Abstract
Stromal-derived growth factors are required for normal epithelial growth but are also implicated in tumour progression. We have observed inactivation of the retinoblastoma protein (Rb), through phosphorylation, in cancer-associated fibroblasts in oro-pharyngeal cancer specimens. Rb is well known for its cell-autonomous effects on cancer initiation and progression; however, cell non-autonomous functions of Rb are not well described. We have identified a cell non-autonomous role of Rb, using three-dimensional cultures, where depletion of Rb in stromal fibroblasts enhances invasive potential of transformed epithelia. In part, this is mediated by upregulation of keratinocyte growth factor (KGF), which is produced by the depleted fibroblasts. KGF drives invasion of epithelial cells through induction of MMP1 expression in an AKT- and Ets2-dependent manner. Our data identify that stromal fibroblasts can alter the invasive behaviour of the epithelium, and we show that altered expression of KGF can mediate these functions.
Keywords: invasion, keratinocyte growth factor, MMP1, retinoblastoma, stroma
Introduction
Data from retinoblastoma protein (Rb)-knockout mice and chimeric embryos indicate that Rb has both autonomous and non-autonomous functions. Mice that are null for the Rb die by embryonic day 14.5 from lack of development of the placenta. If development of the placenta is maintained, or chimeric mice are generated, then embryos are carried to term with only partial defects compared to those observed in null animals (Maandag et al, 1994; Williams et al, 1994; Lipinski et al, 2001; Whyatt and Grosveld, 2002; de Bruin et al, 2003; Wu et al, 2003). In particular, the elevated apoptosis and loss of differentiation in various tissues are not detected in chimeric embryos suggesting that Rb expression in one cell is able to regulate the growth of neighbouring cells. Relatively little is known about the cell non-autonomous functions of Rb, although a number of interpretations of how Rb regulates neighbouring cells include: secretion of apoptotic stimuli or the regulation of growth factor expression (Whyatt and Grosveld, 2002), although these factors have not been identified.
In cancer, the interplay between stromal cells and the epithelium is altered, with the stroma playing an important part in cancer development through stimulation of proliferation, invasion and angiogenesis (Bhowmick et al, 2004; Littlepage et al, 2005; Ostman and Augsten, 2009). Fibroblasts are a major cellular component of the stroma, and in various tumours they are able to promote the proliferation and malignant growth of epithelial cells, through altered expression of growth and survival factors, extracellular matrix proteins and proteases (Bhowmick et al, 2004; Kalluri and Zeisberg, 2006; Orimo and Weinberg, 2006). Previous work has shown that Rb is inactivated by phosphorylation in isolated breast cancer-associated fibroblasts (CAFs; Mercier et al, 2008), and in addition increased activation of the AKT pathway has been observed within the stroma (Cully et al, 2006; Bergamaschi et al, 2008). We have recently shown that Rb regulates the AKT pathway within the epithelium (Menges et al, 2006); however, the function of Rb in the stromal compartment is not known, and is a focus of this study.
Here, we show that Rb and Rb-dependent pathways are commonly inactivated in CAFs in oro-pharyngeal cancers; recapitulation of this observation in organotypic raft cultures results in immortalised epithelial cells becoming invasive when cultured with Rb-inactivated fibroblasts. We further identify stromal-derived keratinocyte growth factor (KGF) as a key mediator of epithelial invasion, which regulates invasion via an Akt-Ets2-MMP1-dependent pathway.
Results
Disruption of Rb-dependent pathways in CAFs
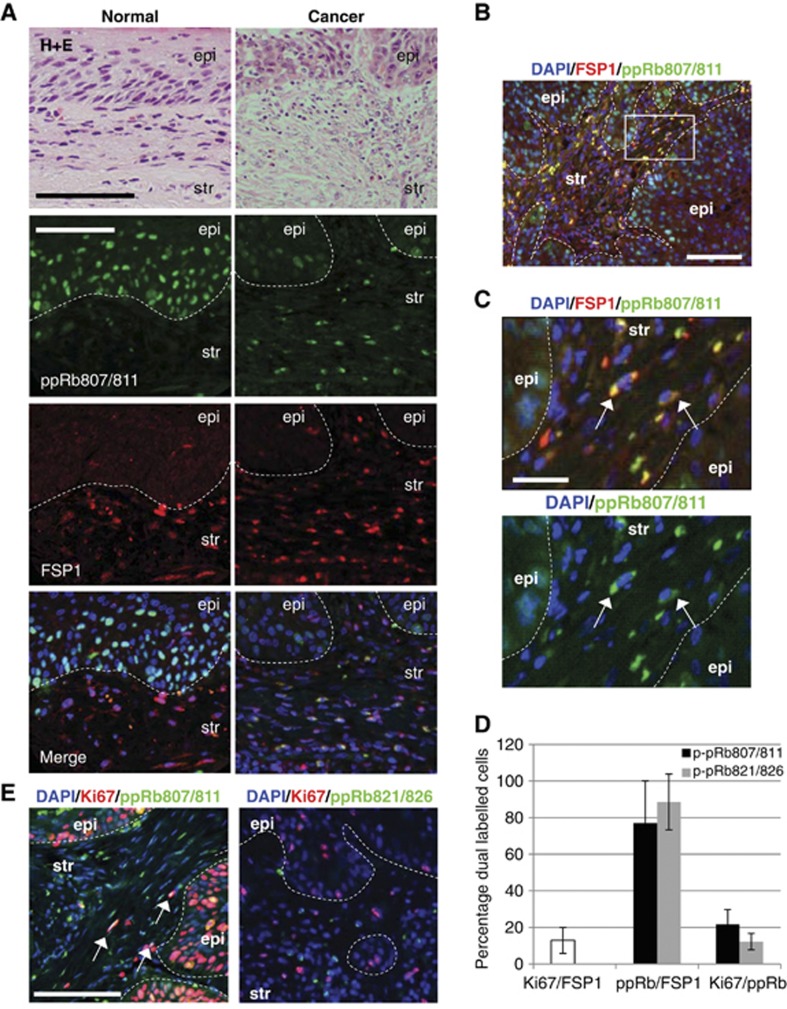
Previous work has shown that Rb is inactivated by phosphorylation in isolated breast CAFs (Mercier et al, 2008), although the implications of this modification on the growth of the epithelium is unclear. To establish the activation status of Rb in CAFs in situ, oro-pharyngeal cancer specimens were analysed by immunohistochemical staining for phosphorylated (inactive) Rb (p-pRb) at serines 807 and 811, and the stromal marker vimentin. The specificity of the phosphorylated Rb antibodies used in this study were assessed using cultured human fibroblasts and a human osteosarcoma cell line SAOS2, which lacks Rb expression (Supplementary Figure 1). In the stroma associated with normal epithelium morphology, relatively little phosphorylated Rb (p-pRb807/811) was observed (Supplementary Figure 2A). However, in stroma associated with cancerous epithelium, large numbers of stromal cells stained positive for phosphorylated Rb and vimentin, suggesting that Rb is inactivated within the stroma (Supplementary Figure 2A). Vimentin stains extensively within the stroma, however, since it is known that distinct subpopulations of fibroblasts exist within the stroma and are not always identified by vimentin staining (Sugimoto et al, 2006); two additional fibroblast markers, smooth muscle actin and the fibroblast-specific protein-1 (FSP1) and also the macrophage marker CD68, were used to localise the stromal phosphorylated Rb. Both smooth muscle actin and FSP1 stained a subpopulation of stromal cells (Supplementary Figure 2B). Smooth muscle actin identified mainly blood vessels and fibroblasts associated with these blood vessels, and showed little colocalisation with phospho-Rb, whereas FSP-1 stained only a subpopulation of stromal cells. Many CAFs that stained positive for FSP-1 contained phosphorylated Rb in comparison to the FSP-1-positive fibroblasts associated with normal tissue in the same sections (Figure 1A). FSP1-positive fibroblasts are key in promoting carcinogenesis through their ability to mediate macrophage infiltration and chronic inflammation (Zhang et al, 2011). However, staining with the macrophage marker CD68 showed that phospho-Rb did not frequently colocalise with these cells (Supplementary Figure 2C). The degree of phosphorylated Rb staining within the stroma, and its colocalisation with FSP1, was quantified for 34 oro-pharyngeal cancer specimens (Table I). p-pRb was strongly detected within the cancer-associated stroma, compared to matched normal samples, with strong colocalisation with the FSP-1-positive fibroblast population. A similar pattern of staining was observed when using an antibody raised to Rb phosphorylated at threonine residues 821 and 826 (p-pRB821/826), with large proportions of FSP-1-positive fibroblasts also staining positive for p-pRB821/826 in the tumour-associated stroma (Supplementary Figure 3A and Supplementary Table 1). The stromal staining of phosphorylated Rb was observed in both HPV-positive and HPV-negative cancers suggesting this inactivating modification is independent of HPV status (Table I). In fact, FSP-1-positive cells were also positive for both phosphorylated modifications (Supplementary Figure 1D).
