Abstract
Stomach contractions are initiated and coordinated by an underlying electrical activity (slow waves), and electrical dysrhythmias accompany motility diseases. Electrical recordings taken directly from the stomach provide the most valuable data, but face technical constraints. Serosal or mucosal electrodes have cables that traverse the abdominal wall, or a natural orifice, causing discomfort and possible infection, and restricting mobility. These problems motivated the development of a wireless system. The bidirectional telemetric system constitutes a front-end transponder, a back-end receiver and a graphical user interface. The front-end module conditions the analog signals, then digitizes and loads the data into a radio for transmission. Data receipt at the back-end is acknowledged via a transceiver function. The system was validated in a bench-top study, then validated in-vivo using serosal electrodes connected simultaneously to a commercial wired system. The front-end module was 35×35×27 mm3 and weighed 20 g. Bench-top tests demonstrated reliable communication within a distance range of 30 m, power consumption of 13.5 mW, and 124-hour operation when utilizing a 560-mAh, 3-V battery. In-vivo, slow wave frequencies were recorded identically with the wireless and wired reference systems (2.4 cycles/min), automated activation time detection was modestly better for the wireless system (5% vs 14% false positive rate), and signal amplitudes were modestly higher via the wireless system (462 vs 386 μV; p<0.001). This telemetric system for slow wave acquisition is reliable, power efficient, readily portable and potentially implantable. The device will enable chronic monitoring and evaluation of slow wave patterns in animals and patients.
Keywords: wireless monitoring, gastric electrical activity, spikes, dysrhythmia, electrogastrography
1. Introduction
Stomach contractions are initiated and coordinated by an underlying bioelectrical activity, termed slow waves. Slow waves are generated and propagated by a specialized cell type in the gastrointestinal (GI) tract wall, termed interstitial cells of Cajal (ICCs) (Huizinga and Lammers, 2009). Aberrant slow wave patterns (dysrhythmias) have been associated with gastric dysmotility in several significant gastric disorders, notably gastroparesis and functional dyspepsia (Lin et al., 2010),(Leahy et al., 1999).
Chronic studies in awake animals and patients, in fasted and fed states, are needed to better elucidate the pathophysiological significance of gut electrical abnormalities (Ver Donck et al., 2006),(O'Grady et al., 2011a),(Song et al., 2011). Slow wave recordings taken directly from the stomach provide more reliable and descriptive data than cutaneous recordings (electrogastrography; EGG), however, are invasive, and therefore face greater technical constraints. One important issue is that serosal or mucosal recording systems currently transmit signals through lead wires traversing either through the abdominal wall or a natural orifice (Du et al., 2009),(Lin et al., 2011),(Coleski and Hasler, 2009). These wires can act as a conduit for infection, induce discomfort, and connect to bulky acquisition systems that restrict mobility (Ver Donck et al., 2006),(Albert et al., 2010).
Wireless technology could eliminate the need for wired recording systems, offering a better solution for in-vivo monitoring. Telemetric systems are established in other fields including electroencephalography, electromyography, and electrocardiography (Farajidavar et al., 2011),(Joshi et al., 2005). A capsule telemetry system was recently developed for acquiring recordings from the luminal surface of the small intestine, during transit through the GI tract (Woo and Cho, 2010), and a telemetry system for cutaneous EGG has previously been presented (Haddab and Laghrouche, 2009). However, to the best of our knowledge, no wireless system presently exists that is suitable for use in chronic invasive slow wave recordings, such as that could allow for the reliable identification and monitoring of gastric dysrhythmias.
An ideal wireless system should be small, portable, implantable and power efficient, while reliable and dependable. To these ends, we have developed and validated a telemetric system prototype for recording gastric slow wave data.
