Abstract
Parathyroid hormone (PTH) has variable actions on bone. Chronically increased PTH is catabolic leading to osteoporosis, yet intermittent administration is anabolic and increases bone mass. PTH deficiency is associated with decreased bone remodeling and increased bone mass. However, the effects of PTH replacement therapy on bone in hypoparathyroidism are not well known. We discontinued calcitriol therapy and treated five hypoparathyroid subjects (2 adults and 3 adolescents) with synthetic human PTH 1-34 (hPTH 1-34), injected 2-3 times daily for 18 months, with doses individualized to maintain serum calcium at 1.9-2.25 mmol/L. Biochemical markers and bone density (BMD) were assessed every 6 months; iliac-crest biopsies were performed before and after 1 year of treatment. hPTH 1-34 therapy significantly increased bone markers to supranormal levels. Histomorphometry revealed that treatment dramatically increased cancellous bone volume and trabecular number and decreased trabecular separation. Changes in trabecular width were variable, suggesting that the increase in trabecular number was due to the observed intratrabecular tunneling. Cortical width remained unchanged, however, hPTH 1-34 treatment increased cortical porosity. Cancellous bone remodeling was also stimulated, inducing significant changes in osteoid, mineralizing surface, and bone formation rate. Similar changes were seen in endocortical and intracortical remodeling. BMD Z-scores were unchanged at the spine and femoral neck. Total hip Z-scores increased, however, total body BMD Z-scores decreased during the first 6 months of treatment and then stabilized, remaining significantly decreased compared to baseline. Radial Z-scores also decreased with treatment; this was most pronounced in the growing adolescent. Daily hPTH 1-34 therapy for hypoparathyroidism stimulated bone turnover, increased bone volume, and altered bone structure in the iliac crest. These findings suggest that treatment with hPTH 1-34 in hypoparathyroid adults and adolescents has varying effects in the different skeletal compartments, leading to an increase in trabecular bone and an apparent trabecularization of cortical bone.
Introduction
Hypoparathyroidism due to inadequate production or secretion of parathyroid hormone (PTH) leads to hypocalcemia, hyperphosphatemia and, in some cases, hypercalciuria. At the level of the bone, PTH deficiency results in decreased bone turnover and, oftentimes, increased bone mass as measured by DXA and histomorphometry.(1,2) Hypoparathyroidism is one of the few remaining hormone deficiencies for which hormone replacement therapy is rarely used. Rather, conventional therapy involves the use of calcium supplements and highly active vitamin D analogues such as calcitriol. There have been several studies demonstrating that the hypocalcemia of hypoparathyroidism can be successfully managed using synthetic human PTH 1-34 (hPTH 1-34) in lieu of vitamin D analogues.(3,4) These studies have also shown that 3-year treatment of hypoparathyroidism with hPTH 1-34 markedly increases biochemical markers of bone turnover well above the normal range compared to patients treated with calcitriol, suggesting that hPTH 1-34 replacement therapy converts the bone from a low-turnover state to a high-turnover state. Despite this, non-invasive measures of bone density did not reveal significant changes from the calcitriol-treated group over three years.(4) The explanation for this apparent paradox and the implications for overall bone health with long-term PTH replacement treatment in hypoparathyroidism are not clear. Studies using full length recombinant human PTH 1-84 (rhPTH 1-84) as a fixed dose of 100 μg daily or every other day as adjuvant therapy, rather than full replacement therapy, have also demonstrated increases in bone turnover markers and cortical porosity with variable effects on DXA and iliac crest bone biopsies.(5-8) Understanding the effects of long-term treatment with PTH is important, especially given the black box warning advising against PTH use for greater than two years in osteoporosis. This is the first study to investigate the effects of individually titrated hPTH 1-34 full replacement therapy on bone using histomorphometric analyses of double-labeled iliac crest bone biopsies.
Methods
Subjects
Five subjects, aged 15 - 49 years, with hypoparathyroidism for greater than one year, were referred to the National Institutes of Health (NIH) for inclusion in a study evaluating the effects of hPTH 1-34 replacement therapy on the skeleton. Subjects were excluded if they had significant liver or kidney disease (eGFR < 25 mL/min/1.73 m2), chronic disease known to affect mineral metabolism, a history of chronic steroid or bisphosphonates use, active thyroid cancer, pregnancy, or allergy to tetracycline antibiotics. This protocol was approved by the Institutional Review Board of the National Institute of Dental and Craniofacial Research (NIDCR). Written informed consent was obtained from adult subjects or the parents of adolescent subjects; written assent was obtained from the adolescents.
Therapy of Hypoparathyroidism
Following an optimization period on conventional therapy, calcitriol was discontinued and the subjects were randomly assigned to receive twice (n = 4) or thrice (n = 1) daily subcutaneous injections of synthetic human hPTH 1-34, beginning the morning after the last dose of calcitriol. Three additional subjects were enrolled and randomized to therapy (one to BID, two to TID); however, only data from subjects who underwent baseline and 1-year post PTH biopsies were included in this analysis. hPTH 1-34 lyophilized powder was purchased from Bachem, Inc. (Torrance, CA) and then prepared and packaged by the NIH Pharmaceutical Development Service as previously described.(3) The mean starting dose was 0.23 mcg/kg/dose in the BID group and 0.06 mcg/kg/dose in the TID subject. However, the doses were rapidly adjusted as needed to maintain the serum calcium level at or slightly less than the lower limit of normal (7.6 – 9 mg/dL, 1.9 – 2.25 mmol/L). Calcium intake through diet or supplements while on hPTH 1-34 was maintained at 1000-2000 mg/day. Subjects found to be Vitamin D deficient were treated with ergocalciferol or cholecalciferol to maintain the 25-hydroxy Vitamin D level above 62.5 nmol/L (25 ng/mL).
