Abstract
Non-motile primary cilium is an antenna-like structure whose defect is associated with a wide range of pathologies, including developmental disorders and cancer. Although mechanisms regulating cilia assembly have been extensively studied, how cilia disassembly is regulated remains poorly understood. Here, we report unexpected roles of Dishevelled 2 (Dvl2) and interphase polo-like kinase 1 (Plk1) in primary cilia disassembly. We demonstrated that Dvl2 is phosphorylated at S143 and T224 in a manner that requires both non-canonical Wnt5a ligand and casein kinase 1 epsilon (CK1ε), and that this event is critical to interact with Plk1 in early stages of the cell cycle. The resulting Dvl2–Plk1 complex mediated Wnt5a–CK1ε–Dvl2-dependent primary cilia disassembly by stabilizing the HEF1 scaffold and activating its associated Aurora-A (AurA), a kinase crucially required for primary cilia disassembly. Thus, via the formation of the Dvl2–Plk1 complex, Plk1 plays an unanticipated role in primary cilia disassembly by linking Wnt5a-induced biochemical steps to HEF1/AurA-dependent cilia disassembly. This study may provide new insights into the mechanism underlying ciliary disassembly processes and various cilia-related disorders.
Keywords: Cilia, CK1, Dvl2, Plk1, Wnt
Introduction
Primary cilia, observed in most stromal and epithelial cells, are microtubule-based cellular sensing structures important for transducing extracellular signals. Aberrant ciliary function leads to many ciliopathies, such as renal cysts, hypertension, diabetes, neuronal, visual, respiratory, and other developmental disorders (Davenport and Yoder, 2005; Fliegauf et al, 2007; Adams et al, 2008; Sharma et al, 2008; Berbari et al, 2009). Unlike motile cilia, primary cilia lack a pair of central microtubules and dynein arms (9+0 arrangements of microtubules), and thus do not show wave motion. Primary cilia undergo a dynamic cycle of assembly and disassembly throughout the cell cycle. They assemble from basal body/mother centriole in G0- or G1-phase and rapidly disassemble prior to mitosis entry (Dawe et al, 2007; Pan and Snell, 2007; Santos and Reiter, 2008; Seeley and Nachury, 2010). Over the years, various components important for primary cilia assembly have been isolated and mechanisms underlying this event have begun to emerge. However, how the ciliary disassembly process is regulated remains poorly understood. Given that cancer cells commonly lack primary cilia (Michaud and Yoder, 2006; Plotnikova et al, 2008), pathways regulating cilia assembly/disassembly processes could be tightly linked to the mechanisms regulating tumourigenesis.
Polo-like kinase 1 (Plk1) plays a pivotal role during the M-phase of the cell cycle (Barr et al, 2004; van de Weerdt and Medema, 2006; Petronczki et al, 2008; Archambault and Glover, 2009). Plk1 specifically localizes to centrosomes, kinetochores, and midbody through the function of the C-terminal non-catalytic polo-box domain (PBD) (Park et al, 2010), which forms a conserved phospho-Ser/Thr (pS/pT)-binding module (Cheng et al, 2003; Elia et al, 2003). Plk1 is highly overexpressed in various human cancers and is thought to promote tumourigenesis (Strebhardt and Ullrich, 2006). Although Plk1 does not appear to be required for proper ciliogenesis (Soung et al, 2009), whether it contributes to the ciliary disassembly process is not known.
The Wnt signalling pathway is involved in a wide range of developmental processes, such as cell migration, proliferation, and polarity. Deregulation of Wnt signalling is associated with various human diseases, including cancer (Logan and Nusse, 2004; Moon et al, 2004; Clevers, 2006). Binding of various Wnt ligands to Frizzled (Fz), a family of G protein-coupled cell surface receptors, triggers signalling cascades, and Dishevelled (Dvl) plays a key role as a signal mediator in both canonical and non-canonical Wnt pathways (Moon et al, 2002; Nusse, 2005; Angers and Moon, 2009; Gao and Chen, 2010). Activation of the Wnt canonical pathway leads to stabilization of β-catenin, which turns on a set of genes by binding to LEF/TCF family transcriptional factors and regulates cell proliferation. Non-canonical pathways, which do not involve the function of β-catenin, also regulate various physiologically important processes, including planar cell polarity, axon guidance, and intracellular Ca++ release (Logan and Nusse, 2004; Zhang et al, 2007; Angers and Moon, 2009; Gao and Chen, 2010). Although molecular mechanisms are yet to be clarified, a flurry of evidence suggests that Wnt pathways are tightly associated with cilia assembly/disassembly processes (see reviews Gerdes and Katsanis, 2008; He, 2008).
Casein kinase 1 (CK1) is a member of the Ser/Thr kinase superfamily with seven mammalian CK1 isoforms (α, β, γ1, γ2, γ3, δ, and ε) characterized to date (Knippschild et al, 2005). Among them, CK1ε exhibits a high enzyme-substrate specificity for Dvl phosphorylation in vitro and its closest relative CK1δ (82% identity in the entire amino-acid sequence and 96% identity in the kinase domain) also phosphorylates Dvl in vitro (McKay et al, 2001; Gao et al, 2002; Price, 2006; Dahlberg et al, 2009). Interestingly, both CK1δ and CK1ε appear to be required for Wnt5a-induced Dvl2 phosphorylation in vivo (Bryja et al, 2007). However, whether these phosphorylation events are physiologically significant, and whether CK1δ and CK1ε have distinct cellular functions remains largely elusive.
In this study, we provide evidence that both non-mitotic Plk1 and Dvl2 have unanticipated roles in primary cilia disassembly. The PBD of Plk1 binds to the CK1ε-phosphorylated, conserved S143 and T224 sites of Dvl2, the most abundant isoform of the mammalian Dvl1–3 family (Lee et al, 2008), and this event is crucial in mediating the Wnt5a–CK1ε–Dvl2-dependent primary cilia disassembly pathway at the early stage of the cell cycle (note that S143 of Dvl2 is different from the previously identified S142 of Dvl1, which is analogous to S158 of Dvl2 (Klimowski et al, 2006)). Furthermore, the Dvl2–Plk1 complex appears to modulate HEF1 stability and, therefore, HEF1/Aurora-A (AurA)-dependent primary cilia disassembly, likely through the regulation of Smad3-dependent HEF1 degradation (Liu et al, 2000; Nourry et al, 2004). Given the close relationship between primary cilia disassembly and the cell cycle (Quarmby and Parker, 2005; Plotnikova et al, 2008; Seeley and Nachury, 2010) and the crucial role of Plk1 in promoting cell proliferation, we propose that Plk1 plays a key role in coupling primary cilia disassembly with cell-cycle progression.
Results
Identification of Dvl2 as a novel Plk1 PBD-binding protein
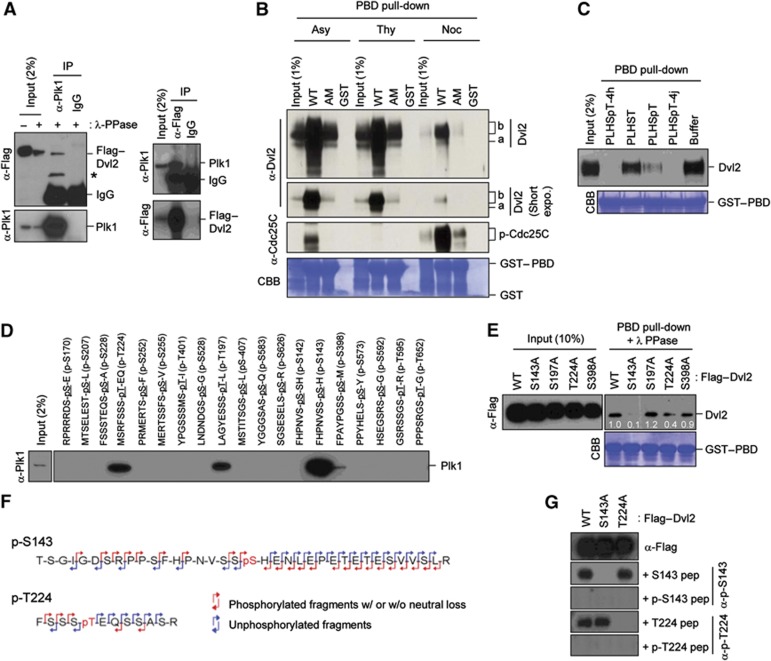
To investigate new functions of Plk1 at the centrosome, we attempted to identify previously uncharacterized Plk1 PBD-binding proteins by performing PBD pull-downs with various centrosomal proteins expressed in asynchronously growing HeLa cells. Among the proteins that efficiently bind to the PBD was Dvl2, a component that plays a central role in mediating both canonical and non-canonical Wnt signalling pathways. Subsequent studies on the interaction between endogenous Plk1 and transfected Flag-tagged Dvl2 (Flag–Dvl2) showed that these two proteins were co-immunoprecipitated in a reciprocal manner (Figure 1A). Furthermore, GST–PBD efficiently interacted with both fast-migrating (i.e., underphosphorylated; marked ‘a’) and slow-migrating (i.e., hyperphosphorylated; marked ‘b’) Dvl2 forms in HeLa lysates prepared from asynchronously (Asy)-growing or thymidine (Thy)-treated (S-phase) cells (Figure 1B). The somewhat reduced level of Dvl2 bound to GST–PBD in nocodazole (Noc)-treated (M-phase) cells in Figure 1B appears to be due to inefficient detection of the hyperphosphorylated form of Dvl2, which was greatly improved by λ phosphatase treatment (Supplementary Figure S1). The respective GST–PBD (H538A K540M) mutant (PBD (AM)), defective in phospho-dependent binding (Elia et al, 2003), interacted with Dvl2 only marginally (Figure 1B). Consistent with this observation, provision of a previously characterized PBD-binding phosphopeptide, PLHSpT (Yun et al, 2009) or its high-affinity N-alkylphenyl derivatives, 4h and 4j (Liu et al, 2011), but not the non-phospho control PLHST peptide, disrupted the PBD–Dvl2 interaction (Figure 1C). Thus, Plk1 interacts with Dvl2 in a phospho-dependent manner throughout the cell cycle.
