Abstract
Background
Stroke survivors have less muscle mass in their paretic limbs compared to non-paretic limbs, which may or may not be accompanied by changes in regional and/or whole body fat mass.
Aim
To examine the current evidence regarding differences in regional fat mass between paretic and non-paretic limbs and changes in whole body fat mass over time in stroke survivors.
Methods
A systematic search of relevant databases. Studies measuring whole body or regional fat mass using dual energy x-ray absorpiometry (DEXA), computed tomography (CT) or magnetic resonance imaging (MRI) were included.
Results
Eleven trials were identified. Fat mass differences between paretic and non-paretic limbs and change in fat mass over time were not consistent. Meta-analyses were conducted using DEXA-derived data from 10 trials (n=324). There were no differences in fat mass between paretic and non-paretic legs (pooled mean difference 31.4g, 95% CI −33.9 to 96.6, p<0.001), and slightly greater fat mass in the paretic arms compared to non-paretic arms (pooled mean difference 84.0g, 95% CI 30.7 to 137.3, p=0.002). Whole body fat mass did not increase significantly between one month and six months post-stroke (pooled mean difference 282.3g, 95% CI −824.4 to 1389, p=0.62), but there was an increase between six and 12 months post- stroke (pooled mean difference 1935g, 95% CI 1031 to 2839, p<0.001).
Conclusions
There were inconsistent findings regarding changes in fat mass after stroke. Large, well-designed studies are required to further investigate the impact of body composition changes on the health of stroke survivors.
Keywords: Stroke, body composition, fat mass, dual energy x-ray absorpiometry
Introduction
A recent systematic review (1) found strong evidence that people later after stroke (at least six months) have significantly less muscle mass in their paretic limbs; in the order of 4% in the legs and 8% in the arms. An increase in lipid deposition in and around muscle fibres is a feature of muscular atrophy (2). Furthermore, muscle biopsy studies have found infiltration of lipids into muscle fibres in paretic limbs of people after stroke (3). Other studies have demonstrated an increase in the amount of low-attenuation muscle tissue on computed tomography (CT) slices of the paretic thigh (4), which is indicative of increased fat content within muscles (5). Therefore it was hypothesized that stroke survivors may have an increase in regional fat mass in the paretic limb later after stroke, and/or an increase in whole body fat mass over time.
The composition of muscles, particularly muscle fibre type and fat content, directly impacts on the ability of the body to metabolize glucose. Loss of muscle mass as well as increased fat content within muscles has been linked to greater insulin resistance, poor physical fitness and greater risk of cardiovascular disease (5–7). More than 80% of stroke survivors have impaired glucose metabolism (8) and this has been linked to a two to three-fold increase in the risk of recurrent stroke (9). Amongst healthy, sedentary adults with a range of body mass index scores, insulin stimulated glucose disposal is negatively correlated with body fat mass, visceral fat, subcutaneous abdominal fat and thigh fat (5). However, increased fat content within the thigh musculature (as evidenced by low-attenuation tissue on CT slices of the mid-thigh) has been shown to be the single strongest correlate of insulin resistance (5). Others (6) also found a positive correlation between mid-thigh low attenuation tissue and fasting concentration of glucose in healthy sedentary women, but this was no longer significant after controlling for total body adiposity and age.
Before effective interventions to address changes in body composition after stroke can be developed, the incidence, rate and magnitude of the changes in both fat and muscle must be known. We recently published a systematic review examining the current evidence for changes in muscle mass in stroke survivors (1).
Aim
The aim of this review was to examine the available evidence for changes in whole body and regional fat mass in people after stroke.
Methods
We searched the following databases from inception until 2nd May 2010: Ovid (Medline, Ageline, Embase classic and Embase, AMED), Cumulative Index of Nursing and Allied Health, Cochrane Library and Web of Knowledge. Search terms were based on the population of interest (e.g. stroke, cerebrovascular accident, brain injury, hemiplegia), and the construct of interest (e.g. muscle or lean mass, fat mass, intramuscular, body composition, body dimensions). Search terms were truncated according to each database and combined. Searches were limited to humans and English language. The full search strategy for Ovid databases is included in Appendix 1. We sent the final list of included studies to five content experts for validation. We examined the reference lists of all included studies and the reference lists of all related literature or systematic reviews retrieved in the search. Grey literature and other unpublished sources were not searched.
Studies that had measures of fat mass or fat volume, derived from adult human stroke survivors were included. Only measures of fat mass or volume using gold standard methods (dual energy x-ray absorptiometry [DEXA], CT or magnetic resonance imaging [MRI]) were included whereas body composition determinations by bioelectric impedance analysis or anthropometry were excluded. In cases where DEXA scans were reported but fat mass data were not reported, authors were contacted and invited to share raw data. There were no restrictions on study design. For intervention studies, only baseline or control group data were considered.
