Abstract
Objective
The benefits of cochlear implantation for children with developmental delays (DD) are often unclear. We compared cognition, adaptive behavior, familial stress, and communication in children with and without DD.
Study Design
Retrospective review
Setting
Two tertiary care pediatric hospitals
Patients
204 children who underwent cochlear implantation assessed before and >1 year after implantation
Main Outcome Measures
The Mullen Scales of Early Learning (MSEL), Vineland Adaptive Behavior Scales (VABS), Parental Stress Index (PSI), and Preschool Language Scale (PLS).
Results
We developed a specific definition of DD for hearing-impaired children based upon DSM-IV criteria for mental retardation; 60 children met the criteria for DD and 144 children did not. Prior to implantation, multiple linear regression demonstrated that children with DD had lower scores in every domain of the MSEL and VABS (p<0.05) but no differences in any domains of the PSI and PLS (p>0.1) compared to children without DD. After implantation, children without DD demonstrated significant improvements in intelligence as measured by the MSEL, age-appropriate improvements in adaptive behavior as evaluated by the VABS, and their familial stress levels were not increased after cochlear implantation. In contrast, children with DD underwent implantation at a later age and demonstrated less comprehensive developmental improvements after cochlear implantation and higher stress levels. However, when the age differences were taken into account using multiple linear regression analyses, the differences between two cohorts were reduced.
Conclusions
These data indicate that our definition of DD is a reliable method of stratifying deaf children. While children with DD have a normal developmental rate of adaptive behavior after cochlear implantation, their developmental rate of intelligence is lower and they have higher stress levels than children without DD. However, our data suggest that if children with DD could be implanted as early as children without DD, their intelligence and stress outcomes would be improved.
Introduction
Developmental delay (DD) is a pattern of persistently slow learning of basic motor and language skills during childhood. The definition of DD includes diminished intelligence (the capacity for learning, reasoning, and understanding) and diminished adaptive abilities (the ability to function and cope within the environment) (1). The many different causes include syndromes (Down, Fragile X, etc.), other genetic conditions, problems during pregnancy (fetal alcohol syndrome, maternal rubella infection, etc.), hypoxia at birth, CNS infections (CMV, meningitis, etc.), toxic exposure (lead, mercury, etc.), cretinism, malnutrition, brain trauma during delivery, and psycho-social disadvantages. Premature infants are at particularly high risk of DD(2). Roughly 20–25% of children born at <28 weeks gestational age have DD(3–4). Compared to those born at term, children with birth weights <750g have a significantly higher risk of having DD diagnosed by school-age (21% vs. 2%). Oxygen dependence at 36 weeks of age (bronchopulmonary dysplasia) was also associated with DD (odds ratio 4.5)(5–6).
Approximately 2–3 in 1000 children are born with a significant degree of sensorineural hearing loss (7–8). It is estimated that about 25% of the cases of congenital hearing loss are attributed to identifiable prenatal or postnatal disease or trauma, 18% to undiagnosed genetic factors, 15% to autosomal dominant genetic mutations, 40% to autosomal recessive genetic mutations, and 2% to sex-linked genetic mutations(9). Indigent patients are at a higher risk of neonatal hearing loss than the average U.S. population (10). Prematurity and low birth weight are also risk factors for sensorineural hearing loss (10–13).
Because DD and hearing loss are both extremely common (the first and fourth most common developmental disorders in the U.S., respectively(14)), there are many children with DD who have hearing loss severe enough to be potential candidates for cochlear implantation. While pediatric cochlear implantation has become the most popular treatment for deaf children with normal cognition, it is used only sporadically for deaf children with DD. This is because the traditional clinical aim of auditory habilitation of patients with congenital hearing loss is to achieve oral communication. However, children with DD have reduced ability to use auditory information to learn speech and language. For a child with both hearing loss and DD, the DD is likely to be the ultimate limiting disability affecting his/her long-term outcomes(15).
Several studies have evaluated speech and language development following cochlear implantation in populations of multiply handicapped children(16–22). Overall, these studies indicated that a baseline level of linguistic skill was attainable, but that progress and skill acquisition were both slower and more limited than that seen in patients who experienced only hearing loss. However, because there is no standard way to define DD in deaf children, there is substantial variability among the patients enrolled in the different studies. As well, standardized speech and language tests may not accurately assess benefit in this patient population, because children with DD often remain at the floor on the range of test scores and it is difficult to demonstrate changes after implantation.
The definition of DD is even more difficult to make in children younger than 2 or 3 years of age because many of the standardized tests are not validated for children this young. This is critical because cochlear implantation has been shown to be more effective the younger the child(23). Even in older age children, it is difficult to perform many of these tests because they require that the child be able to hear in order to take the test.
One component of our routine pediatric hearing loss and cochlear implant evaluation and follow-up process includes psychological testing to measure cognitive skills, assessments that are not performed routinely by most pediatric centers. Over the years, we have noticed that some children with DD demonstrated surprising improvements in cognitive development after cochlear implantation. Therefore, we hypothesized that cochlear implantation provides important benefits to cognitive development in deaf children with DD. We performed a retrospective study of pediatric patients who underwent cochlear implantation to address this issue. Our goal was to first develop a definition of DD for children with severe-to-profound hearing loss in which the value of a cochlear implant would be significantly questioned in many cochlear implant centers in the United States. We then used this definition to compare those with and without DD before and after cochlear implantation.
