Summary
Chemokine-dependent migration of T lymphocytes assures recirculation of naïve T cells to secondary lymphoid organs, and tissue specific trafficking of memory-effector T cells. Previous studies carried out in rodents, have demonstrated age-associated modulation of the expression of chemokine receptors such as CXCR4 and CCR5, however, little is known about the molecular mechanisms that regulate receptor expression and turnover in T cells, during advancing age in humans. Our recent results demonstrating increased chemotactic migration in response to CXCL12 in CD4+ T cells obtained from the elderly, as compared to those from young donors, led us to hypothesize that increase in surface expression, due to altered endocytic regulation of CXCR4 on T cells during aging, might be directly responsible for increased migration towards CXCL12. Studies presented here demonstrate a significant increase in the surface expression of CXCR4 in CD4+ T cells from elderly human donors, relative to those from the young. Additionally, CXCL12-mediated endocytosis of CXCR4 was differentially regulated during aging, which could be attributed to alterations in the ubiquitination of CXCR4. Thus, altered ubiquitination of CXCR4 may contribute to the increased surface expression and enhanced T cell migration to chemotactic stimuli in the elderly.
Introduction
Studies have well documented that aging significantly attenuates the ability of an individual to mount appropriate immune responses (Arnold et al., 2011; Baum et al., 2011; Ponnappan & Ponnappan, 2011). Most of the studies dealing with immunity during advancing age in humans have focused on the impact of age on antigen-specific TCR-repertoire diversity, antigenic reactivity and T cell-dependent antibody generation in response to vaccination (Caruso et al., 2005; Brunner et al., 2011; Deeks, 2011; den Elzen et al., 2011), with only a limited number focusing on trafficking and/or chemotactic migration. Studies in murine models of aging that have reported on chemotactic migration, demonstrate a significantly higher response to the chemokine CXCL12 in T cells from aged mice than those from younger cohorts (Steeber et al., 1996; Stohlawetz et al., 1996; Toichi et al., 1997; Nyugen et al., 2010). As migration and trafficking of memory and naïve T cells are critical to mounting an effective immune response, any defect in these properties is likely to negatively impact this ability. Therefore, understanding the impact of aging on chemokines and chemokine receptors in regulating migration is essential to delineating immune senescence.
Chemokines and chemokine receptors have been shown to exquisitely regulate T cell migration (Estes et al., 2004; Contento et al., 2008; Bai et al., 2009; Booth et al., 2010; Bunting et al., 2011). Amongst these receptors, CXCR4, a member of the G-protein coupled receptor (GPCR) family, has received a great deal of attention due to its vital role in T lymphocyte development, migration and cytokine secretion. The functions attributed to CXCR4 in T lymphocytes are linked to its surface expression, which is regulated by ligand-dependent endocytosis and degradation of the endocytosed receptor. Abnormal expression or function of CXCR4 has been reported in several diseases such as cancer, cardiovascular disease, inflammatory allergic disease, HIV and neuroinflammatory disease (Feng et al., 1996; Abu El-Asrar et al., 2001; Rauer et al., 2002; Burger et al., 2003; Walter et al., 2005). Ligand-dependent stimulation of CXCR4 has been demonstrated to result in endocytosis and degradation, following ubiquitination and lysosomal targeting. Three sites for ubiquitination have been identified on CXCR4 that are linked to lysosomal degradation (Marchese & Benovic, 2001; Marchese et al., 2003; Bhandari et al., 2007; Bhandari et al., 2009; Malik & Marchese, 2010; Caballero & Marchese, 2011). Interestingly, mono-ubiquitination of CXCR4 is not required for endocytosis and trafficking, but is essential for receptor degradation. Agonist mediated activation appears to promote mono-ubiquitination and lysosomal degradation of CXCR4 in HEK293 cell line via a mechanism dependent on ubiquitin ligase, AIP4,(Marchese et al., 2003; Bhandari et al., 2009). Indeed, activation of CXCR4 in HEK293 cells expressing catalytically inactive AIP4 mutants or depleted of AIP4 by siRNA, results in reduction, but not complete abrogation of ubiquitination and degradation. This partial inhibition of mono-ubiquitination and degradation allowed us to posit that other mechanisms, perhaps poly-ubiquitination by alternative ligases and proteasome-mediated degradation, may also be involved in CXCR4 regulation during aging (Lapham et al., 2002; Marchese, 2009; Berlin et al., 2010). Support for this hypothesis is based on studies in which pretreatment with lactacystin, a cell permeable proteasome specific inhibitor, increased the surface expression of CXCR4 in T lymphocytes. Therefore, it is likely that CXCL12-induced CXCR4 internalization and/or degradation involves multiple lysine-dependent mono-ubiquitination or poly-ubiquitination events. Additionally, deubiquitination events, primarily attributed to USP14, appear to be critical in controlling ligand-dependent CXCR4 internalization, degradation and chemotaxis. While it was reported, that over-expression of USP14 followed by CXCL12 treatment resulted in a significant reduction of CXCR4-dependent migration (Mines et al., 2009), little is known about the impact of USP14 inhibition on CXCR4 surface expression in T cells in general and specifically during aging. We now report that age-associated alteration in the expression of CXCR4 in T cells from the elderly appears to underlie increased migration observed in response to CXCL12. Employing a small molecule inhibitor of USP14 and analysis of ubiquitination of CXCR4, we now demonstrate age-associated alteration in the ubiquitination status of CXCR4, which may directly or indirectly regulate the expression and/or internalization of the receptor.
Results
1. CD4+ T cells demonstrate increased surface expression of CXCR4 during aging
As our previous studies clearly demonstrated that CD4+ T cells from the elderly have higher migratory index in response to CXCL12 (Cane et al., 2011), we next evaluated the surface expression of CXCR4 in CD4+ T cells obtained from both young and elderly donors. Expression of CXCR4 was analyzed in primary human CD4+ T cells, as well as in CD4+CD45RO+ and RA+ T cell subsets, since aging is often accompanied by a shift in population of CD4+ naïve T cells to a predominantly CD4+ memory T cell subset.