2. Methods
2.1 System Design
A bidirectional wireless system was developed constituting a front-end transponder, a back-end receiver and a custom-made graphical user interface (GUI). Figure 1(a) shows a block diagram of the system. The front-end module of the prototype was designed to acquire data from four channels, and consisted of an analog board, and a wireless system on-chip (nRF24Le1, Nordic Semiconductor), which includes an analog-to-digital converter (ADC), a micro-controller (μC) and a 2.4-GHz transceiver. Signals passed through a high-pass filter (0.05 Hz) to an instrumentation amplifier (INA333; Texas Instruments) with a gain of 10, then to a second-order band-pass filter (0.05 - 0.3 Hz) with a gain of 196 (Figure 1(b)).
Figure 1.
The new telemetric system. (a) Schematic block diagram of the system design. AI0-4: electrode channels; ADC: analog-to-digital converter; μC: micro-controller; (b) Analog front-end comprising filters and amplifiers; (c) The fabricated front-end in comparison to a U.S. quarter; (d) Flowchart for bidirectional transmission.
The filtered signals were sampled at 8 Hz and digitized, then loaded into data packets and sent by the transceiver, which utilized Gaussian frequency-shift keying (GFSK) modulation. The fabricated front-end is shown in Figure 1(c) compared to a U.S. quarter. Packets were received in the back-end transceiver and the microcontroller unloaded the data and sent them to a computer via UART (universal asynchronous receiver/transmitter; 500 kBaud) communication, while transmitting a receipt acknowledgement packet back to the transmitter. A GUI was designed to process and display the signals in real time, and data was stored for off-line analysis.
To increase reliability, the microcontroller on both ends executed a series of commands shown in Figure 1(d). After the microcontroller in the front-end acquired data from ADC (step 1), it loaded the data into the transceiver radio (step 2). It took 130 μs for the radio to turn into the transmitting mode and send the data packets wirelessly (step 3). The radio then returned back to the receiving mode, which took another 130 μs (step 4), while simultaneously the back-end radio looked for data packets (step 5). If the data packet was received, the device turned into the transmitting mode again and sent an acknowledgement to the front-end (step 6), while loading the packet onto the UART to be sent to the computer (step 7) as the microcontroller on the front-end prepared the next packet. If the packet was not received by the back-end, the microcontroller in the front-end loaded the same packet into the radio to be sent again (back to step 3) until attainment was confirmed. The retransmitting process mainly depended on the sampling rate in the front-end and the time for each packet to travel on-air (TOA), where TOA = (Packet length)/(Air data rate).
Each packet was composed of one preamble byte, 3–5 address bytes, payload of up to 32 bytes, and 1–2 bytes for cyclic redundancy check. The air data rate and the data packet length were chosen to be 1 Mbps and 4 bytes, respectively; hence, the TOA was 96 μs maximum, which is much shorter than the sampling period of 125 ms.
2.2 Validation Methods
The system was first validated in a bench-top study, and then in a separate in-vivo study in an anesthetized canine model. In the bench-top experiments, sinusoidal waveforms of known amplitudes and frequencies were fed into the transmitter and recorded at the receiver.
Ethical approval for the in-vivo dog study was granted by the University of Mississippi Medical Center (UMMC) review committee. The canine model was chosen because canine gastric slow wave activity resembles human gastric electrical activity in pattern and morphology (Lammers et al 2009). A male hound dog of weight 23 kg was used. The surgical and anesthetic methods were the same as those recently described in detail in another recent study (Egbuji et al., 2010). The anesthetic was administered after an overnight fast, and an upper midline laparotomy was performed. Vital signs were continuously monitored and temperature was maintained in the physiological range by the use of a heating pad and blankets. The animal was euthanized at the end of the study while still under anaesthesia.
