Abstract
Axon regeneration is an essential process to rebuild functional connections between injured neurons and their targets. Regenerative axonal growth requires alterations in axonal microtubule dynamics, but the signalling mechanisms involved remain incompletely understood. Our results reveal that axon injury induces a gradient of tubulin deacetylation, which is required for axon regeneration both in vitro and in vivo. This injury-induced tubulin deacetylation is specific to peripheral neurons and fails to occur in central neurons. We found that tubulin deacetylation is initiated by calcium influx at the site of injury, and requires protein kinase C-mediated activation of the histone deacetylase 5 (HDAC5). Our findings identify HDAC5 as a novel injury-regulated tubulin deacetylase that plays an essential role in growth cone dynamics and axon regeneration. In addition, our results suggest a mechanism for the spatial control of tubulin modifications that is required for axon regeneration.
Keywords: axon regeneration, calcium signalling, histone deacetylase, microtubule, protein kinase C
Introduction
Lack of robust axon regeneration represents one of the major barriers to recovery of neurological functions following injury to the central nervous system (CNS). The extent of axon regeneration not only depends on the presence or absence of inhibitory cues in the environment, but also on the intrinsic growth capacity of damaged neurons. Indeed, blocking extracellular inhibitory influences alone is not sufficient to allow complete axon regeneration (Lee et al, 2010) emphasizing the need for a better understanding of the mechanisms controlling the intrinsic regenerative ability of injured neurons. Unlike their counterpart in the CNS, injured peripheral neurons activate intrinsic signalling pathways that enable axonal regrowth. The fundamental principles that increase neuronal growth capacity and govern axon regeneration include both cell body responses and local axonal responses (Liu et al, 2011). The local axonal responses allow injured neurons to transform their damaged axonal end into a new growth cone-like structure that is essential to initiate regeneration. Indeed, neurons within the CNS often fail to reform a growth cone and instead form a retraction bulb, the hallmark of non-regenerating axons (Erturk et al, 2007). Growth cone formation and axon regeneration appear to critically depend on cytoskeleton dynamics. In particular, a precise balance between stable and dynamic microtubules is required for growth cone formation and axon regeneration (Erturk et al, 2007; Hellal et al, 2011; Sengottuvel et al, 2011). Various signal transduction pathways control cytoskeletal dynamics to regulate axon growth and guidance during development (O'Donnell et al, 2009), but the detailed signalling pathways that control growth cone formation and dynamics following injury remain poorly characterized.
The microtubule cytoskeleton represents a fundamental structural element that controls neuronal morphology and supports the establishment and maintenance of axonal connections with their targets. The dynamic properties of microtubules are largely believed to arise from post-translational modifications of tubulin, the molecular building block of microtubules (Hammond et al, 2008). For example, acetylated tubulin is enriched in older, stable microtubules along the axon shaft, correlating with microtubule stability (Janke and Kneussel, 2010). In contrast, tyrosinated tubulin is enriched in the growth cone area, correlating with the highly dynamic properties of microtubules in that region (Dent and Gertler, 2003). Like axon growth during development, axon injury induces microtubule remodelling to form a new growth cone and to promote axonal elongation (Erturk et al, 2007). For instance, following axotomy of the giant lamprey central neurons, acetylated microtubule levels are reduced, while levels of tyrosinated microtubules are increased (Hall et al, 1991; Mullins et al, 1994).
Histone deacetylases (HDACs) are enzymes that catalyse deacetylation not only from histones but also from various cytoplasmic proteins such as α-tubulin. HDAC6 (Hubbert et al, 2002) and Sirt2 (North et al, 2003) represent the two known tubulin deacetylases. While many studies have focussed on the nuclear functions of HDACs, their function in the cytoplasm is less well understood. However, recent studies indicate that inhibition of HDACs promotes axonal outgrowth of (1) dorsal root ganglia (DRG) neurons grown on inhibitory substrates and (2) cerebellar granule (CGN) neurons via transcription-dependent (Gaub et al, 2010) and -independent mechanisms (Rivieccio et al, 2009).
To further understand the role of HDACs in axon regeneration, we examined the mechanisms underlying tubulin deacetylation after injury. We found that axon injury decreases the levels of acetylated tubulin and stable microtubules in proximity of the injury site in peripheral but not in central neurons. We also established that tubulin deacetylation is necessary for growth cone dynamics and axon regeneration in vitro and in vivo. In addition, we identified a signalling pathway in injured axons that involves local calcium influx and activation of calcium-dependent protein kinase C (PKC), which in turn regulates HDAC5 activity to promote tubulin deacetylation. Blocking calcium influx, PKC or HDAC5 activity prevented tubulin deacetylation, thereby preventing axon regeneration. Therefore, our current findings identify HDAC5 as a novel injury-regulated tubulin deacetylase responsible for axon regeneration. In addition, these results suggest a mechanism for the spatial control of tubulin modification that is required to promote regeneration.
Results
Axon injury induces tubulin deacetylation in peripheral but not in central neurons
To further understand the role of microtubule dynamics in axon regeneration, we investigated the changes in microtubule post-translational modifications induced by nerve injury. Mouse sciatic nerves were ligated to induce injury and nerve segments proximal and distal to the ligation site were analysed by western blot 24 h later. Injury decreased the levels of acetylated tubulin, without affecting the total level of α-tubulin (Figure 1A and B; Supplementary Figure S1A and B). We also observed an increase in the levels of tyrosinated tubulin (Figure 1A and B). Using immunohistochemistry, injury-induced decreases in acetylated tubulin proximal to the ligation site were also observed. These data indicate that tubulin deacetylation occurred mostly in axons (Figure 1C).
Figure 1.
Injury causes microtubule deacetylation in peripheral but not in central axons. (A) Representative western blot of unligated nerve (U) and proximal (P) and distal (D) ligated nerve. Ac-tub: acetylated tubulin, Tyr-tub: tyrosinated tubulin. (B) Quantification of (A). Acetylated and tyrosinated tubulin levels were normalized to total α-tubulin (mean±s.e.m., ***P<0.001, n=8). (C) Longitudinal sections of sciatic nerves were stained with the indicated antibodies. Note that in ligated samples, the increased IgG levels in the nerve crossreact with the secondary antibody used and generate background staining for the acetylated tubulin. Importantly, the specific signal in axons stained with α-tubulin is decreased (white arrowheads). Bar, 200 μm. (D) Representative western blot of unligated (U) and proximal (P) ligated optic nerve. (E) Quantification of (D) (n=4, NS, not significant). (F) Optic nerve longitudinal sections were stained with the indicated antibodies. Bar, 200 μm. (G) Schematic illustration of three consecutive sciatic nerve segments. (H) Representative western blot of three consecutive 3 mm sciatic nerve segments. (I) Sciatic nerves were ligated and analysed by western blot with the indicated antibodies at different time points after ligation. (J) Quantification of (I) (n=4, mean±s.e.m.).
We then tested whether injury-induced tubulin deacetylation is specific to peripheral nervous system (PNS) neurons. Optic nerves were ligated and nerve segments proximal to the ligation site were analysed 24 or 48 h later by western blot (Figure 1D and E; Supplementary Figure S1C and D) and by immunohistochemistry (Figure 1F). Injury in optic nerves had no significant effects on tubulin acetylation levels at the two time points examined. In addition, injury-induced decreases in acetylated tubulin levels failed to occur in injured DRG dorsal column (Supplementary Figure S1E). These results indicate that injury-induced tubulin deacetylation is specific to peripheral axons and fails to occur in central axons.
To determine whether tubulin deacetylation and tyrosination occur near the injury site, the proximal part of ligated sciatic nerves was analysed over a 9-mm segment. Injury-induced tubulin modifications occurred primarily within the first 3 mm immediately proximal to the injury site (Figure 1G and H). Within this 3 mm nerve segment, we observed gradients of acetylated and tyrosinated tubulin (Supplementary Figure 1F and G). We next determined the time course of injury-induced tubulin modifications (Figure 1I and J). Tubulin deacetylation and tyrosination underwent significant changes 6 h after injury, and gradually reached a plateau at 48 h. In this experiment, we used a 10-mm nerve segment proximal to the ligation site. It is possible that tubulin modifications occur more rapidly but that the sample size analysed prevented detection of earlier changes. Indeed, and as discussed below, tubulin modifications can occur as rapidly as 3.5 h following injury. Together, these results indicate that nerve injury induces local changes in tubulin post-translational modifications, with a decrease in tubulin acetylation and an increase in tubulin tyrosination. Moreover, tubulin deacetylation is specific to PNS neurons. Tubulin post-translational modifications in response to injury may underlie the difference between central and peripheral axons’ ability to regenerate. Because injury-induced tubulin deacetylation is believed to play a role in microtubule stability (Takemura et al, 1992), we focussed on this process.