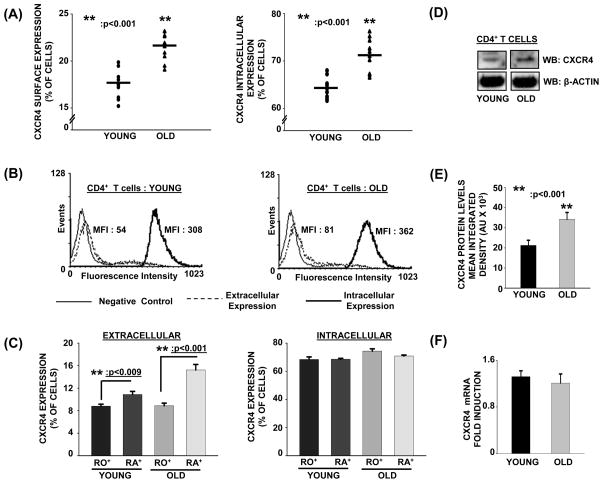
As shown in figure 1A and 1B, levels of both the surface as well as intracellular expression of CXCR4 appeared to be higher in CD4+ T cells obtained from elderly donors as compared to those from the young. Further, as shown in figure 1C, CD4+CD45RA+ naïve T cell subset appeared to have higher levels of surface expression of CXCR4 relative to CD4+CD45RO+ memory subset, derived from both young and elderly donors. Additionally, irrespective of the age of the donor, intracellular levels of CXCR4 were far greater than those detected on the surface of T cells. To determine if the observed increase in CXCR4 expression in T cells from the elderly correlated with an overall increase in CXCR4 protein and/or mRNA expression, we next assessed the protein and mRNA levels of CXCR4, in CD4+ T cells obtained from young and elderly donors. As shown in figure 1D and 1E, total CXCR4 levels in CD4+ T cells from elderly donors were significantly higher relative to those from young donors. Examination of mRNA expression of CXCR4 employing qRT-PCR in CD4+ T cells, shown in figure 1F, demonstrate similar levels of CXCR4 mRNA in CD4+ T cells from both young and elderly donors.
Fig. 1. Increased surface expression of CXCR4 in CD4+ T cells during aging.
(A). Left panel: Surface expression of CXCR4 in CD4+ T cells from young and elderly donors. CD4+ T cells obtained from young and elderly donors were labeled using antibody to CXCR4, followed by Alexa Fluor 488-conjugated secondary antibody staining and then fixed with 2% PFA. Right panel: Intracellular expression of CXCR4 in CD4+ T cells obtained from young and elderly donors. CD4+ T cells obtained from young and elderly donors were fixed using 2% PFA, and then permeabilized with 1xPBS buffer containing 0.1% Saponin. Cells were then stained using an antibody to CXCR4, followed by Alexa Fluor 488-conjugated secondary antibody. Cell surface and intracellular expression of CXCR4 were detected by flow cytometry. Cumulative data of percent cells expressing CXCR4 obtained from 10 independent donor pairs are provided. ** denotes statistical significance at p<0.001.
(B). Flow cytometry of CD4+ T cells from young and elderly donors stained for intracellular and extracellular CXCR4 expression, using antibody to CXCR4, followed by secondary antibody coupled to fluorochrome. Representative data from one young (Left panel)) and elderly (Right panel) donor pair are provided. Intracellular CXCR4 staining was carried out using fixed, permeabilized CD4+ T cells, while extracellular staining was carried out on unfixed cells. Isotype stained cells served as negative controls. MFI indicates mean fluorescence intensity.
(C). Surface and intracellular expression of CXCR4 in CD4+CD45RO+ and RA+ T cell subsets from young and elderly donors. Cumulative data of percent cells expressing CXCR4 obtained from 10 independent donor pairs are provided.
(D). Increased levels of CXCR4 in CD4+ T cells from the elderly. A representative western blot of CXCR4 in CD4+ T cell lysates obtained from young and elderly donors are provided. β-actin was used as a protein loading control. Representative data from one donor pair out of 10 pairs tested are provided.
(E). Quantitation of the specific band corresponding to CXCR4 protein was carried out by densitometry. Values represent mean integrated density ± SE obtained from 10 independent donor pairs. ** denotes statistical significance at p<0.001.
(F). qRT-PCR analysis of CXCR4 mRNA from total RNA isolated from CD4+ T cells obtained from young and elderly donors. Data obtained from 10 donor pairs are provided. β-actin and GAPDH were used as reference genes and for normalization. Data are presented as fold induction.
2. Minimal impact of aging on the surface expression of CCR5 in resting and activated CD4+ T cells
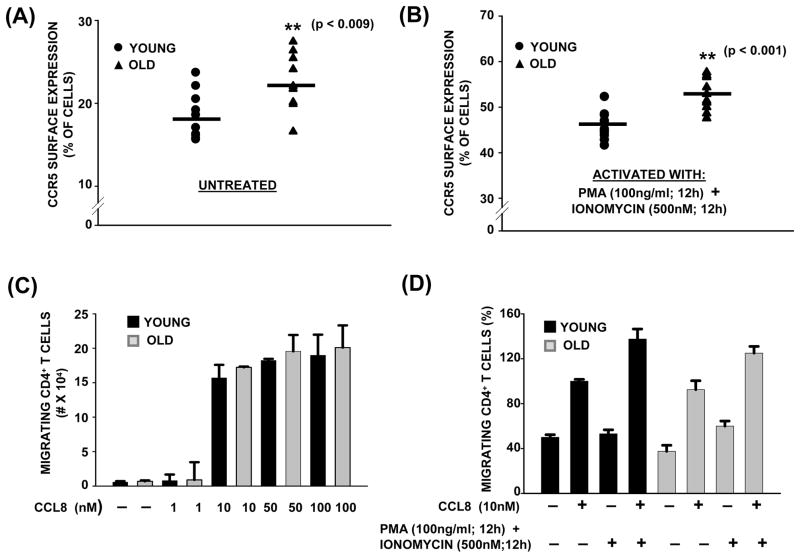
To evaluate if the increase in expression of CXCR4 observed in CD4+ T cells from elderly donors was exclusive to this chemokine receptor and not to a generalized increase in the surface expression of all chemokine receptors during aging, we next assessed the expression of CCR5. CCR5 was chosen for the study since it has been demonstrated to be constitutively expressed in T lymphocytes and has been shown to synergize with CXCR4 in controlling the migration of CD14+ monocytes and CD3+ T cells (Desmetz et al., 2007; Contento et al., 2008; Gouwy et al., 2011). Additionally, CCR5 has also been reported to increase and sustain CXCR4 ligand-induced ERK phosphorylation and activation, necessary for gene transcription and cytokine production in response to CXCL12 (Yopp et al., 2004; Gouwy et al., 2011). As shown in figure 2A and 2B, surface expression of CCR5 showed a consistent but minimal increase between both the age groups, under resting and as well as under activated (PMA + Ionomycin) conditions. Since CCR5 is known to regulate chemotactic migration in T cells, we next examined the effect of different concentrations of CCL8, ligand for CCR5, on CD4+ T cell migration using a trans-well assay. As shown in figure 2C, a dose-dependent increase in migration was observed in CD4+ T cells obtained from both young and elderly donor groups, with the dose of 10nM of CCL8 eliciting the maximum migration index. Thus, employing CCL8 at 10nM, we next assessed the migration of CD4+ T cells from both young and elderly donors. As shown in figure 2D, CD4+ T cells obtained from both young and elderly donors migrated towards CCL8, showing little impact of age on migration. In CD4+ T cells that were pretreated with PMA + Ionomycin, the migration index increased, demonstrating a direct correlation with increased cell surface expression of CCR5. Interestingly, unlike our results obtained in response to CXCL12, the age of the CD4+ T cell donor had little impact on the ability of CD4+ T cells to migrate towards CCL8. Thus, a specific increase in the surface expression of CXCR4, and not a generalized increase in overall expression of chemokine receptors, is attributable to the increase in CXCL12-dependent migration observed in T cells from the elderly.
