Abstract
Inhibitory control and incentive processes underlie decision-making, yet few studies have explicitly examined their interaction across development. Here, the effects of potential rewards and losses on inhibitory control in sixty-four adolescents (13-17-year-olds) and forty-two young adults (18-29-year-olds) were examined using an incentivized antisaccade task. Notably, measures were implemented to minimize age-related differences in reward valuation and potentially confounding motivation effects. Incentives affected antisaccade metrics differently across the age groups. Younger adolescents generated more errors than adults on reward trials, but all groups performed well on loss trials. Adolescent saccade latencies also differed from adults across the range of reward trials. Overall, results suggest persistent immaturities in the integration of reward and inhibitory control processes across adolescence.
Adaptive decision-making requires the effective integration of cognitive control and reward processes (Balleine, Doya, O’Doherty, & Sakagami, 2007; Ernst & Paulus, 2005; Rangel, Camerer, & Montague, 2008). Basic aspects of cognitive control, including working memory and response inhibition, enable an individual to maintain and update internal representations of choices and goals and to resist responding to goal-irrelevant information or stimuli. Reward processes generate value estimates for each available choice or action based on preference or need, thus contributing to an efficient prioritization of behavior (Schultz, 2000). During the developmental span of adolescence, these component processes undergo continued maturation (Ernst, et al., 2005; Luna, Garver, Urban, Lazar, & Sweeney, 2004). Understanding how these still-maturing processes interact in a normative adolescent population may provide insight on basic mechanisms contributing to the emergence of risk taking, a serious health concern for this age group often considered the result of suboptimal decision-making (Arnett, 1992; Dahl, 2004; Steinberg, 2004). In the current study, we focus on characterizing the effects of various incentive (reward, loss) contingencies on performance in a response inhibition task.
Response inhibition, or voluntary response suppression, refers to the ability to suppress task-irrelevant responses and is a key component of the voluntary control of behavior (Fuster, 1989; Miller & Cohen, 2001). One well-studied behavioral index of response inhibition is performance on the antisaccade task (Hallett, 1978). In this oculomotor paradigm, participants are typically asked to fixate a central target on a computer screen. A stimulus (often a small filled circle) is then presented at an unpredictable horizontal location. Participants are instructed to refrain from making a rapid eye movement (saccade) towards the stimulus when it appears (i.e., inhibitory response) and instead make a voluntary saccade to the opposite location in space. Work from our laboratory and several others consistently demonstrate that response inhibition continues to improve into adolescence, with mature levels of responding stabilizing by 14-15 years (Fischer, Biscaldi, & Gezeck, 1997; Klein & Foerster, 2001; Luna, et al., 2004; Luna, et al., 2001; Munoz, Broughton, Goldring, & Armstrong, 1998). These findings are consistent with those from a range of other inhibitory control paradigms (e.g., go/no go, stop signal), which also indicate continued improvement into adolescence (Levin, Culhane, Hartmann, Evankovich, & Mattson, 1991; Liston, et al., 2006; Luciana & Nelson, 1998; Paus, Babenko, & Radil, 1990; Ridderinkhof, Band, & Logan, 1999; Ridderinkhof & van der Molen, 1997; Tipper, Bourque, Anderson, & Brehaut, 1989; Williams, Ponesse, Schachar, Logan, & Tannock, 1999). Functional magnetic resonance imaging (fMRI) data support these behavioral findings and indicate that continued development in underlying brain systems underlie improvements in inhibitory control (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Luna, et al., 2001; Rubia, Smith, Taylor, & Brammer, 2007; Velanova, Wheeler, & Luna, 2008), including the processes involved in establishing an inhibitory response state (i.e., the ability to have sustained and consistent inhibitory responding) (Dosenbach, et al., 2006; Fair, et al., 2007; Logan & Gordon, 2001).
While significant progress has been made in understanding the development of inhibitory control, less is known about its interaction with reward processing, especially during adolescence. Initial behavioral studies indicate that when a reward contingency is added to AS trials, both adolescents and adults generate fewer inhibitory errors (Hardin, Schroth, Pine, & Ernst, 2007; Jazbec, et al., 2006; Jazbec, McClure, Hardin, Pine, & Ernst, 2005; Blaukopf & DiGirolamo, 2006; Duka & Lupp, 1997). However, adolescents show greater effects of rewards on other measures, such as latencies of AS errors, suggesting differential effects of reward on aspects of performance across age groups (Jazbec, et al., 2006). Recent work from our laboratory combining eye tracking and fMRI has extended these initial behavioral findings. Behaviorally (in the MR environment), we found that adolescents (aged 13-17 years) but not adults (18-30 years) generated significantly fewer errors on rewarded relative to neutral antisaccade trials. Both age groups generated significantly lower latencies on rewarded vs. neutral trials. Imaging results showed that adolescents had attenuated brain activation in ventral striatum (VS) during early assessment of a reward cue, but heightened VS activation during response preparation and reward anticipation, relative to adults (Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010). These data are in line with other developmental fMRI studies indicating that adolescents show a heighted response to reward anticipation and receipt in subcortical systems, prominently the ventral striatum (Ernst, et al., 2005; Eshel, Nelson, Blair, Pine, & Ernst, 2007; Galvan, et al., 2006; van Leijenhorst, Crone, & Bunge, 2006)(but also see (Bjork, et al., 2004)). Importantly, results from Geier et al. (2010) also indicate that adolescents had enhanced activation (relative to adults) during a response preparation and reward anticipation period in oculomotor control regions, including the superior precentral sulcus (presumed human frontal eye field, FEF). This result suggests a possible mechanism for observed behavioral improvements as the FEF is an area known to support response preparation, a key element in the ability to resist inappropriate responding in the antisaccade task (Curtis & Connolly, 2008). In Geier et al. (2010), however, only reward and neutral contingencies were presented and the reward did not vary in magnitude. Thus, initial findings examining the interaction between inhibitory control and reward processing using rewarded antisaccade tasks need to be replicated and explored across a broader range of potential incentive contingencies.