HDAC enzymatic activity is required for injury-induced tubulin deacetylation
Tubulin deacetylation is mediated by HDACs (Hubbert et al, 2002; North et al, 2003). To investigate whether HDACs deacetylate tubulin in injured axons in vivo, we performed sciatic nerve ligation in the presence of the HDAC inhibitor scriptaid. We selected scriptaid over trichostatin A for its specificity and low toxicity (Su et al, 2000). Compared with vehicle-treated control, scriptaid treatment blocked tubulin deacetylation in injured nerves (Figure 2A–C). As a positive control for the scriptaid effect, we observed the expected increase in acetyl-histone H3 levels (Figure 2A), which likely originates from non-neuronal, Schwann cell nuclei that are present in the nerve.
Figure 2.
Injury-induced tubulin deacetylation requires HDAC activity. (A) Sciatic nerves were treated with vehicle or scriptaid (1 mg/kg of weight). Unligated and proximal ligated samples were analysed by western blot with the indicated antibodies. Acetylated histone H3 (Ac-histone H3) is used as a positive control for scriptaid. (B) Quantification of (A) (mean±s.e.m., n=8, NS: not significant). (C) Sciatic nerves were treated with vehicle or scriptaid as in (A) and longitudinal sections stained with the indicated antibodies. Bar, 100 μm. (D) Embryonic DRGs were axotomized at DIV7 in the presence of vehicle or 5 μM scriptaid and immunostained with the indicated antibodies. White dotted line points to the axotomy site. Bar, 100 μm. (E) The intensity of acetylated tubulin was normalized to α-tubulin and plotted as a function of distance, from proximal to distal. Intensity was set arbitrarily to 1 at the top of each image. (F) Average intensity plot of normalized acetylated tubulin (n=7, −Ax; n=5, vehicle; n=6, scriptaid; mean±s.e.m.) over a 300-μm segment proximal to the axotomy line. 0 refers to the axotomy site. (G) The absolute value of the slope of the acetylated ratio (‘|Slope|’) over a 300-μm distance was calculated: −axotomy (−Ax), 3.5±0.7 (× 10−4); +axotomy (+Ax) with vehicle (V), 22.0±3.5 (× 10−4); +axotomy with scriptaid (S), 2.8±1.2 (× 10−4) (***P<0.001, mean±s.e.m.).
To further study the mechanisms regulating injury-induced tubulin deacetylation, we adapted an in-vitro DRG neuron culture model (Sasaki et al, 2009). Cultured DRG neurons were seeded within a defined area, allowing their axons to extend in a nearly parallel manner. DRGs were immunostained 3.5 h following axotomy with acetylated tubulin and α-tubulin antibodies (Figure 2D). The ratio of acetylated tubulin to α-tubulin was calculated from the proximal to distal end of the axon growth axis (Figure 2E). In control axons, we observed a shallow gradient of acetylated tubulin, with less acetylated tubulin towards the distal growing end (Figure 2F and G). We then measured the acetylated/α-tubulin ratio along a 300-μm axonal section proximal to the axotomy site, plotted this ratio as a function of the distance, and calculated the absolute value of the slope to determine the extent of tubulin deacetylation. In the absence of axotomy, the slope value was 3.5±0.6 (× 10−4) (Figure 2G). Axotomy caused a significant decrease in tubulin acetylation, with a steep gradient on the proximal side of the axotomy and an almost complete absence of acetylated tubulin distal to the axotomy line. Proximal to the axotomy site, the gradient of acetylated/α-tubulin ratio displayed a greatly increased slope value of 22.0±3.8 (× 10−4), ∼6-fold higher than in uninjured axons (Figure 2F and G; Supplementary Figure S2). Scriptaid treatment blocked axotomy-induced tubulin deacetylation in both the proximal and the distal part of the axon (Figure 2D and E), and restored the slope value to a level comparable to the uninjured control with a value of 2.8±1.2 (× 10−4) (Figure 2F and G).
Together, these results indicate that injury-induced tubulin deacetylation in axons requires HDAC enzymatic activity both in vivo and in vitro and that a steep gradient of tubulin deacetylation is created by injury, with higher levels of deacetylation closer to the injury site.
HDAC inhibition restricts axonal growth and regeneration in vitro
Modulation of microtubule dynamics is an essential process for axonal growth (Tanaka and Kirschner, 1995) and tubulin post-translational modifications represent one of the fundamental mechanisms regulating microtubule stability (Schulze et al, 1987). Thus, we tested whether HDAC-mediated tubulin deacetylation affects DRG axonal growth in vitro and in vivo.
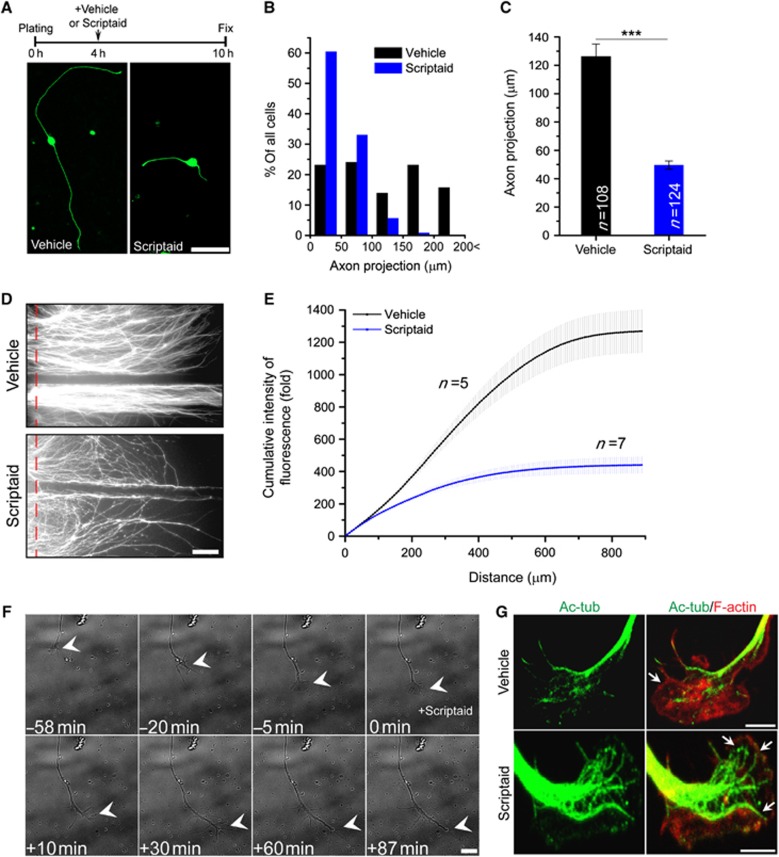
For in-vitro experiments, embryonic DRG neurons were cultured in the presence or absence of scriptaid, fixed and stained with the axonal marker SMI-31. To measure the extent of axon outgrowth, we calculated the distance between the cell body to the tip of the longest axon (‘axon projection length’), as previously described (Abe et al, 2010). Scriptaid significantly inhibited axonal growth compared with vehicle control (Figure 3A). Only 6.4% of scriptaid-treated DRG neurons had >100 μm axon projection length, whereas 52.7% of control showed over 100 μm length (Figure 3B). The average length of control and scriptaid-treated axons was 126.2±8.8 and 49.6±2.9 μm, respectively (Figure 3C). Prolonged treatment with scriptaid also significantly inhibited axon growth (Supplementary Figure S3). To test whether scriptaid delivery specifically to the axon is sufficient to delay axon growth, DRG neurons were plated in Campenot chambers (Campenot, 1977) and infected with lentivirus encoding GFP on DIV1. We added scriptaid to the left axon chamber and vehicle control to the right axon chamber on DIV2 and fixed on DIV4 (Figure 3D). The cumulative fluorescence intensity along a 900-μm section distal to each chamber barrier was measured and normalized to the intensity at the barrier (Figure 3E). If scriptaid delayed axon growth via a transcription-dependent role, we would expect axon growth to be delayed in both left and right chambers. Instead, we observed that only axons in the scriptaid-treated chamber showed delayed growth, suggesting that scriptaid’s effect is at least in part, transcription independent.
Figure 3.