Fig. 2. CCR5 surface expression in resting and activated CD4+ T cells during aging.
(A& B). Surface expression of CCR5 in CD4+ T cells from young and elderly donors. CD4+ T cells obtained from young and elderly donors were either left untreated (A) or activated with PMA/Ionomycin for 12h (B), stained using antibody to CCR5 followed by Alexa Fluor 555-conjugated secondary antibody. Surface expression of CCR5 was detected by flow cytometry. Cumulative data obtained from a minimum of 8 independent donor pairs are provided. ** denotes statistical significance at p<0.001; * denotes statistical significance at p<0.009.
(C). Dose dependent migration of CD4+ T cells from young and elderly donors to CCL8 gradient. CD4+ T cells (3×105) from young and elderly donors were placed in the upper chamber of a trans-well migration plate (5μM pore) and allowed to migrate for 4h at 37°C towards a gradient generated by either 1, 10, 50 or 100nM of CCL8. The number of migrating cells present in the lower chamber was assessed by Trypan Blue staining, and the percentage of migrating cells is provided. Values represent mean ± SE from 4 independent donor pairs.
(D). Chemotactic migration of CD4+ T cells from young and elderly donors to CCL8 gradient. CD4+ T cells were either left untreated or pretreated with PMA/Ionomycin for 12h. Data are representative of 10 independent donor pairs and represent percentage of migrating CD4+ T cells from young and elderly donors in response to CCL8.
3. Differential ubiquitination of CXCR4 occurs in CD4+ T cells during aging and may contribute to increased surface expression
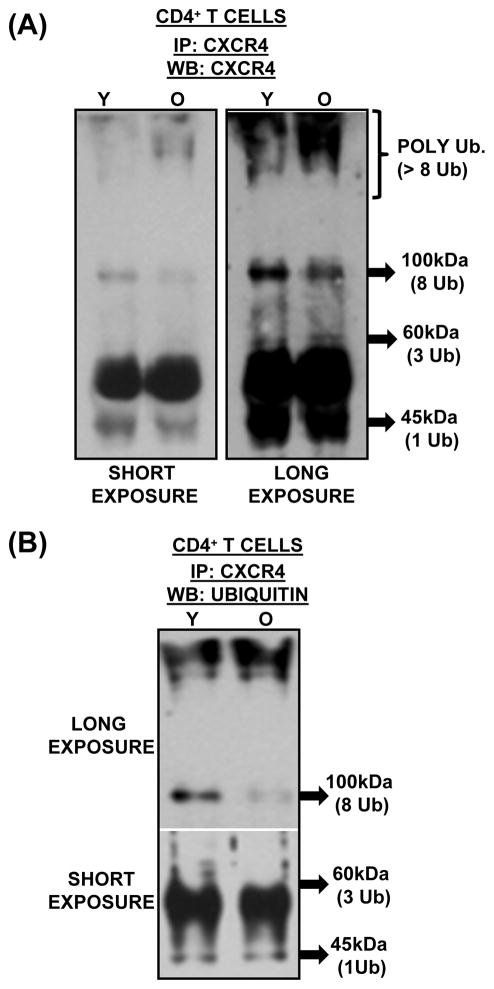
To understand the molecular mechanism/s that contributes to the observed increase in CXCR4 surface expression in CD4+ T cells from elderly donors, we next evaluated the stability of CXCR4 expression during aging. In fact, as previous studies have demonstrated that ubiquitination at the C-terminal domain of CXCR4 is required for lysosome-mediated receptor degradation, we focused on ubiquitination of CXCR4 (Marchese & Benovic, 2001; Bhandari et al., 2007; Malik & Marchese, 2010; Caballero & Marchese, 2011). Additionally, previous studies have also shown that ligand-induced stimulation of CXCR4 results in increased ubiquitination, mediated by a complex formed by E3 ubiquitin ligase AIP4, underscoring the importance of ubiquitination (Marchese et al., 2003; Bhandari et al., 2009). As shown in figure 3A and 3B, immuno-precipitation of CXCR4 followed by western blot with antibody to CXCR4 demonstrated several CXCR4 isoforms in CD4+ T cell lysates from both young and elderly donors (3A, long-exposure). The appearance of several isoforms of CXCR4 (antibody reactive bands) is in agreement with observations made in other cell types. Interestingly, CXCR4 isoforms at 45kDa, 62kDa and 100kDa, appear to be lower in the immunoprecipitates of lysates derived from CD4+ T cells obtained from the elderly relative to those from the young. In contrast, the isoform/s appearing above 150 kDa is more abundant in the elderly relative to that seen in immunoprecipitates from the young. As the appearance of these isoforms may be attributable to differential ubiquitination of CXCR4, we next tested the lysates for ubiquitin-dependent modification of CXCR4, following immunoprecipitation with anti-CXCR4. As seen in figure 3b, CXCR4 is indeed differentially ubiquitinated in lysates obtained from the young, relative to those from the elderly (3b, long exposure). Additionally, the presence of heavily polyubiquitinated CXCR4 in the immunoprecipitates from the elderly, appearing at > 150kDa molecular weight, but not from the young, suggests the involvement of the proteasome in the accumulation, and perhaps in down-regulating, CXCR4 molecules. As our laboratory has previously reported age-associated deficits in proteasomal catalytic function in T cells from the elderly (Das et al., 2007; Ponnappan et al., 2007; Ponnappan & Ponnappan, 2011), accumulation of high molecular weight polyubiquitinated-CXCR4 may be an outcome of such a defect. This observation is further supported by independent reports that employed chemical inhibition of the proteasome, resulting in increased surface expression of CXCR4 in lymphocytes. Thus, results presented here demonstrate that alterations in the ubiquitination of CXCR4 and /or proteasome function, may account for the increased surface expression of CXCR4 observed in CD4+ T cells during aging.