An important consideration related to investigations of the interaction between reward and inhibitory control systems is whether a given reward for which a participant is working provides the same motivational drive across age group. A powerful motivator, money is ubiquitously used in the human reward literature, including developmental studies of adolescence (Ernst, et al., 2005; Eshel, et al., 2007; Galvan, et al., 2006; van Leijenhorst, et al., 2006; Bjork, et al., 2004; Bjork, Smith, Danube, & Hommer, 2007). Certainly, monetary accrual is attractive to most participants and is experimentally advantageous given that money is easily quantified, represented, and manipulated. However, the use of money, or any incentive that is fixed across subjects, raises the thorny issue of how different age groups might vary in terms of the value they attribute to a set dollar amount. How valuable or desirable a given dollar amount may be perceived is likely based on myriad factors (e.g., past experience with money, current employment and financial status) that may be markedly different between adolescents and adults. The reward offered to participants is a critically important variable to consider in experimental designs given that differences in reward valuation may contribute to differences in task motivation and performance. In the case of the rewarded antisaccade task, a more valued reward might lead to greater motivation, which in turn may affect inhibitory behavior differently and potentially confound developmental interpretations.
It is important to be clear in our definitions of ‘motivation’ and ‘value’. ‘Value’ is conceptualized here as the subjective worth of an object, including factors such as reward magnitude (Delgado, Locke, Stenger, & Fiez, 2003; Leon & Shadlen, 1999; Roesch & Olson, 2004; Wallis & Miller, 2003), subjective preference (Hassani, Cromwell, & Schultz, 2001; Tremblay & Schultz, 1999), time between action and reward delivery (Tsujimoto & Sawaguchi, 2005), probability of attainment (O’Doherty, 2004), and an organism’s state of satiety (Critchley & Rolls, 1996). ‘Motivation’ is conceptualized as the effort an organism puts forth in order to gain a reward or avoid a loss (Roesch & Olson, 2004; Stellar & Stellar, 1985). Supporting a distinction between these two constructs are elegant single-cell studies in non-human primates indicating that neurons in regions of orbitofrontal cortex respond to differences in reward values, while neurons in premotor cortex, regions more closely associated with the response that leads to reward receipt, appear to code for the motivation to respond for a reward or avoid loss or punishment (Roesch & Olson, 2003, 2004).
In the current study, we used an incentivized antisaccade (AS) task with potential reward and loss trials to examine the effects of varying magnitudes and different incentive types on response inhibition, while introducing several methods aimed at reducing potential differences across age groups in reward value and subsequent motivation. These methods included having each participant choose one out of several possible reward options for which he or she would be working. In this manner, we aimed to maximize subjective preference, one dimension of a reward’s value (Schultz, 2000). Second, instead of direct monetary accrual, participants had the opportunity to gain (or lose) a fixed number of points that could later be redeemed for the desired item (Daw, O’Doherty, Dayan, Seymour, & Dolan, 2006). We hypothesized that when participants are given a specific range of points that are indirectly related to reward, this would distance the monetary value and encourage a readjustment to a new “fixed economy” where value is adjusted to the range of points (which is the same for all participants) and not to the subjectively laden monetary value. Note that this situation is different from using monetary rewards, in which the relative range and ‘maximum’ amount differs across individuals. These simple methods allowed us to examine any developmental differences in the effects of incentives on response inhibition, while minimizing potential confounding differences in reward value and subsequent motivation.
Method
Participants
Sixty-four adolescents ranging in age from 13-17 years (M=15.06, SD=1.39; 34 females) and forty-two adults ranging in age from 18-29 years (M=20.9, SD=2.82; 23 females) were included in this study. Given that antisaccade performance rates can vary from early to later adolescence (e.g., Luna et al., 2004), and that no consistent standard exists for defining adolescent subgroups in the literature, we used a straightforward median split to divide the adolescent sample into ‘younger’ and ‘older’ subgroups. The ‘younger’ adolescents subgroup (N=32, 18 females; M=13.96 years, SD=0.82) included eleven 13 year olds, eleven 14 year olds, and the ten youngest 15 year olds (based on date of birth). The ‘older’ adolescent subgroup (N=32, 16 females; M=16.16 years, SD=0.88) included the ten oldest 15 year olds, seven 16 year olds, and fifteen 17 year olds. Our previous findings based on a large sample of adolescents indicated that performance on the antisaccade task begins to reach adult-levels by mid-adolescence (approximately 14-15 years) (Luna et al., 2004). By using a median split to define adolescent subgroups here, we were able to examine subgroups with mean ages slightly younger and older than this data-determined change point, while still utilizing the entire sample of adolescents.