Scriptaid treatment inhibits DRG axon growth. (A) Experimental scheme and representative DRG neuron images stained with the axonal marker SMI-31. Bar, 50 μm. (B) The axon projection length was calculated and the distribution of axon projection lengths plotted (vehicle, n=108; scriptaid 5 μM, n=124). (C) The average length of axon projections was calculated (***P<0.001, mean±s.e.m.). (D) Representative image of GFP-expressing embryonic DRG neurons cultured in a Campenot chamber 2 days after treatment with vehicle or scriptaid. Vehicle and scriptaid (5 μM) were added simultaneously on DIV2 to the left and right axon chambers, respectively. Bar, 100 μm. (E) Cumulative intensity of GFP fluorescence along a 900-μm distance was measured and normalized to the intensity at the barrier (red dashed line) (vehicle, n=5; scriptaid, n=7; mean±s.e.m.). (F) Live imaging of a DIV1 DRG growth cone. In all, 5 μM scriptaid was added at time 0. Bar, 10 μm. (G) Growth cones of DIV1 DRG neurons were treated with vehicle or 5 μM scriptaid for 1 h and stained with acetylated tubulin and rhodamine-phalloidin. Arrows point to the cortical actin. Bar, 5 μm.
We also performed live-cell imaging to observe the effect of scriptaid on growth cone motility (Figure 3F; Supplementary Movies S1 and S2). The axon tips of DIV1 cultured DRG were highly motile and displayed dynamic changes in morphology, alternating between filopodia and lamellipodia. This growth cone motility was suppressed within 30 min of scriptaid treatment (Figure 3F; Supplementary Movie S1). Moreover, the overall morphology of the growth cone dramatically changed, with long spike-like structures replacing lamellipodia. Immunofluorescence revealed that microtubules are invading the lamellipodia to a much higher extent in scriptaid-treated neurons than in vehicle-treated neurons, with acetylated tubulin colocalizing with the actin-enriched area (Figure 3G). These results indicate that HDAC inhibition suppresses growth cone dynamics and impairs axonal growth.
HDAC inhibition restricts axon regeneration and target innervation in vivo
We further tested whether HDAC inhibition blocks regenerative axon growth in cultured DRG neurons. We visualized axon regrowth by live-cell fluorescence imaging after in-vitro axotomy using DRG spot cultures expressing GFP (Figure 4A). To assess the regenerative capacity of injured axons, we measured the number of axons crossing the axotomy line 12 and 40 h after axotomy. Axotomized axons displayed robust regeneration with 33.7±5.6 axons/mm crossing over the axotomized line after 12 h, whereas scriptaid treatment suppressed axon regeneration, with only 8.5±2.7 axons/mm growing past the axotomy line (Figure 4B). This suppression of regenerating capacity was maintained at 40 h (Figure 4B). The inhibitory effect of scriptaid on axon regeneration was not due to a non-specific effect on neuronal number or survival, as both the number of axons and the number of neurons were unchanged by scriptaid treatment over a 40-h time period (Supplementary Figure S4A–C).
Figure 4.
Scriptaid treatment inhibits axon regeneration in vitro and in vivo. (A) GFP-expressing DRGs were axotomized (red dashed line) and axon regeneration monitored over time by live-fluorescence microscopy. Images were acquired at 12 and 40 h post axotomy. Bar, 100 μm. (B) Average axon numbers regenerating past the axotomy line (n=4 for each, **P<0.01, mean±s.e.m.). (C) A regeneration index was calculated from the images acquired 40 h post axotomy. The fluorescence intensity of a square area (2.7 × 0.1 mm) at 0.1 mm distal to the axotomy line was measured and normalized to the similar area 0.1 mm proximal to the axotomy line. (D) Whole mount images of injured sciatic nerve of thy1-YFP16 mice. Sciatic nerve was crushed and fixed after 24 h. YFP intensity was measured along a 700-μm segment and normalized to the intensity at the crush site (red dotted line). Bar, 100 μm. (E) Average intensity of normalized YFP fluorescence along a 700-μm segment past the axotomy site (vehicle, n=4; scriptaid, n=5; mean±s.e.m.). (F) Confocal images of whole mounted EHL muscles of thy1-YFP16 mice stained with α-bungarotoxin (α-BTX) at the indicated time after sciatic nerve injury. Bar, 10 μm. A single scriptaid treatment locally at the time of injury delays axon regeneration. (G) Quantification of re-innervated endplates (8 mice; vehicle, n=395; scriptaid, n=399; mean±s.e.m., **P<0.01). (H) As in (E), but scriptaid was administered at the time of injury and thereafter every 3 days for 21 days. Repeated injection of scriptaid, but not vehicle, blocked axon regeneration into the endplates of EHL muscle. Bar, 50 μm. (I) Quantification of re-innervation (8 mice; vehicle, n=345; scriptaid, n=360; ***P<0.001, mean±s.e.m.). The dose of scriptaid is 5 μM in (A) and 1 mg/kg of weight in (D), (F) and (H).
To determine whether scriptaid also inhibited axon regeneration in vivo, we used thy1-YFP16 mice that express YFP in DRG and motor neurons (Feng et al, 2000). Sciatic nerves were crushed over a 1-mm window, resulting in loss of YFP fluorescence in that area within 2 h (Supplementary Figure S5A). Axon regeneration was evaluated by measuring YFP intensity over the crush line 24 h after injury (Figure 4D), as previously described (Abe et al, 2010). Axon regeneration was markedly impaired in the presence of scriptaid since 50% of the YFP intensity had recovered 581.8 μm past the crush site in control nerves, whereas YFP intensity had recovered only 150.8 μm in scriptaid-treated nerves (Figure 4E). We obtained similar results when measuring axon regeneration using GAP-43 staining (Supplementary Figure S5B and C).
Finally, we investigated whether scriptaid treatment affects axon re-innervation in the extensor hallucis longus (EHL) muscle 3 weeks after sciatic nerve crush. In uninjured mice, 100% of endplates were occupied by axons (Figure 4F). Seven days after nerve crush, the EHL muscles showed no intact axons at endplates in either vehicle or scriptaid-treated mice, indicating that Wallerian degeneration was not affected by scriptaid (Figure 4F). Twenty-one days after crush, the EHL muscles in vehicle-treated mice showed a robust re-innervation with 82.8±3.2% of endplates re-occupied by axons. In contrast, scriptaid-treated mice showed only 39.6±7.9% of endplates occupied by axons (Figure 4F and G). The reduced number of axons that regenerated into the EHL muscle following a single treatment of scriptaid at the time of nerve crush suggests that scriptaid delayed but did not completely prevent axon regeneration. However, a repeated treatment of scriptaid locally at the site of the nerve crush every 3 days over a 3-week period almost completely abolished re-innervation (Figure 4H and I). Together, these results indicate that HDAC inhibition impairs growth cone dynamics and leads to delayed axon regeneration both in vitro and in vivo.
HDAC5 mediates injury-induced tubulin deacetylation
To determine which HDAC is responsible for injury-induced tubulin deacetylation, the class II HDACs (HDAC4, HDAC5 or HDAC6) or the class I HDACs (HDAC1, HDAC2 or HDAC3) were knocked down prior to DRG axotomy (Figure 5A; Supplementary Figure S6A and B). Knockdown of HDAC4 or the class I HDACs did not prevent axotomy-induced microtubule deacetylation (Figure 5A; Supplementary Figure 7A–C), whereas HDAC5 and HDAC6 knockdown did but to varying degrees (Figure 5C). In contrast to scriptaid treatment (Figure 2G), HDAC5 or HDAC6 knockdown did not block tubulin deacetylation in proximity of the axotomy site (0–50 μm region). Remaining low levels of HDAC5 in axons (Supplementary Figure S6C) may be responsible for the local tubulin deacetylation following axotomy. Alternatively, tubulin deacetylation in regenerating axonal tips may require other, scriptaid-sensitive deacetylases. We thus calculated the slope of the acetylated/total tubulin ratio in the adjacent 250 μm (50–300 μm) (Figure 5C; Supplementary Figure S7C). There was no significant difference in slope between no axotomy control and axotomy with HDAC4 knockdown (Figure 5C). HDAC5 knockdown had a more pronounced effect on preventing injury-induced tubulin deacetylation than HDAC6 (Figure 5C). Expression of an shRNA-resistant human GFP-HDAC5 restored the axotomy-induced tubulin deacetylation to control levels (Supplementary Figure S8A–D).
Figure 5.
