Abstract
Neurons are critically dependent on mitochondrial integrity based on specific morphological, biochemical, and physiological features. They are characterized by high rates of metabolic activity and need to respond promptly to activity-dependent fluctuations in bioenergetic demand. The dimensions and polarity of neurons require efficient transport of mitochondria to hot spots of energy consumption, such as presynaptic and postsynaptic sites. Moreover, the postmitotic state of neurons in combination with their exposure to intrinsic and extrinsic neuronal stress factors call for a high fidelity of mitochondrial quality control systems. Consequently, it is not surprising that mitochondrial alterations can promote neuronal dysfunction and degeneration. In particular, mitochondrial dysfunction has long been implicated in the etiopathogenesis of Parkinson’s disease (PD), based on the observation that mitochondrial toxins can cause parkinsonism in humans and animal models. Substantial progress towards understanding the role of mitochondria in the disease process has been made by the identification and characterization of genes causing familial variants of PD. Studies on the function and dysfunction of these genes revealed that various aspects of mitochondrial biology appear to be affected in PD, comprising mitochondrial biogenesis, bioenergetics, dynamics, transport, and quality control.
Keywords: mitochondria, parkin, Parkinson, PINK1, synuclein
Introduction
Parkinson’s disease (PD) is a heterogeneous neurodegenerative disease entity typically diagnosed by its cardinal motor symptoms, including bradykinesia, hypokinesia, rigidity, resting tremor, and postural instability, which are subsumed under the syndrome of parkinsonism. The motor manifestations are attributable to the degeneration of dopaminergic (DA) neurons within the substantia nigra pars compacta (SNc), resulting in dopamine depletion and derangements of neuronal circuits in the basal ganglia target regions of these neurons. Another pathological hallmark of PD is the presence of α-synuclein-containing deposits in neuronal perikarya (Lewy bodies) and processes (Lewy neurites). The role of Lewy bodies in the pathogenic process is discussed controversially. Parkinsonism can occur in the absence of Lewy bodies, for instance in some cases of familial PD or in drug-induced parkinsonism (Davis et al, 1979; Langston et al, 1999; Nuytemans et al, 2010). On the other hand, Lewy body pathology is sometimes found at autopsy in individuals without reported symptoms of parkinsonism (Jellinger, 2009; Adler et al, 2010). The manifestation of non-motor symptoms, some of which even precede the motor symptoms, reflect the fact that the neurodegenerative process is not limited to the SNc but has a much wider impact. Non-motor symptoms, such as autonomic dysfunction, sleep abnormalities, depression, and dementia, can contribute considerably to disability, as they usually are not responsive to dopamine replacement therapy.
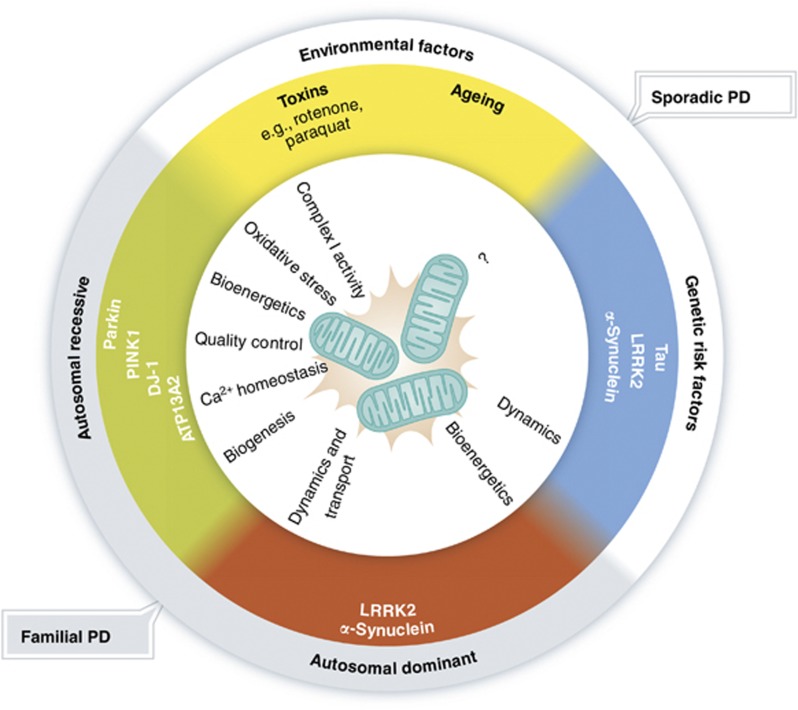
The etiopathogenesis of sporadic PD, the most common form of parkinsonism, is complex with variable contributions of genetic susceptibility and environmental factors (Figure 1). Ageing is one of the most important risk factors for sporadic PD. Given the demographic trend towards an aged population, the prevalence of PD and thus its socioeconomic burden will increase dramatically in the next decades. Over the last 15 years enormous effort has been taken to unravel the role of genetics in PD pathogenesis. Linkage analyses discovered six genes associated with Mendelian forms of parkinsonism, and genome-wide association studies identified susceptibility genes contributing to the risk for sporadic PD. Strikingly, there is an overlap between Mendelian genes and risk genes in the case of α-synuclein and leucine-rich repeat kinase 2 (LRRK2), blurring the traditional boundaries between familial and sporadic PD. The identification of genes associated with parkinsonism has had a major impact on PD research, allowing to dissect molecular pathways implicated in the pathogenesis. From genetic cellular and animal models, it emerged that mitochondrial alterations, oxidative stress, and impaired clearance of misfolded proteins and damaged organelles by proteasomal and lysosomal degradation pathways contribute to the disease process (reviewed in Dawson et al, 2010; Corti et al, 2011; Martin et al, 2011; and Shulman et al, 2011). Moreover, there is increasing evidence that sporadic and familial variants of PD share some common pathways that converge at mitochondria (reviewed in Abou-Sleiman et al, 2006; Lin and Beal, 2006; Mandemakers et al, 2007; Bogaerts et al, 2008; Henchcliffe and Beal, 2008; Schapira, 2008; Vila et al, 2008; Van Laar and Berman, 2009; Bueler, 2010; Burbulla et al, 2010; Winklhofer and Haass, 2010; and Schon and Przedborski, 2011). In the following, we will review our current knowledge on the role of mitochondria in PD pathogenesis and how these insights have changed our conceptional thinking and may eventually be translated into novel neuroprotective approaches.
Figure 1.
Aetiology of Parkinson’s disease (PD) and possible links to mitochondrial integrity. Familial PD is caused by mutations in genes identified by linkage analyses that are inherited in an autosomal recessive or dominant manner. Sporadic PD is considered to be a complex neurodegenerative disease entity with both genetic susceptibility and environmental factors contributing to the etiopathogenesis. Recent genome-wide association studies have identified susceptibility loci, which in two cases (α-synuclein and LRRK2) overlap with classical PD genes, linking the aetiology of familial parkinsonism with that of sporadic PD. Both genetic and environmental factors influence various mitochondrial aspects, such as bioenergetics, dynamics, transport, and quality control.
Mitochondrial dysfunction in sporadic PD
The role of complex I deficiency and mitochondrial DNA mutations
The first link between parkinsonism and mitochondria became evident in the early 1980s, when it was discovered that a neurotoxin causing a parkinsonian syndrome inhibits mitochondrial respiration. MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), a contaminant of an illicit opioid preparation which was used intravenously by drug addicts, can cross the blood–brain barrier and is taken up by DA neurons via the dopamine transporter after oxidation to MPP+ (Langston et al, 1983; Nicklas et al, 1985). Within DA neurons, MPP+ accumulates in mitochondria and inhibits complex I (NADH ubiquinone oxidoreductase) of the electron transport chain. Although MPTP-induced parkinsonism results from an acute toxic insult and therefore differs from the slow and progressive disease process in sporadic PD, the impact of MPTP has been far reaching. In particular, MPTP and other complex I inhibitors such as rotenone are still being used to model PD in animals and to evaluate therapeutic approaches (reviewed in Hirsch, 2007; Bezard and Przedborski, 2011; and Cannon and Greenamyre, 2011). Interestingly, consumption of fruit and herbal teas from plants of the Annonceae family, containing the complex I inhibitor annonacin, has been linked to the high frequency of atypical parkinsonism in Guadeloupe (Caparros-Lefebvre and Elbaz, 1999; Lannuzel et al, 2003; Champy et al, 2004), further substantiating a causal role of mitochondrial dysfunction in the pathogenesis of at least some parkinsonian syndromes. In support of a direct or indirect role of complex I, the activity of complex I has been reported to be reduced (in the range of 30%) in the SNc and frontal cortex of PD patients at autopsy (Schapira et al, 1989; Parker et al, 2008). In mitochondrial preparations from PD frontal cortex samples, complex I subunits derived from both mitochondrial and nuclear genomes were found to be oxidatively damaged, reflected by an increase in protein carbonyls (Keeney et al, 2006). This study also reported that the levels of an 8-kDa subunit of complex I were reduced by 33% in PD frontal cortex, suggesting that oxidative damage may cause misassembly or reduced stability of complex I subunits. Remarkably, in about 25% of PD patients analysed complex I activities were found to be reduced also in platelets (reviewed in Schapira, 2008). This finding may indicate a systemic complex I defect in a subfraction of PD patients due to genetic and/or environmental causes. In a mouse model of mild complex I deficiency induced by the dopamine neuron-specific loss of the Ndufs4 subunit, increased striatal dopamine turnover rates and decreased dopamine release from striatal axon terminals have been observed (Sterky et al, 2012). These alterations in striatal dopamine homeostasis may be caused by a reduced vesicular uptake of dopamine due to ATP deficiency followed by enhanced cytosolic dopamine metabolism, suggesting that impaired dopamine release may be an early consequence of mitochondrial impairment (Choi et al, 2011; Sterky et al, 2012).
Mitochondrial oxidative phosphorylation depends on both mitochondrial and nuclear DNA-encoded proteins. Mitochondrial DNA (mtDNA) encodes 13 proteins that are all subunits of respiratory chain complexes, 22 tRNAs, and 2 rRNAs. Mutations in mtDNA can be either inherited maternally or acquired and typically cause variable phenotypes in cells with high energy demands, such as neurons and muscle cells. Mouse models with defects in genes essential for the maintenance of mtDNA support the notion that alterations in the mitochondrial genome cause respiratory chain deficiencies and phenotypes associated with ageing and age-related diseases (reviewed in Reeve et al, 2008; Larsson, 2010; and Park and Larsson, 2011). Transgenic mice expressing a proofreading-deficient version of the mtDNA polymerase γ (POLG) accumulate mtDNA mutations and display features of premature ageing (Trifunovic et al, 2004; Kujoth et al, 2005). Notably, cosegregation of parkinsonism with mutations in the human POLG1 gene has been reported in several families (reviewed in Orsucci et al, 2011). In support of a causative role of mitochondrial dysfunction in PD, mice with a DA neuron-specific deletion of the mitochondrial transcription factor TFAM, which is essential for mitochondrial transcription and maintenance of mtDNA, develop a parkinsonian phenotype reproducing key features of PD: adult onset, progressive impairment of motor functions responsive to L-DOPA therapy, and loss of midbrain DA neurons (Ekstrand et al, 2007). Similarly, expression of mitochondrially targeted PstI endonuclease in DA neurons, which induces double-strand breaks in mtDNA, causes progressive neuronal degeneration and striatal dopamine depletion (Pickrell et al, 2011).
An age-dependent increase in mtDNA deletions has been found in individual DA neurons dissected from the SNc of post mortem human brain (Bender et al, 2006; Kraytsberg et al, 2006). Neurons harbouring >60% of mtDNA molecules with deletions showed a significant decrease in cytochrome c oxidase, three catalytic subunits of which are encoded by mtDNA. Different types of mtDNA deletions were found in the same individual, but each neuron contained only a single mtDNA mutation, indicating that the mutation was acquired and clonally expanded. In comparison with age-matched controls, the amount of mtDNA mutations was slightly higher in DA neurons from PD patients (Bender et al, 2006). Moreover, SNc neurons seem to be particularly vulnerable to mtDNA mutations, since hippocampal neurons or pyramidal cortical neurons of aged individuals did not contain high levels of mtDNA mutations.
There is currently no strong evidence that mtDNA mutations are a major primary cause of PD. However, it seems quite plausible that mtDNA mutations accumulate in the course of the disease as a consequence of an increase in cellular stress and mitochondrial replication errors along with a decrease in the fidelity of quality control systems. Once the mtDNA mutations surpass a critical threshold, the resulting respiratory deficiency may contribute to neuronal degeneration and cell death. Of note, complex I is particularly vulnerable to mtDNA damage, since seven of its subunits are encoded by mtDNA.
Mitochondrial dysfunction and oxidative stress
According to a widespread concept, inhibition of complex I decreases mitochondrial ATP production and increases the formation of reactive oxygen species (ROS), which damage mtDNA, components of the respiratory chain and other mitochondrial factors, thereby triggering a vicious circle between mitochondrial impairment and oxidative stress (reviewed in Abou-Sleiman et al, 2006; Lin and Beal, 2006; and Henchcliffe and Beal, 2008). This model has been particularly popular to explain the increased vulnerability of SNc DA neurons, since this neuronal population is characterized by a high oxidative burden and a low anti-oxidant capacity. Mitochondria can be both a source and a target of ROS (reviewed in Starkov, 2008 and Murphy, 2009). However, an obligatory link between mitochondrial dysfunction and increased ROS production has been questioned based on weak experimental support for such a scenario in vivo (reviewed in Fukui and Moraes, 2008; Gems and Doonan, 2009; and Park and Larsson, 2011). For example, rotenone toxicity has been reported to be caused by spare respiratory capacity rather than oxidative stress. In primary neurons, rotenone does increase the formation of mitochondrial superoxide, however, trapping superoxide fails to reduce rotenone toxicity (Yadava and Nicholls, 2007). In addition, various mouse models with severe respiratory chain deficiency display increased apoptotic cell death but not increased ROS formation or oxidative stress (Wang et al, 2001; Kujoth et al, 2005; Trifunovic et al, 2005; Kruse et al, 2008). Moreover, ROS are not always harmful agents, they also act as important signal transducers in a variety of biological processes.
Mitochondrial effects of genes associated with PD
Parkin: a versatile neuroprotective E3 ubiquitin ligase
The parkin gene has been identified in 1998 as a causative gene for autosomal recessive parkinsonism (Kitada et al, 1998). More than 100 pathogenic parkin mutations have been reported, accounting for the majority of autosomal recessive parkinsonism. The parkin gene encodes a cytosolic 465 amino-acid protein with a ubiquitin-like (UBL) domain at the N-terminus and an RBR (RING-between-RING) domain close to the C-terminus. The RBR domain is composed of two RING fingers that flank an in-between RING (IBR) domain and coordinates six zinc ions. An additional RING finger domain (RING0) has been identified between the UBL and RBR motifs, which contributes to the binding of zinc ions (Hristova et al, 2009). Coordination of zinc ions (eight Zn2+ in total) is essential for parkin to adopt and maintain its correct three-dimensional structure, consistent with the observation that pathogenic mutations within the zinc-binding motifs are inactivated by misfolding (Cookson et al, 2003; Gu et al, 2003; Sriram et al, 2005; Wang et al, 2005b, 2007). Moreover, the cysteine-rich RBR domain renders parkin vulnerable to inactivation by severe oxidative stress (Winklhofer et al, 2003; LaVoie et al, 2007; Wong et al, 2007; Schlehe et al, 2008). Oxidatively modified, misfolded parkin has indeed been found in the brains of PD patients, suggesting that inactivation of parkin may also play a role in sporadic PD (Pawlyk et al, 2003; Chung et al, 2004; Yao et al, 2004; LaVoie et al, 2005; Wang et al, 2005a). In support of this notion, the c-Abl tyrosine kinase has been reported to be activated in DA neurons of sporadic PD patients, leading to the phosphorylation and inactivation of parkin (Ko et al, 2010; Imam et al, 2011).
The presence of the RBR domain suggested that parkin acts as an E3 ubiquitin ligase, mediating the covalent attachment of ubiquitin moieties to substrate proteins (Shimura et al, 2000; Zhang et al, 2000). Considerable evidence has been accumulated indicating that parkin can catalyse various modes of ubiquitination, including poly-ubiquitination with different lysine linkages or mono-ubiquitination. The linkage type determines the fate of the ubiquitinated protein; ubiquitin chain linkage via Lys48 typically targets substrates for proteasomal degradation, whereas linkage involving other lysine residues and mono-ubiquitination or multiple mono-ubiquitination are implicated in numerous regulatory processes, such as signal transduction, trafficking, DNA damage response, DNA repair, and autophagy (reviewed in Komander, 2009; Ikeda et al, 2010; and Behrends and Harper, 2011). There are two major classes of E3 ubiquitin ligases: HECT ligases transiently accept ubiquitin from an E2 conjugating enzyme at a cysteine residue within the HECT domain to form a thioester, whereas RING-type ligases act as bridging proteins that bring the ubiquitin-charged E2 in close proximity to the substrate, but are not ubiquitinated themselves (reviewed in Deshaies and Joazeiro, 2009). It was recently shown that parkin functions as an RING/HECT hybrid: RING1 binds to a ubiquitin-charged E2 conjugating enzyme, which transfers ubiquitin to a conserved cysteine residue in RING2, thereby forming a thioester between parkin and ubiquitin (Wenzel et al, 2011). Ubiquitin is then discharged to a lysine residue of the substrate protein. So far, this mechanism has been demonstrated in vitro with recombinant parkin and auto-ubiquitination of parkin as a surrogate substrate, but undoubtedly, these findings have a major impact on our understanding of the substrate specificity of RBR E3 ligases.
