Abstract
Background
Identifying individuals at risk for chronic kidney disease (CKD) is critical for timely treatment initiation to slow progression of the disease. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) are known biomarkers of acute kidney injury, but it is unknown whether these markers are associated with incident CKD stage 3 in the general population.
Study Design
Matched case-control study
Setting and Participants
African American and Caucasian participants from the Atherosclerosis Risk in Communities (ARIC) study who at baseline had an estimated glomerular filtration rate ≥ 60 ml/min/1.73 m2 and urinary albumin-creatinine ratio (UACR) ≤ 30 mg/g. A total of 143 controls were matched on age, sex and race to 143 cases of incident CKD stage 3 after 8.6 years of follow-up.
Predictors
Quartile of NGAL and KIM-1.
Outcomes & Measurements
Incident CKD stage 3 (eGFR < 60 ml/min/1.73 m2 at follow-up and a decrease in eGFR from baseline to follow-up of ≥25%)
Results
Both NGAL (p=0.05) and KIM-1 (p<0.001) were positively correlated with baseline UACR; neither was associated with baseline eGFR. Participants with NGAL concentrations in the fourth quartile had more than 2-fold higher odds (adjusted OR, 2.11, 95% CI, 0.96–4.64) of incident CKD stage 3 compared to participants in the first quartile after multivariable adjustment (p-trend=0.03). Adjustment for urinary creatinine and albumin resulted in a non-significant association (highest quartile adjusted OR. 1.52; 95% CI, 0.64–3.58; p=0.2). No significant association between KIM-1 levels and incident CKD was observed in crude or adjusted models.
Limitations
The relatively small sample size of the study limits precision and power to detect weak associations.
Conclusions
Higher NGAL levels, but not KIM-1 levels, were associated with incident CKD stage 3. Adjustment for urinary creatinine and albumin concentration attenuated this association. Additional studies are needed to confirm these findings and assess the utility of urinary NGAL as a marker of CKD risk.
Chronic kidney disease (CKD) affects nearly 20 million people in the United States and increases risk for cardiovascular disease, kidney failure and mortality. [1] Identification of individuals at higher risk of early kidney function decline is critical to the timely initiation of treatment to prevent progression of CKD and its associated outcomes.
Proteinuria is a marker of kidney damage and strongly predicts progression of kidney disease. [2–4] Many patients with CKD, however, progress to kidney failure despite having only mildly elevated levels of urinary protein excretion, and many with significant proteinuria do not progress. [5] Data on the clinical significance of urinary proteins specific to kidney dysfunction and disease are limited.
Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) have been identified as potential markers of acute kidney injury. [6, 7] It is unknown, however, whether urinary levels of these markers are associated with incident CKD stage 3. We therefore evaluated the potential associations of urinary NGAL and KIM-1 levels with incident CKD stage 3 in a case-control study nested within the Atherosclerosis Risk in Communities (ARIC) Study and ARIC Carotid MRI Study.
Methods
ARIC Study and ARIC Carotid MRI Study
The ARIC study is a population-based, multicenter, prospective cohort study of 15,792 men and women aged 45–64 years recruited from four US communities (Forsyth County, NC; suburban Minneapolis, MN; Washington County, MD; and Jackson, MS). [8] Study enrollment commenced in 1987 and continued through 1989. Participants attended 3 follow-up study visits approximately 3 years apart. Carotid artery intima-media thickness (IMT) was measured using B-mode ultrasound at each visit. Urine was collected at the fourth ARIC visit (1996–1998), which serves as the baseline visit for this analysis. We selected 286 participants free of CKD (defined as estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 and/or urinary albumin-creatinine ratio (UACR) > 30 mg/g) at the fourth ARIC visit for this nested case-control study.
The ARIC Carotid MRI Study was conducted between 2004 and 2005 and aimed at identifying novel correlates of plaque and early pathologic changes in the carotid arteries that may increase risk for coronary heart disease and stroke. [9] The goal of the study was to recruit 1,200 participants with high values of maximum carotid artery IMT at their most recent ultrasound examination (ARIC exam 3 or 4, 1993–95 or 1996–98) and 800 individuals randomly sampled from the remaining eligible participants. After refusals (n=1403) and exclusions (n=837), 2066 individuals participated in the study.