HDAC5 mediates injury-induced tubulin deacetylation. (A) HDAC4, HDAC5 or HDAC6 was knocked down using lentivirus delivery of shRNA. DRG neurons were axotomized 4 days following lentiviral infection and stained with the indicated antibodies 3.5 h after axotomy. Bar, 100 μm. (B) Average intensity plot of normalized acetylated tubulin values (mean±s.e.m.). (C) The slope values of normalized acetylated tubulin intensity over a 50- to 300-μm distance was calculated: shControl, −Ax, 3.5±1.4 (× 10−4), +Ax, 19.9±2.1 (× 10−4); shHDAC4, −Ax, 3.2±0.7 (× 10−4), +Ax, 19.2±1.2 (× 10−4); shHDAC5, −Ax, 3.0±0.7 (× 10−4), +Ax, 5.0±1.4 (× 10−4); shHDAC6, −Ax, 4.5±1.2 (× 10−4), +Ax, 10.8±0.7 (× 10−4) (***P<0.001, mean±s.e.m.). (D) In-vitro tubulin deacetylase assay. The indicated Flag–HDAC was immunoprecipitated from HEK293T cells and incubated with sciatic nerve extract. The supernatant was then analysed for remaining levels of acetylated tubulin. (E) Quantification of remaining acetylated tubulin normalized to α-tubulin (n=4, ***P<0.001, *P<0.05, mean±s.e.m.). (F) In-vitro tubulin deacetylase assay was performed as in (D). FLAG–HDAC5-expressing HEK293T cells were stimulated with or without phorbol ester (PMA). Immunoprecipitated HDAC5 was then incubated with or without calf intestinal phosphatase (CIP, 5 units/reaction) prior to incubation with sciatic nerve extract. Cell lysate before immunoprecipitation, the immunoprecipitated material and the supernatant were analysed by western blot with the indicated antibodies. (G) Quantification of remaining acetylated tubulin normalized to α-tubulin (n=5, *P<0.05, ***P<0.001, mean±s.e.m.).
To directly test whether HDAC5 deacetylates tubulin, we performed an in-vitro tubulin deacetylation assay. Flag-tagged HDAC4, HDAC5 and HDAC6, a known tubulin deacetylase (Hubbert et al, 2002), were incubated with sciatic nerve lysates, which were then analysed for remaining levels of acetylated tubulin (Figure 5D and E; Supplementary Figure S9A). In contrast to earlier reports showing that HDAC5 does not deacetylate α-tubulin derived peptides (Zhang et al, 2003), HDAC5 efficiency was comparable to HDAC6 (Figure 5E). HDAC5-mediated tubulin deacetylation was inhibited by scriptaid in vitro (Supplementary Figure S9B and C) and was not due to the co-precipitation of HDAC6 with HDAC5 (Supplementary Figure S9D). HDAC4 displayed a weak efficiency that was not significantly different from control.
HDAC5 localization in the cytoplasm is known to be mediated by PKC phosphorylation (McKinsey et al, 2000; Chawla et al, 2003; Vega et al, 2004; Ha et al, 2008). We thus tested whether activation of PKC can enhance HDAC5 tubulin deacetylase activity. Flag–HDAC5 expressing cells were treated with PMA to stimulate the PKC pathway (Supplementary Figure S9E) and immunoprecipitated Flag–HDAC5 was then treated with vehicle or calf intestine phosphatase (CIP) before incubation with sciatic nerve lysates. PMA treatment activated PKC and enhanced HDAC5 phosphorylation (Figure 5F). Flag–HDAC5 immunoprecipitated from PMA-treated cells displayed enhanced tubulin deacetylase activity (Figure 5F and G). Treatment of immunoprecipitated Flag–HDAC5 with CIP completely blocked its deacetylase activity (Figure 5G). These results suggest that HDAC5 tubulin deacetylase activity is regulated by phosphorylation and that non-phosphorylated HDAC5 displays no activity. Interestingly, we observed that HDAC5 knockdown in cultured DRG neurons did not increase the basal level of acetylated tubulin (Supplementary Figure S10A and B), confirming that in basal conditions, HDAC5 does not play a significant role in tubulin acetylation levels. This is in contrast to HDAC6 knockdown, which increased the basal levels of acetylated tubulin (Supplementary Figure S10A and B) confirming previous reports (Rivieccio et al, 2009). These results indicate that HDAC5 is a novel tubulin deacetylase regulated by phosphorylation and that unlike HDAC6, HDAC5 does not appear to play a major role in tubulin acetylation under basal conditions.
Injury induces HDAC5 phosphorylation and the formation of an HDAC5 gradient
To determine whether HDAC5 is phosphorylated in axons in vivo, we examined the levels and localization of phosphorylated HDAC5 (p-HDAC5) in control and injured sciatic nerves. Immunofluorescence analysis showed that p-HDAC5 is present in injured, but not in uninjured axons, stained with the axonal marker βIII tubulin (TUJ1) (Figure 6A). Western blot analyses also revealed that p-HDAC5 levels in the nerve are increased by injury (Figure 6B). Since we used the PKC activator PMA to stimulate HDAC5 phosphorylation (Figure 5F), we next tested whether HDAC5 phosphorylation in vivo depends on PKC activity. We observed that the general PKC inhibitor Gö6983 prevented HDAC5 phosphorylation in injured nerves (Figure 6B and C).
Figure 6.
Injury induces HDAC5 phosphorylation and the formation of an HDAC5 gradient. (A) Longitudinal sections of unligated or ligated sciatic nerve stained with p-HDAC5 and the axon marker βIII tubulin (TUJ1). Colocalization of p-HDAC5 with TUJ1 demonstrates HDAC5 axonal localization. (B) Sciatic nerves were treated with vehicle or the PKC inhibitor Gö6983 (1 mg/kg of weight) and ligated for 2 h. The level of p-HDAC5 was analysed by western blot in ligated and unligated sciatic nerves. (C) Quantification of normalized p-HDAC5 to total HDAC5 intensity (n=4, **P<0.01, mean±s.e.m.). (D) DRG neurons were axotomized at DIV7 (white dotted line) and stained with HDAC5, p-HDAC5 and HDAC6 antibodies 3.5 h after axotomy. Bar, 100 μm. (E) Average intensity plot of HDAC5, p-HDAC5 or HDAC6 with the corresponding average intensity plot of acetylated tubulin (n=6 for each; plot, mean±s.e.m.). (F) Cultured DIV7 DRG neurons were treated with or without ionomycin or Gö6983. HDAC5 was immunoprecipitated and analysed by western blot with anti-kinesin-1 and anti-HDAC5 antibodies (TCL: total cell lysate prior to immunoprecipitation). (G) Sciatic and optic nerves were analysed by western blot for HDAC5. No HDAC5 can be detected in the optic nerve.
We next tested whether the gradient of tubulin acetylation in injured axons results from a gradient of HDAC5 along the axons. We quantified the localization of HDAC5 and p-HDAC5 in injured, cultured DRG axons and we found that both HDAC5 and p-HDAC5 localized in a gradient in injured axons, with increased levels closer to the injury site (Figure 6D). Quantification of HDAC5 and p-HDAC5 levels revealed that their gradient correlates with the tubulin acetylation gradient that is formed following injury (Figure 6E). In contrast, HDAC6 failed to accumulate in injured axons and HDAC6 localization did not correlate with the tubulin deacetylation gradient (Figure 6D and E). In cultured DRG neurons, the gradient of HDAC5 was measured over a distance of 300 μm proximal to the injury site (Figure 6D and E), whereas in vivo, the level of HDAC5 was determined in a 3-mm segment of sciatic nerve (Figure 6B), thus preventing the detection of increased levels of HDAC5 in that nerve segment. If axonal localization of HDAC5 mediates tubulin deacetylation in response to injury, then HDAC5 should be absent from the optic nerve, in which we showed absence of injury-induced tubulin deacetylation (Figure 1D–F; Supplementary Figure S1C and D). We indeed observed that in contrast to the sciatic nerve, HDAC5 is not detected in the optic nerve (Figure 6G). HDAC5 localization in axons thus appears to be specific to PNS neurons.
To understand the mechanisms responsible for the formation of an HDAC5 gradient, we examined whether HDAC5 represents a cargo for the anterograde molecular motor kinesin-1. HDAC5 was immunoprecipitated from cultured DRG neurons and analysed by western blot for kinesin-1. Kinesin-1 co-immunoprecipitated with HDAC5 but not with an IgG control (Figure 6F). DRG treatment with ionomycin to promote PKC activation via increased calcium enhanced the interaction between kinesin-1 and HDAC5, while treatment with the PKC inhibitor Gö6983 decreased it (Figure 6F). These results suggest that kinesin-1-dependent transport of p-HDAC5 contributes to the generation of the HDAC5 gradient along injured axons. Taken together, these data suggest that activation of PKC regulates the formation of an HDAC5 gradient in injured axons.
Calcium-mediated PKC activation functions upstream of HDAC5
We next investigated the upstream signalling events induced by injury and leading to HDAC5-dependent tubulin deacetylation. Injury-induced calcium influx is known to mediate the transformation of a cut axon tip into new growth cones in invertebrates (Gitler and Spira, 1998; Kamber et al, 2009; Ghosh-Roy et al, 2010). Axotomy is also known to increase axonal calcium concentrations in rat cortical neurons (Mandolesi et al, 2004). To test whether axotomy of mouse DRG neurons similarly alters intracellular calcium concentrations, DRG axons were laser axotomized and the changes in calcium concentration monitored using a calcium indicator Fluo-4AM. Axotomy caused an immediate increase in axonal calcium concentration and initiated a rapid calcium wave propagating from the axotomy site back to the cell body (Figure 7A and B; Supplementary Figure S11A; Supplementary Movie S3). We then tested whether injury-induced tubulin deacetylation is calcium dependent. We found that calcium chelation by EGTA at the time of nerve injury prevented tubulin deacetylation, without affecting tubulin tyrosination (Supplementary Figure S11B). These observations indicate that tubulin deacetylation in response to axonal injury in DRG neurons requires local calcium influx.