Methods
Patient population
Potential study candidates were identified from the two university pediatric hearing loss clinics with large cochlear implant programs. IRB approval was obtained from both institutions. All patients with severe-to-profound sensorineural hearing loss receive a complete history and physical exam, a medical and genetic test battery, and audiological, speech/language, and psychological testing, as part of their initial hearing loss evaluation(24). Thus, pre-implant evaluations were done at whatever age the child presented to our clinic for evaluation. However, children did not undergo psychological or speech/language testing until at least 9 months of age in order to improve test reliability.
For this research study, we only included children who were implanted <8 years of age. This was done in an effort to exclude those children who were deaf for many years and were well past the critical window for language development(8). We did not include children who did not undergo cochlear implantation because our programs do not routinely perform psychological testing on deaf children that our team members did not feel would be good candidates for cochlear implantation. This decision was based upon the clinical expertise of the team members and was in accordance with the limited level of clinical understanding available.
Not all test data are available for every patient because we did not perform all tests when we first started this process, and we gradually evolved to include these specific measures(25). As well, in our clinical paradigm, follow-up exams after cochlear implantation are recommended on an annual basis. However, these are not required and are left to the parent to schedule. Therefore, not all children got follow-up testing. Children who did return for follow-up testing received tests identical to those they had pre-implant for easy comparison of outcomes.
Psychological testing
These tests were administered by licensed psychologists and psychological associates within our programs. They also scored and interpreted the responses.
The Mullen Scales of Early Learning (MSEL) (American Guidance Service, Inc, Circle Pines, MN) is an objective standardized test designed for young children that was used to estimate intelligence. The age range for the Mullen is birth to 5 years, 8 months, though it can be used in older children with significant cognitive impairment such as those included in this study. Items administered for this test load onto five different domains (Visual Reception, Gross Motor, Fine Motor, Expressive Language, and Receptive Language). Because verbal and nonverbal domains are assessed separately, this measure is particularly useful in the assessment of children who have suspected language delays and/or hearing loss. We used the raw scores (rather than age-standardized scores) in order to have the most sensitivity in detecting changes in patients with DD who tend to cluster at the low end of the percentile range.
The Vineland Adaptive Behavior Scales (VABS) (American Guidance Service, Inc, Circle Pines, MN) was used to measure adaptive behavior. This describes the child’s ability to cope and function effectively within his/her environment. This standardized measure is a structured parent interview and can be performed with caregivers of children from birth to adulthood. In its entirety, the measure provides information regarding a child’s functioning across several domains including Communication, Socialization, Motor Skills, and Daily Living Skills.
The Parenting Stress Index (PSI) (Psychological Assessment Resources, Inc, Odessa, FL) was used to objectively measure familial stress and quality of life. It identifies factors that increase stress in the child-parent system according to parental perception, and we use this test as a measure of quality of life. It can be performed in children from one month to twelve years of age, is only available in English, and requires that the parents have at least a 5th grade reading level. The PSI Total Stress score is comprised of two domain scores. The parent domain measures the degree to which the stress of parenting is impacting a caretaker’s ability to function, and the child domain measures child behaviors that are likely to impact familial stress. Stress scores of 188–258 are considered average (mean=223, SD=37), and lower scores represent less stress.
Speech and language testing
The only standardized test of speech and language included in this study is the Preschool Language Scale (PLS). The PLS is used in patients with minimal vocabulary, was designed specifically for use with young children, and has been validated from birth to 6 years, 11 months of age. This test was performed by licensed pediatric speech pathologists within our programs who specialize in working with children with hearing loss.
Definition of developmental delay (DD)
Because many centers will not routinely implant children with mental retardation, we used this as the starting point for creating our definition of DD. However, it is impossible (and inappropriate) to diagnose young children with mental retardation because of substantial inter-patient variability in developmental rate. Children who have significant delays may occasionally catch up over time.
Nevertheless, mental retardation is a well-established term defined by Diagnostic and Statistical Manual-Fourth Edition: Text Revised (DSM-IV-TR, American Psychiatric Association, Washington, DC) as requiring both diminished intelligence and adaptive abilities(1). Specifically, the patient must meet three criteria: 1) their intelligence is >2SD below the mean, 2) they have significant limitations in two or more adaptive skill areas, and 3) the condition is present from childhood (defined as age 18 or less). The reduced adaptive functioning has to be present in at least in two domain areas, but the amount of reduction required is not specifically stated.
For our definition of DD, we chose to use the MSEL - Visual Reception domain score as the measure of intelligence. It tests nonverbal problem solving, and while this can be affected by severe visual problems, the level of retinopathy of prematurity that is typically found in afflicted NICU patients does not affect this score. We did not use the other domain scores in making the diagnosis of DD because of the potential that confounding disabilities could impact the results. For example, the Gross and Fine Motor scores are reduced by other physical disabilities often found in patients with central nervous system disease and the Expressive and Receptive Language scores are reduced by the hearing loss. When identifying a child >2SD below the mean, the scaled score was used (mean=50, SD=10).
For similar reasons, we chose to use the Vineland Adaptive Behavior Scales Daily Living Skills domain score as the primary measure of adaptive abilities. The Communication and Socialization domains are impacted by the hearing loss and the Motor Skills domain is reduced by physical disabilities. As no specific level was set by DSM-IV-TR criteria, we chose to include children whose scaled score was > 1SD below the mean (mean=100, SD=15). Thus, patients had to have both the Daily Living Skills domain as well as one additional domain from the VABS below this threshold.