Selection of Cases and Controls
Participants were eligible for selection if information was available on serum creatinine at ARIC visit 4 (baseline) and the ARIC Carotid MRI Study visit (follow-up). Individuals with eGFR < 60 ml/min/1.73 m2 or UACR > 30 mg/g at baseline were excluded. Incident CKD stage 3 was defined as an eGFR < 60 ml/min/1.73 m2 at follow-up and a decrease in eGFR from baseline to follow-up of at least 25%. Controls were frequency matched to cases by race, sex, and 5-year age categories.
Kidney function measurement
A modified kinetic Jaffe method was used to measure serum creatinine at both visits and the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation was used to estimate glomerular filtration rate. [10] Spot urine samples were collected and stored at −70°C. Urinary albumin concentration was measured using nephelometry on either the Dade Behring BN100 or the Beckman Image Nephelometer. Urinary creatinine was measured using the modified Jaffe method. UACR was calculated to quantify albumin excretion.
NGAL and KIM-1 Measurements
Measurement of NGAL and KIM-1 was conducted using an immunoassay on a fluorescent bead-based platform (Rules Based Medicine, www.rulesbasedmedicine.com) after centrifugation at 4000 rpm for 5 minutes. The assay for NGAL has an inter-assay coefficient of variation (CV) of 13% at a concentration of 18.1 ng/mL and 5% at a concentration of 1070 ng/mL. The assay for KIM-1 has an inter-assay CV of 13% at a concentration of 0.035 ng/mL and a CV of 4% at a concentration of 2.9 ng/mL. Ten participants had undetectable NGAL levels and were assigned the smallest value in study (2.7 ng/mL). Thirteen participants had undetectable KIM-1 levels and were assigned the smallest value in the study (0.0065 ng/mL).
Covariate assessment
Diabetes mellitus was defined as a fasting glucose of ≥126 mg/dL, use of oral hypoglycemic medication or insulin, or non-fasting glucose of ≥200 mg/dL or self-reported physician diagnosis of diabetes mellitus. Plasma cholesterol, triglycerides and high-density lipoprotein (HDL) cholesterol were determined using enzymatic methods. Blood pressure was measured using a random-zero sphygmomanometer and the average of the second and third readings of three seated blood pressure measurements was used in analyses. Body mass index (BMI) was defined as a participant's weight in kilograms divided by height in meters squared. Current smoking status and race were defined by self-report.
Statistical analysis
T-tests and chi-square tests were used to test differences between continuous and categorical variables, respectively. Correlations between NGAL and KIM-1 and age, BMI, systolic blood pressure (SBP), HDL, glucose, eGFR, and ln(UACR) were assessed by Pearson correlation coefficients.
Concentrations of NGAL and KIM-1 were skewed and therefore were natural log-transformed to achieve approximate normality. The association between the natural log-transformed values of these markers and incidence of CKD was examined continuously and by quartile of each marker. Conditional logistic regression was used to calculate odds ratios (OR) associated with eGFR < 60 ml/min/1.73 m2 at follow-up and a decrease in eGFR from baseline to follow-up of at least 25%. Model 1 adjusted for the ARIC Carotid MRI Study sampling variables of center and IMT strata. Model 2 adjusted for Model 1 + baseline eGFR, SBP, hypertension medication use, diabetes, HDL cholesterol, BMI, smoking status (Model 2). Model 3 adjusted for the variables in model 2 and the ln(UACR). Model 4 adjusted for the variables in model 2 and the natural log of urinary albumin and the natural log of urinary creatinine.
The association between continuous NGAL and KIM-1 levels and CKD was displayed graphically using logistic regression models, including cubic spine terms, with knots at the 25th, 50th, and 75th percentile of NGAL and KIM-1 concentration. Statistical analyses were conducted using STATA, version 10 (Stata Corp, College Station, TX).
Results
Baseline Study Characteristics
A total of 286 participants were included in these analyses. The mean age of study participants was 64.7 years at baseline (Table 1). Approximately 53% of the participants were women and 22% were African-American. Cases had a higher mean BMI than controls. The prevalence of diabetes, current smoking, hypertension, and hypertension medication use at baseline did not differ by case status. UACR and eGFR values also were similar for cases and controls.
Table 1.