Figure 7.
Calcium-dependent PKC activation after injury promotes tubulin deacetylation and decreases microtubule stability. (A) Calcium influx induced by laser axotomy in DRG neurons was visualized over a 60-s time period using FM-4AM dye. The standard colour palette varies from blue (minimal intensity of fluorescence) to white (maximum intensity of fluorescence). Bar, 10 μm. (B) Maximum fold changes in calcium influx (Fmax/F0) in axons and cell bodies (n=8, **P<0.01, mean±s.e.m.). (C) Ligated and unligated sciatic nerves were treated with vehicle, Gö6983 (1 mg/kg of weight) or EGTA (10 mM) and analysed by western blot. (D) p-PKCμ intensity was normalized to total PKCμ (n=4, **P<0.01, mean±s.e.m.). (E) Longitudinal sections of ligated sciatic nerve treated with vehicle or Gö6983. White dotted line indicates the site of ligation. Bar, 200 μm. (F) Ligated and unligated sciatic nerves were treated with vehicle, Gö6983 or KN-93 (1 mg/kg of weight) and analysed by western blot. (G) Normalized acetylated tubulin intensity (n=6, **P<0.01, mean±s.e.m.). (H) PKC was inhibited with Gö6983 (10 μM) prior to axotomy and DRG neurons were stained with the indicated antibodies 3.5 h following axotomy. Bar, 200 μm. (I) Average intensity plot of normalized acetylated tubulin in Gö6983-treated DRG neurons with or without axotomy (mean±s.e.m.). (J) Slopes values of acetylated tubulin intensity over a 50- to 300-μm distance: −Ax, 7.8±1.2 (× 10−4); +Ax, 4.3.0±1.7 (× 10−4) (mean±s.e.m.). (K) In-vitro microtubule polymerization assay. Sciatic nerves were treated with vehicle, scriptaid or Gö6983 (1 mg/kg of weight) before ligation. Ligated sciatic nerves were dissected after 24 h. Microtubules prepared from sciatic nerve extract were repolymerized in vitro and precipitated by ultracentrifugation. Input, supernatant (S) or pellet (P) was analysed by SDS–PAGE. GAPDH is used as a control. (L) Quantification of the tubulin ratio (P/S) (n=4, **P<0.01, mean±s.e.m.).
One important signalling molecule that senses and responds to changes in intracellular calcium is PKC (Clapham, 2007) and our data indicate that PKC phosphorylates HDAC5 in response to injury (Figure 6B and C). We thus analysed PKC activation after axotomy in vitro and in vivo. Spot-cultured DRG neurons were axotomized and analysed by immunofluorescence. Active, phosphorylated PKC (p-PKC) was present in axotomized axons, but not in control axons (Supplementary Figure S11C). We then tested whether PKCμ, the PKC isoform mediating downstream signal transduction (Zugaza et al, 1996; Kunkel et al, 2007) is activated by injury in sciatic nerves. We observed a two-fold increase in p-PKCμ levels, which could be blocked by Gö6983 or EGTA (Figure 7C and D). To determine whether blocking PKC activation prevents tubulin deacetylation, nerves were pretreated with Gö6983. Only Gö6983, but not KN-93, a Ca2+/calmodulin kinase (CaMK) inhibitor, blocked tubulin deacetylation after nerve injury (Figure 7F and G). Immunohistochemistry also revealed the inhibitory effect of Gö6983 on injury-induced tubulin deacetylation in sciatic nerve (Figure 7E). To confirm and extend these results, we used in-vitro cultured DRG neurons to assess the role of PKCμ in tubulin deacetylation in the presence of Gö6983 (Figure 7H) or PKCμ shRNA (Supplementary Figure S12A). Both Gö6983 treatment and PKCμ knockdown prevented axotomy-induced tubulin deacetylation (Figure 7I; Supplementary Figure S12B). There were no significant differences in slope between the axotomy control and axotomy with PKCμ inhibition or knockdown (Figure 7J; Supplementary Figure S12C). Similarly to HDAC5 knockdown (Figure 5B), we observed that PKCμ inhibition or knockdown did not prevent tubulin deacetylation in the first 50 μm immediately proximal to the axotomy site (Figure 7I; Supplementary Figure S12B). HDAC5 may be sufficiently active at the site of injury in the absence of PKC signalling or other kinases may activate HDAC5. Alternatively, other deacetylases or changes in the cytoskeleton (e.g., tyrosination) may be involved.
We next investigated whether the injury-induced decrease in tubulin acetylation underlies a decrease in microtubule stability. To test whether microtubule stability was altered in injured nerves, we measured the steady-state level of microtubule polymerization, as previously described (Lewcock et al, 2007). Extracts derived from ligated nerves had reduced polymerized microtubule levels (i.e., less tubulin in the pellet fraction) compared with unligated nerves. The presence of scriptaid or Gö6983 in ligated nerves restored the levels of polymerized microtubule to the level of uninjured controls (Figure 7K and L). These observations suggest that nerve injury decreases microtubule stability via HDAC5- and PKC-dependent tubulin deacetylation. The observed changes in microtubule properties likely arise from axonal microtubules, as we demonstrate that tubulin within the sciatic nerve mostly localizes to axons rather than to Schwann cells (Supplementary Figure S13A and B).
Taken together, these results suggest that nerve injury induces PKCμ activation through a calcium-dependent pathway and that PKCμ and HDAC5 activation are required for tubulin deacetylation as well as the decreased microtubule stability observed following nerve injury.
HDAC5 is required for growth cone dynamics and regenerative growth
Our data showed that HDAC activity is required for axonal growth and regeneration and that HDAC5 is the primary HDAC mediating tubulin deacetylation specifically in injured axons. To test whether HDAC5 knockdown blocks regenerative axon growth in cultured DRG neurons, we visualized axon regrowth by live-cell imaging after in-vitro axotomy and quantified the number and percentage of axons crossing the axotomy line 40 h after axotomy. HDAC5 knockdown markedly suppressed axon regeneration (Figure 8A and B). Expression of human HDAC5, which is resistant to shRNA silencing, partially rescued axon regeneration. In contrast, HDAC6 knockdown had no significant effect on axon regeneration, displaying instead a tendency to increase rather than to decrease axon regeneration. Surprisingly, we found that HDAC5 overexpression also inhibited axon regeneration (Figure 8B). We used live-cell imaging to determine whether the inhibitory effects of HDAC5 knockdown or overexpression resulted from altered growth cone dynamics (Figure 8D; Supplementary Movies S4, S5 and S6). Compared with controls, HDAC5 knockdown significantly reduced growth cone dynamics, similarly to what we observed with scriptaid treatment (Figure 3F). In contrast, HDAC5 overexpression enhanced growth cone dynamics above control levels (Figure 8E). These results suggest that a moderate level of HDAC5 activity is required to optimize growth cone dynamics and axon regeneration and that deviation from a moderate situation leads to impaired axon growth.
Figure 8.
HDAC5 regulates growth cone dynamics and axon regeneration. (A) In-vitro axon regeneration assay. HDAC5 or HDAC6 was knocked down, or human HDAC5 (hHDAC5) overexpressed using lentiviral infection on DIV3. Four days after lentiviral infection, axons were axotomized (red dotted arrow) and axon regeneration visualized 40 h after axotomy. Bar, 100 μm. (B) Average axon numbers regenerating past the axotomy line (n=7 for each; ***P<0.001, mean±s.e.m.). (C) The regeneration index from data shown in (A) was calculated. (D) DRG neurons were infected at DIV1 with lentivirus expressing GFP (control), GFP and HDAC5shRNA (KD) or GFP and hHDAC5 (OE). Live imaging of GFP-expressing DRG growth cones was performed at DIV3. Images at 0 and +690 s were shown. ‘t-Projection’ images are projection of all frames. Bar, 5 μm (Control, shControl; shHDAC5, KD; HDAC5, OE). (E) Average rate of change in growth cone area (n=7 for control and HDAC5 OE, n=8 for HDAC5 KD; *P<0.05, mean±s.e.m.). (F) Proposed model for the generation of a tubulin deacetylation gradient in injured axons. Following axotomy, local calcium influx activates PKC, which in turn phosphorylates HDAC5. p-HDAC5 displays enhanced activity towards tubulin and promotes local tubulin deacetylation. The retrograde propagation of calcium along the axon enhances HDAC5 interaction with kinesin-1 in more proximal axonal locations and leads to anterograde transport of HDAC5 towards the injury, leading to the formation of a gradient of HDAC5, and a resulting gradient of tubulin deacetylation.