Figure 1.
Interaction between Plk1 PBD and the p-S143 and p-T224 epitopes of Dvl2. (A) HeLa cells transfected with Flag–Dvl2 were subjected to reciprocal immunoprecipitations. Immunoprecipitates were treated with λ phosphatase (PPase) to eliminate phosphorylated and slow-migrating forms and then immunoblotted. Asterisk, degradation product of Flag–Dvl2. (B) Total lysates prepared from asynchronously (Asy) growing, thymidine (Thy)-treated, or Noc-treated HeLa cells were subjected to pull-downs with bead-associated control GST, GST–PBD WT, or GST–PBD (H538A K540M) (AM). Precipitates were immunoblotted and the membrane was stained with Coomassie (CBB). Note that both fast- and slow-migrating Dvl2 forms (arbitrarily referred to as a and b forms, respectively) efficiently bound to GST–PBD (WT), but not to GST–PBD (AM). (C) Lysates from asynchronously growing HeLa cells were mixed with nonphospho-T78 peptide (PLHST), phospho-T78 peptide (PLHSpT), high-affinity PLHSpT derivatives 4h or 4j (Liu et al, 2011), or control buffer. The resulting lysates were then subjected to PBD pull-down as in (B). (D) Bead-immobilized Dvl2-derived peptides were incubated with HeLa lysates, precipitated, and then immunoblotted. (E) HEK293T cells transfected with the indicated Dvl2 constructs were subjected to PBD pull-downs, treated with λ phosphatase (PPase), and analysed. Numbers, relative amounts of Dvl2 bound to GST–PBD. (F) Anti-Flag–Dvl2 immunoprecipitates prepared from transfected HeLa cells arrested with thymidine for 18 h were subjected to mass spectrometry analysis to determine in-vivo phosphorylation site. The phosphorylation site was determined by the phosphorylated (red) MS/MS fragment ions with (w/) or without (w/o) neutral loss of phosphate and unphosphorylated (blue) fragment ions. The full results are shown in Supplementary Figure S2B. (G) HEK293T cells transfected with the indicated Dvl2 constructs were immunoblotted in the presence of 5 μg/ml of the indicated S143 or T224 peptide to determine the specificity of the phospho-antibodies. Figure source data can be found with the Supplementary data.
Visual scanning of the Dvl2 primary sequence revealed multiple potential PBD-binding sequences that fall into the consensus PBD-binding motif (Φ/P-Φ-T/Q/H/M-S-pS/pT-P/X; X, any amino-acid residue; Φ, hydrophobic residue) (Elia et al, 2003). Testing of the phosphorylated peptides derived from these sequences showed that both p-S143 and p-T224 peptides efficiently interacted with Plk1, while p-T197 and p-S398 peptides interacted with Plk1 at a lesser level (Figure 1D). Among these four sites, mutation of S143 to A severely crippled the PBD–Dvl2 interaction, while mutation of T224 to A mildly weakened the interaction (Figure 1E). Mass spectrometry analyses with immunoprecipitated Flag–Dvl2 revealed that both S143 and T224 residues are phosphorylated in vivo (Figure 1F; Supplementary Figure S2A and B), which was further confirmed by immunoblotting analyses with phospho-specific antibodies generated against the two phospho-epitopes (Figure 1G). In line with the importance of S143 and T224 for PBD binding, both of these residues are highly conserved among vertebrates and also in different human isoforms (Supplementary Figure S2C and D).
CK1δ and CK1ε induce the Dvl2–Plk1 interaction by phosphorylating the S143 and T224 residues on Dvl2
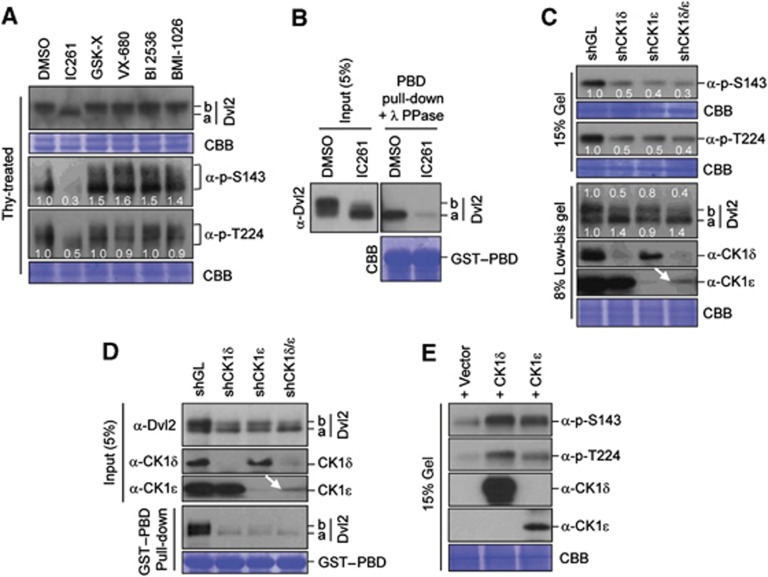
To determine the kinase(s) responsible for the phosphorylation of the S143 and T224 residues, HeLa cells were treated with previously characterized inhibitors. Among them, a CK1δ/ε-specific inhibitor, IC261 (Gao et al, 2002; Tillement et al, 2008), induced a fast-migrating Dvl2 form (a form) and greatly diminished the levels of both p-S143 and p-T224 epitopes (Figure 2A). In contrast, inhibition of GSK-3 (GSK inhibitor X), AurA and AurB (VX-680), Plk1 (BI 2536), or Cdc2 (BMI-1026) failed to significantly alter the levels of the p-S143 and p-T224 epitopes. Consistent with these findings, treatment of cells with IC261 abolished the Plk1 PBD–Dvl2 interaction (Figure 2B).
Figure 2.
CK1δ/ε-dependent phosphorylation of Dvl2 at S143 and T224. (A) Thymidine-arrested HeLa cells were treated with the indicated compounds to inhibit CK1δ/ε (IC261), GSK-3β (GSK Inhibitor X), AurA (VX-680), Plk1 (BI 2536), or Cdk (BMI-1026), respectively, and the resulting samples were subjected to immunoblotting analyses. Note only IC261 generated the fast-migrating ‘a’ form and greatly diminished the levels of the p-S143 and p-T224 epitopes. Numbers in (A) indicate relative signal intensities. (B) HeLa cells were treated with control DMSO or IC261 for 3 h and then subjected to PBD pull-downs. Precipitates were treated with λ phosphatase (PPase), immunoblotted, and the resulting membrane was stained with Coomassie (CBB). (C–E) Asynchronously growing hTERT-RPE cells were either silenced for control luciferase (GL), CK1δ, CK1ε, or both CK1δ and CK1ε by lentivirus-based shRNA (C, D) or transfected with CK1δ or CK1ε and treated with thymidine for 18 h (E). The resulting cells were then immunoblotted with the indicated antibodies (C, E) or subjected to PBD pull-downs (D). Where indicated, samples were separated by 15% SDS–PAGE to condense the Dvl2 signals for easy quantification or 8% low-bis-acrylamide gel to reveal hyperphosphorylated, slow-migrating proteins. Failure to induce an additive effect of shCK1δ and shCK1ε on the generation of the p-S143 and p-T224 epitopes (C) and in the PBD binding (D) could be in part attributable to the inefficient depletion of CK1ε (arrows). Numbers in (C) indicate signal intensities relative to the signal in the shGL sample. Note that the transfected CK1δ and CK1ε in (E) were overexpressed over their endogenous proteins ∼40 times and 10 times, respectively (data not shown). Figure source data can be found with the Supplementary data.
Subsequent analyses showed that depletion of either CK1δ or CK1ε greatly diminished the levels of the p-S143 and p-T224 epitopes on Dvl2 (Figure 2C). However, significant levels of the p-S143 and p-T224 epitopes were still detectable in the cells depleted of both CK1δ and CK1ε (shCK1δ/ε). This could be due to the incomplete depletion of CK1δ or CK1ε (arrow) or to the presence of other unidentified kinase(s) that phosphorylates these sites. Notably, depletion of CK1δ induced the Dvl2, a form much more efficiently than depletion of CK1ε (Figure 2C; compare lane 2 with lane 3 in the 8% low-bis gel Dvl2 panel). This finding suggests that CK1δ and CK1ε-dependent Dvl2 phosphorylation sites do not completely overlap and that CK1δ and CK1ε may have distinct functions yet to be characterized. Consistent with the importance of CK1δ or CK1ε in the generation of the p-S143 and p-T224 epitopes, depletion of these kinases markedly impaired the PBD–Dvl2 binding (Figure 2D).
In a second experiment, we observed that CK1δ and CK1ε directly phosphorylated and generated the p-S143 and p-T224 epitopes in vitro (Supplementary Figure S2E) and induced these phospho-epitopes in vivo (Figure 2E), thus corroborating the critical role of these kinases in the induction of the Dvl2–Plk1 complex.
Plk1 promotes primary cilia disassembly in a kinase activity-dependent manner
Since Dvl2 mediates both canonical and non-canonical Wnt pathways, we first investigated whether Plk1 contributes to the β-catenin-dependent canonical pathway. However, Plk1 activity neither altered the degree of β-catenin ubiquitination nor influenced the level of β-catenin-dependent transcription activity (Supplementary Figure S3), thereby diminishing the likelihood that Dvl2-bound Plk1 contributes to the canonical pathway.
