Abstract
The selective 5-HT1 receptor agonist sumatriptan is an effective therapeutic for migraine pain yet the antimigraine mechanisms of action remain controversial. Pain-responsive fibres containing calcitonin gene-related peptide (CGRP) densely innervating the cranial dura mater are widely believed to be an essential anatomical substrate for the development of migraine pain. 5HT1 receptors in the dura colocalize with CGRP fibres in high density and thus provide a possible peripheral site of action for sumatriptan. In the present study, we used high-resolution optical imaging selectively within individual mouse dural CGRP nociceptive fibre terminations and found that application of sumatriptan caused a rapid, reversible dose-dependent inhibition in the amplitude of single action potential evoked Ca2+ transients. Pre-application of the 5-HT1 antagonist GR127935 or the selective 5-HT1D antagonist BRL 15572 prevented inhibition while the selective 5-HT1B antagonist SB 224289 did not, suggesting this effect was mediated selectively through the 5-HT1D receptor subtype. Sumatriptan inhibition of the action potential evoked Ca2+ signaling was mediated selectively through N-type Ca2+ channels. Although the T-type Ca2+ channel accounted for a greater proportion of the Ca2+ signal it did not mediate any of the sumatriptan inhibition. Our findings support a peripheral site of action for sumatriptan in inhibiting the activity of dural pain fibres selectively through a single Ca2+ channel subtype. This finding adds to our understanding of the mechanisms that underlie the clinical effectiveness of 5HT1 receptor agonists such as sumatriptan and may provide insight for the development of novel peripherally targeted therapeutics for mitigating the pain of migraine.
Keywords: migraine, Ca2+ signaling, pain, imaging, 5-HT1, dura
1.INTRODUCTION
The serotonin 5-HT1 receptor agonist sumatriptan and the other so-called triptans are selective and effective therapies for the acute treatment of migraine pain (Bigal et al., 2009; Moskowitz and Cutrer, 1993; Sprenger and Goadsby, 2009; Tfelt-Hansen et al., 2000; Tfelt-Hansen and Koehler, 2011). However, despite the success of the triptans in the clinical setting, the anatomical locus of their antimigraine activity remains unresolved, and both peripheral and central nervous system sites of action are likely (Ahn and Basbaum, 2005; Akerman et al., 2011; Bartsch et al., 2004; Durham and Russo, 2002; Humphrey and Feniuk, 1991; Lambert, 2010; Levy et al., 2004; Mehrotra et al., 2008; Tfelt-Hansen, 2010). Beyond providing insight into migraine neuropathology, understanding the anatomical locus of their action may lead to the development of novel therapeutics to better manage the disorder, and so continues to be an area of intense investigation.
The neuropeptide calcitonin gene-related peptide (CGRP) is hypothesized to play an important role in migraine pathology (Durham, 2008; Ho et al., 2010). Serum levels of CGRP are increased during migraine attack (Goadsby et al., 1990) (however, see (Tvedskov et al., 2005); migraine patients infused with CGRP develop a delayed headache, fulfilling the criteria of a migraine (Lassen et al., 2002); and infusion of CGRP receptor antagonists have been shown to effectively treat migraine pain (Durham and Vause, 2010; Fischer, 2010; Hoffmann and Goadsby, 2011). Importantly, treatment of migraine headache pain with sumatriptan has been shown to correlate with normalization of CGRP levels –an effect that paralleled migraine resolution (Edvinsson and Ho, 2010; Goadsby and Edvinsson, 1993; Sarchielli et al., 2006; Stepien et al., 2003).
CGRP fibres that originate from the trigeminal ganglion densely innervate the intracranial meninges, in particular the cranial dura mater (Messlinger et al., 1993; Strassman et al., 2004) and this innervation is hypothesized to be the essential anatomical substrate for the development of migraine pain (Messlinger, 2009; Olesen et al., 2009; Pietrobon and Striessnig, 2003). 5-HT1 receptors colocalize with CGRP immunoreactive fibres in high density in the cranial dura and the antimigraine actions of sumatriptan may involve the inhibition of CGRP release from these terminals (Hargreaves, 2007; Harriott and Gold, 2008; Ho et al., 2010; Longmore et al., 1997). Consistent with this proposal, both in animal models and in humans, elevated CGRP levels after stimulation of the trigeminal ganglion are normalized with sumatriptan treatment (Buzzi et al., 1991; Goadsby and Edvinsson, 1993; Limmroth et al., 2001) and in primary cultures from rat trigeminal ganglia, CGRP secretion under conditions simulating migraine pathology is inhibited by sumatriptan (Durham and Russo, 1999).