Together, these studies identify HDAC5 as a novel injury-regulated tubulin deacetylase, which functions downstream of a calcium-PKC signalling cascade to control growth cone dynamics and axon regeneration. Furthermore, our work suggests a model in which a signalling pathway initiated locally at the site of injury establishes a gradient of tubulin deacetylation to promote axon regeneration (Figure 8E).
Discussion
Alterations in tubulin post-translational modifications after injury have been reported over two decades ago (Hall et al, 1991) and the role of microtubule stability in axon regeneration has recently emerged (Erturk et al, 2007; Hellal et al, 2011; Sengottuvel et al, 2011). However, the signalling pathways initiated by injury and regulating microtubule post-translational modifications remain poorly understood. Here, we reveal a detailed molecular mechanism that controls the formation of a gradient of tubulin deacetylation specifically in injured peripheral, but not in central axons, which is required for growth cone dynamics and axon regeneration.
Injured axons need to transform their damaged axonal tip into a new growth cone-like structure, a critical step for the initiation of regeneration. Formation of a new growth cone after injury results in part from the re-organization of the local microtubule cytoskeleton (Erez et al, 2007) and disorganized microtubules underlie the formation of retraction bulbs and the failure of axon regeneration (Erturk et al, 2007). The injury-induced, calcium-dependent tubulin deacetylation and decreased microtubule stability that we observed in proximity of the injury site may mark the new end of microtubules and contribute to de-novo growth cone formation. Previous studies suggested that microtubule destabilization strongly promotes plasma membrane resealing after axotomy (Xie and Barrett, 1991) and that calcium is needed for membrane sealing after severing neurites in differentiated PC12 cells (Detrait et al, 2000). Most axons within the spinal root reseal their damaged axon tip within 2 h in vivo (Howard et al, 1999) and resealing of cultured rat septal neurons occurs within 30–60 min (Xie and Barrett, 1991). Although we did not directly determine whether tubulin deacetylation occurs prior to plasma membrane resealing, our in-vitro results suggest that tubulin modification occurs early in the regeneration process, at the time when the growth cone needs to be reformed. Our findings that injury induces tubulin deacetylation specifically in PNS but not in CNS axons points to the important role of injury-induced tubulin modifications in growth cone formation and axon regeneration.
Our findings revealed a signalling pathway downstream of calcium influx involving PKC and HDAC5 that alters microtubule stability and promotes axon regeneration. Our data are consistent with previous findings in Aplysia (Kamber et al, 2009) and in DRG neurons (Chierzi et al, 2005) reporting that local calcium influx is essential for growth cone formation. Downstream of calcium influx we found that tubulin deacetylation is controlled by PKC activity. Our results are also in agreement with previous work reporting that PKC inhibition blocks neurite outgrowth in many different types of neurons in vitro (Campenot et al, 1994; Theodore et al, 1995; Kolkova et al, 2000; Tsai et al, 2007) and that pharmacological activation of PKC enhances neurite outgrowth in retinal ganglion cells (Wu et al, 2003). However, others have reported that inhibition of PKC increases CGN neurons axonal regeneration capacity and survival on inhibitory substrates (Sivasankaran et al, 2004). The precise role of PKC in axon regeneration may thus depend on the type of neuron and its environment.
Downstream of PKC, HDAC5 is activated to deacetylate tubulin and to promote axon regeneration. Surprisingly, we found that HDAC5 knockdown or overexpression prevented axon regeneration to a similar extent. To gain insights into the mechanism of this effect, we examined growth cone dynamics and observed that while HDAC5 knockdown greatly reduced growth cone dynamics, HDAC5 overexpression had the opposite effect. These results suggest that HDAC5 precisely controls microtubule stability and growth cone dynamics to optimize growth. Any significant deviations from moderate levels of HDAC5 activity lead to impaired axonal growth. Our results confirm and extend recent reports indicating that moderate microtubule stability is required to promote growth and regeneration (Erturk et al, 2007; Hellal et al, 2011; Hur et al, 2011; Sengottuvel et al, 2011). Indeed, while our work showed that HDAC5-mediated decrease of microtubule acetylation and stability is essential for DRG axonal growth, others have shown that a moderate increase in microtubule stability can enhance the growth capacity of CNS neurons. For example, low concentrations of Taxol, a microtubule-stabilizing drug, prevent the formation of retraction bulbs (Erturk et al, 2007) and promote the regenerative growth of retinal ganglion neurons (Sengottuvel et al, 2011) as well as DRG central branches (Erturk et al, 2007; Hellal et al, 2011). However, these positive effects disappear when the Taxol concentration is increased. The beneficial effect of Taxol in CNS axon regeneration is not solely mediated by its activity in axons, but is also proposed to arise from Taxol’s action on the surrounding glia (Sengottuvel et al, 2011). Similarly to low doses of Taxol, inhibition of HDAC6 increases microtubule acetylation and enhances the capacity of DRG neurons to grow on inhibitory substrates (Rivieccio et al, 2009). A precise control of microtubule stability thus appears essential for the growth capacity of a given neuronal population. Our results suggest that PNS neurons require less stable microtubules for axon regeneration than CNS neurons, which may result in part from the permissivity of the environment.
Tubulin acetylation is known to correlate with microtubule stability and our findings revealed that PKC and HDAC5 activity regulates both microtubule stability and tubulin acetylation. However, tubulin acetylation can also regulate the affinity of microtubule-associated proteins that may play a role in axon regeneration. The calcium-independent increase in microtubule tyrosination we observed may provide an additional control over microtubule stability or the affinity for microtubule-associated proteins to promote axon regeneration.
Transcription-dependent and -independent HDAC mechanisms appear to govern axon growth and survival. Our data indicate that tubulin deacetylation is regulated in axons by PKC and HDAC5, suggesting a local, extranuclear role of HDAC5 in axon regeneration. Furthermore, our data suggest that tubulin represents one key substrate of HDAC5 in axons. The localization of HDAC5 in DRG axons described here is in agreement with previous literature reporting cytoplasmic localization of HDAC5 in hippocampal neurons (Chawla et al, 2003). Using a compartmentalized chamber culture system, we show that the HDAC inhibitor scriptaid applied selectively to the axon chamber is sufficient to delay axon regeneration, further suggesting a local, axonal role of HDAC5 in peripheral axon regeneration. The extranuclear roles of HDACs in axon regeneration are also supported by earlier findings demonstrating that inhibition of HDAC6 promotes regeneration of DRG neurons grown on inhibitory substrates in a transcription-independent manner (Rivieccio et al, 2009). Similarly, inhibition of HDAC1 activity or nuclear export was shown to protect cerebellar neurons from degeneration in a mouse model of multiple sclerosis (Kim et al, 2010). In addition to these extranuclear roles, HDACs are known to repress axon regeneration at the transcriptional level. For example, inhibition of HDACs activates a pro-regenerative gene expression program in CNS neurons (Gaub et al, 2010, 2011). HDAC nuclear activity is also involved in promoting retinal ganglion cell death following optic nerve injury (Pelzel et al, 2010).
These previous findings point to a negative role of HDACs in axon regeneration in both central and peripheral neuronal models. In contrast, our results reveal that HDAC5 activity is required for axon regeneration of peripheral DRG neurons and that HDAC6 plays no significant role under these conditions. Like HDAC6, HDAC5 catalyses the deacetylation of tubulin in an in-vitro assay whereas in cultured neurons, HDAC5 deacetylates tubulin only following injury. HDAC5 and HDAC6 may also deacetylate other yet unknown substrates as HDAC6 was shown to deacetylate cortactin in addition to tubulin (Zhang et al, 2007). Therefore, the apparent differential role of HDAC5 and HDAC6 in DRG neurons regeneration may also in part result from different substrate specificity beyond microtubules. Our findings emphasize the distinct roles played by HDAC5 and HDAC6 in tubulin deacetylation and axon regeneration.
Our work suggests that a gradient of deacetylated microtubules is formed following injury and is important for proper regenerative growth. Enhancing deacetylation in proximity but not further away from the injury site creates a deacetylation gradient with less stable microtubules near the site of injury. We show that the deacetylation gradient correlates with a gradient of HDAC5 in distal axons, which is likely formed by anterograde transport of HDAC5 bound to kinesin-1. These data suggest that a gradient of HDAC5 activity in injured axons assists with the creation of a tubulin deacetylation gradient, which in turn promotes axonal growth. Indeed, during axon developmental growth, consolidation of the axon shaft was proposed to be mediated in part by microtubule stabilization, ensuring that the growth cone remains on the tip of the axon (Lewcock et al, 2007). A tubulin deacetylation gradient may thus balance the dynamic properties needed at the leading growth cone, while consolidating the axon shaft. Future studies will determine how neurons maintain such a gradient over time to sustain long-distance regeneration.