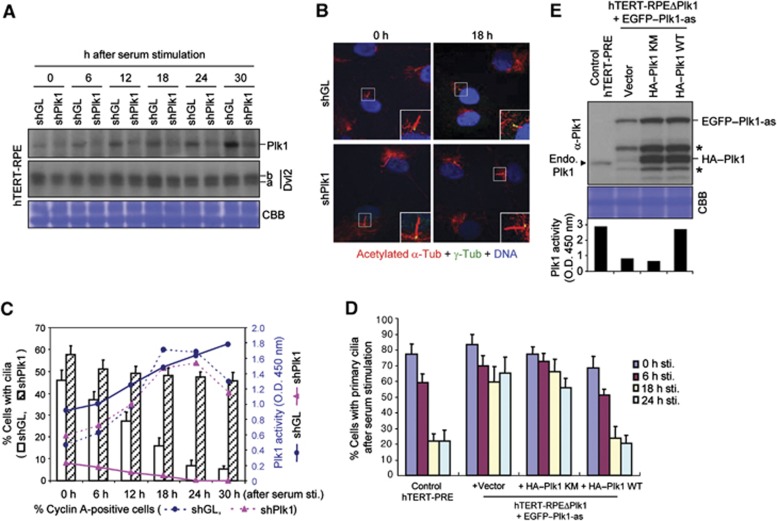
It has been suggested that Dvl2 regulates the apical docking and planar polarization of basal body in multiciliated epithelial cells and promotes ciliogenesis (Park et al, 2008). Thus, we investigated whether the formation of the Dvl2–Plk1 complex contributes to the processes of primary cilia assembly. Surprisingly, however, we observed that depletion of Dvl2 in hTERT-immortalized retinal pigment epithelial (hTERT-RPE) cells induced substantially longer primary cilia in most cells, whereas overexpression of Dvl2 induced markedly shorter cilia in a fewer cell population (Supplementary Figure S4A–E; see also Figure 4B–D, below). Similar results were obtained with NIH 3T3 mouse fibroblast cells (Supplementary Figure S4F–H). These findings suggest that Dvl2 facilitates the disassembly of primary cilia. In line with this view, depletion of Dvl2 in cells derived from a patient suffering from premature chromatid separation (PCS) syndrome also resulted in a longer primary cilia (Miyamoto et al, 2011). Therefore, we investigated whether Plk1 contributes to primary cilia disassembly and whether the formation of the Dvl2–Plk1 complex is important for this event. To this end, hTERT-RPE cells were infected with control shLuciferase (shGL) or shPlk1 lentivirus, and then serum starved for 48 h to allow the cells to generate primary cilia. The resulting cells were then stimulated with serum to induce primary cilia disassembly, harvested at various time points after stimulation, and then analysed. In the control shGL cells, the level of Plk1 became progressively abundant as the cells proceeded through the cell cycle, whereas the level of Plk1 remained low in the shPlk1 cells (Figure 3A). Under these conditions, the shGL cells gradually resorbed primary cilia over time. Twenty-four hours after serum stimulation, most (∼90%) of the population completely resorbed primary cilia, while the remaining population displayed much-shortened (∼2 μm) primary cilia (Figure 3B and C) (note that cells infected with lentivirus exhibited somewhat less efficient primary cilia formation in comparison to those in Figure 3D below; see also Supplementary Figure S4I). In stark contrast, only a small fraction (∼7%) of the shPlk1 population detectably resorbed primary cilia during the same period of time, and the rest displayed substantially longer (∼5 μm) cilia than the control shGL cells (Figure 3B and C; see also Figure 4D below). Consistent with a role of Plk1 in primary cilia disassembly, a significantly higher shPlk1 population exhibited primary cilia in Figure 3C. A separate experiment with much narrower time intervals suggested that Plk1 is required for proper primary cilia disassembly from as early as 4–6 h after serum stimulation in a sustained manner (Supplementary Figure S5A). Both of the shGL and shPlk1 cells entered the cell cycle efficiently following serum stimulation, as evidenced by the accumulated Cyclin A-positive population (Figure 3C) and by flow cytometry analyses (Supplementary Figure S5B; note that the shPlk1 cells do not show a detectable level of cell-cycle delay until ∼12–18 h after serum stimulation). These observations suggest that the observed delay in primary cilia disassembly in shPlk1 cells is not the consequence of an altered cell cycle in these cells. Measurement of Plk1 kinase activity in these cells revealed that the shGL cells, but not the shPlk1 cells, exhibited a gradually increasing level of Plk1 activity as the cells proceeded through the cell cycle (Figure 3C).
Figure 3.
Requirement of Plk1 activity for proper disassembly of primary cilia. (A, B) hTERT-RPE cells were infected with lentiviruses expressing shGL or shPlk1. Forty-eight hours after serum starvation, the cells were stimulated with serum, harvested, and then subjected to immunoblotting (A) and immunostaining (B) analyses. Magnified images of cilia are shown in the enlarged boxes in (B). (C) From the immunostained samples in (B), cells with primary cilia were quantified (bar graphs). A fraction of the total lysates in (A) was used to determine Plk1 activity using an ELISA-based assay (solid lines) (Park et al, 2009). To monitor cell-cycle progression, Cyclin A-positive cells were quantified (dotted lines). Error bars, standard deviation from more than three independent experiments. Note that a low but significant level of Plk1 activity was detectable even at the early stages after serum stimulation. (D) Control parental hTERT-RPE cells or hTERT-RPE Plk1-as cells lacking endogenous Plk1 but expressing the indicated constructs were starved for 48 h and stimulated with serum. The resulting cells were fixed at the indicated time points after stimulation and immunostained as in (B) to quantify the cells with primary cilia. Error bars, standard deviation. (E) Total lysates prepared from the 24-h samples in (D) were subjected to immunoblotting analyses and ELISA-based kinase assays (graph) to determine the level of Plk1 activity. Arrowhead indicates endogenous Plk1 that is not detectable in the hTERT-RPE Plk1-as cells deleted of the genomic PLK1 locus. Asterisks, EGFP–Plk1 degradation products. For graphs in (C, D), >300 cells were counted from the samples at each time point. Figure source data can be found with the Supplementary data.
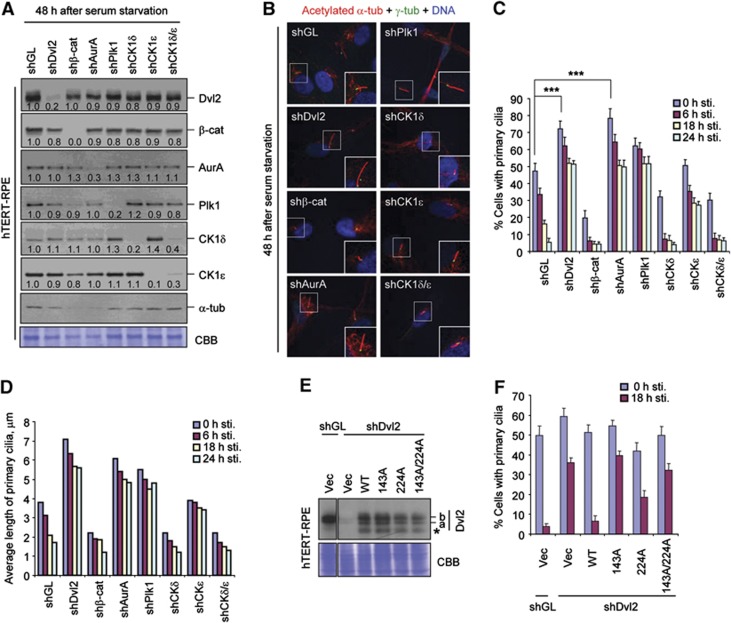
Figure 4.
Induction of primary cilia disassembly by the CK1ε-dependent Plk1–Dvl2 p-S143/p-T224 complex. (A–D) hTERT-RPE cells infected with the indicated sh-lentiviruses were first starved for 48 h. A set of the resulting cells was harvested for immunoblotting (A) or immunostaining (B) analyses. Enlarged images of cilia are shown in the boxes at the bottom right corner (B). Another set of the starved cells was subsequently stimulated with serum, fixed at the indicated time point, and immunostained. The cells with primary cilia were then counted (C) and the average lengths of primary cilia among cilia-positive cells were quantified (D). Numbers in (A) indicate signal intensities relative to α-tubulin signals. Both anti-α-tubulin immunoblotting and Coomassie (CBB) staining were carried out for loading controls. Error bars, standard deviation from more than three independent experiments. Statistics: ***P<0.001 (unpaired two-tailed t-test). (E, F) hTERT-RPE cells expressing the indicated constructs were infected with lentivirus expressing either control shGL or shDvl2, and then immunoblotted (E). The resulting cells were starved for 48 h and then stimulated with serum. The cells harvested at the indicated time points after serum stimulation were immunostained and quantified (F). Note that cells infected with lentiviruses (expression or RNAi viruses) exhibited less efficient primary cilia formation. Asterisk, degradation product. Error bars, standard deviation from more than three independent experiments. For samples in (C, D, F), >300 cells were counted for each sample. Figure source data can be found with the Supplementary data.
To directly determine whether Plk1 kinase activity is required for primary cilia disassembly, we expressed control vector, kinase-inactive Plk1 (K82M) (Lee et al, 1995), or Plk1 wild-type (WT) in the hTERT-RPE Plk1-as mutant cells lacking both copies of the PLK1 locus but expressing a greatly deactivated Plk1-as allele (Burkard et al, 2007) (see also Figure 3E, below). We observed that the Plk1-as mutant expressing either control vector or Plk1 (K82M) exhibited a considerably delayed primary cilia disassembly (Figure 3D). By contrast, Plk1-as cells expressing WT Plk1, which exhibited Plk1 kinase activity at a level similar to that of control hTERT-RPE WT cells, disassembled primary cilia as efficiently as the control WT cells (Figure 3D and E). It has been reported that a mitotic kinase, AurA, promotes primary cilia disassembly through the interaction with a pro-metastatic scaffolding protein, HEF1 (Pugacheva et al, 2007). Remarkably, treatment of cells with a Plk1 inhibitor, BI 2536, attenuated the ciliary disassembly process as effectively as that of an AurA inhibitor, VX-680 (Supplementary Figure S6A–C), further corroborating the notion that Plk1 activity is critically required for proper primary cilia disassembly.
