimental animals (6–9,14,21). In terms of mechanisms for IGF-I regulation, experimental data from genetic mouse models lacking IGF-I or GH revealed that GH-independent mechanisms predominantly contribute to bone acquisition during prepubertal growth while GH-dependent mechanisms contribute predominantly to bone acquisition during both pubertal and postpubertal growth periods (7). There are a number of potential explanations for the limited contribution of GH during the prepubertal period compared to the pubertal growth period. First, serum levels of GH increase several fold during the pubertal growth spurt. Thus, lower levels of GH during prepubertal growth may contribute to reduced GH effects on target tissues. Second, studies have shown that the relatively low contribution could be due to GH insensitivity during the prepubertal growth period. In this regard, we found that the gain in bone in response to GH therapy is several fold greater when GH was administered during the pubertal growth period compared to the prepubertal growth period, thus suggesting that GH effect on bone acquisition is growth-period dependent (12). In terms of the mechanism for the reduced GH sensitivity in target tissues during the prepubertal growth period, studies by Waxman et al. (45) have shown that certain liver factors necessary for mediating GH effects are absent in prepubertal rats. Third, a cooperative crosstalk between IGF-I and estrogen receptor signaling has been well documented (46) which could contribute to greater GH action during pubertal and postpubertal growth periods.
In this study, we demonstrate that TH-dependent mechanisms contribute to prepubertal regulation of IGF-I action and bone acquisition. Accordingly, the magnitude of the skeletal deficit is approximately 3 times greater in TH-deficient mice compared to GH-deficient mice at the end of prepubertal growth period. The findings that mice deficient in both GH and TH did not exhibit greater BMD deficits than mice deficient in TH alone indicate that TH and GH may be acting via a common pathway to regulate bone density. The skeletal phenotype data from genetic mouse models deficient in TH and/or GH action have provided unequivocal evidence that there is a critical time period prior to puberty, when the effects of GH are small and TH plays a key role in the regulation of skeletal growth.
The findings that treatment of TH deficient mice with daily administration of T3/T4 during the 10 day period (5 to 14 days of age) when serum T3 levels are elevated rescues deficiencies in body weight, bone length as well as bone density suggest that the deficits in both somatic and bone growth in TH deficient mice were a consequence of the lack of an increase in T3 production during the prepubertal growth period. The findings from TRα1 knock-in mutant mice also provide evidence for a direct T3 effect on bone (25,47). Neither TH nor GH treatment alone rescued the skeletal deficit in the double-mutant mice lacking both GH and TH. While combined treatment with both GH and TH rescued the bone density deficit in double- mutant mice, it did not completely rescue body weight or bone length. By contrast, GH or TH treatment alone reduced body fat in double-mutant mice. In terms of the question why TH alone was ineffective in stimulating bone acquisition in TH/GH double mutant mice, there is now a considerable amount of clinical data that demonstrate normal TH action is necessary for the optimal response to both endogenous and recombinant hGH substitution (48). Thus, TH may sensitize GH effects in bone. If all of the TH effect is dependent on GH, we hypothesized that GH-deficiency would result in a similar reduction in bone density at the end of the prepubertal growth period. However, the current studies do not support this hypothesis. Thus, TH exerts direct GH-independent effects on bone in addition to sensitizing skeletal responses to GH.
A key finding is that TH regulates IGF-I gene transcription via a TRE located in intron 1 of the IGF-I gene. Consistent with direct effects of TH on IGF-I gene expression, TH treatment during the prepubertal growth period rescued the deficit in IGF-I mRNA level in both liver and bone. IGF-I in serum is short lived unless it is bound to the IGFBP-3/ALS complex. Our findings also provide the first experimental evidence that TH increases ALS expression in the liver, thus suggesting that the rise in serum levels of IGF-I during the prepubertal growth period may be mediated via both a TH-dependent increase in IGF-I as well as ALS expression. Surprisingly, combined treatment with GH and TH in the double-mutant mice for two weeks did not increase either IGF-I nor ALS expression in the liver. Accordingly, serum IGF-I levels remained unchanged in response to GH+TH treatment in the double-mutant mice. These data suggest that the mechanisms that mediate effects of TH and GH on target genes are complex.
