Abstract
Background
Late-onset sepsis is an important cause of morbidity and mortality in infants. Diagnosis of late-onset sepsis can be challenging. The complete blood cell count and differential have been previously evaluated as diagnostic tools for late-onset sepsis in small, single-center reports.
Objective
We evaluated the diagnostic accuracy of the complete blood count and differential in late-onset sepsis in a large multicenter population.
Study design
Using a cohort of all infants with cultures and complete blood cell count data from a large administrative database, we calculated odds ratios for infection, as well as sensitivity, specificity, positive and negative predictive values, and likelihood ratios for various commonly used cut-off values.
Results
High and low white blood cell counts, high absolute neutrophil counts, high immature-to-total neutrophil ratios, and low platelet counts were associated with late-onset sepsis. Associations were weaker with increasing postnatal age at the time of the culture. Specificity was highest for white blood cell counts <1000/mm3 and >50,000/mm3 (>99%). Positive likelihood ratios were highest for white blood cell counts <1000/mm3 (4.1) and platelet counts <50,000/mm3 (3.5).
Conclusion
No complete blood count index possessed adequate sensitivity to reliably rule out late-onset sepsis in this population.
Keywords: neonatal, late-onset sepsis, blood cell count
Late-onset sepsis (LOS) is common in infants admitted to neonatal intensive care units (NICUs).1,2 Mortality from LOS is high and ranges from 7% in near-term infants to 39% in preterm infants infected with Gram-negative organisms.3,4 In addition, there is significant morbidity among survivors, including poor neurodevelopmental outcomes.5
Unfortunately, the clinical presentation of LOS in infants is often subtle.3 The usefulness of blood cultures, the diagnostic standard, is limited by the time needed to isolate the organism and by difficulties in obtaining adequate blood volume to reliably isolate organisms.6,7 The complete blood cell (CBC) count is a rapid, inexpensive, and widely available diagnostic test. In a survey of NICU providers, 99% obtained a CBC count when evaluating an infant for sepsis.8 However, the diagnostic accuracy of the CBC count is not well-defined in this setting and may be affected by physiologic changes, especially in very-low-birth-weight (<1500 g) infants.9 Studies evaluating the usefulness of the CBC count have yielded conflicting results (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/B170, which lists previously published studies of CBC count indices in LOS) but were limited by sample size. We sought to determine the diagnostic utility of the CBC count for diagnosis of LOS in infants admitted to the NICU using a large, multicenter, contemporary database.
MATERIALS AND METHODS
Patient Population
We obtained CBC counts and culture data from infants admitted to 293 NICUs in the United States managed by the Pediatrix Medical Group from 1996–2009. The data were obtained from an administrative database that prospectively captures information from daily progress notes generated by clinicians on all neonates cared for by the Pediatrix Medical Group. Data on multiple aspects of care are entered into the system to generate admission notes, daily progress notes, procedure notes, and discharge summaries. Information is collected regarding maternal history and demographics, medications, laboratory results, culture results, diagnoses, and other aspects of clinical care. All infants who had cultures obtained during their hospitalization between days of life (DOL) 4 and 120 were included in the analysis. Infants without a culture between DOL 4 and 120 were excluded. Sepsis episodes for organisms considered contaminants were excluded.
Definitions
LOS was defined as a positive culture (blood, urine collected by catheterization or suprapubic tap, or cerebrospinal fluid [CSF]) between DOL 4 and 120. When multiple cultures with the same organism were obtained within a 21-day period, they were considered to be a single infectious episode. Cultures positive with >1 organism were treated as separate positive cultures. All negative cultures were included in the analysis, regardless of the presence of a prior positive culture. If one of the organisms was considered a contaminant, we counted only the true organism as a positive culture and ignored the likely contaminant.
Coagulase-negative Staphylococcus (CoNS) infections were divided into 3 categories: definite, probable, and possible. We defined a definite CoNS infection as 2 positive cultures drawn on the same day; probable CoNS infection as 2 positive cultures within a 4-day period, 3 positive cultures within a 7-day period, or 4 positive cultures within a 10-day period; and possible CoNS infection as a culture positive for CoNS that did not meet criteria for definite or probable CoNS sepsis. We included definite and probable CoNS infections in our analysis. We excluded all negative cultures obtained after a positive culture in the same patient, given the likelihood of a false-negative result. If multiple cultures were obtained on the same day, we kept 1 entry in order of preference: positive culture over negative culture and blood culture over urine culture over CSF culture.