We have recently introduced a new experimental approach that, for the first time, allows examination of the peripheral signaling processes selectively within individual CGRP terminal pain fibres in the dura (Baillie et al., 2011). In the present study, we use high-resolution optical imaging to directly test the hypothesis that 5-HT1 receptor activation by sumatriptan has a peripheral site of action by inhibiting action potential mediated Ca2+ signaling in terminals of CGRP pain fibres.
2. MATERIALS AND METHODS
2.1 Dissection
This work was approved by the University of Saskatchewan’s Animal Research Ethics Board, and adhered to the Canadian Council on Animal Care guidelines for humane animal use. 41 Transgenic mice Tg (Calca-EGFP) (GENSAT Project at Rockefeller University) (1 month-adult) were dissected as previously described (Baillie et al., 2011). Briefly, the head from an anaesthetized animal was removed from the body at the atlanto–occipital joint. The skin was cut along the skull sagittally and gently moved laterally on both sides. Dissection scissors were then inserted into the foramen magnum and the skull with brain was cut laterally below the occipital and parietal bones on both sides. The two lateral cuts were joined sagitally at the ventral section of the frontal bone. The remaining skull and brain was placed in a bath of physiological solution and the brain carefully removed from the skull leaving intact dura mater and arachnoid mater layers attached to the skull. The interparietal bone was removed by cutting along the lambdoidal suture and the frontal bone removed by cutting along the coronal suture. Two separate and complete parietal bone-dural layer preparations were obtained with a remaining cut along the sagittal suture. Individual dural-skull preparations were placed dural layer up in a microscope chamber and continuously perfused with physiological saline consisting of (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 10 Glucose, 2 MgCl2, 1.25 NaH2PO4, and 2 CaCl2.
2.2 Calcium indicator application
All functional imaging experiments were performed on unmyelinated nociceptive fibres (Baillie et al., 2011). Afferent nociceptive fibres were selectively loaded with the membrane permeant high affinity Ca2+ indicator Rhod-2 AM (Biotium) by applying a small piece of indicator solution soaked Surgifoam® to the rostral area of the preparation for 10 - 20 s. The 220 μM indicator solution was made up fresh each day in physiological solution containing 5% Pluronic F-127/DMSO.
2.3 Immunocytochemistry
Dural skull preparations were dissected as above and placed in 10% formalin for 15 minutes. Preparations were then rinsed in 0.01M phosphate buffered saline (PBS) 3 times for 10 minutes each before being placed in a blocking solution consisting of 10% goat serum, 1% bovine serum albumin (BSA) and 0.01M PBS containing 0.3% triton for 60 minutes. A custom primary antibody for the 5-HT1D receptor (Potrebic et al., 2003) was diluted to 1:60000 with 5% goat serum, 1% BSA, and 0.01M PBS containing 0.3% triton and preparations incubated at room temperature for 48 hours. Preparations were then rinsed in 0.01M PBS containing 1% goat serum 3 times for 10 minutes each. The secondary antibody, Alexa Fluor 594 goat anti-rabbit IgG (Invitrogen) was diluted to 1:500 with 0.01M PBS containing 1% goat serum and the preparations incubated at room temperature for 1 h. The preparations were then rinsed 3 times for 10 minutes each before imaging.
2.4 Widefield epifluorescence microscopy
We used the X-Cite 120PC system (EXFO Electro-Optical Engineering Inc.) light source (12% intensity) directly coupled to an Olympus BX51WIF upright research microscope by a 3 m liquid light guide. The light intensity was attenuated with two neutral density filters in series (Olympus N.D.25 and N.D.6) and shutter controlled with a ProScan II Controller (Prior Scientific). The illumination and fluorescence light was filtered using a TRITC filter set, a FITC filter set, or a Texas Red filter set. Epifluorescence was detected with two high sensitivity cooled CCD cameras. The 16-bit, 512 × 512 ImagEM EM CCD Camera (C9100-13, Hamamatsu) cooled to −65 °C and the 16-bit, 1344 × 1024 ORCA-R2 CCD camera (C10600-10B, Hamamatsu) cooled to −35 °C.
2.5 Analysis of calcium signals
Images were acquired at 20 frames/s using 2 × 2 binning and a minimum number of total images taken to reduce photodynamic damage. Increasing fluorescence baseline, steadily diminishing transients, and/or changes in fibre morphology were considered indicative of photodynamic damage, and fibres showing these changes were discarded. Fluorescence signals were converted to relative fluorescence changes over time and expressed in percentages, defined as ΔF/F = ((F1 − B1) − (F0 − B0))/(F0 − B0), where F1 and F0 are fluorescence in the terminal fibre at any given time point and at the beginning of the experiment, respectively, and B1 and B0 are the background fluorescence at any given time point and at the beginning of the experiment, respectively. Background values were taken from an adjacent area located at least 10 μm from imaged areas. To quantify the magnitude of fluorescence change, the peak amplitude of the transient was measured. During drug application, responses were considered stable if < 5% variability was observed over ~ 10 min control period and baseline fluorescence did not change. The average magnitude of the Ca2+ transients before drug application was set as 100%, and the average of four Ca2+ transients in stable drug and wash conditions normalized to predrug conditions was taken as the magnitude of drug effect and drug recovery, respectively. Results are shown as means ± s.e.m. Statistical analysis was done using an independent group t - test (two-tailed); significance was achieved when P < 0.01.