Our in vitro findings from experiments evaluating the consequence of TRα knockdown as well as overexpression on IGF-I mRNA levels in MC3T3-E1 osteoblasts, and the findings from EMSA experiments that TRα1 bound to a TRE in the IGF-I gene, indicate that ligand bound TRα1 is an activator of IGF-I gene transcription. While TRα may be the predominant receptor involved in mediating TH effects in skeletal cells because it is expressed at higher levels than TRβ, these studies do not exclude a similar role for TRβ in target tissues such as the liver where it is highly expressed. If TRα1 is a positive regulator of IGF-I expression, we predict that mice lacking TRα1 would exhibit reduced serum IGF-I levels compared to control mice. However, TRα0/0 mice have elevated serum IGF-I levels. Since TRα0/0 KO mice are euthyroid (31), this unexpected finding may result from the systemic consequences of the global deletion of TRα0/0. Furthermore, results from in vitro studies on TH effects on IGF-I gene expression in serum-free conditions may not recapitulate the effects of TH in vivo. Alternatively, in the absence of TRα, it is possible that the TRE in intron 1 of the IGF-1 gene may be occupied by other transcription factors such as AP1 and SF-1 that result in enhanced IGF-1 transcription (49,50).
In an earlier study, O’Shea et al. (47) reported diminished expression of GH receptor, IGF-I and IGF-I receptor in the growth plates of heterozygous TRα1PV mutant mice carrying the PV mutation targeted to TRα1. The mutant TRα1PV protein acts as a potent dominant negative receptor that interferes with transcriptional activities of WT TRα and TRβ receptors. Thus, the findings in TRα1PV mice compared to TRα0/0 KO mice may result from additional effects of the PV mutant protein that are similar to the effect of hypothyroidism in which unliganded TRα acts as a repressor of target gene expression.
By contrast, circulating IGF-1 and hepatic IGF-1 expression were decreased in TRβ KO mice. TRβ KO mice have chronically elevated circulating T4 and T3 levels due to disruption of the pituitary thyroid negative feedback axis (32,51). The lack of TRβ expression in the liver in TRβ KO mice results in reduced hepatic IGF-I expression despite elevated systemic TH levels because the liver, in contrast to the bone, is a TRβ responsive target tissue. Thus, in the skeleton, elevated TH in TRβ KO mice increased IGF-I expression via TRα transactivation.
The findings that inhibition of IGF-I action only partially reduced TH effects on osteoblast differentiation and the rate of metatarsal bone mineralization indicate that TH acts via other pathways in addition to IGF-I. Consistent with this, it is known that TH influences FGF receptor signaling in bone and interacts with the Wnt/β-catenin and Ihh/PTHrP signaling pathways to regulate endochondral ossification (52–54). Wang et al. (54) have demonstrated that TH stimulates IGF-I expression in growth plate chondrocytes to induce proliferation. Furthermore, they also have shown that the TH effect on chondrocyte hypertrophy is mediated via activation of Wnt/β-catenin signaling which is in part initiated by IGF-I signaling. Future studies are needed to determine whether a similar mechanism is involved in mediating TH effects on osteoblast differentiation.
The limitations of this study include the inability of the methods used to differentiate between different bone (cortical/trabecular) and fat (white/brown) compartments, the short window of time used to study the growth phenotype and the single dose of T3/T4 used for rescue experiments. Despite these limitations, our studies using different mouse mutants show that TH acting via IGF-I-dependent and -independent mechanisms plays a more critical role than GH in the regulation of skeletal growth during the prepubertal growth period.
ACKNOWLEDGEMENTS
We thank Sheila Pourteymoor, Heather Davidson, Catrina Alarcon, Anil Kapoor, Moira Cheung, Yan Lu and Joe Rung-Aroon for technical assistance. This research was supported by National Institutes of Health Grant AR 048139 to SM and Medical Research Council grants G0800261 to JHDB and GRW and G0800359 to M. Cheung and GRW. The research work was performed at facilities provided by the Department of Veterans Affairs.