Therefore, patients were considered to have DD if they met two neurocognitive criteria: (1) their intelligence was 2 SD or more below the mean as measured by the visual reception domain score in the MSEL and (2) their adaptive behavior was 1 SD or more below the mean as measured by the Daily Living Scale domain and one other domain of the VABS. Since all patients were children, they already met the third DSM-IV-TR criteria for mental retardation.
Statistical Analysis
All data were organized in Excel (2007, Microsoft, Seattle, WA). Paired and non-paired Student’s T-Tests, as well as simple linear regression, were performed in Excel. The paired T-test was used to compare changes in test scores from pre-op to post-op, whereas the non-paired T-test was used to compare means among different patient populations. Multiple linear regression analyses were performed in SPSS (IBM, Armonk, NY). All plotting was done using Sigmaplot (11.0, Systat Software, San Jose, CA). The threshold for statistical significance was set to be p<0.05, and for clarity we have provided the actual p-values for every analysis throughout the paper.
Results
Stratification of patients by DD status
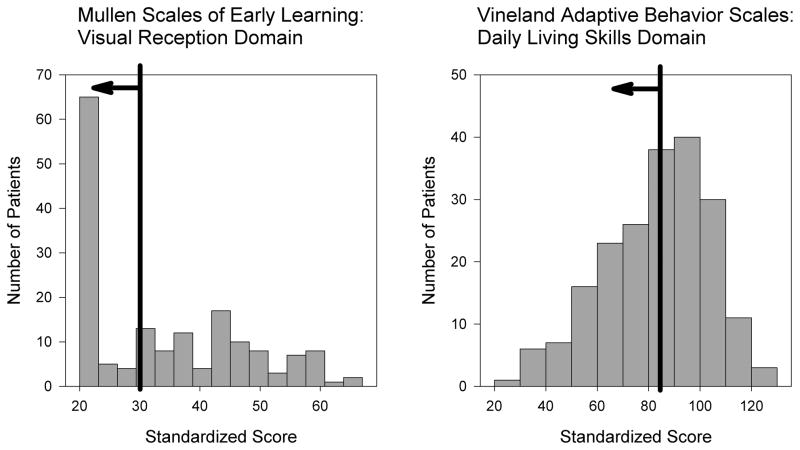
We collected baseline neurocognitive data on 204 children being evaluated for cochlear implantation as part of their candidacy process. These patients were then stratified into either having DD or not having DD based on our definition. Of these children, 71 (35%) met the first criterion (intelligence 2 SD or more below the mean) and 96 (47%) met the second criterion (adaptive behavior 1 SD or more below the mean). Together, 60 children (29%) met both criteria and thus were considered to have DD whereas the remaining 144 children (71%) were considered to not have DD (Figure 1).
Figure 1. Selection of study candidates based on intelligence and adaptive behavior scores.
Histograms of the MSEL-visual reception scaled T-scores (left) and VABS-daily living skills standard scores (right) from the baseline evaluations are shown. Patients to the left of the black line meet the criteria for developmental delay (DD).
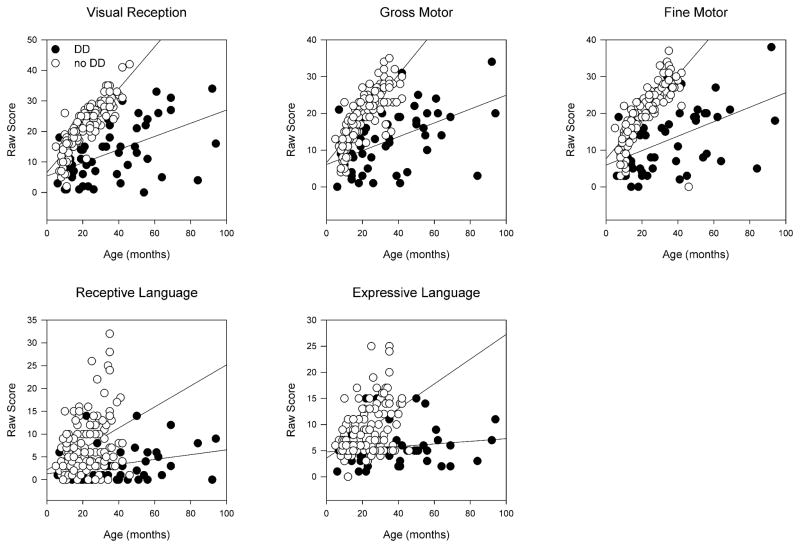
Analyses of the baseline data were performed to calculate the standard deviations and age-dependence for each domain for children with and without DD (Figure 2). We fit simple linear regression lines of domain score versus age to the two cohorts of children (slopes are given in the figure legend). We then performed multiple linear regression analyses using patient age and the presence or absence of DD as independent variables. The measures of intelligence assessed by the MSEL demonstrated reduced regression line slopes for every domain when the patient had DD (p-values given in figure legend). Thus, even though the visual reception domain was the only domain used in the definition of DD, the other domains also showed remarkable reductions.
Figure 2. Baseline MSEL domain raw scores.
These scores are not corrected for age. Children with DD had statistically lower scores in every domain, after accounting for age. The slope of each line (change in score/change in months) and the p-value comparing patients with DD to those without DD after performing multiple linear regression analyses is given for each domain. Visual Reception (DD: 0.216, no DD: 0.686, p<0.001); Gross Motor (DD: 0.189, no DD: 0.598, p<0.001); Fine Motor (DD: 0.197, no DD: 0.569, p<0.001); Receptive Language (DD: 0.0526, no DD: 0.23, p<0.001); Expressive Language (DD: 0.0255, no DD: 0.237, p<0.001).