Monitoring
Subjects were evaluated at the Clinical Center of the National Institutes of Health in Bethesda, Maryland every 6 months. Testing included measurement of routine blood and urine biochemistries, 25-hydroxy Vitamin D, 1,25-dihydroxy Vitamin D, bone-specific alkaline phosphatase (BSAP) (immunoenzymatic assay, Mayo Medical Labs, Rochester, MN), osteocalcin (OC) (electrochemiluminescence, NIH, Bethesda, MD), 24-hour urine pyridinoline (PD) and deoxypyridinoline (DPD) (HPLC, Quest Diagnostics, San Juan Capistrano, CA), and 24-hour urine collagen cross-linked N-telopeptide (NTX) (Vitros ECi competitive assay, Mayo Medical Labs, Rochester, MN). Bone density (% coefficient of variation, 95% confidence level) of the lumbar spine (1.1 %), total body (1.1%), 1/3 distal radius (1.6 %), femoral neck (1.8 %) and total hip (0.9 %) were assessed by dual-energy X-ray Absorptiometry (DXA, Hologic, Bedford, MA) every 6 months. Z-scores were calculated using published normative databases for the adolescents(9) and the Hologic-provided normative database for adults. Between visits to the NIH, serum and urine mineral metabolism was monitored periodically at the subjects’ local laboratories and hPTH 1-34 doses were adjusted to maintain the serum calcium level at or slightly below the lower limit of normal (7.6 – 9 mg/dL, 1.9 – 2.25 mmol/L).
Bone Biopsies and Histomorphometry
Following double-labeling with tetracycline (250 mg four times a day) or demeclocycline (150 mg four times a day) using a standard regimen of 3 days on antibiotic:12 days off antibiotic:3 days on antibiotic: 4 days off antibiotic with biopsy on the 5th day, a transcortical iliac crest bone biopsy was obtained just prior to starting hPTH 1-34 and repeated after one year of hPTH 1-34 treatment on the contralateral side. Bone specimens were embedded in methyl methacrylate and prepared for analysis according to a standard protocol.(10) Quantitative digital analysis was performed using OsteoMeasure™ (Osteometrics, Atlanta, GA). Histomorphometric parameters are expressed according to standardized nomenclature.(11) Normative data were derived from independent analysis of samples from studies(12,13) and published databases.(14,15)
Statistics
All data are presented as mean ± SD, baseline vs. last measured time-point. Comparison of means was done using paired t-tests and repeated measures ANOVA. Post-hoc Holm-Sidak or Tukey tests were performed for pairwise multiple comparisons. Significance is considered at p < 0.05.
Results
Subjects
Baseline characteristics and diagnoses are listed in Table 1. The two subjects with post-surgical hypoparathyroidism were on thyroid hormone replacement; subject #1 had a history of thyroid cancer and had a therapeutically suppressed TSH during the study. Subject #4 had significant nephrocalcinosis, moderate renal insufficiency and hypertension at baseline; she had been intermittently treated with hydrocholorothiazide and losartan. All adolescents had normal growth and pubertal development. The adolescent girls (subjects #2 and #4) had achieved near-final adult height prior to starting hPTH 1-34. However, Subject #3 experienced his pubertal growth spurt in the 2 years prior to hPTH 1-34 treatment; he grew approximately 6.2 cm the year prior to treatment and 2.5 cm during the 18 months on hPTH 1-34, maintaining the 25th percentile in height and a normal growth velocity for age. The two adult women (subjects #1 and #5) were pre-menopausal.
Table 1.
Baseline Subject Characteristics
| Subject | Symbol | Sex | Etiology | Duration of HypoPTH | Age at start of hPTH 1-34 | Regimen | Serum Ca (2.05-2.5 mmol/L) | Serum Mg (0.75-1 mmol/L) | Serum Phos (0.8-1.55 mmol/L)* | Intact PTH (16-87 pg/mL | 25-OH Vit D (>50 nmol/L) | NC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | • | F | PS-PTC | 3 | 46 | BID | 2.2 | 0.88 | 1.72 | < 3 | 165 | no |
| 2 | ○ | F | DiGeorge | 15 | 15 | BID | 1.92 | 0.69 | 1.61 | < 3 | 55 | no |
| 3 | □ | M | idiopathic | 15 | 15 | TID | 2.1 | 0.87 | 2.5 | < 3 | 57.5 | no |
| 4 | △ | F | CaR | 16 | 16 | BID | 2.1 | 0.74 | 1.52 | < 3 | 122.5 | yes |
| 5 | ■ | F | PS-MNG | 3 | 48 | BID | 2.1 | 0.8 | 1.74 | 4.3 | 97.5 | no |
PS-PTC = post-surgical-papillary thyroid carcinoma, CaR = activating mutation of the calcium-sensing receptor, PS-MNG = post-surgical-multinodular goiter, HypoPTH = hypoparathyroidism, hPTH 1-34, NC = nephrocalcinosis by renal CT.
Normal range of serum phosphorus in adolescents: 0.97-1.94 mmol/L)
hPTH 1-34 Therapy
The mean total daily dose of hPTH 1-34 required to maintain serum calcium within the target range was 0.57 ± 0.24 mcg/kg/d. The injections were well-tolerated by all the subjects.
Serum and Urine Bone Markers (Fig 1)
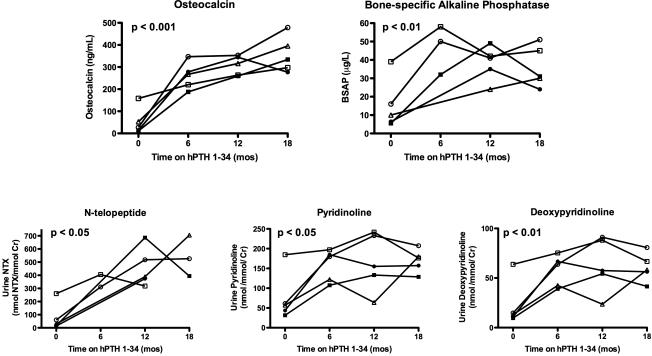
Figure 1.