Author’s roles: W.X., K.G., and S.M. designed experiments and performed data analysis and interpretation. C.K. and J.E.W. assisted in the skeletal phenotype measurements. CL assisted in the TH receptor work. AG and AW assisted in the gene expression work involving TRα and TRβ KO mice. L.R.D. contributed to data involving Duox2 KO mice. W.X. and S.M. wrote the manuscript with assistance from G.R.W. All authors provided critical revisions for the manuscript. S.M. conceived and oversaw the entire the project.
Footnotes
Conflict of interest: All the authors state that they have no conflict of interest.
REFERENCES
- 1.Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, Weaver C. Peak bone mass. Osteoporos Int. 2000;11(12):985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 2.Libanati C, Baylink DJ, Lois-Wenzel E, Srinvasan N, Mohan S. Studies on the potential mediators of skeletal changes occurring during puberty in girls. J Clin Endocrinol Metab. 1999;84(8):2807–2814. doi: 10.1210/jcem.84.8.5905. [DOI] [PubMed] [Google Scholar]
- 3.Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement. 2000;17(1):1–45. [PubMed] [Google Scholar]
- 4.Hansen MA, Overgaard K, Riis BJ, Christiansen C. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ. 1991;303(6808):961–964. doi: 10.1136/bmj.303.6808.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui SL, Slemenda CW, Johnston CC., Jr The contribution of bone loss to postmenopausal osteoporosis. Osteoporos Int. 1990;1(1):30–34. doi: 10.1007/BF01880413. [DOI] [PubMed] [Google Scholar]
- 6.Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology. 2007;148(12):5706–5715. doi: 10.1210/en.2007-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohan S, Richman C, Guo R, Amaar Y, Donahue LR, Wergedal J, Baylink DJ. Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology. 2003;144(3):929–936. doi: 10.1210/en.2002-220948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walenkamp MJ, Karperien M, Pereira AM, Hilhorst-Hofstee Y, van Doorn J, Chen JW, Mohan S, Denley A, Forbes B, van Duyvenvoorde HA, van Thiel SW, Sluimers CA, Bax JJ, de Laat JA, Breuning MB, Romijn JA, Wit JM. Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J Clin Endocrinol Metab. 2005;90(5):2855–2864. doi: 10.1210/jc.2004-1254. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277(46):44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 10.LeRoith D. Clinical relevance of systemic and local IGF-I: lessons from animal models. Pediatr Endocrinol Rev. 2008;5(Suppl 2):739–743. [PubMed] [Google Scholar]
- 11.Ohlsson C, Mohan S, Sjogren K, Tivesten A, Isgaard J, Isaksson O, Jansson JO, Svensson J. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30(5):494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasukawa Y, Baylink D, Guo R, Mohan S. Evidence that sensitivity to growth hormone (GH) is growth period and tissue type dependent: studies in GH deficient (lit/lit) mice. Endocrinology. 2003;144(9):3950–3957. doi: 10.1210/en.2002-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savage MO, Burren CP, Rosenfeld RG. The continuum of growth hormone-IGF-I axis defects causing short stature: diagnostic and therapeutic challenges. Clin Endocrinol (Oxf) 2010;72(6):721–728. doi: 10.1111/j.1365-2265.2009.03775.x. [DOI] [PubMed] [Google Scholar]
- 14.Walenkamp MJ, Wit JM. Genetic disorders in the GH IGF-I axis in mouse and man. Eur J Endocrinol. 2007;157(Suppl 1):S15–S26. doi: 10.1530/EJE-07-0148. [DOI] [PubMed] [Google Scholar]
