Abstract
Hypothesis
Biofilm formation in otopathogenic of P. aeruginosa (OPPA) strains is inhibited by ethylenediaminetetraacetic acid (EDTA).
Background
EDTA, a widely used chelating agent, has been shown to inhibit biofilm formation in a number of bacteria. Since EDTA may be a well-tolerated reagent to inhibit biofilm formation in cases of suppurative otitis media, we asked if it might be effective in all OPPA strains isolated from chronically infected cholesteatomas.
Methods
OPPA strains were isolated from patients with infected cholesteatomas. These strains were grown into log phase then were placed in minimal media with varying concentrations of EDTA, and incubated for varying periods. Biofilm production was measured colorimetrically by staining with crystal violet.
Results
Without added EDTA, most otopathogenic PA exhibited a distinct, but varying, time-course of biofilm formation and dissolution with peak production at 12–18 hours. Addition of 1 mM EDTA resulted in a delay in the time to peak biofilm formation for most strains, although the amount of biofilm was not decreased. In contrast, some strains showed greater biofilm production with 1 mM EDTA compared to the untreated bacteria. Addition of 10 mM EDTA resulted in a similar effect. Some strains increased biofilm production over controls. Moreover, EDTA inhibited planktonic growth of all OPPA strains at the concentrations studied.
Conclusion
Our hypothesis was disproven: EDTA tends to delay biofilm development while it consistently inhibits planktonic growth. Since EDTA does not cause suppression of biofilm production in all isolates of OPPA, usefulness as an antimicrobial is questioned.
Keywords: Pseudomonas aeruginosa, cholesteatoma, biofilm, EDTA
INTRODUCTION
In aqueous environments in both the natural world and the medical setting, bacteria can be observed to exist in a visible slime form termed a biofilm. From common dental plaque to the gelatinous material within an infected cholesteatoma, this microbial phenotype is ubiquitous and offers the resident microorganisms many survival advantages. Despite its disorganized appearance, early microscopic investigation revealed a highly complex and regulated structure, containing interwoven channels and distinct bacterial sub-populations.(1) Encased in a protective self-produced hydrated gel matrix, these bacteria are often resistant to the effects of synthetic antimicrobials. Speculated mechanisms of resistance include slowed diffusion within the biofilm matrix, altered microenvironments leading to local antimicrobial deactivation, and possibly dormant bacterial sub-populations that are less susceptible to antibiotics targeting metabolic processes.(2) Similarly, the natural immune system is often unable to fully eradicate biofilms from biological systems. In fact, cellular and humoral immune responses may ultimately be counterproductive, leading to inflammatory damage in the adjacent tissues. (3)
This phenomenon of resistance has far-reaching implications across medical fields. In particular, the relapsing-remitting course of several otolaryngologic disease processes may well be explained by the presence of biofilms, which can serve as a long-term nidus of infection. A mature biofilm is marked by the process of detachment, in which individual bacteria can transition from the biofilm matrix and re-enter a planktonic, or freely mobile, state, thereby seeding the local environment.(4) In that way, though acute episodes of local or systemic infection may be medically managed with antibiotics, relapse will ultimately occur due to the persistence of viable organisms within the biofilm. Early studies identified biofilms in device infections, chronic otitis media, and chronic mucosally-based head and neck infections caused by a variety of infectious organisms.(5) In particular, aural cholesteatomas demonstrate chronic and recurrent biofilm infections, most commonly due to Pseudomonas aeruginosa (PA).(6) These infections are frequently resistant to both topical and systemic antimicrobials, responding only to surgical removal of the infected nidus.
Defining the molecular mechanisms of biofilm formation by PA may help shed light on the pathogenesis of cholesteatoma infection and aid in the development of non-surgical treatment options. Many of the steps and factors involved in the process of biofilm development have been well documented. Nonetheless, controversy still exists in the literature around several aspects, notably the role of micronutrients in biofilm development and stability. In this study, biofilm formation by several strains of PA was investigated using an in vitro static assay previously described.(7) The common laboratory strain PA-01 was compared to otopathogenic PA variants (OPPA) originally obtained from human cholesteatoma specimens and treatment with EDTA was used to assess the reaction of the various strains to cation chelation.