2.6 Electrical stimulation
A Master 8 - CP, software controlled 8-channel pulse stimulator and ISO-Flex stimulus isolator unit (A.M.P.I.) were used to deliver electrical stimulations via 1 μm bipolar tungsten electrodes (WPI). Stimulation intensity was kept just above threshold to elicit action potentials (140 – 180 μA for a duration of 100 μs) and was not adjusted throughout the course of an experiment.
2.7 Drugs
Sumatriptan (500 nM - 40 μM) (Sigma), GR 127935 (300 nM) (Tocris), BRL 15572 (10 nM) (Tocris), SB 224289 (10 nM) (Tocris), Nifedipine (10 μM) (Sigma), ω-Conotoxin GVIA (1 μM) (Alomone labs), ω-Agatoxin IVA (200 nM) (Alomone labs), and NNC 55-0396 Dihydrochloride (20 μM) (Tocris) were all bath applied.
3. RESULTS
3.1 Sumatriptan inhibition of action potential evoked Ca2+ transient amplitude
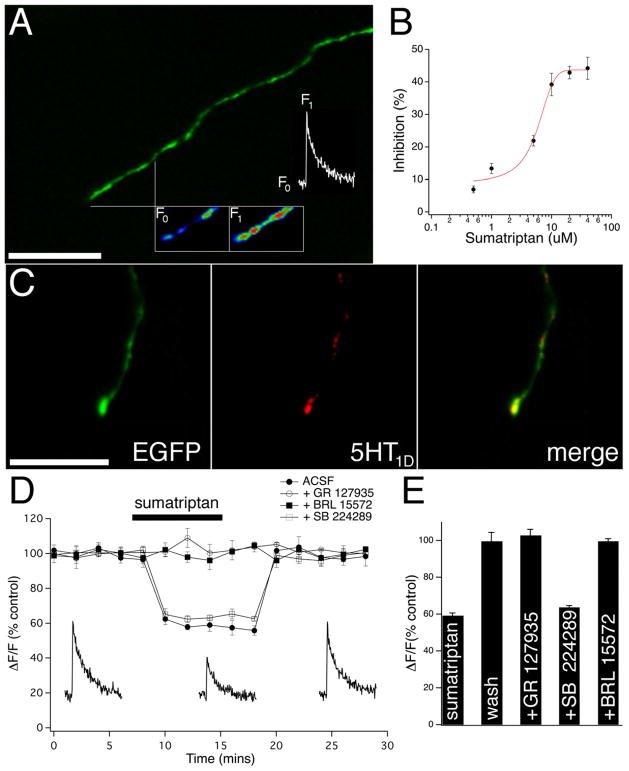
To study Ca2+ signaling in the terminals of CGRP containing nociceptive fibres we selectively identified individual fibres using a fluorescent transgenic CGRP-EGFP mouse (Baillie et al., 2011). Single action potential mediated Ca2+ transients were evoked by electrical stimulation (single pulse, 140 – 180 μA; 100 μs) at distances greater than 500 μm proximal to the distal fibre terminations (Fig. 1A). We find that bath application of sumatriptan caused a dose-dependent inhibition in the amplitude of the evoked Ca2+ transient (500 nM - 40μM; n=5 for each concentration) (Fig. 1B). The lowest concentration of sumatriptan to achieve maximal inhibition (20 μM) was used in all subsequent experiments. We used a custom primary antibody for the 5-HT1D receptor (Potrebic et al., 2003) because the serotonin 5-HT1D receptor subtype has been shown to be selectively expressed in primary afferent neurons and not in peripheral tissues (as the 5-HT1B receptor) (Longmore et al., 1997), and we found punctate 5HT1D immunoreactive co-localized labeling in the CGRP terminating nociceptive fibres (Fig. 1C). We performed Ca2+ transient amplitude timecourse experiments and found that bath application of sumatriptan caused a rapid reversible inhibition in the amplitude of the Ca2+ transient (40.8 ± 1.4%; n=7) (Fig. 1D+E). Below the time course traces are examples of action potential evoked Ca2+ transients before, during and after washout of sumatriptan. Sumatriptan mediated inhibition in the amplitude of the Ca2+ transient was prevented by pre-application of the 5-HT1 antagonist GR127935 (300 nM) or the selective 5-HT1D antagonist BRL 15572 (10 nM); Ca2+ transients remained at 102.7 ± 3.3%; n=5 and 99.7 ± 1.4%; n=5 of control conditions respectively (Fig. 1D+E). Pre-application of the selective 5-HT1B antagonist SB 224289 (10 nM) did not block the sumatriptan mediated inhibition in the amplitude of the Ca2+ transient; Ca2+ transients decreased to 63.7 ± 1.0%; n=5 of control conditions (Fig. 1D+E). It should be noted that sumatriptan does not affect the baseline (un-evoked) Ca2+ signal in terminal CGRP fibres (98.9 ± 0.5% of control; n=6) in contrast to studies performed in primary cultures of trigeminal neurons, where a large sustained Ca2+ influx was noted (Durham and Russo, 1999, 2003).