Baseline characteristics, by case status
| Control (n=143) | Case (n=143) | p-value | |
|---|---|---|---|
| age, years* | 64.6 +/− 5.7 | 64.7 +/− 5.5 | - |
| Female,* | 53.9 | 53.9 | - |
| Black,* | 22.4 | 22.4 | - |
| Diabetes, | 14.7 | 18.2 | 0.4 |
| SBP, mmHg | 127.7 +/− 18.1 | 134.2 ± 19.6 | 0.003 |
| Hypertension medication use, | 37.1 | 40.4 | 0.5 |
| BMI, kg/m2 | 27.6 ±4.7] | 29.0±5.4] | 0.02 |
| weight, kg | 78.1 ±14.6] | 81.9 ±17.5] | 0.05 |
| Current Smoking, | 14.0 | 12.7 | 0.8 |
| HDL-C, mg/dL | 51.6±18.3] | 49.9±18.0] | 0.4 |
| urinary albumin, mg/L | 2.0[1.0, 5.0] | 3.0[1.0, 6.0] | 0.1 |
| SCr, mg/dL | 0.72 ±0.17] | 0.73 ±0.17] | 0.6 |
| Urinary creatinine, mg/dL | 80 [40, 123] | 95 [52, 147] | 0.1 |
| UACR, mg/g | 3.1 [1.6, 6.7] | 3.5 [1.3, 7.7] | 0.3 |
| NGAL (ng/mL) | 18 [9.3, 35.0] | 24 [12.0, 42.0] | 0.02 |
| KIM-1 (ng/mL) | 0.14 [0.05, 0.27] | 0.14 [0.06, 0.33] | 0.5 |
| baseline eGFR* (mL/min/1.73 m2) | 83.2 ±15.7] | 81.6 ±14.0] | 0.4 |
| follow-up eGFR (mL/min/1.73 m2) | 80.8 ±19.3] | 48.4 ±7.6] | - |
Values for continuous variables shown as mean ± SD or median [25th, 75th] percentile; values for categorical variables given as percentage.
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; SCr, serum creatinine; UACR, urinary albumin-creatinine ratio; NGAL, neutrophil gelatinase-associatec lipocalin; KIM-1, kidney injury molecule 1; SBP, systolic blood pressure.
Matching characteristics
The median concentration of NGAL at baseline was higher in cases than in controls (18.0 vs. 24.0 ng/mL; p=0.02). There was no significant difference in KIM-1 concentration between cases and controls (0.14 vs. 0.14; p=0.5). Both NGAL (p=0.05) and KIM-1 (p<0.001) were positively correlated with ln(UACR). The only other statistically significant correlation was between ln(NGAL) and BMI (p=0.04) (Table 2).
Table 2.
Pearson correlation coefficients between NGAL and KIM-1 and risk factors
| Age | BMI | SBP | HDL-C | Glucose | eGFR | ln(uAlb) | ln(uCr) | ln(UACR) | |
|---|---|---|---|---|---|---|---|---|---|
| ln(NGAL) | −0.11 (0.08) | 0.12 (0.04) | 0.09 (0.1) | 0.08 (0.2) | 0.03 (0.7) | 0.09 (0.1) | 0.21 (<0.001) | 0.22 (<0.001) | 0.12 (0.05) |
| ln(KIM-1) | 0.07 (0.3) | −0.05 (0.4) | −0.04 (0.5) | −0.07 (0.3) | 0.01 (0.9) | −0.01 (0.8) | 0.42 (<0.001) | 0.12 (0.04) | 0.38 (<0.001) |
| ln(uAlb) | 0.10 (0.1) | 0.10 (0.1) | 0.13 (0.03) | −0.02 (0.7) | 0.04 (0.5) | 0.02 (0.7) | 0.66 (<0.001) | 0.30 (<0.001) | 0.76 (<0.001) |
Note: P values are shown in parentheses
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; uAlb, urinary albumin; uCr, urinary creatinine; UACR, urinary albumin-creatinine ratio. NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule 1. SBP, systolic blood pressure.
Association of NGAL with Incident CKD Stage 3
The distribution of NGAL levels was skewed to the right, with the skew being more prominent among cases than controls (Figure 1). Higher NGAL quartiles were significantly associated with incident CKD stage 3 (Table 3). The odds ratio (OR) of incident CKD stage 3 was 1.30 (95% CI, 0.67–2.53) for participants with NGAL levels in the second quartile, 2.20 (95% CI, 1.12–4.35) for those in the third quartile, and 2.36 (95% CI, 1.12–5.01) for those in the fourth quartile (p-trend= 0.01) as compared to participants with NGAL concentrations in the first quartile (Model 1). Adjustment for baseline eGFR, SBP, hypertension medication use, diabetes, HDL cholesterol, BMI, and current smoking did not change the associations appreciably (Model 2). The results remained similar after additional adjustment for ln(UACR) (Model 3). Adjustment for ln(urinary albumin) and ln(urinary creatinine) separately attenuated the association of interest and the linear trend across quartiles was not significant (p=0.2; Model 4).