Biofilm response to EDTA of several bacterial species, including PA has been variably reported in the literature, with some authors finding increased formation and others finding significant suppression.(8–19) Most of these studies, however, have assessed only a single time-point for biofilm growth and EDTA exposure which has not been consistent between various authors. In this investigation, we measured biofilm growth over time in various strains of PA including otopathogenic strains. We then evaluated the response of each strain to EDTA, in both the biofilm phenotype and the planktonic phenotype over time.
METHODS
Bacterial Strains
The bacterial cultures used in this study included the common lab variant of Pseudomonas aeruginosa, PA-01, as well as several clinical isolates of OPPA (OPPA1, 6–10, 14–15) obtained from surgical specimens of resected cholesteatomas.(6) The strains were maintained at −80°C in 20% glycerol and were plated onto Luria-Bertani (LB) agar for amplification. Isolated colonies were inoculated into LB broth and incubated with shaking at 225 rpm at 37°C for 16–18 hours. The collection of the otopathogenic strains from human subjects was approved by the Washington University Institutional Review Board.
Planktonic Growth
Planktonic growth was measured by using the bacterial cultures described above after uniform adjustment to an absorbance of 0.3 at 600nm. A 1:1000 dilution was made in LB, appropriate concentrations of EDTA were added, and 5mL was incubated at 37C rocking at 225 rpm. Growth was measured by recording absorbance at 600nm of 1:10 dilutions. Measurements were stopped when absorbance began trending down and cultures showed evidence of bacterial death.
Biofilm Assay
Biofilm formation was assayed by crystal violet staining using a modification of a previously described assay.(7) Briefly, the absorbance of an overnight bacterial culture grown in LB broth was assessed by measuring the optical density at 600 nm using a 1:10 dilution. All cultures were adjusted with sterile LB to bring this absorbance measurement to 0.3 (Barnstead Thermolyne Turner Spetrophotometer Model 340, Dubuque, IA, USA). These cultures were then diluted 1:1000 in M63 minimal media supplemented with MgCl2 (1 mM), D-glucose (0.2%) and casamino acids (0.5%).(20) 100 μL of this dilution was placed in each well of a 96-well polyvinyl chloride microtiter plate (Co-Star flat-bottom, Corning, Inc. Corning NY, USA), with six replicates for each bacteria to control for well-to-well variation. To create a hydration chamber, ensuring uniform water vapor at the air-liquid interface, each plate was placed in a sealed polyethylene bag along with a damp paper towel. After the appropriate concentration of EDTA was added, the plate was incubated at 30°C for the assigned time-points. To assess biofilm formation, plates were washed in tap water three times by submerging and inverting the plate. After drying, 125 μL of 0.1% crystal violet (Sigma) was added and incubated at room temperature for 15 minutes. The wells were then washed five times with tap water. To extract the crystal violet, 200 μL of 33% glacial acetic acid was added to each well and incubated at room temperature for 15 minutes. After mixing using a pipette, the amount of crystal violet eluted was determined using a microplate reader (A595 nm) (Biotek Synergy HT, Winooski, VT, USA).
For data analysis, the mean of the biofilm production in the presence of EDTA was divided by the production without EDTA and the log of the ratio was tabulated for each time point assessed (6, 12, 18,24, 48 and 72 hours).
Approvals
The collection of specimens from human subjects was approved by the Washington University Institutional Review Board. (IRB ID#: 201104239)
RESULTS
We assessed the in vitro activity of 1 mM and 10 mM EDTA against both the planktonic growth and the biofilm formation of the common lab strain PA-01 as well as 8 of our clinical OPPA variants.
Planktonic Growth
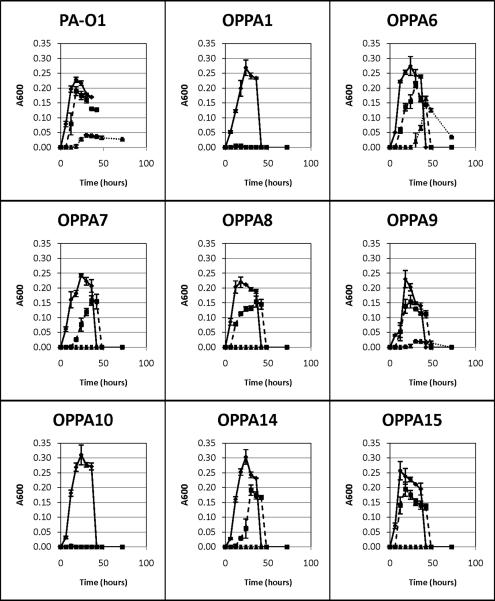
Growth of planktonic cultures were universally suppressed by the addition of EDTAGrowth of all strains was significantly suppressed by the addition of 10 mM EDTA with only PA-01, OPPA6 and OPPA9 exhibiting even minimal growth. Replication of OPPA1 and OPPA10 were exquisitely sensitive, demonstrating complete inhibition of growth with even 1 mM EDTA. (Fig.1)
Figure 1.