The full search results were reviewed by title and abstract by one reviewer. The full texts of all potentially eligible articles were independently reviewed by two reviewers. Any disagreements were resolved by discussion. Included articles were critically appraised for risk of bias by two reviewers. Four key criteria considered to have potential to introduce bias were: risk of bias in recruitment, whether measurement of body composition was the primary aim of the study, whether assessors or those interpreting imaging data were blinded to either the aim of the study or the side of hemiplegia, and whether adequate steps were taken to minimise sources of error in the measurement technique. These criteria are identical to those used in our previous systematic review of changes in muscle mass after stroke (1). Data from all studies were extracted and synthesized narratively.
Where there were sufficient data available; either published or provided by authors, meta-analyses were performed using the review software package Revman5. The generic inverse variance method of meta-analysis was used as data were paired (11). This involved imputing an assumed correlation between measures taken from the paretic and non-paretic limbs. Based on raw data provided (11,12), a correlation of 0.9 was imputed for the leg comparisons and a correlation of 0.8 imputed for the arm comparisons. The statistical heterogeneity of each meta-analysis was assessed using the I2 statistic. Where I2 > 50%, sensitivity analyses were performed to investigate sources of heterogeneity. Where medians and inter-quartile ranges were reported rather than means and standard deviations, standard methods for conversion were used.
Results
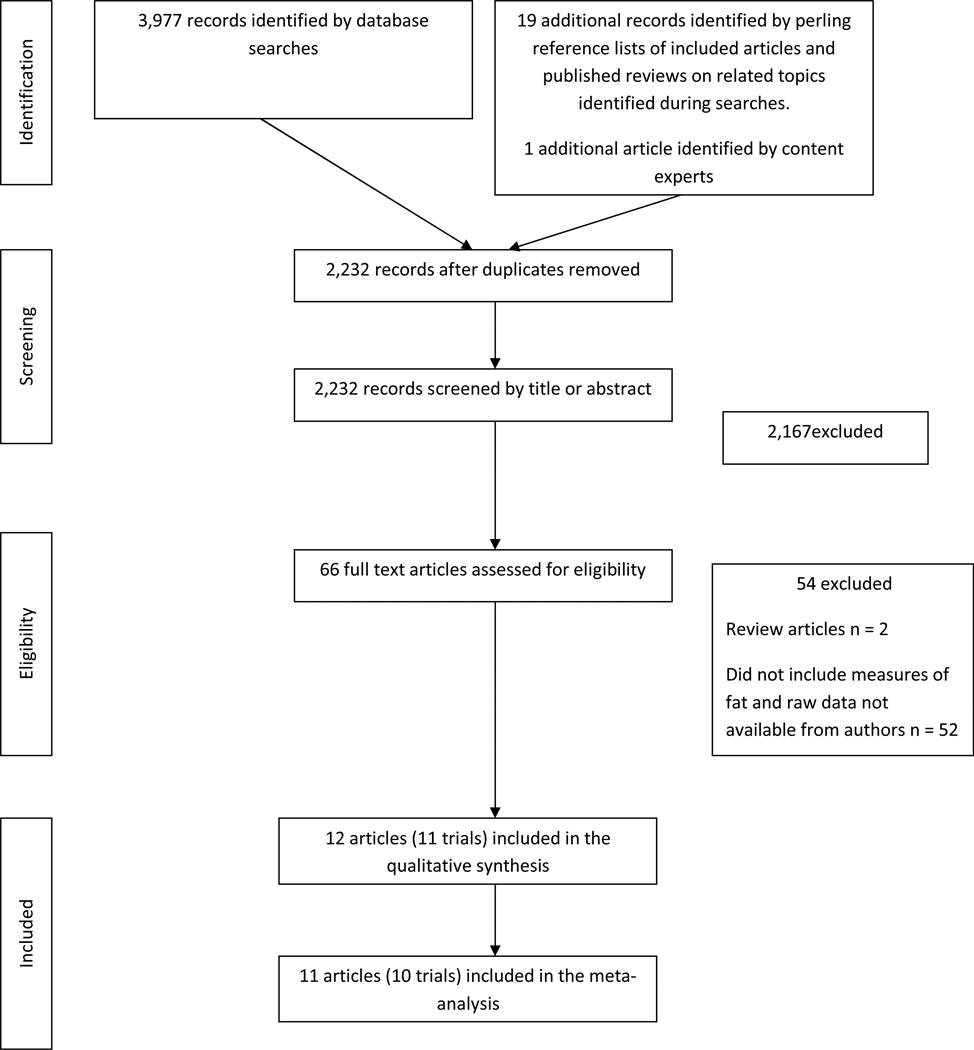
Figure 1 shows the flow of trial selection. A total of 2,232 records were examined by title and abstract. Of these, 66 potential articles were reviewed by full text. A total of 54 trials were excluded leaving 12 articles (11 trials) to be included in the review. Two of these articles reported from the same trial (13–14). Table 1 summarizes the included studies. There were a total of 352 participants in the 11 trials, with mean ages ranging from 62.5 to 75.0 years (4, 7, 11–20). Seven of the trials were cross-sectional, where measures were taken from the paretic and non-paretic limbs at one point in time (4, 7, 11–14, 16, 19). In the remaining four trials measures of fat mass were taken at several time points relative to stroke onset (15, 17, 18, 20). In two of these trials (15, 18) fat mass was reported for the paretic and non-paretic limbs separately at each time point. Mean stroke latency of participants in the cross-sectional trials ranged from six months (16, 19) to five years (11, 13, 14). In the majority of cross-sectional trials, only people who were able to walk were included (4, 7, 11, 13, 14) in all other trials either the level of participant functional ability was not reported (16, 19) or people with a range of abilities were included (12, 17). One trial included only people still unable to walk at three months post-stroke (18). In the four longitudinal studies measures of body composition were made at various time points including one week (17), one month (20), three months (18) six to seven months (15, 17, 18, 20) and one year (17, 18, 20) post-stroke. In all trials DEXA scans were used to measure body composition. In one trial (7) CT scans of the mid-thigh were also taken and the area of low-density lean tissue was measured.