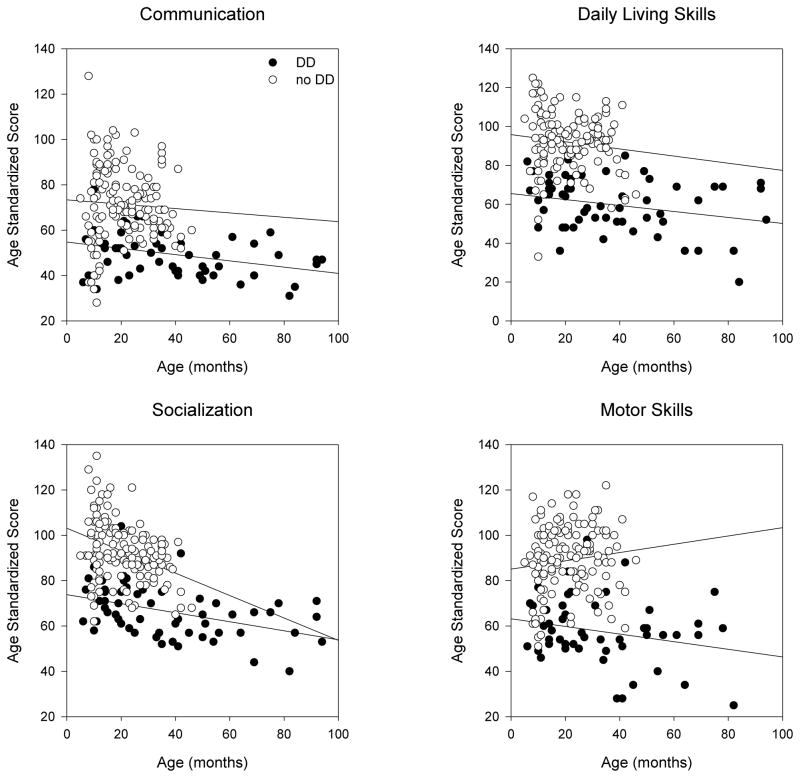
The VABS is age-standardized and so normal development is indicated by a flat line for each domain (i.e. slope=0). Delayed development is indicted by a line with a negative slope. We performed multiple linear regression analyses using the presence or absence of DD as an independent variable. Even though age should have been accounted for by the test scoring process itself, we analyzed the data two different ways, with and without using age as an additional independent variable. Both analytic techniques demonstrated that all domains of the VABS were significantly reduced in children with DD (Figure 3).
Figure 3. Baseline VABS domain scores.
These scores are corrected for age. Children with DD had statistically lower scores in every domain. Thus, even though the slopes of the two lines are not as obviously different as in the MSEL domains, there is a clear gap between the lines along the vertical dimension. The slope of each line (change in score/change in months) and the p-value comparing patients with DD to those without DD after performing multiple linear regression analyses is given for each domain. Communication (DD: 0.0252, no DD: −0.096, p<0.001); Daily Living Skills (DD: 0.00569, no DD: −0.184, p<0.001); Socialization (DD: −0.0678, no DD: −0.494, p<0.001); Motor Skills (DD: −0.168, no DD: 0.183, p<0.001).
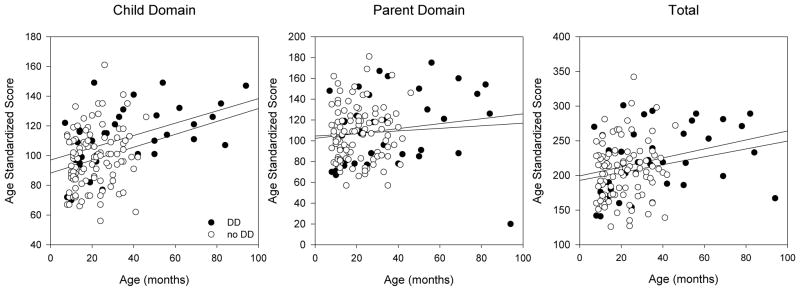
The PSI does not change with the age of the child and we performed identical statistical analyses. We found that there were no differences in either the child or the parent domain scores (Figure 4). Thus, there were similar levels of stress in families that have children with DD compared to those that have children without DD
Figure 4. Baseline PSI domain scores.
These scores are not corrected for age and lower values indicate less stress. Children with DD did not have any domains with statistically-significant differences compared to children without DD. The slope of each line (change in score/change in months) and the p-value comparing patients with DD to those without DD after performing multiple linear regression analyses is given for each domain. Child Domain (DD: 0.289, no DD: 0.434, p=0.180); Parent Domain (DD: 0.0079, no DD: 0.123, p=0.373); Total (DD: 0.298, no DD: 0.749, p=0.994).
For the speech and language evaluations, the PLS test raw scores are not age-adjusted. Our analyses including age as an independent variable demonstrated that children with DD had similar regression line slopes compared to children without DD (Figure 5). Thus, a subject’s DD status had no measureable impact on how the PLS test estimated his/her speech and language abilities in our population of children with severe-to-profound hearing loss.
Figure 5. Baseline PLS domain raw scores.