Planktonic growth of various PA strains as assessed by changes in the absorbance at 600 nm over time. Data points are the mean of 2 separate trials and error bars represent ± 1 SD from the mean. ◆ Control, ∎ 1mM EDTA, ▴ 10 mM EDTA
Biofilm Growth
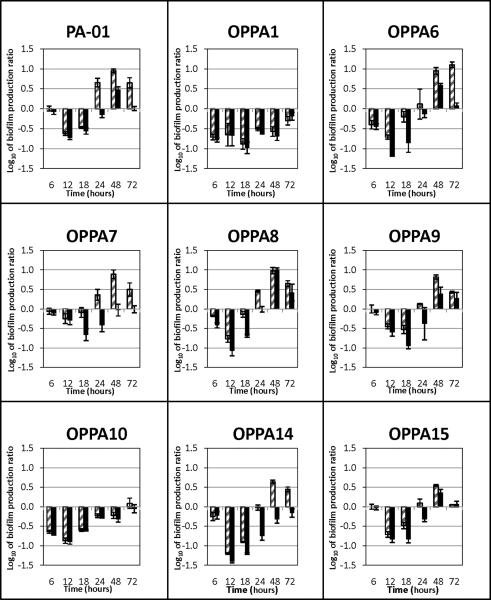
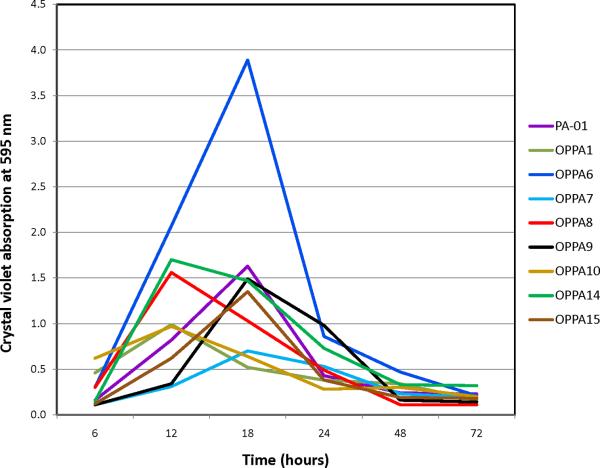
Without the addition of EDTA, all strains grew well and formed robust biofilms on 96-well microtitre plates. Without EDTA, all bacteria demonstrated similar biofilm growth-and-dissolution time-courses with a peak at 12–18 hours and dissolution around 24–48 hours. Addition of EDTA led to a shift in the time-course, with a peak around 48 hours for most clinical isolates. Not all PA strains were affected equally, however (Fig. 2–3). OPPA1 and OPPA10, in particular, demonstrated significant suppression of biofilm formation. In general, 10mM EDTA led to a less robust biofilm than 1mM EDTA. For most PA isolates, however, biofilm formation with 10 mM EDTA at 48 hours was still several-fold higher than without the addition of EDTA.(Fig. 4)
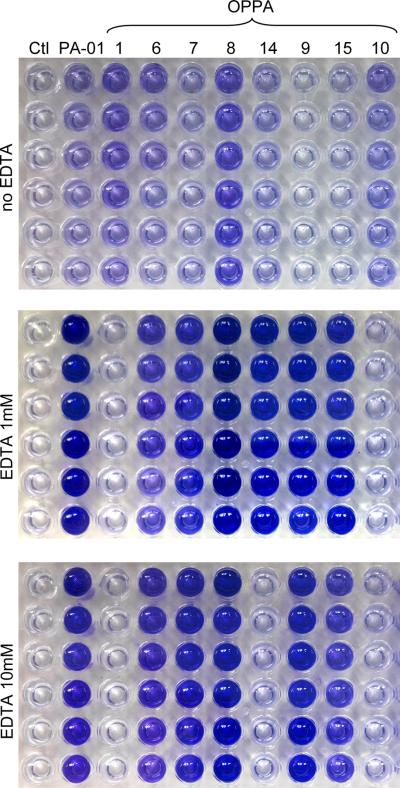
Figure 2.