All participants had far visual acuity of at least 20/40 (corrected or uncorrected) and medical histories that revealed no neurological disease, brain injury, and no history of personal or first-degree relative major psychiatric illness (determined by interview). Participants were assessed for IQ using the Wechsler Abbreviated Scale of Intelligence (WASI, full-scale). Adolescent and adult IQs were both in the normal range (adults: M=117.10, SD=10.97; adolescents: M=106.56, SD=10.50) but were significantly different, t(104)=4.96, p<0.001). However, IQ was not significantly correlated with any outcome measure in either adolescents or adults and so was not included in subsequent analyses. Participants did not differ in parental income, U=754.5, Z= −1.661, p=0.097, or education levels, U=770.5, Z=-1.536, p=0.124, indicating similar socioeconomic backgrounds. Participants or their legal guardians provided informed consent or assent prior to participating in this study. All experimental procedures in this study were approved by the Institutional Review Board at the University of Pittsburgh. Participants were paid US $50 for their participation in the study in addition to any performance-related earnings.
Minimizing Differences in ‘Value’ of Rewards across Age Groups
Prior to the eye tracking session, each participant completed a brief questionnaire asking him or her to choose one of several potential rewards for which they would be working. Reward options included either a pre-paid Visa debit card or a $25.00 gift card redeemable at various restaurants and businesses. The determination of which gift cards were offered, which included iTunes®, Home Depot®, McDonald’s®, Max & Erma’s®, Starbucks®, Footlocker®, Best Buy®, Aeropostale®, AMC Theatres®, Barnes & Noble®, Old Navy®, was based on informal interviews with adolescents and young adults asking them to identify gift cards they would find appealing. The debit or gift card was offered in addition to a standard participation payment, which was received regardless of performance. By making available various options, each participant could choose a reward with the highest relative subjective preference. Each participant was then asked to rate how valuable (7-point Likert scale, where 1 = extremely valuable and 7 = not valuable) he or she considered their chosen reward to be and to write down at least one item that they might purchase with the card as a means to increase its motivational salience. Participants were instructed that they could potentially win (‘reward’ trials) or lose (‘loss’ trials) points throughout the experiment depending on their performance and that these points would be tallied at the end of the session to determine their reward. Participants were told that they would begin with 100 points and assured that no debt could be accrued from loss trials. Participants were also explicitly instructed that they would be remunerated based on the number of points earned out of a maximum total of 220.
Incentive-mediated Antisaccade Task
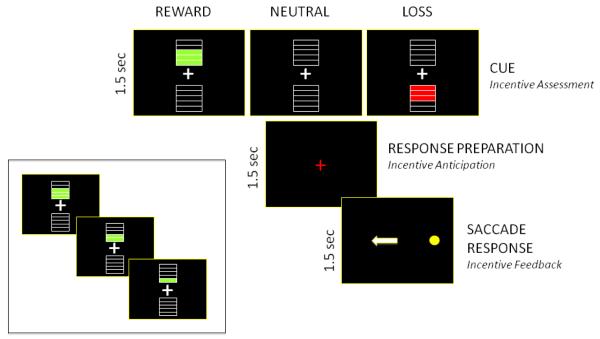
Each trial began with the presentation of one out of eleven possible incentive cues (1.5 sec) (Figure 1). Green filled bars (1, 2, 3, 4, or 5 colored bars, see inset of Figure 1) above a central white fixation indicated how many points the subject would win if he or she correctly performed the trial (potential ‘reward’ trial). An incorrect response on reward trials did not result in point loss, rather no gain. Red filled bars (1-5) below the white fixation indicated how many points the subject would lose if an error were made (potential ‘loss’ trial). A correct response during loss trials did not result in a point gain, rather no loss. In order to minimize the engagement of working memory systems, known to have a protracted development (Luna et al., 2001), participants were instructed not to keep score and that the computer would do so for them. Empty bars (5 each) above and below the fixation indicated that no points were at stake on that trial and that the participant’s total points would remain the same regardless of performance (‘neutral’ trial). Participants were encouraged to perform as best they could on all trials regardless of whether points were at stake. Next, the incentive cue image disappeared and the white central fixation turned red (1.5 sec), prompting participants to prepare a response during this time. Finally, a peripheral stimulus (yellow dot subtending approximately 0.5 degrees/visual angle) appeared at an unpredictable horizontal location (± 4 and 8 degrees/visual angle) (1.5 sec). Participants were instructed not to look at the stimulus when it appeared but instead gaze to the mirror location. The background screen was black at all times. During the saccade response epoch, eye movement data were scored on-line using interfaced E-Prime (Psychology Software Tools, Inc., Pittsburgh, PA) and ASL (Applied Science Laboratories, Bedford, MA) eye tracking software. A script detected if anytime during the first 1000msec of the response epoch the participant generated an eye movement toward the peripheral target or if no eye movement was generated. If an eye movement was made toward the target, an auditory tone (1163Hz peak frequency; ‘D’) was played for 400msec to indicate an incorrect response. If instead the participant initially looked toward the mirror location of the target during this 1000msec window, a correct response, a 400msec sound of a cash register (‘cha-ching’) was played (1516Hz peak frequency, ‘F-sharp’). Auditory tones were modified using Audacity, an open-source sound-editing program (http://audacity.sourceforge.net). Finally, between all trials a white fixation point appeared in the center of the screen (1.5 sec). There were a total of 40 reward and 40 loss trials with eight opportunities for each valence (reward, loss) magnitude (four possible target locations, +/− 4, 8 degrees/visual angle, each presented twice) and 40 total opportunities for neutral trials.
Figure 1.
Incentive mediated antisaccade task. Different amounts of possible point gain or loss were indicated by 1, 2, 3, 4, or 5 filled bars either above or below the central fixation (exemplified in inset).