To date, about 30 putative parkin substrates have been reported and both degradative and non-degradative ubiquitination were attributed to parkin (reviewed in Dawson and Dawson, 2010). These substrates do not fit into a common pathway that could unravel the function of parkin. However, from a plethora of studies in cellular and animal models it emerged that parkin has a remarkably wide protective capacity. The increased expression of parkin both in vitro and in vivo protects against cell death in various stress paradigms, such as mitochondrial stress, endoplasmic reticulum (ER) stress, excitotoxicity, and proteotoxic stress (reviewed in Moore, 2006 and Pilsl and Winklhofer, 2012). Vice versa, parkin-deficient cells are characterized by an increased vulnerability to stress-induced cell death. Surprisingly, the sensitivity of parkin knockout (KO) mice to neurotoxins, such as MPTP or 6-OHDA seems not to be increased; nigral degeneration in parkin KO mice has only been reported after inflammatory stimulation by lipopolysaccaride (Perez et al, 2005; Thomas et al, 2007; Frank-Cannon et al, 2008). In line with parkin playing a role in the cellular stress response, parkin gene expression is considerably upregulated under cellular stress. ATF4 and p53 have been shown to increase parkin expression, whereas c-Jun and N-myc act as transcriptional repressors of parkin (West et al, 2004; Bouman et al, 2011; Zhang et al, 2011).
Several viability pathways were reported to be influenced by parkin, including JNK, PI3K, and NF-κB signalling, p53 transcriptional activity, or Bax activation (Cha et al, 2005; Yang et al, 2005; Fallon et al, 2006; Henn et al, 2007; Hasegawa et al, 2008; da Costa et al, 2009; Sha et al, 2010; Johnson et al, 2012). Parkin has recently been shown to induce the proteasomal degradation of PARIS (parkin-interacting substrate), which acts as a transcriptional repressor of PGC-1α (peroxisome proliferator-activated receptor gamma-co-activator 1-alpha) (Shin et al, 2011). PGC-1α stimulates mitochondrial biogenesis as a co-activator of various transcription factors, such as NRF (nuclear respiratory factor)-1 and -2 (reviewed in Scarpulla, 2011). Thus, loss of parkin function suppresses mitochondrial biogenesis through an accumulation of PARIS. This study not only provided an important link between the protective activity of parkin and mitochondria but also implicated a transcriptional program in mediating the effects of parkin. Moreover, by generating conditional parkin KO mice Dawson and coworkers could show for the first time that loss of parkin function in adult mice leads to a progressive degeneration of DA neurons which can be suppressed by silencing PARIS expression (Shin et al, 2011). This finding supports the notion that developmental compensation accounts for the absence of major phenotypic alterations in germline parkin KO mice.
Parkin at the interface of neurodegeneration and cancer
In an attempt to characterize FRA6E, one of the most active common fragile sites in the human genome located at chromosome 6q25-q27, the parkin genomic structure was found to span a large region of FRA6E (Cesari et al, 2003; Denison et al, 2003a). Common fragile sites are specific loci that are susceptible to chromosomal breaks and rearrangements and seem to play a role in oncogenesis. Studies to detect genomic copy number variations in human ovarian and breast carcinomas identified a common minimal region of loss located within the parkin gene. Indeed, decreased or absent parkin expression was observed in various malignancies (Cesari et al, 2003; Denison et al, 2003a, 2003b; Picchio et al, 2004; Wang et al, 2004; Agirre et al, 2006; Fujiwara et al, 2008; Ikeuchi et al, 2009; Poulogiannis et al, 2010; Veeriah et al, 2010; Mehdi et al, 2011). Several studies have now provided considerable evidence that parkin might be a bona fide tumour suppressor gene (TSG). Heterozygous deletion of parkin accelerated the development of intestinal adenoma in mice expressing mutant APC, a regulator of Wnt signalling (Poulogiannis et al, 2010). Upon γ-irradiation parkin KO mice developed lymphomas in the spleen with a shorter tumour latency compared with wild-type mice (Zhang et al, 2011). In one line of parkin KO mice lacking exon 3, enhanced hepatocyte proliferation and development of hepatic tumours has been observed (Fujiwara et al, 2008). Ectopic expression of parkin in parkin-deficient tumour cells lines (glioma cells or lung cancer cells) resulted in reduced tumour growth after injection of these cells as xenografts into nude mice (Picchio et al, 2004; Veeriah et al, 2010). Some studies reported that parkin overexpression inhibits cell proliferation, albeit this is not a consistent finding (Picchio et al, 2004; Poulogiannis et al, 2010; Tay et al, 2010; Veeriah et al, 2010).
The mechanism underlying the tumour suppressor activity of parkin is not well understood. A previous study identified cyclin E as a substrate of parkin for ubiquitination and proteasomal degradation in neuronal cells (Staropoli et al, 2003); therefore, it is tempting to speculate that a decrease in parkin expression results in an accumulation of cyclin E, a cell-cycle regulator required for the transition from G1 to S phase. Increased levels of cyclin E have been observed in some but not all parkin-deficient primary tumours and cancer cell lines (Ikeuchi et al, 2009; Tay et al, 2010; Veeriah et al, 2010; Yeo et al, 2012). Based on the effect of parkin on mitochondrial bioenergetics as reviewed further below, it is also conceivable that parkin exerts its tumour suppressor activity via influencing tumour metabolism. Evidence for such a scenario was recently provided by Feng and coworkers (Zhang et al, 2011). A hallmark of tumour cells is the switch from mitochondrial energy production to aerobic glycolysis, which is known as the Warburg effect (reviewed in Vander Heiden et al, 2009 and Cairns et al, 2011). To compensate for the lower efficiency of ATP production by glycolysis compared with mitochondrial respiration, tumour cells increase glucose uptake and utilization. An important role in regulating energy metabolism plays the TSG p53, a transcription factor that promotes mitochondrial respiration and reduces glycolysis via transcription of specific target genes. Parkin was recently identified as a p53 target gene, which can mediate effects of p53 on energy metabolism and antioxidant defense (Zhang et al, 2011). Parkin deficiency activates glycolysis and reduces mitochondrial respiration in human lung cancer cells and mouse embryonic fibroblasts, thereby contributing to the Warburg effect. Remarkably, parkin can also affect lipid metabolism by regulating fatty acid uptake. In wild-type mice, parkin expression is robustly upregulated upon exposure to a high fat and cholesterol diet (HFD), inducing the stabilization of the fatty acid transporter CD36, whereas parkin KO mice are resistant to weight gain and hepatic insulin resistance under HFD feeding (Kim et al, 2011).
Whether germline pathogenic mutations in the parkin gene can increase the risk for cancer is difficult to assess given that parkin-linked parkinsonism is rare and a large number of cases would be required for a statistically robust epidemiological study (reviewed in Plun-Favreau et al, 2010 and Devine et al, 2011).
PINK1: a mitochondrial kinase of complex regulation and processing
The PINK1 (PTEN-induced putative kinase 1) gene was linked to autosomal recessive early onset PD in 2004 (Valente et al, 2004). It encodes a ubiquitously expressed 581 amino-acid protein with an N-terminal mitochondrial targeting sequence (MTS), a transmembrane domain and a highly conserved serine/threonine kinase domain with homology to the Ca2+/calmodulin family. About 30 pathogenic PINK1 mutations have been identified, among them missense, non-sense, or frameshift mutations, deletions or genomic rearrangements (reviewed in Deas et al, 2009; Nuytemans et al, 2010; and Corti et al, 2011). Most PINK1 mutations have been described to impair its kinase activity or reduce the stability of the protein, in line with a loss of function mechanism.
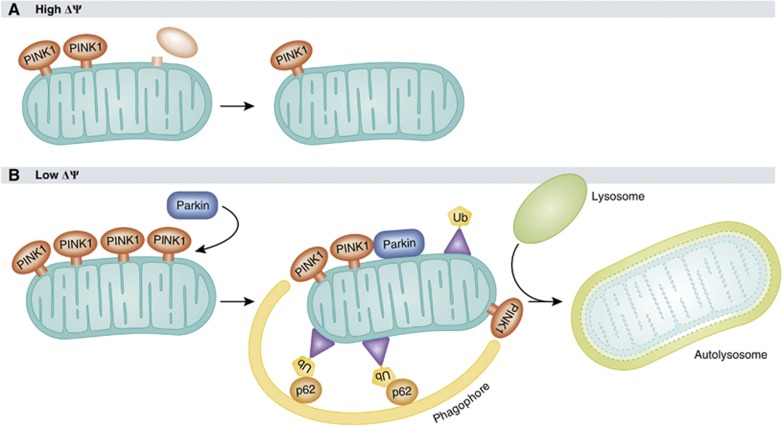
The subcellular localization of PINK1 is still debated. PINK1 has been found at the outer and inner mitochondrial membrane and in the cytosol (Silvestri et al, 2005; Muqit et al, 2006; Haque et al, 2008; Lin and Kang, 2008; Weihofen et al, 2009; Jin et al, 2010; Narendra et al, 2010b; Murata et al, 2011; Shi et al, 2011). From recent research, a complex mechanism of PINK1 targeting and processing has emerged that could provide an explanation for the different observations regarding PINK1 localization. PINK1 seems to be imported via the TOM/TIM23 complexes at the outer/inner mitochondrial membrane in a membrane potential-dependent manner for cleaving off its MTS by the mitochondrial processing protease (Greene et al, 2012). PINK1 exposing its kinase domain to the intermembrane space could then be released from the transport pore by lateral diffusion to be further processed by a protease giving rise to a PINK1 fragment, which is subsequently degraded by an MG132-sensitive protease, possibly in the cytoplasm. How this retrotranslocation is mediated, is not known. It has been hypothesized that proteolytic cleavage of a PINK1 import intermediate still associated with the TOM complex leads to a C-terminal PINK1 fragment that reaches the cytoplasm by reverse translocation (Meissner et al, 2011). PARL (presenilin-associated rhomboid like protease) has recently been identified as a protease promoting PINK1 cleavage under basal conditions to keep mitochondrial PINK1 levels low (Whitworth et al, 2008; Jin et al, 2010; Deas et al, 2011; Meissner et al, 2011; Shi et al, 2011). When the mitochondrial membrane potential is dissipated, PINK1 mitochondrial import and processing by PARL is inhibited, leading to the integration of PINK1 into the outer mitochondrial membrane, which is a prerequisite to recruit parkin for the induction of mitophagy (Jin et al, 2010; Matsuda et al, 2010; Narendra et al, 2010b; Meissner et al, 2011) (see below). In depolarized mitochondria, endogenous PINK1 is associated with the TOM complex, which may allow rapid reimport of PINK1 after repolarization to switch off the mitophagy pathway (Lazarou et al, 2012). Interestingly, PINK1 can be processed in the absence of PARL resulting in a slightly different cleavage pattern, thus, PARL is apparently not the only protease capable of PINK1 processing (Narendra et al, 2010b; Shi et al, 2011; Greene et al, 2012).
PINK1 can increase the resistance to diverse cellular stressors in a kinase-dependent manner (Petit et al, 2005; Haque et al, 2008; Wood-Kaczmar et al, 2008; Gandhi et al, 2009; Morais et al, 2009; Sandebring et al, 2009; Klinkenberg et al, 2010; Murata et al, 2011; Wang et al, 2011b). Of note, PINK1 deficiency in mice, as demonstrated in germline PINK1 KO mice or in shRNA-mediated PINK1 knockdown mice, significantly increases sensitivity of DA neurons to systemic MPTP treatment (Haque et al, 2012). Nigrostriatal degeneration in these models can be prevented by virally expressed parkin or DJ-1, suggesting that these genes are either downstream or act in parallel pathways to confer stress protection. To get insight into the mechanism underlying the protective activity of PINK1, efforts have been intensified to identify its substrates. Pridgeon et al (2007) reported that PINK1 phosphorylates TRAP1 (TNF receptor-associated protein 1), a mitochondrial chaperone of the Hsp90 family also known as Hsp75. Phosphorylation of TRAP1 was shown to be essential for the protective activity of PINK1 against oxidative stress. Plun-Favreau et al (2007) identified the mitochondrial serine protease HtrA2/Omi as a PINK1 interactor. A direct interaction between PINK1 and HtrA2/Omi favours the phosphorylation of HtrA2/Omi, which seems to be mediated by the p38 stress kinase pathway. Phosphomimetic HtrA2/Omi mutants show an increased protease activity along with the ability to protect against mitochondrial toxins. In another approach to identify PINK1-interacting proteins by immunopurification, the mitochondrial outer membrane Rho-like GTPase Miro2 and the adaptor protein Milton were found to form a complex with PINK1 (Weihofen et al, 2009). By binding to both Miro (two human homologues Miro1 and Miro2) and kinesin heavy chain, Milton links mitochondria to microtubules for axonal transport (Guo et al, 2005; Glater et al, 2006). The physical interaction of PINK1 and Miro therefore suggested that PINK1 has an impact on mitochondrial transport. In a recent study employing cultured rat hippocampal neurons or Drosophila larval neurons, it was indeed observed that the overexpression of either PINK1 or parkin causes an arrest of mitochondrial transport (Wang et al, 2011d). Based on the known interaction between PINK1 and Miro, Schwarz and coworkers followed up the idea that PINK1 and parkin may directly modify Miro. Miro1 was shown to be a direct target of PINK1 for phosphorylation, and Miro1 phosphorylated at serine 156 in turn is degraded by the proteasome in a parkin-dependent manner. This observation fits into a model in which degradation of Miro induced by the PINK1/parkin pathway stops mitochondrial movement and helps to sequester damaged mitochondria prior to their elimination by mitophagy (see below). A recent study from Bingwei Lu’s laboratory showed that PINK1 and Miro have opposing effects on mitochondrial flux and net velocity in Drosophila larval motor neurons (Liu et al, 2012). Overexpression of PINK1 or Miro RNAi inhibited axonal mitochondrial transport in both anterograde and retrograde directions, and downregulation of Miro rescued PINK1 mutant phenotypes in Drosophila muscles and DA neurons. Liu et al also found that overexpression of PINK1 and parkin reduces Miro1 levels in HeLa cells, but phosphorylation of Miro1 at serine 156 was not required for this effect. Intriguingly, whereas Miro protein levels were increased in PINK1 mutant flies, the opposite effect was observed in mammalian models of PINK1 deficiency. In mouse embryonic fibroblasts from PINK1 KO mice or in PINK1 RNAi HeLa cells, Miro protein levels were significantly reduced, possibly reflecting differences between Drosophila and mammalian cells to respond to and compensate for PINK1 loss of function. An impact of PINK1 on mitochondrial transport was also observed by Mandelkow and coworkers who found that MARK2 (microtubule affinity-regulating kinase 2) phosphorylates N-terminally truncated PINK1 thereby increasing its kinase activity (Matenia et al, 2012). Whereas N-terminally truncated PINK1 overexpressed in chicken retinal ganglion cells promotes anterograde mitochondrial transport and increases the fraction of stationary mitochondria, full-length PINK1 enhances retrograde transport, suggesting that PINK1 may act as a molecular switch between anterograde and retrograde mitochondrial transport depending on the mitochondrial membrane potential.
The mechanisms of protection mediated by PINK1 are not completely understood. It has been suggested that effects on mitochondrial bioenergetics, quality control, and calcium homeostasis are involved. In various PINK1 loss of function models, mitochondrial impairment has been observed, such as alterations in mitochondrial morphology and dynamics, a decrease in mitochondrial membrane potential, and respiration defects (Exner et al, 2007; Hoepken et al, 2007; Gautier et al, 2008; Piccoli et al, 2008; Dagda et al, 2009, 2011; Gegg et al, 2009; Gispert et al, 2009; Lutz et al, 2009; Sandebring et al, 2009; Cui et al, 2010; Yuan et al, 2010; Amo et al, 2011; Heeman et al, 2011). Decreased complex I enzymatic activity was found in fly and mouse models lacking PINK1 and suggested to be the primary event leading to synaptic dysfunction under increased energy demand, based on the observation that the mobilization of reserve pool synaptic vesicles during rapid stimulation is impaired at the neuromuscular junction in PINK1-deficient flies and can be rescued by supplementing synapses with ATP (Morais et al, 2009). Moreover, downregulation of a complex I component in flies phenocopies several PINK1 mutant phenotypes (Vilain et al, 2012). An alternative explanation was provided by Abramov and coworkers who observed an imbalance of calcium homeostasis in PINK1-deficient cells (Gandhi et al, 2009). When PINK1 is downregulated by RNAi in neurons derived from human embryonic stem cells, cellular stress results in mitochondrial calcium overload due to the dysfunction of the Na+/Ca2+ exchanger. Mitochondrial Ca2+ overload in turn stimulates ROS production, inhibits the glucose transporter and reduces respiratory functions.
Parkin and PINK1: team players in the removal of damaged mitochondria
Surprisingly, neither parkin nor PINK1 KO mice show an overt morphological or behavioural phenotype, which may be explained by developmental compensation or functional redundancy. However, both parkin and PINK1-deficient cells are characterized by an increased vulnerability to mitochondrial damage (Casarejos et al, 2006; Rosen et al, 2006; Henn et al, 2007; Paterna et al, 2007; Haque et al, 2008, 2012; Sandebring et al, 2009), indicating that mitochondrial alterations can be compensated under basal but not under stress conditions. Remarkably, parkin or PINK1 mutant flies display a striking and surprisingly similar phenotype, including reduced life span, male sterility, and apoptotic flight muscle degeneration. In flies and cultured cells, parkin can rescue PINK1 deficiency but not vice versa, indicating that parkin is acting downstream of PINK1 (Clark et al, 2006; Park et al, 2006; Yang et al, 2006; Exner et al, 2007; Lutz et al, 2009).