An example of biofilm formation in 96 well culture plates. Biofilm formation by our various PA strains after 48 hours of incubation with no EDTA (top), 1 mM EDTA (middle), and 10 mM EDTA (bottom). All wells have been stained with 0.1% crystal violet and dissolved with 33% acetic acid. Vertical columns are replicates with a negative control on the far left. Increased intensity of crystal violet incorporation indicated increased biofilm formation.
Figure 4.
Biofilm production by various PA strains in the presence of EDTA was divided by production without EDTA to determine a ratio for each time point assessed. The graphs below show the log of this ratio. Negative values represent suppression of biofilm growth by EDTA, while positive values demonstrate increased biofilm formation with EDTA exposure. The data show the mean of three separate trials, each having six replicates, and error bars are ± 1 SEM. Pattern filled bars, 1 mM EDTA, Solid bars10 mM EDTA
DISCUSSION
The implication that biofilm formation acts as an essential protective virulence factor in many infectious disease processes has lead to considerable interest in developing anti-biofilm agents. In particular, the chronic relapsing nature of cholesteatoma, combined with work by Chole et al. (21) demonstrating biofilm formation in resected cholesteatoma specimens, suggests that biofilm development is a virulence factor in the pathogenesis of this disease process. Furthermore, evidence that infection increases the virulence of cholesteatomas (22) makes the development of topical antimicrobial therapy for cholesteatomas of particular importance.
As a potential topical therapeutic agent in the treatment of cholesteatoma, we have chosen to explore the in vitro effects of EDTA due to its low cost, presumed low mucosal toxicity, and significant evidence in the literature of its antimicrobial and anti-biofilm properties. Various investigators, however, have used different concentrations of EDTA and a range of exposure times, leading to some inconsistency in the reported effects on biofilm development. For our study, given the low solubility of EDTA, we have chosen to explore concentrations of 1 mM and 10 mM to mimic the likely effective concentration at the mucosal surface if EDTA were to be used as a topical agent. Higher concentrations have been investigated by others but dilution of the EDTA in a biologic environment makes much more concentrated solutions less likely to represent the true physiologic situation. In this study, we demonstrate that biofilms of several PA clinical isolates have distinct growth and dissolution time-courses (Fig. 2), which are significantly altered by exposure to EDTA. Although biofilm development is initially suppressed, incubation with EDTA leads to delayed biofilm formation in most strains. Growth in planktonic culture, on the other hand, is universally suppressed. Several authors, however, have described the efficacy of EDTA to prevent and disrupt PA biofilms in culture. Banin et al. (8) demonstrated that treatment of established PA-O1 biofilms in a flow cell system with 50 mM EDTA resulted in biofilm dissolution when followed over a 2.5-hour time course. When the authors assessed biofilm organization by confocal microscopy, they observed intact biofilm structure but a large amount of cell killing. Similarly, Al-Bakri et al.(23) reported that that incubating established PA biofilms with 27 mM EDTA for four hours lead to an almost 99% decrease in viable cell counts. Percival et al.,(12) as well, found a significant reduction in viable cells when established PA biofilms were treated with 137 mM EDTA for 21 hours. On the basis of the present study, cell death observed in these studies is expected given the high concentrations of EDTA used. These authors, however, only assessed the effects of EDTA on biofilms over an abbreviated time-course. With few viable cells remaining, delayed biofilm induction may subsequently occur, as was seen in our study. In another report, using static cultures and biofilm quantification by crystal violet staining, O'May et al.(11) investigated the effects of various iron chelators on PA-01 biofilm growth. The authors showed that concentrations of EDTA up to 2.5 mM lead to dose-dependent decreases in biofilm formation which were reversible by the addition of Fe3+ to solution. Only a 24-hour time-point was assessed, however, and the place at which the results were gathered on the natural biofilm growth-and-dissolution curve cannot be determined. Our findings suggest that there may have been further biofilm formation at later time points.