Figure 1.
Flow chart of study inclusion
Table 1.
Characteristics of included studies
| Study ID | n | Study design | Participant characteristics BMI (kg/m2) mean (SD) |
Age in years Mean (SD or range) |
Number (%) male |
Type of stroke |
Stroke latency |
Measurement tool |
Body part |
Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Billinger et al 2009 | 12 | Cross- sectional |
Able to walk independently Excluded people with diabetes BMI 29.7 (5.2) |
60.6 (14.5) | 5 (42%) | 9 (75%) ischemic 3 (25%) hemorrhagic |
5.8 (2.4) years |
DEXA | Legs and arms |
No significant difference in fat mass between sides |
| Carin-Levy et al 2006 | 17 | Longitudinal | Baseline median FIM score = 107 BMI 24.6 (7.8) |
66 (11.5) | 10 (59%) |
8 (47%) ischemic 5 (29%) hemorrhagic 4 (33%) normal CT |
3 weeks and 6 months |
DEXA | Whole body |
Significant increase in whole body fat mass over time. |
| Celik et al 2008 | 35 | Cross- sectional |
71% of participants Brunnstrom motor stage of 3 or less (ie did not have ability to perform isolated movements) BMI not reported |
62.7 (9.5) | 18 (51%) |
Not reported |
1.3 (1.9) years |
DEXA | Legs | No significant difference in fat mass between sides |
| Iversen et al 1989 | 15 | Cross- sectional |
Not reported BMI not reported |
62.5 (39– 78) |
8 (53%) | Not reported |
Approx 6 months |
DEXA | Arms and legs |
Significantly more fat mass in the paretic vs non- paretic limbs |
| Jørgensen and Jakobsen 2001 | 28 | Longitudinal | Unable to walk at baseline BMI not reported |
75.0 (7.0) | 18 (64%) |
Not reported |
1 week, 2 months, 7 months and 1 year |
DEXA | Legs | Significant increase in fat mass only in the paretic limb of people still unable to walk at 2 months |
| Lazoura et al 2010 | 58 | Longitudinal | Unable to walk at 3 months BMI not reported |
64.1 (SD not reported) |
36 (62%) |
Not reported |
3, 6 and 12 months |
DEXA | Legs | Significant increase in fat mass in the paretic, and to lesser extent non- paretic leg |
| Okabe et al 2004 | 24 | Cross- sectional |
Not reported BMI not reported |
68.9 (1.8) | 14 (58%) |
Not reported |
6.2 (1.1) months |
DEXA | Legs | Significantly less fat in the paretic vs non-paretic legs |
| Pang et al 2005 / Pang and Eng 2005* | 58/ 56 |
Cross- sectional |
Able to walk independently BMI not reported |
65.5 (8.8) | 35 (60%)/3 4(61%) |
Not reported |
5.6 (5.1) years / 4.1 (4) years |
DEXA | Legs/A rms |
Significantly more fat mass in the paretic vs non- paretic legs and arms |
| Ramnemark et al 1999 | 19 | Longitudinal | Less than antigravity power in either arm or leg on paretic side BMI males 24.9 (3.3) BMI females 23.7 (3.8) |
74.9 (8.2) | 12 (63%) |
16 (84%) infarct 3 (16%) hemorrhagic |
1 month, 4 months, 7 months and 12 months |
DEXA | Whole body | No significant change in fat mass over time |
| Ryan et al 2000 | 26 | Cross- sectional |
Able to walk independently |
66.0 (9.0) | 22 (85%) |
All infarct | 3.2 (4.7) years |
DEXA | Leg | Significantly more fat mass in the paretic vs non- paretic legs |
| Ryan et al 2002 | 60 | Cross- sectional |
Able to walk independently BMI 27.7 (4.3) |
65.0 (9.0) | 47 (78%) |
All infarct | 3.0 (3.7) years |
DEXA and CT | Arm, leg and thigh (DEXA) Mid thigh (CT) |
No significant difference in fat mass between sides. Trend toward greater mid-thigh low density tissue in paretic leg. |
DEXA = dual energy x-ray absorpiometry, FIM = functional independence measure, BMI = body mass index
these 2 papers reported the results of one trial with the same participants.