We collected demographic data including sex, race, birth weight, gestational age (GA), inborn status, and Apgar score at 5 minutes. If multiple CBC counts were obtained on the same day for a patient, we included the highest value for white blood cell (WBC) count, absolute neutrophil count (ANC), immature-to-total neutrophil ratio (I/T ratio), and platelet counts. We included CBC counts obtained on the day of culture or on the day prior to culture if no CBC count was obtained on the day of culture. Mean CBC count indices were compared between infants with positive and negative cultures using t-tests with P<0.05 considered statistically significant.
Statistical analysis
The association of CBC count indices (WBC count, ANC, I/T ratio, and platelet count) with culture results was evaluated using logistic regression with and without controlling for GA as an independent variable. For each regression, we divided the WBC counts, ANC, I/T ratio, and platelet counts into 5% increments (vintiles). We obtained odds ratios (OR) for each vintile compared with the median vintile. Given the wide range of postnatal age at the time of culture, we divided our cohort into cultures obtained during the first week of life (DOL 4–7), the first month of life (DOL 8–30), and beyond the first month of life (DOL>30) and repeated the logistic regressions. Finally, we repeated the logistic regression on a subset of the data including only blood cultures.
We calculated sensitivities, specificities, positive and negative predictive values, and positive and negative likelihood ratios (+LR and –LR) for previously defined diagnostic cut-off values of WBC counts, ANC, I/T ratio, and platelet counts.10,11 We determined the area under the receiver operating characteristics (ROC) curve for WBC counts, ANC, I/T ratio, and platelet counts. For WBC counts, we calculated ROC curves separately for WBC counts >15,000/mm3 and WBC counts <15,000/mm3. We multiplied values for ANC, WBC counts <15,000/mm3, and platelet counts by (–1) to generate positive ROC curves. We repeated curves by GA category as well as by postnatal age at the time of culture. We conducted the analysis with STATA 10 (College Station, TX). This study was approved by the Duke Institutional Review Board.
RESULTS
We identified 37,826 infants with 69,854 cultures: 63,160 (90.4%) blood cultures, 2525 (3.6%) urine cultures, and 4169 (6.0%) CSF cultures. The mean GA of all infants was 29.6 weeks (5th, 95th percentile: 24.0, 39.0), and the mean birth weight was 1439 g (560, 3459) (see Table, Supplemental Digital Content 2, http://links.lww.com/INF/B171, which shows demographics). Of all cultures, 16,527 (23.7%) were obtained between DOL 4 and 7, 37,791 (54.1%) were obtained between DOL 8 and 30, and 15,536 (22.2%) were obtained after DOL 30. There were 9656 (13.8%) positive cultures in 7951 infants (21.0%). Of the 9656 positive cultures, 7783 (80.6%) were from blood, 1718 (17.8%) from urine, and 155 (1.6%) from CSF (see Table, Supplemental Digital Content 3, http://links.lww.com/INF/B172, which shows organisms by culture site). The following cultures were excluded from the analysis as their organisms were considered to be contaminants: non-speciated streptococci (n=150), Bacillus sp. (n=81), Corynebacterium sp. (n=18), diphtheroids sp. (n=9), Gram-positive rods (not including Listeria sp.) (n=24), Lactobacillus sp. (n=2), Micrococcus sp. (n=21), Stomatococcus sp. (n=3), and Bacteroides sp. (n=3).
The mean WBC count was 15,287/mm3 (5th, 95th percentile: 4200/mm3, 33,800/mm3) and 15,214/mm3 (5400/mm3, 32,400/mm3) for positive and negative culture results, respectively (P=0.48) (Table 1). The mean ANC was 9420/mm3 (1504/mm3, 24,510/mm3) for positive cultures and 8582/mm3 (1584/mm3, 24,510/mm3) for negative cultures (P<0.01). The mean I/T ratios were 0.26 (0.03, 0.67) for positive cultures and 0.20 (0.02, 0.57) for negative cultures (P<0.01). The mean platelet count for positive cultures was 222,510/mm3 (40,000/mm3, 504,000/mm3) and for negative cultures was 273,700/mm3 (70,000/mm3, 550,000/mm3) (P<0.01). For infants with positive cultures and values for all CBC indices, 14.4% (1219/8472) were noted to have completely “normal” CBC indices (WBC count 5000/mm3–19,000/mm3, ANC ≥1500/mm3, I/T ratio <0.2, and platelet count 150,000/mm3–399,999/mm3).