These scores are not corrected for age. Children with and without DD had similar domain scores after accounting for age. The slope of each line (change in score/change in months) and the p-value comparing patients with DD to those without DD after performing multiple linear regression analyses is given for each domain. Auditory Comprehension (DD: 0.0859, no DD: 0.0854, p=0.310); Expressive Communication (DD: 0.115, no DD: 0.141, p=0.342).
Effect of cochlear implantation in children with and without DD
We collected follow-up cognitive and stress data in children who subsequently underwent cochlear implantation to assess for changes in the test scores as a result of this intervention. A total of 36 patients from the original 204 patients (18%) returned for follow-up testing. The follow-up rate for each cohort was similar, including 12 with DD (20%) and 24 without DD (17%). However, children with DD were initially implanted at an older age than children without DD (25 ± 4 months vs. 16 ± 2 months, respectively; p=0.026). This difference reflects the increased complexity of medical care for children with DD early in life, in which they often required many surgical procedures for other disorders such as feeding, airway, and craniofacial abnormalities. Thus, by the time they presented to our clinic they tended to be older than children without DD.
We stratified these data so that the outcomes of patients with DD and without DD could be assessed independently. The average follow-up time for children with DD was 32 ± 5 months whereas it was 22 ± 2 months for children without DD. Because of this difference and because the follow-up times between patients within each group were variable, we normalized the change for each patient to a two-year follow-up period. This was done by calculating the change in domain score per month, and then multiplying by 24 months.
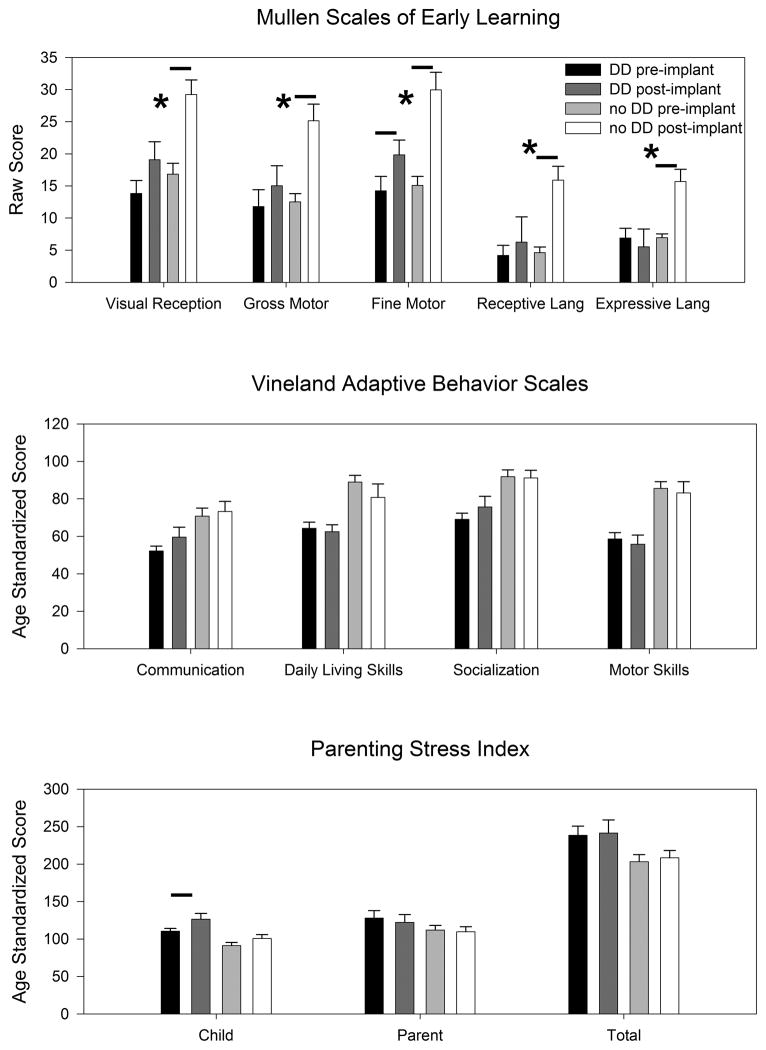
Children without DD had statistically-significant improvements in every domain of the MSEL (Figure 6). This is what one would expect because raw scores (which are not corrected for age), were used in analyses. In contrast, children with DD demonstrated statistically-significant improvements only in the Fine Motor domain. As well, the changes in every domain score for the MSEL were significantly larger in those children without DD compared to those with DD. With the VABS, neither group of children demonstrated changes in any domain score. Because the scores are corrected for age, this is consistent with a normal developmental trajectory. As measured by the PSI, children with DD demonstrated increased stress in the Child domain, whereas children without DD did not. This suggests that children with DD exhibited an increase in problematic behaviors affecting the parent-child relationship.
Figure 6. Cochlear implant cognitive and stress outcomes stratified by the presence or absence of developmental delay.
Data are normalized to a two-year follow-up period. Small horizontal bars indicate statistically-significant changes in the scores from pre-implant to post-implant (p<0.05). The asterisks indicate when the change was statistically larger in children without DD compared to those with DD. Note that the Vineland Adaptive Behavior Scales is normalized for age. Thus, the lack of change in a domain score indicates that the child is developing at an age-appropriate rate.