Interaction of Plk1 with the S143 or T224 motif of Dvl2 is required for proper primary cilia disassembly
We next examined whether CK1δ/CK1ε-dependent regulation of the Dvl2–Plk1 interaction is important for primary cilia disassembly by depleting each of the components involved in this event. hTERT-RPE cells silenced for β-catenin and AurA were also included for comparison. Following depletion, cells were serum starved for 48 h and then subjected to immunoblotting analyses. Depletion of each of the components did not significantly alter the levels of other proteins (Figure 4A). Under these conditions, the cells depleted of Dvl2, AurA, or Plk1 exhibited substantially longer primary cilia than the cells depleted of control shGL, while the cells depleted of CK1ε displayed moderately longer cilia than the control cells (Figure 4B). Consistent with these observations, depletion of any one of Dvl2, AurA, Plk1, and CK1ε greatly retarded primary cilia disassembly following serum stimulation (Figure 4C and D; see also Supplementary Figure S7). Notably, in comparison to the control shGL cells, a significantly increased fraction of the shDvl2, shAurA, shPlk1, and shCK1ε cells displayed primary cilia at the 0 time point (Figure 4C). Given that primary cilia are regulated through dynamic assembly and disassembly processes, this could be due to impaired primary cilia disassembly process in these cells even under serum-starved conditions. Although the effect of the depletion of β-catenin or CK1δ on primary cilia disassembly was difficult to determine due to an inefficient cilia generation after serum starvation (Figure 4C and D), these components did not appear to significantly contribute to this process. Rather, they may play a role in primary cilia assembly process.
Since CK1ε directly phosphorylates Dvl2 at S143 and T224 and generates Plk1 docking sites, we then investigated whether CK1ε-dependent Dvl2 phosphorylation is important for the Dvl2–Plk1 complex-dependent primary cilia disassembly. To this end, we generated hTERT-RPE cells stably expressing various forms of Dvl2, which were then silenced for either control Luciferase (shGL) or Dvl2 (shDvl2) (Figure 4E). Expression of Dvl2 forms partially sensitive to shDvl2 (containing one base mismatch to shDvl2; see Materials and methods for details) was necessary to express these proteins at a level similar to that of endogenous Dvl2 after shDvl2 treatment. Upon serum starvation, the shGL cells expressing control vector efficiently assembled primary cilia, which were then almost completely resorbed 18 h after serum stimulation (Figure 4F). As expected, the shDvl2 cells expressing control vector showed a significant impairment in primary cilia disassembly, and expression of WT Dvl2 rescued this defect efficiently (Figure 4F). Under these conditions, cells expressing the S143A or S143A/T224A double mutant exhibited a severely delayed cilia resorption. However, cells expressing the T224A mutant (which contains the WT S143 residue) displayed only a moderate level of defect in this process (Figure 4F). Consistent with these findings, even under serum-starved conditions, Plk1 PBD interacted with Dvl2 in a manner that required the S143 and, to a less degree, T224 residues (Supplementary Figure S8). This interaction was greatly enhanced 3 h after serum stimulation (Supplementary Figure S8A). Since the S143 and T224 residues are also similarly required for PBD binding in asynchronously growing cells (Figure 1E), the observed Dvl2–Plk1 complex formed under serum-starved conditions may likely persist throughout the entire cell cycle to promote primary cilia disassembly. Analyses of the shDvl2 cells expressing shRNA-insensitive Dvl2 constructs (containing five base mismatches to shDvl2; see Materials and methods for details) yielded similar results (Supplementary Figure S9A and B). Notably, mutation of S143 or T224 to either aspartic acid or glutamic acid to mimic the negatively charged phosphate group failed to enhance the Dvl2–Plk1 interaction (Supplementary Figure S9C). This finding suggests that a phosphorylated serine or threonine residue is critically required for PBD binding, as observed previously (Kang et al, 2006; Soung et al, 2009; Johmura et al, 2011).
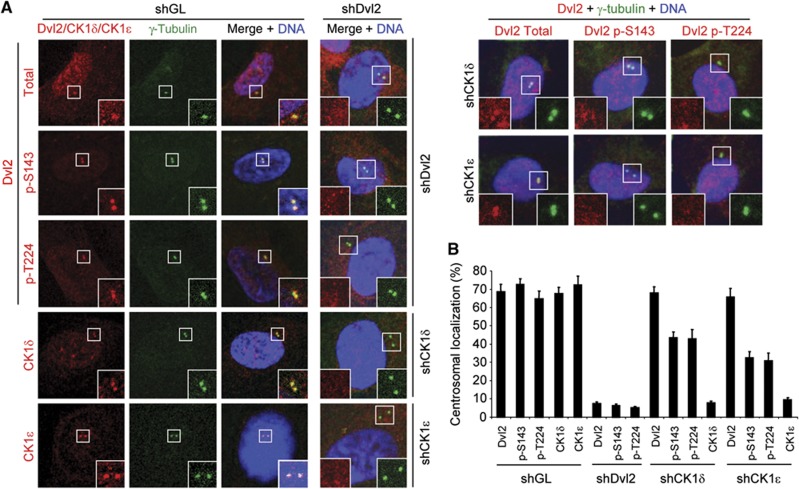
We next examined the subcellular localization patterns of Dvl2 and its phosphorylated forms in hTERT-RPE cells. Our results showed that Dvl2 localizes to interphase centrosomes and also weakly to nucleus, while the Dvl2 p-S143 and p-T224 epitopes were found to be tightly associated with centrosomes (Figure 5A). In addition, both CK1δ- and CK1ε-specific fluorescent signals were clearly associated with interphase centrosomes, although the CK1δ signals were frequently less prominent than the CK1ε signals, particularly at the late stage of the cell cycle (see below). Consistent with the CK1δ- and CK1ε-dependent Dvl2 phosphorylation (Figure 2), depletion of either CK1δ or CK1ε greatly diminished the levels of centrosomally localized p-S143 and p-T224 epitopes without altering the level of Dvl2 at centrosomes (Figure 5A and B).
Figure 5.
Centrosomal localization of Dvl2 p-S143/p-T224, CK1δ, and CK1ε in hTERT-RPE cells. (A, B) Asynchronously growing hTERT-RPE cells were silenced for either control luciferase (shGL), shDvl2, shCK1δ, or shCK1ε, and the resulting cells were then immunostained with the indicated antibodies after extraction with 0.5% Triton X-100 (A) and quantified (B). More than 300 cells from each of three independent experiments were counted for each sample. Error bars, standard deviation.
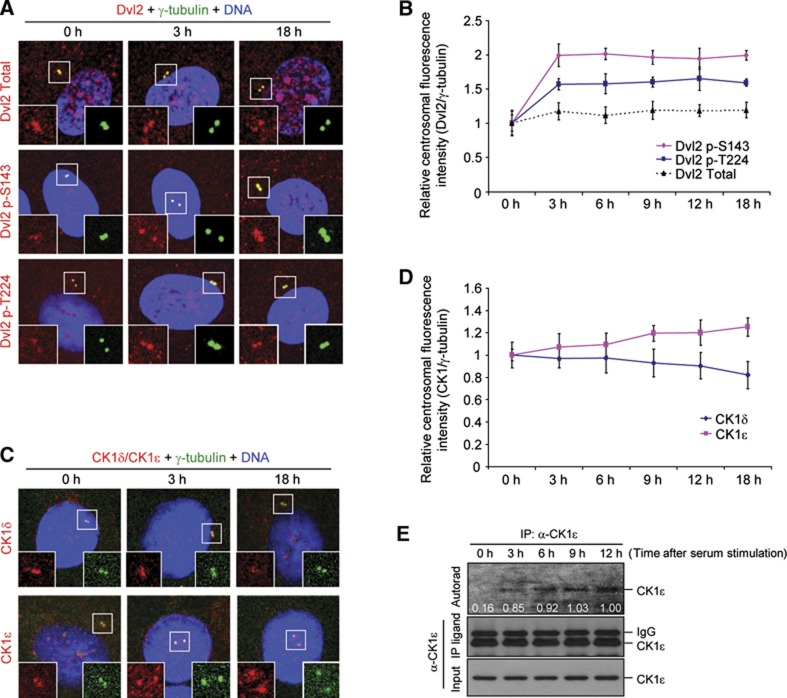
To closely examine the localization patterns of these proteins during the cell cycle, we immunostained hTERT-RPE cells following serum stimulation. The results showed that both centrosome-associated p-S143 and p-T224 epitopes were greatly enhanced shortly (as early as 3 h) after serum stimulation, although the level of total Dvl2 at the centrosomes remained largely unchanged (Figure 6A and B). As expected if CK1ε were primarily responsible for the promotion of primary cilia disassembly (Figure 4C and D), the level of centrosome-associated CK1ε signals gradually increased following the addition of serum and became most abundant at mitotic centrosomes (Figure 6C and D; Supplementary Figure S10), the stage where primary cilia are completely resorbed. In contrast, the level of CK1δ signals at this site steadily decreased over time and remained low at mitotic centrosomes (Figure 6C and D; Supplementary Figure S10). To examine whether the sharp increase in the p-S143 and p-T224 epitopes at the 3-h time point in Figure 6B could be attributable to the activation of CK1ε following serum stimulation, hTERT-RPE cells were starved for 48 h and stimulated with serum. Subsequent in-vitro immunoprecipitation kinase assays using total cellular lysates revealed that the autophosphorylation activity of CK1ε was markedly increased 3 h after serum stimulation and then was largely remained at similar levels (Figure 6E). Taken in aggregates, the above findings indicate that total CK1ε population becomes activated in response to serum stimulation, and that centrosome-localized CK1ε, but not CK1δ, plays a critical role in primary cilia disassembly.
Figure 6.