Biochemical markers of bone formation increase in hypoparathyroid subjects treated with hPTH 1-34 replacement therapy for 18 months. P-values determined by repeated measures ANOVA. Normal values are as follows: Osteocalcin (OC), 9-40 ng/mL (adults); Bone-specific Alkaline Phosphatase (BSAP), 0-14 mcg/L (premenopausal women); Collagen cross-linked N-telopeptide (NTX), 102 -1048 nmol/mmol Cr (14-18 y/o male), 55-378 nmol/mmol Cr (14-18 y/o female), 19-63 nmol/mmol Cr (adult females); Pyridinoline (PD), 42-200 nmol/mmol Cr (15-17 y/o), 22.1-88.9 nmol/mmol Cr (adult females); Deoxypyridinoline (DPD), < 58.7 nmol/mmol Cr (15-17 y/o), 4.4-21.1 nmol/mmol Cr (adult females). See Table 1 for key to subject symbols.
Subjects exhibited excellent control of serum calcium on replacement hPTH 1-34, with no significant changes from baseline in mean fasting serum calcium, magnesium and phosphorus and 24-hour urinary calcium excretion at any time point (data not shown). With the exception of the adolescent male coming off of his pubertal growth spurt (Fig 1, open square), bone formation (osteocalcin and BSAP) and resorption markers (NTX, pyridinoline, and deoxypyridinoline) were suppressed or within the normal range prior to starting hPTH 1-34 therapy. After initiation of therapy, bone markers rose significantly and continued to be increased from baseline after 18 months of hPTH 1-34 replacement therapy: osteocalcin, 53±61 vs. 356±82 ng/mL, p < 0.001; BSAP, 15±14 vs. 36±11 μg/L, p < 0.01; NTX, 76±104 vs. 542±156 nmol BCE/mmol Cr, p < 0.05, pyridinoline, 76±62 vs. 170±29 nmol/mmol/Cr, p < 0.05; deoxypyridinoline, 22±23 vs. 61±14 nmol/mmol/cr, p < 0.01. While osteocalcin levels continued to rise during hPTH 1-34 therapy, BSAP, NTX, PD, and DPD appeared to peak within the first 12 months and then stabilized or declined.
Bone Densitometry (Fig 2)
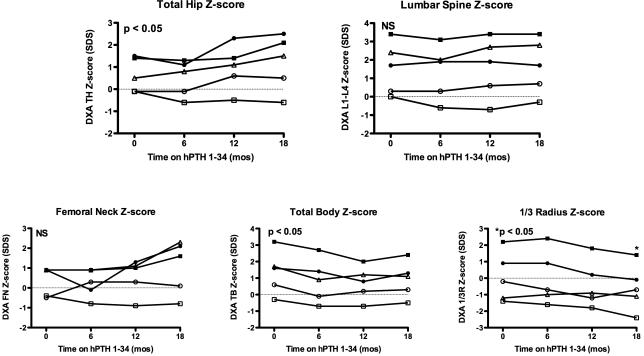
Figure 2.
Bone mineral density Z-scores measured by dual-energy X-ray absorptiometry (DXA) in hypoparathyroid subjects treated with hPTH 1-34 replacement therapy for 18 months. P-values determined by repeated measures ANOVA. Radial Z-scores significantly different only at 18 month time-point. See Table 1 for key to subject symbols.
hPTH 1-34 replacement therapy resulted in increased BMD Z-scores in the total hip (0.66±0.8 vs. 1.2±1.2, p < 0.05) but did not significantly affect the lumbar spine or femoral neck, although there was an upward trend in the femoral neck Z-scores for the oldest subjects at 18 months. There was a significant decline in mean whole body BMD Z-score at 6 months, which then stabilized but was still significantly lower than baseline after 18 months of hPTH 1-34 (1.36±1.31 vs. 0.92±1.09, p < 0.05). Additionally, there was a gradual decline in the mean distal radius BMD Z-score that reached statistical significance by 18 months (0.06±1.51 vs. -0.58±1.39, p <0.05). In subject #3 (the adolescent male, open square), the Z-score in his distal radius declined to -2.4.