Figure 1. Sumatriptan causes a dose dependent decrease in action potential evoked Ca2+ transient amplitude.
(A) A CGRP-EGFP terminating nociceptive fibre showing a typical region from which fluorescent transients were acquired and quantified, along with a pseudocolour inset of baseline Rhod-2 fluorescence (F0), and a single action potential evoked signal (F1) with the corresponding transient above displayed. (B) The sumatriptan dose-response curve shows that the amplitude of the Ca2+ transient is inhibited with increasing concentrations of sumatriptan. (C) 5-HT1D receptors are co-localized with terminating CGRP nociceptors. (Left) CGRP-EGFP terminating nociceptive fibre shows punctate immunoreactive labeling with a 5-HT1D antibody (middle and merge). (D) Graph of Ca2+ transient amplitude timecourse experiments showing the effect of sumatriptan application in control conditions (solid circles), in the presence of the selective 5-HT1 receptor antagonist GR127935 (hollow circles), the selective 5-HT1D antagonist BRL 15572 (solid squares), and the selective 5-HT1B antagonist SB 224289 (hollow squares). Individual example transients during control, sumatriptan, and wash conditions taken at the relative timepoints on the graph are shown below. (E) Bar graph summary showing that the sumatriptan reduction in the amplitude of the Ca2+ transient was completely reversible with wash, blocked by GR 127935 and BRL 15572, but not by SB 224289. Scale bars = 20 μm.
3.2 Sumatriptan inhibition of N-type Ca2+ channel mediated signaling
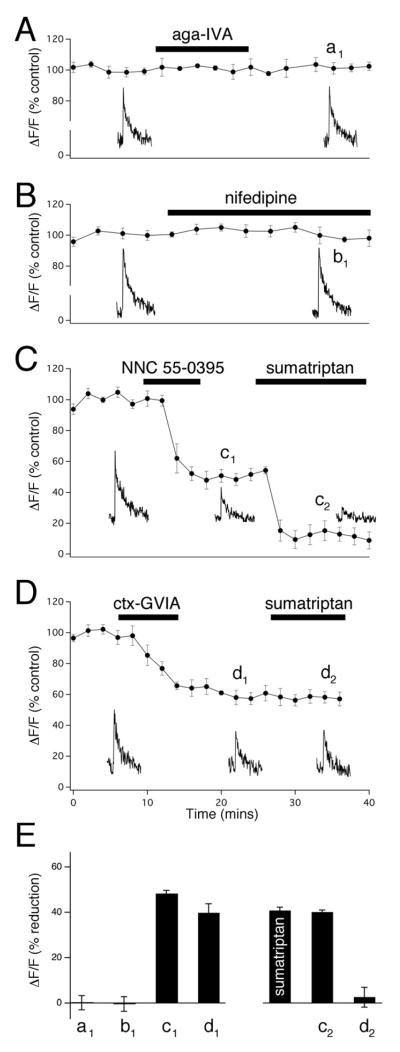
We have previously shown that action potential evoked Ca2+ signaling in the terminals of CGRP containing nociceptive fibres was dependent on extracellular Ca2+ suggesting influx through Ca2+channels (Baillie et al., 2011). To examine the inhibitory Ca2+ signaling action of 5HT1 receptor activation by sumatriptan we first performed a series of selective Ca2+ channel block experiments to determine which Ca2+ channels mediate the action potential evoked Ca2+ signaling. We found that block of P/Q-type Ca2+ channels by ω-agatoxin IVA (200 nM) and block of L-type Ca2+ channels by nifedipine (10 μM) had no effect on action potential evoked Ca2+ transients (101.1 ± 1.5%; n=7 and 100.5 ± 1.4%; n=7 respectively; Fig 2A, B+E). The low voltage activated T-type Ca2+ channel is known to exist in high density in nociceptors and is likely involved in central and peripheral nociceptive processing (Todorovic and Jevtovic-Todorovic, 2006, 2011). We find that application of the selective T-type Ca2+ channel antagonist NNC 55-0396 (20 μM) caused a large irreversible decrease in the amplitude of the Ca2+ transient (48.8 ± 1.2%; n=6, Fig. 2C+E). Application of sumatriptan in the presence of the T-type Ca2+ channel antagonist caused a further decrease in amplitude (40.1 ± 0.9%; n=6, Fig. 2C+E), similar to the reduction with sumatriptan in control conditions (40.8 ± 1.4; n=7; Fig. 1D+E), suggesting that the T-type Ca2+ channel does not mediate sumatriptan inhibition (Fig. 2C+E). A large body of work suggests that the N-type Ca2+ channel controls neurotransmitter release from peripheral sensory neurons (Altier et al., 2007; Snutch, 2005). We found that brief application of the selective N-type Ca2+ channel antagonist ω-conotoxin GVIA (1 μM) caused a large irreversible reduction in Ca2+ transient amplitude (40.7 ± 1.0%; n=6; Fig 2D+E). Application of sumatriptan in the presence of the N-type Ca2+ channel antagonist did not cause a further decrease in the amplitude of the Ca2+ transient. This suggests that N-type Ca2+ channels mediate sumatriptan inhibition of Ca2+ signaling in the terminals of CGRP containing nociceptive fibres.