Evidence for a linear PINK1/parkin mitochondrial pathway was first provided by Richard Youle’s laboratory. Narendra et al (2008) observed that parkin is targeted to damaged mitochondria in a PINK1-dependent manner and induces their degradation by mitophagy, a selective mode of autophagy to dispose of dysfunctional mitochondria. When CCCP (carbonyl cyanide 3-chlorophenylhydrazone), an uncoupling agent that dissipates the mitochondrial membrane potential, is added to HeLa cells that do not express endogenous parkin, overexpressed parkin is recruited to mitochondria within 1 h, and mitochondria are cleared from parkin-expressing cells upon prolonged exposure to CCCP. As outlined above, dissipation of the mitochondrial membrane potential by CCCP leads to the accumulation of full-length PINK1 at the outer mitochondrial membrane, which is essential for the mitochondrial translocation of parkin (Figure 2; Geisler et al, 2010a; Narendra et al, 2010b; Vives-Bauza et al, 2010; Seibler et al, 2011). Strikingly, the presence of membrane-localized PINK1 is sufficient to recruit parkin, since PINK1 targeted to peroxisomes or lysosomes recruits and activates parkin on the respective organelles (Lazarou et al, 2012). In an effort to elucidate the mechanism of parkin-induced mitophagy, several cofactors of this pathway have been identified. Selective autophagy requires specific labelling of the substrates destined for degradation, such as ubiquitination, and subsequent binding of adaptor proteins to recruit the autophagic machinery to the tagged cargo (reviewed in Kirkin et al, 2009). The signalling adaptor protein p62 (also termed as SQSTM1, sequestosome-1), which links ubiquitinated cargo to the autophagic machinery by binding to both ubiquitin and LC3, has been described as an essential factor for parkin-induced mitophagy (Ding et al, 2010; Geisler et al, 2010a; Lee et al, 2010b). However, two studies reported that only perinuclear clustering of mitochondria, but not mitophagy was impaired in fibroblasts from p62 KO mice (Narendra et al, 2010c; Okatsu et al, 2010). The ubiquitin-binding deacetylase HDAC6 has also been implicated in parkin-mediated mitophagy by promoting autophagosome–lysosome fusion (Lee et al, 2010b). Ambra1 (activating molecule in Beclin-regulated autophagy) was recently identified as a parkin-interacting protein that promotes autophagic clearance of mitochondria by activating class III PI3K, which is essential for the formation of phagophores (Van Humbeeck et al, 2011).
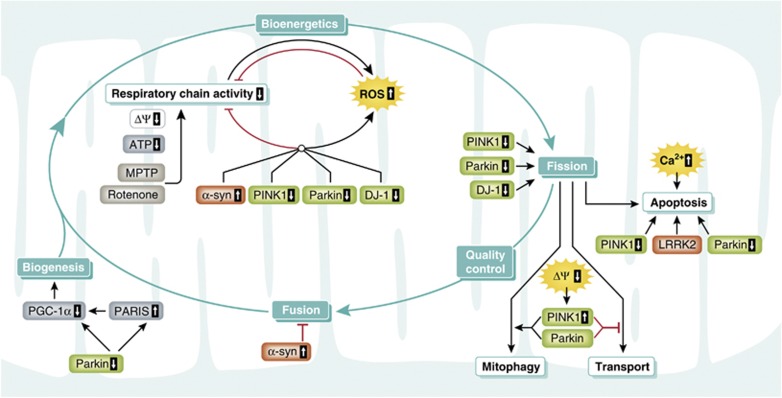
Figure 2.
Mechanism of PINK1- and parkin-induced mitophagy. (A) When the mitochondrial membrane potential is high, PINK1 is imported into mitochondria, proteolytically processed and rapidly degraded, resulting in mitochondria with low levels of endogenous PINK1 under basal conditions. (B) Under conditions of low membrane potential, for example, after uncoupling with CCCP, full-length PINK1 accumulates on the mitochondrial surface, which is essential for the translocation of parkin to mitochondria. Parkin then ubiquitinates mitochondrial proteins at the outer membrane, followed by the recruitment of adaptor proteins, such as p62, which link ubiquitinated cargo to the autophagic machinery. Damaged mitochondria are engulfed by phagophores, which mature into autophagosomes and fuse with lysosomes to autolysosomes, which eventually degrade their content.
Is parkin-mediated ubiquitination required for mitophagy and which target(s) and mode(s) of ubiquitination are implicated in this process? An increase in ubiquitinated proteins has indeed been detected at mitochondria upon CCCP treatment in parkin-expressing cells but not in cells lacking functional parkin (Geisler et al, 2010a; Lee et al, 2010b; Matsuda et al, 2010; Okatsu et al, 2010; Chan et al, 2011). A quantitative proteomic approach revealed a significant increase in both Lys48- and Lys63-linked poly-ubiquitination at mitochondria of CCCP-treated HeLa cells overexpressing parkin (Chan et al, 2011). Mitochondrial proteins most severely reduced in these cells included the fusion proteins Mfn1 and Mfn2, the mitochondrial transport proteins Miro1 and Miro2, and the import receptor subunit TOM70 (Chan et al, 2011). Removal of Mfn1/2 may facilitate the sequestration and clearance of mitochondria by increasing mitochondrial fission, which precedes mitophagy (Twig et al, 2008; Tanaka et al, 2010). Degradation of Miro1 and Miro2 blocks mitochondrial transport and thus favours clustering of damaged mitochondria (Wang et al, 2011d; Liu et al, 2012). Mfn2 has been shown to be directly involved in mitochondrial transport by interacting with Miro1/2 and Milton (Misko et al, 2010), thus by decreasing Mfn1/2 and Miro1/2 protein levels parkin can affect both mitochondrial dynamics and transport. Notably, proteasomal degradation of outer mitochondrial membrane proteins seems to be a prerequisite of parkin-dependent mitophagy, as inhibition of the proteasome abrogates mitophagy (Tanaka et al, 2010; Chan et al, 2011; Yoshii et al, 2011). However, degradative ubiquitination of Mfn1 and Mfn2 (Tanaka et al, 2010) or non-degradative ubiquitination of VDAC1 (Geisler et al, 2010a) is not essential for parkin-induced mitophagy, since this pathway is not impaired in fibroblasts from Mfn1/2 or VDAC1/3 KO mice, indicating that either the essential substrate(s) for parkin-mediated ubiquitination has yet to be identified, or rather widespread ubiquitination of mitochondrial proteins facilitates mitophagy (Narendra et al, 2010a; Tanaka et al, 2010; Chan et al, 2011). In conclusion, remodelling of the outer mitochondrial membrane by ubiquitin in response to CCCP treatment serves at least two functions: proteasomal degradation of mitochondrial proteins and attracting ubiquitin-binding proteins that recruit the autophagic machinery. Whether PINK1 and parkin interact directly, and if PINK1 phosphorylates parkin and/or parkin ubiquitinates PINK1 is a controversial issue. Some studies provided evidence for such a scenario, others not (Moore, 2006; Kim et al, 2008; Shiba et al, 2009; Um et al, 2009; Sha et al, 2010; Vives-Bauza et al, 2010; Lazarou et al, 2012).
The pathophysiological relevance of mitophagy in PD
In favour of a relevant role of mitophagy in the pathogenesis of PD, pathogenic mutations in both parkin and PINK1 compromise distinct steps in the mitophagic pathway (Geisler et al, 2010a, 2010b; Kawajiri et al, 2010; Lee et al, 2010b; Matsuda et al, 2010; Narendra et al, 2010b, 2010c; Okatsu et al, 2010; Vives-Bauza et al, 2010; Chan et al, 2011; Seibler et al, 2011). However, most of the studies on PINK1/parkin-induced mitophagy used the protonophore CCCP or the ionophore valinomycin to induce mitophagy in PINK1/parkin-overexpressing tumour cells lines or mouse embryonic fibroblasts. Thus, the major critical issues are whether this phenomenon occurs under pathophysiological stress conditions with endogenous expression levels of parkin and PINK1 and whether an impairment of mitophagy contributes to neuronal dysfunction and cell death in PD. So far, studies addressing these important questions did not provide conclusive and coherent answers. It has been reported that parkin is recruited to mitochondria in cultured cells overexpressing a catalytically inactive form of the mitochondrial helicase Twinkle, suggesting that parkin can target mitochondria with mtDNA deletions (Suen et al, 2010). In the same study, it was observed that long-term overexpression of parkin in heteroplasmic cybrid cells induces selective elimination of mitochondria with mutations in the cytochrome c oxidase subunit I gene (Suen et al, 2010). To test for parkin-induced mitophagy in vivo, Larsson and coworkers made use of a mouse model of progressive parkinsonism caused by DA neuron-specific loss of the mitochondrial transcription factor TFAM, which is essential for mtDNA maintenance and transcription initiation at mtDNA promoters (Ekstrand et al, 2007). These mice display severe respiratory chain deficiency and accumulation of mitochondrial aggregates, however, no evidence was found for mitochondrial translocation of parkin or parkin-induced mitophagy (Sterky et al, 2011).
Another crucial aspect concerning the mitophagy pathway seem to be the bioenergetic status of the cells analysed. Berman and coworkers observed that after CCCP treatment parkin efficiently translocates to uncoupled mitochondria in tumour cells, but not in rat primary cortical or striatal/midbrain neurons (Van Laar et al, 2011). Typically, tumour-derived cultured cells, such as HeLa cells, do not depend on oxidative phosphorylation, as they generate ATP preferentially by aerobic glycolysis, which can provide a biosynthetic advantage during cell proliferation (reviewed in Vander Heiden et al, 2009 and Cairns et al, 2011). Remarkably, when HeLa cells are forced into dependence on oxidative phosphorylation by culturing in glucose-free galactose cell culture medium, parkin no longer translocates to uncoupled mitochondria (Van Laar et al, 2011). In contrast to the latter study, accumulation of overexpressed YFP-parkin at axonal mitochondria was recently observed in rat hippocampal neurons treated with the complex III inhibitor antimycin A (Wang et al, 2011d). As already mentioned above, Schwarz and coworkers could demonstrate that the mitochondrial recruitment of parkin decreases mitochondrial motility as a consequence of parkin-dependent proteasomal degradation of Miro. This mitochondrial arrest may represent an early step of mitophagy, however, whether arrested mitochondria in this model are indeed eliminated by the mitophagy pathway has not been demonstrated. As a conclusion, in neurons that depend on mitochondrial respiration for energy production induction of mitophagy may be more restrictive and possibly regulated in a more complex manner in comparison with tumour cells.
Parkin, PINK1, and mitochondrial dynamics: an impact on fusion or fission?
Although mitochondria appear as solitary, static, bean-shaped double-membrane units in electron micrographs, life-cell imaging revealed that mitochondria are highly dynamic organelles which can change their shape, size, and subcellular localization. These dynamic processes are regulated by mitochondrial fusion, fission, and transport along cytoskeletal tracks. Depending on whether fusion or fission predominates, mitochondria form highly interconnected tubular networks or fragmented, rod-like/spherical structures. The last decade has witnessed remarkable progress in the identification and characterization of the basic mechanisms that govern mitochondrial fusion and fission. The core machinery mediating mitochondrial fusion and fission consists of dynamin-like GTPases, which are tightly regulated at various levels (reviewed in Detmer and Chan, 2007; Westermann, 2010) (Soubannier and McBride, 2009; Campello and Scorrano, 2010; and Oettinghaus et al, 2012). Mitofusins (Mfn1 and Mfn2) induce outer mitochondrial membrane fusion, whereas OPA1 mediates fusion of the inner membrane. Mitochondrial fission is dependent on the cytosolic GTPase Drp1, which is recruited to prospective fission sites upon activation, and involves additional fission factors, such as Mff, Mief1, and Fis1. In addition to characterizing the fusion and fission machinery at a molecular level, the impact of mitochondrial dynamics on key cellular processes, such as bioenergetics, apoptosis, autophagy, quality control and stress response pathways is still being explored. Beyond any doubt, dysregulation of mitochondrial dynamics can cause or at least contribute to the pathogenesis of various neurological disorders (reviewed in Chan, 2006; Knott et al, 2008; Oettinghaus et al, 2012; and Pilsl and Winklhofer, 2012). Notably, germline mutations in the Mfn2 gene are responsible for Charcot-Marie-Tooth type 2A, a peripheral neuropathy affecting both sensory and motor neurons (Zuchner et al, 2004), whereas mutations in the OPA1 gene cause autosomal dominant optic atrophy (Alexander et al, 2000; Delettre et al, 2000).
Alterations in mitochondrial dynamics have been documented in several genetic PD models. Specifically, in cultured mammalian cells and primary neurons the acute loss of parkin or PINK1 function causes Drp1-dependent mitochondrial fragmentation along with a decrease in the mitochondrial membrane potential and ATP production (Exner et al, 2007; Dagda et al, 2009; Lutz et al, 2009; Sandebring et al, 2009; Weihofen et al, 2009; Cui et al, 2010; Wang et al, 2011a). This phenotype can be rescued by increasing mitochondrial fusion (enhanced expression of Mfn2 or OPA1) or decreasing fission (enhanced expression of dominant negative Drp1) (Dagda et al, 2009; Lutz et al, 2009; Sandebring et al, 2009; Cui et al, 2010). In contrast to mammalian models, the phenotype of parkin- or PINK1-deficient flies can be reverted by increasing mitochondrial fission or decreasing fusion (Deng et al, 2008a; Poole et al, 2008; Yang et al, 2008). From the observations in the fly model, it was concluded that parkin and PINK1 promote mitochondrial fission. However, in mammalian cells the increased expression of parkin or PINK1 efficiently protects the mitochondrial network from fragmentation induced by cellular stress or Drp1 overexpression (Lutz et al, 2009; Sandebring et al, 2009; Bouman et al, 2011). A possible explanation for the obvious discrepancies are differences between mammals and Drosophila to cope with the loss of parkin or PINK1 function. Of note, mitochondrial fragmentation is an immediate response to parkin or PINK1 silencing and is more pronounced after a transient knockdown. Thus, it appears that compensatory strategies can be induced to prevent irreversible cellular damage in parkin- or PINK1-deficient cells. In line with this scenario, we observed that mitochondrial fragmentation occurs in cultured Drosophila S2 cells early upon parkin or PINK1 silencing, but is rapidly followed by hyperfusion (Lutz et al, 2009). Mitochondrial fusion may be activated in an attempt to dilute dysfunctional mitochondria and to achieve complementation with functional mitochondria. However, this mode of compensation does not promote elimination of damaged mitochondria, explaining why postmitotic cells in tissues with high energy demands are affected in parkin- or PINK1-deficient flies. We did not observe a hyperfusion phenotype upon knocking down parkin or PINK1 expression in mammalian cells; thus, the compensatory mechanisms seem to be different to those in fly cells. In this context, it is important to note that a stable loss of parkin or PINK1 in mammalian cells can obviously be compensated under basal conditions, consistent with the fact that parkin- or PINK1-deficient mice do not show major alterations in mitochondrial morphology. However, parkin- or PINK1-deficient cells including patients’ fibroblasts are much more vulnerable to stress-induced mitochondrial fragmentation, suggesting that the compensatory strategies are not sufficient to prevent mitochondrial damage under cellular stress (Hoepken et al, 2007; Mortiboys et al, 2008; Grunewald et al, 2010).
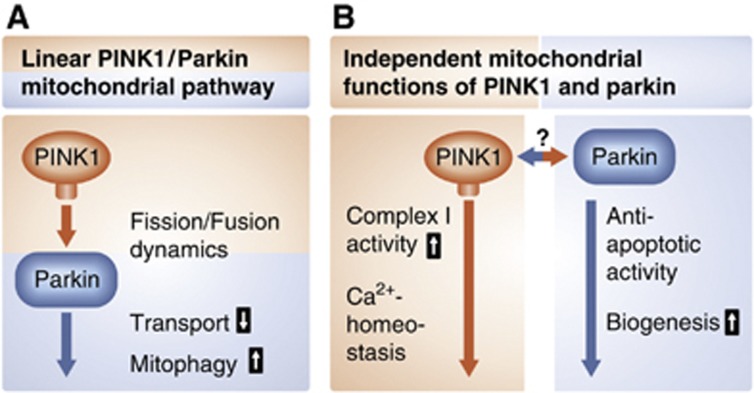
The effects of PINK1 and parkin on mitochondrial dynamics may be secondary to alterations in mitochondrial bioenergetics and/or mitochondrial depolarization (Morais et al, 2009; Sandebring et al, 2009). In line with this notion, many phenotypes of PINK1-deficient flies, such as male sterility, flight muscle degeneration, and synaptic transmission defects, are rescued by Ndi1p, a yeast NADH dehydrogenase that can bypass electron transport in complex I in mammalian cells. Complex I deficiency in PINK1 mutant flies is not rescued by increasing mitochondrial fission, indicating that PINK1-associated complex I dysfunction acts at least partly upstream of mitochondrial remodelling (Vilain et al, 2012). Interestingly, the loss of parkin function in flies does not compromise complex I activity and Ndi1p has no effect on the phenotype of parkin-deficient flies, although parkin can rescue the PINK1 phenotype (Vilain et al, 2012). These findings add evidence to the idea that in addition to a simple linear PINK1/parkin pathway, there may exist parallel pathways and independent functions of parkin and PINK1 (Figure 3). Indeed, parkin can prevent cytochrome c release induced by pro-apoptotic BH3 domains, whereas PINK1 does not have this activity (Berger et al, 2009).
Figure 3.
Mitochondrial functions of PINK1 and parkin. (A) A linear pathway with PINK1 acting upstream of parkin is implicated in mediating degradation of damaged mitochondria via mitophagy. (B) PINK1 and parkin also affect mitochondrial functions via parallel pathways, possibly by acting on mitochondria independently from each other.
Parkin has been reported to directly influence the mitochondrial fusion and fission machinery, which makes it even more difficult to decipher the net effect of parkin on mitochondrial dynamics. In the context of parkin-induced mitophagy, it has been observed that parkin mediates ubiquitination and proteasomal degradation of Mfn1 and Mfn2 in response to mitochondrial uncoupling (Poole et al, 2008; Gegg et al, 2010; Tanaka et al, 2010; Ziviani et al, 2010; Chan et al, 2011; Glauser et al, 2011). In contrast to these studies, parkin has been found to induce the proteasomal degradation of the fission-promoting proteins Drp1 or Fis1 (Cui et al, 2010; Wang et al, 2011a). How can these discrepant observations be explained? Conceptually, parkin may modulate mitochondrial dynamics in a context-specific and possibly cell type-specific manner. An attractive model would be that under moderate stress conditions with only minor mitochondrial damage parkin prevents mitochondrial fragmentation and favours mitochondrial fusion with the remaining healthy mitochondrial population, allowing functional complementation. However, when mitochondria are irreversibly damaged in response to severe stress, parkin may promote mitochondrial fission, which facilitates sequestration and elimination of damaged mitochondria via mitophagy.