Iliac Crest Histomorphometry
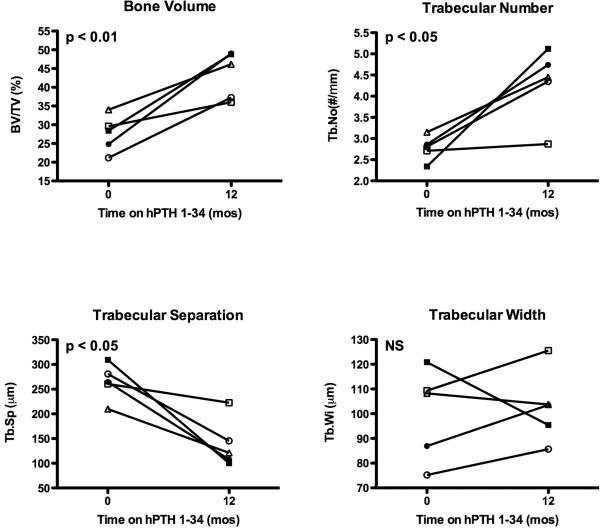
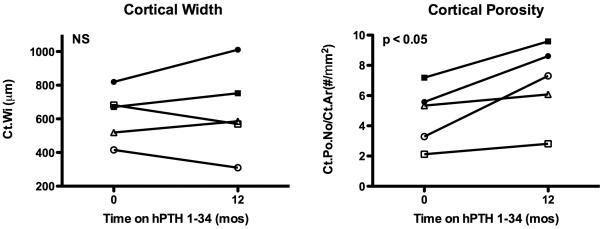
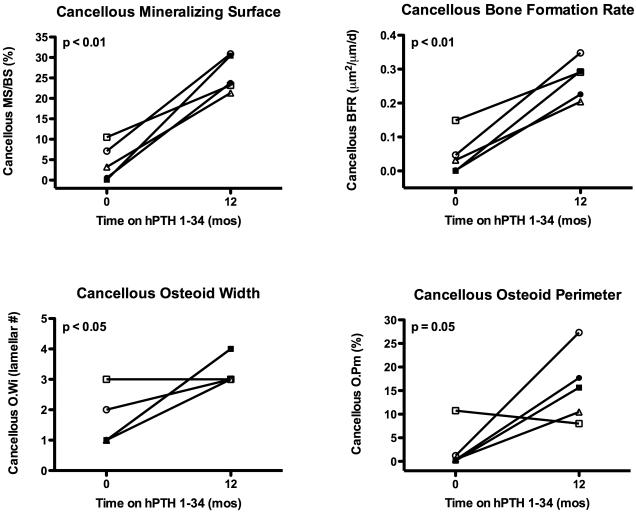
Prior to starting hPTH 1-34, cancellous bone volume/total volume (BV/TV) and trabecular number (Tb.No) were increased, and trabecular separation (Tb.Sp) was decreased in several subjects compared to published normal ranges. One year of hPTH 1-34 replacement therapy induced a marked increase in BV/TV (27.6±4.9 vs. 43.5±6.3%, p < 0.01, Fig 3). This appeared to be due to an increase in Tb.No (2.8±0.3 vs. 4.3±0.9 #/mm, p < 0.05, Fig 3) and decrease in Tb.Sp (265±36 vs. 139±50 μm, p < 0.05, Fig 3). Changes in trabecular width (Tb.Wi) were variable between subjects (Figs 3 and 4) but were not statistically significant for the group. Thus, the increase in Tb.No appeared to be due to intratrabecular tunneling (Fig 5), seen in all five subjects; this phenomenon has been described previously in both hypoparathyroid(8,16) and postmenopausal women treated with PTH.(17,18) Cortical width (Ct.Wi) did not change, however, cortical porosity/cortical area (Ct.Po.No/Ct.Ar) increased with hPTH 1-34 therapy (4.7±2.0 vs. 6.9±2.6 #/mm2, p < 0.05, Figs 4 and 5).
Figure 3.
Static cancellous histomorphometric changes in iliac crest bone biopsies from hypoparathyroid subjects at baseline and after 12 months of hPTH 1-34 replacement therapy. P-values determined by repeated measures ANOVA. Normal values for age expressed as mean ± SD are as follows: BV/TV, 21.5 ± 2.8 % (45-54 y/o female), 25.7 ± 5.3% (14-16 y/o), 27.8 ± 4.5% (17-23 y/o); Tb.No, 2.0 ± 0.5 #/mm (45-54 y/o female), 1.6 ± 0.2 #/mm (14-16 y/o), 1.8 ± 0.3 #/mm (17-23); Tb.Sp, 432 ± 170 μm (45-54 y/o female), 461 ± 70 μm (14-16 y/o), 404 ± 77 μm (17-23 y/o); Tb.Wi, 114 ± 28 μm (45-54 y/o female), 157 ± 22 μm (14-16 y/o), 153 ± 24 μm (17-23).
Figure 4.
Cortical width (Ct.Wi) and cortical porosity (Ct.Po.No/Ct.Ar.) in iliac crest bone biopsies from hypoparathyroid subjects at baseline and after 12 months of hPTH 1-34 replacement therapy. P-values determined by paired t-test. Normal values for age expressed as mean ± SD are as follows: Ct.Wi, 557 ± 141 μm (45-54 y/o female), 1180 ± 350 μm (14-16 y/o), 1010 ± 200 μm (17-23 y/o); Ct.Po.No/Ct.Ar., not available. See Table 1 for key to subject symbols.
Figure 5.
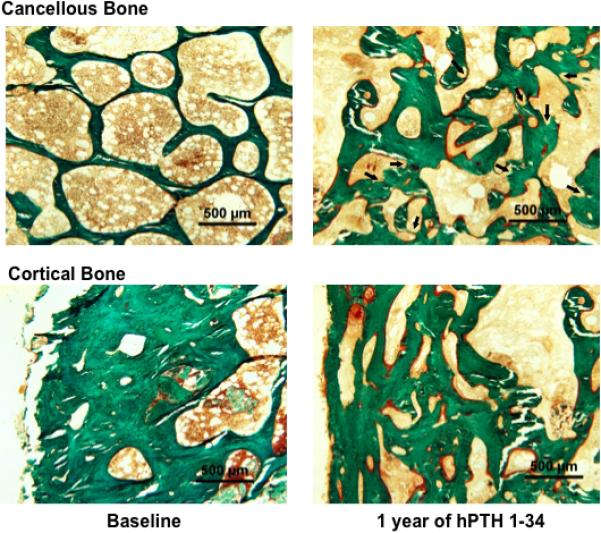
Iliac crest bone biopsy from Subject # 1 (Table 1) before and after 12 months of hPTH 1-34 replacement therapy. hPTH 1-34 increased cancellous bone volume (top row) with increased trabecular number and width, decreased marrow space, and increased osteoid surface (red-stained seams on bone surface). Numerous sites of trabecular tunneling (arrows) are seen within thick trabeculae, which increased trabecular number. (Goldner's trichrome stain. Original magnification x40)
Double labeling with tetracycline or demeclocycline prior to biopsy allowed for assessment of dynamic histomorphometry. Prior to starting hPTH 1-34, bone turnover was very low as evidenced by decreased presence of single and double antibiotic labels (Fig 6). Cancellous mineralizing surface and bone formation rate increased dramatically with hPTH 1-34 therapy (MS/BS, 4±4 vs. 26±4%, p < 0.01; BFR (BS), 0.046±0.061 vs. 0.273±0.058 μm2/mm/d, p<0.01, Fig 6 and 7). Similar increases were seen in endocortical MS/BS (9.4 ± 9.9 vs. 44.3±19.3%, p < 0.01), endocortical BFR (0.120 ± 0.133 vs. 0.481 ± 0.273 μm2/mm/d, p < 0.05), intracortical MS/BS (17.1 ± 14.9 vs. 40.5 ± 8.1%, p < 0.05), and intracortical BFR (0.216 ± 0.223 vs. 0.462 ± 0.113 μm2/mm/d, p < 0.05). Increases in cancellous osteoid width (O.Wi, 1.60 ± 0.89 vs. 3.40 ± 0.55 lamellar# p < 0.05) and osteoid perimeter (O.Pm, 2.6 ± 4.6 vs. 15.8 ± 7.5%, p = 0.05) were also seen in most patients (Figs. 5 and 7).