Eye Tracking
Participants were seated in a darkened room with their head comfortably positioned on a chin rest with a Velcro head restraint. Eye movements were obtained using a near-infrared table-mounted ASL eye-tracking system (Model 504; Applied Science Laboratories, Bedford, MA, USA) that recorded eye position by pupil and corneal reflection. At the beginning of each eye-tracking session, a nine-point calibration procedure was performed. Stimuli were presented using E-Prime displayed on a PC monitor positioned 56cm directly in front of the participant.
In addition to the on-line scoring used for auditory feedback (which recorded a binary ‘correct’ or ‘incorrect’), eye data were scored off-line by a technician for various saccade metrics, including latency of correct antisaccades and error rates, using ILAB software (Gitelman, 2002) and an in-house scoring suite written in MATLAB (Math Works, Inc., Natic, MA) running on a Dell Dimension 8300 PC. Saccades were identified using a velocity algorithm in ILAB employing a 30-degrees/sec criterion. A ‘correct’ response in the antisaccade task was one in which the first saccade following peripheral target onset was towards the mirror location of the peripheral cue and extended beyond a 2.5 degrees/visual angle central fixation zone. An error was defined as occurring when the first saccade following peripheral target onset was directed toward the target and exceeded the central fixation zone. For each participant, error rate was calculated for a given trial type (e.g., 1-point reward trials) as the number of errors divided by the number of opportunities. Trials during which no eye movements were generated (<1% of trials) were excluded from further analyses.
Statistical Analyses
We first examined how different magnitudes of incentives affected antisaccade error rates and latencies (correct trials only) in adolescents and adults. Error rate data were analyzed in two ways. First, we analyzed the raw error rate values across magnitudes for reward and loss trials. Second, we adjusted the error rates on reward and loss trials across individuals based on their neutral trial performance, using the formula: (REWARD - NEUTRAL)/(NEUTRAL). In this manner, we assessed the affect of incentives on performance controlling for differences in “baseline” (neutral) error rate between adolescents and adults. We decided to analyze both raw and adjusted error rates because it is not clear whether responding during neutral trials is reflective of a true baseline; that is, free of incentive context for both adolescents and adults (see discussion below). For latency data, we made a direct comparison between adolescents and adults correct response latencies on neutral trials and found that they did not differ. As such, adjusting the valenced trials by the baseline (neutral) was not necessary. Error rates (raw and adjusted) and latencies were separately examined with repeated measures analysis of variance (ANOVA). For each model, age group (‘younger’ adolescents, ‘older’ adolescents, adults) was used as a categorical between-subjects factor and point magnitude (1-5 points) was used as the within-subjects factor. Separate models were run for reward and loss trials. Unless otherwise noted, Greenhouse-Geisser levels of significance are reported when assumptions of sphericity are violated, determined by a significant Mauchly’s test. Significant main effects and interactions from ANOVA analyses were further investigated with t-tests (with Bonferroni correction). Trend analysis was used to further examine effects of magnitude. Effect sizes for ANOVA results are reported as partial eta2 (ηP2).
In a second analysis step, we examined the effects of different types of incentive contingencies on task performance. For latency and error rates, separate repeated measures ANOVA were run with age group as the categorical between subjects factor and incentive type (reward, loss, or neutral) as the within subjects factor. In these models, unadjusted measures of performance on reward and loss trials were collapsed across point magnitudes.
Results
Variability in Reward Choice
Most participants (75%) selected the pre-paid debit card (money) as their reward. However, 18 adolescents (10 ‘younger’, 8 ‘older’) and 8 adults selected an alternative gift card, indicating variability in what participants subjectively viewed as rewarding. Importantly, adolescents and adults did not differ (p>0.1) in how “valuable” they rated their chosen reward (adults: M=2.14, SD=1.39; adolescents combined: M=2.57, SD=1.64; ‘younger’ adolescents: M=2.81, SD=1.62; ‘older’ adolescents: M=2.32, SD=1.66).
Error Rates and Latencies across Reward and Loss Magnitudes
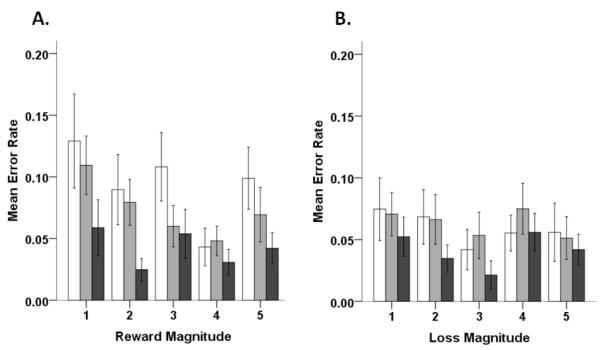
Mean error rates (raw) and latencies for reward, loss, and neutral trials are reported in Table 1. In terms of raw error rates on reward trials (Figure 2a), we observed a main effect of magnitude, F(4,412)=3.70, p<0.01, ηP2=0.04, and a main effect of age group, F(2,103)=4.71, p<0.01, ηP2=0.08, but no significant interaction. Pairwise comparisons showed that, overall, younger adolescents made significantly more errors than adults, t(72)=2.78, p<0.01, and that overall more errors were made on 1-point reward trials compared to 4-point reward trials, t(105)=3.45, p<0.001. Trend analysis showed that error rates decreased linearly with magnitude, F(1,103)=4.08, p<0.05 ηP2=0.04. There was also a significant quadratic component, F(1,103)=4.01, p<0.05 ηP2=0.04, reflecting the slight increase in error rate on 5-point trials (Figure 2a). On potential loss trials, no significant differences in error rates were observed (Figure 2b). When we examined adjusted error rate data to control for neutral trial performance, no significant effects of age group or magnitude were observed for either reward or loss trials (data not shown).