TABLE 1.
Complete Blood Cell Count Indices According to Culture Results
| Positive (n=9834) | Negative (n=62,702) | |
|---|---|---|
| WBC count, /mm3 | ||
| <5000 | 7% | 4% |
| 5000–19,999 | 70% | 76% |
| 20,000–39,999 | 20% | 18% |
| ≥40,000 | 3% | 2% |
| Platelet count, /mm3 | ||
| <50,000 | 8% | 2% |
| 50,000–99,999 | 15% | 9% |
| 100,000–149,999 | 15% | 12% |
| 150,000–399,999 | 50% | 58% |
| ≥400,000 | 12% | 19% |
| ANC, /mm3 | ||
| <500 | <1% | <1% |
| 500–1499 | 4% | 4% |
| ≥1500 | 95% | 96% |
| I/T ratio | ||
| <0.20 | 46% | 62% |
| 0.20–0.24 | 10% | 8% |
| 0.25–0.29 | 8% | 7% |
| ≥0.30 | 36% | 23% |
WBC indicates white blood cell; ANC, absolute neutrophil count; I/T, immature-to-total neutrophils.
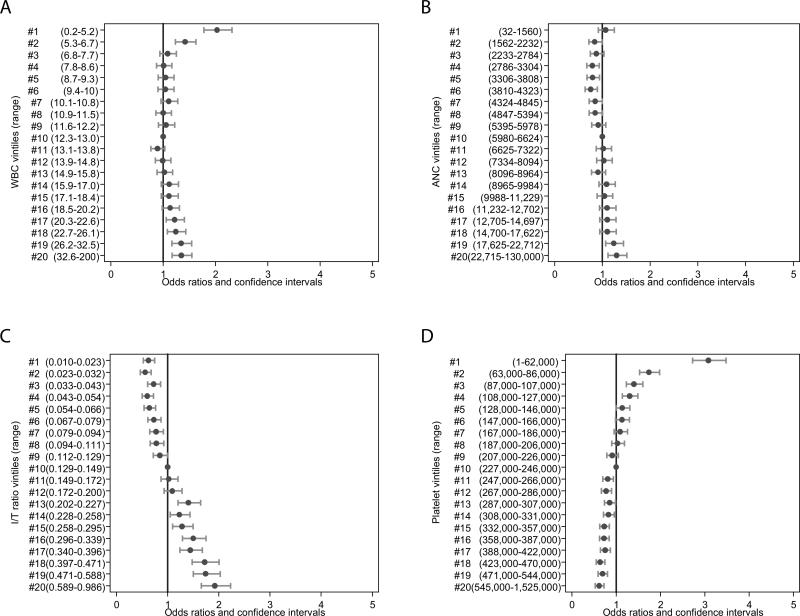
High WBC counts were associated with statistically significant ORs for infection observed from the seventeenth to the twentieth vintile, corresponding to a WBC count >20,300/mm3 (Figure 1a). Low WBC counts <6800/mm3 (first and second vintile) were also associated with increasing odds of infection (Figure 1a). ANCs >17,670/mm3 (nineteenth and twentieth vintiles) were associated with increasing odds of infection (Figure 1b), while low ANCs <4865/mm3 (≤7th vintile) were associated with decreased odds of infection. An I/T ratio of ≤0.11 (≤9th vintile) was associated with decreased odds of infection. High I/T ratios >0.2 (≥13th vintile) were associated with progressively increasing odds of infection (Figure 1c). Platelet counts above the ninth vintile (≥228,000/mm3) were associated with decreased odds of infection, while lower platelet counts ≤130,000/mm3 (≤5th vintile) were associated with increasing odds of infection (Figure 1d). These associations remained unchanged when the analysis was repeated on a subset of the data that included only blood cultures.
FIGURE 1.
Association between complete blood cell count indices and late-onset sepsis (>72 hours). A. WBC vintiles and ORs; B. ANC vintiles and ORs; C. I/T ratio vintiles and ORs; D. Platelet vintiles and ORs. WBC indicates white blood cell; ANC, absolute neutrophil count; I/T ratio, immature-to-total neutrophil ratio; OR, odds ratio.