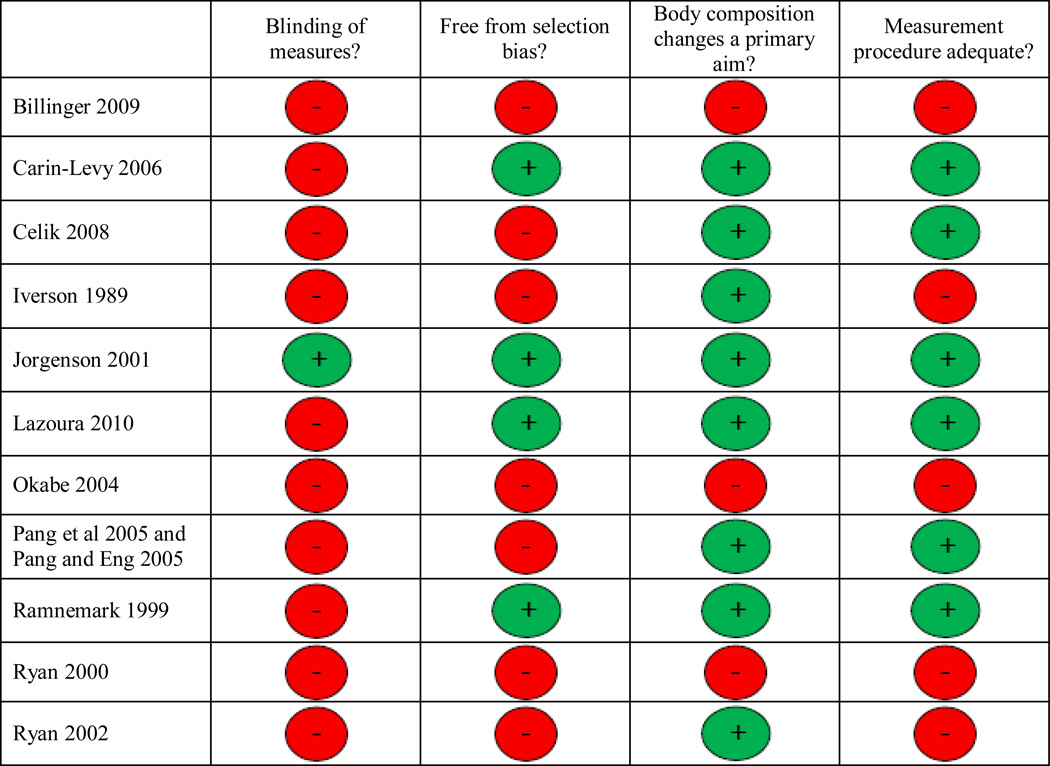
The risk of bias in the included trials was rated as moderate to high. Blinding of the technicians taking the measures to the side of hemiparesis was reported in only one trial (17) and also occurred in two others (4, 7). Only four of the 11 trials could be considered free of selection bias (i.e. used consecutive or random sampling methods). In the majority of trials (9 out of 11), investigation of either changes in body composition over time or differences between paretic and non-paretic limbs was a primary aim of the study. Finally, eight of the 11 trials either adequately reported or later confirmed the methods by which error was minimized in the measurement procedures (for example by reporting use of the standard positioning, the same technician, averages of two scans or determination of the coefficient of variation of DEXA scans). Figure 2 shows the risk of bias assessment for each trial.
Figure 2.
Risk of bias of included trials
Differences between the fat mass in paretic and non-paretic limbs were not consistent across studies. In three trials fat mass was greater in the paretic arm compared to the non-paretic arm (11, 13, 16), which contrasts with one trial where fat mass was greater in the non-paretic arm (15). In five trials fat mass was greater in the paretic leg compared to the non-paretic leg (7, 13–15, 18) but in three trials fat mass was greater in the non-paretic leg (11, 12, 19). In one trial, there were no differences found between sides (4).