Furthermore, we performed multiple linear regression analyses on these data to assess the impact of the differences in the average age at implantation between the two cohorts (Table). Both the presence of a developmental delay and the age at implantation were independent variables and the change in each of the domain scores for the MSEL, the VABS, and the PSI were dependent variables. This demonstrated that the age at implantation was a confounding variable for the MSEL, in that the change in every domain score was either statistically correlated (p<0.05) or suggestive of an association without meeting the threshold for statistical significance (0.05<p<0.1). However, there was no effect of the age at implantation on the domains of the VABS or PSI. The presence of a developmental delay was only statistically significant for the fine motor domain of the MSEL (a negative correlation, i.e. the presence of DD was associated with decreased fine motor development), and was not a factor for any domain of the VABS or the PSI. Thus, the multiple linear regression analyses corroborated the above mentioned lack of an effect of DD status on adaptive behavior, but suggested that the larger improvements in the intelligence in children without DD appear to be due predominantly to the fact that they were implanted earlier than children with DD. Similarly, elevated stress in children with DD appears to be related to their later age of implantation.
Table.
multiple linear regression analyses of changes after cochlear implantation. The dependent variables are listed in the leftmost column, and the presence of a developmental delay and the age at implantation were independent variables. Statistically significant values are in bold font.
| Presence of Developmental Delay | Age at Implantation | |
|---|---|---|
| MSEL | ||
| Visual Reception | 0.145 | 0.073 |
| Gross Motor | 0.248 | 0.002 |
| Fine Motor | 0.001 | 0.005 |
| Receptive Language | 0.818 | 0.062 |
| Expressive Language | 0.170 | 0.078 |
| VABS | ||
| Communication | 0.579 | 0.587 |
| Daily Living Skills | 0.764 | 0.259 |
| Socialization | 0.571 | 0.587 |
| Motor Skills | 0.937 | 0.781 |
| PSI | ||
| Child Domain | 0.427 | 0.727 |
| Parent Domain | 0.900 | 0.602 |
| Total | 0.866 | 0.890 |
Discussion
Children with special needs require complex, individualized therapy to maximize their long-term quality of life. Herein, we show that our objective definition of DD is a reliable method of stratifying deaf children because those who meet the criteria for DD have lower test scores in every domain area of the MSEL and the VABS. Using this definition, we compared cognitive and stress outcomes after cochlear implantation in patients with and without DD. Children without DD demonstrated significant improvements in intelligence as measured by the MSEL, age-appropriate improvements in adaptive behavior as evaluated by the VABS, and their familial stress levels were not increased after cochlear implantation. In contrast, children with DD underwent implantation at a later age and demonstrated less comprehensive developmental improvements after cochlear implantation. Thus, children with DD not only started off with a lower level of intelligence than those without DD, but also had a lower rate of intellectual development. In contrast, children with DD started at a lower level of adaptive behavior than those without DD but after implantation, they did not fall further behind during the time we followed them. As an assessment of quality of life, the PSI demonstrated that families of children with DD experienced elevated stress, even though they started with a similar stress level to families of children without DD. However after statistically accounting for the later age at implantation, the effect of having developmental delays on the change in intelligence and stress resolved. Taken together, we conclude that cochlear implantation does not adversely affect development in children with DD. Indeed, a normal developmental acquisition rate of adaptive behavior occurs and if implanted early enough, a normal rate of cognitive development may be anticipated. However, one consequence of cochlear implantation is the potential risk of increased familial stress, particularly if implanted at a later age.
However, there are several caveats to consider. While of course the age of the child at implantation is critical to developmental outcomes, it is not a simple matter to implant children with DD earlier. In this study, children with DD were implanted at a later age than children without DD, and this was because they had multiple co-morbidities that delayed their presentation to our cochlear implant centers. It may not be feasible to implant these children as early as children without DD are typically implanted. As well, it is possible that the data we present herein may not be representative of the majority of deaf children with DD. This is because the children with DD that we studied in this report formed a select group that we thought, based on the clinical experience of our team members, had the best chance of benefiting from cochlear implantation. A better control group would be a group of children with DD that do not undergo implantation. While our team did not follow the rest of the children with DD who did not undergo cochlear implantation, we would expect them to have worse outcomes had they undergone cochlear implantation. A randomized, prospective, controlled clinical trial would effectively answer this question of whether or not to perform cochlear implantation in children with DD. We have started such a trial and are actively enrolling patients at our two sites (ClinicalTrials.gov Identifier: NCT01256229) (26).
It should be noted that children with DD generally had domain scores lower than children without DD and that these average domain scores were all statistically different. This validated our definition of DD in subdividing our patient population. As well, these preliminary analyses demonstrate that children with DD did not experience floor effects on the chosen measures. Thus, we were able to detect improvements or declines in each group.
Caution is warranted, however, when using these tests to stratify children into categories. Many children had domain scores that were surprisingly better or worse than expected. For example, many of the unfilled dots (children without DD) in Figures 2–5 are mixed in with the filled dots (children with DD) and vice versa. This heterogeneity likely describes how children can develop in individual patterns and along variable timelines. Thus, it is critical to not rigorously adhere to strict criteria when deciding whether or not to implant a child that potentially has DD. Rather, we have found a variety of factors influencing ultimate outcome following CI including, but not limited to, familial support, quality and frequency of speech/language therapy, utilization of an Auditory-Verbal approach to speech/language therapy. However, many of these factors are difficult to reliably assess in an objective fashion.