Serosal slow wave data were acquired from the canine distal corpus using a flexible printed circuit board (PCB) array, with circular gold contacts with a 0.3 mm diameter and copper connection lines embedded in a polyimide ribbon base (Du et al., 2009),(O'Grady et al., 2011b). The array was positioned flush with the serosa on the anterior stomach, midway between the curvatures, and just proximal to the corpus-antrum border (as defined by the nerves of Laterjet). The flexible PCB was attached to a ribbon cable, which was connected simultaneously to the novel wireless system and a commercial system (BioSemi, The Netherlands), through a signal splitter. After placement of the recording array, the wound edges were opposed and sutured closed, in order to evaluate for potential signal attenuation by absorption and scattering in the tissue layers surrounding the device.
Analysis was performed in the Gastrointestinal Electrical Mapping Suite (GEMS v1.4) (Yassi et al., 2012). Wired data was down-sampled to 30 Hz and pre-processed with moving median and Savitzky-Golay filters to control drift and high-frequency noises (Paskaranandavadivel et al., 2011). Individual slow wave events were automatically detected with the Falling-Edge Variable Threshold (FEVT) method, followed by manual review and correction (Erickson et al., 2010). Slow wave events were marked at the point of maximal negative gradient (the ‘activation time’), which corresponds to the arrival of the wavefront directly under the electrode (O’Grady 2012). Outcomes from the wireless device and the commercial wired device were compared by frequency, FEVT false-positive (FP) detection rate, activation times, and signal amplitudes, to provide information on the accuracy, reliability and quality of recordings. Slow wave frequencies were determined by fast Fourier transform. Student’s t-test was used to evaluate statistical difference (significance threshold p<0.05).
3. Results
The encapsulated front-end module was 35×35×27 mm3 and weighed 20 g with a battery. Figure 1(c) shows the top-view of the device.
3.1 Bench-Top Study Outcomes
In the bench-top experiments, transmission and receipt of signals were successfully achieved at a distance up to 30 m. The device consumed 13.5 mW and functioned consistently for 124 hours based on a 560-mAh, 3-V cell coin battery. Various sinusoidal waveforms were fed into the transmitter and recorded in the receiving station. Figure 2 shows the resultant system spectral response when the signal amplitude was chosen as 500 μV and the frequency was varied from 10 mHz – 1 Hz. The system had an acceptable band-pass response in the range of 0.05-0.3 Hz, which matched the design.
Figure 2.
Bode plot of the wireless system response to sinusoidal waveforms generated in various frequencies.
3.2 In-vivo Study Outcomes
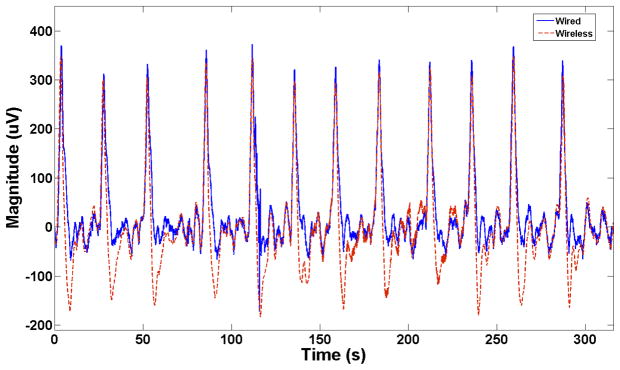
The wireless and wired systems were used to acquire the slow wave signals side-by-side for 90 minutes. The results showed that the novel wireless system could record signals comparable to the commercial wired device. An overlay from a representative segment of 315 s in the signals recorded by both devices is shown in Figure 3. The wirelessly recorded events showed a slightly steeper down-stroke than those recorded via the wired system, meaning that the FEVT algorithm consistently marked activation times slightly earlier than for the wired device data (mean absolute error = 0.57 s). Extracellular amplitudes were modestly higher for the wireless system (462 (SD 37) vs 386 (SD 47) μV; p<0.001).
Figure 3.
Wired (blue / solid line) and wireless (red / dashed line) in-vivo signal overlay showing a very similar performance between the two devices.