For longitudinal studies, there were also inconsistent findings. In one trial no significant changes in whole body fat mass were found between measures taken at one month post-stroke and measures taken at 12 months post-stroke (20). However, in two other trials an increase in whole body fat mass was found between three weeks and six months post-stroke (15) and between three months and six months (18) and to a lesser extent after a longer period of follow up (six and 12 months post-stroke) (18). Lazoura et al reported that both the paretic and non-paretic legs increased in fat mass over time, with no difference in the amount of fat mass gain between sides. In contrast, Jorgensen et al reported an increase in fat mass in the paretic leg only, and only in those participants still unable to walk at two months post-stroke.
Meta-analyses
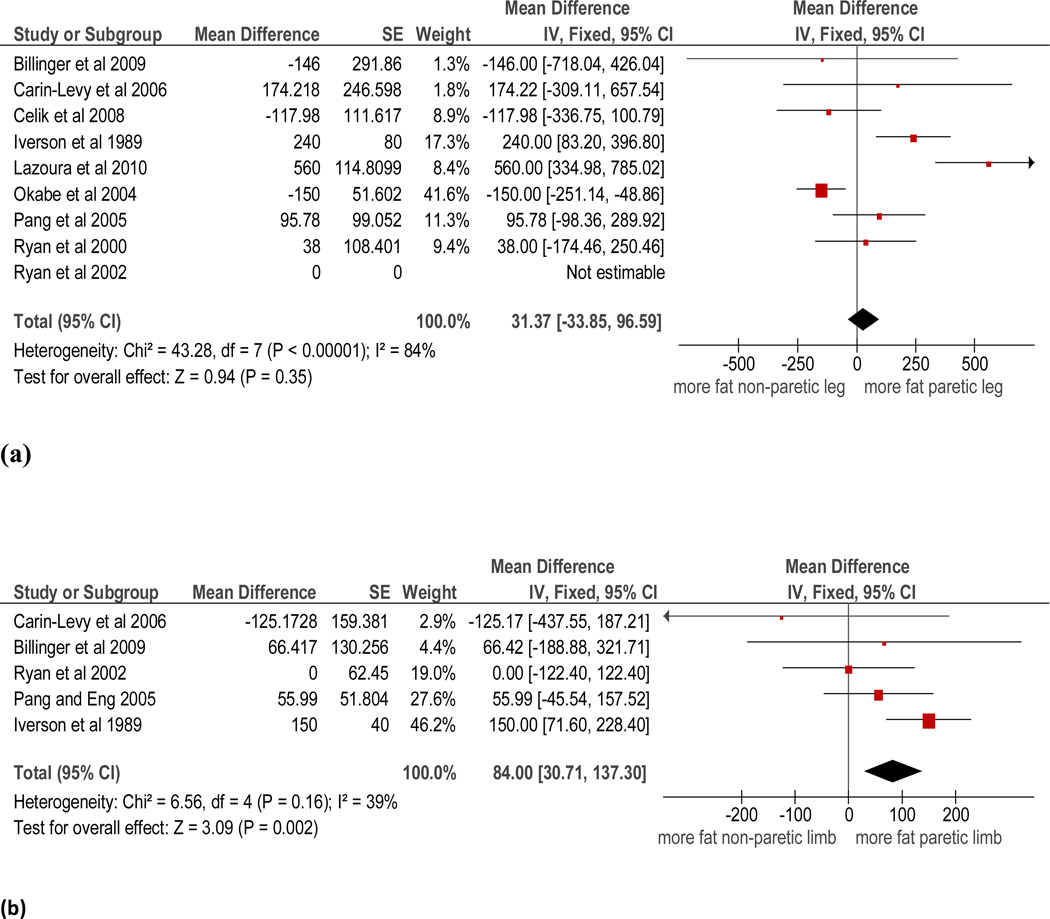
Data was pooled for the comparison between paretic and non-paretic limbs in participants later after stroke for DEXA measures of fat mass. The results of the meta-analysis of the nine trials (n=305) in which fat mass in the paretic and non-paretic legs was reported showed an overall non-significant difference between sides (pooled mean difference 31.4g, 95% CI −33.9 to 96.6, p<0.001 see Figure 3). The statistical heterogeneity of this analysis was high (I2=84%). Sensitivity analyses revealed that no single trial contributed significantly to this heterogeneity. The results of the meta-analysis of the five trials (n=160) in which fat mass in the paretic and non-paretic arms was measured showed an overall statistically significant, but very small difference in favor of greater fat mass in the paretic arm (pooled mean difference 84.0g, 95% CI 30.7 to 137.3, p=0.002 see Figure 3). The statistical heterogeneity of this analysis was acceptable (I2=39%).
Figure 3.