DJ-1: an ROS-sensitive and ROS-protective protein
Loss-of-function mutations in the DJ-1 gene are a rare cause of autosomal recessive parkinsonism (Bonifati et al, 2003). DJ-1, a ubiquitously expressed protein of 189 amino acids, belongs to an evolutionarily conserved superfamily and shares structural similarities to the stress-inducible E. coli chaperone Hsp31 (Lee et al, 2003; Lucas and Marin, 2007). DJ-1 forms dimers with extensive contacts covering 35% of the molecular surface of each molecule (Wilson et al, 2003). A wide range of obviously unrelated DJ-1 functional activities have been reported, however, there is consensus on the fact that DJ-1 is responsive to and protective against oxidative stress (reviewed in Kahle et al, 2009 and Cookson, 2010a). Oxygen species react with the sulphydryl group of a conserved cysteine residue at position 106 to form sulphinic acid, giving rise to a shift in the isoelectric point towards more acidic values (Mitsumoto et al, 2001; Canet-Aviles et al, 2004). Importantly, this cysteine residue is essential for the stress-protective activity of DJ-1 (Canet-Aviles et al, 2004; Taira et al, 2004; Meulener et al, 2006; Blackinton et al, 2009).
DJ-1 KO mice do not display nigrostriatal degeneration, however, they are impaired in scavenging mitochondrial H2O2, which has been attributed to the function of DJ-1 as an atypical peroxiredoxin-like peroxidase (Andres-Mateos et al, 2007). DJ-1 is mostly found in the cytoplasm, but oxidative stress can induce its translocation to the outer mitochondrial membrane (Canet-Aviles et al, 2004; Blackinton et al, 2009; Junn et al, 2009). Moreover, cell fractionation and immunogold electron microscopy revealed that endogenous Drp1 can be detected in the mitochondrial matrix and intermembrane space (Zhang et al, 2005). It has been suggested that parkin, PINK1, and DJ-1 form a complex with E3 ligase activity to degrade misfolded proteins (Xiong et al, 2009); however, size exclusion chromatography did not support such a scenario, as parkin, PINK1, and DJ-1 were separated into distinct complexes (Thomas et al, 2011). Concerning the maintenance of mitochondrial integrity, DJ-1 shares some features with PINK1 and parkin. Similarly to PINK1 and parkin, DJ-1 increases resistance against mitochondrial toxins and decreases mitochondrial fragmentation in response to mitochondrial damage (Canet-Aviles et al, 2004; Kim et al, 2005; Zhang et al, 2005; Irrcher et al, 2010; Krebiehl et al, 2010; Thomas et al, 2011). DJ-1 deficiency in cultured cells, primary neurons and patients’ fibroblasts or lymphoblasts causes mitochondrial fragmentation and depolarization, which can be reverted by parkin or PINK1, but DJ-1 cannot rescue the PINK1- or parkin-deficient phenotype (Exner et al, 2007; Irrcher et al, 2010; Krebiehl et al, 2010; Thomas et al, 2011). Mitochondrial alterations induced by DJ-1 loss of function can be prevented by antioxidants, indicating that increased levels of oxidative stress account for the mitochondrial phenotypes (Irrcher et al, 2010; Thomas et al, 2011). Indeed, brain or skeletal muscle mitochondria from DJ-1 KO mice display increased formation of ROS (Andres-Mateos et al, 2007; Irrcher et al, 2010).
Notably, DJ-1 maintains its protective activity in the absence of PINK1, thus DJ-1 appears to work rather independently from PINK1 and parkin in a parallel pathway (Thomas et al, 2011; Haque et al, 2012).
α-Synuclein: a protein of structural plasticity that links sporadic and genetic PD
α-Synuclein is of particular relevance to the etiopathogenesis of PD. The identification of mutations in the gene encoding α-synuclein (SNCA) as a cause of familial PD launched the ‘molecular era’ of PD research (Polymeropoulos et al, 1997). Shortly after this discovery, α-synuclein was found to be a major component of Lewy bodies (Spillantini et al, 1997). So far, three missense mutations in the α-synuclein gene and genomic duplications or triplications have been identified in patients with autosomal dominant parkinsonism (Polymeropoulos et al, 1997; Kruger et al, 1998; Singleton et al, 2003; Chartier-Harlin et al, 2004; Farrer et al, 2004; Ibanez et al, 2004; Zarranz et al, 2004). In addition, genome-wide association studies revealed that several single-nucleotide polymorphisms in the SNCA gene are strongly associated with PD risk (Satake et al, 2009; Simon-Sanchez et al, 2009; Edwards et al, 2010; Hamza et al, 2010; International Parkinson Disease Genomics Consortium et al, 2011).
α-Synuclein is a 140 amino-acid protein that is abundantly expressed in the central nervous system of vertebrates. It belongs to the synuclein family, which also includes β- and γ-synuclein. At the N-terminal region it harbours 7 imperfect repeats of 11 amino acids containing a KTKGEV motif which mediate formation of amphipathic α-helices upon membrane binding (Davidson et al, 1998; Eliezer et al, 2001). The central NAC (non-amyloid β component of Alzheimer’s disease amyloid plaques) domain is responsible for the aggregation propensity of α-synuclein (Giasson et al, 2001). The acidic C-terminal domain contains several phosphorylation sites and influences the aggregation behaviour of α-synuclein (Okochi et al, 2000; Fujiwara et al, 2002; Tofaris et al, 2006). α-Synuclein is an intrinsically disordered protein in aqueous solution, however, depending on the environment it displays considerable structural flexibility, including membrane-bound α-helical structures, oligomers, protofibrils, and amyloid fibrils, characterized by a cross-β-sheet structure (reviewed in Volles and Lansbury, 2003; Beyer, 2007; and Uversky, 2010). It has recently been reported that endogenous α-synuclein isolated from cultured cells and red blood cells under non-denaturing conditions adopts a helically folded tetrameric structure with enhanced lipid-binding capacity and markedly reduced aggregation propensity in comparison with α-synuclein monomers, suggesting that α-synuclein tetramers are the physiological species (Bartels et al, 2011). Formation of stable tetramers with helical secondary structure was also described for recombinantly expressed human α-synuclein in the absence of lipid bilayers or micelles (Wang et al, 2011c). However, these findings have been disputed in a joint publication of six different groups (Fauvet et al, 2012). Using α-synuclein standards of well-characterized conformational and oligomeric states and applying various methods such as native and denaturing gel electrophoresis techniques, size exclusion chromatography, and an oligomer-specific ELISA, the authors of this study demonstrated that native α-synuclein from human, rat, and mouse brains, several mammalian cell lines, and human red blood cells exhibit identical migration patterns and apparent molecular weight as unfolded monomeric recombinant α-synuclein. Thus, the results of the latter study question the existence of physiological α-synuclein tetramers, a critical issue for the development of α-synuclein-specific therapeutic strategies, which mostly aim at preventing α-synuclein oligomerization.
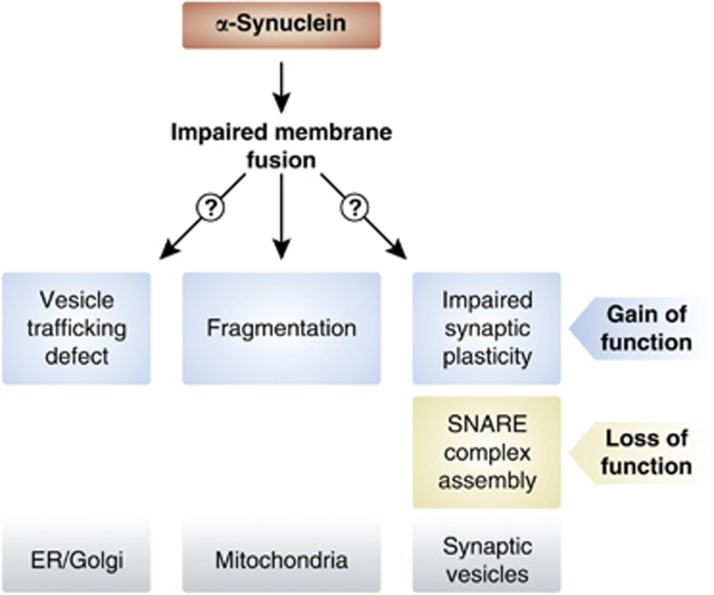
It has now widely been accepted that not the final fibrillar α-synuclein aggregates but rather oligomeric prefibrillar folding intermediates are the major toxic species (reviewed in Lansbury and Lashuel, 2006; Cookson and van der Brug, 2008; and Winklhofer et al, 2008). However, the mechanisms of α-synuclein-induced toxicity are still under discussion, hampered by the difficulties in discriminating primary and specific effects from secondary and/or unspecific effects caused by overloading cells with a misfolding-prone protein. Upon overexpression of wild-type or mutant α-synuclein in cellular and animal models alterations in various cellular processes have been reported, including proteasomal and lysosomal degradation, autophagy, and vesicular transport (reviewed in Cookson, 2009; Waxman and Giasson, 2009; Sulzer, 2010; Venda et al, 2010; and Vekrellis et al, 2011). The physiological function of α-synuclein has also largely remained elusive. Its enrichment in presynaptic terminals and its binding to vesicles suggests a role in vesicle dynamics and synaptic plasticity (reviewed in Auluck et al, 2010). A common theme emerging from studies on the function and dysfunction of α-synuclein is that it appears to affect membrane fusion events (Figure 4). Mice lacking α-synuclein were reported to show increased dopamine release upon stimulation, pointing to a role of α-synuclein in negatively regulating dopamine neurotransmission (Abeliovich et al, 2000; Yavich et al, 2004). Similarly, in α-, β-, and γ-synuclein triple KO mice, an elevated evoked release of dopamine in the nigrostriatal system was observed (Anwar et al, 2011). When α-synuclein is overexpressed in PC12 cells evoked catecholamine release is inhibited and docked vesicles accumulate at the plasma membrane (Larsen et al, 2006). In primary hippocampal neurons, mild overexpression of α-synuclein in the range of gene duplication or triplication decreases neurotransmitter release, probably by reducing the size of the synaptic vesicle recycling pool (Nemani et al, 2010). Similarly, virus-mediated expression of α-synuclein in the SN of adult rats causes a reduction in striatal dopamine release (Lundblad et al, 2012). α-Synuclein has been reported to cooperate with presynaptic cysteine-string protein-α (CSPα) to maintain neuronal integrity (Chandra et al, 2005). CSPα is a co-chaperone of the Hsp40 family involved in the assembly of SNARE complexes, the core components of a conserved machinery mediating synaptic vesicle membrane fusion during exocytosis (Sharma et al, 2011). Strikingly, neurodegeneration observed in CSPα-deficient mice could be rescued by transgenic expression of α-synuclein, whereas deletion of endogenous α-synuclein enhanced the neurodegenerative phenotype (Chandra et al, 2005). Concerning the mechanism of this functional interaction it has been demonstrated that α-synuclein directly promotes the assembly of SNARE complexes by simultaneous binding to phospholipids via its N-terminal region and to synaptobrevin-2 via its C-terminal domain (Burre et al, 2010; Figure 4).
Figure 4.
Physiological and pathophysiological functions of α-synuclein on membrane dynamics. Effects of α-synuclein on membrane fusion events can explain why both an increase of α-synuclein expression and a loss of α-synuclein have adverse effects. Overexpression of α-synuclein causes ER/Golgi vesicle trafficking defects and mitochondrial fragmentation, whereas α-synuclein deficiency affects SNARE complex assembly.
In vitro, α-synuclein preferentially binds to small vesicles containing acidic phospholipids and induces ordering of phospholipids at sites of packing defects in the bilayer caused by curvature stress (Davidson et al, 1998; Kamp and Beyer, 2006). Local defects in a membrane are required for the formation of a fusion stalk, thus, annealing of α-synuclein to those defects should prevent premature membrane fusion. Indeed, fusion of small unilamellar vesicles is inhibited in the presence of recombinant α-synuclein, and overexpression of α-synuclein in cultured cells and C. elegans reduces the fusion rate of mitochondria (Kamp et al, 2010). Notably, mitochondrial fragmentation induced by overexpression of α-synuclein can be prevented by PINK1, parkin, or DJ-1 but not by their pathogenic mutants (Kamp et al, 2010). Mitochondrial fragmentation upon increased α-synuclein expression was also observed in another study using cultured cells and primary neurons (Nakamura et al, 2011). In this study, it was reported that α-synuclein causes an increase in Drp1-independent mitochondrial fission, which is not accompanied by alterations in the morphology of other cellular organelles. Interestingly, α-synuclein-induced mitochondrial fragmentation is not a consequence of mitochondrial depolarization or respiration deficits, it occurs without overt toxicity and is independent of proteins of the mitochondrial fusion and fission machinery (Kamp et al, 2010; Nakamura et al, 2011). In conclusion, both the increased expression of α-synuclein and the loss of α-synuclein function may impact on membrane fusion, explaining the observed effects of α-synuclein on neurotransmitter release. Similarly, the ER-to-Golgi vesicle trafficking defect observed in several models of α-synuclein overexpression could be attributed to impaired fusion of vesicles at the Golgi membrane (Gitler et al, 2008; Figure 4).
There is evidence for a preferential binding of α-synuclein to mitochondria (Nakamura et al, 2008), and localization of α-synuclein at mitochondria or even within mitochondria has been reported (Li et al, 2007; Cole et al, 2008; Devi et al, 2008; Parihar et al, 2008; Shavali et al, 2008; Zhang et al, 2008; Kamp et al, 2010; Nakamura et al, 2011). In various models of α-synuclein overexpression structural alterations of mitochondria, increased oxidative stress, and/or bioenergetic defects have been observed (Hsu et al, 2000; Martin et al, 2006; Stichel et al, 2007; Devi et al, 2008; Parihar et al, 2008; Liu et al, 2009; Chinta et al, 2010; Loeb et al, 2010; Su et al, 2010; Choubey et al, 2011). α-Synuclein KO mice are resistant or less sensitive to mitochondrial toxins (Dauer et al, 2002; Schluter et al, 2003; Klivenyi et al, 2006) and were reported to have qualitative and quantitative lipid abnormalities with a decrease in cardiolipin content (Ellis et al, 2005). Vice versa, α-synuclein-overexpressing mice are more vulnerable to mitochondrial toxins (Orth et al, 2003; Song et al, 2004; Shavali et al, 2008), albeit this is not a consistent finding (Rathke-Hartlieb et al, 2001; Dong et al, 2002). α-Synuclein toxicity in cellular models and in Drosophila is mitigated by the mitochondrial chaperone TRAP1, supporting the notion that α-synuclein affects mitochondrial function (Butler et al, 2012). α-Synuclein has also been reported to increase autophagy and mitophagy in primary cortical neurons, and depletion of parkin showed beneficial effects on neuronal survival, which led the authors to conclude that inhibition of excess mitophagy is protective in this model (Choubey et al, 2011). This observation would be in line with a double transgenic mouse model in which the absence of parkin delayed the neurodegenerative phenotype caused by A30P α-synuclein overexpression (Fournier et al, 2009). It should be mentioned that α-synuclein has also been found to impair autophagy (Winslow et al, 2010). The effect of parkin on α-synuclein-induced pathology is controversial as well: in various models, a protective effect of parkin on α-synuclein-induced alterations was observed (Petrucelli et al, 2002; Yang et al, 2003; Lo Bianco et al, 2004; Yasuda et al, 2007; Khandelwal et al, 2010). Furthermore, in another mouse model with a combined transgenic expression of A30P/A53T α-synuclein and a targeted deletion of parkin, mitochondrial pathology was more prominent in comparison with single transgenic mice (Stichel et al, 2007).
A striking feature of α-synuclein is its transmissibility to neighbouring cells (reviewed in Brundin et al, 2010; Frost and Diamond, 2010; Steiner et al, 2011; and Hansen and Li, 2012). The first evidence for this phenomenon came from studies in PD patients that received neural grafts derived from fetal midbrain tissue. At autopsy between 10 and 16 years after transplantation, Lewy body pathology was not only observed in the host striatal neurons but also in the grafted neurons of some PD patients (Kordower et al, 2008a, 2008b; Li et al, 2008, 2010; Mendez et al, 2008). Studies in mice have essentially supported the phenomenon of intercellular α-synuclein transfer. Wild-type neuronal stem cells or embryonic neurons grafted into the brain of α-synuclein-overexpressing transgenic mice can take up host α-synuclein and develop inclusions (Desplats et al, 2009; Hansen et al, 2011). How can α-synuclein spread from cell to cell? Obviously, α-synuclein is secreted from cultured cells by exocytotic vesicles (Lee et al, 2005; Jang et al, 2010) or via exosomes, which are endosome-derived vesicles secreted during fusion of multivesicular bodies with the plasma membrane (Emmanouilidou et al, 2010; Alvarez-Erviti et al, 2011). The mechanism by which α-synuclein is taken up by recipient cells is largely unknown. Passive diffusion, endocytosis, and exosome-mediated uptake have been observed, and it is highly likely that the mode of cell entry depends on the type of α-synuclein species to be taken up (Sung et al, 2001; Desplats et al, 2009; Park et al, 2009; Emmanouilidou et al, 2010; Hansen et al, 2011). Internalized α-synuclein fibrils generated from recombinant α-synuclein promote aggregation of endogenous α-synuclein in mouse primary hippocampal neurons by a seeding activity and cause synaptic dysfunction and ultimately cell death (Volpicelli-Daley et al, 2011). Remarkably, in young asymptomatic A53T α-synuclein transgenic mice, intracerebral injection of brain homogenates from older symptomatic transgenic mice accelerates α-synuclein aggregation and neuronal degeneration, even at regions far beyond injection sites, a phenomenon also observed after injection of synthetic α-synuclein fibrils (Luk et al, 2012). The propagation of misfolded protein species by similar mechanisms has also been observed for other proteins associated with neurodegenerative disease, like tau, Aβ peptide and huntingtin, and thus may reflect a unifying mechanism of disease progression.
LRRK2: a GTPase-regulated kinase or a kinase-regulated GTPase?