Figure 2. N-type Ca2+ channels mediate Sumatriptan inhibition of Ca2+ signaling.
(A–D) Graphs of timecourse experiments showing the effects of Ca2+ channel blockers on the single action potential evoked Ca2+ transient amplitudes. Individual example transients taken at the relative timepoints on the graphs are shown below. (A + B) Neither the P/Q Ca2+channel blocker Agatoxin-IVA (200 nm) nor the L-type Ca2+ channel blocker Nifedipine (10 μM) decreased the amplitude of the Ca2+ transient. (C + D) The T-type Ca2+ channel blocker NNC 55-0936 (20 μM) as well as the N-type Ca2+ channel blocker Conotoxin-GVIA (1μM) significantly inhibited the Ca2+ transient amplitude. C) The amplitude of the T-type Ca2+ channel mediated inhibition was further reduced by the application of sumatriptan, while the amplitude of the N-type Ca2+ channel mediated inhibition remained unaffected with sumatriptan application (D). (E) Bar graph summaries under the experimental conditions seen in (A – D) showing the inhibition of Ca2+ transient amplitudes ( F/F; % reduction) at the timepoints shown on the graphs (a1 − d1). The magnitude of the sumatriptan mediated inhibition alone was found to be equal to the magnitude of the sumatriptan mediated inhibition in the presence of the T-type Ca2+ channel blocker (c2), while in the presence of the N-type Ca2+ channel blocker, the sumatriptan mediated inhibition was completely occluded (d2).
4. DISCUSSION
In the present study we performed high-resolution functional imaging selectively within individual dural CGRP nociceptive fibre terminations and found that sumatriptan has a peripheral site of action mediated through N-type Ca2+ channels to inhibit action potential evoked Ca2+ signaling. CGRP is hypothesized to play an important role in migraine pathology (Ho et al., 2010). Our finding here that sumatriptan causes selective inhibition of N-type Ca2+ channel mediated Ca2+ signaling in dural CGRP fibres is consistent with this hypothesis. Though we do not have a measure of CGRP release from the terminals, we can infer that given the significant contribution of the N-type Ca2+ channel to the Ca2+ signal (~40%), selective inhibition through 5-HT1 receptor activation should dramatically inhibit neurotransmitter release, since release shows a highly non-linear dependence on Ca2+ influx through voltage dependent Ca2+ channels (Bollmann and Sakmann, 2005; Dodge and Rahamimoff, 1967; Katz and Miledi, 1965; Schneggenburger and Neher, 2005). As well, N-type Ca2+ channels are heavily clustered at synaptic sites associated with exocytotic proteins and a large body of work suggests that this channel controls functional coupling between action potentials and evoked neurotransmitter release from nociceptive terminals (Altier et al., 2007; Altier and Zamponi, 2004; Snutch, 2005; Zamponi et al., 2009). It is also well established that N-type Ca2+ channels are strongly regulated by G protein coupled receptors that allow precise control of neurotransmitter release through inhibition of the N-type channel (Currie, 2010; Weiss, 2009).
To the best of our knowledge, this is the first study to show that the activation of 5-HT1 receptors in trigeminal neurons causes inhibition of Ca2+ signaling selectively through a single Ca2+ channel subtype; the N-type. The N-type Ca2+ channel subtype in particular over other subtypes has been shown to be an attractive target for therapeutic intervention concerning chronic and neuropathic pain conditions (Snutch, 2005; Zamponi et al., 2009). Indeed, the highly selective N-type Ca2+ channel peptide antagonist ziconotide (Prialt®) is a non-opioid analgesic that has recently been used clinically for the amelioration of severe and chronic pain even in morphine-unresponsive situations and without the serious opioid-related side effects such as respiratory depression, addiction, and tolerance (Snutch, 2005). The results presented in the current study suggest that the development of novel N-type Ca2+ channel selective therapeutics may have implications for the treatment of migraine pain. A major advantage for developing compounds that selectively target the dural N-type Ca2+ channel and regulate neurotransmitter release is that the medication would not have to cross the blood brain barrier for therapeutic action, thus eliminating serious central nervous system side effects.