Fig. 3. CXCR4 is differentially ubiquitinated in CD4+ T cells during aging.
(A). Lowered expression of low molecular weight CXCR4 isoforms in CD4+ T cells from the elderly. CD4+ T cell lysates obtained from a pool of 5 young and 5 elderly donors were immunoprecipitated with an antibody to CXCR4. The immunoprecipitate was resolved using SDS-PAGE and detected using an antibody to CXCR4. Representative blot from one immune-precipitation assay is provided. Arrows depict approximate molecular weight of the CXCR4 isoforms derived from molecular weight standards, and approximate number of ubiquitin tags that could account for the increase in molecular weight.
(B). Decreased monoubiquitination and increased polyubiquitination (>8Ub-linkages) of CXCR4 in CD4+ T cells, accompanies aging. Cell lysates of CD4+ T cells obtained from 3 independent pools of 5, 3 and 3 young and elderly donors were immunoprecipitated with an antibody to CXCR4. Resolved immunoprecipitates were immunoblotted with antibody to ubiquitin. Long exposure indicates extended exposure of the film to demonstrate the appearance of intense high molecular weight CXCR4 isoforms denoting polyubiquitination. Arrows indicate ubiquitinated species attributable to 1, 3 and 8 ubiquitin linkages and are based on approximately 8.5kDa increments in molecular weight.
4. Inhibition of USP14 results in increased surface expression of CXCR4
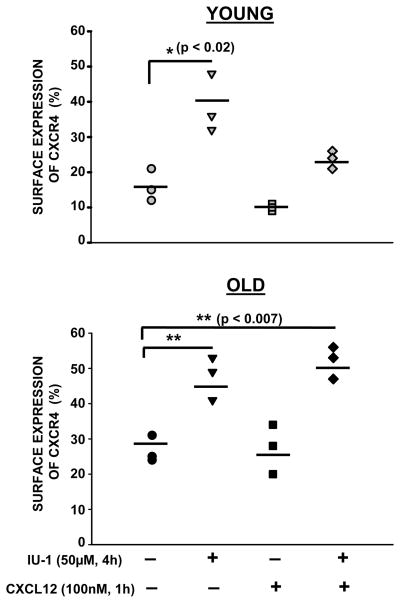
As previous experiments indicated increased polyubiquitinated CXCR4 isoforms occurring in CD4+ T cells from the elderly, implying alteration in the ubquitination/deubiquitination cycle of CXCR4, we next evaluated the effect of inhibition of a deubiquitinating enzyme, USP14, on the surface expression of CXCR4. We chose to target USP14, since previous studies have reported a critical role for USP14 in CXCR4 degradation and chemotaxis mediated by CXCL12 (Mines et al., 2009). CD4+ T cells from young and elderly donors were either left untreated or pretreated with a small molecule inhibitor of USP14, IU-1, and then activated with CXCL12. At the end of the treatment, surface expression of CXCR4 was evaluated by flow cytometry employing a CXCR4 specific antibody. As shown in figure 4, increase in the surface expression of CXCR4 upon IU-1 treatment occurred in CD4+ T cells obtained from both young and elderly donors, suggesting that USP14 specific deubiquitination activity is essential for CXCR4 internalization. Interestingly, we also observed that pretreatment with IU-1 partially blocked CXCL12-mediated CXCR4 down-modulation/degradation in CD4+ T cells from young donors but not in those from the elderly, indicating an age associated defect in ligand-induced degradation of CXCR4 in CD4+ T cells. Thus, inhibition of USP14 results in increased surface expression of CXCR4 and negatively impacts CXCL12 mediated CXCR4 internalization and degradation, by regulating the ubiquitination/deubiquitination cycle.
Fig. 4. Inhibition of USP14 results in increased CXCR4 surface expression in CD4+ T cells.
Surface expression of CXCR4 in CD4+ T cells from young and elderly donors. CD4+ T cells obtained from young and elderly donors were either left untreated or pretreated with IU-1 (50μM; 4h), and then either activated with CXCL12 (100nM; 1h), or not. T cells were then stained using an antibody to CXCR4, followed by Alexa Fluor 488-conjugated secondary antibody. Surface expression of CXCR4 was detected by flow cytometry. Data obtained from 3 independent donor pairs are provided. ** denotes statistical significance at p<0.009; * denotes statistical significance at p<0.05.