Differential regulation of centrosome-localized Dvl2 p-S143/p-T224, CK1δ, and CK1ε signals following serum stimulation. (A–D) Forty-eight hours after serum starvation, hTERT-RPE cells were stimulated with serum for the indicated lengths of time. The resulting cells were then immunostained (A, C) and centrosomal fluorescence intensities were quantified (B, D). Signal intensities from >50 cells in (B, D) were quantified for each time point from three independent experiments. Error bars, standard deviation from three independent experiments. (E) Cells prepared similarly as in (A–D) were subjected to immunoprecipitation kinase assays in the presence of [γ-32P]ATP, and the resulting samples were analysed by autoradiogram and immunoblotting analyses. Figure source data can be found with the Supplementary data.
Wnt5a-induced Dvl2–Plk1 complex regulates HEF1/AurA-dependent primary cilia disassembly
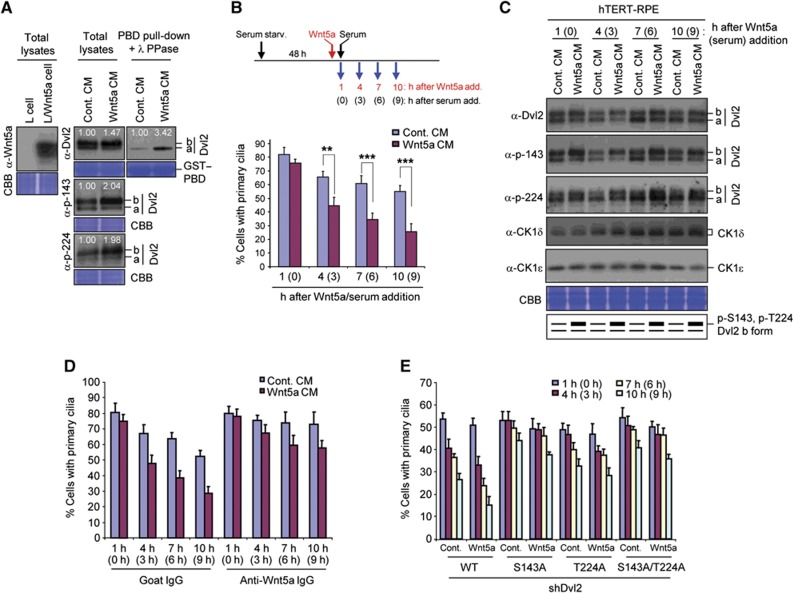
We then investigated how the CK1ε-dependent Dvl2–Plk1 complex is regulated and how it brings about primary cilia disassembly. Since Plk1 does not appear to contribute to the canonical Wnt pathway (Supplementary Figure S3), we first examined whether the CK1ε–Dvl2–Plk1 axis-dependent cilia disassembly pathway can be triggered by the activation of the non-canonical Wnt pathway. To this end, hTERT-RPE cells were treated with either a control conditioned medium (CM) or CM containing Wnt5a, one of the non-canonical pathway ligands, for 6 h, and then analysed. As reported previously (Bryja et al, 2007), treatment of hTERT-RPE cells with Wnt5a modestly induced hyperphosphorylated Dvl2 form (b form) and significantly increased the levels of the p-S143 and p-T224 epitopes (Figure 7A). Consequently, the Wnt5a treatment enhanced the Dvl2–Plk1 PBD interaction more than three-fold (Figure 7A) and substantially accelerated primary cilia disassembly (Figure 7B) in a manner that required CK1ε (Supplementary Figure S11A). As would be expected if the CK1ε-dependent generation of the p-S143 and p-T224 epitopes were important for mediating the Wnt5a-dependent processes, the levels of these two phospho-epitopes were increased as rapidly as 1 h after the Wnt5a treatment (Figure 7C). Remarkably, neutralization of the Wnt5a activity by the addition of an anti-Wnt5a-specific antibody into the CM greatly delayed primary cilia disassembly (Figure 7D), suggesting that Wnt5a is one of the critical ligands that stimulate this process. The S143A mutation markedly decelerated the Wnt5a-stimulated cilia disassembly, while the T224A mutation delayed this process at a somewhat reduced level (Figure 7E). It is notable that the defect in primary cilia disassembly in the S143A mutant was manifest as early as 3–6 h after serum stimulation (4–7 h after Wnt5a treatment), thus diminishing the likelihood of an indirect effect by an altered cell cycle. Taken together, the above observations suggest that Wnt5a induces primary cilia disassembly via the S143- and T224-dependent formation of the Dvl2–Plk1 complex. In line with the role of Wnt5a in primary cilia disassembly, Wnt5a became abundant following serum stimulation (Supplementary Figure S11B).
Figure 7.
Regulation of primary cilia disassembly by Wnt5a-dependent formation of the Dvl2–Plk1 complex. (A) Total cellular lysates prepared from mouse L fibroblasts expressing either control vector or Wnt5a were subjected to immunoblotting analyses (left). hTERT-RPE cells serum starved for 24 h were treated with either control or Wnt5a-containing CM for 6 h, harvested, and subjected to PBD pull-downs. Precipitates were treated with λ phosphatase (PPase) before analyses (right). Numbers indicate the levels of the Dvl2 b form or total Dvl2 bound to PBD. (B, C) hTERT-RPE cells serum starved for 48 h were treated with either control or Wnt5a CM 1 h prior to serum stimulation. The cells harvested at the indicated time points after the stimulation were immunostained and quantified (B) or immunoblotted (C). Statistics: **P<0.01, ***P<0.001 (unpaired two-tailed t-test). Note that treatment of cells with Wnt5a CM enhances the levels of the p-S143 and p-T224 epitopes as early as 1 h after the treatment. Error bars, standard deviation from more than three independent experiments. (D) hTERT-RPE cells serum starved for 48 h were treated with control or Wnt5a CM pre-incubated with either control IgG or anti-Wnt5a IgG. One hour after the treatment, cells were stimulated with serum, fixed according to the schedule in (B), immunostained, and quantified. Error bars, standard deviation. (E) hTERT-RPE cells expressing various Dvl2 constructs were depleted of endogenous Dvl2, serum starved for 48 h, and treated with control or Wnt5a CM for 1 h prior to serum stimulation. The cells were fixed according to the schedule in (B), immunostained, and quantified. The efficiency of primary cilia generation was somewhat diminished in the cells doubly infected with lentiviruses (for expression and RNAi viruses). For the quantification in (B, D, E), >300 cells were counted for each sample. Error bars, standard deviation from more than three independent experiments. Figure source data can be found with the Supplementary data.
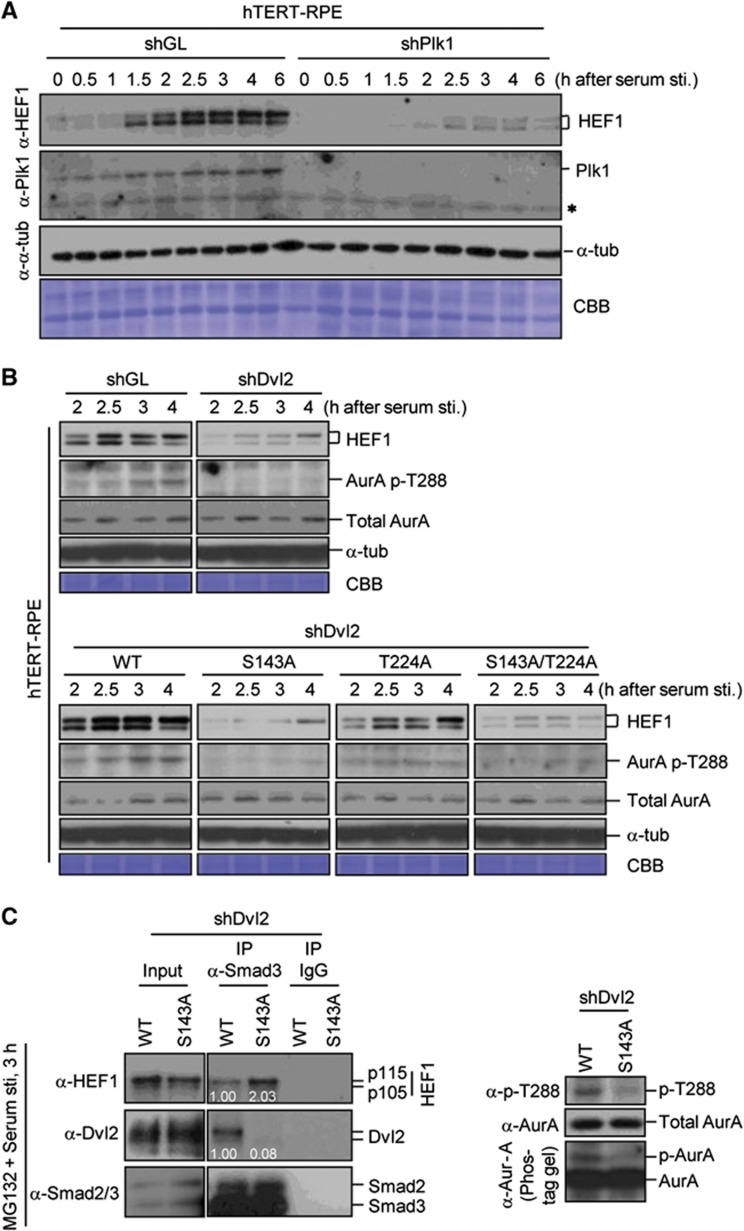
It has been shown that HEF1 binds to AurA and activates the latter, which in turn activates HDAC6 to promote primary cilia disassembly (Pugacheva et al, 2007). HEF1 is phosphorylated by multiple kinases and its protein level is cell cycle regulated (Law et al, 1998; Liu et al, 2000). Thus, we investigated whether the Dvl2–Plk1 complex induces primary cilia disassembly by regulating the level of HEF1. We observed that the level of HEF1 becomes abundant, as cells enter the cell cycle following serum stimulation, and depletion of Plk1 drastically decreased the level of HEF1 (Figure 8A). Depletion of Dvl2 (shDvl2) or impairment of the Dvl2-dependent Plk1 function by the S143A or T224A mutation also resulted in the diminished level of HEF1 without altering the level of the HEF1 transcript (Figure 8B; Supplementary Figure S12A). Provision of WT Dvl2, but not the S143A mutant, into the Dvl2 RNAi cells restored the level of HEF1 (Figure 8B; Supplementary Figure S12B) and, consequently, the level of activated AurA (i.e., AurA p-T288 epitope) (Figure 8B). As expected, acute inhibition of Plk1 activity substantially diminished the HEF1 stability (Supplementary Figure S12C). Hence, Dvl2-dependent Plk1 activity is required for proper HEF1 stability and, therefore, HEF1/AurA-dependent primary cilia disassembly.