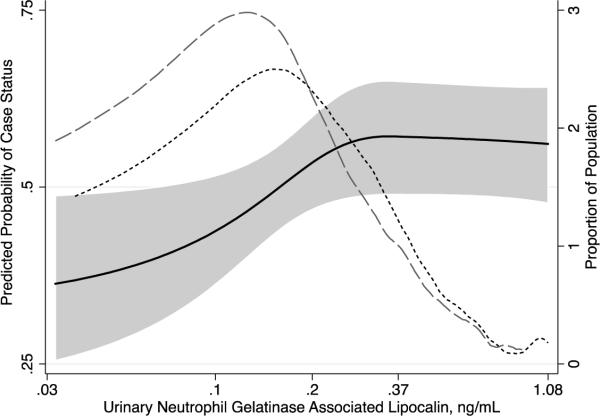
Figure 1.
Predicted probability of case status by urinary neutrophil gelatinase-associated lipocalin (NGAL). Dashed lines represent distribution of NGAL concentration among controls (broken line) and cases (dashed line).
Table 3.
Odds incident CKD stage 3 comparing quartiles 2–4 with quartile 1 of marker
| NGAL | Quartile 1 (<13 ng/mL) | Quartile 2 (13–21 ng/mL) | Quartile 3 (22–39 ng/mL) | Quartile 4 (≥40 ng/mL) | P-trend |
|---|---|---|---|---|---|
| Model 1 | 1.00 (Reference) | 1.30 (0.67, 2.53) | 2.20 (1.12, 4.35) | 2.36 (1.12, 5.01) | 0.01 |
| Model 2 | 1.00 (Reference) | 1.27 (0.64–2.53) | 1.96 (0.96–3.98) | 2.09 (0.95–4.59) | 0.03 |
| Model 3 | 1.00 (Reference) | 1.29 (0.64–2.56) | 1.98 (0.97–4.04) | 2.11 (0.96–4.64) | 0.03 |
| Model 4 | 1.00 (Reference) | 1.08 (0.53, 2.22) | 1.58 (0.75, 3.36) | 1.52 (0.64, 3.58) | 0.2 |
| KIM-1 | Quartile 1 (<0.06 ng/mL) | Quartile 2 (0.06–0.14 ng/mL) | Quartile 3 (0.15–0.30 ng/mL) | Quartile 4 (>0.30 ng/mL) | P-trend |
|---|---|---|---|---|---|
| Model 1 | 1.00 (Reference) | 0.85 (0.44, 1.65) | 1.18 (0.60, 2.29) | 1.51 (0.72, 3.17) | 0.2 |
| Model 2 | 1.00 (Reference) | 0.88 (0.44–1.76) | 1.26 (0.63–2.52) | 1.59 (0.74–3.42) | 0.2 |
| Model 3 | 1.00 (Reference) | 0.90 (0.45–1.82) | 1.34 (0.65–2.74) | 1.81 (0.78–4.21) | 0.1 |
| Model 4 | 1.00 (Reference) | 0.89 (0.44, 1.81) | 1.21 (0.59, 2.51) | 1.22 (0.48, 3.08) | 0.5 |
| Urinary Albumin | Quartile 1 (<1 mg/L) | Quartile 2 (1–2 mg/L) | Quartile 3 (2.1–6 mg/L) | Quartile 4 (≥6.1 mg/L) | P-trend |
|---|---|---|---|---|---|
| Model 1 | 1.00 (Reference) | 1.49 (0.58, 3.81) | 1.38 (0.76, 2.49) | 1.80 (0.95, 3.43) | 0.07 |
| Model 2 | 1.00 (Reference) | 1.90 (0.69–5.21) | 1.43 (0.77–2.65) | 1.64 (0.84–3.21) | 0.1 |
| Model 4* | 1.00 (Reference) | 1.83 (0.66–5.06) | 1.28 (0.68–2.41) | 1.34 (0.67–2.70) | 0.4 |
| Urinary Creatinine | Quartile 1 (<49 mg/dL) | Quartile 2 (50–87 mg/dL) | Quartile 3 (88–134 mg/dL) | Quartile 4 (≥135 mg/dL) | P-trend |
|---|---|---|---|---|---|
| Model 1 | 1.00 (Reference) | 1.42 (0.70, 2.88) | 2.62 (1.22, 5.62) | 2.43 (1.10, 5.39) | 0.01 |
| Model 2 | 1.00 (Reference) | 1.21 (0.58–2.52) | 2.30 (1.03–5.10) | 2.36 (1.03–5.43) | 0.02 |
| Model 4** | 1.00 (Reference) | 1.12 (0.53, 2.38) | 2.12 (0.94, 4.80) | 2.11 (0.89, 5.01) | 0.04 |
Except where indicated, values shown are unadjusted Odds Ratio (95% CI).
NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule 1; UACR, urinary albumin-creatinine ratio; CKD, chronic kidney disaese
Model 1 – Adjusted for ARIC Carotid MRI Study sampling variables of center and IMT strata
Model 2 - Adjusted for Model 1 + estimated glomerular filtration rate, systolic blood pressure, anti-hypertensive medication use, diabetes, high density lipoprotein cholesterol, body mass index, current smoking
Model 3 - Adjusted for Model 2 + ln(UACR)
Model 4 – Except where indicated, adjusted for Model 2 + ln(urinary albumin) + ln(urinary creatinine)
Adjusted for model 2 + ln(urinary creatinine)
Adjusted for model 2 + In(urinary albumin)
Higher NGAL concentration was associated with a greater probability of being a case in a log-linear manner (Figure 1). The odds of incident CKD stage 3 were 1.31 times greater for each standard deviation higher ln(NGAL) concentration (OR, 1.31; 95% CI, 1.00–1.72) after adjustment for covariates including ln(UACR). Adjustment for covariates and ln(urinary albumin) and ln(urinary creatinine) separately attenuated this association (OR, 1.19; 95% CI, 0.90–1.58).
Standardization of NGAL to the concentration of urinary creatinine resulted in multivariable (Model 4) odds ratio for incident CKD stage 3 of 1.59 (95% CI, 0.77–3.26) for quartile 2, 1.74 (95% CI, 0.79–3.80) for quartile 3, and 1.28 (95% CI, 0.58–2.84) for quartile 4 compared to quartile 1.
Association of KIM-1 with Incident CKD Stage 3
Higher KIM-1 quartiles were not significantly associated with incident CKD Stage 3 (Table 3). The unadjusted odds ratio for incident CKD stage 3 was 0.85 (95% CI, 0.44–1.65) for individuals with KIM-1 levels in the second quartile, 1.18 (95% CI, 0.60–2.29) for the third quartile, and 1.51 (95% CI, 0.72–3.17) for the fourth quartile (p-trend = 0.2) as compared to participants with KIM-1 concentrations in the first quartile (Table 3; Model 1). These associations did not change appreciably after adjustment.
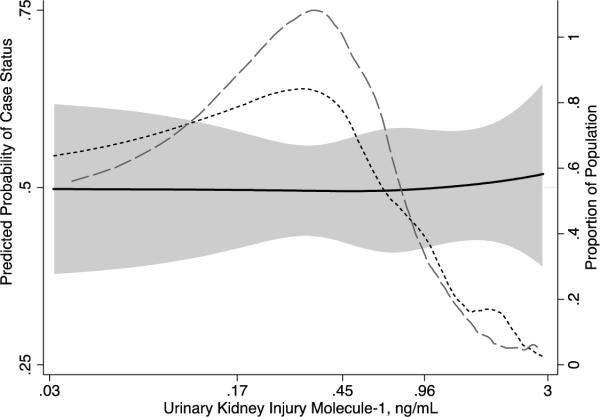
The continuous relationship between KIM-1 and incident CKD stage 3 is shown in Figure 2. A total of 6% of controls and 9% of cases were at the lower limit of detection of the assay (0.028 ng/mL) for KIM-1. Overall the continuous relationship between KIM-1 and incidence of CKD Stage 3 was relatively flat. The odds of incident CKD stage 3 were 1.18 times greater for each standard deviation higher ln(KIM-1) concentration (OR, 1.18; 95% CI, 0.89–1.57) after adjustment for covariates including ln(UACR) and 1.07 times greater (OR, 1.07; 95% CI, 0.80–1.43) after adjustment for covariates and ln(urinary albumin) and ln(urinary creatinine).
Figure 2.
Predicted probability of case status by urinary concentration of Kidney Injury Molecule -1 (KIM-1). Dashed lines represent distribution of KIM-1 concentration among controls (broken line) and cases (dashed line).