Figure 1.
Phosphorylated Rb staining in stromal fibroblasts associated with cancerous epithelium. (A) Oro-pharyngeal cancer sections and associated normal tissue from the same patient were stained using antibodies against the FSP1 and Rb phosphorylated at residues 807 and 811 (p-pRb (807/811)). Phospho-Rb stained strongly in normal epithelium (epi) but only in a small number of cells in the stroma (str). Compared to stroma associated with normal epithelium, cancer-associated stroma contained many more phosphorylated Rb-positive cells. (B) A large proportion of stromal cells staining positive for phospho-Rb and also FSP1 (yellow colour) suggesting Rb is phosphorylated within CAFs. Scale bars represent 100 μm. (C) The area boxed in B was magnified in C and showed that in a proportion of CAFs phospho-Rb was mislocalised to the cytoplasm (arrows). Scale bars represent 25 μm. (D) Percentages of dual stained cells in the stroma associated with oro-pharyngeal cancers, error bars represent s.d. (E) Representative images of cancer-associated stroma dual stained with phosphorylated Rb and Ki67. Examples of stromal cells positive for both phospho-Rb and Ki67 are indicated by arrows. Scale bars represent 100 μm.
Table 1. Scores determining the ratio of phospho-Rb (Ser807/811) staining within the FSP1 population of fibroblasts associated with normal and cancerous head and neck epithelium.
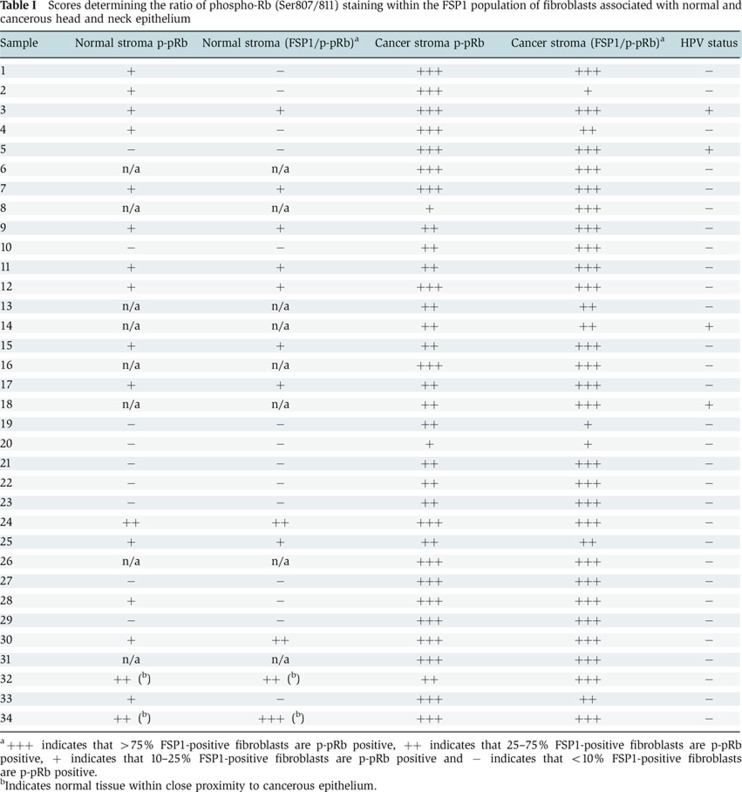
a+++ indicates that >75% FSP1-positive fibroblasts are p-pRb positive, ++ indicates that 25–75% FSP1-positive fibroblasts are p-pRb positive, + indicates that 10–25% FSP1-positive fibroblasts are p-pRb positive and − indicates that <10% FSP1-positive fibroblasts are p-pRb positive.
bIndicates normal tissue within close proximity to cancerous epithelium.
Interestingly, phosphorylated Rb staining was also observed in the cytoplasm of numerous stromal fibroblasts (Figure 1B and C), and since hyperphosphorylated Rb has decreased affinity for the nuclear compartment (Mittnacht and Weinberg, 1991) and has been found to be mislocalised in various epithelial cells (Jiao et al, 2008), this provided further evidence that Rb is inactivated in these cells. Costaining of the oro-pharyngeal cancers with an antibody to total Rb protein further identified that Rb is mislocalised in cells where phosphorylated Rb was detected in the cytoplasm (Supplementary Figure 3B).
As Rb is inactivated by phopshorylation during the cell cycle, the enhanced staining observed in the stroma associated with cancerous epithelium may be representative of the enhanced proliferative nature of the tumour-associated fibroblasts, which has also been observed in some head and neck cancers (Liu et al, 2006). Phosphorylation of residues 807/811 and 821/826 are detected in isolated fibroblasts in culture that are in S-phase, as detected by BrdU incorporation, suggesting that these events are required for proliferation. However, phosphorylation at threonine 821/826 did not appear to be present in all S-phase cells unlike the serine 807/811 phosphorylation (Supplementary Figure 1C). The degree of proliferation was assessed in the FSP-1-positive population in cancer specimens, by assessing the number of positive cells that costained with Ki67 and this ranged from 0–3% in the stroma associated with normal epithelium and 2–40% (12.9% mean) in the stroma associated with cancerous epithelium (Supplementary Figure 4A). These levels were much lower than phosphorylated Rb staining that was observed in 25–100% of FSP-1-positive cells (mean 75%±23 for p-pRb807/811 and mean 88%±15 for p-pRb821/826) in the stroma associated with cancer epithelium (Figure 1D). To further establish whether the phospho-Rb-positive cells in the stroma were proliferating, whole sections were costained with phospho-Rb and Ki67. Of the p-pRb807/811-positive cells within the stroma, 20±8% were also Ki67 positive, and of the p-pRb821/826-positive cells, 12±4% were Ki67 positive, suggesting that only a small proportion of the phospho-Rb-positive cells are proliferating (Figure 1D and E; Supplementary Figure 4B and C).
To further examine whether the Rb-regulated pathways are inactivated in the stroma, the same sections were stained for PCNA, which is a downstream target of E2F and normally inhibited by Rb. Within the stroma associated with cancerous epithelium, strong PCNA staining was observed in comparison to the stroma of matched normal epithelium (Supplementary Figure 5). The level of PCNA staining was also high in tumour tissue but low in normal epithelium where it was only detected in the proliferating basal layers. This may represent the enhanced proliferation observed in the stroma but may also be a reflection of Rb activity.