We found in dural CGRP fibre terminations that the T-type Ca2+ channel accounted for a greater proportion of the Ca2+ signal (~50%) than the N-type channel but did not mediate any of the sumatriptan inhibition. Application of sumatriptan in the presence of the T-type Ca2+ channel antagonist caused a decrease in the amplitude of the Ca2+ signal to the same extent as the reduction seen with sumatriptan in control conditions (~40%). Although T-type Ca2+ channels are thought to play a central role in peripheral pain processing, where they are believed to regulate subthreshold excitability in the peripheral nociceptive fibres, they are not thought to control neurotransmitter release (Iftinca and Zamponi, 2009; Jevtovic-Todorovic and Todorovic, 2006; Todorovic and Jevtovic-Todorovic, 2006, 2007, 2011). Consistent with this, it was recently shown that T-type Ca2+ channels did not contribute to high potassium-induced CGRP release measured by an enzyme-linked immunoassay in an ex vivo rat dural-skull preparation similar to the mouse preparation used in our study (Amrutkar et al., 2011). It is again interesting to speculate on the development of novel antimigraine therapeutics. Compounds that selectively target the T-type Ca2+ channel could be specific enough to ameliorate the pathophysiological activation and/or sensitization mechanisms of the dural nociceptive fibres that are thought to occur during migraine, without modifying neurotransmitter release mechanisms that may have remained unimpaired during the migraine event.
We found that block of P/Q- and L-type Ca2+ channels had no effect on action potential evoked Ca2+ transients in dural CGRP nociceptive fibre terminations. If we assume that Ca2+ influx through P/Q- and L-type Ca2+ channels controls the release of CGRP, then our findings here contrast those of Amrutkar et al. (2011), who found that block of P/Q- and L-type Ca2+ channel subtypes each decreased 60mM potassium-induced CGRP release (Amrutkar et al., 2011). The differences between the two studies may be due to the different stimulation protocols and/or the different concentrations of the Ca2+ channel antagonists used (Sidach and Mintz, 2000).
5. CONCLUSION
The antimigraine activity of the 5-HT1 receptor agonist sumatriptan is well established yet the anatomical locus and mechanisms of action remain unresolved. In the present study we have shown that sumatriptan acts peripherally at individual dural CGRP nociceptive fibre terminations to inhibit action potential evoked Ca2+ signaling selectively through N-type Ca2+ channels. This finding provides insight for the development of novel peripherally targeted therapeutics for mitigating the pain of migraine.
The 5-HT1 receptor agonist sumatriptan is an effective migraine therapeutic.
The antimigraine anatomical locus and mechanisms of action remain unresolved.
We show sumatriptan acts peripherally at individual dural CGRP pain fibre terminals.
N-type Ca2+ channels selectively mediate sumatriptan inhibition of Ca2+ signaling.
Findings provide insight for developing novel peripherally targeted therapeutics.
Acknowledgments
This work was supported by a grant to S.J.M. from the Canadian Institutes of Health Research and the Saskatchewan Regional Partnership Program and the NIH NS066091 to A.H.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Landon D. Baillie, Email: ldb874@mail.usask.ca.
Andrew H. Ahn, Email: andrew.ahn@neurology.ufl.edu.