Mutations in the gene encoding LRRK2 cause autosomal dominant parkinsonism and are the most common cause of familial PD (Paisan-Ruiz et al, 2004; Zimprich et al, 2004). Moreover, genome-wide association studies identified genetic variants in the LRRK2 gene as risk factors for sporadic PD (Satake et al, 2009; Simon-Sanchez et al, 2009; Hamza et al, 2011; International Parkinson Disease Genomics Consortium et al, 2011). LRRK2 is a 2257 amino-acid protein that belongs to the ROCO protein family, characterized by an Roc (Ras of complex proteins) domain with GTPase activity and a COR (C-terminal of Roc) domain. A kinase domain with sequence similarities to RIPKs (receptor-interacting serine/threonine protein kinases) and MLKs (mixed lineage kinases), a subclass of the MAPKKK (mitogen-activated protein kinase kinase kinases) family, is located C-terminal to the COR domain. In addition, LRRK2 harbours several protein–protein interaction domains, such as a WD40 domain, leucine-rich repeats (LRRs), and an ankyrin domain. To date, six missense mutations have been demonstrated to segregate with familial PD, which cluster to the Roc, COR, and kinase domain.
The modular structure of LRRK2 suggests a role as a signalling protein, which can act as a kinase, GTPase, and/or scaffolding protein. LRRK2 forms dimers, and dimer formation appears to be essential for full catalytic activity (Deng et al, 2008b; Gotthardt et al, 2008; Greggio et al, 2008; Sen et al, 2009). LRRK2 shows a weak kinase activity in vitro mediating autophosphorylation and phosphorylation of generic substrates (i.e., myelin basic protein) or putative substrates, such as members of the ERM protein family (ezrin/radixin/moesin) that anchor the actin cytoskeleton to the plasma membrane, kinases of the MAP and Ste20 serine/threonine kinase families, 4E-BP, the eukaryotic translation initiation factor 4E-binding protein, or the forkhead box transcription factor FoxO1 (West et al, 2005; Gloeckner et al, 2006, 2009; Jaleel et al, 2007; Imai et al, 2008; Parisiadou et al, 2009; Kanao et al, 2010; Zach et al, 2010). It has been reported that the kinase activity is regulated by the GTPase activity, however, the Roc domain can be phosphorylated by the kinase domain, suggesting the possibility that not the kinase but the GTPase activity is the main output of LRRK2 and/or that both activities are regulated in a reciprocal manner (Greggio et al, 2008, 2009; Kamikawaji et al, 2009; Gloeckner et al, 2010; Liu et al, 2010; Webber et al, 2011). This hypothesis is in line with the recent identification of a GTPase-activating protein, ArfGAP1, which enhances the GTPase activity of LRRK2 but is also phosphorylated by LRRK2 (Stafa et al, 2012; Xiong et al, 2012).
The physiological and pathological functions of LRRK2 are still incompletely understood. It has been reported that pathogenic LRRK2 mutations increase kinase activity and/or decrease GTPase activity. Overexpression of pathogenic LRRK2 mutants in cultured cells, primary neurons or rodents causes cellular toxicity, which is dependent on the kinase activity (Greggio et al, 2006; Smith et al, 2006; West et al, 2007; Ho et al, 2009; Dusonchet et al, 2011). Indeed, inhibitors of LRRK2 kinase are protective in in-vitro and in-vivo models of LRRK2-induced neurodegeneration (Lee et al, 2010a; Liu et al, 2011a). From diverse cellular and animal models of LRRK2 loss and gain of function it emerged that LRRK2 can influence neurite outgrowth, cytoskeleton dynamics, vesicle trafficking, translational control, endocytosis, autophagy, mitochondrial function, MAPK and Wnt signalling, and extrinsic (TNFα/FasL) or intrinsic apoptosis pathways (reviewed in Cookson, 2010b; Berwick and Harvey, 2011; Daniels et al, 2011; and Tsika and Moore, 2012). Which of these pathways are relevant to the pathogenesis of PD requires further investigation. Notably, flies expressing the pathogenic G2019S LRRK2 mutant exhibit late-onset loss of DA neurons in selected clusters accompanied by locomotion deficits (Liu et al, 2008; Ng et al, 2009). In this fly LRRK2 model, coexpression of human parkin protects against DA neurodegeneration induced by ageing or rotenone treatment (Ng et al, 2009).
An interesting immunoregulatory function of LRRK2 was recently reported based on genome-wide association studies identifying LRRK2 as a major susceptibility gene for inflammatory bowel disease (Barrett et al, 2008; Franke et al, 2010). LRRK2-deficient mice display an increased susceptibility to experimentally induced colitis (Liu et al, 2011b). LRRK2 which is expressed in macrophages, dendritic cells, and B lymphocytes can inhibit nuclear translocation of NFAT, a transcription factor regulating immune responses, by increasing its interaction with a cytoplasmic NFAT repressor (Gardet et al, 2010; Liu et al, 2011b). Notably, this activity of LRRK2 does not involve phosphorylation of NFAT.
So far, little is known about a possible impact of LRRK2 on mitochondria. Upon overexpression in cultured cells, about 10% of LRRK2 was found at the outer mitochondrial membrane (West et al, 2005). Mitochondrial localization was also shown for endogenous LRRK2 in mammalian brain tissue by confocal imaging, subcellular fractionation, and electron microscopy (Biskup et al, 2006). Mitochondrial pathology has been observed in aged G2019S LRRK2 transgenic mice (Ramonet et al, 2011), and the expression of this mutant aggravates mitochondrial alterations in inducible A53T α-synuclein transgenic mice (Lin et al, 2009). However, a similar approach using double transgenic mice did not provide support for a pathophysiological interaction of LRRK2 and α-synuclein (Daher et al, 2012). Skin fibroblasts derived from five patients carrying the G2019S mutation show a decrease in mitochondrial membrane potential and total ATP production and a trend towards increased mitochondrial interconnectivity (Mortiboys et al, 2010). A recent study reported that overexpression of wild-type LRRK2 and pathogenic LRRK2 mutants in cultured cells and primary cortical neurons cause mitochondrial fragmentation by increasing mitochondrial recruitment of Drp1 (Wang et al, 2012). Notably, LRRK2-induced toxicity could be blocked by decreasing mitochondrial fission or increasing fusion. Further studies are needed to determine whether these mitochondrial effects of LRRK2 are direct or indirect and what the underlying mechanism might be.
Controversies and unanswered questions
Why are SNc DA neurons particularly vulnerable?
A key challenge in the neurodegeneration field has been to understand the selective vulnerability of neuronal sub-populations. SNc DA neurons are considered special in terms of oxidative stress management and calcium homoeostasis. Sources of ROS in DA neurons are the enzymatic and non-enzymatic metabolism of cytoplasmic dopamine. Vesicular dopamine is protected from oxidation, thus, an increase in oxidation-prone cytoplasmic dopamine is toxic to neurons. VMAT2 is the vesicular monoamine transporter responsible for dopamine uptake into presynaptic vesicles, and dysfunction of VMAT, for example as a consequence of decreased mitochondrial ATP production, can induce nigrostriatal degeneration (Caudle et al, 2007). Moreover, the amount of iron, which can trigger ROS formation via the Fenton reaction, has been reported to be high in SNc DA neurons, whereas antioxidative components, such as reduced glutathione, are expressed at low levels (Kish et al, 1985; Chinta and Andersen, 2008). Oxidative stress may also arise from excitotoxicity and mitochondrial dysfunction, which can trigger a vicious circle. Excitotoxicity results from overstimulation of glutamate receptors and elicits a cascade of harmful events including calcium influx that can challenge the storage capacity of the ER and mitochondria. SNc DA neurons are vulnerable to excitotoxicity based on rich glutamatergic input from the subthalamic nucleus. A decrease in ATP production, for example as a consequence of mitochondrial dysfunction, can compromise the ATP-dependent regulation of glutamate receptors favouring their overstimulation.
Calcium toxicity has been suggested as another determinant of selective vulnerability. Adult ventrolateral SNc DA neurons engage L-type Cav1.3 calcium channels for rhythmic pacemaking, in contrast to less vulnerable DA neurons of the ventral tegmental area which use sodium channels for pakemacing activity (Chan et al, 2007). Reliance on these L-type calcium channels increases with age and leads to sustained elevated cytoplasmic calcium levels, which may stimulate mitochondrial respiration and ROS production. An imbalance in calcium homeostasis can be enhanced by mitochondrial and ER stressors or genetic mutations in stress-protective genes, resulting in mitochondrial calcium overload, which impairs ATP synthesis and consequently, ATP-dependent cellular processes.
Clearly, none of the current hypotheses can sufficiently explain the selective vulnerability of SNc DA neurons, rather, a combination of the phenomena described above as well as some yet unknown factors are responsible for the preferential susceptibility of this neuronal population.
Is mitochondrial complex I inhibition a causal factor for PD?
Generic complex I inhibitors, such as MPTP, rotenone, or annonacin, can induce parkinsonian syndromes in humans and animal models, however, it is not clear whether their mode of action is restricted to complex I inhibition. Compatible with the complex I inhibition hypothesis, ectopic expression of Ndi1p, a yeast single-subunit NADH dehydrogenase that is insensitive to MPTP and rotenone, has been shown to protect DA neurons from MPTP and rotenone toxicity in vitro and in vivo (Sherer et al, 2003; Seo et al, 2006; Richardson et al, 2007; Marella et al, 2008). The role of complex I inhibition in PD has been challenged by a study using mice that lack functional Ndufs4, a gene encoding a subunit implicated in assembly and function of complex I. Ndufs4 KO mice show neuronal degeneration in the cerebellum, olfactory bulb, and vestibular nuclei, whereas DA neurons are not affected (Kruse et al, 2008; Quintana et al, 2010; Choi et al, 2011). Moreover, midbrain primary cultures derived from Ndufs4 KO mice are not protected against MPP+ or rotenone, suggesting that toxicity of these drugs is not caused by complex I inhibition (Choi et al, 2008). Ndufs4-deficient DA neurons are even more sensitive to rotenone, and complex I-independent mechanisms have been reported to contribute to the toxicity of rotenone, such as microtubule depolymerization (Ren et al, 2005; Choi et al, 2011). However, it was recently shown that complex I activity is only partially decreased but not abolished in Ndufs4 KO mice due to the formation of respiratory supercomplexes with a stabilizing effect of complex III (Calvaruso et al, 2012; Sterky et al, 2012). This finding can explain why Ndufs4-deficient neurons are still sensitive to MPTP and rotenone toxicity. DA neuron-specific conditional Ndufs4 KO mice do not show overt nigrostriatal degeneration, only a mild decrease (7.5%) in tyrosine hydroxylase-positive neurons at 24 months of age (Sterky et al, 2012). Nevertheless, striatal dopamine turnover is increased and dopamine release is decreased in Ndufs4-deficient mice, which may reflect an early consequence of mitochondrial dysfunction. These findings support the notion that complex I deficiency can contribute to the pathogenesis of PD.
The most convincing link between PD genes and complex I activity was provided for PINK1. In fact, there is strong experimental evidence for a central role of complex I in PINK1-associated mitochondrial pathology. Complex I enzymatic activities are reduced in PINK1-deficient mice and flies (Gautier et al, 2008; Morais et al, 2009). In flies, expression of the yeast complex I equivalent Ndi1p rescues several PINK1-deficient phenotypes, and downregulation of complex I subunits phenocopies some PINK1-associated alterations (Vilain et al, 2012). A recent screen for genetic modifiers in PINK1-deficient flies identified Heix, a gene encoding a prenyltransferase involved in vitamin K2 biosynthesis (Vos et al, 2012). In the fly model, vitamin K2 was able to rescue mitochondrial morphological defects and to maintain ATP production, which has been attributed to its ability to serve as a mitochondrial electron carrier. The molecular mechanism of these interactions and its significance in mammalian models requires further investigation.
How can discrepant findings in Drosophila and mammalian PD models be explained?
The Drosophila model has contributed valuable insights into PD pathogenesis. Importantly, it has been helpful to characterize the function of PD-associated genes and to identify genetic modifiers, thereby focusing research activities on key biochemical pathways. The strikingly similar phenotype of PINK1 and parkin mutant flies has revealed that parkin acts downstream of PINK1 to maintain mitochondrial integrity. Various effects of PINK1 and parkin on mitochondria have been described in different models, including mitochondrial morphology, dynamics, bioenergetics, transport, and quality control. Rather than a single linear pathway integrating all these effects described, diverse pathways account for these manifold activities that converge on mitochondria. Consistent with this notion, there are different activities of PINK1 and parkin that are either dependent or independent on each other.
What is still causing confusion in the field is the fact that opposing effects are reported from genetic fly and mammalian models. For example, the PINK1 or parkin mutant phenotypes in Drosophila can be rescued by decreasing mitochondrial fusion and increasing fission, whereas the acute loss of PINK1 or parkin in cellular mammalian models is reverted by increasing mitochondrial fusion or decreasing fission. A similar phenomenon has been reported for affecting mitochondrial transport. In PINK1 mutant flies, Miro levels are increased and Miro RNAi can rescue PINK1 mutant phenotypes (Liu et al, 2012). However, in human cells Miro levels are decreased in the absence of PINK1 and Miro overexpression can revert PINK1 deficiency (Weihofen et al, 2009; Liu et al, 2012). One plausible explanation for these seemingly discrepant findings is that mammalian cells have elaborate compensatory strategies that may be absent or different in flies. In fact, compensation can at least partly explain why genetic PD mouse models lack a striking phenotype and why patients with mutations in PD genes develop symptoms only after some decades when coping mechanisms may be less efficient due to ageing.
Does dysfunctional mitophagy cause PD?
A role of parkin and PINK1 in mitochondrial quality control is appealing and can explain beneficial mitochondrial effects of these PD genes in different models. Beyond any doubt, parkin-induced autophagic removal of mitochondria is a robust phenomenon in cultured cells treated with mitochondrial uncouplers, such as CCCP. Parkin-induced mitophagy is strictly dependent on PINK1 expression, since parkin cannot translocate to uncoupled mitochondria in the absence of PINK1. There is an ongoing discussion as to whether PINK1/parkin-mediated mitophagy is a physiologically and pathophysiologically relevant pathway in vivo. The main criticism is that CCCP is a drastic unphysiological stressor that causes uncoupling of all mitochondria within seconds. Parkin-induced mitophagy has mostly been studied in established tumour cell lines or mouse embryonic fibroblasts, which preferentially produce ATP by glycolysis. In contrast, neurons depend on oxidative phosphorylation for ATP production and therefore may not easily discard their mitochondria. In parkin- or PINK1-deficient flies, enlarged aggregated mitochondria have been observed, however, this is not necessarily a consequence of defective mitochondrial clearance. In fact, it has not been demonstrated conclusively that the phenotypic alterations in parkin or PINK1 mutant flies are caused by an impairment of mitophagy. Moreover, parkin can rescue PINK1 mutant flies, which argues against defective mitophagy being responsible for the PINK1-deficient phenotype, given that parkin cannot promote mitophagy in the absence of PINK1.
Perspective and future directions
Research into the function and dysfunction of PD-associated genes revealed that at least some of these genes interface with pathways regulating various aspects of mitochondrial biology (Figure 5). In fact, there is compelling evidence that mitochondrial dysfunction is a common denominator of sporadic and familial PD. Genome-wide association studies indicated that genetic variants of the classical PD genes α-synuclein and LRRK2 increase the risk of developing sporadic PD, adding to the notion that sporadic and familial disease entities share common pathways. Emerging evidence suggests that bioenergetic deficits and dysregulation of the mitochondrial quality control rather than oxidative stress alone are relevant players in the pathogenesis of PD. Hence, a plethora of challenging issues remains to be addressed. First, it will be important to disentangle direct from indirect mitochondrial effects. Second, results from in-vitro studies should be validated in suitable in-vivo models to prove the physiological or pathophysiological relevance of the observed effects. Third, we need to increase our understanding of the interaction of disease or susceptibility genes with environmental factors in triggering and/or promoting neurodegeneration. Most importantly, the ultimate aim would be to translate basic research into clinical practice by exploiting mitochondrial pathways as a rationale to intervene early in the pathogenic process. To this aim, it would be helpful to have reliable biomarkers or imaging techniques to identify patients, which are likely to benefit from mitochondria-based strategies.
Figure 5.
PD genes are implicated in various aspects of mitochondrial biology. Products of autosomal recessive PD genes (shown in green) or autosomal dominant PD genes (shown in red) directly or indirectly affect a wide spectrum of mitochondrial processes, including their life cycle, bioenergetic capacity, quality control, dynamic changes of morphology and connectivity (fusion, fission), subcellular distribution (transport), and the regulation of cell death pathways.