Functional Rb is required in fibroblasts to prevent invasion of transformed epithelium
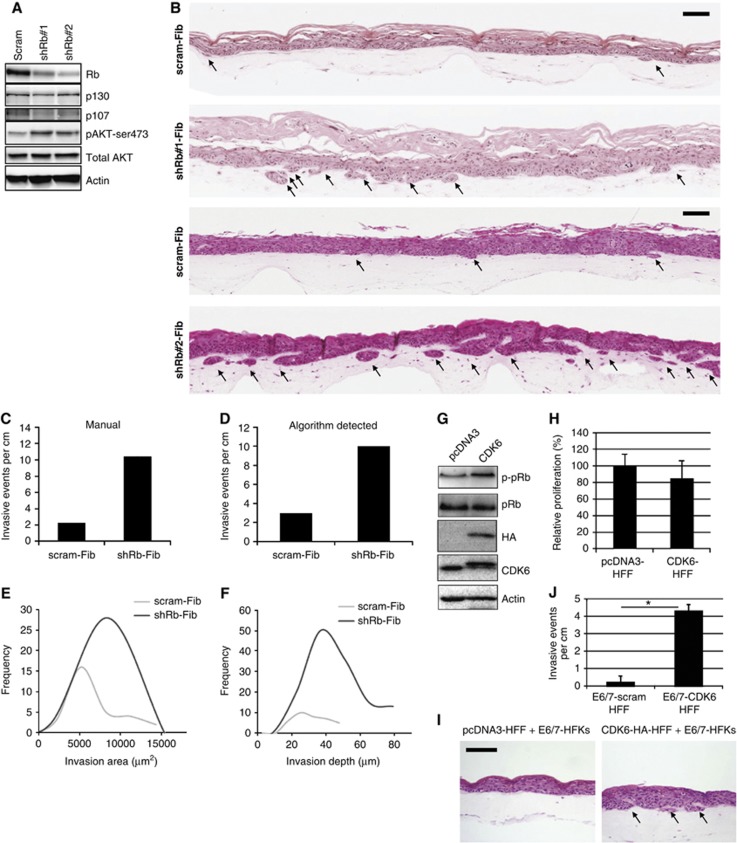
In order to establish the role of Rb inactivation within the stroma, we utilised organotypic cultures of stratified epithelium on a collagen base. This culture system forms a skin-like structure with a basement membrane laid down between the epithelium and stroma. Rb levels were stably depleted in primary human foreskin fibroblasts (HFFs) by retroviral-mediated expression of shRNA targeting Rb and then embedded into the collagen base (Figure 2A). Rb depletion increased AKT activation, as we have previously observed within the epithelium (Menges et al, 2006). Primary human foreskin keratinocytes (HFKs) immortalised with the human papillomavirus type 16 (HPV-16) proteins E6 and E7 (E6/7-HFKs) were grown in organotypic cultures using Rb-depleted fibroblasts as feeder cells. E6/7-HFKs do not differentiate and exhibit enhanced proliferation compared to primary keratinocytes when grown as organotypic rafts (McCance, 2005). No differences in the differentiation or proliferation of the epithelium was observed when E6/E7-HFKs were cultured with Rb-depleted fibroblasts compared to control fibroblasts; however, there were increased numbers of epithelial invasions into the underlying collagen in the former. When invasions were observed with control HFFs, they were infrequent and small as observed in two independently generated epithelial cell lines shown in Figure 2B. Quantification showed that the frequency of invasive events (invasions per cm) is significantly increased in cultures fed with Rb-depleted HFFs (Figure 2B and C). To further assess the invasive phenotype, an algorithm developed in Matlab employing adaptive thresholding and trigonometric functions (Supplementary methods and Supplementary Figure 6) was applied to whole-slide images (WSI) obtained using an Aperio ScanScope CS Slide Scanner. The invasions were then measured in terms of frequency of events, area and depth of invasion (Figure 2D–F). There was a good agreement between manually and algorithm-detected events. This invasive phenotype was observed with three independently generated E6/7-transformed HFK lines when cultured with different Rb-depleted HFFs lines (three independent lines). Similarly, an HPV-negative cervical epithelial cell line C33a, an immortalised keratinocyte cell line HaCat and the breast cancer cell line MDA-MB-231 exhibited invasion in the presence of Rb-depleted HFFs (Supplementary Figure 7). As Rb depletion can result in enhanced proliferation, fibroblasts were pretreated with mitomycin C for 2 h prior to incorporating them into collagen plugs, this did not alter the invasive potential of the epithelium (Supplementary Figure 8A and B), suggesting that these events do not result from enhanced proliferation of the fibroblast population. Indeed, assessment of BrdU incorporation in normal fibroblasts in the organotypic rafts demonstrated very low levels of proliferation (∼1.5%, Supplementary Figure 8C). In order to further assess the role of Rb in fibroblasts in the control of epithelial growth and invasion in a manner more representative of the hyperphosphorylation of Rb observed in human stromal fibroblasts, we exogenously expressed CDK6 in HFFs. Exogenously expressed CDK6 induced hyperphosphorylation of Rb (Figure 2G), but interestingly did not result in enhanced proliferation of the fibroblasts (Figure 2H), and caused an increase in epithelial invasion to a similar degree as Rb depletion (Figure 2I and J), suggesting that the Rb pathway within the stroma plays an essential role containing epithelial homoeostasis.
Figure 2.
Depletion of Rb in fibroblasts triggers aggressive invasion of immortalised epithelial cells. (A) Knockdown of Rb in primary foreskin fibroblasts and the concomitant increase in active AKT. (B) Primary foreskin keratinocytes immortalised with the viral oncoproteins E6 and E7 (E6/7-HFK) invade aggressively into collagen-containing Rb-depleted fibroblasts (shRb#1 and #2-Fib) compared to collagen-containing control fibroblasts (scram-Fib). Invasions are indicated by arrows. (C) Shows quantification of the number of invasive events across the organotypic cultures per cm, determined manually and automatically (D) from two independent cultures. (E) and (F) Graphs of the size and depth, respectively, of epithelial invasions into collagen. (G) HA-tagged CDK6 overexpression in HFFs and the concomitant increase in Rb phosphorylation at serine residues 807 and 811. (H) Proliferation as assessed by BrdU incorporation in three independently generated fibroblast lines with exogenous CDK6 expression. Error bars represent s.d. (I) Representative organotypic cultures of E6/7-HFKs with control (pcDNA3) or pcDNA-CDK6 overexpressing fibroblasts. Scale bars represent 100 μm. Invasions are indicated by arrows and quantified from three independent experiments in (J). Experiments were carried out at least three times with different batches of HFKs and HFFs. Error bars represent s.e. *P<0.01.
Stromal-derived KGF regulates epithelial invasion
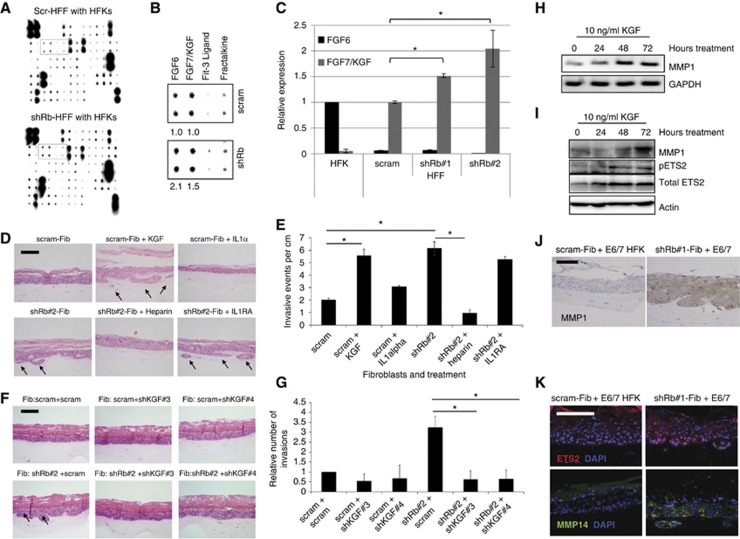
Multiple cytokines mediate cross-talk between fibroblasts of the stroma and epithelial cells, and are crucial in regulating the growth, development and repair of the epithelium. In order to identify factors that mediate the invasive phenotype of Rb, depleted HFFs in monolayer cocultures with HFKs were analysed by cytokine arrays to determine differences in secreted factors (Figure 3A). Fibroblast growth factor 6 (FGF6) and FGF7 (also known as KGF) were shown to be increased in cocultures containing Rb-depleted HFFs (Figure 3B). In order to identify the source of the differential expression, real-time PCR was conducted in isolated keratinocytes and fibroblasts (control and Rb-depleted). The differential secretion of FGF6 can only be a result of altered expression in the keratinocyte population as fibroblasts express very little FGF6 (detected at >40 cycles in HFFs) while the reciprocal is true for KGF, therefore the elevated levels of KGF observed result from Rb depletion within the fibroblast population (Figure 3C). We selected KGF as a potential mediator of epithelial cell invasion as it is well known to regulate the growth of the epithelium and since a role for KGF in mediating invasion has been postulated (Zheng et al, 1996; Ropiquet et al, 1999; Shin et al, 2002; Niu et al, 2007). To evaluate whether KGF contributes to the invasion in organotypic cultures of E6/7-HFKs, raft cultures containing E6/E7-HFKs and control fibroblasts were treated with 10 ng/ml KGF, which resulted in a significant increase in the number of invasions (Figure 3D and E). We also established that IL1α was also elevated in Rb-depleted fibroblasts (unpublished observation) and therefore we tested whether IL1α influenced invasiveness. Treatment of control cultures with IL1α resulted in a small but nonsignificant increase in the number of invasions; however, blocking the IL1-receptor antagonist (IL1RA) did not inhibit invasion induced by Rb-depleted fibroblasts (Figure 3D and E). Organotypic cultures containing Rb-depleted HFFs were grown in the presence of heparin, to block KGF binding to its receptor and this significantly reduced the number of invasions (Figure 3D and E). However, heparin is known to block numerous ligand/receptor interactions (Ron et al, 1993); so in order to establish whether KGF was specifically mediating the invasions in these cultures, E6/7-HFKs were grown with KGF-depleted HFFs. KGF knockdown within Rb-depleted HFFs effectively abolished invasion by the epithelium (Figure 3F and G).