References
- Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115:1–4. doi: 10.1016/j.pain.2005.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci. 2011;12:570–584. doi: 10.1038/nrn3057. [DOI] [PubMed] [Google Scholar]
- Altier C, Dale CS, Kisilevsky AE, Chapman K, Castiglioni AJ, Matthews EA, Evans RM, Dickenson AH, Lipscombe D, Vergnolle N, Zamponi GW. Differential role of N-type calcium channel splice isoforms in pain. J Neurosci. 2007;27:6363–6373. doi: 10.1523/JNEUROSCI.0307-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Zamponi GW. Targeting Ca2+ channels to treat pain: T-type versus N-type. Trends Pharmacol Sci. 2004;25:465–470. doi: 10.1016/j.tips.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Amrutkar DV, Ploug KB, Olesen J, Jansen-Olesen I. Role for voltage gated calcium channels in calcitonin gene-related peptide release in the rat trigeminovascular system. Neuroscience. 2011;172:510–517. doi: 10.1016/j.neuroscience.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Baillie LD, Hagen V, Gardner KM, Mulligan SJ. Functional imaging within individual pain fibres ex vivo with optical microscopy. J Neurosci Methods. 2011;198:274–279. doi: 10.1016/j.jneumeth.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Bartsch T, Knight YE, Goadsby PJ. Activation of 5-HT(1B/1D) receptor in the periaqueductal gray inhibits nociception. Ann Neurol. 2004;56:371–381. doi: 10.1002/ana.20193. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Krymchantowski AV, Ho T. Migraine in the triptan era: progresses achieved, lessons learned and future developments. Arq Neuropsiquiatr. 2009;67:559–569. doi: 10.1590/s0004-282x2009000300040. [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B. Control of synaptic strength and timing by the release-site Ca2+ signal. Nat Neurosci. 2005;8:426–434. doi: 10.1038/nn1417. [DOI] [PubMed] [Google Scholar]
- Buzzi MG, Carter WB, Shimizu T, Heath H, 3rd, Moskowitz MA. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology. 1991;30:1193–1200. doi: 10.1016/0028-3908(91)90165-8. [DOI] [PubMed] [Google Scholar]
- Currie KP. G protein modulation of CaV2 voltage-gated calcium channels. Channels (Austin) 2010;4:497–509. doi: 10.4161/chan.4.6.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham PL. Inhibition of calcitonin gene-related peptide function: a promising strategy for treating migraine. Headache. 2008;48:1269–1275. doi: 10.1111/j.1526-4610.2008.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham PL, Russo AF. Regulation of calcitonin gene-related peptide secretion by a serotonergic antimigraine drug. J Neurosci. 1999;19:3423–3429. doi: 10.1523/JNEUROSCI.19-09-03423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham PL, Russo AF. New insights into the molecular actions of serotonergic antimigraine drugs. Pharmacol Ther. 2002;94:77–92. doi: 10.1016/s0163-7258(02)00173-0. [DOI] [PubMed] [Google Scholar]
- Durham PL, Russo AF. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J Neurosci. 2003;23:807–815. doi: 10.1523/JNEUROSCI.23-03-00807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham PL, Vause CV. Calcitonin gene-related peptide (CGRP) receptor antagonists in the treatment of migraine. CNS Drugs. 2010;24:539–548. doi: 10.2165/11534920-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Ho TW. CGRP receptor antagonism and migraine. Neurotherapeutics. 2010;7:164–175. doi: 10.1016/j.nurt.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MJ. Calcitonin gene-related peptide receptor antagonists for migraine. Expert Opin Investig Drugs. 2010;19:815–823. doi: 10.1517/13543784.2010.490829. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Hargreaves R. New migraine and pain research. Headache. 2007;47(Suppl 1):S26–43. doi: 10.1111/j.1526-4610.2006.00675.x. [DOI] [PubMed] [Google Scholar]
- Harriott AM, Gold MS. Serotonin type 1D receptors (5HTR) are differentially distributed in nerve fibres innervating craniofacial tissues. Cephalalgia. 2008;28:933–944. doi: 10.1111/j.1468-2982.2008.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6:573–582. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- Hoffmann J, Goadsby PJ. New Agents for Acute Treatment of Migraine: CGRP Receptor Antagonists, iNOS Inhibitors. Curr Treat Options Neurol. 2011 doi: 10.1007/s11940-011-0155-4. [DOI] [PubMed] [Google Scholar]
- Humphrey PP, Feniuk W. Mode of action of the anti-migraine drug sumatriptan. Trends Pharmacol Sci. 1991;12:444–446. doi: 10.1016/0165-6147(91)90630-b. [DOI] [PubMed] [Google Scholar]
- Iftinca MC, Zamponi GW. Regulation of neuronal T-type calcium channels. Trends Pharmacol Sci. 2009;30:32–40. doi: 10.1016/j.tips.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Todorovic SM. The role of peripheral T-type calcium channels in pain transmission. Cell Calcium. 2006;40:197–203. doi: 10.1016/j.ceca.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. The Effect of Calcium on Acetylcholine Release from Motor Nerve Terminals. Proc R Soc Lond B Biol Sci. 1965;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Lambert GA. The lack of peripheral pathology in migraine headache. Headache. 2010;50:895–908. doi: 10.1111/j.1526-4610.2010.01669.x. [DOI] [PubMed] [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A. 2004;101:4274–4279. doi: 10.1073/pnas.0306147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limmroth V, Katsarava Z, Liedert B, Guehring H, Schmitz K, Diener HC, Michel MC. An in vivo rat model to study calcitonin gene related peptide release following activation of the trigeminal vascular system. Pain. 2001;92:101–106. doi: 10.1016/s0304-3959(00)00475-9. [DOI] [PubMed] [Google Scholar]