There may be improvements associated with cochlear implantation that we did not assess with our testing paradigm. We did not use more complex outcome measures (such as the Reynell Developmental Language Scales and others) (23, 27) because it was clear from our clinical evaluation that these children with DD would barely rise above the lowest score. However, there are non-standard testing paradigms available to assess more subtle forms of communication. For example, video recordings of speech therapy sessions can be analyzed and coded for very subtle forms of verbal and non-verbal communication (such as grunts and eye movements) using editing and linking capabilities in multiplatform software such as CLAN (www.childes.psy.cmu.edu/clan/), ELAN (www.latmpi.eu/tools/elan/), and PRAAT (www.fon.hum.uva.nl/praat/). Compilation of data using this technique is allowing for analysis of nonverbal behavior that has been identified as an important indicator of subsequent communication ability in difficult-to-test populations (28–29). We used the MSEL, VABS, and PSI for this study because these tests are commonly used in deaf children and vary in a linear fashion with age (30–33).
Because of the lower cortical processing ability of a child with DD, the value of the auditory information provided by a cochlear implant is questionable. Multiple handicaps, such as hearing loss and cognitive delay, interact synergistically to magnify the adverse effects on development. It has been proposed that all areas of development are interrelated and that deficiencies in one area, such as communication, can have broader effects on each of the others (34). Such a phenomenon has been termed a “pseudo-handicap effect” (16). The pseudo-handicap effect suggests that the presence of one handicap, especially a neurosensory handicap, can potentiate the effects of other handicaps by reducing a child’s ability to interact with the environment, further limiting his/her potential for cognitive growth. This is concerning because it suggests that if a child with DD also has an inadequately-treated hearing loss, his/her cognitive delays may worsen.
However, there is little compelling evidence supporting the idea that cochlear implantation provides benefit to children who do not have the cognitive potential to develop speech and language. As well, pediatric cochlear implantation does entail a certain level of risk and a substantial allocation of medical, educational, and family resources. In the end, risk-to-benefit and cost-to-benefit analyses drive many centers to not implant deaf children if they have severe cognitive delays. Prior to beginning our prospective clinical trial, we used the clinical judgment of our cochlear implant team members when deciding whether or not to offer cochlear implantation as a treatment option to any child. We now strive to enroll every child with DD into the above-mentioned study. Even if the parents of a child with a DD decline to be randomized, we are enrolling that child in a prospective longitudinal follow-up cohort to assess whether their developmental rate is indeed normal after implantation, if they are implanted early enough. No matter what the developmental status of a child, we still consider it mandatory that the parents have appropriate expectations regarding outcomes after cochlear implantation. We consider appropriate parental expectations after cochlear implantation of a child with DD to be improved sound awareness and slightly better interactions with their surrounding environment, but not necessarily the development oral communication skills.
Appropriate expectations are particularly critical in situations where a child has DD, because it is not unusual for the parents to focus on the inaccurate conception that providing their children with hearing will solve the multitude of other disabilities they face. This is only human, and is to be expected when a parent is facing such a devastating situation such as having a child with special needs. However, we believe that with appropriate counseling from a multi-disciplinary team (ours includes otolaryngology, audiology, speech/language pathology, psychology, social work, physical therapy, developmental pediatrics, genetics, and ophthalmology), many parents can move past this wishful yet inaccurate conception to more realistic and appropriate expectations about long-term outcome. When they reach this point, we offer cochlear implantation as a potential treatment option.
Acknowledgments
This work was supported by NIH R01 DC010075. E. O’Brian Smith provided biostatistical support.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. Task Force on DSM-IV. [Google Scholar]
- 2.Hack M, Taylor HG, Drotar D, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. Jama. 2005;294:318–25. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz JM. The outcome of extreme prematurity. Seminars in perinatology. 2001;25:348–59. doi: 10.1053/sper.2001.27164. [DOI] [PubMed] [Google Scholar]
- 4.Msall ME, Buck GM, Rogers BT, et al. Risk factors for major neurodevelopmental impairments and need for special education resources in extremely premature infants. The Journal of pediatrics. 1991;119:606–14. doi: 10.1016/s0022-3476(05)82415-1. [DOI] [PubMed] [Google Scholar]
- 5.Hack M, Taylor HG, Klein N, et al. School-age outcomes in children with birth weights under 750 g. N Engl J Med. 1994;331:753–9. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- 6.Taylor HG, Klein N, Drotar D, et al. Consequences and risks of <1000-g birth weight for neuropsychological skills, achievement, and adaptive functioning. J Dev Behav Pediatr. 2006;27:459–69. doi: 10.1097/00004703-200612000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Haggard MP, Pullan CR. Staffing and structure for paediatric audiology services in hospital and community units. Br J Audiol. 1989;23:99–116. doi: 10.3109/03005368909077828. [DOI] [PubMed] [Google Scholar]