Acknowledgments
This work is supported by the Deutsche Forschungsgemeinschaft (SFB 596 ‘Molecular Mechanisms of Neurodegeneration’), the German Ministry for Education and Research (NGFN plus ‘Functional Genomics of Parkinson’s Disease’), the Helmholtz Alliance ‘Mental Health in an Ageing Society’, and the Center for Integrated Protein Science Munich (CIPSM). CH is supported by a ‘Forschungsprofessur’ provided by the LMU Excellence Program.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239–252 [DOI] [PubMed] [Google Scholar]
- Abou-Sleiman PM, Muqit MM, Wood NW (2006) Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci 7: 207–219 [DOI] [PubMed] [Google Scholar]
- Adler CH, Connor DJ, Hentz JG, Sabbagh MN, Caviness JN, Shill HA, Noble B, Beach TG (2010) Incidental Lewy body disease: clinical comparison to a control cohort. Mov Disord 25: 642–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B (2000) OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26: 211–215 [DOI] [PubMed] [Google Scholar]
- Agirre X, Roman-Gomez J, Vazquez I, Jimenez-Velasco A, Garate L, Montiel-Duarte C, Artieda P, Cordeu L, Lahortiga I, Calasanz MJ, Heiniger A, Torres A, Minna JD, Prosper F (2006) Abnormal methylation of the common PARK2 and PACRG promoter is associated with downregulation of gene expression in acute lymphoblastic leukemia and chronic myeloid leukemia. Int J Cancer 118: 1945–1953 [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM (2011) Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 42: 360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amo T, Sato S, Saiki S, Wolf AM, Toyomizu M, Gautier CA, Shen J, Ohta S, Hattori N (2011) Mitochondrial membrane potential decrease caused by loss of PINK1 is not due to proton leak, but to respiratory chain defects. Neurobiol Dis 41: 111–118 [DOI] [PubMed] [Google Scholar]
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL (2007) DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA 104: 14807–14812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar S, Peters O, Millership S, Ninkina N, Doig N, Connor-Robson N, Threlfell S, Kooner G, Deacon RM, Bannerman DM, Bolam JP, Chandra SS, Cragg SJ, Wade-Martins R, Buchman VL (2011) Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J Neurosci 31: 7264–7274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auluck PK, Caraveo G, Lindquist S (2010) alpha-Synuclein: membrane interactions and toxicity in Parkinson’s disease. Annu Rev Cell Dev Biol 26: 211–233 [DOI] [PubMed] [Google Scholar]
- Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP et al. (2008) Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet 40: 955–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels T, Choi JG, Selkoe DJ (2011) alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477: 107–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends C, Harper JW (2011) Constructing and decoding unconventional ubiquitin chains. Nat Struct Mol Biol 18: 520–528 [DOI] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38: 515–517 [DOI] [PubMed] [Google Scholar]
- Berger AK, Cortese GP, Amodeo KD, Weihofen A, Letai A, LaVoie MJ (2009) Parkin selectively alters the intrinsic threshold for mitochondrial cytochrome c release. Hum Mol Genet 18: 4317–4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DC, Harvey K (2011) LRRK2 signaling pathways: the key to unlocking neurodegeneration? Trends Cell Biol 21: 257–265 [DOI] [PubMed] [Google Scholar]
- Beyer K (2007) Mechanistic aspects of Parkinson’s disease: alpha-synuclein and the biomembrane. Cell Biochem Biophys 47: 285–299 [DOI] [PubMed] [Google Scholar]
- Bezard E, Przedborski S (2011) A tale on animal models of Parkinson’s disease. Mov Disord 26: 993–1002 [DOI] [PubMed] [Google Scholar]
- Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL (2006) Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol 60: 557–569 [DOI] [PubMed] [Google Scholar]
- Blackinton J, Lakshminarasimhan M, Thomas KJ, Ahmad R, Greggio E, Raza AS, Cookson MR, Wilson MA (2009) Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1. J Biol Chem 284: 6476–6485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaerts V, Theuns J, van Broeckhoven C (2008) Genetic findings in Parkinson’s disease and translation into treatment: a leading role for mitochondria? Genes Brain Behav 7: 129–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299: 256–259 [DOI] [PubMed] [Google Scholar]
- Bouman L, Schlierf A, Lutz AK, Shan J, Deinlein A, Kast J, Galehdar Z, Palmisano V, Patenge N, Berg D, Gasser T, Augustin R, Trumbach D, Irrcher I, Park DS, Wurst W, Kilberg MS, Tatzelt J, Winklhofer KF (2011) Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ 18: 769–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P, Melki R, Kopito R (2010) Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol 11: 301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueler H (2010) Mitochondrial dynamics, cell death and the pathogenesis of Parkinson’s disease. Apoptosis 15: 1336–1353 [DOI] [PubMed] [Google Scholar]
- Burbulla LF, Krebiehl G, Kruger R (2010) Balance is the challenge—the impact of mitochondrial dynamics in Parkinson’s disease. Eur J Clin Invest 40: 1048–1060 [DOI] [PubMed] [Google Scholar]
- Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC (2010) Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329: 1663–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EK, Voigt A, Lutz AK, Toegel JP, Gerhardt E, Karsten P, Falkenburger B, Reinartz A, Winklhofer KF, Schulz JB (2012) The mitochondrial chaperone TRAP1 mitigates a-synuclein toxicity. PLoS Genet 8: e1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11: 85–95 [DOI] [PubMed] [Google Scholar]
- Calvaruso MA, Willems P, van den Brand M, Valsecchi F, Kruse S, Palmiter R, Smeitink J, Nijtmans L (2012) Mitochondrial complex III stabilizes complex I in the absence of NDUFS4 to provide partial activity. Hum Mol Genet 21: 115–120 [DOI] [PubMed] [Google Scholar]
- Campello S, Scorrano L (2010) Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep 11: 678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR (2004) The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA 101: 9103–9108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT (2011) The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci 124: 225–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparros-Lefebvre D, Elbaz A (1999) Possible relation of atypical parkinsonism in the French West Indies with consumption of tropical plants: a case-control study. Caribbean Parkinsonism Study Group. Lancet 354: 281–286 [DOI] [PubMed] [Google Scholar]
- Casarejos MJ, Menendez J, Solano RM, Rodriguez-Navarro JA, Garcia de Yebenes J, Mena MA (2006) Susceptibility to rotenone is increased in neurons from parkin null mice and is reduced by minocycline. J Neurochem 97: 934–946 [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW (2007) Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci 27: 8138–8148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari R, Martin ES, Calin GA, Pentimalli F, Bichi R, McAdams H, Trapasso F, Drusco A, Shimizu M, Masciullo V, D’Andrilli G, Scambia G, Picchio MC, Alder H, Godwin AK, Croce CM (2003) Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25–q27. Proc Natl Acad Sci USA 100: 5956–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha GH, Kim S, Park J, Lee E, Kim M, Lee SB, Kim JM, Chung J, Cho KS (2005) Parkin negatively regulates JNK pathway in the dopaminergic neurons of Drosophila. Proc Natl Acad Sci USA 102: 10345–10350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champy P, Hoglinger GU, Feger J, Gleye C, Hocquemiller R, Laurens A, Guerineau V, Laprevote O, Medja F, Lombes A, Michel PP, Lannuzel A, Hirsch EC, Ruberg M (2004) Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: possible relevance for atypical parkinsonism in Guadeloupe. J Neurochem 88: 63–69 [DOI] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ (2007) ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 447: 1081–1086 [DOI] [PubMed] [Google Scholar]
- Chan DC (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125: 1241–1252 [DOI] [PubMed] [Google Scholar]
- Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, Hess S, Chan DC (2011) Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet 20: 1726–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC (2005) Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123: 383–396 [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364: 1167–1169 [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Andersen JK (2008) Redox imbalance in Parkinson’s disease. Biochim Biophys Acta 1780: 1362–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Mallajosyula JK, Rane A, Andersen JK (2010) Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett 486: 235–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Kruse SE, Palmiter RD, Xia Z (2008) Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci USA 105: 15136–15141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Palmiter RD, Xia Z (2011) Loss of mitochondrial complex I activity potentiates dopamine neuron death induced by microtubule dysfunction in a Parkinson’s disease model. J Cell Biol 192: 873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey V, Safiulina D, Vaarmann A, Cagalinec M, Wareski P, Kuum M, Zharkovsky A, Kaasik A (2011) Mutant A53T alpha-synuclein induces neuronal death by increasing mitochondrial autophagy. J Biol Chem 286: 10814–10824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM (2004) S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science 304: 1328–1331 [DOI] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441: 1162–1166 [DOI] [PubMed] [Google Scholar]
- Cole NB, Dieuliis D, Leo P, Mitchell DC, Nussbaum RL (2008) Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp Cell Res 314: 2076–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR (2009) alpha-Synuclein and neuronal cell death. Mol Neurodegener 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR (2010a) DJ-1, PINK1, and their effects on mitochondrial pathways. Mov Disord 25: Suppl 1S44–S48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR (2010b) The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat Rev Neurosci 11: 791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR, Lockhart PJ, McLendon C, O’Farrell C, Schlossmacher M, Farrer MJ (2003) RING finger 1 mutations in Parkin produce altered localization of the protein. Hum Mol Genet 12: 2957–2965 [DOI] [PubMed] [Google Scholar]
- Cookson MR, van der Brug M (2008) Cell systems and the toxic mechanism(s) of alpha-synuclein. Exp Neurol 209: 5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti O, Lesage S, Brice A (2011) What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol Rev 91: 1161–1218 [DOI] [PubMed] [Google Scholar]
- Cui M, Tang X, Christian WV, Yoon Y, Tieu K (2010) Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. J Biol Chem 285: 11740–11752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa CA, Sunyach C, Giaime E, West A, Corti O, Brice A, Safe S, Abou-Sleiman PM, Wood NW, Takahashi H, Goldberg MS, Shen J, Checler F (2009) Transcriptional repression of p53 by parkin and impairment by mutations associated with autosomal recessive juvenile Parkinson’s disease. Nat Cell Biol 11: 1370–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Cherra SJ 3rd, Kulich SM, Tandon A, Park D, Chu CT (2009) Loss of PINK1 Function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem 284: 13843–13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Gusdon AM, Pien I, Strack S, Green S, Li C, Van Houten B, Cherra SJ 3rd, Chu CT (2011) Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson’s disease. Cell Death Differ 18: 1914–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher JP, Pletnikova O, Biskup S, Musso A, Gellhaar S, Galter D, Troncoso JC, Lee MK, Dawson TM, Dawson VL, Moore DJ (2012) Neurodegenerative phenotypes in an A53T alpha-synuclein transgenic mouse model are independent of LRRK2. Hum Mol Genet 21: 2420–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels V, Baekelandt V, Taymans JM (2011) On the road to leucine-rich repeat kinase 2 signalling: evidence from cellular and in vivo studies. Neurosignals 19: 1–15 [DOI] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R (2002) Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci USA 99: 14524–14529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson WS, Jonas A, Clayton DF, George JM (1998) Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem 273: 9443–9449 [DOI] [PubMed] [Google Scholar]
- Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, Kopin IJ (1979) Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res 1: 249–254 [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL (2010) The role of parkin in familial and sporadic Parkinson’s disease. Mov Disord 25: Suppl 1S32–S39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL (2010) Genetic animal models of Parkinson’s disease. Neuron 66: 646–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deas E, Plun-Favreau H, Gandhi S, Desmond H, Kjaer S, Loh SH, Renton AE, Harvey RJ, Whitworth AJ, Martins LM, Abramov AY, Wood NW (2011) PINK1 cleavage at position A103 by the mitochondrial protease PARL. Hum Mol Genet 20: 867–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deas E, Plun-Favreau H, Wood NW (2009) PINK1 function in health and disease. EMBO Mol Med 1: 152–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP (2000) Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 26: 207–210 [DOI] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M (2008a) The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci USA 105: 14503–14508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Lewis PA, Greggio E, Sluch E, Beilina A, Cookson MR (2008b) Structure of the ROC domain from the Parkinson’s disease-associated leucine-rich repeat kinase 2 reveals a dimeric GTPase. Proc Natl Acad Sci USA 105: 1499–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison SR, Callahan G, Becker NA, Phillips LA, Smith DI (2003a) Characterization of FRA6E and its potential role in autosomal recessive juvenile parkinsonism and ovarian cancer. Genes Chromosomes Cancer 38: 40–52 [DOI] [PubMed] [Google Scholar]
- Denison SR, Wang F, Becker NA, Schule B, Kock N, Phillips LA, Klein C, Smith DI (2003b) Alterations in the common fragile site gene Parkin in ovarian and other cancers. Oncogene 22: 8370–8378 [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Joazeiro CA (2009) RING domain E3 ubiquitin ligases. Annu Rev Biochem 78: 399–434 [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA 106: 13010–13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer SA, Chan DC (2007) Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8: 870–879 [DOI] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK (2008) Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283: 9089–9100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine MJ, Plun-Favreau H, Wood NW (2011) Parkinson’s disease and cancer: two wars, one front. Nat Rev Cancer 11: 812–823 [DOI] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, Dorn GW 2nd, Yin XM (2010) Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem 285: 27879–27890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Ferger B, Feldon J, Bueler H (2002) Overexpression of Parkinson’s disease-associated alpha-synucleinA53T by recombinant adeno-associated virus in mice does not increase the vulnerability of dopaminergic neurons to MPTP. J Neurobiol 53: 1–10 [DOI] [PubMed] [Google Scholar]
- Dusonchet J, Kochubey O, Stafa K, Young SM Jr, Zufferey R, Moore DJ, Schneider BL, Aebischer P (2011) A rat model of progressive nigral neurodegeneration induced by the Parkinson’s disease-associated G2019S mutation in LRRK2. J Neurosci 31: 907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, Wang L, Zuchner S, Konidari I, Wang G, Singer C, Nahab F, Scott B, Stajich JM, Pericak-Vance M, Haines J, Vance JM, Martin ER (2010) Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet 74: 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, Larsson NG (2007) Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci USA 104: 1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliezer D, Kutluay E, Bussell R Jr, Browne G (2001) Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol 307: 1061–1073 [DOI] [PubMed] [Google Scholar]
- Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barcelo-Coblijn GC, Nussbaum RL (2005) Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol Cell Biol 25: 10190–10201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K (2010) Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30: 6838–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner N, Treske B, Paquet D, Holmstrom K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken HH, Gasser T, Kruger R, Winklhofer KF, Vogel F, Reichert AS, Auburger G, Kahle PJ, Schmid B, Haass C (2007) Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci 27: 12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon L, Belanger CM, Corera AT, Kontogiannea M, Regan-Klapisz E, Moreau F, Voortman J, Haber M, Rouleau G, Thorarinsdottir T, Brice A, van Bergen En Henegouwen PM, Fon EA (2006) A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol 8: 834–842 [DOI] [PubMed] [Google Scholar]
- Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D, Langston JW (2004) Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol 55: 174–179 [DOI] [PubMed] [Google Scholar]
- Fauvet B, Kamdem MM, Fares MB, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, Eliezer D, Moore DJ, Schneider B, Aebischer P, El-Agnaf OM, Masliah E, Lashuel HA (2012) Alpha-synuclein in the central nervous system and from erythrocytes, mammalian cells and E. coli exists predominantly as a disordered monomer. J Biol Chem 287: 15345–15364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M, Vitte J, Garrigue J, Langui D, Dullin JP, Saurini F, Hanoun N, Perez-Diaz F, Cornilleau F, Joubert C, Ardila-Osorio H, Traver S, Duchateau R, Goujet-Zalc C, Paleologou K, Lashuel HA, Haass C, Duyckaerts C, Cohen-Salmon C, Kahle PJ et al. (2009) Parkin deficiency delays motor decline and disease manifestation in a mouse model of synucleinopathy. PLoS One 4: e6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Trevino I, O’Brien DE, Casey B, Goldberg MS, Tansey MG (2008) Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci 28: 10825–10834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A et al. (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet 42: 1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Diamond MI (2010) Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci 11: 155–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T (2002) alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4: 160–164 [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Marusawa H, Wang HQ, Iwai A, Ikeuchi K, Imai Y, Kataoka A, Nukina N, Takahashi R, Chiba T (2008) Parkin as a tumor suppressor gene for hepatocellular carcinoma. Oncogene 27: 6002–6011 [DOI] [PubMed] [Google Scholar]
- Fukui H, Moraes CT (2008) The mitochondrial impairment, oxidative stress and neurodegeneration connection: reality or just an attractive hypothesis? Trends Neurosci 31: 251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY (2009) PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell 33: 627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardet A, Benita Y, Li C, Sands BE, Ballester I, Stevens C, Korzenik JR, Rioux JD, Daly MJ, Xavier RJ, Podolsky DK (2010) LRRK2 is involved in the IFN-gamma response and host response to pathogens. J Immunol 185: 5577–5585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier CA, Kitada T, Shen J (2008) Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci USA 105: 11364–11369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW (2010) Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 19: 4861–4870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Schapira AH, Taanman JW (2009) Silencing of PINK1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS One 4: e4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W (2010a) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12: 119–131 [DOI] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Treis A, Skujat D, Weber SS, Fiesel FC, Kahle PJ, Springer W (2010b) The PINK1/Parkin-mediated mitophagy is compromised by PD-associated mutations. Autophagy 6: 871–878 [DOI] [PubMed] [Google Scholar]