Paretic versus non-paretic fat mass in the (a) leg and (b) arm
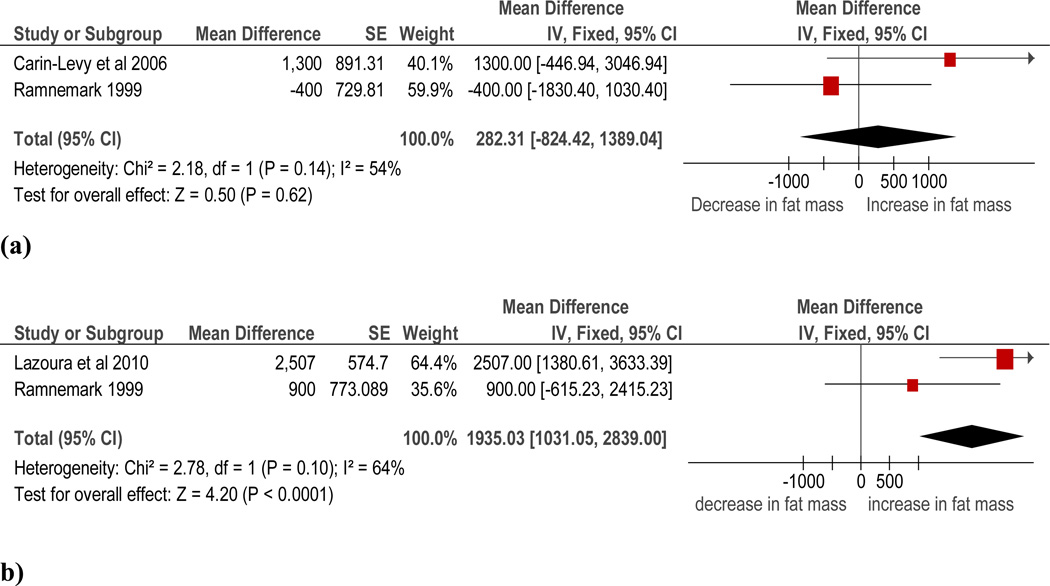
Data were pooled from two trials (n=37) (15, 18, 20) for the change in whole body fat mass between one month and six months post-stroke. Combining data from these two trials resulted in a non-significant change (pooled mean difference 282.3g, 95% CI −824.4 to 1389, p=0.62), with a statistical heterogeneity of I2=54% (see Figure 4). Finally, data were pooled from two trials (n=77) (18, 20) for change in whole body fat mass between six (18) or seven (20) months and 12 months post-stroke. The meta-analysis of these two trials found a significant increase in whole body fat mass (pooled mean difference 1935g, 95% CI 1031 to 2839, p<0.001) with a statistical heterogeneity of I2=64%.
Figure 4.
Change in whole body fat mass between (a) one month and six months post-stroke and (b) six months and 12 months post-stroke
Discussion
The results of this review demonstrated that there is little difference in regional fat mass between paretic and non-paretic limbs later after stroke. While there was a statistically significant finding in relation to more fat mass in the paretic arm, the magnitude of this difference is likely too small to be of clinical relevance. However, because the findings of individual trials regarding leg fat mass were conflicting, the statistical heterogeneity of this analysis was unacceptably high and therefore the results should be interpreted with some caution. The trials included in the review were of reasonably high quality. It is possible that risk of bias may have been overestimated due to lack of reporting of details such as blinding technicians to the side of paresis, use of standard positioning and coefficients of variation.
There may be several reasons for the disparity in results between trials. It may be that changes in body composition after stroke are highly variable between individuals. However, this cannot fully explain the results; if this were the case, all trials would have reported high variability in their data and an overall non-statistically significant difference either over time or between limbs. Changes in body composition are likely influenced by a variety of factors including nutritional status, level of physical activity (which in turn is strongly influenced by stroke severity), levels of circulating hormonal and inflammatory factors (21, 22). However, no trials reported associations between physical fitness, activity levels or other potential confounders, so their impact on results cannot be determined.
Similarly, the disparity in results of the longitudinal trials may possibly be explained by differences in the level of physical ability among participants. It is well known that people after stroke participate in very low levels of physical activity both in the early weeks (23) and in the months and years following stroke (24). However, it is likely that those people who retained, or quickly regained the ability to walk after stroke are less at risk of negative changes in body composition including increases in fat mass. This theory is supported by the results of the trial by Jorgensen et al who found an increase in fat mass only in the paretic legs of people with persistent walking disability. Currently, it is not possible to speculate on the degree to which changes in body composition are independently related to stroke itself, versus being related to post-stroke consequences, in particular inactivity and a reduction in contractile properties of the hemiparetic limbs. Further work is needed to tease out the relationships between degree of stroke related disability, physical activity levels and changes in body composition. Poor nutritional status and the presence and degree of control of diabetes mellitus may also play a role.