- Longmore J, Shaw D, Smith D, Hopkins R, McAllister G, Pickard JD, Sirinathsinghji DJ, Butler AJ, Hill RG. Differential distribution of 5HT1D- and 5HT1B-immunoreactivity within the human trigemino-cerebrovascular system: implications for the discovery of new antimigraine drugs. Cephalalgia. 1997;17:833–842. doi: 10.1046/j.1468-2982.1997.1708833.x. [DOI] [PubMed] [Google Scholar]
- Mehrotra S, Gupta S, Chan KY, Villalon CM, Centurion D, Saxena PR, MaassenVanDenBrink A. Current and prospective pharmacological targets in relation to antimigraine action. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:371–394. doi: 10.1007/s00210-008-0322-7. [DOI] [PubMed] [Google Scholar]
- Messlinger K. Migraine: where and how does the pain originate? Exp Brain Res. 2009;196:179–193. doi: 10.1007/s00221-009-1756-y. [DOI] [PubMed] [Google Scholar]
- Messlinger K, Hanesch U, Baumgartel M, Trost B, Schmidt RF. Innervation of the dura mater encephali of cat and rat: ultrastructure and calcitonin gene-related peptide-like and substance P-like immunoreactivity. Anat Embryol (Berl) 1993;188:219–237. doi: 10.1007/BF00188214. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Cutrer FM. SUMATRIPTAN: a receptor-targeted treatment for migraine. Annu Rev Med. 1993;44:145–154. doi: 10.1146/annurev.me.44.020193.001045. [DOI] [PubMed] [Google Scholar]
- Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4:386–398. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- Potrebic S, Ahn AH, Skinner K, Fields HL, Basbaum AI. Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: implications for the selective antimigraine action of triptans. J Neurosci. 2003;23:10988–10997. doi: 10.1523/JNEUROSCI.23-34-10988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchielli P, Pini LA, Zanchin G, Alberti A, Maggioni F, Rossi C, Floridi A, Calabresi P. Clinical-biochemical correlates of migraine attacks in rizatriptan responders and non-responders. Cephalalgia. 2006;26:257–265. doi: 10.1111/j.1468-2982.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Presynaptic calcium and control of vesicle fusion. Curr Opin Neurobiol. 2005;15:266–274. doi: 10.1016/j.conb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Sidach SS, Mintz IM. Low-affinity blockade of neuronal N-type Ca channels by the spider toxin omega-agatoxin-IVA. J Neurosci. 2000;20:7174–7182. doi: 10.1523/JNEUROSCI.20-19-07174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snutch TP. Targeting chronic and neuropathic pain: the N-type calcium channel comes of age. NeuroRx. 2005;2:662–670. doi: 10.1602/neurorx.2.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger T, Goadsby PJ. Migraine pathogenesis and state of pharmacological treatment options. BMC Med. 2009;7:71. doi: 10.1186/1741-7015-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien A, Jagustyn P, Trafny EA, Widerkiewicz K. Suppressing effect of the serotonin 5HT1B/D receptor agonist rizatriptan on calcitonin gene-related peptide (CGRP) concentration in migraine attacks. Neurol Neurochir Pol. 2003;37:1013–1023. [PubMed] [Google Scholar]
- Strassman AM, Weissner W, Williams M, Ali S, Levy D. Axon diameters and intradural trajectories of the dural innervation in the rat. J Comp Neurol. 2004;473:364–376. doi: 10.1002/cne.20106. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs. 2000;60:1259–1287. doi: 10.2165/00003495-200060060-00003. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen PC. Does sumatriptan cross the blood-brain barrier in animals and man? J Headache Pain. 2010;11:5–12. doi: 10.1007/s10194-009-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tfelt-Hansen PC, Koehler PJ. One hundred years of migraine research: major clinical and scientific observations from 1910 to 2010. Headache. 2011;51:752–778. doi: 10.1111/j.1526-4610.2011.01892.x. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Jevtovic-Todorovic V. The role of T-type calcium channels in peripheral and central pain processing. CNS Neurol Disord Drug Targets. 2006;5:639–653. doi: 10.2174/187152706779025490. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Jevtovic-Todorovic V. Regulation of T-type calcium channels in the peripheral pain pathway. Channels (Austin) 2007;1:238–245. doi: 10.4161/chan.4953. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Jevtovic-Todorovic V. T-type voltage-gated calcium channels as targets for the development of novel pain therapies. Br J Pharmacol. 2011;163:484–495. doi: 10.1111/j.1476-5381.2011.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol. 2005;58:561–568. doi: 10.1002/ana.20605. [DOI] [PubMed] [Google Scholar]
- Weiss N. Regulation of N-type calcium channels by G-proteins: multiple pathways to control calcium entry into neurons. Channels (Austin) 2009;3:219–220. doi: 10.4161/chan.3.4.9255. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Lewis RJ, Todorovic SM, Arneric SP, Snutch TP. Role of voltage-gated calcium channels in ascending pain pathways. Brain Res Rev. 2009;60:84–89. doi: 10.1016/j.brainresrev.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]