Controlling for GA, we observed similar results. For WBC counts, only the nineteenth and twentieth vintiles and the first and second vintiles were associated with increasing odds of infection. For ANC, lower vintiles (4th–6th vintile) were associated with lower odds of infection. Platelet counts below the fourth vintile (<127,000/mm3) were associated with increased odds of infection. The results for the I/T ratio remained unchanged.
Limiting the regressions to cultures obtained on DOL 4–7, we observed no increase in the odds of infection with high WBC counts or low ANC. Increased odds of infection were observed only for platelet counts <89,000/mm3 and high I/T ratio (>0.36). Between DOL 8 and 30, results were similar to those over the entire study period (DOL 4–120). For cultures obtained after DOL 30, we observed increased odds of infection with high WBC counts only at the twentieth vintile (WBC >27,100/mm3, OR 1.66 [5th, 95th percentile: 1.28, 2.14]), increased odds of infection for high I/T ratio only above the fifteenth vintile (I/T ratio >0.26), and no evidence of higher odds of infection with low ANC.
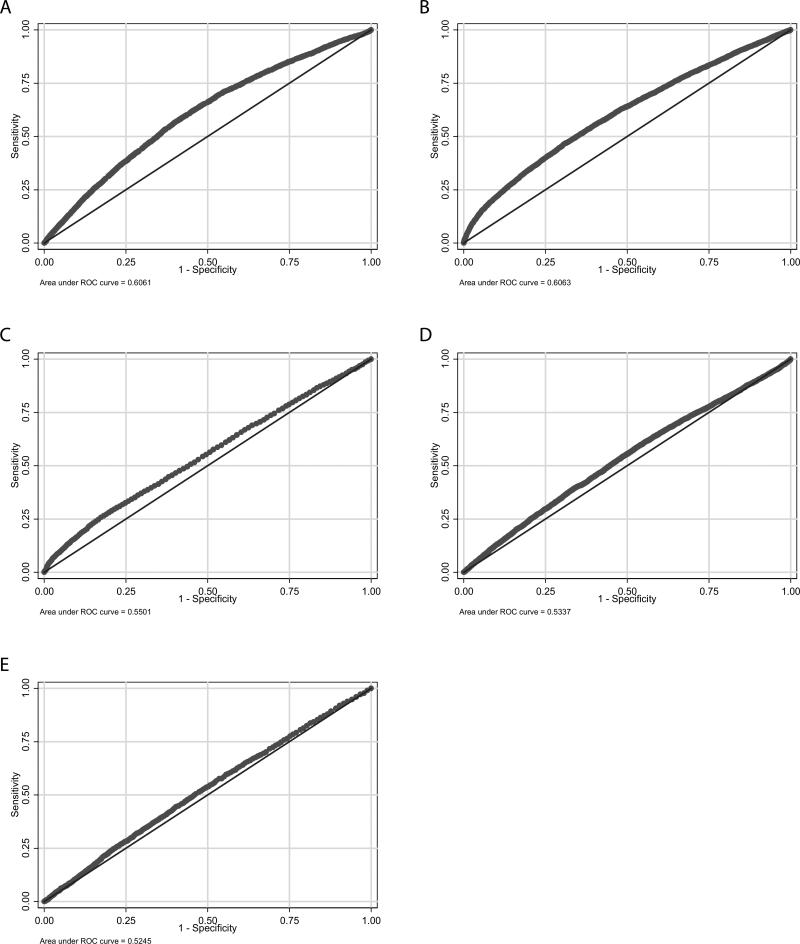
Test characteristics for commonly used CBC index cut-offs were evaluated for the entire cohort and by GA categories (see Table, Supplemental Digital Content 4, http://links.lww.com/INF/B173, which shows test characteristics of various WBC, ANC, I/T ratio, and platelet count values). For infants <34 weeks GA, sensitivity was low (<55%) for all indices analyzed, while specificity was high (>95%) for multiple values. For infants 34–36 weeks GA, all CBC index cut-offs had similarly poor sensitivity. Specificity was again highest for low and high WBC counts, low ANCs, and low platelet counts. Sensitivity remained poor for infants >36 weeks GA for all CBC index cut-offs analyzed, while specificity was high for multiple values. The greatest area under the ROC curve (AUC) was observed for I/T ratios (AUC=0.606) (Figure 2a) and platelet count (AUC=0.606) (Figure 2b). Areas under the ROC curve for WBC counts <15,000/mm3 (Figure 2c) and ANC (Figure 2d) were similar (AUC=0.550 and AUC=0.534, respectively). The smallest area under the ROC curve were seen for WBC counts >15,000/mm3 (AUC=0.525) (Figure 2e). Areas under the ROC curves were similar for cultures obtained on DOL 4–7, DOL 8–30, and DOL >30 and for different GA categories (Table 2).