Standardization of KIM-1 to the concentration of urinary creatinine resulted in multivariable (Model 4) odds ratio for incident CKD stage 3 of 0.52 (95% CI, 0.25–1.10) for quartile 2, 0.52 (95% CI, 0.24–1.12) for quartile 3, and 0.61 (95% CI, 0.28–1.35) for quartile 4 compared to quartile 1.
Association of Urinary Albumin and Urinary Creatinine with Incident CKD Stage 3
Higher quartiles of urinary albumin were not significantly associated with incident CKD stage 3 in multivariable adjusted models (Table 3; Model 4 – excluding adjustment for ln(urinary albumin). Higher quartiles of urinary creatinine were associated with incident CKD stage 3 (p-trend = 0.04) (Model 4 – excluding adjustment for ln(urinary creatinine)).
Discussion
Higher baseline urinary concentration of NGAL was associated with incident CKD stage 3 in this matched case-control study of ARIC participants, whereas urinary KIM-1 levels at baseline were not significantly associated with development of CKD stage 3. Both NGAL and KIM-1 levels were positively correlated with UACR levels at baseline. Multivariable adjustment attenuated these associations somewhat and additional adjustment for baseline urinary albumin and urinary creatinine resulted in nonsignificant associations.
NGAL and KIM-1 are known markers of AKI. Both of these markers are up-regulated after ischemic damage in animal studies. [7, 11] NGAL can be produced in response to damaged epithelial cells of the lungs or gastrointestinal tract [12, 13], or locally in the proximal tubules following ischemic or nephrotoxic injury in animals and humans. [14, 15] KIM-1 is a transmembrane protein that is undetectable in healthy kidney tissue but becomes overexpressed in differentiated tubule cells after tubulointerstitial damage. [16] Recent studies in adults and children who have undergone cardiac procedures or kidney transplant indicate that NGAL and KIM-1 levels rise before currently used markers of AKI, such as serum creatinine, and that elevated concentrations of these markers predict AKI. [14, 17–24] [25].
Recent literature suggests that NGAL may be a marker of prevalent CKD as well. [26–35] Urinary NGAL is correlated with markers currently used to assess kidney function, such as serum creatinine and eGFR [26, 27, 31, 33–35], and has good diagnostic properties to detect progression of CKD. [29, 31] There are fewer studies on the association between KIM-1 and prevalent CKD. One study reported significantly higher urinary KIM-1 levels in 53 patients with various forms of kidney disease than in 11 apparently healthy controls. [16]
Neither NGAL nor KIM-1 levels in our study were significantly correlated with eGFR at baseline, but both were significantly correlated with baseline ln(UACR). The reasons for this are speculative. The baseline eGFR levels in these studies were in the normal range by design, as opposed to previous studies in participants with established CKD. [29, 31, 32] These markers may not be sensitive to mildly decreased kidney function. Estimates of GFR also lack precision in this range. A rise in urinary NGAL and KIM-1 levels may indicate actively inflamed tubular cells, potentially associated with mildly increased urinary albumin excretion, whereas elevated serum creatinine and decreased GFR may be an indicator of accumulated loss of functional nephrons in the glomeruli and be relatively insensitive to early damage. [36]
The association between NGAL concentration and progression of prevalent CKD was investigated previously in a study of 96 patients with CKD (biopsy-confirmed glomerulonephritis, diabetic nephropathy, autosomal polycystic kidney disease, or other kidney diseases) without kidney failure (eGFR ≥15 mL/min/1.73 m2) and 14 controls. Each 10 ng/mL higher urinary NGAL or serum NGAL was associated with a 3% (HR, 1.03; 95% CI, 1.03–1.04) and 2% (HR, 1.02; 95% CI, 1.01–1.03) higher risk for progression of CKD (31 cases), defined as doubling of serum creatinine or development of ESRD, respectively, after adjustment for age and baseline eGFR. [29] No previous studies to our knowledge have examined the association between KIM-1 and incident CKD stage 3.