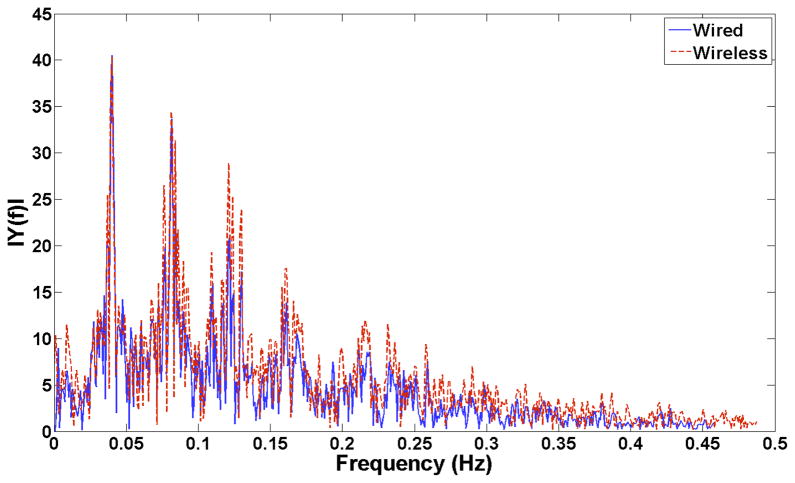
Across a continuous representative 600s-segment of in-vivo data, the dominant slow wave frequency for the wired device, by fast Fourier transform, was 0.04 Hz (2.4 cycles/min), which was identical to the frequency recorded by the wireless system (2.4 cycles/min; p>0.99) (Figure 4). The FEVT detection algorithm performance was higher for the wireless device because the designated sampling and filtering parameters effectively reduced competing noises (5% vs 14% FP rate).
Figure 4.
Fast-Fourier transform analysis of the in-vivo wired (blue / solid line) and wireless (red / dashed line) data showing the dominant 0.04 Hz signal frequency (recorded identically between the two systems) and associated harmonics.
4. Discussion
A bidirectional telemetry system has been developed and validated for monitoring gastric slow wave activity. This device is the first to enable slow wave recordings to be transmitted wirelessly through the tissues and skin from the stomach, with a sufficiently high signal quality for detecting individual slow wave activation times. The system was shown to be highly reliable and power efficient, and its small size makes it portable and suitable for implantation.
Importantly, the transmitted data closely matched the reference wired data, without signal distortion due to tissue absorption (Christ et al., 2006). The signal integrity of the slow wave recordings was shown by validation to be of high quality, and reliability was ensured by the acknowledgement function in transmission. The low sampling rate provides a 125 ms quiet time for the transceiver operation, in which there were a 260-μs delay in the radio mode switching and a 96-μs period for TOA, meaning data could potentially be retransmitted up to 350 times before the next set of data appears to guarantee reception.
The first filter stage prevented saturation in the INA333 amplifier. The inbuilt low-pass filter setting of 0.3 Hz was found to be adequate for slow wave sampling, and automated slow wave detection was shown to be highly accurate. Another type of gastric electrical activity, termed ‘spikes’ also occurs in GI smooth muscles, being episodes of sharp Ca2+ influx associated with more vigorous contractions, particularly occurring in the fed state (Lammers et al., 2009),(Sanders, 2008). The present device was not designed to evaluate spike potentials, because it is slow waves and not spikes that have been primarily associated with aberrant electrical activity in studies (Lammers et al., 2008b),(O'Grady et al., 2011a). However, the low-pass filter could be tuned to allow spike detection or to detect a wider frequency band of slow wave data, if desirable in the future design. A higher sampling frequency may also be needed to effectively detect spikes (Lammers et al., 2008a), which could be accommodated by reducing the number of retransmissions.