We identified only one study, which measured muscle tissue using CT – in this case mid-thigh slices (4). Use of CT imaging allows quantification of low-density lean tissue, which reflects fat content in and around muscle fibres (5). In this study, a significantly greater amount of low-density lean tissue was found in the paretic versus non-paretic leg. Low density lean tissue has been related to insulin resistance in healthy men and older women (5, 6) but no studies to date have investigated the impact of increased low density lean tissue of the thigh in stroke survivors on health.
While DEXA is the most commonly used modality to measure whole body and regional fat mass, it has some limitations, including its reliance on estimations of fat mass based on a two-compartment body composition model of fat and fat-free tissue, being less accurate than CT or MRI measures, and not being able to differentiate between fat stored intramuscularly or subcutaneously.
There is some evidence that aerobic exercise can reverse some of the negative changes in body composition after stroke. A randomised controlled trial of a four week program of robot-assisted treadmill walking demonstrated a significant increase in muscle mass and decrease in fat mass in the intervention group, but not the control group (25). Others have shown that programs of treadmill training improve leg strength (26), cardiovascular fitness (27) and walking ability (28). It is therefore logical to assume that encouraging physical activity very early after stroke may also help ameliorate changes in body composition, but there is as yet no evidence to support this hypothesis.
There is a need for more longitudinal trials involving frequent and accurate measures of body composition starting within the first few weeks after stroke. Such trials should also examine the interactions between stroke severity, physical activity levels and fitness on changes in body composition. Links between changes in body composition and the metabolic regulation of glucose and how this may impact on risk of future stroke could also be examined. Information gleaned from such trials will inform the development of interventions to ameliorate negative body composition changes.
Acknowledgments
Acknowledgements and funding
The authors would like to acknowledge the following people who contributed raw data for the meta-analysis; Dr Sandra A Billinger, Dr Gillian Mead and Associate Professor Berna Celik.
Dr English is supported by a National Health and Medical Research Council Research Training Fellowship (#610312). Dr. Ryan was supported by a VA Research Career Scientist Award, National Institute on Aging (NIA) grants RO1-AG030075, and Claude D. Pepper Older Americans Independence Center P30-AG028747.
Appendix 1
Full search strategy for Ovid databases
| Search terms | Field | Limits |
|---|---|---|
| (stroke or brain injur$ or hemip$ or cerebrovascular accident$ or cerebrovascular disorder$) |
Title | Human |
| AND | English language |
|
| (muscle$ or muscul$ or cross-sectional area or muscle thickness or lean mass or fat or intramuscular or adipos$ or body composition$ or anthropometry$) |
Abstract | |
| NOT | ||
| (cardi$ or heart or swim$ or angina or cerebral palsy or child$) |
Abstract |
Footnotes
Conflict of interest: None declared.
References
- 1.English C, McLennan H, Thoirs K, Coates A, Bernhardt J. Loss of skeletal muscle mass after stroke: a systematic review. Int J Stroke. 2010;5:395–402. doi: 10.1111/j.1747-4949.2010.00467.x. [DOI] [PubMed] [Google Scholar]
- 2.Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543–559. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scelsi R, Lotta S, Lommi G, Poggi P, Marchetti C. Hemiplegic atrophy. Morphological findings in the anterior tibial muscle of patients with cerebral vascular accidents. Acta Neuropathol. 1984;62:324–331. doi: 10.1007/BF00687615. [DOI] [PubMed] [Google Scholar]
- 4.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83:1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 5.Ryan AS, Nicklas BJ, Berman DM. Aerobic exercise is necessary to improve glucose utilization with moderate weight loss in women. Obesity. 2006;14:1064–1072. doi: 10.1038/oby.2006.122. [DOI] [PubMed] [Google Scholar]
- 6.Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord. 1999;23(2):126–132. doi: 10.1038/sj.ijo.0800777. [DOI] [PubMed] [Google Scholar]
- 7.Ryan A, Dobrovolny C, Silver K, Smith G, Macko R. Cardiovascular fitness after stroke: Role of muscle mass and gait deficit severity. Jnl Stroke and Cerebrovasc Dis. 2000;9(4):185–191. doi: 10.1053/jscd.2000.7237. [DOI] [PubMed] [Google Scholar]