The role of NGAL in CKD is not completely understood. Some postulate that NGAL upregulation promotes apoptosis [37–40] while others suggest that NGAL aids in cell survival. [41, 42] Even less is known about the role of KIM-1 in CKD. Circulating KIM-1 may play a positive role in repair of acute kidney injury, while the attendant excessive cell proliferation may be related to a decrease in kidney function over a longer time period.. [43–46]
Higher urinary creatinine concentration, but not urinary albumin concentration, was independently associated with incident CKD Stage 3 in our analyses. Adjustment for urinary creatinine concentration attenuated the observed association between NGAL incident CKD stage 3. Urinary creatinine concentration reflects lower urine volume output, as well as greater creatinine production through higher muscle mass. An implicit assumption of standardizing biomarkers to urinary creatinine is that production of urinary creatinine is uniform over all participants, which may not hold in this study population. [47] It is noteworthy that UACR also did not predict incident CKD stage 3 in this selected population, possibly reflecting limited power and a limited range of albuminuria.
Limitations of this study include the case-control design, which excludes potential participants due to deaths and losses to follow-up between the baseline and follow-up visits. This may attenuate the associations of interest. The relatively small sample size of the study limits the precision with which we may estimate the associations and provide limited power to detect associations of moderate strength. Follow-up was based on a single serum creatinine measurement, but the requirement of a decrease in eGFR of >25% from baseline reduces the potential bias due to imprecision in GFR estimation from serum creatinine. The inability to distinguish monomeric from dimeric forms of NGAL in the urine can have an appreciable impact on the performance of the assay and on results. Specifically, measuring nonmonomeric forms of NGAL may prevent elucidation of the source of NGAL- specifically, if NGAL is being produced by tubular epithelial cells or neutrophils, potentially indicating different causes of CKD. [48].
In summary, we found that concentration of urinary NGAL, but not urinary KIM-1, was associated with incident CKD stage 3. Additional studies are needed with larger populations to confirm our findings and to assess the utility of NGAL and KIM-1 as markers of CKD risk.
Acknowledgements
The authors thank the staff and participants of the ARIC Study for their important contributions.
Support: The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C) with the ARIC Carotid MRI examination funded by U01HL075572-01. N.A.B. was supported by the National Institutes of Health/NHLBI grant T32HL07024 and by the Agency for Healthcare Research and Quality grant T32HS019488.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
financial disclosure: The authors declare that they have no relevant financial interests.
REFERENCES
- 1.US Renal Data System . USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2009. [Google Scholar]
- 2.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33(5):1004–10. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- 3.Ruggenenti P, et al. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN) Kidney Int. 1998;53(5):1209–16. doi: 10.1046/j.1523-1755.1998.00874.x. [DOI] [PubMed] [Google Scholar]
- 4.Rossing P, et al. Impact of arterial blood pressure and albuminuria on the progression of diabetic nephropathy in IDDM patients. Diabetes. 1993;42(5):715–9. doi: 10.2337/diab.42.5.715. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, Chertow GM, Curhan GC. Methodological issues in studying the epidemiology of mild to moderate chronic renal insufficiency. Kidney Int. 2002;61(5):1567–76. doi: 10.1046/j.1523-1755.2002.00299.x. [DOI] [PubMed] [Google Scholar]
- 6.Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol. 2007;156:203–12. doi: 10.1159/000102085. [DOI] [PubMed] [Google Scholar]
- 7.Han WK, et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 8.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 9.Wasserman BA, et al. MRI measurements of carotid plaque in the atherosclerosis risk in communities (ARIC) study: methods, reliability and descriptive statistics. J Magn Reson Imaging. 2010;31(2):406–15. doi: 10.1002/jmri.22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Mishra J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarkerfor ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 12.Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45(1):17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- 13.Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta. 2000;1482(1–2):272–83. doi: 10.1016/s0167-4838(00)00152-7. [DOI] [PubMed] [Google Scholar]
- 14.Mori K, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115(3):610–21. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt-Ott KM, et al. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15(4):442–9. doi: 10.1097/01.mnh.0000232886.81142.58. [DOI] [PubMed] [Google Scholar]
- 16.van Timmeren MM, et al. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212(2):209–17. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 17.Bachorzewska-Gajewska H, et al. Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am J Nephrol. 2006;26(3):287–92. doi: 10.1159/000093961. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch R, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22(12):2089–95. doi: 10.1007/s00467-007-0601-4. [DOI] [PubMed] [Google Scholar]