- 15.Edderkaoui B, Baylink DJ, Beamer WG, Wergedal JE, Porte R, Chaudhuri A, Mohan S. Identification of mouse Duffy antigen receptor for chemokines (Darc) as a BMD QTL gene. Genome Res. 2007;17(5):577–585. doi: 10.1101/gr.6009507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ralston SH, Uitterlinden AG. Genetics of osteoporosis. Endocr Rev. 2010;31(5):629–662. doi: 10.1210/er.2009-0044. [DOI] [PubMed] [Google Scholar]
- 17.Rosen CJ, Beamer WG, Donahue LR. Defining the genetics of osteoporosis: using the mouse to understand man. Osteoporos Int. 2001;12(10):803–810. doi: 10.1007/s001980170030. [DOI] [PubMed] [Google Scholar]
- 18.Richman C, Kutilek S, Miyakoshi N, Srivastava AK, Beamer WG, Donahue LR, Rosen CJ, Wergedal JE, Baylink DJ, Mohan S. Postnatal and pubertal skeletal changes contribute predominantly to the differences in peak bone density between C3H/HeJ and C57BL/6J mice. J Bone Miner Res. 2001;16(2):386–397. doi: 10.1359/jbmr.2001.16.2.386. [DOI] [PubMed] [Google Scholar]
- 19.Collett-Solberg PF. Update in growth hormone therapy of children. J Clin Endocrinol Metab. 2011;96(3):573–579. doi: 10.1210/jc.2010-1131. [DOI] [PubMed] [Google Scholar]
- 20.Wit JM, Reiter EO, Ross JL, Saenger PH, Savage MO, Rogol AD, Cohen P. Idiopathic short stature: management and growth hormone treatment. Growth Horm IGF Res. 2008;18(2):111–135. doi: 10.1016/j.ghir.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Bassett JH, Williams GR. The skeletal phenotypes of TRalpha and TRbeta mutant mice. J Mol Endocrinol. 2009;42(4):269–282. doi: 10.1677/JME-08-0142. [DOI] [PubMed] [Google Scholar]
- 22.Gogakos AI, Duncan Bassett JH, Williams GR. Thyroid and bone. Arch Biochem Biophys. 2010;503(1):129–136. doi: 10.1016/j.abb.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Gothe S, Wang Z, Ng L, Kindblom JM, Barros AC, Ohlsson C, Vennstrom B, Forrest D. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev. 1999;13(10):1329–1341. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kindblom JM, Gothe S, Forrest D, Tornell J, Vennstrom B, Ohlsson C. GH substitution reverses the growth phenotype but not the defective ossification in thyroid hormone receptor alpha 1−/−beta−/− mice. J Endocrinol. 2001;171(1):15–22. doi: 10.1677/joe.0.1710015. [DOI] [PubMed] [Google Scholar]
- 25.Bassett JH, Nordstrom K, Boyde A, Howell PG, Kelly S, Vennstrom B, Williams GR. Thyroid status during skeletal development determines adult bone structure and mineralization. Mol Endocrinol. 2007;21:1893–1904. doi: 10.1210/me.2007-0157. [DOI] [PubMed] [Google Scholar]
- 26.Kawai M, Devlin MJ, Rosen CJ. Fat targets for skeletal health. Nat Rev Rheumatol. 2009;5(7):365–372. doi: 10.1038/nrrheum.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo M, Hall JA, Correa-Medina M, Ueta C, Kang HW, Cohen DE, Bianco AC. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes. 2011;60(4):1082–1089. doi: 10.2337/db10-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin X, Strong DD, Baylink DJ, Mohan S. Structure-function analysis of the human insulin-like growth factor binding protein-4. J Biol Chem. 1998;273(36):23509–23516. doi: 10.1074/jbc.273.36.23509. [DOI] [PubMed] [Google Scholar]
- 29.Kinugawa K, Jeong MY, Bristow MR, Long CS. Thyroid hormone induces cardiac myocyte hypertrophy in a thyroid hormone receptor alpha1-specific manner that requires TAK1 and p38 mitogen-activated protein kinase. Mol Endocrinol. 2005;19(6):1618–1628. doi: 10.1210/me.2004-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson KR, Marden CC, Ward-Bailey P, Gagnon LH, Bronson RT, Donahue LR. Congenital hypothyroidism, dwarfism and hearing impairment caused by a missense mutation in the mouse dual oxidase 2 gene, Duox2. Mol Endocrinol. 2007 doi: 10.1210/me.2007-0085. [DOI] [PubMed] [Google Scholar]