In contrast, other investigators have found that treatment with EDTA leads to either no effect or an apparent increase in PA biofilm formation. Liaqat et al.(9) found that EDTA at concentrations up to 25 μM elicited no significant effect on PA biofilm growth as assessed by crystal violet staining. However, biofilm formation was only evaluated at a single time point (72-hours). Similarly, when Yakandawala et al.(14) reported that treatment of static PA-01 cultures with 342 μM EDTA elicited no significant effect on biofilm growth, only a 16–18 hour exposure was assessed. Finally, a significant increase in biofilm formation was reported by Liu et al.(24) when a static PA-01 culture was treated with 17 μM EDTA. Once again, though, only a 24-hour time-point was assessed. Similarly, Mulcahy et al.(18) using only a 24-hour exposure reported that adding EDTA at concentrations up to 8.55 mM to a static culture of PA-O1 increased biofilm growth over baseline as assessed by crystal violet staining
Therefore, previous studies of the effects of EDTA on PA biofilms seem inconsistent because different time points were used to assess the extent of biofilm formation and varying concentrations of EDTA were used. Our study demonstrates that EDTA lengthens the time of biofilm formation. Hence, the apparent effects of EDTA will be drastically different depending on whether a short or long time of exposure was assayed.
These findings are also likely not limited to PA, as work using other bacterial species has demonstrated similar results. The biofilm literature using other microorganisms is comparably inconsistent in regards to concentration and time of exposure to EDTA. Chang et al. (25) demonstrated suppression of Listeria monocytogenes biofilms in static culture in response to EDTA concentrations as low as 0.1 mM. These authors, however, only assessed a 48 hour time-point. Similarly, using six clinical isolates of Staphylococcus epidermidis from human nasopharynx, Juda et al. (16) reported inhibition of biofilm formation after 72 hours by treatment with 2 mM EDTA. Again, growth was only assessed at a single time-point. On the other hand, although Yakandawala et al. (14) reported biofilm inhibition by low levels of EDTA for a Staphylococcus epidermidis isolate, the authors found no significant effect of EDTA biofilm growth for several other bacterial species, including Enterococcus faecalis and Escherichia coli when exposed to EDTA for 16–18 hours. Finally, Liaqat et al.(9) found that incubation of both Bacillus cereus and Bacillus subtilis isolates with 25 μM EDTA for 72 hours resulted in a statistically significant increase in biofilm formation as compared to controls.
In conclusion, our results help to clarify the anti-biofilm and antimicrobial properties of EDTA. Although several PA clinical isolates demonstrated both biofilm and planktonic suppression in response to EDTA treatment, our other PA variants showed a delayed but robust biofilm production even though their corresponding planktonic growth was significantly inhibited. This time-delay suggests that the chelating action of EDTA leads to the activation of cellular machinery which takes time to convert cellular metabolism to a biofilm phenotype. Furthermore, since not all strains demonstrated this response, there is likely a strain-specific genetic basis for susceptibility to EDTA. Given the increasing interest in use of EDTA as an antimicrobial, our data suggest caution in its clinical use and our time-course results shed new light on the interpretation of the effects of antimicrobials on biofilm production in previous and future in vitro studies. Both biofilm development over time, as well as the response by several different clinical isolates, must be used to fully evaluate the effects of anti-biofilm agents in future studies.
Figure 3.
Biofilm formation, as measured with the crystal violet incorporation method, in variious strains of P. aeruginosa. The peak biofilm formation varies between 12 and 18 hours.
Acknowledgments
Disclosure of funding: NIH: P30 DC004665-11 (RAC) R01 DC000263-24 (RAC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 3.Hoiby N, Fomsgaard A, Jensen ET ea. The immune response to bacterial biofilms. In: Lappin-Scott HM, Costerton JW, editors. Microbial Biofilms. Cambridge University Press; Cambridge: 1995. pp. 233–50. [Google Scholar]
- 4.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 5.Post JC, Hiller NL, Nistico L, et al. The role of biofilms in otolaryngologic infections: update 2007. Curr Opin Otolaryngol Head Neck Surg. 2007;15:347–51. doi: 10.1097/MOO.0b013e3282b97327. [DOI] [PubMed] [Google Scholar]