FIGURE 2.
Receiver operating characteristic (ROC) curves for complete blood cell count indices. A. I/T ratio (AUC=0.606); B. Platelet count (AUC=0.606); C. WBC count <15,000/mm3 (AUC=0.550); D. ANC (AUC=0.534); E. WBC count >15,000/mm3 (AUC=0.525). WBC indicates white blood cell; ANC, absolute neutrophil count; I/T ratio, immature-to-total neutrophil ratio; AUC, area under the curve.
TABLE 2.
Area Under the Receiver Operating Characteristics Curve for Various Complete Blood Cell Count Indices by Day of Life and Gestational Age
| All | DOL 4–7 | DOL 8–30 | DOL >30 | GA <34 weeks | GA 34–36 weeks | GA >36 weeks | |
|---|---|---|---|---|---|---|---|
| WBC count <15,000/mm3 | 0.550 | 0.533 | 0.578 | 0.568 | 0.551 | 0.540 | 0.545 |
| WBC count >15,000/mm3 | 0.525 | 0.487 | 0.535 | 0.540 | 0.523 | 0.512 | 0.513 |
| ANC | 0.534 | 0.503 | 0.536 | 0.537 | 0.529 | 0.551 | 0.545 |
| I/T ratio | 0.606 | 0.656 | 0.598 | 0.588 | 0.599 | 0.628 | 0.594 |
| Platelet count | 0.606 | 0.592 | 0.614 | 0.638 | 0.616 | 0.577 | 0.484 |
DOL indicates day of life; GA, gestational age; WBC, white blood cell; ANC, absolute neutrophil count; I/T, immature-to-total neutrophils.
DISCUSSION
In the present study, we report the largest series of CBC counts in infants with LOS admitted to the NICU. Although we observed increased odds of infection for high (>20,300/mm3) and low WBC count (<6800/mm3), high ANC (>17,670/mm3), high I/T ratio (>0.2), and low platelet count (<130,000/mm3), none of these CBC index cut-offs were associated high sensitivity or +LRs.
The high proportion of infants with normal CBC count indices suffering from LOS may be related to the heterogeneity of our cohort. GA can affect CBC index responses as a consequence of maternal risk factors and non-infectious comorbidities that are similar to those seen with bacteremia.12 More specifically, very-low-birth-weight infants are known to develop physiologic late-onset (≥72 hours of life) neutropenia, which does not correlate with sepsis.9 The pathogenesis of organisms responsible for LOS may also play a role. Elevated I/T ratios were significantly more common in Gram-negative versus Gram-positive infections.13,14 In our cohort, only 28% of cultured organisms were Gram-negative, possibly accounting for our high observed proportion of normal I/T ratios.
Our ORs and test characteristic results are similar to those of previously published, smaller studies evaluating the WBC count,15,16 ANC,13,15 I/T ratio,14,16 and platelet count.15,17 While other studies have failed to demonstrate increased odds of infection for abnormal WBC counts18 and have shown higher sensitivity for diagnosing LOS,17 these studies had smaller sample sizes (<2416 subjects), defined a lower WBC count cut-off as abnormal (>9000/mm3), and had a higher prevalence of LOS (23.7%). When examining the predictive value of the platelet count for specific organisms only, we found that the odds of candidemia were increased (OR 5.17 [5th, 95th percentile: 4.52, 5.92]) for platelet counts below 100,000/mm3, similar to previously published results (OR 3.56 [5th, 95th percentile: 2.68, 4.74]).19 The odds of CoNS infection were also increased (OR 1.99 [5th , 95th percentile: 1.79, 2.21]), though not as significantly as previously published results, possibly due the different definition of CoNS bacteremia used in that study.20 Lastly, the odds of a Gram-negative rod infection were also increased when the platelet count was below 100,000/mm3 (OR 2.32 [5th, 95th percentile: 2.12, 2.53]). The mean platelet count in Gram-negative rod infections was higher in our cohort (230,000/mm3) compared with previously published results, though this may be related to the patient population that consisted of very-low-birth-weight infants only compared with all infants in our study.21 However, none of the studies of CBC count indices have demonstrated sensitivities that approach those of serum biomarkers such as procalcitonin (>80% in certain studies).22,23