Figure 8.
Regulation of HEF1 stability and AurA activity by the Dvl2–Plk1 complex. (A) hTERT-RPE cells silenced for either control luciferase (shGL) or Plk1 (shPlk1) were serum starved for 48 h. Following serum stimulation, samples were harvested at the indicated time points and immunoblotted. The same membrane was stained with Coomassie (CBB). (B) Various hTERT-RPE cells silenced for either control luciferase (shGL) or Dvl2 (shDvl2) were serum starved for 48 h. Following serum stimulation, cells were harvested for immunoblotting analyses. Note that the levels of HEF1 and the AurA p-T288 epitope were greatly diminished in the shDvl2, the S143A, and the S143A/T224A double mutant cells. (C) hTERT-RPE cells expressing WT Dvl2 or the S143A mutant were depleted of endogenous Dvl2, serum starved for 48 h, and treated with MG132 for 2 h prior to serum stimulation. Cells harvested 3 h after serum stimulation were subjected to immunoprecipitation analyses (left). Smad2 was detectable in the Smad3 immunoprecipitates because of the interaction between these two proteins. Total lysates were immunoblotted (right) to determine the level of AurA phosphorylation by 10% SDS–PAGE without (upper and middle) or with 50 μM Phos-tag (0.2 mmol/l MnCl2(H2O)4 and 0.02 mmol/l Phos-tag acrylamide AAL-107) (Zeng et al, 2010) (lower). Figure source data can be found with the Supplementary data.
It has been reported that Smad3 binds to HEF1 directly and also interacts with APC10 to form an ubiquitin-dependent degradation complex for HEF1 (Liu et al, 2000; Nourry et al, 2004). To understand the underlying mechanism of how the Dvl2–Plk1 complex modulates the HEF1 stability, we first investigated whether the Dvl2–Plk1 complex directly phosphorylates and enhances the HEF1 stability. To this end, we generated the HEF1(7A) mutant lacking 7 in-vivo phosphorylation sites that are also phosphorylated by Plk1 in vitro (Supplementary Figure S13A–C). However, the 7A mutations neither influenced the level of Smad3 binding nor significantly altered the HEF1 stability in serum-stimulated hTERT-RPE cells (Supplementary Figure S13D and E).
Since Smad3 also forms a complex with Dvls (Warner et al, 2003, 2005), we then investigated whether the Dvl2–Plk1 complex restricts Smad3-dependent HEF1 degradation by enhancing the formation of the Smad3–Dvl2 complex. Using the Dvl2 RNAi cells expressing either WT Dvl2 or the Dvl2(S143A) mutant, we found that Smad3 interacted with both WT Dvl2 and HEF1 (Figure 8C, left). Notably, failure to detect the Dvl2–HEF1 interaction under the same conditions suggests that Smad3 forms two distinct complexes with either Dvl2 or HEF1. Strikingly, in cells expressing the Plk1-binding incompetent Dvl2(S143A) mutant, the Smad3–Dvl2(S143A) interaction was drastically diminished, whereas the Smad3–HEF1 interaction was significantly enhanced (Figure 8C, left). The inverse correlation between the level of the Smad3–Dvl2 complex and that of the Smad3–HEF1 complex suggests that the formation of these two complexes is regulated in a reciprocal manner. As expected, cells expressing WT Dvl2, which does not favour the formation of the Smad3–HEF1 degradation complex, exhibited a significantly higher level of the T288-phosphorylated, activated AurA than did the S143A mutant (Figure 8C, right). We also observed that inhibition of the kinase activity of Plk1 (BI 2536) or CK1δ/CK1ε (IC261) substantially enhanced the Smad3–HEF1 interaction, whereas it decreased the Smad3–Dvl2 interaction (Supplementary Figure S13F), suggesting that the kinase activity of these enzymes is important to downregulate the Smad3–HEF1 complex and therefore to promote the HEF1 stability.
Discussion
In this study, we demonstrate the existence of a non-mitotic function of Plk1 in primary cilia disassembly and an unexpected role of Dvl2 in this process. Our results showed that, in addition to the well-studied Plk1 functions during mitosis, Plk1 regulates primary cilia disassembly through its interaction with Wnt5a–CK1ε-dependent p-S143 and p-T224 epitopes of Dvl2. The formation of the Dvl2–Plk1 complex appears to be critical for the stabilization of HEF1 and activation of the HEF1/AurA complex, consequently triggering HEF1/AurA-dependent primary cilia disassembly as cells enter the cell cycle (Figure 9). Thus, Plk1 provides the missing link between Wnt5a–CK1ε-induced Dvl2 phosphorylation and HEF1/AurA-dependent primary cilia disassembly. Consistent with the role of Plk1 during this process, a low but significant level of Plk1 activity was detectable sufficiently earlier than the stage during which HEF1 began to accumulate following serum stimulation (Figures 3C and 8A). This finding suggests that a low level of Plk1 activity could be sufficient to stabilize HEF1 and to bring about HEF1/AurA-dependent primary cilia disassembly at the early stages of the cell cycle. Since AurA functions in a positive-feedback loop to phosphorylate and activate Plk1 at the time of mitotic entry (Macůrek et al, 2008; Seki et al, 2008), Plk1-dependent activation of the HEF1/AurA complex may in turn activate Plk1 in a reciprocal manner (Figure 9), thereby allowing a rapid accumulation of local Plk1 and AurA activities to trigger this event.
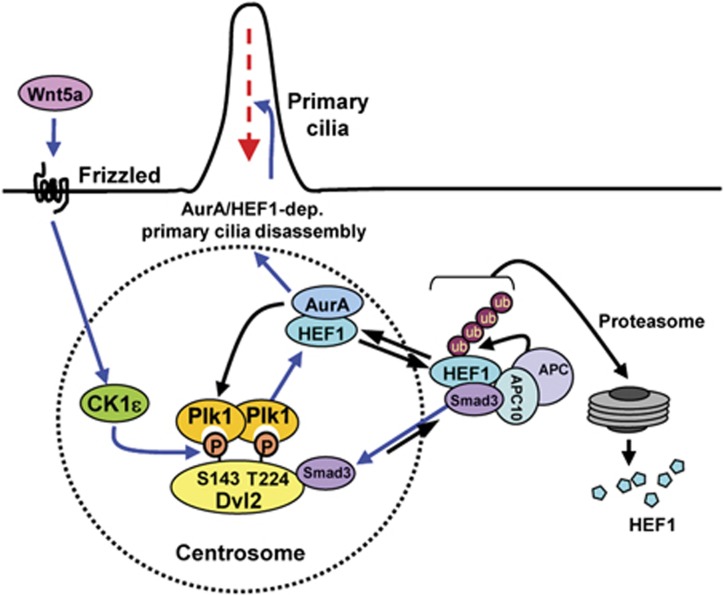
Figure 9.
Model illustrating primary cilia disassembly by the Wnt5a–CK1ε–Dvl2–Plk1 axis. Wnt5a stimulation triggers CK1ε-dependent phosphorylation of Dvl2 at S143 and T224, and induces the formation of the Dvl2–Plk1 complex. Dvl2-bound Plk1 enhances the Dvl2–Smad3 interaction, thus diminishing the level of the HEF1 population associating with the Smad3/APC10-containing APC complex (Liu et al, 2000; Nourry et al, 2004) (see the unequal blue arrow). This event leads to the stabilization of HEF1, and therefore activation of HEF1/AurA-dependent cilia disassembly pathway. Loss of the p-S143/p-T224-dependent Dvl2–Plk1 interaction reverses this event by enhancing the Smad3–HEF1 interaction and the Smad3–APC10-dependent HEF1 degradation. Since AurA has been shown to phosphorylate and activate Plk1 (Macůrek et al, 2008; Seki et al, 2008), the AurA/HEF1 complex may activate Plk1 in a positive-feedback loop. Blue arrows denote Wnt5a-induced primary cilia disassembly pathway.
Our results showed that both CK1δ and CK1ε are required for proper generation of the Dvl2 p-S143 and p-T224 epitopes. Interestingly, however, depletion of CK1ε, but not CK1δ, significantly impaired primary cilia disassembly. This finding suggests that, although CK1δ and CK1ε appear to exhibit similar biochemical properties, they are functionally distinct at the basal bodies/centrosomes during the cell cycle. In support of this view, CK1ε gradually accumulated at centrosomes as cells proceeded through the cell cycle, whereas CK1δ progressively disappeared from these structures (Figure 6C and D; Supplementary Figure S10). Consistent with the critical role of CK1ε-dependent Dvl2 phosphorylation in the regulation of primary cilia disassembly, CK1ε was greatly activated after serum stimulation, and that both CK1ε and Dvl2 were colocalized to basal bodies (Supplementary Figure S14A). In-vitro binding analysis suggested that CK1ε interacts with Dvl2 directly (Supplementary Figure S14B), as has been hinted previously (Corbit et al, 2008).
Our results showed that at the centre of the mechanism of Plk1-mediated primary cilia disassembly lies the formation of the Dvl2–Plk1 complex. Consistently, the degree of primary cilia assembly appeared to be inversely correlated with the level of Dvl2 (Figure 4B–D; Supplementary Figure S4A–E). Although depletion of Dvl2 substantially increased the fraction of cells with primary cilia, however, none of the mitotic shDvl2 cells possessed primary cilia (Supplementary Figure S15A). This finding is in line with the previous observation that primary cilia disassembly is a prerequisite for mitotic entry (Pan and Snell, 2007; Santos and Reiter, 2008).