Figure 6.
Cancellous bone remodeling in iliac crest bone biopsies from hypoparathyroid subjects at baseline and after 12 months of hPTH 1-34 replacement therapy. P-values determined vy paired t-test. Normal values for age expressed as mean ± SD are as follows: MS/BS, 6.5 ± 3.9% (45-54 y/o female), 12.5 ± 3.4% (14-16 y/o), 7.9 ± 2.7% (17-23); BFR (BS), 0.042 ± 0.025 μm2/μm/d (45-54 y/o female), 0.101 ± 0.028 μm2/μm/d (14-16 y/o), 0.061 ± 0.025 μm2/μm/d (17-23 y/o). See Table 1 for key to subject symbols.
Figure 7.
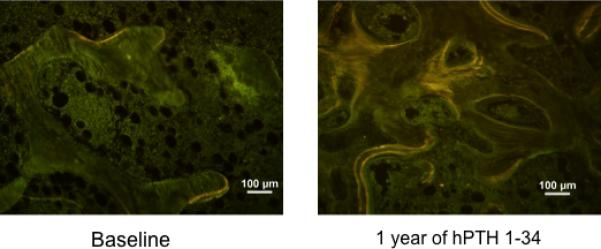
Tetracycline labels in cancellous bone from Subject # 1 (Table 1) before and after 12 months of hPTH 1-34 replacement therapy. Compared to baseline, the treatment dramatically increased bone formation rate (BFR/BS) with increased mineralizing surface, as indicated by the increased extent of single and double labels. (Unstained, original magnification x100)
Discussion
Without calcitriol therapy, appropriate serum calcium levels were well-maintained in five subjects with hypoparathyroidism using individually-titrated full replacement dose hPTH 1-34. While no significant changes in routine biochemistries were noted, at the level of the skeleton, 18 months of hPTH 1-34 replacement therapy converted hypoparathyroid bone from a low/normal turnover state to a high turnover state as evidenced by dramatic increases in biochemical markers of bone turnover. Double-labeled iliac crest bone biopsies before and after 12 months of hPTH 1-34 treatment confirmed that, in this anatomical site, increased bone formation rates resulted in increased cancellous bone volume, trabecular number, and osteoid associated with a decrease in trabecular separation. Despite the marked increase in bone volume, changes in trabecular width were variable within the group; thus, the increase in bone volume was reflected as an increase in trabecular number, formed via intratrabecular tunneling. We also noted an increase in cortical porosity without an increase in cortical thickness. Despite these significant changes in bone turnover and bone volume, BMD Z-scores increased significantly only at the total hip, and not at the lumbar spine or femoral neck. Total body BMD decreased initially and then stabilized, while Z-scores gradually declined in the distal radius. These changes in Z-scores were seen despite normal growth and development in the adolescent subjects, suggesting that the effect was due to hPTH 1-34 treatment.
The anabolic skeletal effects of recombinant human PTH 1-34 (rhPTH 1-34), which is structurally identical to synthetic human PTH 1-34 (hPTH 1-34), in euparathyroid women with post-menopausal osteoporosis has been examined closely. Daily rhPTH 1-34, given as a 20 mcg subcutaneous injection for up to 2 years, resulted in significantly increased BMD by DXA in the lumbar spine and femoral neck. Decreases in the distal radius BMD have also been reported although these decreases have not been associated with an increase in distal radius fractures in this population.(19) Iliac crest bone biopsies and micro-CT demonstrated an increase in cortical thickness without an increase in cortical porosity suggesting that, in post-menopausal women, PTH has an anabolic effect on cortical bone as well as cancellous bone.(20) Using quantitative backscattered electron imaging (qBEI), it was determined that the increases seen by DXA were due to PTH-stimulated bone remodeling, resulting in absolute increases in hypomineralized new bone matrix.(21) Bone turnover markers were also increased initially in the osteoporosis population, peaking within the first year of therapy, but then gradually declined during the second year of treatment.(22,23) Furthermore, during the 12 months after discontinuation of rhPTH 1-34, the bone turnover markers returned to baseline and the BMD effects appeared to be reversing in post-menopausal women.(22)
The skeletal effects of hPTH 1-34 replacement therapy in hypoparathyroidism are less well understood. Adults with chronic hypoparathyroidism develop hypermineralized, dense bones that are abnormal in their geometric, histomorphometric, and microarchitectural features, presumably due to chronic lack of bone turnover.(1,2,24) Cortical width and cancellous bone volume are increased, while dynamic indices of bone turnover such as bone formation rate and mineral apposition rate are decreased.(1) Short-term studies comparing once daily with twice daily hPTH 1-34 replacement therapy in hypoparathyroidism showed that markers of bone turnover were markedly elevated above the normal range in response to both treatment regimens, however, the effect was less marked with twice daily dosing suggesting that this dose regimen increased bone turnover to a lesser extent than once daily hPTH 1-34.(25) A similar study in children also revealed increased bone markers with hPTH 1-34 therapy, without significant differences between the daily and BID group.(26) A three-year randomized study comparing calcitriol with replacement dose hPTH 1-34 in hypoparathyroid adults demonstrated that hPTH 1-34 increased bone markers, at levels at least 2-fold greater than normal, and that these increases persisted throughout treatment. Despite this increase in bone turnover, there was no significant difference in the BMD of the total body, femoral neck, lumbar spine or distal radius compared to the calcitriol group as measured by DXA.