Materials and methods
Surgeries, chemical treatments and sample preparations
All surgical procedures were approved by the Washington University in St Louis, School of Medicine Animal Studies Committee. Sciatic nerve injury experiments were performed as described previously (Abe et al, 2010). Briefly, mice sciatic nerve was ligated, crushed or axotomized unilaterally and dissected at the indicated time after surgery. For CNS experiments, mouse spinal cord was injured by hemisection and dissected after 24 h, or the optic nerve was ligated and dissected after 24 h. For biochemical studies, sciatic or optic nerves were homogenized in lysis buffer (Tris–HCl at pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4) with protease inhibitor cocktail (Roche). For immunohistochemistry, sciatic or optic nerves, or spinal cord was fixed for 1 h in 4% paraformaldehyde in PBS, incubated overnight in 30% sucrose, embedded in OCT solution (Tissue-Tek) and frozen in dry ice-cooled methylbutane. For chemical treatments, sciatic nerves were soaked with DMSO-dissolved chemicals in Surgifoam (Johnson and Johnson) for 30 min prior injury. Drug concentrations were as follow: scriptaid and Gö6983: 1 mg/kg of mouse weight; EGTA: 10 mM.
DRG culture and in-vitro axotomy assay
DRGs from E13.5 mice were dissociated with trypsin-EDTA. Dissociated DRG neurons were plated on poly-D-lysine/laminin-coated dishes with culture medium (Neurobasal medium without phenol red), with Glutamax I, PenStrep and B-27 containing nerve growth factor (Invitrogen). DRG axons were axotomized on DIV7 with a blade (FST, 10035-10), fixed after 3.5 h and stained with acetylated tubulin and α-tubulin antibodies. Images were acquired using a confocal microscope (Nikon, 20 × MI/0.75). The intensity ratio of acetylated tubulin to α-tubulin was measured with ImageJ: the intensity was obtained by scanning the image with a measurement line of one pixel-height parallel to the axotomized line, from the top to the bottom of the images (1024 × 1024 pixels), the ratio for each line calculated and plotted in function of the distance to the axotomy site. The slope value was calculated as follow: slopes from individual images were calculated by linear regression. X value: distance from proximal to the cell body to the axotomy site; Y value: normalized fold intensity of acetylated tubulin. For live imaging of axon regeneration, GFP expressing DRG neurons were axotomized and visualized by fluorescence microscopy (Nikon, 10 × /0.25) at the indicated time. Axon regeneration was quantified by counting the number of axons crossing the axotomy line. A regeneration index was calculated from the images acquired 40 h post axotomy. The fluorescence intensity of a square area (2.7 × 0.1 mm) at 0.1 mm distal to the axotomy line was measured and normalized to the similar area 0.1 mm proximal to the axotomy line. Campenot chambers were used for compartmentalized culture as described (Campenot, 1977). When indicated, drug concentrations used in culture DRG neurons were as follow: scriptaid and Gö6983: 5 μM.
Antibodies and lentiviruses
Acetylated tubulin (T7451), tyrosinated tubulin (T9028), FLAG (F7425), HDAC4 (H9536), HDAC5 (H8163), α-tubulin (ab52866), GAP-43 (ab16053), PKC (ab23511) from Abcam; histone H3 (9715), p-PKC (9371), p-PKCμ (2051, 2054), PKCμ (2052) from Cell signalling; SMI-31 (Covance SMI-31R), HDAC6 (Biovision 3606-100), p-HDAC5 (Santa Cruz sc-101692), acetyl-histone H3 (Millipore 06-599). To knockdown HDACs and PKCμ, pLKO.1 shRNA lentiviral constructs from TRC (The RNAi Consortium) shRNA libraries and pGIPZ lentiviral constructs (Openbiosystems) were used and Lentivirus produced following the TRC manual.
Growth cone live imaging, calcium imaging and single axons laser axotomy
To measure growth cone dynamics, DRG neurons were infected at DIV1 with lentivirus expressing GFP (control), GFP and HDAC5 shRNA (KD) or GFP and hHDAC5 (OE). At DIV3, growth cone movements were monitored using confocal microscopy (LSM510M, 63 × /1.4, Zeiss). Live images were acquired every 22 s for a total of 690 s. Image sequences from individual growth cones were analysed using ImageJ macro. GFP images from each sequence were binarized. The area of each binarized growth cone image was measured to calculate the average rate of change in growth cone area.
For calcium imaging, neurons were incubated in medium containing 2 μM Fluo-4AM (Invitrogen). After 20 min, cells were washed twice and incubated for an additional 20 min. Glass dishes were mounted on a two-photon microscope chamber, at 37°C and 5% CO2 atmosphere (LSM510M, Zeiss). Single axons were axotomized by laser (Mai-Tai, Spectra-Physics), with 800 nm wavelength, 100% emission power and 10 iterations for 0.64 ms. Live imaging was performed using plan-apochromat 63 × /1.4 oil objectives with 60–100 ms acquisition time.
In-vivo regeneration assay
Sciatic nerve was crushed, dissected after 24 h and analysed as previously described using GAP43 immunostaining (Abe et al, 2010). The sciatic nerve of thy1-YFP16 was dissected 24 h after crush injury, mounted onto a glass slide and images acquired using confocal microscopy (Nikon, 10 × /0.25). Re-innervation assay was performed as described previously (Magill et al, 2007). Briefly, the tibialis anterior muscle as well as the distal 5 mm of the deep peroneal nerve from thy1-YFP16 mice was removed and the EHL muscle was dissected, fixed and stained with Alexa Fluor 647-conjugated α-bungarotoxin (Invitrogen) and mounted for confocal imaging.
In-vitro tubulin deacetylation assay
The tubulin deacetylation assay was performed as described previously (Hubbert et al, 2002; North et al, 2003). FLAG–HDAC4, HDAC5 and HDAC6 were expressed in HEK293T cells. Cells were lysed in lysis buffer (50 mM Tris–HCl at pH 7.6, 150 mM NaCl, 0.5 mM EDTA, 1% Triton X-100) with protease inhibitors (Roche) and phosphatase inhibitors (Sigma) and immunoprecipitated using anti-FLAG antibody. Mouse sciatic nerves were homogenized in tubulin deacetylation assay buffer (10 mM Tris–HCl at pH 8.0, 10 mM NaCl with protease inhibitors), clarified by centrifugation at 13 000 r.p.m. for 20 min and the supernatant was collected. In all, 25 μg of sciatic nerve extract was incubated with immunoprecipitated Flag–HDAC at room temperature for 4 h. The supernatant and immunoprecipitant were analysed by SDS–PAGE. To obtain hyper-phosphorylated HDAC5, HEK293T cells were stimulated by 10 μM PMA for 1 h before lysis. When indicated, immunoprecipitated HDAC5 was treated with CIP prior to incubation with sciatic nerve extract.
In-vitro microtubule polymerization
Preparation of polymerized microtubule in vitro was performed as described previously (Sun et al, 2011). Ligated or unligated sciatic nerves were homogenized in PEM buffer (50 mM PIPES, 1 mM EGTA, 1 mM MgSO4 and protease inhibitors, pH 6.9). The homogenate was centrifuged at 100 000 g for 30 min at 4°C. Glycerol (20%), GTP (1 mM) and Taxol (20 μM) were added to lysates and incubated for 30 min at 37°C. Polymerized microtubules were precipitated by centrifugation at 100 000 g for 30 min at 22°C. Supernatant and pellet were collected for western blot analysis.
Statistics
Western blot were scanned and quantified by ImageJ and t-test was used for statistical analysis. ImageJ macro was used to measure fluorescence intensity in confocal images. To calculate the average acetylation slope, slope values were averaged and presented as ±s.e.m. ANOVA followed by Tukey test and t-test was used for statistical analysis between experimental sets.
Supplementary Material
Acknowledgments
We thank Drs V Klyachko and K O’Malley for critical reading of the manuscript. We are grateful to Tammy Kershner for technical assistance and to Jung Eun Shin for assistance with surgical procedures. We thank Dennis Oakley for assistance with confocal and two-photon imaging at The Bakewell NeuroImaging Laboratory. This work was supported in part by National Institutes of Health Grant RO1NS060709 from NINDS, the Edward Mallinckrodt Jr Foundation and the International Institute for Research In Paraplegia (IRP) Geneva (to VC), a P30 NIH Neuroscience Core grant (NS057105) to Washington University and a Children's Discovery Institute grant CDI-LI-2010-94.