The focus of the present study was on the technical development and validation of the wireless device, including its transmission capabilities and accuracy. A short in-vivo testing period was therefore sufficient for the current work, however, in future, the front-end of the device will be hermetically sealed in order to conduct experiments in chronic preparations. In the current study, the recording included a period of bradygastric activity; 2.4 cycles/min being lower than the typical canine frequency of around 5 cycles/min (Lammers et al., 2009). Stable bradygastric activity of this type is known to occur in dogs, and may be spontaneous or may reflect the experimental conditions (Qian et al., 2003). Although tachygastria was not evaluated in the present study, it is anticipated that the adjustability in frequency response of our system will also be well suited for detecting high-frequency slow wave activities (e.g. Figure 2).
Invasive acquisition from the target organ provides a superior quantity and quality of data to cutaneous electrogastrography (O'Grady et al., 2010),(Lammers et al., 2008b), and it is anticipated that this wireless system will be usefully applied in animal and clinical studies. Large animal recordings have previously been conducted using wires tunneled percutaneously and performed during periods when the animals were confined (e.g. (Ver Donck et al., 2006),(Xing et al., 2003)). An implantable telemetry system will enable continuous monitoring in versatile conditions. The current battery life of 5 days could be extended by inductive recharging or a stand-by mode in the transceiver to facilitate more prolonged studies.
The greatest potential application that we perceive for this system is in patient diagnostic monitoring. Dysrhythmias have been associated with several motility disorders, however their functional and symptomatic significance remains incompletely understood (Parkman et al., 2003). Current investigations have been mainly limited to wired studies via oral, nasogastric, transcutaneous, or PEG placement (Coleski and Hasler, 2009),(Lin et al., 1998),(Ayinala et al., 2005). With further miniaturization, which is technically feasible, the device could be coupled to endoscopic recording electrodes (e.g. (Coleski and Hasler, 2009),(Ayinala et al., 2005)), introduced into the patient’s stomach via minimally-invasive endoscopic intubation, and attached securely to the mucosa by endoclips (e.g. (Deb et al., 2012)). In this way, the device could allow routine minimally-invasive patient recordings for several days in both fasted and fed states.
Currently, the device is limited to four-channel recording, validated by individual channel recordings. Four-channel capacity is sufficient to provide useful localized information on slow wave frequency, amplitude and velocity, in the form of a ‘miniature-activation map’, so as to guide dysrhythmia identification in experimental and clinical studies (O'Grady et al., 2009). However, recent work has shown that dysrhythmic slow wave patterns may be spatially complex, such that high-resolution (multi-electrode) analyses are most ideal for their proper characterization (Lammers et al., 2008b), (O'Grady et al., 2011a). For this purpose, communication channels can be extended to 8 or 16 channels in the transceiver chip used in this prototype allowing multi-electrode signal transduction since there is sufficient bandwidth. Each transceiver can also be programmed with different individual identification; therefore, multiple modules can be implanted within the body for recording from 16, 32, and even 128 electrodes.
Acknowledgments
This work was supported by Intel Corp., Texas Norman Hackerman Advanced Research Program, NZ Health Research Council and the NIH (R01 DK64775). We thank Mr. Peng Du and Mr. Nira Paskaranandavadivel (University of Auckland) for their expert technical assistance, and the animal care facility staff of the University of Mississippi Medical Center.