(4) Z-scores were not reported and the total hip site, which increased in our patients, was not analyzed in their 3-year study, thus, proper comparisons cannot be made. In a comparable pediatric study, BMD Z-scores were not different between the hPTH 1-34 and the calcitriol groups, with the exception of a statistically significant decrease in the 1/3 radius site in the children receiving hPTH 1-34,(26) as seen in our growing adolescent male subject. This same group reported a 20 year-old hypoparathyroid woman with a calcium sensing receptor (CaR) mutation treated with hPTH 1-34 for 14 years; bone biopsies performed in adulthood revealed dramatic increases in cancellous bone volume yet decreased mineralization by qBEI and decreased radial BMD by DXA.(16) Similarly, biopsies before and after one year of hPTH 1-34 in two hypoparathyroid adolescents revealed an increase in cancellous bone volume despite a decrease in mineralization.(16) While the decreases in radial BMD and increases in cancellous bone volume are consistent with our results, these findings cannot be generalized to adults due to the young age of these patients at the time of PTH initiation. Given the suggestion that BID hPTH 1-34 therapy stimulates bone turnover less than daily therapy, there is emerging evidence that continuous hPTH 1-34 therapy may stimulate bone turnover even less than intermittent therapy. A recent, randomized, cross-over study comparing 3 months of continuous subcutaneous hPTH 1-34 infusion with twice daily subcutaneous injections demonstrated that, while there were no statistically significant differences in bone formation markers (alkaline phosphatase and osteocalcin), bone resorption markers were less elevated during continuous infusion therapy.(27) Effects on bone with long-term therapy have not been studied. Thus, this remains an open question.
Studies using subcutaneous full-length recombinant human PTH 1-84 (hPTH 1-84) as adjuvant therapy, rather than full replacement therapy, dosed at 100 mcg every other day for 2 years in adults with hypoparathyrodism, also showed that PTH therapy increased bone turnover. In these studies, bone turnover markers peaked at 5-9 months of therapy and then appeared to decline; most markers were still elevated compared to baseline at 2 years. BMD of the lumbar spine increased while BMD of the 1/3 radius decreased in these subjects.(6) Similar to our study, iliac crest biopsies after 1 year of therapy revealed increased Tb.N, increased MS/BS, increased BFR, and increased cortical porosity without change in cortical thickness.(5) Despite these findings, and unlike our subjects, the authors did not detect an increase in BV/TV or a decrease in Tb.Sp. In the subset of subjects who were biopsied at baseline and 2 years, differences from baseline were present but the changes were not as marked, suggesting that subjects were returning towards baseline in keeping with their declining bone markers.(5) In a recent placebo-controlled trial by Sikjaer, et al., hypoparathyroid adults received 100 mcg rhPTH 1-84 or placebo daily, rather than every other day, for 24 weeks, while calcitriol doses were slowly weaned. Similar to our study and the studies by Rubin et al., subjects receiving daily rhPTH 1-84 had elevated bone turnover markers during the 24 weeks of treatment. In contrast, they experienced decreases in areal BMD (aBMD) by DXA at the hip, lumbar spine, and whole body, while they did not detect a change in the distal radius.(7) Despite these apparent decreases in aBMD, volumetric measurements by quantitative computed tomography (vBMD) revealed increases in vBMD in the lumbar spine with decreases in vBMD at the hip, suggesting variable responsiveness to PTH in different skeletal compartments.(8) Additionally, while they similarly noted an increase in cortical porosity and the presence of intratrabecular tunneling, the rhPTH 1-84 - treated patients experienced a decrease in trabecular thickness and trabecular density, without a change in trabecular number or separation.(8) Given the frequency of hypercalcemia in this study, it is possible that the treatment dose used was too high, thus inducing a transient hyperparathyroid state. In addition, unlike the other studies, the study by Sikjaer et al.(7) did not exclude women within 5 years of menopause, which may have contributed to bone loss; however, that this study was placebo-controlled makes this factor less likely. The differences in bone turnover, BMD, and biopsy data seen in our subjects and studies receiving rhPTH 1-84 may be related to the inclusion of adolescents, different PTH peptide used, more frequent injections, and individually titrated doses consistent with full replacement therapy, rather than adjuvant therapy.
The primary limitations of this study are the small and heterogeneous nature of our patient population (age, sex, etiology of hypoparathyroidism) and relatively short duration of therapy. Although the changes in cancellous bone volume, trabecular number, trabecular separation, bone turnover, osteoid, and cortical bone appeared to be quite uniform, individual variability was seen in some indices. While the increase in cortical porosity most likely led to the decrease in total body and radial BMD by DXA, other factors may have contributed, including a potential decrease in mineralization density, as has been reported in postmenopausal osteoporosis(21) and hypoparathyroid adolescents(16) and, possibly, an increase in bone area which has been seen in ovariectomized animals(28) but has not been definitively documented in humans treated with PTH(29). One of the adult subjects was on suppressive doses of thyroid hormone, which is associated with bone loss; however, as shown in the figures, this subject (subject #1, solid circle) did not experience significant decreases in BMD or bone volume compared to the other subjects. Rather, she experienced a robust increase in BMD.