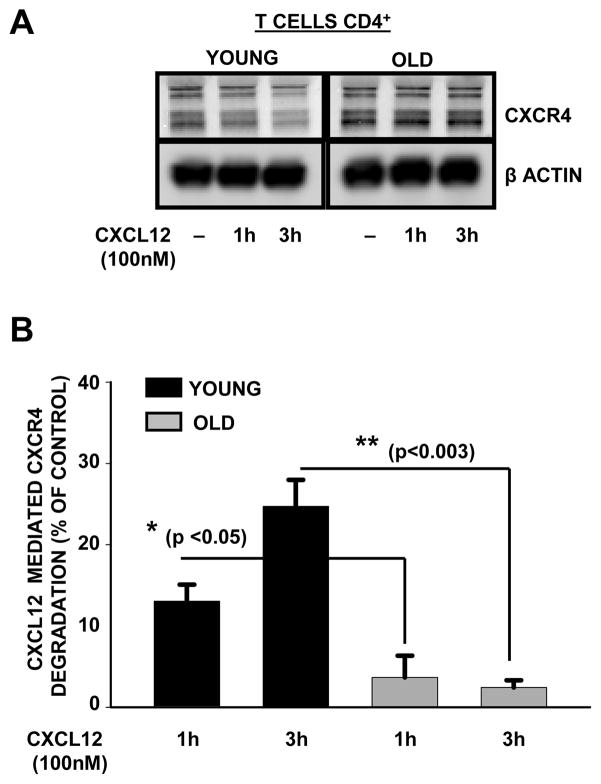
5. Decreased ligand-induced degradation of CXCR4 in CD4+ T cells during aging
Upon stimulation with CXCL12, CXCR4 is internalized and sorted through the endosomal compartment, where it is either recycled back to plasma membrane or is degraded. The observed decrease in mono-ubiquitination of CXCR4 in resting CD4+ T cells obtained from the elderly donors, prompted us to evaluate whether CXCL12-dependent degradation of CXCR4 was also impaired in CD4+ T cells from the elderly. CD4+ T cells obtained from both young and elderly donors were either left untreated or activated with CXCL12 (100nM) for 1h and 3h. As shown in figure 5A and 5B, we observed a time-dependent decrease in CXCR4 levels in lysates of CD4+ T cells obtained from young donors, but not in those from the elderly, where the levels of CXCR4 appeared to be minimally affected, irrespective of the length of activation with CXCL12. Thus, our results suggest that ligand-dependent CXCR4 degradation is impaired in CD4+ T cells from elderly donors.
Fig. 5. Decreased ligand-induced degradation of CXCR4 in CD4+ T cells accompanies aging.
(A). Representative western blot of the predominant CXCR4 isoforms in CD4+ T cell lysates obtained from young and elderly donors that were either left untreated or treated with CXCL12 for 1h and 3h. β-actin was used as a control for equal protein loading.
(B). Percent degradation of CXCR4 following CXCL12 treatment at 1 and 3h relative to control (untreated) obtained from young and elderly donors are presented. Values represent mean ±S.E. obtained from a minimum of 6 independent donor pairs. ** denotes statistical significance at p<0.003; * denotes statistical significance at p<0.05.
Discussion
Chemokine-dependent migration is a fundamental feature of T cell function which ensures homing of naïve T cells to secondary lymphoid organs and recirculation of memory-effector T cells to the periphery (Friedman et al., 2005; Contento et al., 2008; Bunting et al., 2011). CXCL12 and its receptor CXCR4 have garnered a great deal of interest due to their central role in inflammation, homeostasis, migration in the immune system, and in a wide variety of cancers due to their increased association with cancer progression and tumor cell proliferation (Zlotnik et al., 2011). Additionally, the recognition of CXCR4 as a co-receptor for HIV infection has provided greater significance for its expression within T cell subsets (Feng et al., 1996). Recent studies on WHIM syndrome, a congenital immune deficiency, have demonstrated mutations of the intracellular carboxy terminus of CXCR4. WHIM leukocytes have enhanced responses to CXCL12 due to receptor desensitization (Bachelerie, 2010). Thus, any biological process that alters the level and/or function of chemokine receptors will likely impact the ability of the immune system, not only to mount appropriate responses to invading pathogens, but also affect immune homeostasis.
Limited information regarding changes of chemokine receptors during human aging, coupled together with our previous observation of increased chemotactic migration towards CXCL12 gradient in CD4+ T cells from the elderly, prompted us to evaluate the expression, function and regulation of CXCR4 in CD4+ T cells from young and elderly donors. Here, we provide evidence that CD4+ T cells from the elderly have increased surface expression of CXCR4, which may partly contribute to the increased migration towards a gradient generated by CXCL12, previously described by our laboratory (Cane et al., 2011). While the overall protein levels of CXCR4 is also significantly increased in CD4+ T cells during aging, the mRNA levels remain unaffected, suggesting a role for post translational regulation. Our results of increased CXCR4 expression is in partial agreement with previously published results by Yung and colleagues in a murine model of aging employing splenic lymphocytes (Mo et al., 2003). To determine whether a generalized upregulation of chemokine receptor expression accompanies aging, we next evaluated CCR5 expression. Interestingly, we found that, unlike CXCR4, surface expression of CCR5 on CD4+ T cells showed only a minimal increase in cells from the elderly, both under resting conditions, as well as upon activation with PMA / Ionomycin. Thus, the expression of CXCR4 to a greater extent and CCR5 to a lesser extent was affected by advancing age. As cells exposed to a chemotactic gradient generated by CXCL12 demonstrated increased migratory index in T cells from the elderly, we next assessed migration of T cells towards CCL8, the ligand for CCR5. In a chemotactic gradient generated by CCL8, CD4+ T cells obtained from both young and elderly donors demonstrated similar migratory indices in a trans-well assay, suggesting that the minimal change in CCR5 expression did not significantly impact migration towards CCL8. These results led us to conclude that the increase in CXCR4 expression with age is not due to a generalized age-asociated increase in chemokine receptor expression and function, but rather to a specific increase in CXCR4.