- 31.Gauthier K, Plateroti M, Harvey CB, Williams GR, Weiss RE, Refetoff S, Willott JF, Sundin V, Roux JP, Malaval L, Hara M, Samarut J, Chassande O. Genetic analysis reveals different functions for the products of the thyroid hormone receptor alpha locus. Mol Cell Biol. 2001;21(14):4748–4760. doi: 10.1128/MCB.21.14.4748-4760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauthier K, Chassande O, Plateroti M, Roux JP, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J. Different functions for the thyroid hormone receptors TRalpha and TRbeta in the control of thyroid hormone production and post-natal development. Embo J. 1999;18(3):623–631. doi: 10.1093/emboj/18.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortiga-Carvalho TM, Shibusawa N, Nikrodhanond A, Oliveira KJ, Machado DS, Liao XH, Cohen RN, Refetoff S, Wondisford FE. Negative regulation by thyroid hormone receptor requires an intact coactivator-binding surface. J Clin Invest. 2005;115(9):2517–2523. doi: 10.1172/JCI24109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohan S, Baylink DJ. Development of a simple valid method for the complete removal of insulin-like growth factor (IGF)-binding proteins from IGFs in human serum and other biological fluids: comparison with acid-ethanol treatment and C18 Sep-Pak separation. J Clin Endocrinol Metab. 1995;80(2):637–647. doi: 10.1210/jcem.80.2.7531716. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Xing W, Wergedal J, Chan JY, Mohan S. Targeted disruption of nuclear factor erythroid-derived 2-like 1 in osteoblasts reduces bone size and bone formation in mice. Physiol Genomics. 2010;40(2):100–110. doi: 10.1152/physiolgenomics.00105.2009. [DOI] [PubMed] [Google Scholar]
- 36.Xing W, Singgih A, Kapoor A, Alarcon CM, Baylink DJ, Mohan S. Nuclear factor-E2-related factor-1 mediates ascorbic acid induction of osterix expression via interaction with antioxidant-responsive element in bone cells. J Biol Chem. 2007;282(30):22052–22061. doi: 10.1074/jbc.M702614200. [DOI] [PubMed] [Google Scholar]
- 37.Mohan S, Kapoor A, Singgih A, Zhang Z, Taylor T, Yu H, Chadwick RB, Chung YS, Donahue LR, Rosen C, Crawford GC, Wergedal J, Baylink DJ. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. J Bone Miner Res. 2005;20(9):1597–1610. doi: 10.1359/JBMR.050406. [DOI] [PubMed] [Google Scholar]
- 38.Amaar YG, Baylink DJ, Mohan S. Ras-Association Domain Family 1 Protein, RASSF1C, Is An IGFBP-5 Binding Partner And A Potential Regulator Of Osteoblast Cell Proliferation. J Bone Miner Res. 2005;20(8):1430–1439. doi: 10.1359/JBMR.050311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xing W, Kim J, Wergedal J, Chen ST, Mohan S. Ephrin B1 Regulates Bone Marrow Stromal Cell Differentiation and Bone Formation by Influencing TAZ Transactivation via Complex Formation with NHERF1. Mol Cell Biol. 2010;30(3):711–721. doi: 10.1128/MCB.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukherjee A, Rotwein P. Akt promotes BMP2-mediated osteoblast differentiation and bone development. J Cell Sci. 2009;122(Pt 5):716–726. doi: 10.1242/jcs.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu WX, Du GG, Kopp P, Rentoumis A, Albanese C, Kohn LD, Madison LD, Jameson JL. The thyrotropin (TSH) receptor transmembrane domain mutation (Pro556-Leu) in the hypothyroid hyt/hyt mouse results in plasma membrane targeting but defective TSH binding. Endocrinology. 1995;136(7):3146–3153. doi: 10.1210/endo.136.7.7789342. [DOI] [PubMed] [Google Scholar]