Only one of the subjects randomized to TID therapy had a biopsy at baseline and after one year of hPTH 1-34 therapy (subject #3, open square); he was also the only male, and the only adolescent who had been experiencing a pubertal growth spurt during the 2 years prior to therapy. Thus, it is not clear if some of the changes seen were due to the TID regimen, male sex, or pubertal effects. The baseline elevation in bone markers seen in this subject is expected for a pubertal boy. Previous studies suggest that the degree of bone turnover marker elevation seen with hPTH 1-34 therapy appears to be related to the frequency of subcutaneous hPTH 1-34 exposure, with more frequent exposure resulting in reduced elevation of bone marker(25-27). Thus, both the higher bone markers at baseline and the TID dosing could explain the less marked changes seen in his bone turnover markers and his divergence from the group in the other indices. Peak bone mineral acquisition occurs the year after the peak pubertal growth spurt(30) and transient undermineralization of the male adolescent skeleton would be expected in mid-puberty,(31) during the period of the pubertal growth spurt; therefore, declines seen in the distal radius Z-score during the year following his pubertal growth spurt are unexpected and not physiological.
This is the first study to examine iliac crest histomorphometry before and after one year of individually-titrated hPTH 1-34 full replacement therapy in adults and adolescents with hypoparathyroidism. Biopsy findings were consistent with an anabolic effect of hPTH 1-34 in cancellous bone. However, the increase in cortical porosity together with the decrease in total body and 1/3 radius Z-scores suggest that hPTH 1-34 therapy in hypoparathyroidism may have catabolic effects in cortical bone, with apparent trabecularization of the cortex and a redistribution of cortical bone towards cancellous bone. Further study of the long-term skeletal effects of hPTH 1-34 replacement as well as evaluation of the skeletal changes that occur after withdrawal of hPTH 1-34 therapy are critical in assessing the therapeutic potential of PTH replacement, particularly in growing children who would require decades of therapy.
Acknowledgements
This research was supported by the Division of Intramural Research at the National Institute of Dental and Craniofacial Research, IRP, National Institutes of Health, DHHS.
Footnotes
Disclosure
All authors state that they have no conflicts of interest.
Requests for materials should be addressed to Rachel I. Gafni, MD.
Authors’ roles: Study design: RIG, MHK, BAB, and MTC. Study conduct: RIG, JSB, PA, MHK, BAB, MTC. Data analysis and interpretation: RIG, NB, JCR, HZ, DWD, MTC. Drafting manuscript: RIG and MTC. Revising manuscript content: all authors. Approving final version of the manuscript: RIG and MTC.
References
- 1.Rubin MR, Dempster DW, Zhou H, Shane E, Nickolas T, Sliney J, Jr., Silverberg SJ, Bilezikian JP. Dynamic and structural properties of the skeleton in hypoparathyroidism. J Bone Miner Res. 2008;23(12):2018–24. doi: 10.1359/JBMR.080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langdahl BL, Mortensen L, Vesterby A, Eriksen EF, Charles P. Bone histomorphometry in hypoparathyroid patients treated with vitamin D. Bone. 1996;18(2):103–8. doi: 10.1016/8756-3282(95)00443-2. [DOI] [PubMed] [Google Scholar]
- 3.Winer KK, Yanovski JA, Cutler GB., Jr Synthetic human parathyroid hormone 1-34 vs calcitriol and calcium in the treatment of hypoparathyroidism. Jama. 1996;276(8):631–6. [PubMed] [Google Scholar]
- 4.Winer KK, Ko CW, Reynolds JC, Dowdy K, Keil M, Peterson D, Gerber LH, McGarvey C, Cutler GB., Jr Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1-34) versus calcitriol and calcium. J Clin Endocrinol Metab. 2003;88(9):4214–20. doi: 10.1210/jc.2002-021736. [DOI] [PubMed] [Google Scholar]
- 5.Rubin MR, Dempster DW, Sliney J, Jr., Zhou H, Nickolas TL, Stein EM, Dworakowski E, Dellabadia M, Ives R, McMahon DJ, Zhang C, Silverberg SJ, Shane E, Cremers S, Bilezikian JP. PTH(1-84) administration reverses abnormal bone-remodeling dynamics and structure in hypoparathyroidism. J Bone Miner Res. 2011;26(11):2727–36. doi: 10.1002/jbmr.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin MR, Sliney J, Jr., McMahon DJ, Silverberg SJ, Bilezikian JP. Therapy of hypoparathyroidism with intact parathyroid hormone. Osteoporos Int. 2010;21(11):1927–34. doi: 10.1007/s00198-009-1149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sikjaer T, Rejnmark L, Rolighed L, Heickendorff L, Mosekilde L. The effect of adding PTH(1-84) to conventional treatment of hypoparathyroidism: a randomized, placebo-controlled study. J Bone Miner Res. 2011;26(10):2358–70. doi: 10.1002/jbmr.470. [DOI] [PubMed] [Google Scholar]
- 8.Sikjaer T, Rejnmark L, Thomsen JS, Tietze A, Bruel A, Andersen G, Mosekilde L. Changes in 3-dimensional bone structure indices in hypoparathyroid patients treated with PTH(1-84): A randomized controlled study. J Bone Miner Res. 2011;26(10):2358–70. doi: 10.1002/jbmr.1493. [DOI] [PubMed] [Google Scholar]
- 9.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J.Clin.Endocrinol.Metab. 2007;92(6):2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 10.Dempster DW, Shane ES. Bone quantification and dynamics of bone turnover by histomorphometric analysis. In: Becker KL, editor. Principles and Practice of Endocrinology and Metabolism. 2nd ed. JB Lippincott Co.; Philadelphia: 1995. pp. 491–498. [Google Scholar]