Table 1. Mean Percent Error Rates and Latencies (msec) of Correct Antisaccades for Potential Reward Trials. Standard Deviation Values Are Shown in Parentheses.
| Magnitude | Age Group | Error Rate | Latency (msec) | ||
|---|---|---|---|---|---|
| Reward | Loss | Reward | Loss | ||
| 1 | Younger Adolescents |
12.87 (21.61) |
7.36 (14.22) |
324.67 (92.26) |
359.97 (52.30) |
| Older Adolescents | 10.80 (13.41) |
6.93 (9.79) |
381.86 (83.58) |
383.21 (83.71) |
|
| Adults | 5.84 (14.66) |
5.16 (10.22) |
381.57 (74.90) |
373.31 (60.06) |
|
| 2 | Younger Adolescents |
8.94 (16.08) |
6.80 (12.44) |
376.06 (71.10) |
363.99 (45.22) |
| Older Adolescents |
7.85 (10.47) |
6.51 (11.20) |
389.33 (64.43) |
380.62 (71.90) |
|
| Adults | 2.42 (5.77) |
3.42 (7.03) |
360.64 (53.01) |
367.79 (60.95) |
|
| 3 | Younger Adolescents |
10.74 (15.65) |
4.11 (9.13) |
379.72 (84.13) |
358.86 (49.74) |
| Older Adolescents |
5.89 (9.40) |
5.30 (10.60) |
392.62 (90.84) |
373.56 (61.31) |
|
| Adults | 5.63 (12.82) |
2.13 (7.33) |
367.15 (48.12) |
367.07 (53.10) |
|
| 4 | Younger Adolescents |
4.27 (8.58) |
5.48 (8.20) |
350.71 (55.37) |
351.83 (43.43) |
| Older Adolescents |
4.72 (6.70) |
7.43 (11.59) |
393.44 (78.09) |
377.99 (72.38) |
|
| Adults | 3.02 (6.72) |
5.58 (9.91) |
361.94 (69.57) |
366.84 (48.25) |
|
| 5 | Younger Adolescents |
9.83 (14.18) |
5.55 (13.27) |
342.67 (49.45) |
359.42 (40.85) |
| Older Adolescents |
6.88 (12.44) |
5.04 (9.69) |
362.04 (68.78) |
372.05 (63.00) |
|
| Adults | 4.15 (8.19) |
4.11 (7.95) |
352.12 (57.32) |
357.99 (55.37) |
|
| Neutral | Younger Adolescents |
6.91 (8.51) |
380.14 (80.17) |
||
| Older Adolescents | 7.38 (7.58) |
382.33 (70.37) |
|||
| Adults | 4.00 (4.72) |
362.46 (53.43) |
|||
Figure 2.
Mean error rates for reward (A) and potential loss (B) trials across different magnitudes. For each graph, younger adolescents are represented by white bars, older adolescents are represented by gray bars, and adult are represented by black bars. Error bars represent +/− 1 SEM.
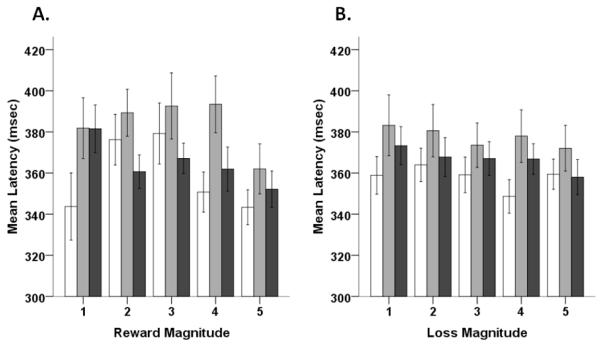
In terms of latencies on reward trials, we observed a significant main effect of magnitude, F(4,404)=5.16, p<0.001, ηP2=0.05, and an age group by magnitude interaction, F(8,404)=2.34, p<0.05, ηP2=0.04, but no main effect of age group (Figure 3a). Trend analysis of magnitude showed significant linear, F(1,101)=6.99, p<0.01, ηP2=0.07, and quadratic, F(1,101)=8.32, p<0.005, ηP2=0.08, components. Significant quadratic, F(2,101)=3.33, p<0.05, ηP2=0.01, and cubic, F(2,101)=4.10, p<0.05 ηP2=0.08, trends of magnitude on age group (magnitude by age interaction) were also observed. Latencies for younger and older adolescents showed a quadratic trend with faster responses at low and high magnitudes, while adults showed a more cubic trend (Figure 3a). Finally, similar to error rate findings, no significant effects of age group or magnitude were observed on loss trials (Figure 3b).
Figure 3.
Mean latencies for reward (A) and potential loss (B) trials across different magnitudes. For each graph, younger adolescents are represented by white bars, older adolescents are represented by gray bars, and adult are represented by black bars. Error bars represent +/− 1 SEM.
Finally, given the high overall performance of adults, we examined whether incentives had an effect on adult behavior by a using non-parametric sign test. Results showed that 22 of 42 (52.3%) adults generated more errors on neutral compared to 3-point loss trials (binomial distribution, p<0.001). For latencies of correct AS, we found that 28 of 42 (66.67%) adults generated an overall higher mean latency (slower response times) on potential loss trials compared to neutral trials. We also found that 29 of 42 (69%) adults had longer latencies on 3-point reward compared to neutral trials, Z=-2.32, p<0.05.