References
- Albert NM, Hancock K, Murray T, et al. Cleaned, ready-to-use, reusable electrocardiographic lead wires as a source of pathogenic microorganisms. Am J Crit Care. 2010;19:e73–80. doi: 10.4037/ajcc2010304. [DOI] [PubMed] [Google Scholar]
- Ayinala S, Batista O, Goyal A, et al. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005;61:455–461. doi: 10.1016/s0016-5107(05)00076-3. [DOI] [PubMed] [Google Scholar]
- Christ A, Klingenbock A, Samaras T, Goiceanu C, Kuster N. The dependence of electromagnetic far-field absorption on body tissue composition in the frequency range from 300 MHz to 6 GHz. IEEE Trans Micro Theory Tech. 2006;54:2188–2195. [Google Scholar]
- Coleski R, Hasler WL. Coupling and propagation of normal and dysrhythmic gastric slow waves during acute hyperglycaemia in healthy humans. Neurogastroenterol Motil. 2009;21:492–9. e1–2. doi: 10.1111/j.1365-2982.2008.01235.x. [DOI] [PubMed] [Google Scholar]
- Deb S, Tang SJ, Abell TL, et al. An endoscopic wireless gastrostimulator (with video) Gastrointest Endosc. 2012;75:411–415.e1. doi: 10.1016/j.gie.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du P, O'Grady G, Egbuji JU, et al. High-resolution mapping of in vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: methodology and validation. Ann Biomed Eng. 2009;37:839–846. doi: 10.1007/s10439-009-9654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbuji JU, O'Grady G, Du P, et al. Origin, propagation and regional characteristics of porcine gastric slow wave activity determined by high-resolution mapping. Neurogastroenterol Motil. 2010;22:e292–300. doi: 10.1111/j.1365-2982.2010.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JC, O'Grady G, Du P, et al. Falling-edge, variable threshold (FEVT) method for the automated detection of gastric slow wave events in serosal high-resolution electrical recordings. Ann Biomed Eng. 2010;38:1511–1529. doi: 10.1007/s10439-009-9870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajidavar A, Seifert JL, Bell JE, et al. A wireless system for monitoring transcranial motor evoked potentials. Ann Biomed Eng. 2011;39:517–523. doi: 10.1007/s10439-010-0152-x. [DOI] [PubMed] [Google Scholar]
- Haddab S, Laghrouche M. Microcontroller-based system for electrogastrography monitoring through wireless transmission. Measurement Science Review. 2009;9:122–126. [Google Scholar]
- Huizinga JD, Lammers WJEP. Gut peristalsis is coordinated by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:1–8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- Joshi AK, Kowey PR, Prystowsky EN, et al. First experience with a Mobile Cardiac Outpatient Telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol. 2005;95:878–881. doi: 10.1016/j.amjcard.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Lammers WJ, Michiels B, Voeten J, Ver Donck L, Schuurkes JA. Mapping slow waves and spikes in chronically instrumented conscious dogs: automated on-line electrogram analysis. Med Biol Eng Comput. 2008a;46:121–129. doi: 10.1007/s11517-007-0294-7. [DOI] [PubMed] [Google Scholar]
- Lammers WJ, Ver Donck L, Stephen B, Smets D, Schuurkes JA. Origin and propagation of the slow wave in the canine stomach: the outlines of a gastric conduction system. Am J Physiol Gastrointest Liver Physiol. 2009;296:1200–1210. doi: 10.1152/ajpgi.90581.2008. [DOI] [PubMed] [Google Scholar]
- Lammers WJEP, Ver Donck L, Stephen B, Smets D, Schuurkes JAJ. Focal activities and re-entrant propagations as mechanisms of gastric tachyarrhythmias. Gastroenterology. 2008b;135:1601–1611. doi: 10.1053/j.gastro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Leahy A, Besherdas K, Clayman C, Mason I, Epstein O. Abnormalities of the electrogastrogram in functional gastrointestinal disorders. American Journal of Gastroenterology. 1999;94:1023–1028. doi: 10.1111/j.1572-0241.1999.01007.x. [DOI] [PubMed] [Google Scholar]