- 6.Wang EW, Jung JY, Pashia ME, et al. Otopathogenic Pseudomonas aeruginosa strains as competent biofilm formers. Arch Otolaryngol Head Neck Surg. 2005;131:983–9. doi: 10.1001/archotol.131.11.983. [DOI] [PubMed] [Google Scholar]
- 7.O'Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–61. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 8.Banin E, Brady KM, Greenberg EP. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl Environ Microbiol. 2006;72:2064–9. doi: 10.1128/AEM.72.3.2064-2069.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liaqat I, Bachmann RT, Sabri AN, et al. Isolate-specific effects of patulin, penicillic Acid and EDTA on biofilm formation and growth of dental unit water line biofilm isolates. Curr Microbiol. 2010;61:148–56. doi: 10.1007/s00284-010-9591-8. [DOI] [PubMed] [Google Scholar]
- 10.Martineau L, Dosch HM. Biofilm reduction by a new burn gel that targets nociception. J Appl Microbiol. 2007;103:297–304. doi: 10.1111/j.1365-2672.2006.03249.x. [DOI] [PubMed] [Google Scholar]
- 11.O'May CY, Sanderson K, Roddam LF, et al. Iron-binding compounds impair Pseudomonas aeruginosa biofilm formation, especially under anaerobic conditions. J Med Microbiol. 2009;58:765–73. doi: 10.1099/jmm.0.004416-0. [DOI] [PubMed] [Google Scholar]
- 12.Percival SL, Kite P, Eastwood K, et al. Tetrasodium EDTA as a novel central venous catheter lock solution against biofilm. Infect Control Hosp Epidemiol. 2005;26:515–9. doi: 10.1086/502577. [DOI] [PubMed] [Google Scholar]
- 13.Reid DW, O'May C, Roddam LF, et al. Chelated iron as an anti-Pseudomonas aeruginosa biofilm therapeutic strategy. J Appl Microbiol. 2009;106:1058. doi: 10.1111/j.1365-2672.2008.03955.x. [DOI] [PubMed] [Google Scholar]
- 14.Yakandawala N, Gawande PV, Lovetri K, et al. Effect of ovotransferrin, protamine sulfate and EDTA combination on biofilm formation by catheter-associated bacteria. J Appl Microbiol. 2007;102:722–7. doi: 10.1111/j.1365-2672.2006.03129.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Stewart PS. Biofilm removal caused by chemical treatments. Water Res. 2000;34:4229–33. [Google Scholar]
- 16.Juda M, Paprota K, Jaloza D, et al. EDTA as a potential agent preventing formation of Staphylococcus epidermidis biofilm on polichloride vinyl biomaterials. Ann Agric Environ Med. 2008;15:237–41. [PubMed] [Google Scholar]
- 17.Muller E, Al-Attar J, Wolff AG, et al. Mechanism of salicylate-mediated inhibition of biofilm in Staphylococcus epidermidis. J Infect Dis. 1998;177:501–3. doi: 10.1086/517386. [DOI] [PubMed] [Google Scholar]
- 18.Mulcahy H, Lewenza S. Magnesium limitation is an environmental trigger of the Pseudomonas aeruginosa biofilm lifestyle. PLoS One. 2011;6:e23307. doi: 10.1371/journal.pone.0023307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shanks RM, Sargent JL, Martinez RM, et al. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol Dial Transplant. 2006;21:2247–55. doi: 10.1093/ndt/gfl170. [DOI] [PubMed] [Google Scholar]
- 20.Kolodkin-Gal I, Romero D, Cao S, et al. D-amino acids trigger biofilm disassembly. Science. 2010;328:627–9. doi: 10.1126/science.1188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chole RA, Faddis BT. Evidence for microbial biofilms in cholesteatomas. Arch Otolaryngol Head Neck Surg. 2002;128:1129–33. doi: 10.1001/archotol.128.10.1129. [DOI] [PubMed] [Google Scholar]
- 22.Jung JY, Lee DH, Wang EW, Nason R, Sinnwell TM, Vogel JP, Chole RA. P. aeruginosa infection increases morbidity in experimental cholesteatomas. Laryngoscope. 2011;121:2449–54. doi: 10.1002/lary.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Bakri AG, Othman G, Bustanji Y. The assessment of the antibacterial and antifungal activities of aspirin, EDTA and aspirin-EDTA combination and their effectiveness as antibiofilm agents. J Appl Microbiol. 2009;107:280–6. doi: 10.1111/j.1365-2672.2009.04205.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Yang L, Molin S. Synergistic activities of an efflux pump inhibitor and iron chelators against Pseudomonas aeruginosa growth and biofilm formation. Antimicrob Agents Chemother. 2010;54:3960–3. doi: 10.1128/AAC.00463-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang Y, Gu W, McLandsborough L. Low concentration of ethylenediaminetetraacetic acid (EDTA) affects biofilm formation of Listeria monocytogenes by inhibiting its initial adherence. Food Microbiol. 2012;29:10–7. doi: 10.1016/j.fm.2011.07.009. [DOI] [PubMed] [Google Scholar]