- 19.Mishra J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 20.Mishra J, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21(6):856–63. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- 21.Parikh CR, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6(7):1639–45. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 22.Trachtman H, et al. Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatr Nephrol. 2006;21(7):989–94. doi: 10.1007/s00467-006-0146-y. [DOI] [PubMed] [Google Scholar]
- 23.Wagener G, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiacc surgery. Anesthesiology. 2006;105(3):485–91. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Han WK, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73(7):863–9. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liangos O, et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18(3):904–12. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- 26.Bolignano D, et al. Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol. 2007;27(4):373–8. doi: 10.1159/000103912. [DOI] [PubMed] [Google Scholar]
- 27.Bolignano D, et al. Pathological and prognostic value of urinary neutrophil gelatinase-associated lipocalin in macroproteinuric patients with worsening renal function. Kidney Blood Press Res. 2008;31(4):274–9. doi: 10.1159/000151665. [DOI] [PubMed] [Google Scholar]
- 28.Bolignano D, et al. Neutrophil gelatinase-associated lipocalin reflects the severity of renal impairment in subjects affected by chronic kidney disease. Kidney Blood Press Res. 2008;31(4):255–8. doi: 10.1159/000143726. [DOI] [PubMed] [Google Scholar]
- 29.Bolignano D, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(2):337–44. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding H, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin Immunol. 2007;123(2):227–34. doi: 10.1016/j.clim.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Malyszko J, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in non-diabetic patients with stage 2–4 chronic kidney disease. Ren Fail. 2008;30(6):625–8. doi: 10.1080/08860220802134607. [DOI] [PubMed] [Google Scholar]
- 32.Malyszko J, et al. Neutrophil gelatinase-associated lipocalin is a new and sensitive marker of kidney function in chronic kidney disease patients and renal allograft recipients. Transplant Proc. 2009;41(1):158–61. doi: 10.1016/j.transproceed.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 33.Poniatowski B, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in patients with chronic heart failure and coronary artery disease. Kidney Blood Press Res. 2009;32(2):77–80. doi: 10.1159/000208989. [DOI] [PubMed] [Google Scholar]
- 34.Brunner HI, et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2577–84. doi: 10.1002/art.22008. [DOI] [PubMed] [Google Scholar]
- 35.Mitsnefes MM, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol. 2007;22(1):101–8. doi: 10.1007/s00467-006-0244-x. [DOI] [PubMed] [Google Scholar]
- 36.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71(10):967–70. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 37.Stoesz SP, et al. Heterogeneous expression of the lipocalin NGAL in primary breast cancers. Int J Cancer. 1998;79(6):565–72. doi: 10.1002/(sici)1097-0215(19981218)79:6<565::aid-ijc3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 38.Furutani M, et al. Identification of a neutrophil gelatinase-associated lipocalin mRNA in human pancreatic cancers using a modified signal sequence trap method. Cancer Lett. 1998;122(1–2):209–14. doi: 10.1016/s0304-3835(97)00391-1. [DOI] [PubMed] [Google Scholar]
- 39.Friedl A, et al. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J. 1999;31(7):433–41. doi: 10.1023/a:1003708808934. [DOI] [PubMed] [Google Scholar]
- 40.Yan L, et al. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J BiolChem. 2001;276(40):37258–65. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 41.Mishra J, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15(12):3073–82. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 42.Tong Z, et al. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391(Pt 2):441–8. doi: 10.1042/BJ20051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramer AB, et al. Reduction of proteinuria in adriamycin-induced nephropathy is associated with reduction of renal kidney injury molecule (Kim-1) over time. Am J Physiol Renal Physiol. 2009;296(5):F1136–45. doi: 10.1152/ajprenal.00541.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuehn EW, et al. Kidney injury molecule-1 expression in murine polycystic kidney disease. Am J Physiol Renal Physiol. 2002;283(6):F1326–36. doi: 10.1152/ajprenal.00166.2002. [DOI] [PubMed] [Google Scholar]
- 45.Han WK, et al. Human kidney injury molecule-1 is a tissue and urinary tumor marker of renal cell carcinoma. J Am Soc Nephrol. 2005;16(4):1126–34. doi: 10.1681/ASN.2004070530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huo W, et al. Kidney injury molecule-1 (KIM-1): a novel kidney-specific injury molecule playing potential double-edged functions in kidney injury. Transplant Rev (Orlando) 2010;24(3):143–6. doi: 10.1016/j.trre.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78(5):486–94. doi: 10.1038/ki.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai L, et al. The origin of multiple molecular forms in urine of HNL/NGAL. Clinical journal of the American Society of Nephrology: CJASN. 2010;5(12):2229–35. doi: 10.2215/CJN.00980110. [DOI] [PMC free article] [PubMed] [Google Scholar]