- Gems D, Doonan R (2009) Antioxidant defense and aging in C. elegans: is the oxidative damage theory of aging wrong? Cell Cycle 8: 1681–1687 [DOI] [PubMed] [Google Scholar]
- Giasson BI, Murray IV, Trojanowski JQ, Lee VM (2001) A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem 276: 2380–2386 [DOI] [PubMed] [Google Scholar]
- Gispert S, Ricciardi F, Kurz A, Azizov M, Hoepken HH, Becker D, Voos W, Leuner K, Muller WE, Kudin AP, Kunz WS, Zimmermann A, Roeper J, Wenzel D, Jendrach M, Garcia-Arencibia M, Fernandez-Ruiz J, Huber L, Rohrer H, Barrera M et al. (2009) Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One 4: e5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, Barlowe C, Lindquist S (2008) The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci USA 105: 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL (2006) Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol 173: 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser L, Sonnay S, Stafa K, Moore DJ (2011) Parkin promotes the ubiquitination and degradation of the mitochondrial fusion factor mitofusin 1. J Neurochem 118: 636–645 [DOI] [PubMed] [Google Scholar]
- Gloeckner CJ, Boldt K, von Zweydorf F, Helm S, Wiesent L, Sarioglu H, Ueffing M (2010) Phosphopeptide analysis reveals two discrete clusters of phosphorylation in the N-terminus and the Roc domain of the Parkinson-disease associated protein kinase LRRK2. J Proteome Res 9: 1738–1745 [DOI] [PubMed] [Google Scholar]
- Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O’Neill E, Meitinger T, Kolch W, Prokisch H, Ueffing M (2006) The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet 15: 223–232 [DOI] [PubMed] [Google Scholar]
- Gloeckner CJ, Schumacher A, Boldt K, Ueffing M (2009) The Parkinson disease-associated protein kinase LRRK2 exhibits MAPKKK activity and phosphorylates MKK3/6 and MKK4/7, in vitro. J Neurochem 109: 959–968 [DOI] [PubMed] [Google Scholar]
- Gotthardt K, Weyand M, Kortholt A, Van Haastert PJ, Wittinghofer A (2008) Structure of the Roc-COR domain tandem of C. tepidum, a prokaryotic homologue of the human LRRK2 Parkinson kinase. EMBO J 27: 2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA (2012) Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep 13: 378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR (2006) Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis 23: 329–341 [DOI] [PubMed] [Google Scholar]
- Greggio E, Taymans JM, Zhen EY, Ryder J, Vancraenenbroeck R, Beilina A, Sun P, Deng J, Jaffe H, Baekelandt V, Merchant K, Cookson MR (2009) The Parkinson’s disease kinase LRRK2 autophosphorylates its GTPase domain at multiple sites. Biochem Biophys Res Commun 389: 449–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggio E, Zambrano I, Kaganovich A, Beilina A, Taymans JM, Daniels V, Lewis P, Jain S, Ding J, Syed A, Thomas KJ, Baekelandt V, Cookson MR (2008) The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J Biol Chem 283: 16906–16914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald A, Voges L, Rakovic A, Kasten M, Vandebona H, Hemmelmann C, Lohmann K, Orolicki S, Ramirez A, Schapira AH, Pramstaller PP, Sue CM, Klein C (2010) Mutant Parkin impairs mitochondrial function and morphology in human fibroblasts. PLoS One 5: e12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu WJ, Corti O, Araujo F, Hampe C, Jacquier S, Lucking CB, Abbas N, Duyckaerts C, Rooney T, Pradier L, Ruberg M, Brice A (2003) The C289G and C418R missense mutations cause rapid sequestration of human Parkin into insoluble aggregates. Neurobiol Dis 14: 357–364 [DOI] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE (2005) The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron 47: 379–393 [DOI] [PubMed] [Google Scholar]
- Hamza TH, Chen H, Hill-Burns EM, Rhodes SL, Montimurro J, Kay DM, Tenesa A, Kusel VI, Sheehan P, Eaaswarkhanth M, Yearout D, Samii A, Roberts JW, Agarwal P, Bordelon Y, Park Y, Wang L, Gao J, Vance JM, Kendler KS et al. (2011) Genome-wide gene-environment study identifies glutamate receptor gene GRIN2A as a Parkinson’s disease modifier gene via interaction with coffee. PLoS Genet 7: e1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H (2010) Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet 42: 781–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P (2011) alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest 121: 715–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Li JY (2012) Beyond alpha-synuclein transfer: pathology propagation in Parkinson’s disease. Trends Mol Med 18: 248–255 [DOI] [PubMed] [Google Scholar]
- Haque ME, Mount MP, Safarpour F, Abdel-Messih E, Callaghan S, Mazerolle C, Kitada T, Slack RS, Wallace V, Shen J, Anisman H, Park DS (2012) Inactivation of Pink1 in vivo sensitizes dopamine producing neurons to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and can be rescued by autosomal recessive PD genes, Parkin or DJ-1. J Biol Chem (advance online publication, 17 April 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque ME, Thomas KJ, D’Souza C, Callaghan S, Kitada T, Slack RS, Fraser P, Cookson MR, Tandon A, Park DS (2008) Cytoplasmic Pink1 activity protects neurons from dopaminergic neurotoxin MPTP. Proc Natl Acad Sci USA 105: 1716–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Treis A, Patenge N, Fiesel FC, Springer W, Kahle PJ (2008) Parkin protects against tyrosinase-mediated dopamine neurotoxicity by suppressing stress-activated protein kinase pathways. J Neurochem 105: 1700–1715 [DOI] [PubMed] [Google Scholar]
- Heeman B, Van den Haute C, Aelvoet SA, Valsecchi F, Rodenburg RJ, Reumers V, Debyser Z, Callewaert G, Koopman WJ, Willems PH, Baekelandt V (2011) Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J Cell Sci 124: 1115–1125 [DOI] [PubMed] [Google Scholar]
- Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4: 600–609 [DOI] [PubMed] [Google Scholar]
- Henn IH, Bouman L, Schlehe JS, Schlierf A, Schramm JE, Wegener E, Nakaso K, Culmsee C, Berninger B, Krappmann D, Tatzelt J, Winklhofer KF (2007) Parkin mediates neuroprotection through activation of IkappaB kinase/nuclear factor-kappaB signaling. J Neurosci 27: 1868–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC (2007) Animal models in neurodegenerative diseases. J Neural Transm 72: Suppl87–90 [DOI] [PubMed] [Google Scholar]
- Ho CC, Rideout HJ, Ribe E, Troy CM, Dauer WT (2009) The Parkinson disease protein leucine-rich repeat kinase 2 transduces death signals via Fas-associated protein with death domain and caspase-8 in a cellular model of neurodegeneration. J Neurosci 29: 1011–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepken HH, Gispert S, Morales B, Wingerter O, Del Turco D, Mulsch A, Nussbaum RL, Muller K, Drose S, Brandt U, Deller T, Wirth B, Kudin AP, Kunz WS, Auburger G (2007) Mitochondrial dysfunction, peroxidation damage and changes in glutathione metabolism in PARK6. Neurobiol Dis 25: 401–411 [DOI] [PubMed] [Google Scholar]
- Hristova VA, Beasley SA, Rylett RJ, Shaw GS (2009) Identification of a novel Zn2+-binding domain in the autosomal recessive juvenile Parkinson-related E3 ligase parkin. J Biol Chem 284: 14978–14986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E (2000) alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol 157: 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A (2004) Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet 364: 1169–1171 [DOI] [PubMed] [Google Scholar]
- Ikeda F, Crosetto N, Dikic I (2010) What determines the specificity and outcomes of ubiquitin signaling? Cell 143: 677–681 [DOI] [PubMed] [Google Scholar]
- Ikeuchi K, Marusawa H, Fujiwara M, Matsumoto Y, Endo Y, Watanabe T, Iwai A, Sakai Y, Takahashi R, Chiba T (2009) Attenuation of proteolysis-mediated cyclin E regulation by alternatively spliced Parkin in human colorectal cancers. Int J Cancer 125: 2029–2035 [DOI] [PubMed] [Google Scholar]
- Imai Y, Gehrke S, Wang HQ, Takahashi R, Hasegawa K, Oota E, Lu B (2008) Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J 27: 2432–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam SZ, Zhou Q, Yamamoto A, Valente AJ, Ali SF, Bains M, Roberts JL, Kahle PJ, Clark RA, Li S (2011) Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: implications for Parkinson’s disease. J Neurosci 31: 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Parkinson Disease Genomics Consortium, Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simon-Sanchez J, Schulte C, Lesage S, Sveinbjornsdottir S, Stefansson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB, Wood NW (2011) Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet 377: 641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrcher I, Aleyasin H, Seifert EL, Hewitt SJ, Chhabra S, Phillips M, Lutz AK, Rousseaux MW, Bevilacqua L, Jahani-Asl A, Callaghan S, MacLaurin JG, Winklhofer KF, Rizzu P, Rippstein P, Kim RH, Chen CX, Fon EA, Slack RS, Harper ME et al. (2010) Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet 19: 3734–3746 [DOI] [PubMed] [Google Scholar]
- Jaleel M, Nichols RJ, Deak M, Campbell DG, Gillardon F, Knebel A, Alessi DR (2007) LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. Biochem J 405: 307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ (2010) Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem 113: 1263–1274 [DOI] [PubMed] [Google Scholar]
- Jellinger KA (2009) A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta 1792: 730–740 [DOI] [PubMed] [Google Scholar]
- Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ (2010) Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 191: 933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BN, Berger AK, Cortese GP, Lavoie MJ (2012) The ubiquitin E3 ligase parkin regulates the proapoptotic function of Bax. Proc Natl Acad Sci USA 109: 6283–6288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junn E, Jang WH, Zhao X, Jeong BS, Mouradian MM (2009) Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res 87: 123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle PJ, Waak J, Gasser T (2009) DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med 47: 1354–1361 [DOI] [PubMed] [Google Scholar]
- Kamikawaji S, Ito G, Iwatsubo T (2009) Identification of the autophosphorylation sites of LRRK2. Biochemistry 48: 10963–10975 [DOI] [PubMed] [Google Scholar]
- Kamp F, Beyer K (2006) Binding of alpha-synuclein affects the lipid packing in bilayers of small vesicles. J Biol Chem 281: 9251–9259 [DOI] [PubMed] [Google Scholar]
- Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, Nuscher B, Bartels T, Giese A, Beyer K, Eimer S, Winklhofer KF, Haass C (2010) Inhibition of mitochondrial fusion by alpha-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J 29: 3571–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanao T, Venderova K, Park DS, Unterman T, Lu B, Imai Y (2010) Activation of FoxO by LRRK2 induces expression of proapoptotic proteins and alters survival of postmitotic dopaminergic neuron in Drosophila. Hum Mol Genet 19: 3747–3758 [DOI] [PubMed] [Google Scholar]
- Kawajiri S, Saiki S, Sato S, Sato F, Hatano T, Eguchi H, Hattori N (2010) PINK1 is recruited to mitochondria with parkin and associates with LC3 in mitophagy. FEBS Lett 584: 1073–1079 [DOI] [PubMed] [Google Scholar]
- Keeney PM, Xie J, Capaldi RA, Bennett JP Jr (2006) Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci 26: 5256–5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal PJ, Dumanis SB, Feng LR, Maguire-Zeiss K, Rebeck G, Lashuel HA, Moussa CE (2010) Parkinson-related parkin reduces alpha-Synuclein phosphorylation in a gene transfer model. Mol Neurodegener 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Stevens MV, Akter MH, Rusk SE, Huang RJ, Cohen A, Noguchi A, Springer D, Bocharov AV, Eggerman TL, Suen DF, Youle RJ, Amar M, Remaley AT, Sack MN (2011) Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J Clin Invest 121: 3701–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW (2005) Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A 102: 5215–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Park J, Kim S, Song S, Kwon SK, Lee SH, Kitada T, Kim JM, Chung J (2008) PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem Biophys Res Commun 377: 975–980 [DOI] [PubMed] [Google Scholar]
- Kirkin V, McEwan DG, Novak I, Dikic I (2009) A role for ubiquitin in selective autophagy. Mol Cell 34: 259–269 [DOI] [PubMed] [Google Scholar]
- Kish SJ, Morito C, Hornykiewicz O (1985) Glutathione peroxidase activity in Parkinson’s disease brain. Neurosci Lett 58: 343–346 [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392: 605–608 [DOI] [PubMed] [Google Scholar]
- Klinkenberg M, Thurow N, Gispert S, Ricciardi F, Eich F, Prehn JH, Auburger G, Kogel D (2010) Enhanced vulnerability of PARK6 patient skin fibroblasts to apoptosis induced by proteasomal stress. Neuroscience 166: 422–434 [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante RJ, Kowall NW, Abeliovich A, Beal MF (2006) Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis 21: 541–548 [DOI] [PubMed] [Google Scholar]
- Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E (2008) Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9: 505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HS, Lee Y, Shin JH, Karuppagounder SS, Gadad BS, Koleske AJ, Pletnikova O, Troncoso JC, Dawson VL, Dawson TM (2010) Phosphorylation by the c-Abl protein tyrosine kinase inhibits parkin’s ubiquitination and protective function. Proc Natl Acad Sci USA 107: 16691–16696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komander D (2009) The emerging complexity of protein ubiquitination. Biochem Soc Trans 37: 937–953 [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW (2008a) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14: 504–506 [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB (2008b) Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord 23: 2303–2306 [DOI] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 38: 518–520 [DOI] [PubMed] [Google Scholar]
- Krebiehl G, Ruckerbauer S, Burbulla LF, Kieper N, Maurer B, Waak J, Wolburg H, Gizatullina Z, Gellerich FN, Woitalla D, Riess O, Kahle PJ, Proikas-Cezanne T, Kruger R (2010) Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson’s disease-associated protein DJ-1. PLoS One 5: e9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18: 106–108 [DOI] [PubMed] [Google Scholar]
- Kruse SE, Watt WC, Marcinek DJ, Kapur RP, Schenkman KA, Palmiter RD (2008) Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metab 7: 312–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484 [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219: 979–980 [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D (1999) Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol 46: 598–605 [DOI] [PubMed] [Google Scholar]
- Lannuzel A, Michel PP, Hoglinger GU, Champy P, Jousset A, Medja F, Lombes A, Darios F, Gleye C, Laurens A, Hocquemiller R, Hirsch EC, Ruberg M (2003) The mitochondrial complex I inhibitor annonacin is toxic to mesencephalic dopaminergic neurons by impairment of energy metabolism. Neuroscience 121: 287–296 [DOI] [PubMed] [Google Scholar]
- Lansbury PT, Lashuel HA (2006) A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature 443: 774–779 [DOI] [PubMed] [Google Scholar]
- Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D (2006) Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci 26: 11915–11922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson NG (2010) Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem 79: 683–706 [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Cortese GP, Ostaszewski BL, Schlossmacher MG (2007) The effects of oxidative stress on parkin and other E3 ligases. J Neurochem 103: 2354–2368 [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ (2005) Dopamine covalently modifies and functionally inactivates parkin. Nat Med 11: 1214–1221 [DOI] [PubMed] [Google Scholar]
- Lazarou M, Jin SM, Kane LA, Youle RJ (2012) Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell 22: 320–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BD, Shin JH, VanKampen J, Petrucelli L, West AB, Ko HS, Lee YI, Maguire-Zeiss KA, Bowers WJ, Federoff HJ, Dawson VL, Dawson TM (2010a) Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson’s disease. Nat Med 16: 998–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Patel S, Lee SJ (2005) Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci 25: 6016–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP (2010b) Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol 189: 671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Kim SJ, Kim IK, Ko J, Jeong CS, Kim GH, Park C, Kang SO, Suh PG, Lee HS, Cha SS (2003) Crystal structures of human DJ-1 and Escherichia coli Hsp31, which share an evolutionarily conserved domain. J Biol Chem 278: 44552–44559 [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14: 501–503 [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Widner H, Rehncrona S, Bjorklund A, Lindvall O, Brundin P (2010) Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov Disord 25: 1091–1096 [DOI] [PubMed] [Google Scholar]
- Li WW, Yang R, Guo JC, Ren HM, Zha XL, Cheng JS, Cai DF (2007) Localization of alpha-synuclein to mitochondria within midbrain of mice. Neuroreport 18: 1543–1546 [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443: 787–795 [DOI] [PubMed] [Google Scholar]
- Lin W, Kang UJ (2008) Characterization of PINK1 processing, stability, and subcellular localization. J Neurochem 106: 464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Parisiadou L, Gu XL, Wang L, Shim H, Sun L, Xie C, Long CX, Yang WJ, Ding J, Chen ZZ, Gallant PE, Tao-Cheng JH, Rudow G, Troncoso JC, Liu Z, Li Z, Cai H (2009) Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron 64: 807–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zhang C, Yin J, Li X, Cheng F, Li Y, Yang H, Ueda K, Chan P, Yu S (2009) alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci Lett 454: 187–192 [DOI] [PubMed] [Google Scholar]
- Liu M, Dobson B, Glicksman MA, Yue Z, Stein RL (2010) Kinetic mechanistic studies of wild-type leucine-rich repeat kinase 2: characterization of the kinase and GTPase activities. Biochemistry 49: 2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Sawada T, Lee S, Yu W, Silverio G, Alapatt P, Millan I, Shen A, Saxton W, Kanao T, Takahashi R, Hattori N, Imai Y, Lu B (2012) Parkinson’s Disease-Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria. PLoS Genet 8: e1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Hamamichi S, Lee BD, Yang D, Ray A, Caldwell GA, Caldwell KA, Dawson TM, Smith WW, Dawson VL (2011a) Inhibitors of LRRK2 kinase attenuate neurodegeneration and Parkinson-like phenotypes in Caenorhabditis elegans and Drosophila Parkinson’s disease models. Hum Mol Genet 20: 3933–3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Lee J, Krummey S, Lu W, Cai H, Lenardo MJ (2011b) The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol 12: 1063–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang X, Yu Y, Li X, Wang T, Jiang H, Ren Q, Jiao Y, Sawa A, Moran T, Ross CA, Montell C, Smith WW (2008) A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci USA 105: 2693–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Bianco C, Schneider BL, Bauer M, Sajadi A, Brice A, Iwatsubo T, Aebischer P (2004) Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson’s disease. Proc Natl Acad Sci USA 101: 17510–17515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb V, Yakunin E, Saada A, Sharon R (2010) The transgenic overexpression of alpha-synuclein and not its related pathology associates with complex I inhibition. J Biol Chem 285: 7334–7343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JI, Marin I (2007) A new evolutionary paradigm for the Parkinson disease gene DJ-1. Mol Biol Evol 24: 551–561 [DOI] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM (2012) Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med 209: 975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M, Decressac M, Mattsson B, Bjorklund A (2012) Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proc Natl Acad Sci USA 109: 3213–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lammermann K, Brunner B, Kurz-Drexler A, Vogel F, Reichert AS, Bouman L, Vogt-Weisenhorn D, Wurst W, Tatzelt J, Haass C, Winklhofer KF (2009) Loss of Parkin or PINK1 Function Increases Drp1-dependent Mitochondrial Fragmentation. J Biol Chem 284: 22938–22951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandemakers W, Morais VA, De Strooper B (2007) A cell biological perspective on mitochondrial dysfunction in Parkinson disease and other neurodegenerative diseases. J Cell Sci 120: 1707–1716 [DOI] [PubMed] [Google Scholar]