Figure 3.
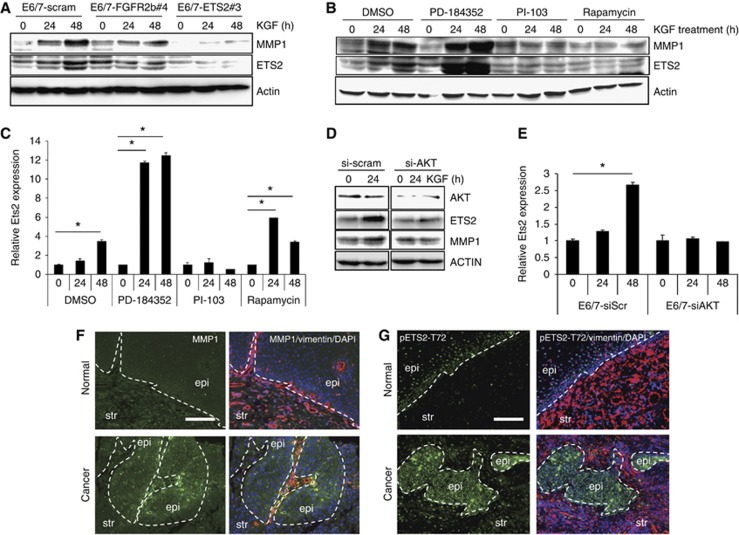
KGF induces invasion of epithelial cells. (A) Cytokine arrays from cocultures of HFKs with control or shRb HFFs. (B) Highlight of region in A showing elevated levels of FGF6 and FGF7 (KGF) that are secreted in cocultures with Rb-depleted fibroblasts, relative levels are shown below blots. (C) Real-time PCR identified enhanced expression of KGF but not FGF6 in Rb-depleted fibroblasts. FGF6 is only expressed in keratinocytes (HFK) while KGF is only expressed in fibroblasts (HFF); experiments were carried out using three independently generated fibroblast lines. Error bars represent s.d. (D) Representative organotypic cultures containing control fibroblasts (scram-Fib) treated with KGF or IL1α, and Rb-depleted fibroblasts (shRb#2-Fib) treated with heparin or the IL1RA. (E) Quantification of invasive events from triplicate analysis KGF unlike IL1α-induced invasion of the epithelium, furthermore the invasive phenotype induced by Rb-depleted fibroblasts could not be inhibited by IL1RA. Error bars represent s.e. (F) E6/7-HFKs could be prevented from invading into collagen through depletion of KGF levels within fibroblasts. (G) Quantification of the invasion frequency from three independent experiments. * Indicates P<0.05 determined by Students t-test. Error bars represent s.e. (H) and (I) E6/7-HFKs plated on 100 μg/ml collagen were treated with KGF and RNA, and proteins were harvested at the indicated times. RT–PCR (H) and western blotting (I) showed that over the time course MMP1 expression was induced by KGF as was the known regulator of MMP1 expression, Ets2 and the activated phosphorylated form (pETS2). (J) Imunohistochemical detection of MMP1 identified enhanced expression throughout the epithelium cultured with Rb-depleted fibroblasts. (K) Ets2 and MMP14 (MT1-MMP) were increased in epithelium cultured with Rb-depleted fibroblasts. Scale bars represent 100 μm.
KGF induces expression of MMP proteins and the Ets2 transcription factor in keratinocytes
To establish how KGF mediates invasion by E6/7-HFKs, the epithelial cells were plated on a collagen matrix and treated with KGF to determine the levels of matrix metalloproteinases. The expression of various MMPs was assessed first by RT–PCR. Of the MMPs assessed, only MMP1, 2, 9 and 14 were expressed in the epithelial cells, whereas MMP7 and 13 were not (data not shown), and over the time course of KGF treatment only MMP1 expression was induced, both, at mRNA and protein level (Figure 3H and I, respectively). Furthermore, the levels of MMP1 protein expression in the epithelium of E6/7-HFK rafts cocultured with Rb-depleted HFFs were also significantly increased (Figure 3J). Following KGF treatment, the expression and phosphorylation of the transcription factor, Ets2, was found to increase (Figure 3I). Ets2 is a known activator of MMP1 transcriptional expression (Buttice et al, 1996) and it was expressed at high levels in the invasive regions of organotypic cultures grown with Rb-depleted HFFs (Figure 3K). In addition, the collagenase MMP14 (MT1-MMP) was also elevated in the invasive areas (Figure 3K); however, MMP14 was not induced upon KGF treatment and may represent sequential activation of MMPs during the invasion process as MMP14 is rate limiting to the breakdown of collagen (Friedl and Wolf, 2008; Kessenbrock et al, 2010).
KGFR- and Ets2-dependent signalling pathways control epithelial invasion
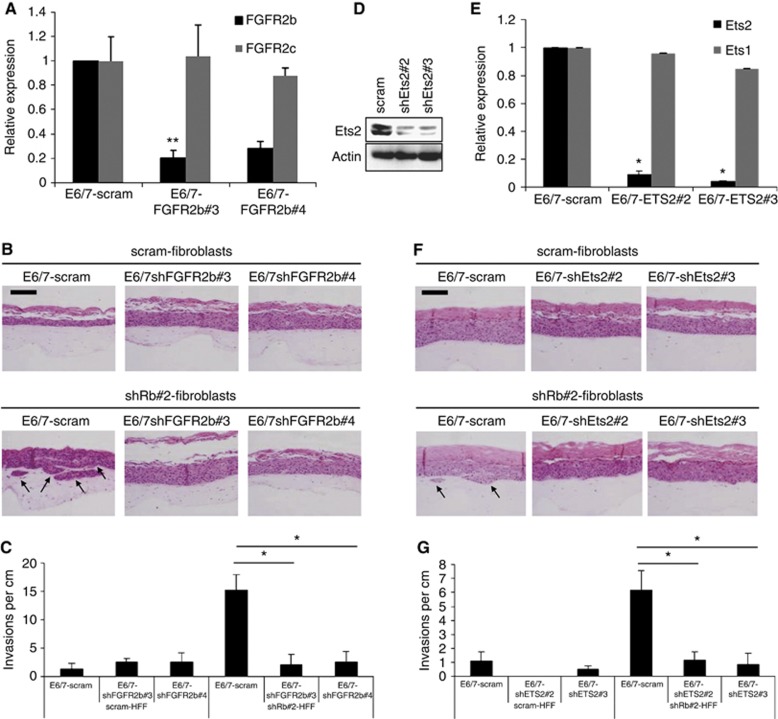
To elucidate the importance of KGF signalling in mediating invasive events, the levels of the KGF receptor, FGFR2b, were depleted in the E6/7-HFKs. shRNA designed to exon 8 of the FGFR2 gene were used to specifically deplete FGFR2b. As there is limited availability of antibody reagents to specifically detect the protein derived from the FGFR2b isoform, qPCR was used to confirm knockdown of FGFR2b (Figure 4A). Importantly, knockdown of FGFR2b did not affect expression of FGFR2c in these cells. E6/7-HFKs depleted of FGFR2b were rafted with control and Rb-depleted HFFs and while FGFR2b depletion did not alter the growth of the epithelium cultured with control HFFs, when cultured with Rb-depleted HFFs the number of invasions were reduced compared to control levels (Figure 4B and C). These results implicate a KGF-activated signalling pathway in mediating invasions induced by Rb-depleted HFFs. As we had previously observed Ets2 to be induced by KGF treatment, we assessed whether Ets2 is an effector of KGF-mediated signalling. Ets2 levels were depleted in the E6/7-HFKs by shRNA expression, which was confirmed by western blotting (Figure 4D) and qPCR (Figure 4E). Depletion of Ets2 did not alter expression of Ets1, but led to a reduction in the invasive potential of E6/7-HFKs cultured with Rb-depleted fibroblasts, suggesting that Ets2 transduces signalling mediated by KGF/FGFR2b (Figure 4F and G). To test this hypothesis, KGF was added to FGFR2b or Ets2 knockdown E6/7-HFKs, and western blot analysis showed that in both FGFR2b and Ets2 knockdown cells, KGF mediated induction of Ets2 and MMP1 was stunted (Figure 5A). This was also observed in organotypic rafts of Ets2-knockdown E6/7-HFKs cultured with Rb-depleted fibroblasts (Supplementary Figure 8D).