- 8.Yoon PJ. Pediatric cochlear implantation. Curr Opin Pediatr. 2011;23:346–50. doi: 10.1097/MOP.0b013e32834618ec. [DOI] [PubMed] [Google Scholar]
- 9.Steel KP, Brown SD. Genetics of deafness. Current opinion in neurobiology. 1996;6:520–5. doi: 10.1016/s0959-4388(96)80059-6. [DOI] [PubMed] [Google Scholar]
- 10.Oghalai JS, Chen L, Brennan ML, et al. Neonatal hearing loss in the indigent. The Laryngoscope. 2002;112:281–6. doi: 10.1097/00005537-200202000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Cristobal R, Oghalai JS. Hearing loss in children with very low birth weight: current review of epidemiology and pathophysiology. Arch Dis Child Fetal Neonatal Ed. 2008;93:F462–8. doi: 10.1136/adc.2007.124214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oghalai JS. Cochlear hearing loss. In: Jackler RK, Brackmann DE, editors. Neurotology. 2. Philadelphia, Pa: Mosby; 2005. pp. 589–606. [Google Scholar]
- 13.Cone-Wesson B, Vohr BR, Sininger YS, et al. Identification of neonatal hearing impairment: infants with hearing loss. Ear and hearing. 2000;21:488–507. doi: 10.1097/00003446-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Bhasin TK, Brocksen S, Avchen RN, et al. Prevalence of four developmental disabilities among children aged 8 years--Metropolitan Atlanta Developmental Disabilities Surveillance Program, 1996 and 2000. MMWR Surveill Summ. 2006;55:1–9. [PubMed] [Google Scholar]
- 15.Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American Deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006;11:303–21. doi: 10.1093/deafed/enj042. [DOI] [PubMed] [Google Scholar]
- 16.Lesinski A, Hartrampf R, Dahm MC, et al. Cochlear implantation in a population of multihandicapped children. The Annals of otology, rhinology & laryngology. 1995;166:332–4. [PubMed] [Google Scholar]
- 17.Isaacson JE, Hasenstab MS, Wohl DL, et al. Learning disability in children with postmeningitic cochlear implants. Arch Otolaryngol Head Neck Surg. 1996;122:929–36. doi: 10.1001/archotol.1996.01890210009003. [DOI] [PubMed] [Google Scholar]
- 18.Waltzman SB, Scalchunes V, Cohen NL. Performance of multiply handicapped children using cochlear implants. The American journal of otology. 2000;21:329–35. doi: 10.1016/s0196-0709(00)80040-x. [DOI] [PubMed] [Google Scholar]
- 19.Hamzavi J, Baumgartner WD, Egelierler B, et al. Follow up of cochlear implanted handicapped children. Int J Pediatr Otorhinolaryngol. 2000;56:169–74. doi: 10.1016/s0165-5876(00)00420-1. [DOI] [PubMed] [Google Scholar]
- 20.Yang HM, Lin CY, Chen YJ, et al. The auditory performance in children using cochlear implants: effects of mental function. Int J Pediatr Otorhinolaryngol. 2004;68:1185–8. doi: 10.1016/j.ijporl.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Wiley S, Jahnke M, Meinzen-Derr J, et al. Perceived qualitative benefits of cochlear implants in children with multi-handicaps. Int J Pediatr Otorhinolaryngol. 2005;69:791–8. doi: 10.1016/j.ijporl.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Donaldson AI, Heavner KS, Zwolan TA. Measuring progress in children with autism spectrum disorder who have cochlear implants. Arch Otolaryngol Head Neck Surg. 2004;130:666–71. doi: 10.1001/archotol.130.5.666. [DOI] [PubMed] [Google Scholar]
- 23.Niparko JK, Tobey EA, Thal DJ, et al. Spoken language development in children following cochlear implantation. JAMA. 2010;303:1498–506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerry J, Oghalai JS. Towards an etiologic diagnosis: assessing the patient with hearing loss. Advances in oto-rhino-laryngology. 2011;70:28–36. doi: 10.1159/000322468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin JW, Chowdhury N, Mody A, et al. Comprehensive diagnostic battery for evaluating sensorineural hearing loss in children. Otol Neurotol. 2011;32:259–64. doi: 10.1097/MAO.0b013e31820160fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Outcomes In Children With Developmental Delay And Deafness. Available at: Clinicaltrials.gov. Accessed.
- 27.Lin FR, Wang NY, Fink NE, et al. Assessing the use of speech and language measures in relation to parental perceptions of development after early cochlear implantation. Otol Neurotol. 2008;29:208–13. doi: 10.1097/mao.0b013e31812f6fa6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mundy P, Kasari C, Sigman M, et al. Nonverbal communication and early language acquisition in children with Down syndrome and in normally developing children. J Speech Hear Res. 1995;38:157–67. doi: 10.1044/jshr.3801.157. [DOI] [PubMed] [Google Scholar]
- 29.Mundy P, Crowson M. Joint attention and early social communication: implications for research on intervention with autism. J Autism Dev Disord. 1997;27:653–76. doi: 10.1023/a:1025802832021. [DOI] [PubMed] [Google Scholar]
- 30.Kutz W, Wright C, Krull KR, et al. Neuropsychological testing in the screening for cochlear implant candidacy. The Laryngoscope. 2003;113:763–6. doi: 10.1097/00005537-200304000-00035. [DOI] [PubMed] [Google Scholar]
- 31.Pierson SK, Caudle SE, Krull KR, et al. Cognition in children with sensorineural hearing loss: etiologic considerations. The Laryngoscope. 2007;117:1661–5. doi: 10.1097/MLG.0b013e3180ca7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kushalnagar P, Krull K, Hannay J, et al. Intelligence, parental depression, and behavior adaptability in deaf children being considered for cochlear implantation. J Deaf Stud Deaf Educ. 2007;12:335–49. doi: 10.1093/deafed/enm006. [DOI] [PubMed] [Google Scholar]
- 33.Barker DH, Quittner AL, Fink NE, et al. Predicting behavior problems in deaf and hearing children: the influences of language, attention, and parent-child communication. Dev Psychopathol. 2009;21:373–92. doi: 10.1017/S0954579409000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stark RE. Speech-language habilitation of hearing-impaired children: basis for assessment and training. The American journal of otology. 1991;12 (Suppl):62–6. [PubMed] [Google Scholar]