Effects of Incentive Type
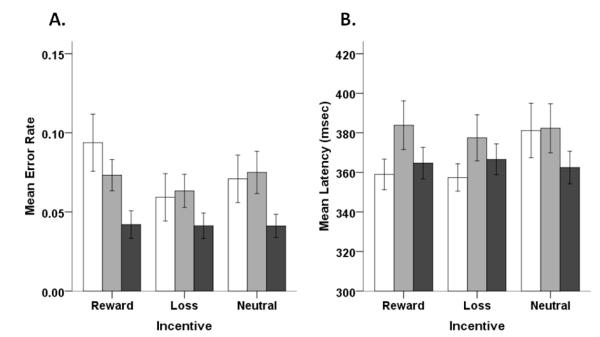
A comparison of mean error rates across incentive types showed a main effect of incentive, F(2, 206)=3.68, p<0.05, ηP2=0.03, and age group, F(2, 103)=3.28, p<0.05, ηP2=0.06, but no age group by incentive type interaction (Figure 4a). Pairwise comparisons showed that overall significantly more errors were made on reward trials relative to loss trials, t(105)=2.62, p<0.01. As can be seen in Figure 4a, this effect was driven by higher error rates in the adolescent subgroups, and in particular by the younger adolescents. For latency, we observed an age group by incentive interaction, F(4, 206)=3.43, p<0.01, ηP2=0.06, but no main effects of age group or incentive (Figure 4b).
Figure 4.
(A) Mean error rates for adolescent subgroups and adults across different incentive types (white bars = ‘younger’ adolescent subgroup, gray bars = ‘older’ adolescent subgroup, black bars = adults). (B) Latency of correct responses for adolescent subgroups and adults (white bars = younger adolescents, gray bars = older adolescents, black bars = adults). For both plots, error bars represent +/− 1 SEM.
To further explore effects related to incentive condition, we separately examined each age group’s response across the three incentive conditions. Younger adolescents made significantly more errors on reward compared to loss trials, t(31)=3.35, p<0.005. Younger adolescents also showed a trend for making more errors on reward compared to neutral trials, t(31)=1.99, p=0.056. Interestingly, younger adolescents also showed trends for making slower responses on neutral trials compared to reward, t(31)=1.87, p=0.071, and loss, t(31)=2.01, p=0.053).
Discussion
We examined the effects of varying magnitudes of potential reward and loss contingencies, and the effects of different types of incentives, on antisaccade task performance in healthy adolescents and young adults. Notably, we implemented several measures intended to minimize potentially confounding differences in reward value and subsequent motivation effects across age groups. Within this context, we found that incentive contingencies differentially affected error rates and latencies across age groups. Our findings are discussed in detail below.
Effects of Age Group and Reward Magnitude on Error Rates
For reward trials, we found that raw error rates varied with magnitude and also with age, but there was no age by magnitude interaction. In terms of age effects, younger adolescents made significantly more inhibitory errors than adults across reward magnitudes (Figure 2A). Younger adolescents also exhibited higher mean error rates across most of the magnitudes compared to older adolescents (Table 1), although these within-adolescence differences failed to reach statistical significance. Overall, this pattern of results is highly consistent with findings from previous developmental antisaccade studies indicating continued improvement in the ability to suppress task-inappropriate responses (i.e., response inhibition) with age (Fischer, et al., 1997; Klein & Foerster, 2001; Luna, et al., 2004; Munoz, et al., 1998).
As reward magnitude increased, error rates (collapsed across age group) tended to decrease, as indicated by a significant linear trend. Interestingly, error rates also increased slightly at the highest reward magnitude (quadratic trend). This minor increase could be reflective of a speed-accuracy trade-off as the fastest responses were also made on 5-point trials (Figure 3A). Our finding of decreased inhibitory errors on trials with a reward contingency is consistent with previous behavioral work and suggests that rewards can modulate inhibitory control systems (Geier, et al., 2010; Hardin, et al., 2007; Jazbec, et al., 2006; Jazbec, et al., 2005). Interestingly, previous work from Hardin et al. (2007) also used different magnitudes of reward and losses in an incentivized antisaccade task, but did not observe effects of magnitude in adolescents or adults. Our use of a broader range of reward magnitudes (5 levels compared to 3 in Hardin et al., 2007), as well as the measures implemented to increase reward salience and minimize differences in value across age groups, may have collectively contributed to the effects observed in this study.
Strikingly, on trials indicating a potential point loss, younger and older adolescents’ error rates dropped relative to reward trials. In fact, adolescent error rates on potential loss trials were on par with those of adults. One interpretation of these results is that adolescents may be particularly sensitive to motivation from potential losses. Interestingly, several adolescent participants anecdotally reported after the task that they found that “trying to avoid the buzz sound on loss trials” was particularly motivating. The qualitative shift observed between reward and loss trial performance also suggests that the influence of motivation on adolescent’s inhibitory control may be highly context specific.