Figure 4.
Depletion of FGFR2b or Ets2 in the epithelium inhibits invasion. (A) Depletion of FGFR2b in E6/7-HFKs was confirmed by qPCR and knockdown of FGFR2b did not affect the expression levels of FGFR2c. Error bars represent s.d. (B) FGFR2b depletion in the epithelium significantly inhibited the ability of E6/7-HFKs to invade in the presence of Rb-depleted fibroblasts. Error bars represent s.e. (C) Quantification of the frequency of invasion. Likewise when Ets2 is depleted in E6/7-HFKs (confirmed by western blot (D) and qPCR (E) Error bars represent s.d.). Rb-depleted fibroblasts did not induce invasion of Ets2-depleted epithelium (F, G). Error bars represent s.e. All experiments were conducted in triplicate with independently generated cell lines. * Indicates P<0.05 determined by Students t-test. Scale bars represent 100 μm.
Figure 5.
KGF induces MMP1 through a FGFR2b-AKT-Ets2 pathway. (A) Western blots of FGFR2b- and Ets2-depleted epithelial cells following KGF treatment demonstrate that FGFR2b depletion inhibits KGF induction of Ets2 and MMP1. Similarly, in Ets2-depleted cells, MMP1 was only weakly induced by KGF. Densitometry of the MMP1 band normalised to actin is shown below the blots (normalised to untreated controls) (B) Inhibition of the MEK-ERK pathway using PD-184352 resulted in elevated expression of MMP1 and Ets2, whereas inhibition of the PI3 K-AKT using PI-103 inhibited induction of both Ets2 and MMP1. Treatment with rapamycin, an inhibitor of the mTOR pathway, also prevents induction of Ets2 protein but did not inhibit transcriptional induction of Ets2 as measured by qPCR (C) suggesting that the AKT pathway induces expression of Ets2. Error bars represent s.e. (D) E6/7-expressing keratinocytes were treated with siRNA targeting AKT; after 48 h, cells were then treated with KGF. KGF induced Ets2 and MMP1 protein levels in control (siScram) E6/7-HFKs but not in siAKT cells. (E) Similarly depletion of AKT prevented the induction of Ets2 transcription. Experiments were carried out at least three times with different batches of cells. Error bars represent sd. * Indicates P<0.05 determined by Students t-test. (F) MMP1 expression is elevated in the epithelium and stroma in head and neck cancer specimens compared to matched normal tissue (8/8 samples tested), similarly (G) activation of Ets2 is strongly observed in the epithelium of these cancers (8/8 samples tested). Scale bars represent 100 μm.
KGF induction of Ets2 is via an AKT-dependent pathway
It has been reported that both ERK and AKT kinase pathways regulate Ets2 activity (Fowles et al, 1998; Smith et al, 2000; Weng et al, 2002). To determine whether the induction of Ets2 by KGF is dependent on the ERK or AKT pathway, E6/7-HFKs were pretreated with the MEK inhibitor PD-184352 or the PI-3 kinase inhibitor PI-103 1 h prior to KGF treatment. Western blot analysis showed that in the presence of the PI-3 kinase inhibitor the induction of Ets2 and MMP1 by KGF was inhibited, surprisingly pretreatment with the MEK inhibitor enhanced expression of both Ets2 and MMP1 (Figure 5B). The increased expression of Ets2 and MMP1 when treated with PD-184352 may be due to cross-talk between the AKT and MEK pathways (Zimmermann and Moelling, 1999; Westbrook et al, 2002). These effects were observed using a range of inhibitor concentrations (data not shown). PI-103 is also known to inhibit mTOR, and so when rapamycin was used to specifically inhibit mTOR, this treatment inhibited Ets2 and MMP1 protein production; however, rapamycin did not inhibit transcription of Ets2 mRNA (Figure 5C) suggesting that the AKT pathway is responsible for the induction of Ets2 mRNA in response to KGF treatment. Furthermore, siRNA targeting all AKT isoforms within epithelial cells also inhibited KGF-induced expression of Ets2 and MMP1 proteins (Figure 5D) and correlated with inhibited transcription of Ets2 (Figure 5E).
Returning to our cohort of head and neck squamous cell carcinomas, we have attempted to identify altered regulation of this pro-invasive pathway in these specimens. Since staining for KGF and its receptor was limited by reagents, we investigated levels of MMP1 and phospho-ETS2. All the cancer tissues showed increased expression of MMP1, which correlates with previous data showing elevated expression of MMP1 in oro-pharyngeal cancers (Yamashita et al, 2001; Narayan et al, 2007) and phospho-Ets2 compared to cells in normal parts of the epithelium (Figure 5F and G). While there was heterogeneity of staining in tumour cells, all cancers contained high levels of MMP1 and phospho-Ets2 in a proportion of cells, while normal cells contained none or very little MMP1 or phospho-Ets2 (Figure 5F and G).
Discussion
Here, we propose a role for Rb-regulated pathways within the stroma that influence the invasive potential of the epithelium. In oro-pharyngeal squamous cell carcinomas, we observed Rb inactivation by phosphorylation in >90% of the specimens tested, suggesting that Rb is frequently inactivated in stroma associated with cancerous epithelium. Inactivation of Rb in CAFs has previously been described in fibroblasts isolated from breast cancer specimens (Mercier et al, 2008), which also correlates with enhanced KGF expression in breast CAFs (Dataset GSE12622(Finak et al, 2008)). It is not clear how the stromal fibroblast lose Rb function; various factors have been identified that alter the growth, differentiation and gene expression in cells of the stromal compartment (Bhowmick et al, 2004), including the epithelial-derived factor PDGF that has been shown to induce KGF expression in fibroblasts (Brauchle et al, 1994). Such signalling events may lead to reprogramming of the fibroblasts, associated with tumour epithelium, to an activated phenotype (Kalluri and Zeisberg, 2006); however, this may also arise through mutations or loss of heterozygosity within the stroma (Eng et al, 2009). It is clear that the stroma can greatly influence various aspects of tumour development; however, the interplay between the two compartments still requires further investigation. Our work has focused on the role of the stromal compartment in regulating epithelial growth. Rb was found to be inactivated in the FSP1 subpopulation of fibroblasts, and it has been recently shown that these FSP1-positive fibroblasts are key in promoting carcinogenesis through their ability to mediate macrophage infiltration and chronic inflammation (Zhang et al, 2011). It has also been demonstrated that KGF stimulates macrophage infiltration during wound healing (Jameson et al, 2005), and our data implicate inactivation of stromal Rb in the regulation of KGF and control of tumour progression. It is possible that a proportion of FSP1-positive cells arise from epithelial–mesenchymal transition (EMT), as has previously been proposed (Iwano et al, 2002). However, we have stained the oro-pharyngeal cancer for evidence of residual keratin markers within the stromal fibroblasts and were unable to observe positive staining in the FSP1 population.