Author contributions: YC designed, planned, carried out and analysed experiments. YC and VC designed and interpreted the experiments and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abe N, Borson SH, Gambello MJ, Wang F, Cavalli V (2010) Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J Biol Chem 285: 28034–28043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB (1977) Local control of neurite development by nerve growth factor. Proc Natl Acad Sci USA 74: 4516–4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB, Draker DD, Senger DL (1994) Evidence that protein kinase C activities involved in regulating neurite growth are localized to distal neurites. J Neurochem 63: 868–878 [DOI] [PubMed] [Google Scholar]
- Chawla S, Vanhoutte P, Arnold FJ, Huang CL, Bading H (2003) Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J Neurochem 85: 151–159 [DOI] [PubMed] [Google Scholar]
- Chierzi S, Ratto GM, Verma P, Fawcett JW (2005) The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur J Neurosci 21: 2051–2062 [DOI] [PubMed] [Google Scholar]
- Clapham DE (2007) Calcium signaling. Cell 131: 1047–1058 [DOI] [PubMed] [Google Scholar]
- Dent EW, Gertler FB (2003) Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40: 209–227 [DOI] [PubMed] [Google Scholar]
- Detrait ER, Yoo S, Eddleman CS, Fukuda M, Bittner GD, Fishman HM (2000) Plasmalemmal repair of severed neurites of PC12 cells requires Ca(2+) and synaptotagmin. J Neurosci Res 62: 566–573 [DOI] [PubMed] [Google Scholar]
- Erez H, Malkinson G, Prager-Khoutorsky M, De Zeeuw CI, Hoogenraad CC, Spira ME (2007) Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy. J Cell Biol 176: 497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erturk A, Hellal F, Enes J, Bradke F (2007) Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J Neurosci 27: 9169–9180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR (2000) Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28: 41–51 [DOI] [PubMed] [Google Scholar]
- Gaub P, Joshi Y, Wuttke A, Naumann U, Schnichels S, Heiduschka P, Di Giovanni S (2011) The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain 134: 2134–2148 [DOI] [PubMed] [Google Scholar]
- Gaub P, Tedeschi A, Puttagunta R, Nguyen T, Schmandke A, Di Giovanni S (2010) HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ 17: 1392–1408 [DOI] [PubMed] [Google Scholar]
- Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD (2010) Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci 30: 3175–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Spira ME (1998) Real time imaging of calcium-induced localized proteolytic activity after axotomy and its relation to growth cone formation. Neuron 20: 1123–1135 [DOI] [PubMed] [Google Scholar]
- Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, McKinsey TA, Olson EN, Jin ZG (2008) Protein kinase D-dependent phosphorylation and nuclear export of histone deacetylase 5 mediates vascular endothelial growth factor-induced gene expression and angiogenesis. J Biol Chem 283: 14590–14599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GF, Lee VM, Kosik KS (1991) Microtubule destabilization and neurofilament phosphorylation precede dendritic sprouting after close axotomy of lamprey central neurons. Proc Natl Acad Sci USA 88: 5016–5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JW, Cai D, Verhey KJ (2008) Tubulin modifications and their cellular functions. Curr Opin Cell Biol 20: 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Hoogenraad CC, Bradke F (2011) Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331: 928–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MJ, David G, Barrett JN (1999) Resealing of transected myelinated mammalian axons in vivo: evidence for involvement of calpain. Neuroscience 93: 807–815 [DOI] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417: 455–458 [DOI] [PubMed] [Google Scholar]
- Hur EM, Yang IH, Kim DH, Byun J, Saijilafu Xu WL, Nicovich PR, Cheong R, Levchenko A, Thakor N, Zhou FQ (2011) Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proc Natl Acad Sci USA 108: 5057–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Kneussel M (2010) Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci 33: 362–372 [DOI] [PubMed] [Google Scholar]
- Kamber D, Erez H, Spira ME (2009) Local calcium-dependent mechanisms determine whether a cut axonal end assembles a retarded endbulb or competent growth cone. Exp Neurol 219: 112–125 [DOI] [PubMed] [Google Scholar]
- Kim JY, Shen S, Dietz K, He Y, Howell O, Reynolds R, Casaccia P (2010) HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat Neurosci 13: 180–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolkova K, Novitskaya V, Pedersen N, Berezin V, Bock E (2000) Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway. J Neurosci 20: 2238–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel MT, Toker A, Tsien RY, Newton AC (2007) Calcium-dependent regulation of protein kinase D revealed by a genetically encoded kinase activity reporter. J Biol Chem 282: 6733–6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B (2010) Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron 66: 663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewcock JW, Genoud N, Lettieri K, Pfaff SL (2007) The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron 56: 604–620 [DOI] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z (2011) Neuronal Intrinsic mechanisms of axon regeneration. Annu Rev Neurosci 34: 131–13–152 [DOI] [PubMed] [Google Scholar]
- Magill CK, Tong A, Kawamura D, Hayashi A, Hunter DA, Parsadanian A, Mackinnon SE, Myckatyn TM (2007) Reinnervation of the tibialis anterior following sciatic nerve crush injury: a confocal microscopic study in transgenic mice. Exp Neurol 207: 64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandolesi G, Madeddu F, Bozzi Y, Maffei L, Ratto GM (2004) Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. FASEB J 18: 1934–1936 [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Lu J, Olson EN (2000) Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408: 106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins FH, Hargreaves AJ, Li JY, Dahlstrom A, McLean WG (1994) Tyrosination state of alpha-tubulin in regenerating peripheral nerve. J Neurochem 62: 227–234 [DOI] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11: 437–444 [DOI] [PubMed] [Google Scholar]
- O'Donnell M, Chance RK, Bashaw GJ (2009) Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci 32: 383–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzel HR, Schlamp CL, Nickells RW (2010) Histone H4 deacetylation plays a critical role in early gene silencing during neuronal apoptosis. BMC Neurosci 11: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivieccio MA, Brochier C, Willis DE, Walker BA, D’Annibale MA, McLaughlin K, Siddiq A, Kozikowski AP, Jaffrey SR, Twiss JL, Ratan RR, Langley B (2009) HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc Natl Acad Sci USA 106: 19599–19604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Lund FE, Milbrandt J (2009) Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci 29: 5525–5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E, Asai DJ, Bulinski JC, Kirschner M (1987) Posttranslational modification and microtubule stability. J Cell Biol 105: 2167–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D (2011) Taxol facilitates axon regeneration in the mature CNS. J Neurosci 31: 2688–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, He Z (2004) PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci 7: 261–268 [DOI] [PubMed] [Google Scholar]
- Su GH, Sohn TA, Ryu B, Kern SE (2000) A novel histone deacetylase inhibitor identified by high-throughput transcriptional screening of a compound library. Cancer Res 60: 3137–3142 [PubMed] [Google Scholar]
- Sun F, Zhu C, Dixit R, Cavalli V (2011) Sunday Driver/JIP3 binds kinesin heavy chain directly and enhances its motility. EMBO J 30: 3416–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N (1992) Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J Cell Sci 103: Part 4953–964 [DOI] [PubMed] [Google Scholar]
- Tanaka E, Kirschner MW (1995) The role of microtubules in growth cone turning at substrate boundaries. J Cell Biol 128: 127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore L, Derossi D, Chassaing G, Llirbat B, Kubes M, Jordan P, Chneiweiss H, Godement P, Prochiantz A (1995) Intraneuronal delivery of protein kinase C pseudosubstrate leads to growth cone collapse. J Neurosci 15: 7158–7167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Yang LY, Wu CH, Chang SF, Hsu CY, Wei CP, Leu SJ, Liaw J, Lee YH, Tsai MD (2007) Injury-induced Janus kinase/protein kinase C-dependent phosphorylation of growth-associated protein 43 and signal transducer and activator of transcription 3 for neurite growth in dorsal root ganglion. J Neurosci Res 85: 321–331 [DOI] [PubMed] [Google Scholar]
- Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA (2004) Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol 24: 8374–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DY, Zheng JQ, McDonald MA, Chang B, Twiss JL (2003) PKC isozymes in the enhanced regrowth of retinal neurites after optic nerve injury. Invest Ophthalmol Vis Sci 44: 2783–2790 [DOI] [PubMed] [Google Scholar]
- Xie XY, Barrett JN (1991) Membrane resealing in cultured rat septal neurons after neurite transection: evidence for enhancement by Ca(2+)-triggered protease activity and cytoskeletal disassembly. J Neurosci 11: 3257–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, Yao TP, Lane WS, Seto E (2007) HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell 27: 197–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P (2003) HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J 22: 1168–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugaza JL, Sinnett-Smith J, Van Lint J, Rozengurt E (1996) Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J 15: 6220–6230 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.