- 11.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J.Bone Miner.Res. 1987;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 12.Recker RR, Kimmel DB, Parfitt AM, Davies KM, Keshawarz N, Hinders S. Static and tetracycline-based bone histomorphometric data from 34 normal postmenopausal females. J Bone Miner Res. 1988;3(2):133–44. doi: 10.1002/jbmr.5650030203. [DOI] [PubMed] [Google Scholar]
- 13.Clarke BL, Ebeling PR, Jones JD, Wahner HW, O'Fallon WM, Riggs BL, Fitzpatrick LA. Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab. 1996;81(6):2264–70. doi: 10.1210/jcem.81.6.8964862. [DOI] [PubMed] [Google Scholar]
- 14.Parisien M, Cosman F, Morgan D, Schnitzer M, Liang X, Nieves J, Forese L, Luckey M, Meier D, Shen V, Lindsay R, Dempster DW. Histomorphometric assessment of bone mass, structure, and remodeling: a comparison between healthy black and white premenopausal women. J Bone Miner Res. 1997;12(6):948–57. doi: 10.1359/jbmr.1997.12.6.948. [DOI] [PubMed] [Google Scholar]
- 15.Glorieux FH, Travers R, Taylor A, Bowen JR, Rauch F, Norman M, Parfitt AM. Normative data for iliac bone histomorphometry in growing children. Bone. 2000;26(2):103–109. doi: 10.1016/s8756-3282(99)00257-4. [DOI] [PubMed] [Google Scholar]
- 16.Theman TA, Collins MT, Dempster DW, Zhou H, Reynolds JC, Brahim JS, Roschger P, Klaushofer K, Winer KK. PTH(1-34) replacement therapy in a child with hypoparathyroidism caused by a sporadic calcium receptor mutation. J Bone Miner Res. 2009;24(5):964–73. doi: 10.1359/JBMR.081233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Recker RR, Bare SP, Smith SY, Varela A, Miller MA, Morris SA, Fox J. Cancellous and cortical bone architecture and turnover at the iliac crest of postmenopausal osteoporotic women treated with parathyroid hormone 1-84. Bone. 2009;44(1):113–9. doi: 10.1016/j.bone.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Jobke B, Muche B, Burghardt AJ, Semler J, Link TM, Majumdar S. Teriparatide in bisphosphonate-resistant osteoporosis: microarchitectural changes and clinical results after 6 and 18 months. Calcif Tissue Int. 2011;89(2):130–9. doi: 10.1007/s00223-011-9500-6. [DOI] [PubMed] [Google Scholar]
- 19.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 20.Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetic K, Muller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16(10):1846–53. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 21.Misof BM, Roschger P, Cosman F, Kurland ES, Tesch W, Messmer P, Dempster DW, Nieves J, Shane E, Fratzl P, Klaushofer K, Bilezikian J, Lindsay R. Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab. 2003;88(3):1150–6. doi: 10.1210/jc.2002-021988. [DOI] [PubMed] [Google Scholar]
- 22.Leder BZ, Neer RM, Wyland JJ, Lee HW, Burnett-Bowie SM, Finkelstein JS. Effects of teriparatide treatment and discontinuation in postmenopausal women and eugonadal men with osteoporosis. J Clin Endocrinol Metab. 2009;94(8):2915–21. doi: 10.1210/jc.2008-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkelstein JS, Klibanski A, Arnold AL, Toth TL, Hornstein MD, Neer RM. Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1-34): a randomized controlled trial. JAMA. 1998;280(12):1067–73. doi: 10.1001/jama.280.12.1067. [DOI] [PubMed] [Google Scholar]
- 24.Abugassa S, Nordenstrom J, Eriksson S, Sjoden G. Bone mineral density in patients with chronic hypoparathyroidism. J Clin Endocrinol Metab. 1993;76(6):1617–21. doi: 10.1210/jcem.76.6.8501170. [DOI] [PubMed] [Google Scholar]
- 25.Winer KK, Yanovski JA, Sarani B, Cutler GB., Jr A randomized, cross-over trial of once-daily versus twice-daily parathyroid hormone 1-34 in treatment of hypoparathyroidism. J Clin Endocrinol Metab. 1998;83(10):3480–6. doi: 10.1210/jcem.83.10.5185. [DOI] [PubMed] [Google Scholar]
- 26.Winer KK, Sinaii N, Peterson D, Sainz B, Jr., Cutler GB., Jr Effects of once versus twice-daily parathyroid hormone 1-34 therapy in children with hypoparathyroidism. J Clin Endocrinol Metab. 2008;93(9):3389–95. doi: 10.1210/jc.2007-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winer KK, Zhang B, Shrader JA, Peterson D, Smith M, Albert PS, Cutler GB., Jr Synthetic Human Parathyroid Hormone 1-34 Replacement Therapy: A Randomized Crossover Trial Comparing Pump Versus Injections in the Treatment of Chronic Hypoparathyroidism. J Clin Endocrinol Metab. 2011;97(2):391–9. doi: 10.1210/jc.2011-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox J, Miller MA, Newman MK, Recker RR, Turner CH, Smith SY. Effects of daily treatment with parathyroid hormone 1-84 for 16 months on density, architecture and biomechanical properties of cortical bone in adult ovariectomized rhesus monkeys. Bone. 2007;41(3):321–30. doi: 10.1016/j.bone.2007.04.197. [DOI] [PubMed] [Google Scholar]
- 29.Uusi-Rasi K, Semanick LM, Zanchetta JR, Bogado CE, Eriksen EF, Sato M, Beck TJ. Effects of teriparatide [rhPTH (1-34)] treatment on structural geometry of the proximal femur in elderly osteoporotic women. Bone. 2005;36(6):948–58. doi: 10.1016/j.bone.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J.Bone Miner.Res. 1999;14(10):1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Wang XF, Iuliano-Burns S, Ghasem-Zadeh A, Zebaze R, Seeman E. Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. J Bone Miner Res. 2010;25(7):1521–6. doi: 10.1002/jbmr.46. [DOI] [PubMed] [Google Scholar]