- Lin Z, Sarosiek I, Forster J, et al. Association of the status of interstitial cells of Cajal and electrogastrogram parameters, gastric emptying and symptoms in patients with gastroparesis. Neurogastroenterol Motil. 2010;22:56–61. e10. doi: 10.1111/j.1365-2982.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- Lin Z, Sarosiek I, Forster J, et al. Two-channel gastric pacing in patients with diabetic gastroparesis. Neurogastroenterol Motil. 2011;23:912–e396. doi: 10.1111/j.1365-2982.2011.01754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. American Journal of Physiology. 1998;274:G186–91. doi: 10.1152/ajpgi.1998.274.1.G186. [DOI] [PubMed] [Google Scholar]
- O'Grady G, Du P, Cheng LK, et al. The origin and propagation of human gastric slow wave activity defined by high-resolution mapping. Am J Physiol Gastrointest Liver Physiol. 2010;299:585–592. doi: 10.1152/ajpgi.00125.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady G, Du P, Egbuji JU, et al. A novel laparoscopic device for measuring gastrointestinal slow-wave activity. Surg Endosc. 2009;23:2842–2848. doi: 10.1007/s00464-009-0515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady G, Egbuji JU, Du P, et al. High-resolution spatial analysis of slow wave initiation and conduction in porcine gastric dysrhythmia. Neurogastroenterol Motil. 2011a;23:e345–55. doi: 10.1111/j.1365-2982.2011.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady G, Paskaranandavadivel N, Angeli T, et al. A comparison of gold vs silver electrode contacts for high-resolution gastric electrical mapping using flexible printed circuit board electrodes. Physiol Meas. 2011b;32:N13–22. doi: 10.1088/0967-3334/32/3/N02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady G. Gastrointestinal extracellular electrical recordings: fact or artifact? Neurogastroenterol Motil. 2012;24:1–6. doi: 10.1111/j.1365-2982.2011.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkman HP, Hasler WL, Barnett JL, Eaker EY. Electrogastrography: a document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol Motil. 2003;15:89–102. doi: 10.1046/j.1365-2982.2003.00396.x. [DOI] [PubMed] [Google Scholar]
- Paskaranandavadivel N, Cheng LK, Du P, O'Grady G, Pullan AJ. Improved signal processing techniques for the analysis of high resolution serosal slow wave activity in the stomach. Conf Proc IEEE Eng Med Biol Soc. 2011:1737–1740. doi: 10.1109/IEMBS.2011.6090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian LW, Pasricha PJ, Chen JD. Origins and patterns of spontaneous and drug-induced canine gastric myoelectrical dysrhythmia. Dig Dis Sci. 2003;48:508–515. doi: 10.1023/a:1022532515172. [DOI] [PubMed] [Google Scholar]
- Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20:39–53. doi: 10.1111/j.1365-2982.2008.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhong DX, Qian W, Hou XH, Chen JD. Short pulse gastric electrical stimulation for cisplatin-induced emesis in dogs. Neurogastroenterol Motil. 2011;23:468–74. e178. doi: 10.1111/j.1365-2982.2011.01684.x. [DOI] [PubMed] [Google Scholar]
- Ver Donck L, Lammers WJ, Moreaux B, et al. Mapping slow waves and spikes in chronically instrumented conscious dogs: implantation techniques and recordings. Med Biol Eng Comput. 2006;44:170–178. doi: 10.1007/s11517-005-0018-9. [DOI] [PubMed] [Google Scholar]
- Woo SH, Cho JH. Telemetry system for slow wave measurement from the small bowel. Med Biol Eng Comput. 2010;48:277–283. doi: 10.1007/s11517-009-0567-4. [DOI] [PubMed] [Google Scholar]
- Xing J, Brody F, Rosen M, Chen JD, Soffer E. The effect of gastric electrical stimulation on canine gastric slow waves. Am J Physiol Gastrointest Liver Physiol. 2003;284:G956–62. doi: 10.1152/ajpgi.00477.2002. [DOI] [PubMed] [Google Scholar]
- Yassi R, O'Grady G, Paskaranandavadivel N, et al. The Gastrointestinal Electrical Mapping Suite (GEMS): Software for analysing and visualising gastrointestinal multi-electrode recordings. BMC Gastroenterol. 2012 doi: 10.1186/1471-230X-12-60. In Press [doi to follow] [DOI] [PMC free article] [PubMed] [Google Scholar]