Our findings support the hypothesis that the cell non-autonomous functions of Rb are mediated through regulation of growth factor expression (Whyatt and Grosveld, 2002). Stromal fibroblasts are a source of KGF, and our results indicate that Rb regulates KGF expression. This hypothesis is supported by evidence from microarray data comparing mouse embryonic fibroblasts from Rb null mice to litter mate controls that also identify significantly increased expression of KGF with Rb depletion (GEO Dataset GDS3099 (Liu et al, 2009)). The downstream targets of Rb function that regulate KGF expression are at present unclear, although we have observed that interleukin-1 α/β (unpublished observation) are also elevated in Rb-depleted fibroblasts and are known to induce KGF expression (Chedid et al, 1994; Tang and Gilchrest, 1996; Maas-Szabowski et al, 1999). We also demonstrate that Rb depletion leads to activation of AKT, as previously observed in epithelial cells (Menges et al, 2006), and it was recently shown that deletion of the PTEN gene in fibroblasts of mouse mammary glands increased AKT activation and accelerated tumour initiation, progression and transformation (Trimboli et al, 2009). Analysis of microarray data (GEO Dataset GSE16073 (Trimboli et al, 2009)) from the stromal compartment associated with mammary tumours in these mice also indicate that KGF expression is elevated and implies that aberrant AKT activation in the stroma alters epithelial growth (Cully et al, 2006; Bergamaschi et al, 2008) and potentially that AKT-regulated pathways can regulate KGF expression.
Both knockdown of Rb or treatment with KGF was found to induce epithelial invasion that was concomitant with increased MMP1 expression. MMP1 is a key mediator of cancer cell invasion (Kessenbrock et al, 2010) and has been previously shown to be induced following exogenous expression of KGF in prostate epithelial, which led to an invasive phenotype (Ropiquet et al, 1999). The role of KGF and its receptor in cancer is unclear with various reports suggesting that KGF is elevated in cancer (Siddiqi et al, 1995; Watanabe et al, 2000; Finch and Rubin, 2006; Manavi et al, 2007), while others suggest that there is a reduction or no difference in expression (Leung et al, 1997; De Bellis et al, 1998; Knerer et al, 1998). This dichotomy is likely due to the fact that many studies only focus on global expression changes within cancer specimens without differentiating between expression originating from the epithelium or that from the stromal compartment. It is clear that few isolated cancer cell lines actually express KGF (Finch and Rubin, 2006) and therefore any expression differences are likely to arise from changes within the stroma, or from varying stromal content within the cancers analysed. Likewise, expression differences in FGFR2b also vary based on the cellular composition of the cancers and defining its role has been equally difficult. The FGFR2 locus has been found to be amplified in a small proportion of gastric (Kunii et al, 2008) and breast cancers (Takeda et al, 2007), while activating mutations occur in 12% of endometrial cancers, specifically in FGFR2b (Dutt et al, 2008; Turner and Grose, 2010). Our data suggest that both KGF and FGFR2b act to promote tumour progression, and this can be blocked by inhibiting either their interaction or downstream signalling. Intriguingly, cancer patients treated with heparin have improved survival compared to patients treated with other anticoagulants, and further heparin has been shown to reduce metastasis (Borsig, 2010). While we do not suggest that KGF is the only growth factor blocked by heparin, heparin treatment may block KGF signalling in the clinical setting, and therefore KGF and FGFR2b signalling provide an attractive target for therapeutic intervention. Together, our data indicate that the Rb has essential functions in the stromal compartment and propose that inactivation of stromal Rb leads to epithelial invasion.
Materials and methods
Cell culture
Human foreskin keratinocytes were isolated as previously described (Pickard et al, 2010) and grown in Epilife supplemented with human keratinocyte growth supplement (Invitrogen). HFFs were purchased from Cascade Biologics, and were maintained in subconfluent cultures in DMEM supplemented with 10% FBS. Organotypic rafts were grown as previously described (McCance et al, 1988), for up to 14 days and where indicated growth medium was supplemented with 10 ng/ml KGF, 20 ng/ml IL1alpha, 20 μg/ml heparin or 20 ng/ml IL1RA. Rafts were sectioned and H+E stained using standard procedures. Invasions were counted manually or through automated detection (See Supplementary methods), and displayed as invasions per cm.
Generation of stable knockdown fibroblasts and immortalised keratinocytes
HFFs depleted of the indicated proteins were generated by retroviral transduction of shRNA using the phoenix system. Vectors with shRNA targeting Rb have been previously described (Incassati et al, 2006; Pickard et al, 2010), and cells were selected using 1.25 μg/ml puromycin for 3 days. For experiments with knockdown of both Rb and KGF, the shRNA targeting Rb was shuttled into pSuper-retro-neo and pSuper-retro-hygro, and cells selected in 400 μg/ml G418 or 20 μg/ml hygromycin for 2–4 days. shRNA targeting KGF were purchased from Origene (TR312988, puromycin selection, molecules 3 and 4) as were shRNA targeting Ets2 (TG320351, puromycin selection, molecules 2 and 3). To knockdown FGFR2b the following primers were used to generate shRNA, shFGFR2b#3: fwd 5′-GATCCCCAATGCAGAAGTGCTGGCTCTGTTCAAGAGACAGAGCCAGCACTTCTGCATTTTTTTA-3′ rev 5′-AGCTTAAAAAAATGCAGAAGTGCTGGCTCTGTCTCTTGAACAGAGCCAGCACTTCTGCATTGGG-3’ shFGFR2b#4: fwd 5′-GATCCCCTCTCCAATTATATAGGGCAGGCCAATTCAAGAGATTGGCCTGCCCTATA-TAATTGGAGATTTTTA-3′ rev 5′-AGCTTAAAAATCTCCAATTATATAGGGCAGGCCAATCTCTTGA-ATTGGCCTGCCCTATATA-ATTGGAGAGGG-3′. These were then cloned into pGFP-B-RS (Origene), and transfected cells selected using 3 mg/ml blasticidin for 2–4 days. E6/7-expressing keratinocytes were generated as previously described (Menges et al, 2006), and then passaged every 2–3 days at least 30 times, to ensure an immortalised line was generated. During passage, cells were frequently reselected in 1.25 μg/ml puromycin for 2 days.
Immunofluorescence
Immunofluorescent and immunohistochemical detection of protein expression in raft cultures was conducted as previously described (Pickard et al, 2010), following antigen retrieval in citrate buffer (DAKO). Antibodies were incubated on the sections overnight. Immunofluorescent detection of phospho-Rb, MMP1 and phospho-Ets2 in paraffin-embedded sections was achieved by dewaxing in reducing concentrations of alcohol, antigen retrieval using 0.1 M Tris-EDTA pH8.0 for 28 min in a pressure cooker and incubation with primary antibodies overnight at 4°C.
KGF treatment
To assess the effects of KGF on cultures of keratinocytes, all plates were coated with 100 μg/ml rat tail collagen I (BD Biosciences). For inhibitor experiments, cells were pretreated for 1 h at the following concentrations: PD184352 (0.1 μM), PI-103 (1 μM) and rapamycin (10 nM).
Antibodies
In this study, antibodies against the following proteins were used for western blotting: Rb (BD Biosciences, 554136, 1:1000), p130 (BD Biosciences, 610261, 1:1000), p107 (Santa Cruz, sc-318, 1:1000), Ets2 (Santa Cruz, sc-351, 1:500), phospho-Ets2 (Invitrogen, 441105G, 1:500), phospho-AKT (Ser473) (Cell Signaling, 9271, 1:1000), total AKT (Cell Signaling, 9272, 1:1000), MMP1 (Epitomics, 1973–1, 1:250) and beta-actin (Sigma-Aldrich, AC74, 1:10 000); for immunofluorescence/histochemistry with antigen retrieval: Ets2 (Santa Cruz, sc-351, 1:50), MMP1 (Epitomics, 1973–1, 1:100), MMP14 (Chemicon, MAB3329, 1:100), phospho-Rb 807/811 (Cell Signaling, #9308, 1:200), phosphor-Rb 821/826 (Santa Cruz, sc-16669-R, 1:200), vimentin (Thermo, MS-129-P0, 1:200), phospho-Ets2 (Invitrogen, 441105G, 1:200) and FSP1 (Santa Cruz, sc-100784, 1:100).