The molecular basis of how the Dvl2–Plk1 complex regulates the HEF1 stability is beginning to emerge. Studies show that HEF1 is degraded through the Smad3–APC10-dependent ubiquitin-mediated degradation complex (Liu et al, 2000; Nourry et al, 2004). We found that the Dvl2–Plk1 complex enhances the Smad3–Dvl2 interaction in a kinase activity-dependent manner, whereas it diminishes the Smad3–HEF1 interaction and therefore increases the HEF1 stability (Figure 8C; Supplementary Figure S13F). Conversely, disruption of the complex by the S143A mutation reversed all of these events (Figure 8C). Unlike HEF1, however, Dvl2 appears to be stable even under Plk1 RNAi conditions (compare Figure 8A with Figure 3A), suggesting that Smad3 binding to the Dvl2–Plk1 complex may not directly regulate the Dvl2 stability. One possibility is that the formation of the Dvl2–Plk1 complex enables Dvl2 to serve as a scaffold to sequester Smad3 and prevent it from associating with HEF1 for degradation (Figure 9). This model suggests that the Dvl2–Plk1 complex intricately modulates the level of Smad3 binding to either Dvl2 or HEF1 in a reciprocal manner. Although how the Dvl2–Plk1 complex alters these dynamic processes remains unknown at present, the requirement of Plk1 kinase activity suggests that Dvl2-bound Plk1 may phosphorylate either Dvl2 or a third protein to regulate these events.
Then, how is the primary cilia disassembly pathway activated? We observed that a non-canonical ligand, Wnt5a, promotes the Dvl2–Plk1 interaction and facilitates primary cilia disassembly (Figure 7A and B). Other studies have shown that non-canonical Wnt ligands facilitate Rac and Rho-dependent actin polymerization (Habas et al, 2001; Capelluto et al, 2002; Marlow et al, 2002; Veeman et al, 2003; Habas and Dawid, 2005), an event that promotes primary cilia disassembly (Bershteyn et al, 2010; Kim et al, 2010). These observations together with our data reported here underscore the importance of the non-canonical Wnt pathway in regulating various cellular processes, including primary cilia disassembly.
Notably, our results obtained from hTERT-RPE and NIH 3T3 cells suggest that Dvl2 plays a key role in mediating primary cilia disassembly. This finding is consistent with an earlier observation that depletion of Dvl2 leads to a longer primary cilia in cells derived from a PCS syndrome patient (Miyamoto et al, 2011). On the other hand, Park et al (2008)) reported that Dvl promotes ciliogenesis in multiciliated Xenopus embryonic epidermal cells by mediating the docking of basal bodies to the apical plasma membrane. The apparent differences in the role of Dvl2 may stem from dissimilar mechanisms of regulating non-motile primary cilia and motile cilia. Further studies are necessary to better comprehend the differential role of Dvl in regulating different types of cilia in evolutionarily distinct organisms.
Aside from the role of Dvl2 in primary cilia disassembly, Dvl2 appears to have a role in mitosis. Recently, Kikuchi et al (2010) reported that Plk1 interacts with the aa354–423 region of Dvl2 and phosphorylates the latter to regulate proper spindle orientation and stable microtubule-kinetochore attachment. However, none of the three potential PBD-binding motifs found within this region of Dvl2 yielded a significant level of PBD binding (Figure 1D), hinting that the reported interaction between Plk1 and the aa354–423 region of Dvl2 could be phospho-independent. These findings further suggest that the Dvl2–Plk1 complex could regulate distinct physiological events at different stages of the cell cycle. Interestingly, depletion of Dvl2 in HeLa cells frequently leads to a mitotic arrest with multipolar spindles under our experimental conditions (Supplementary Figure S15B). On the other hand, depletion of Dvl2 in hTERT-RPE cells failed to induce multipolar spindle morphologies, and rather diminished the fraction of mitotic cells (Supplementary Figure S15B). Since depletion of Plk1 or its binding target, Dvl2, in hTERT-RPE cells does not significantly delay the early stages of the cell cycle following serum stimulation, defect in primary cilia disassembly observed in the shPlk1 or shDvl2 cells may have caused a delay in mitotic entry until cells completely resorb their primary cilia. It is noteworthy that, unlike hTERT-RPE cells, HeLa cells rarely possess primary cilia (Alieva et al, 1999; Pan and Snell, 2007). Therefore, the apparent discrepancy in the phenotypes associated with the depletion of Dvl2 could be attributable to the fact that HeLa cells can readily proceed into mitosis and reveal the Dvl2 RNAi defect(s) in the absence of a primary cilia-imposed delay into mitosis.
Here, we report identification of a primary cilia disassembly pathway—CK1ε–Dvl2–Plk1–HEF1/AurA, in which a non-canonical Wnt5a (or perhaps other functionally related Wnts) elicits a cascade of phosphorylation-dependent events leading to the activation of HEF1/AurA. However, although this linear pathway appears attractive, considering that cilia assembly/disassembly is intricately regulated by various cellular processes, including actin dynamics, cell–cell adhesion, and vesicle trafficking (Dawe et al, 2007; Kim et al, 2010; Pitaval et al, 2010), these dynamic processes are likely regulated by multiple distinct pathways interconnected to one another. Thus, identification of novel components and additional biochemical processes critical for integrating signals from multiple cellular cues will be a key step to fully comprehend the intricate nature of dynamic primary cilia assembly/disassembly processes. Given the reciprocally inhibitory relationship between ciliogenesis and cell proliferation (Quarmby and Parker, 2005; Plotnikova et al, 2008), further investigations on the role of the Wnt5a–CK1ε–Dvl2–Plk1 axis in the primary cilia disassembly will be important to better understand the mechanisms underlying Dvl and Plk1-mediated oncogenesis and to provide new insights into the therapeutic interventions against cancers and other cilia-related human diseases.
Materials and methods
Plasmid constructions, virus generation and infection, cell culture techniques, flow cytometry (FACS) analyses
Detailed information can be found in the Supplementary data. All the lentivirus-based shRNA and synthetic siRNA target sequences are provided in Supplementary Table S1.
Immunoprecipitation, λ-phosphatase treatment, antibody production, and immunoblotting
Immunoprecipitation was carried out essentially as described previously (Lee et al, 1995). Where indicated, immunoprecipitates were treated with λ phosphatase before further analyses. Details are provided in Supplementary data.
Anti-Dvl2 p-S143 and p-T224 antibodies were generated using synthetic peptides, Ac-SFHPNVSS-pS-HENLEPE-NH2 (p-S143 is underlined) and Ac-MSRFSSS-pT-EQSSASR-NH2 (p-T224 is underlined), respectively (in collaboration with Epitomics Inc., Burlingame, CA), and then affinity purified. To detect specific p-S143 and p-T224 signals, immunoblotting analysis was carried out in the presence of 5 μg/ml of corresponding non-phospho S143 or T224 peptide.
All the antibodies used in this study are summarized in Supplementary Table S2.
In-vitro kinase assays, in-vivo ubiquitinylation assay, ELISA-based Plk1 kinase assay, mass spectrometry analysis
Details are provided in Supplementary data.
GST–PBD binding, peptide-binding, and peptide competition assays
GST–PBD WT and its respective GST–PBD (H538A K540M) phosphopincer mutant (AM) (Elia et al, 2003) (a gift of Michael B Yaffe, Massachusetts Institute of Technology, Cambridge, MA) were expressed in Escherichia coli BL21 (DE3) and purified with GSH-agarose. Details on GST–PBD pull-down assays, and related peptide-binding and peptide competition assays are described in Supplementary data.
Primary cilia disassembly and immunofluorescence studies
Details are provided in Supplementary data.
Supplementary Material
Acknowledgments
We thank Sean B Lee and Jeff R Rubin for critical reading of the manuscript; Eric R Fearon, Chou-Zen Giam, Erica A Golemis, Prasad Jallepalli, Sean B Lee, Randall T Moon, Elena N Pugacheva, Philip A Sharp, Shiela A Stewart, Dave Virshup, and Michael B Yaffe for reagents. This work was supported in part by National Cancer Institute’s (NCI’s) intramural grants (KSL and TDV), US Food and Drug Administration (FDA) grant (L-RY), Korea Basic Science Institute (KBSI)’s International Joint Research programme Grant F30601 (JKB), and the World Class Institute (WCI) research programme of the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology of Korea (BYK). Revision experiments, which yielded several main and supplementary figures, were performed by KHL at his present address. The views presented in this article do not necessarily reflect those of the NCI, US FDA, KBSI, and WCI.