Although, it appears that CXCR4 regulation is altered during aging, the molecular mechanism/s dictating this change in CXCR4 remains to be fully defined. In keeping with previous reports (Zaitseva et al., 1998; Contento et al., 2008), our studies in CD4+ T cells obtained from human donors demonstrate the occurrence of different isoforms of CXCR4, based on cross-reactivity with an antibody specific to CXCR4. Our results for the first time demonstrate an age-associated effect on the nature of the detected CXCR4 isoforms. It appears that CXCR4 isoforms with high molecular weights are more abundant in lysates derived from CD4+ T cells from the elderly relative to those from the young. However, it should be noted that not all high molecular weight isoforms are consistently observed in samples from the elderly. The “ladder-like” high molecular weight bands observed in the elderly, suggested that they might correspond to polyubiquitinated forms of CXCR4. As several studies have shown ubiquitination of CXCR4 on lysine residues located at the C-terminal domain of CXCR4 by the E3 ubiquitin ligase AIP4 is responsible for targeting it to lysosome for degradation, such a modification seemed like a plausible explanation for the occurrence of high molecular weight isoforms (Marchese et al., 2003; Bhandari et al., 2009; Caballero & Marchese, 2011). Indeed, in agreement with previous reports, we now demonstrate that in CD4+ T cells from young donors, several CXCR4 isoforms are differentially ubiquitinated, with fewer polyubiquitinated isoforms. In contrast, relatively higher levels of polyubiquitinated CXCR4 isoforms are observed in CD4+ T cells from the elderly, lending support to our hypothesis. Although, at this time it is technically challenging to experimentally discriminate between polyubiquitinated or multiply-monoubiquitinated CXCR4 isoforms, and whether they exhibit K-48 and K-63 ubiquitin linkages, future studies will attempt to address these issues. One probable underlying basis for the increase in polyubiquitinated species of CXCR4 in T cells from the elderly may be attributable to the altered intracellular redox potential in CD4+ T cells during aging coupled with proteasomal dysfunction (Ponnappan et al., 2007; Ponnappan & Ponnappan, 2011). This increased polyubiquitinated species may confer stability, resulting in increased surface expression, leading to higher chemotactic migratory index towards CXCL12 gradient. Additionally, our findings of altered kinetics of ligand-mediated degradation of CXCR4 in CD4+ T cells from the elderly, strongly support the contention that mechanisms involved in CXCR4 down-modulation may also be altered during aging. Our studies for the first time unravel a role for the deubiqitinating enzyme USP14, in the ubiquitination/deubiquitination cycle of CXCR4. These studies further point to an age-associated alteration of the regulation of USP14. Previous reports of USP14 regulation, have implicated both inhibition and over-expression of USP14 in increased surface expression of CXCR4 (Mines et al., 2009). In keeping with this observation, our studies demonstrate that treatment with a small molecule inhibitor of USP14, IU1, results in a significant increase in CXCR4 levels in CD4+ T cells, irrespective of the donor age. Interestingly, increased levels of USP14 are observed in cells from the elderly, relative to those from young donors (data not shown). We believe that this inherent increase in USP14 in T cells may underlie increased CXCR4 expression observed in the elderly. However, it should be noted that the experiments designed in this study fail to discriminate whether USP14 mediated regulation of CXCR4 expression is either direct or indirect.
In summary, the observations that emerge from these studies begin to shed light on the underlying basis for the increase in migration observed in CD4+ T cells during aging by primarily focusing on CXCR4 regulation. The increased expression and altered post-translational ubiquitination of CXCR4 in CD4+ T cells from the elderly, coupled with altered USP14 activity, may result in the retention of CXCR4 on the surface and thus contribute to increased migration towards a chemotactic gradient generated by CXCL12. Given the roles of redox regulation, proteasome and ubiquitination in CXCR4 regulation, further investigations are clearly warranted to dissect the relationships involving these pathways in the context of aging and immune senescence.
Experimental Procedures
Antibodies and reagents
Horseradish peroxidase-conjugated goat anti-mouse antibody was from BD Biosciences (San Jose, CA). Antibodies to human CCR5, CD4CD45-RO and -RA were from eBiosciences (San Diego, CA). Antibody to β-actin and CXCR4 were from Santa Cruz biotechnology (Santa Cruz, CA). Alexa Fluor 555-conjugated goat anti-mouse secondary antibody and Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody were from Invitrogen (Carlsbad, CA). Antibody to ubiquitin-protein conjugates was from Enzo Life Sciences (Farmingdale, NY). Horseradish peroxidase-conjugated goat anti-rabbit antibody was from Thermo Scientific (Rockford, IL). All fine chemicals, unless otherwise mentioned, were obtained from Sigma-Aldrich (St. Louis, MO). CXCL12 and CCL8 were from ProSpec-Tany Technogene (Rehovot, Israel). Electrophoresis supplies were from BioRad (Hercules, CA). USP14 small molecule inhibitor (IU1) was from BioVision (Mountain View, CA).
Human subjects
Peripheral blood was obtained by venipuncture from healthy young (21–30 years) and elderly (65–89 years) adults enrolled from the greater Little Rock area. Immuno-compromised subjects were excluded, including individuals with asthma and those taking immune-modulating drugs upon consultation with the study geriatrician. Subjects on antibiotics or who self reported symptoms of recent infection (<3 weeks before enrollment) were excluded. All protocols involving human subjects were approved by the UAMS Institutional Review Board and appropriate informed consents were obtained. Blood draw was performed at the Clinical Research Center (CRC) at UAMS.
T lymphocyte isolation
CD4+ T cells were negatively selected from blood using the EasySep CD4+ T-cell enrichment kit per manufacturer’s recommended protocol (StemCell Tech., Vancouver, Canada). Purity of isolated CD4+ T cells was determined by flow cytometry and was consistently found to be 90–95% pure. CD4+CD45RO+ and CD4+CD45RA+ T cells were negatively selected from blood using EasySep Human Memory CD4+ T cell enrichment kit and EasySep Human Naïve CD4+ T cell enrichment kit, respectively, with minor modifications of the manufacturer’s recommended protocol (Stem Cell Tech., Vancouver, Canada). Isolated CD4+CD45RO+/ RA+ T cells were determined to be 90–95% pure by flow cytometry.
Western Blotting and Immunoprecipitation
CD4+T cell lysates, equalized for protein levels, were resolved using Sodium dodecyl sulphate - Polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membrane, immunoblotted with specific antibody and detected using enhanced chemiluminescence.
For immunoprecipitation studies, pre-cleared cell lysates (200μg protein) were incubated with antibody to CXCR4 and protein A/G agarose beads overnight at 4°C with gentle rocking. Protein A/G beads containing the adsorbed immunoprecipitated complex were washed with RIPA buffer, resuspended in 30μl of 2X SDS-sample buffer and heated in a boiling water-bath for 5 min. Protein complexes were resolved by SDS-PAGE and subjected to western blot analyses, as detailed above.
Reverse Transcriptase–Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA). 4μg of total RNA for each sample was reverse-transcribed and the resultant cDNA was amplified using the BioRad iCycler PCR system. Reactions were performed in 96-well PCR reaction plates using 12.5 μL of iQ SYBR Green Supermix (BioRad, Hercules, CA), forward and reverse primers (0.3 nmol each), and cDNA (3 μL) in a final volume of 25 μL. Amplification parameters were denaturation at 95 °C for 10 min followed by 40 cycles at 95 °C for 30 s and 60 °C for 70 s. Samples were analyzed in duplicate for the expression levels of cxcr4. Housekeeping genes, human β-actin and GAPDH, were used for normalization. Fold induction was calculated after normalization using the ΔΔCT method. Dissociation curves indicated that each reaction consisted of a single reaction product. Gene-specific primers were designed by Primer3 software employing human sequences obtained from GenBank. Primer sequences will be provided upon request.