Semiquantitative RT–PCR, real time PCR and ELISA
RT–PCR was conducted using 1 μg RNA isolated using Trizol (Invitrogen). cDNA was generated using random oligos and MLV-reverse transcriptase (Invitrogen). cDNA was diluted 1:10 in dH2O prior to PCR. For RT–PCR, 2.5 μl cDNA was used with the following PCR conditions: 1.5 mM MgCl2, 0.2 μM primers, 0.2 mM dNTPs and 0.1 U Taq (Promega). Real-time PCR was conducted using cDNA generated as above, and conducted using Sybr green (Roche), results were quantified against standard curves for each primer set and normalised to RPLPO. Primers used in this study were: KGF fwd: 5′-CACAATGGATACTGACATGGA-3′ rev: 5′-TCACTCTTATATCCCCTCCTTC-3′; MMP1 fwd: 5′-TTACATCGTGTTGC-GGCTCATGAA-3′ rev: 5′-CGG-ACTTCATCTCTGTCGGCTAAT-3′; RPLPO fwd: 5′-ATCAACGGGTACAAACGAGTC-3′ rev: 5′-CAGATGGATCAGCCAAGAA-GG-3′; and Ets2 fwd: 5′-CAGCGTCACCTACTGCTCTG-3′ rev: 5′-AGTCGTGGTCTTTGGGAGTC-3′. Cytokine arrays used were purchased from RayBiotech, Human Cytokine Array 6 and were conducted according to the manufacturers protocol.
Supplementary Material
Acknowledgments
We would like to thank the following people for reagents and services: Ken Arthur for sectioning organotypic rafts, Dr James Murray for AKT and ERK pathway inhibitors, Dr Eric Yuen for MDA-MB-231 cells and MMP primers, and Dr Stephen McQuaid for CD68 and Ki67 antibodies. Use of human tissues was under ethics approval: 06/NIR01/115. Funding for this work was supported by NIDCR: ROI 15935, Wellcome Trust: 082840/Z/07/Z and Experimental Cancer Medicine Centre.
Author contributions: Adam Pickard—experimental, analysis and writing; Ann-Christin Cichon—experimental; Anna Barry—experimental; Declan Kieran—experimental; Daksha Patel—analysis and conception; Peter Hamilton—conception; Manuel Salto-Tellez—analysis and conception; Jacqueline James—analysis and conception; and Dennis J McCance—conception, analysis and writing.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bergamaschi A, Tagliabue E, Sorlie T, Naume B, Triulzi T, Orlandi R, Russnes HG, Nesland JM, Tammi R, Auvinen P, Kosma VM, Menard S, Borresen-Dale AL (2008) Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J Pathol 214: 357–367 [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL (2004) Stromal fibroblasts in cancer initiation and progression. Nature 432: 332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsig L (2010) Heparin as an inhibitor of cancer progression. Prog Mol Biol Translational Sci 93: 335–349 [DOI] [PubMed] [Google Scholar]
- Brauchle M, Angermeyer K, Hubner G, Werner S (1994) Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene 9: 3199–3204 [PubMed] [Google Scholar]
- Buttice G, Duterque-Coquillaud M, Basuyaux JP, Carrere S, Kurkinen M, Stehelin D (1996) Erg, an Ets-family member, differentially regulates human collagenase1 (MMP1) and stromelysin1 (MMP3) gene expression by physically interacting with the Fos/Jun complex. Oncogene 13: 2297–2306 [PubMed] [Google Scholar]
- Chedid M, Rubin JS, Csaky KG, Aaronson SA (1994) Regulation of keratinocyte growth factor gene expression by interleukin 1. J Biol Chem 269: 10753–10757 [PubMed] [Google Scholar]
- Cully M, You H, Levine AJ, Mak TW (2006) Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev 6: 184–192 [DOI] [PubMed] [Google Scholar]
- De Bellis A, Crescioli C, Grappone C, Milani S, Ghiandi P, Forti G, Serio M (1998) Expression and cellular localization of keratinocyte growth factor and its receptor in human hyperplastic prostate tissue. J Clin Endocrinol Metab 83: 2186–2191 [DOI] [PubMed] [Google Scholar]
- de Bruin A, Wu L, Saavedra HI, Wilson P, Yang Y, Rosol TJ, Weinstein M, Robinson ML, Leone G (2003) Rb function in extraembryonic lineages suppresses apoptosis in the CNS of Rb-deficient mice. Proc Nat Acad Sci USA 100: 6546–6551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt A, Salvesen HB, Chen TH, Ramos AH, Onofrio RC, Hatton C, Nicoletti R, Winckler W, Grewal R, Hanna M, Wyhs N, Ziaugra L, Richter DJ, Trovik J, Engelsen IB, Stefansson IM, Fennell T, Cibulskis K, Zody MC, Akslen LA et al. (2008) Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Nat Acad Sci USA 105: 8713–8717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C, Leone G, Orloff MS, Ostroviski MC (2009) Genomic alterations in tumor stroma. Cancer Res 69: 6759–6764 [DOI] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M (2008) Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14: 518–527 [DOI] [PubMed] [Google Scholar]
- Finch PW, Rubin JS (2006) Keratinocyte growth factor expression and activity in cancer: implications for use in patients with solid tumors. J Natl Cancer Inst 98: 812–824 [DOI] [PubMed] [Google Scholar]
- Fowles LF, Martin ML, Nelsen L, Stacey KJ, Redd D, Clark YM, Nagamine Y, McMahon M, Hume DA, Ostrowski MC (1998) Persistent activation of mitogen-activated protein kinases p42 and p44 and ets-2 phosphorylation in response to colony-stimulating factor 1/c-fms signaling. Mol Cell Biol 18: 5148–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Wolf K (2008) Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res 68: 7247–7249 [DOI] [PubMed] [Google Scholar]
- Incassati A, Patel D, McCance DJ (2006) Induction of tetraploidy through loss of p53 and upregulation of Plk1 by human papillomavirus type-16 E6. Oncogene 25: 2444–2451 [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG (2002) Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL (2005) Gammadelta T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med 201: 1269–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao W, Lin HM, Datta J, Braunschweig T, Chung JY, Hewitt SM, Rane SG (2008) Aberrant nucleocytoplasmic localization of the retinoblastoma tumor suppressor protein in human cancer correlates with moderate/poor tumor differentiation. Oncogene 27: 3156–3164 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev 6: 392–401 [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141: 52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerer B, Formanek M, Schickinger B, Martinek H, Kornfehl J (1998) Different KGF expression in squamous cell carcinomas of the head and neck and in normal mucosa. Acta Otolaryngol 118: 438–442 [DOI] [PubMed] [Google Scholar]
- Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di Bacco A, Elbi C, Lutterbach B (2008) FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res 68: 2340–2348 [DOI] [PubMed] [Google Scholar]
- Leung HY, Mehta P, Gray LB, Collins AT, Robson CN, Neal DE (1997) Keratinocyte growth factor expression in hormone insensitive prostate cancer. Oncogene 15: 1115–1120 [DOI] [PubMed] [Google Scholar]
- Lipinski MM, Macleod KF, Williams BO, Mullaney TL, Crowley D, Jacks T (2001) Cell-autonomous and non-cell-autonomous functions of the Rb tumor suppressor in developing central nervous system. EMBO J 20: 3402–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlepage LE, Egeblad M, Werb Z (2005) Coevolution of cancer and stromal cellular responses. Cancer Cell 7: 499–500 [DOI] [PubMed] [Google Scholar]
- Liu Y, Hu T, Shen J, Li SF, Lin JW, Zheng XH, Gao QH, Zhou HM (2006) Separation, cultivation and biological characteristics of oral carcinoma-associated fibroblasts. Oral Dis 12: 375–380 [DOI] [PubMed] [Google Scholar]