- 8.Ivey FM, Ryan AS, Hafer-Macko CE, Garrity BM, Sorkin JD, Goldberg AP, Macko RF. High prevalence of abnormal glucose metabolism and poor sensitivity of fasting plasma glucose in the chronic phase of stroke. Cerebrovasc Dis. 2006;22(5–6):368–371. doi: 10.1159/000094853. [DOI] [PubMed] [Google Scholar]
- 9.Vermeer SE, Sandee W, Algra A, Koudstaal PJ, Kappelle LJ, Dippel DW. Impaired glucose tolerance increases stroke risk in nondiabetic patients with transient ischemic attack or minor ischemic stroke. Stroke. 2006;37(6):1413–1417. doi: 10.1161/01.STR.0000221766.73692.0b. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Deeks JJ, Altman DG, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration. 2008. Chapter 16: Special topics in statistics. Available from www.cochrane-handbook.org. [Google Scholar]
- 11.Billinger SA, Guo LX, Pohl PS, Kluding PM. Single limb exercise: pilot study of physiological and functional responses to forced use of the hemiparetic lower extremity. Top Stroke Rehabil. 2010;17(2):128–139. doi: 10.1310/tsr1702-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celik B, Ones K, Ince N. (2008) Body composition after stroke. Int J Rehabil Res. 2008;31(1):93–96. doi: 10.1097/MRR.0b013e3282f7521a. [DOI] [PubMed] [Google Scholar]
- 13.Pang MY, Eng JJ. Muscle strength is a determinant of bone mineral content in the hemiparetic upper extremity: implications for stroke rehabilitation. Bone. 2005;37(1):103–111. doi: 10.1016/j.bone.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang MY, Eng JJ, McKay HA, Dawson AS. Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporos Int. 2005;16(12):1769–1779. doi: 10.1007/s00198-005-1925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carin-Levy G, Greig C, Young A, Lewis S, Hannan J, Mead G. Longitudinal changes in muscle strength and mass after acute stroke. Cerebrovasc Dis. 2006;21(3):201–207. doi: 10.1159/000090792. [DOI] [PubMed] [Google Scholar]
- 16.Iversen E, Hassager C, Christiansen C. The effect of hemiplegia on bone mass and soft tissue body composition. Acta Neurol Scand. 1989;79(2):155–159. doi: 10.1111/j.1600-0404.1989.tb03729.x. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen L, Jacobsen BK. Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: a 1 year prospective study. Bone. 2001;28(6):655–659. doi: 10.1016/s8756-3282(01)00434-3. [DOI] [PubMed] [Google Scholar]
- 18.Lazoura O, Papadaki PJ, Antoniadou E, Groumas N, Papadimitriou A, Thriskos P, Fezoulidis IV, Vlychou M. Skeletal and body composition changes in hemiplegic patients. J Clin Densitom. 2010;13(2):175–180. doi: 10.1016/j.jocd.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Okabe R, Inaba M, Sakai S, Ishimura E, Moriguchi A, Shoji T, Nishizawa Y. Increased arterial stiffening and thickening in the paretic lower limb in patients with hemiparesis. Clin Sci. 2004;106(6):613–618. doi: 10.1042/CS20030387. [DOI] [PubMed] [Google Scholar]
- 20.Ramnemark A, Nyberg L, Lorentzon R, Olsson T, Gustafson Y. Hemiosteoporosis after severe stroke, independent of changes in body composition and weight. Stroke. 1999;30(4):755–760. doi: 10.1161/01.str.30.4.755. [DOI] [PubMed] [Google Scholar]
- 21.Hafer-Macko CE, Ryan AS, Ivey FM, Macko RF. Skeletal muscle changes after hemiparetic stroke and potential beneficial effects of exercise intervention strategies. J Rehabil Res Dev. 2008;45(2):261–272. doi: 10.1682/jrrd.2007.02.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CE, McArdle A, Griffiths RD. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr. 2007;26(5):524–534. doi: 10.1016/j.clnu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke. 2004;35(4):1005–1009. doi: 10.1161/01.STR.0000120727.40792.40. [DOI] [PubMed] [Google Scholar]
- 24.Michael K, Allen J, Macko R. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86:1552–1556. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Husemann B, Muller F, Krewer C, Heller S, Koenig E. Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after stroke: a randomized controlled pilot study. Stroke. 2007;38(2):349–354. doi: 10.1161/01.STR.0000254607.48765.cb. [DOI] [PubMed] [Google Scholar]
- 26.Smith GV, Silver KH, Goldberg AP, Macko RF. "Task-oriented" exercise improves hamstring strength and spastic reflexes in chronic stroke patients. Stroke. 1999;30(10):2112–2118. doi: 10.1161/01.str.30.10.2112. [DOI] [PubMed] [Google Scholar]
- 27.Macko RF, Ivey FM, Forrester LW. Task-oriented aerobic exercise in chronic hemiparetic stroke: training protocols and treatment effects. Top Stroke Rehabil. 2005;12(1):45–57. doi: 10.1310/PJQN-KAN9-TTVY-HYQH. [DOI] [PubMed] [Google Scholar]
- 28.Silver KH, Macko RF, Forrester LW, Goldberg AP, Smith GV. Effects of aerobic treadmill training on gait velocity, cadence, and gait symmetry in chronic hemiparetic stroke: a preliminary report. Neurorehabil Neural Repair. 2000;14(1):65–71. doi: 10.1177/154596830001400108. [DOI] [PubMed] [Google Scholar]