Flow Cytometry
For surface staining, CD4+ T cells as well as CD4+ CD45RO+ and RA+ T cell subsets were either incubated with antibody to CXCR4 or CCR5 followed by incubation with an antibody conjugated to Alexa Fluor-488 or Alexa Fluor-555, respectively, for 20 min at 4°C. At the end of incubation, cells were washed with ice cold PBS containing 0.1% BSA and fixed with 2% paraformaldehyde (PFA).
Chemotactic Migration assay
CD4+ T cells (3×105) either untreated or pretreated with PMA (100ng/ml) and Ionomycin (500nM) for 12h, were resuspended in 100μl of media containing 0.2% BSA and placed into the upper chamber of a trans-well migration plate (5μm pore, Costar). In the lower chamber, 600μl of media containing 0.2%BSA, with or without 10nM of CCL8, was added and cells were allowed to migrate for 4h at 37°C. The number of migrated cells was counted following Trypan Blue staining. For dose response experiments, CD4+ T cells (3×105) were resuspended in 100μl of media containing 0.2% BSA and placed into the upper chamber of a trans-well migration plate (5μm pore, Costar). In the lower chamber, 600μl of media containing 0.2%BSA with 1, 10, 50 or 100nM of CCL8 was added and cells were allowed to migrate for 4h at 37°C. The number of migrated cells was counted following Trypan Blue staining.
Assessment of CXCR4 regulation by ligand and a ubiquitin specific protease inhibitor
To determine ligand-induced degradation of CXCR4, freshly isolated CD4+ T cells from both young and elderly donors were incubated either in the presence or absence of CXCL12 (100nM) for 1h and 3h. At the end of incubation the cells were washed twice with cold 1X PBS and cellular lysates were obtained. Percent degradation relative to control is presented.
To evaluate the impact of USP14 inhibition on CXCR4 protein levels, CD4+ T cells from young and elderly donors were pretreated with IU-1, 50μM for 4h, followed by activation with CXCL12 (100nM) for 1h. At the end of the incubation CD4+ T cells were washed twice with cold 0.1%BSA/PBS and stained with a CXCR4 specific antibody to assess the surface expression of CXCR4.
Statistical analyses
Differences between means of the data generated in the study were analyzed using Student’s t-test. Differences were considered significant, if p < 0.05.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants, RO1 AG30599 and AG25220 to UP. Support for the CRC was provided by The National Center for Research Resources Grant UL1RR029884. We thank Mrs. Michela Palmieri for help with coordinating human subjects’ recruitment for the study and for providing technical assistance with the isolation of T lymphocytes.
Footnotes
Author Contributions
Dr U. Ponnappan designed the research and provided oversight. Dr. S. Ponnappan directed and helped with the analyses, presentation of results and editing of the manuscript. Dr. S. Cané performed the research as a part of her dissertation project, analyzed the data and wrote the draft of the manuscript.
References
- Abu El-Asrar AM, Struyf S, Al-Mosallam AA, Missotten L, Van DJ, Geboes K. Expression of chemokine receptors in vernal keratoconjunctivitis. Br J Ophthalmol. 2001;85:1357–1361. doi: 10.1136/bjo.85.11.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold CR, Wolf J, Brunner S, Herndler-Brandstetter D, Grubeck-Loebenstein B. Gain and loss of T cell subsets in old age-age-related reshaping of the T cell repertoire. J Clin Immunol. 2011;31:137–146. doi: 10.1007/s10875-010-9499-x. [DOI] [PubMed] [Google Scholar]
- Bachelerie F. CXCL12/CXCR4-axis dysfunctions: Markers of the rare immunodeficiency disorder WHIM syndrome. Dis Markers. 2010;29:189–198. doi: 10.3233/DMA-2010-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Z, Hayasaka H, Kobayashi M, Li W, Guo Z, Jang MH, Kondo A, Choi BI, Iwakura Y, Miyasaka M. CXC chemokine ligand 12 promotes CCR7-dependent naive T cell trafficking to lymph nodes and Peyer’s patches. J Immunol. 2009;182:1287–1295. doi: 10.4049/jimmunol.182.3.1287. [DOI] [PubMed] [Google Scholar]
- Baum PD, Young JJ, McCune JM. Measurement of absolute T cell receptor rearrangement diversity. J Immunol Methods. 2011;368:45–53. doi: 10.1016/j.jim.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Berlin I, Higginbotham KM, Dise RS, Sierra MI, Nash PD. The deubiquitinating enzyme USP8 promotes trafficking and degradation of the chemokine receptor 4 at the sorting endosome. J Biol Chem. 2010;285:37895–37908. doi: 10.1074/jbc.M110.129411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari D, Robia SL, Marchese A. The E3 ubiquitin ligase atrophin interacting protein 4 binds directly to the chemokine receptor CXCR4 via a novel WW domain-mediated interaction. Mol Biol Cell. 2009;20:1324–1339. doi: 10.1091/mbc.E08-03-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari D, Trejo J, Benovic JL, Marchese A. Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J Biol Chem. 2007;282:36971–36979. doi: 10.1074/jbc.M705085200. [DOI] [PubMed] [Google Scholar]
- Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, Jackson SE, Salmon M, Falciani F, Yong K, Rustin MH, Akbar AN, Vukmanovic-Stejic M. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- Brunner S, Herndler-Brandstetter D, Weinberger B, Grubeck-Loebenstein B. Persistent viral infections and immune aging. Ageing Res Rev. 2011;10:362–369. doi: 10.1016/j.arr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Bunting MD, Comerford I, McColl SR. Finding their niche: chemokines directing cell migration in the thymus. Immunol Cell Biol. 2011;89:185–196. doi: 10.1038/icb.2010.142. [DOI] [PubMed] [Google Scholar]
- Burger M, Glodek A, Hartmann T, Schmitt-Graff A, Silberstein LE, Fujii N, Kipps TJ, Burger JA. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22:8093–8101. doi: 10.1038/sj.onc.1207097. [DOI] [PubMed] [Google Scholar]