Author contributions: KHL, YJ, LRY, JEP, MZ, and KSL designed the experiments; KHL, YJ, YG, JKB, and MZ performed the experiments; KHL, YJ, LRY, JEP, YG, JKB, MZ, TDV, BYK, and KSL analysed the data; KHL, LRY, and KSL wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adams M, Smith UM, Logan CV, Johnson CA (2008) Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J Med Genet 45: 257–267 [DOI] [PubMed] [Google Scholar]
- Alieva IB, Gorgidze LA, Komarova YA, Chernobelskaya OA, Vorobjev IS (1999) Experimental model for studying the primary cilia in tissue culture cells. Membr Cell Biol 12: 895–905 [PubMed] [Google Scholar]
- Angers S, Moon RT (2009) Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10: 468–477 [DOI] [PubMed] [Google Scholar]
- Archambault V, Glover DM (2009) Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol 10: 265–275 [DOI] [PubMed] [Google Scholar]
- Barr FA, Sillje HH, Nigg EA (2004) Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol 5: 429–440 [DOI] [PubMed] [Google Scholar]
- Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK (2009) The primary cilium as a complex signaling center. Curr Biol 19: R526–R535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershteyn M, Atwood SX, Woo WM, Li M, Oro AE (2010) MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell 19: 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Schulte G, Rawal N, Grahn A, Arenas E (2007) Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J Cell Sci 120: 586–595 [DOI] [PubMed] [Google Scholar]
- Burkard ME, Randall CL, Larochelle S, Zhang C, Shokat KM, Fisher RP, Jallepalli PV (2007) Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc Natl Acad Sci USA 104: 4383–4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M (2002) The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature 419: 726–729 [DOI] [PubMed] [Google Scholar]
- Cheng KY, Lowe ED, Sinclair J, Nigg EA, Johnson LN (2003) The crystal structure of the human polo-like kinase-1 polo box domain and its phospho-peptide complex. EMBO J 22: 5757–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF (2008) Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol 10: 70–76 [DOI] [PubMed] [Google Scholar]
- Dahlberg CL, Nguyen EZ, Goodlett D, Kimelman D (2009) Interactions between casein kinase Iepsilon (CKIepsilon) and two substrates from disparate signaling pathways reveal mechanisms for substrate-kinase specificity. PLoS One 4: e4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport JR, Yoder BK (2005) An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol 289: F1159–F1169 [DOI] [PubMed] [Google Scholar]
- Dawe HR, Farr H, Gull K (2007) Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J Cell Sci 120: 7–15 [DOI] [PubMed] [Google Scholar]
- Elia AE, Rellos P, Haire L, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB (2003) The molecular basis for phospho-dependent substrate targeting and regulation of Plks by the polo-box domain. Cell 115: 83–95 [DOI] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H (2007) When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 8: 880–893 [DOI] [PubMed] [Google Scholar]
- Gao C, Chen YG (2010) Dishevelled: The hub of Wnt signaling. Cell Signal 22: 717–727 [DOI] [PubMed] [Google Scholar]
- Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM (2002) Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc Natl Acad Sci USA 99: 1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes JM, Katsanis N (2008) Ciliary function and Wnt signal modulation. Curr Top Dev Biol 85: 175–195 [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB (2005) Dishevelled and Wnt signaling: is the nucleus the final frontier. J Biol 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X (2001) Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107: 843–854 [DOI] [PubMed] [Google Scholar]
- He X (2008) Cilia put a brake on Wnt signalling. Nat Cell Biol 10: 11–13 [DOI] [PubMed] [Google Scholar]
- Johmura Y, Soung NK, Park JE, Yu LR, Zhou M, Bang JK, Kim BY, Veenstra TD, Erikson RL, Lee KS (2011) Regulation of microtubule-based microtubule nucleation by mammalian polo-like kinase 1. Proc Natl Acad Sci USA 108: 11446–11451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YH, Park J-E, Yu L-R, Soung N-K, Yun S-M, Bang JK, Seong Y–S, Yu H, Veenstra TD, Lee KS (2006) Self-regulation of Plk1 recruitment to the kinetochores is critical for chromosome congression and spindle checkpoint signaling. Mol Cell 24: 409–422 [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Niikura Y, Kitagawa K, Kikuchi A (2010) Dishevelled, a Wnt signalling component, is involved in mitotic progression in cooperation with Plk1. EMBO J 29: 3470–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG (2010) Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464: 1048–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimowski LK, Garcia BA, Shabanowitz J, Hunt DF, Virshup DM (2006) Site-specific casein kinase 1epsilon-dependent phosphorylation of Dishevelled modulates beta-catenin signaling. FEBS J 273: 4594–4602 [DOI] [PubMed] [Google Scholar]
- Knippschild U, Gocht A, Wolff S, Huber N, Löhler J, Stöter M (2005) The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal 17: 675–689 [DOI] [PubMed] [Google Scholar]
- Law SF, Zhang YZ, Klein-Szanto AJ, Golemis EA (1998) Cell cycle-regulated processing of HEF1 to multiple protein forms differentially targeted to multiple subcellular compartments. Mol Cell Biol 18: 3540–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Yuan Y-L, Kuriyama R, Erikson RL (1995) Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol Cell Biol 15: 7143–7151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YN, Gao Y, Wang HY (2008) Differential mediation of the Wnt canonical pathway by mammalian Dishevelleds-1, -2, and -3. Cell Signal 20: 443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Park JE, Qian WJ, Lim D, Gräber M, Berg T, Yaffe MB, Lee KS, Burke TR (2011) Serendipitous alkylation of a Plk1 ligand uncovers a new binding channel. Nat Chem Biol 7: 595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Elia AE, Law SF, Golemis EA, Farley J, Wang T (2000) A novel ability of Smad3 to regulate proteasomal degradation of a Cas family member HEF1. EMBO J 19: 6759–6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Macůrek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB, Medema RH (2008) Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 455: 119–123 [DOI] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L (2002) Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol 12: 876–884 [DOI] [PubMed] [Google Scholar]
- McKay RM, Peters JM, Graff JM (2001) The casein kinase I family in Wnt signaling. Dev Biol 235: 388–396 [DOI] [PubMed] [Google Scholar]
- Michaud EJ, Yoder BK (2006) The primary cilium in cell signaling and cancer. Cancer Res 66: 6463–6467 [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Porazinski S, Wang H, Borovina A, Ciruna B, Shimizu A, Kajii T, Kikuchi A, Furutani-Seiki M, Matsuura S (2011) Insufficiency of BUBR1, a mitotic spindle checkpoint regulator, causes impaired ciliogenesis in vertebrates. Hum Mol Genet 20: 2058–2070 [DOI] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N (2002) The promise and perils of Wnt signaling through beta-catenin. Science 296: 1644–1646 [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A (2004) WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 5: 691–701 [DOI] [PubMed] [Google Scholar]
- Nourry C, Maksumova L, Pang M, Liu X, Wang T (2004) Direct interaction between Smad3, APC10, CDH1 and HEF1 in proteasomal degradation of HEF1. BMC Cell Biol 5: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R (2005) Wnt signaling in disease and in development. Cell Res 15: 28–32 [DOI] [PubMed] [Google Scholar]
- Pan J, Snell W (2007) The primary cilium: keeper of the key to cell division. Cell 129: 1255–1257 [DOI] [PubMed] [Google Scholar]
- Park J-E, Li L, Park J, Knecht R, Strebhardt K, Yuspa SH, Lee KS (2009) Direct quantification of polo-like kinase 1 activity in cells and tissues using a highly sensitive and specific ELISA assay. Proc Natl Acad Sci USA 106: 1725–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Soung NK, Johmura Y, Kang YH, Liao C, Lee KH, Park CH, Nicklaus MC, Lee KS (2010) Polo-box domain: a versatile mediator of polo-like kinase function. Cell Mol Life Sci 67: 1957–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB (2008) Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 40: 871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M, Lénárt P, Peters JM (2008) Polo on the Rise-from Mitotic Entry to Cytokinesis with Plk1. Dev Cell 14: 646–659 [DOI] [PubMed] [Google Scholar]
- Pitaval A, Tseng Q, Bornens M, Théry M (2010) Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. J Cell Biol 191: 303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikova OV, Golemis EA, Pugacheva EN (2008) Cell cycle-dependent ciliogenesis and cancer. Cancer Res 68: 2058–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MA (2006) CKI, there’s more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev 20: 399–410 [DOI] [PubMed] [Google Scholar]
- Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA (2007) HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129: 1351–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby LM, Parker JD (2005) Cilia and the cell cycle? J Cell Biol 169: 707–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos N, Reiter JF (2008) Building it up and taking it down: the regulation of vertebrate ciliogenesis. Dev Dyn 237: 1972–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley ES, Nachury MV (2010) The perennial organelle: assembly and disassembly of the primary cilium. J Cell Sci 123: 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki A, Coppinger JA, Jang CY, Yates JR, Fang G (2008) Bora and the kinase Aurora A cooperatively activate the kinase Plk1 and control mitotic entry. Science 320: 1655–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Berbari NF, Yoder BK (2008) Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol 85: 371–427 [DOI] [PubMed] [Google Scholar]
- Soung NK, Park JE, Yu LR, Lee KH, Lee JM, Bang JK, Veenstra TD, Rhee K, Lee KS (2009) Plk1-dependent and -independent roles of an ODF2 splice variant, hCenexin1, at the centrosome of somatic cells. Dev Cell 16: 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebhardt K, Ullrich A (2006) Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer 6: 321–330 [DOI] [PubMed] [Google Scholar]
- Tillement V, Lajoie-Mazenc I, Casanova A, Froment C, Penary M, Tovar D, Marquez R, Monsarrat B, Favre G, Pradines A (2008) Phosphorylation of RhoB by CK1 impedes actin stress fiber organization and epidermal growth factor receptor stabilization. Exp Cell Res 314: 2811–2821 [DOI] [PubMed] [Google Scholar]
- van de Weerdt BC, Medema RH (2006) Polo-like kinases: a team in control of the division. Cell Cycle 5: 853–864 [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT (2003) A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5: 367–377 [DOI] [PubMed] [Google Scholar]
- Warner DR, Greene RM, Pisano MM (2005) Interaction between Smad 3 and Dishevelled in murine embryonic craniofacial mesenchymal cells. Orthod Craniofac Res 8: 123–130 [DOI] [PubMed] [Google Scholar]
- Warner DR, Pisano MM, Roberts EA, Greene RM (2003) Identification of three novel Smad binding proteins involved in cell polarity. FEBS Lett 539: 167–173 [DOI] [PubMed] [Google Scholar]
- Yun SM, Moulaei T, Lim D, Bang JK, Park JE, Shenoy SR, Liu F, Kang YH, Liao C, Soung NK, Lee S, Yoon DY, Lim Y, Lee DH, Otaka A, Appella E, McMahon JB, Nicklaus MC, Burke TR Jr, Yaffe MB et al. (2009) Structural and functional analyses of minimal phosphopeptides targeting the polo-box domain of polo-like kinase 1. Nat Struct Mol Biol 16: 876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng K, Bastos RN, Barr FA, Gruneberg U (2010) Protein phosphatase 6 regulates mitotic spindle formation by controlling the T-loop phosphorylation state of Aurora A bound to its activator TPX2. J Cell Biol 191: 1315–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhu J, Yang GY, Wang QJ, Qian L, Chen YM, Chen F, Tao Y, Hu HS, Wang T., Luo ZG (2007) Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat Cell Biol 9: 743–754 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.