In contrast to the adolescent groups, adults performed at a consistently high level during reward, loss, and neutral trials. These results ran counter to our expectation of increased performance gains on valenced trials. One possibility is that adults were not sensitive to the incentive range offered in this study. However, while adults did not show effects of incentive on error rate, they did show faster latencies to greater incentives indicating that they were at least sensitive to value. An alternative explanation for adults’ high performance rates is that they were better able to establish an inhibitory response set compared to adolescents (Rubia, et al., 2006; Velanova, et al., 2008), thereby facilitating an efficient strategy of maximizing performance across all trials regardless of the associated incentive contingency. Future experiments with visually guided saccade trials (in which participants simply have to look at the peripheral stimulus when it appears) interspersed with antisaccade trials might be able to disrupt the formation of an inhibitory response set and better position us to examine effects of different valenced incentives on AS task behavior in adults.
Interestingly, when error rates were adjusted to account for performance on neutral trials, we no longer observed any effects of age group or magnitude across reward trials. However, this result should be interpreted cautiously. Our a priori expectation was that neutral trials should be perceived as a ‘zero magnitude’ incentive stimulus and thus should be linearly related to performance on the various reward and loss magnitude trials. That is, we expected neutral trial performance to be slightly worse than the 1-point reward and loss trials, with each increase in magnitude resulting in greater differences from neutral. However, for the adolescents tested in this study, we found that error rates increased on 1-point trials relative to neutral. One interpretation of this result is that perhaps the neutral trials were not perceived as truly ‘neutral’ (i.e., free of incentive context) by adolescents. One possibility is that neutral trials may have been viewed as more of a loss (e.g., “I’m not going to win on this trial“) as opposed to a true ‘zero magnitude’ stimulus, at least in the context of this experiment. As such, adjusting for baseline differences in this task based on neutral trial performance may not be optimal. Broadly, this finding is in line with previous suggestions that neutral trials may be highly context dependent (Galvan et al., 2006), particularly across different age groups. One way to handle this issue in future work would be to test a group of participants on a typical antisaccade task in the same session but prior to an incentivized task. Performance on the typical antisaccade trials could be used to better adjust for between-group baseline differences and more accurately assess effects of incentives on task performance.
Effects of Age Group and Reward Magnitude on Latency
In contrast to error rates, we observed an age group by magnitude interaction for latencies on reward trials. For both younger and older adolescents, there was a significant quadratic trend across the various magnitudes. Lower (faster) latencies were observed for both groups at 1-point and 5-point trials. Adults, on the other hand, showed an initial drop in latency from 1-point to 2-point trials but relatively little change from 2 to 5-points. Most notably, all three age groups generated similarly fast responses when the maximum amount of potential points was available. These data suggest that antisaccade latencies in younger and older adolescents may be modulated across a broader range of potential reward magnitudes than adults. Moreover, these data indicate that younger and older adolescents can converge to adult-like speeds of responding when given sufficient motivation. These results are consistent with previous reports showing reduced latencies on rewarded trials in adolescents and adults (Hardin et al., 2007; Geier et al., 2010). Finally, similar to the error rate data, we did not observe any differences across groups on potential loss trials. This result, again, suggests that potential losses against a salient, valued reward might be particularly motivating to adolescents and can ‘push’ them towards mature levels of performance.
Effects of Incentive Type
In a comparison of error rates across incentive types (reward, loss, neutral), we observed main effects of incentive type and age group, but no interaction. When performance was collapsed across age groups, more errors were made on reward compared to loss trials. This effect was driven largely by the relatively high error rates generated by younger adolescents and, conversely, the very low error rates on loss trials across age groups. Neutral trial error rates did not differ with respect to reward or loss trials. This result was surprising as our expectation was that valenced trials (reward, loss) should result in fewer overall errors compared to neutral trials. However, as discussed above, it is not clear in this experiment whether neutral trials were indeed perceived as zero magnitude stimuli. In terms of latencies, we observed an age group by incentive type interaction. For younger adolescents, reward and loss latencies decreased relative to neutral (Figure 4B), while older adolescents and adults showed relatively consistent speeds of responding. This result suggests that incentives may have a greater effect on voluntary responses during younger compared to older adolescence and adulthood. This interpretation is in line with results from the developmental reward literature indicating heightened reward effects in younger adolescents (van Leijenhorst, et al., 2010).
Possible Mechanisms Underlying Motivated Eye Movements across Development
The results presented in this study suggest that incentives can differentially affect performance on the antisaccade task across development. An important next step will be to pursue a more mechanistic explanation of how rewards affect oculomotor and control systems in humans. The neural circuitry supporting rewarded eye movements has already been exquisitely well-characterized at the single cell level in non-human primates (Amador, Schlag-Rey, & Schlag, 2000; Hikosaka, Takikawa, & Kawagoe, 2000; Kawagoe, Takikawa, & Hikosaka, 1998). These studies have clearly shown that dopamine (DA) processing in basal ganglia circuits is crucial for both reward- and punishment-dependent modulation of saccadic eye movements. The involvement of DA is particularly relevant in the context of the current developmental study given converging evidence from human and animal models showing that DA neurotransmission in the striatum and prefrontal cortex (PFC) undergoes continued maturation during adolescence (Galvan, 2010; Spear, 2000). In brief, the available evidence from rodent and human models suggests that DA synthesis, availability, and activity levels all peak during adolescence, suggesting that rewards play a particularly important role in adolescent behavior. One possibility is that the unique constellation of dopamine receptor densities and activity levels in task-essential PFC and BG circuits present during younger and older adolescence compared to adulthood may underlie the different patterns of incentive-modulated behavior observed in this study.