- Caballero A, Marchese A. Ubiquitination of GPCRs. Methods Mol Biol. 2011;746:251–259. doi: 10.1007/978-1-61779-126-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane S, Ponnappan S, Ponnappan U. Impairment of non-muscle myosin IIA in human CD4(+) T cells contributes to functional deficits in the elderly. Cell Mol Immunol. 2011 doi: 10.1038/cmi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso C, Candore G, Colonna-Romano G, Lio D, Franceschi C. Inflammation and lifespan. Science. 2005;307:208–209. doi: 10.1126/science.307.5707.208. [DOI] [PubMed] [Google Scholar]
- Contento RL, Molon B, Boularan C, Pozzan T, Manes S, Marullo S, Viola A. CXCR4-CCR5: a couple modulating T cell functions. Proc Natl Acad Sci USA. 2008;105:10101–10106. doi: 10.1073/pnas.0804286105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Ponnappan S, Ponnappan U. Redox regulation of the proteasome in T lymphocytes during aging. Free Radic Biol Med. 2007;42:541–551. doi: 10.1016/j.freeradbiomed.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Elzen WP, Vossen AC, Cools HJ, Westendorp RG, Kroes AC, Gussekloo J. Cytomegalovirus infection and responsiveness to influenza vaccination in elderly residents of long-term care facilities. Vaccine. 2011;29:4869–4874. doi: 10.1016/j.vaccine.2011.03.086. [DOI] [PubMed] [Google Scholar]
- Desmetz C, Lin YL, Mettling C, Portales P, Noel D, Clot J, Jorgensen C, Corbeau P. Cell surface CCR5 density determines the intensity of T cell migration towards rheumatoid arthritis synoviocytes. Clin Immunol. 2007;123:148–154. doi: 10.1016/j.clim.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Estes JD, Thacker TC, Hampton DL, Kell SA, Keele BF, Palenske EA, Druey KM, Burton GF. Follicular dendritic cell regulation of CXCR4-mediated germinal center CD4 T cell migration. J Immunol. 2004;173:6169–6178. doi: 10.4049/jimmunol.173.10.6169. [DOI] [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Friedman RS, Jacobelli J, Krummel MF. Mechanisms of T cell motility and arrest: deciphering the relationship between intra- and extracellular determinants. Semin Immunol. 2005;17:387–399. doi: 10.1016/j.smim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Gouwy M, Struyf S, Berghmans N, Vanormelingen C, Schols D, Van Damme J. CXCR4 and CCR5 ligands cooperate in monocyte and lymphocyte migration and in inhibition of dual-tropic (R5/X4) HIV-1 infection. Eur J Immunol. 2011;41:963–973. doi: 10.1002/eji.201041178. [DOI] [PubMed] [Google Scholar]
- Lapham CK, Romantseva T, Petricoin E, King LR, Manischewitz J, Zaitseva MB, Golding H. CXCR4 heterogeneity in primary cells: possible role of ubiquitination. J Leukoc Biol. 2002;72:1206–1214. [PubMed] [Google Scholar]
- Malik R, Marchese A. Arrestin-2 interacts with the endosomal sorting complex required for transport machinery to modulate endosomal sorting of CXCR4. Mol Biol Cell. 2010;21:2529–2541. doi: 10.1091/mbc.E10-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A. Ubiquitination of chemokine receptors. Methods Enzymol. 2009;460:413–422. doi: 10.1016/S0076-6879(09)05221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell. 2003;5:709–722. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- Mines MA, Goodwin JS, Limbird LE, Cui FF, Fan GH. Deubiquitination of CXCR4 by USP14 is critical for both CXCL12-induced CXCR4 degradation and chemotaxis but not ERK activation. J Biol Chem. 2009;284:5742–5752. doi: 10.1074/jbc.M808507200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo R, Chen J, Han Y, Bueno-Cannizares C, Misek DE, Lescure PA, Hanash S, Yung RL. T cell chemokine receptor expression in aging. J Immunol. 2003;170:895–904. doi: 10.4049/jimmunol.170.2.895. [DOI] [PubMed] [Google Scholar]
- Nyugen J, Agrawal S, Gollapudi S, Gupta S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol. 2010;30:806–813. doi: 10.1007/s10875-010-9448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnappan S, Ovaa H, Ponnappan U. Lower expression of catalytic and structural subunits of the proteasome contributes to decreased proteolysis in peripheral blood T lymphocytes during aging. Int J Biochem Cell Biol. 2007;39:799–809. doi: 10.1016/j.biocel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Ponnappan S, Ponnappan U. Aging and immune function: molecular mechanisms to interventions. Antioxid Redox Signal. 2011;14:1551–1585. doi: 10.1089/ars.2010.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauer M, Pagenstecher A, Schulte-Monting J, Sauder C. Upregulation of chemokine receptor gene expression in brains of Borna disease virus (BDV)-infected rats in the absence and presence of inflammation. J Neurovirol. 2002;8:168–179. doi: 10.1080/13550280290049741. [DOI] [PubMed] [Google Scholar]
- Steeber DA, Green NE, Sato S, Tedder TF. Lyphocyte migration in L-selectin-deficient mice. Altered subset migration and aging of the immune system. J Immunol. 1996;157:1096–1106. [PubMed] [Google Scholar]
- Stohlawetz P, Kolussi T, Jahandideh-Kazempour S, Kudlacek S, Graninger W, Willvonseder R, Pietschmann P. The effect of age on the transendothelial migration of human T lymphocytes. Scand J Immunol. 1996;44:530–534. doi: 10.1046/j.1365-3083.1996.d01-331.x. [DOI] [PubMed] [Google Scholar]
- Toichi E, Hanada K, Hosokawa T, Higuchi K, Hosokawa M, Imamura S, Hosono M. Age-related decline in humoral immunity caused by the selective loss of TH cells and decline in cellular immunity caused by the impaired migration of inflammatory cells without a loss of TDTH cells in SAMP1 mice. Mech Ageing Dev. 1997;99:199–217. doi: 10.1016/s0047-6374(97)00100-0. [DOI] [PubMed] [Google Scholar]
- Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- Yopp AC, Fu S, Honig SM, Randolph GJ, Ding Y, Krieger NR, Bromberg JS. FTY720-enhanced T cell homing is dependent on CCR2, CCR5, CCR7, and CXCR4: evidence for distinct chemokine compartments. J Immunol. 2004;173:855–865. doi: 10.4049/jimmunol.173.2.855. [DOI] [PubMed] [Google Scholar]
- Zaitseva MB, Lee S, Rabin RL, Tiffany HL, Farber JM, Peden KW, Murphy PM, Golding H. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J Immunol. 1998;161:3103–3113. [PubMed] [Google Scholar]
- Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.