Analysis of our data by postnatal age yielded slightly different results compared with the overall cohort. Given that 54.1% of all cultures were obtained between DOL 8 and 30, results of these regressions were similar to those of the overall cohort. For cultures obtained after DOL 30, only high WBC counts (>27,100/mm3) and high I/T ratios (>0.27) were associated with increased odds of infections, but all ORs were relatively low. This may be comparable to the known poor reliability of the CBC count in diagnosing sepsis in children <3 months of age in an ambulatory setting.24
The strengths of our study include the large cohort size, detailed data on CBC count indices, and complete culture results. In addition, we report results on previously defined CBC count index cut-offs commonly used in clinical practice. This study was limited by the lack information on previous antibiotic exposure (antepartum and empirical). Our description of the urine culture results was limited by the absence of information on colony count. In instances where >1 CBC count was obtained on the same day, we retained only the highest values of CBC count indices for the analysis as we did not have information on time of day in the data. It is important to highlight that our study was not designed to address the issue of using CBC count indices to determine duration of antimicrobial therapy after initiation. We did not differentiate a negative culture obtained on antibiotic therapy from one obtained when the infant was not on antibiotics. We therefore were unable to address the question of whether certain CBC count indices obtained after initiation of antimicrobial can be used to predict the need for continuation of treatment. Lastly, this sample represents only infants admitted to a NICU rather than the population of all infants evaluated with a CBC count.
In summary, a high proportion of infants with LOS have “normal” CBC count indices. We found that high and low WBC counts, high ANCs, high I/T ratios, and high platelet counts were associated with increased odds of LOS. These findings differed only slightly when analyzing CBC index responses at different postnatal and gestational ages. Sensitivity and +LRs were low for all index cut-offs analyzed, while specificity was generally high. Overall, no CBC count index can reliably rule out LOS in infants. Therefore, cultures of blood, urine, and CSF should be obtained and empiric antibiotics initiated without delay in infants with suspected LOS.
Supplementary Material
Acknowledgments
Source of Funding: Dr. Benjamin Jr. receives support from the United States government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C) and the nonprofit organization Thrasher Research Foundation for his work in neonatal candidiasis (www.thrasherresearch.org); he also receives research support from Astellas Pharma US, AstraZeneca, Johnson & Johnson, Pfizer, Biosynexus, and UCB Pharma; consulting fees from Cerexa and Astellas Pharma US.; and other support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr. Smith received receives salary support for research from the NIH and the U.S. Department of Health and Human Services (NICHD 1K23HD060040-01 and DHHS-1R18AE000028-01). Dr. Cohen-Wolkowiez received support from NICHD 1K23HD064814-01.This study used CTSA biostatistical services through the Division of Pediatric Quantitative Sciences (NIH-5UL-1RR024128-01).
Footnotes
Conflicts of Interest Christoph P. Hornik, Daniel K. Benjamin, Jennifer Li, Kristian C. Becker, and Reese H. Clark have no relevant conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121:689–696. doi: 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- 2.van den Hoogen A, Gerards LJ, Verboon-Maciolek MA, Fleer A, Krediet TG. Long-term trends in the epidemiology of neonatal sepsis and antibiotic susceptibility of causative agents. Neonatology. 2010;97:22–28. doi: 10.1159/000226604. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very-low-birth-weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Wolkowiez M, Moran C, Benjamin DK, et al. Early- and late-onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052–1056. doi: 10.1097/inf.0b013e3181acf6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 6.Ng PC. Diagnostic markers of infection in neonates. Arch Dis Child Fetal Neonatal Ed. 2004;89:F229–235. doi: 10.1136/adc.2002.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell TG, Rele M, Cowley D, Buttery JP, Curtis N. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children's hospital. Pediatrics. 2007;119:891–896. doi: 10.1542/peds.2006-0440. [DOI] [PubMed] [Google Scholar]