Conclusions
In this study, healthy adolescents and adults were tested using a novel incentivized antisaccade task. Importantly, we implemented methods aimed at minimizing developmental differences in value of the reward for which participants were working in order to reduce potentially confounding motivation effects. Within this context, we found that the adolescent subgroups showed the most variability in terms of the influence of incentives on antisaccade error rates and latencies. Adults, in contrast, showed consistent levels of performance across incentive conditions. The results of this study indicate that the processes involved in motivated inhibitory control are likely still maturing during adolescence.
Performance on the incentive-mediated antisaccade task presented here is an abstraction from the complexities of adolescent risk-taking behaviors (e.g., participation in extreme sports, risky sexual behavior). However, the systems underlying essential components of this task, response inhibition and incentive processing, are known to be engaged during more complex decision-making. As such, continued investigation of the normative development of the interaction between these basic systems will likely yield a more complete understanding of the emergence of complex behaviors like risk taking.
Acknowledgments
This study was supported by a grant from the National Institute of Mental Health (NIMH, 5-R01 MH080243). The authors thank Melanie Wilds-Liebman, Natalie Nawarawong, Catherine Wright, Barbara Fritz, David Montez, Meg Meachim, Heather Jack, and Alina Vaisleib for their assistance with data collection and other aspects of this study. We also thank three anonymous reviewers for their comments on earlier drafts.
References
- Amador N, Schlag-Rey M, Schlag J. Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. Journal of Neurophysiology. 2000;84(4):2166–2170. doi: 10.1152/jn.2000.84.4.2166. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Developmental Review. 1992;12(4):339–373. [Google Scholar]
- Balleine BW, Doya K, O’Doherty J, Sakagami M. Current trends in decision making. Annals of the New York Academy of Sciences. 2007;1104:xi–xv. doi: 10.1196/annals.1390.2226. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. Journal of Neuroscience. 2007;27(18):4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaukopf CL, DiGirolamo GJ. Differential effects of reward and punishment on conscious and unconscious eye movements. Experimental Brain Research. 2006;174(4):786–792. doi: 10.1007/s00221-006-0685-2. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Rolls ET. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. Journal of Neurophysiology. 1996;75(4):1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. Journal of Neurophysiology. 2008;99(1):133–145. doi: 10.1152/jn.00899.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441(7095):876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cognitive Affective and Behavioral Neuroscience. 2003;3(1):27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Lupp A. The effects of incentive on antisaccades: is a dopaminergic mechanism involved. Behavioral Pharmacology. 1997;8(5):373–382. [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biological Psychiatry. 2005;58(8):597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Biscaldi M, Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Research Reviews. 1997;754(1-2):285–297. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. Raven Press; New York: 1989. [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885–2692. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR. ILAB: a program for postexperimental eye movement analysis. Behavioral Research Methods, Instruments, and Computers. 2002;34(4):605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48(5):446–454. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Cromwell HC, Schultz W. Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. Journal of Neurophysiology. 2001;85(6):2477–2489. doi: 10.1152/jn.2001.85.6.2477. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiological Reviews. 2000;80(3):953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, Ernst M. Age-related influence of contingencies on a saccade task. Experimental Brain Research. 2006;174(4):754–762. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biological Psychiatry. 2005;58(8):632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nature Neurosci. 1998;1(5):411–416. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Klein C, Foerster F. Development of prosaccade and antisaccade task performance in participants aged 6 to 26 years. Psychophysiology. 2001;38(2):179–189. [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron. 1999;24(2):415–425. doi: 10.1016/s0896-6273(00)80854-5. [DOI] [PubMed] [Google Scholar]
- Levin HS, Culhane KA, Hartmann J, Evankovich K, Mattson AJ. Developmental changes in performance on tests of purported frontal lobe functioning. Developmental Neuropsychology. 1991;7:377–395. [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Logan GD, Gordon SE. Executive control of visual attention in dual-task situations. Psychological Review. 2001;108(2):393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36(3):273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Reviews in Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;121(4):391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinions in Neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Paus T, Babenko V, Radil T. Development of an ability to maintain verbally instructed central gaze fixation studied in 8- to 10-year-old children. International Journal of Psychophysiology. 1990;10(1):53–61. doi: 10.1016/0167-8760(90)90045-f. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience. 2008;9(7):545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Band GPH, Logan GD. A study of adaptive behavior: effects of age and irrelevant information on the ability to inhibit one’s actions. Acta Psychologica. 1999;101:315–337. [Google Scholar]
- Ridderinkhof KR, van der Molen MW. Mental resources, processing speed, and inhibitory control: a developmental perspective. Biological Psychology. 1997;45:241–261. doi: 10.1016/s0301-0511(96)05230-1. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. Journl of Neurophysiology. 2003;90(3):1766–1789. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304(5668):307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Revies Neuroscience. 2000;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Stellar JR, Stellar E. The Neurobiology of Motiviation and Reward. Springer-Verlag; New York: 1985. [Google Scholar]
- Tipper SP, Bourque TA, Anderson SH, Brehaut JC. Mechanisms of attention: a developmental study. Journal of Experimental Child Psychology. 1989;48(3):353–378. doi: 10.1016/0022-0965(89)90047-7. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398(6729):704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Sawaguchi T. Neuronal activity representing temporal precition of reward in the primate prefrontal cortex. Journal of Neurophysiology. 2005;93(6):3687–3692. doi: 10.1152/jn.01149.2004. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44(11):2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational Changes in Anterior Cingulate and Frontoparietal Recruitment Support the Development of Error Processing and Inhibitory Control. Cerebral Cortex. 2008;18(11):2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. European Journal of Neuroscience. 2003;18(7):2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35(1):205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]