- 42.Bassett JH, Williams AJ, Murphy E, Boyde A, Howell PG, Swinhoe R, Archanco M, Flamant F, Samarut J, Costagliola S, Vassart G, Weiss RE, Refetoff S, Williams GR. A lack of thyroid hormones rather than excess thyrotropin causes abnormal skeletal development in hypothyroidism. Mol Endocrinol. 2008;22(2):501–512. doi: 10.1210/me.2007-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sprenkle PM, McGee J, Bertoni JM, Walsh EJ. Development of auditory brainstem responses (ABRs) in Tshr mutant mice derived from euthyroid and hypothyroid dams. J Assoc Res Otolaryngol. 2001;2(4):330–347. doi: 10.1007/s101620010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiong S, Chirala SS, Hsu MH, Wakil SJ. Identification of thyroid hormone response elements in the human fatty acid synthase promoter. Proc Natl Acad Sci U S A. 1998;95(21):12260–12265. doi: 10.1073/pnas.95.21.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waxman DJ, O'Connor C. Growth Hormone Regulation of Sex-dependent Liver Gene Expression*. Mol Endocrinol. 2006;20:2613–2629. doi: 10.1210/me.2006-0007. [DOI] [PubMed] [Google Scholar]
- 46.Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, Maggi A, DiAugustine RP, Korach KS. Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem. 2002;277(10):8531–8537. doi: 10.1074/jbc.M109592200. [DOI] [PubMed] [Google Scholar]
- 47.O'Shea PJ, Bassett JH, Sriskantharajah S, Ying H, Cheng SY, Williams GR. Contrasting skeletal phenotypes in mice with an identical mutation targeted to thyroid hormone receptor alpha1 or beta. Mol Endocrinol. 2005;19(12):3045–3059. doi: 10.1210/me.2005-0224. [DOI] [PubMed] [Google Scholar]
- 48.Cacciari E, Cicognani A, Pirazzoli P, Bernardi F, Zappulla F, Salardi S, Mazzanti L, Biasini A, Valenti E. Effect of long-term GH administration on pituitary-thyroid function in idiopathic hypopituitarism. Acta Paediatr Scand. 1979;68(3):405–409. doi: 10.1111/j.1651-2227.1979.tb05028.x. [DOI] [PubMed] [Google Scholar]
- 49.Leone TC, Cresci S, Carter ME, Zhang Z, Lala DS, Strauss AW, Kelly DP. The human medium chain acyl-CoA dehydrogenase gene promoter consists of a complex arrangement of nuclear receptor response elements and Sp1 binding sites. J Biol Chem. 1995;270(41):24622. [PubMed] [Google Scholar]
- 50.Lopez G, Schaufele F, Webb P, Holloway JM, Baxter JD, Kushner PJ. Positive and negative modulation of Jun action by thyroid hormone receptor at a unique AP1 site. Mol Cell Biol. 1993;13(5):3042–3049. doi: 10.1128/mcb.13.5.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, Wehner JM, Curran T. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: evidence for tissue-specific modulation of receptor function. Embo J. 1996;15(12):3006–3015. [PMC free article] [PubMed] [Google Scholar]
- 52.Mackie EJ, Tatarczuch L, Mirams M. The growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011;211(2):109–121. doi: 10.1530/JOE-11-0048. [DOI] [PubMed] [Google Scholar]
- 53.Stevens DA, Harvey CB, Scott AJ, O'Shea PJ, Barnard JC, Williams AJ, Brady G, Samarut J, Chassande O, Williams GR. Thyroid hormone activates fibroblast growth factor receptor-1 in bone. Mol Endocrinol. 2003;17(9):1751–1766. doi: 10.1210/me.2003-0137. [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Shao YY, Ballock RT. Thyroid hormone-mediated growth and differentiation of growth plate chondrocytes involves IGF-1 modulation of beta-catenin signaling. J Bone Miner Res. 2010;25(5):1138–1146. doi: 10.1002/jbmr.5. [DOI] [PMC free article] [PubMed] [Google Scholar]