- 8.Rubin LG, Sanchez PJ, Siegel J, Levine G, Saiman L, Jarvis WR. Evaluation and treatment of neonates with suspected late-onset sepsis: a survey of neonatologists’ practices. Pediatrics. 2002;110:e42. doi: 10.1542/peds.110.4.e42. [DOI] [PubMed] [Google Scholar]
- 9.Omar SA, Salhadar A, Wooliever DE, Alsgaard PK. Late-onset neutropenia in very-low-birth-weight infants. Pediatrics. 2000;106:E55. doi: 10.1542/peds.106.4.e55. [DOI] [PubMed] [Google Scholar]
- 10.Ottolini MC, Lundgren K, Mirkinson LJ, Cason S, Ottolini MG. Utility of complete blood count and blood culture screening to diagnose neonatal sepsis in the asymptomatic at risk newborn. Pediatr Infect Dis J. 2003;22:430–434. doi: 10.1097/01.inf.0000068206.11303.dd. [DOI] [PubMed] [Google Scholar]
- 11.Escobar GJ, Li DK, Armstrong MA, et al. Neonatal sepsis workups in infants ≥2000 grams at birth: a population-based study. Pediatrics. 2000;106:256–263. doi: 10.1542/peds.106.2.256. [DOI] [PubMed] [Google Scholar]
- 12.Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J Pediatr. 1979;95:89–98. doi: 10.1016/s0022-3476(79)80096-7. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar S, Bhagat I, Hieber S, Donn SM. Can neutrophil responses in very-low-birth-weight infants predict the organisms responsible for late-onset bacterial or fungal sepsis? J Perinatol. 2006;26:501–505. doi: 10.1038/sj.jp.7211554. [DOI] [PubMed] [Google Scholar]
- 14.Pauli I, Jr., Shekhawat P, Kehl S, Sasidharan P. Early detection of bacteremia in the neonatal intensive care unit using the new BACTEC system. J Perinatol. 1999;19:127–131. doi: 10.1038/sj.jp.7200124. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez BE, Mercado CK, Johnson L, Brodsky NL, Bhandari V. Early markers of late-onset sepsis in premature neonates: clinical, hematological and cytokine profile. J Perinat Med. 2003;31:60–68. doi: 10.1515/JPM.2003.009. [DOI] [PubMed] [Google Scholar]
- 16.Fanaroff AA, Korones SB, Wright LL, et al. Incidence, presenting features, risk factors and significance of late-onset septicemia in very-low-birth-weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Infect Dis J. 1998;17:593–598. doi: 10.1097/00006454-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Berger C, Uehlinger J, Ghelfi D, Blau N, Fanconi S. Comparison of C-reactive protein and white blood cell count with differential in neonates at risk for septicaemia. Eur J Pediatr. 1995;154:138–144. doi: 10.1007/BF01991918. [DOI] [PubMed] [Google Scholar]
- 18.Makhoul IR, Yacoub A, Smolkin T, Sujov P, Kassis I, Sprecher H. Values of C-reactive protein, procalcitonin, and Staphylococcus-specific PCR in neonatal late-onset sepsis. Acta Paediatr. 2006;95:1218–1223. doi: 10.1080/08035250600554250. [DOI] [PubMed] [Google Scholar]
- 19.Benjamin DK, Jr, DeLong ER, Steinbach WJ, et al. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics. 2003;112(3 Pt 1):543–547. doi: 10.1542/peds.112.3.543. [DOI] [PubMed] [Google Scholar]
- 20.Khashu M, Osiovich H, Henry D, et al. Persistent bacteremia and severe thrombocytopenia caused by coagulase-negative Staphylococcus in a neonatal intensive care unit. Pediatrics. 2006;117:340–348. doi: 10.1542/peds.2005-0333. [DOI] [PubMed] [Google Scholar]
- 21.Guida JD, Kunig AM, Leef KH, McKenzie SE, Paul DA. Platelet count and sepsis in very low birth weight neonates: is there an organism-specific response? Pediatrics. 2003;111(6 Pt 1):1411–1415. doi: 10.1542/peds.111.6.1411. [DOI] [PubMed] [Google Scholar]
- 22.Isidor B, Caillaux G, Gilquin V, et al. The use of procalcitonin in the diagnosis of late-onset infection in neonatal intensive care unit patients. Scand J Infect Dis. 2007;39:1063–1066. doi: 10.1080/00365540701466181. [DOI] [PubMed] [Google Scholar]
- 23.Boo NY, Nor Azlina AA, Rohana J. Usefulness of a semi-quantitative procalcitonin test kit for early diagnosis of neonatal sepsis. Singapore Med J. 2008;49:204–208. [PubMed] [Google Scholar]
- 24.Bachur RG, Harper MB. Predictive model for serious bacterial infections among infants younger than 3 months of age. Pediatrics. 2001;108:311–316. doi: 10.1542/peds.108.2.311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.