- Liu H, Knabb JR, Spike BT, Macleod KF (2009) Elevated poly-(ADP-ribose)-polymerase activity sensitizes retinoblastoma-deficient cells to DNA damage-induced necrosis. Mol Cancer Res 7: 1099–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maandag EC, van der Valk M, Vlaar M, Feltkamp C, O'Brien J, van Roon M, van der Lugt N, Berns A, te Riele H (1994) Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J 13: 4260–4268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas-Szabowski N, Shimotoyodome A, Fusenig NE (1999) Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci 112: Pt 121843–1853 [DOI] [PubMed] [Google Scholar]
- Manavi M, Hudelist G, Fink-Retter A, Gschwandtler-Kaulich D, Pischinger K, Czerwenka K (2007) Gene profiling in Pap-cell smears of high-risk human papillomavirus-positive squamous cervical carcinoma. Gynecol Oncol 105: 418–426 [DOI] [PubMed] [Google Scholar]
- McCance DJ (2005) Human papillomaviruses and cell signaling. Sci STKE 2005: pe29. [DOI] [PubMed] [Google Scholar]
- McCance DJ, Kopan R, Fuchs E, Laimins LA (1988) Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proc Nat Acad Sci USA 85: 7169–7173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges CW, Baglia LA, Lapoint R, McCance DJ (2006) Human papillomavirus type 16 E7 up-regulates AKT activity through the retinoblastoma protein. Cancer Res 66: 5555–5559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier I, Casimiro MC, Wang C, Rosenberg AL, Quong J, Minkeu A, Allen KG, Danilo C, Sotgia F, Bonuccelli G, Jasmin JF, Xu H, Bosco E, Aronow B, Witkiewicz A, Pestell RG, Knudsen ES, Lisanti MP (2008) Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumor suppressor functional inactivation: implications for the response to hormonal therapy. Cancer Biol Ther 7: 1212–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittnacht S, Weinberg RA (1991) G1/S phosphorylation of the retinoblastoma protein is associated with an altered affinity for the nuclear compartment. Cell 65: 381–393 [DOI] [PubMed] [Google Scholar]
- Narayan G, Bourdon V, Chaganti S, Arias-Pulido H, Nandula SV, Rao PH, Gissmann L, Durst M, Schneider A, Pothuri B, Mansukhani M, Basso K, Chaganti RS, Murty VV (2007) Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: identification of candidate amplified and overexpressed genes. Genes Chromosomes Cancer 46: 373–384 [DOI] [PubMed] [Google Scholar]
- Niu J, Chang Z, Peng B, Xia Q, Lu W, Huang P, Tsao MS, Chiao PJ (2007) Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-kappaB transcription factors. J Biol Chem 282: 6001–6011 [DOI] [PubMed] [Google Scholar]
- Orimo A, Weinberg RA (2006) Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5: 1597–1601 [DOI] [PubMed] [Google Scholar]
- Ostman A, Augsten M (2009) Cancer-associated fibroblasts and tumor growth--bystanders turning into key players. Curr Opin Genet Dev 19: 67–73 [DOI] [PubMed] [Google Scholar]
- Pickard A, Wong PP, McCance DJ (2010) Acetylation of Rb by PCAF is required for nuclear localization and keratinocyte differentiation. J Cell Sci 123: 3718–3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Bottaro DP, Finch PW, Morris D, Rubin JS, Aaronson SA (1993) Expression of biologically active recombinant keratinocyte growth factor. Structure/function analysis of amino-terminal truncation mutants. J Biol Chem 268: 2984–2988 [PubMed] [Google Scholar]
- Ropiquet F, Huguenin S, Villette JM, Ronfle V, Le Brun G, Maitland NJ, Cussenot O, Fiet J, Berthon P (1999) FGF7/KGF triggers cell transformation and invasion on immortalised human prostatic epithelial PNT1A cells. Int J Cancer 82: 237–243 [DOI] [PubMed] [Google Scholar]
- Shin EY, Ma EK, Kim CK, Kwak SJ, Kim EG (2002) Src/ERK but not phospholipase D is involved in keratinocyte growth factor-stimulated secretion of matrix metalloprotease-9 and urokinase-type plasminogen activator in SNU-16 human stomach cancer cell. J Cancer Res Clin Oncol 128: 596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi I, Funatomi H, Kobrin MS, Friess H, Buchler MW, Korc M (1995) Increased expression of keratinocyte growth factor in human pancreatic cancer. Biochem Biophys Res Commun 215: 309–315 [DOI] [PubMed] [Google Scholar]
- Smith JL, Schaffner AE, Hofmeister JK, Hartman M, Wei G, Forsthoefel D, Hume DA, Ostrowski MC (2000) ets-2 is a target for an akt (protein kinase B)/jun N-terminal kinase signaling pathway in macrophages of motheaten-viable mutant mice. Mol Cell Biol 20: 8026–8034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Mundel TM, Kieran MW, Kalluri R (2006) Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 5: 1640–1646 [DOI] [PubMed] [Google Scholar]
- Takeda M, Arao T, Yokote H, Komatsu T, Yanagihara K, Sasaki H, Yamada Y, Tamura T, Fukuoka K, Kimura H, Saijo N, Nishio K (2007) AZD2171 shows potent antitumor activity against gastric cancer over-expressing fibroblast growth factor receptor 2/keratinocyte growth factor receptor. Clin Cancer Res 13: 3051–3057 [DOI] [PubMed] [Google Scholar]
- Tang A, Gilchrest BA (1996) Regulation of keratinocyte growth factor gene expression in human skin fibroblasts. J Dermatol Sci 11: 41–50 [DOI] [PubMed] [Google Scholar]
- Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, Naidu S, Wei G, Sharma SM, Stephens JA, Fernandez SA, Gurcan MN, Weinstein MB, Barsky SH, Yee L, Rosol TJ et al. (2009) Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 461: 1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Grose R (2010) Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10: 116–129 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Ishiwata T, Nishigai K, Moriyama Y, Asano G (2000) Overexpression of keratinocyte growth factor in cancer cells and enterochromaffin cells in human colorectal cancer. Pathol Int 50: 363–372 [DOI] [PubMed] [Google Scholar]
- Weng LP, Brown JL, Baker KM, Ostrowski MC, Eng C (2002) PTEN blocks insulin-mediated ETS-2 phosphorylation through MAP kinase, independently of the phosphoinositide 3-kinase pathway. Hum Mol Genet 11: 1687–1696 [DOI] [PubMed] [Google Scholar]
- Westbrook TF, Nguyen DX, Thrash BR, McCance DJ (2002) E7 abolishes raf-induced arrest via mislocalization of p21(Cip1). Mol Cell Biol 22: 7041–7052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt D, Grosveld F (2002) Cell-nonautonomous function of the retinoblastoma tumour suppressor protein: new interpretations of old phenotypes. EMBO Rep 3: 130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BO, Schmitt EM, Remington L, Bronson RT, Albert DM, Weinberg RA, Jacks T (1994) Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J 13: 4251–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, de Bruin A, Saavedra HI, Starovic M, Trimboli A, Yang Y, Opavska J, Wilson P, Thompson JC, Ostrowski MC, Rosol TJ, Woollett LA, Weinstein M, Cross JC, Robinson ML, Leone G (2003) Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 421: 942–947 [DOI] [PubMed] [Google Scholar]
- Yamashita K, Mori M, Kataoka A, Inoue H, Sugimachi K (2001) The clinical significance of MMP-1 expression in oesophageal carcinoma. Br J Cancer 84: 276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen L, Xiao M, Wang C, Qin Z (2011) FSP1+ fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am J Pathol 178: 382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Siren V, Vaheri A (1996) Keratinocyte growth factor enhances urokinase-type plasminogen activator activity in HPV16 DNA-immortalized human uterine exocervical epithelial cells. Eur J Cell Biol 69: 128–134 [PubMed] [Google Scholar]
- Zimmermann S, Moelling K (1999) Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286: 1741–1744 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.