- Marella M, Seo BB, Nakamaru-Ogiso E, Greenamyre JT, Matsuno-Yagi A, Yagi T (2008) Protection by the NDI1 gene against neurodegeneration in a rotenone rat model of Parkinson’s disease. PLoS One 3: e1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Dawson VL, Dawson TM (2011) Recent advances in the genetics of Parkinson’s disease. Annu Rev Genomics Hum Genet 12: 301–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK (2006) Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci 26: 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matenia D, Hempp C, Timm T, Eikhof A, Mandelkow EM (2012) Microtubule affinity regulating kinase 2 (MARK2) turns PTEN-induced kinase 1 (PINK1) on at T313, a mutation site in Parkinson’s disease: Effects on mitochondrial transport. J Biol Chem 287: 8174–8186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189: 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi SJ, Alam MS, Batra S, Rizvi MM (2011) Allelic loss of 6q25–27, the PARKIN tumor suppressor gene locus, in cervical carcinoma. Med Oncol 28: 1520–1526 [DOI] [PubMed] [Google Scholar]
- Meissner C, Lorenz H, Weihofen A, Selkoe DJ, Lemberg MK (2011) The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking. J Neurochem 117: 856–867 [DOI] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O (2008) Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med 14: 507–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulener MC, Xu K, Thomson L, Ischiropoulos H, Bonini NM (2006) Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. Proc Natl Acad Sci USA 103: 12517–12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH (2010) Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 30: 4232–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsumoto A, Nakagawa Y, Takeuchi A, Okawa K, Iwamatsu A, Takanezawa Y (2001) Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic Res 35: 301–310 [DOI] [PubMed] [Google Scholar]
- Moore DJ (2006) Parkin: a multifaceted ubiquitin ligase. Biochem Soc Trans 34: 749–753 [DOI] [PubMed] [Google Scholar]
- Morais VA, Verstreken P, Roethig A, Smet J, Snellinx A, Vanbrabant M, Haddad D, Frezza C, Mandemakers W, Vogt-Weisenhorn D, Van Coster R, Wurst W, Scorrano L, De Strooper B (2009) Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med 1: 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortiboys H, Johansen KK, Aasly JO, Bandmann O (2010) Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology 75: 2017–2020 [DOI] [PubMed] [Google Scholar]
- Mortiboys H, Thomas KJ, Koopman WJ, Klaffke S, Abou-Sleiman P, Olpin S, Wood NW, Willems PH, Smeitink JA, Cookson MR, Bandmann O (2008) Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann Neurol 64: 555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muqit MM, Abou-Sleiman PM, Saurin AT, Harvey K, Gandhi S, Deas E, Eaton S, Payne Smith MD, Venner K, Matilla A, Healy DG, Gilks WP, Lees AJ, Holton J, Revesz T, Parker PJ, Harvey RJ, Wood NW, Latchman DS (2006) Altered cleavage and localization of PINK1 to aggresomes in the presence of proteasomal stress. J Neurochem 98: 156–169 [DOI] [PubMed] [Google Scholar]
- Murata H, Sakaguchi M, Jin Y, Sakaguchi Y, Futami J, Yamada H, Kataoka K, Huh NH (2011) A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: activation of AKT via mTORC2. J Biol Chem 286: 7182–7189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J, Gardner B, Wakabayashi J, Sesaki H, Cheng Y, Finkbeiner S, Nussbaum RL, Masliah E, Edwards RH (2011) Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem 286: 20710–20726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Nemani VM, Wallender EK, Kaehlcke K, Ott M, Edwards RH (2008) Optical reporters for the conformation of alpha-synuclein reveal a specific interaction with mitochondria. J Neurosci 28: 12305–12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Kane LA, Hauser DN, Fearnley IM, Youle RJ (2010a) p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 6: 1090–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183: 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ (2010b) PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8: e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Kane LA, Hauser DN, Fearnley IM, Youle RJ (2010c) p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 6: 1090–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH (2010) Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65: 66–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CH, Mok SZ, Koh C, Ouyang X, Fivaz ML, Tan EK, Dawson VL, Dawson TM, Yu F, Lim KL (2009) Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila. J Neurosci 29: 11257–11262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE (1985) Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci 36: 2503–2508 [DOI] [PubMed] [Google Scholar]
- Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C (2010) Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum Mutat 31: 763–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettinghaus B, Licci M, Scorrano L, Frank S (2012) Less than perfect divorces: dysregulated mitochondrial fission and neurodegeneration. Acta Neuropathol 123: 189–203 [DOI] [PubMed] [Google Scholar]
- Okatsu K, Saisho K, Shimanuki M, Nakada K, Shitara H, Sou YS, Kimura M, Sato S, Hattori N, Komatsu M, Tanaka K, Matsuda N (2010) p62/SQSTM1 cooperates with Parkin for perinuclear clustering of depolarized mitochondria. Genes Cells 15: 887–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi M, Walter J, Koyama A, Nakajo S, Baba M, Iwatsubo T, Meijer L, Kahle PJ, Haass C (2000) Constitutive phosphorylation of the Parkinson’s disease associated alpha-synuclein. J Biol Chem 275: 390–397 [DOI] [PubMed] [Google Scholar]
- Orsucci D, Caldarazzo Ienco E, Mancuso M, Siciliano G (2011) POLG1-related and other ‘mitochondrial Parkinsonisms’: an overview. J Mol Neurosci 44: 17–24 [DOI] [PubMed] [Google Scholar]
- Orth M, Tabrizi SJ, Schapira AH, Cooper JM (2003) Alpha-synuclein expression in HEK293 cells enhances the mitochondrial sensitivity to rotenone. Neurosci Lett 351: 29–32 [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44: 595–600 [DOI] [PubMed] [Google Scholar]
- Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P (2008) Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci 65: 1272–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisiadou L, Xie C, Cho HJ, Lin X, Gu XL, Long CX, Lobbestael E, Baekelandt V, Taymans JM, Sun L, Cai H (2009) Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J Neurosci 29: 13971–13980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CB, Larsson NG (2011) Mitochondrial DNA mutations in disease and aging. J Cell Biol 193: 809–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J (2006) Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441: 1157–1161 [DOI] [PubMed] [Google Scholar]
- Park JY, Kim KS, Lee SB, Ryu JS, Chung KC, Choo YK, Jou I, Kim J, Park SM (2009) On the mechanism of internalization of alpha-synuclein into microglia: roles of ganglioside GM1 and lipid raft. J Neurochem 110: 400–411 [DOI] [PubMed] [Google Scholar]
- Parker WD Jr., Parks JK, Swerdlow RH (2008) Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res 1189: 215–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterna JC, Leng A, Weber E, Feldon J, Bueler H (2007) DJ-1 and Parkin modulate dopamine-dependent behavior and inhibit MPTP-induced nigral dopamine neuron loss in mice. Mol Ther 15: 698–704 [DOI] [PubMed] [Google Scholar]
- Pawlyk AC, Giasson BI, Sampathu DM, Perez FA, Lim KL, Dawson VL, Dawson TM, Palmiter RD, Trojanowski JQ, Lee VM (2003) Novel monoclonal antibodies demonstrate biochemical variation of brain parkin with age. J Biol Chem 278: 48120–48128 [DOI] [PubMed] [Google Scholar]
- Perez FA, Curtis WR, Palmiter RD (2005) Parkin-deficient mice are not more sensitive to 6-hydroxydopamine or methamphetamine neurotoxicity. BMC Neurosci 6: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M, Chen F, Gu Y, Hasegawa H, Salehi-Rad S, Wang L, Rogaeva E, Fraser P, Robinson B, St George-Hyslop P, Tandon A (2005) Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem 280: 34025–34032 [DOI] [PubMed] [Google Scholar]
- Petrucelli L, O’Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, Choi P, Wolozin B, Farrer M, Hardy J, Cookson MR (2002) Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron 36: 1007–1019 [DOI] [PubMed] [Google Scholar]
- Picchio MC, Martin ES, Cesari R, Calin GA, Yendamuri S, Kuroki T, Pentimalli F, Sarti M, Yoder K, Kaiser LR, Fishel R, Croce CM (2004) Alterations of the tumor suppressor gene Parkin in non-small cell lung cancer. Clin Cancer Res 10: 2720–2724 [DOI] [PubMed] [Google Scholar]
- Piccoli C, Sardanelli A, Scrima R, Ripoli M, Quarato G, D’Aprile A, Bellomo F, Scacco S, De Michele G, Filla A, Iuso A, Boffoli D, Capitanio N, Papa S (2008) Mitochondrial respiratory dysfunction in familiar parkinsonism associated with PINK1 mutation. Neurochem Res 33: 2565–2574 [DOI] [PubMed] [Google Scholar]
- Pickrell AM, Pinto M, Hida A, Moraes CT (2011) Striatal dysfunctions associated with mitochondrial DNA damage in dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurosci 31: 17649–17658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsl A, Winklhofer KF (2012) Parkin, PINK1 and mitochondrial integrity: emerging concepts of mitochondrial dysfunction in Parkinson’s disease. Acta Neuropathol 123: 173–188 [DOI] [PubMed] [Google Scholar]
- Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, Harvey K, Deas E, Harvey RJ, McDonald N, Wood NW, Martins LM, Downward J (2007) The mitochondrial protease HtrA2 is regulated by Parkinson’s disease-associated kinase PINK1. Nat Cell Biol 9: 1243–1252 [DOI] [PubMed] [Google Scholar]
- Plun-Favreau H, Lewis PA, Hardy J, Martins LM, Wood NW (2010) Cancer and neurodegeneration: between the devil and the deep blue sea. PLoS Genet 6: e1001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276: 2045–2047 [DOI] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ (2008) The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA 105: 1638–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulogiannis G, McIntyre RE, Dimitriadi M, Apps JR, Wilson CH, Ichimura K, Luo F, Cantley LC, Wyllie AH, Adams DJ, Arends MJ (2010) PARK2 deletions occur frequently in sporadic colorectal cancer and accelerate adenoma development in Apc mutant mice. Proc Natl Acad Sci USA 107: 15145–15150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L (2007) PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol 5: e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Kruse SE, Kapur RP, Sanz E, Palmiter RD (2010) Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc Natl Acad Sci USA 107: 10996–11001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramonet D, Daher JP, Lin BM, Stafa K, Kim J, Banerjee R, Westerlund M, Pletnikova O, Glauser L, Yang L, Liu Y, Swing DA, Beal MF, Troncoso JC, McCaffery JM, Jenkins NA, Copeland NG, Galter D, Thomas B, Lee MK et al. (2011) Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One 6: e18568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathke-Hartlieb S, Kahle PJ, Neumann M, Ozmen L, Haid S, Okochi M, Haass C, Schulz JB (2001) Sensitivity to MPTP is not increased in Parkinson’s disease-associated mutant alpha-synuclein transgenic mice. J Neurochem 77: 1181–1184 [DOI] [PubMed] [Google Scholar]
- Reeve AK, Krishnan KJ, Turnbull D (2008) Mitochondrial DNA mutations in disease, aging, and neurodegeneration. Ann NY Acad Sci 1147: 21–29 [DOI] [PubMed] [Google Scholar]
- Ren Y, Liu W, Jiang H, Jiang Q, Feng J (2005) Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J Biol Chem 280: 34105–34112 [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Guillot TS, Watson JL, Nakamaru-Ogiso E, Seo BB, Sherer TB, Greenamyre JT, Yagi T, Matsuno-Yagi A, Miller GW (2007) ) Obligatory role for complex I inhibition in the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Toxicol Sci 95: 196–204 [DOI] [PubMed] [Google Scholar]
- Rosen KM, Veereshwarayya V, Moussa CE, Fu Q, Goldberg MS, Schlossmacher MG, Shen J, Querfurth HW (2006) Parkin protects against mitochondrial toxins and beta-amyloid accumulation in skeletal muscle cells. J Biol Chem 281: 12809–12816 [DOI] [PubMed] [Google Scholar]
- Sandebring A, Thomas KJ, Beilina A, van der Brug M, Cleland MM, Ahmad R, Miller DW, Zambrano I, Cowburn RF, Behbahani H, Cedazo-Minguez A, Cookson MR (2009) Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS One 4: e5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M et al. (2009) Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet 41: 1303–1307 [DOI] [PubMed] [Google Scholar]
- Scarpulla RC (2011) Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 1813: 1269–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH (2008) Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol 7: 97–109 [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1: 1269. [DOI] [PubMed] [Google Scholar]
- Schlehe JS, Lutz AK, Pilsl A, Lammermann K, Grgur K, Henn IH, Tatzelt J, Winklhofer KF (2008) Aberrant folding of pathogenic Parkin mutants: aggregation versus degradation. J Biol Chem 283: 13771–13779 [DOI] [PubMed] [Google Scholar]
- Schluter OM, Fornai F, Alessandri MG, Takamori S, Geppert M, Jahn R, Sudhof TC (2003) Role of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Neuroscience 118: 985–1002 [DOI] [PubMed] [Google Scholar]
- Schon EA, Przedborski S (2011) Mitochondria: the next (neurode)generation. Neuron 70: 1033–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D (2011) Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci 31: 5970–5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Webber PJ, West AB (2009) Dependence of leucine-rich repeat kinase 2 (LRRK2) kinase activity on dimerization. J Biol Chem 284: 36346–36356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BB, Nakamaru-Ogiso E, Flotte TR, Matsuno-Yagi A, Yagi T (2006) In vivo complementation of complex I by the yeast Ndi1 enzyme. Possible application for treatment of Parkinson disease. J Biol Chem 281: 14250–14255 [DOI] [PubMed] [Google Scholar]
- Sha D, Chin LS, Li L (2010) Phosphorylation of parkin by Parkinson disease-linked kinase PINK1 activates parkin E3 ligase function and NF-kappaB signaling. Hum Mol Genet 19: 352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Burre J, Sudhof TC (2011) CSPalpha promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nat Cell Biol 13: 30–39 [DOI] [PubMed] [Google Scholar]
- Shavali S, Brown-Borg HM, Ebadi M, Porter J (2008) Mitochondrial localization of alpha-synuclein protein in alpha-synuclein overexpressing cells. Neurosci Lett 439: 125–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT (2003) Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci 23: 10756–10764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Lee JR, Grimes DA, Racacho L, Ye D, Yang H, Ross OA, Farrer M, McQuibban GA, Bulman DE (2011) Functional alteration of PARL contributes to mitochondrial dysregulation in Parkinson’s disease. Hum Mol Genet 20: 1966–1974 [DOI] [PubMed] [Google Scholar]
- Shiba K, Arai T, Sato S, Kubo S, Ohba Y, Mizuno Y, Hattori N (2009) Parkin stabilizes PINK1 through direct interaction. Biochem Biophys Res Commun 383: 331–335 [DOI] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25: 302–305 [DOI] [PubMed] [Google Scholar]
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM (2011) PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell 144: 689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6: 193–222 [DOI] [PubMed] [Google Scholar]
- Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, Casari G (2005) Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet 14: 3477–3492 [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H et al. (2009) Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet 41: 1308–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J et al. (2003) alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302: 841. [DOI] [PubMed] [Google Scholar]
- Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA (2006) Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci 9: 1231–1233 [DOI] [PubMed] [Google Scholar]
- Song DD, Shults CW, Sisk A, Rockenstein E, Masliah E (2004) Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp Neurol 186: 158–172 [DOI] [PubMed] [Google Scholar]
- Soubannier V, McBride HM (2009) Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta 1793: 154–170 [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388: 839–840 [DOI] [PubMed] [Google Scholar]
- Sriram SR, Li X, Ko HS, Chung KK, Wong E, Lim KL, Dawson VL, Dawson TM (2005) Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum Mol Genet 14: 2571–2586 [DOI] [PubMed] [Google Scholar]
- Stafa K, Trancikova A, Webber PJ, Glauser L, West AB, Moore DJ (2012) GTPase activity and neuronal toxicity of Parkinson’s disease-associated LRRK2 is regulated by ArfGAP1. PLoS Genet 8: e1002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA (2008) The role of mitochondria in reactive oxygen species metabolism and signaling. Ann NY Acad Sci 1147: 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staropoli JF, McDermott C, Martinat C, Schulman B, Demireva E, Abeliovich A (2003) Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron 37: 735–749 [DOI] [PubMed] [Google Scholar]
- Steiner JA, Angot E, Brundin P (2011) A deadly spread: cellular mechanisms of alpha-synuclein transfer. Cell Death Differ 18: 1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterky FH, Hoffman AF, Milenkovic D, Bao B, Paganelli A, Edgar D, Wibom R, Lupica CR, Olson L, Larsson NG (2012) Altered dopamine metabolism and increased vulnerability to MPTP in mice with partial deficiency of mitochondrial complex I in dopamine neurons. Hum Mol Genet 21: 1078–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterky FH, Lee S, Wibom R, Olson L, Larsson NG (2011) Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc Natl Acad Sci USA 108: 12937–12942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stichel CC, Zhu XR, Bader V, Linnartz B, Schmidt S, Lubbert H (2007) Mono- and double-mutant mouse models of Parkinson’s disease display severe mitochondrial damage. Hum Mol Genet 16: 2377–2393 [DOI] [PubMed] [Google Scholar]
- Su LJ, Auluck PK, Outeiro TF, Yeger-Lotem E, Kritzer JA, Tardiff DF, Strathearn KE, Liu F, Cao S, Hamamichi S, Hill KJ, Caldwell KA, Bell GW, Fraenkel E, Cooper AA, Caldwell GA, McCaffery JM, Rochet JC, Lindquist S (2010) Compounds from an unbiased chemical screen reverse both ER-to-Golgi trafficking defects and mitochondrial dysfunction in Parkinson’s disease models. Dis Model Mech 3: 194–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen DF, Narendra DP, Tanaka A, Manfredi G, Youle RJ (2010) Parkin overexpression selects against a deleterious mtDNA mutation in heteroplasmic cybrid cells. Proc Natl Acad Sci USA 107: 11835–11840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D (2010) Clues to how alpha-synuclein damages neurons in Parkinson’s disease. Mov Disord 25: Suppl 1S27–S31 [DOI] [PubMed] [Google Scholar]
- Sung JY, Kim J, Paik SR, Park JH, Ahn YS, Chung KC (2001) Induction of neuronal cell death by Rab5A-dependent endocytosis of alpha-synuclein. J Biol Chem 276: 27441–27448 [DOI] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H (2004) DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep 5: 213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ (2010) Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol 191: 1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay SP, Yeo CW, Chai C, Chua PJ, Tan HM, Ang AX, Yip DL, Sung JX, Tan PH, Bay BH, Wong SH, Tang C, Tan JM, Lim KL (2010) Parkin enhances the expression of cyclin-dependent kinase 6 and negatively regulates the proliferation of breast cancer cells. J Biol Chem 285: 29231–29238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B, von Coelln R, Mandir AS, Trinkaus DB, Farah MH, Leong Lim K, Calingasan NY, Flint Beal M, Dawson VL, Dawson TM (2007) MPTP and DSP-4 susceptibility of substantia nigra and locus coeruleus catecholaminergic neurons in mice is independent of parkin activity. Neurobiol Dis 26: 312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KJ, McCoy MK, Blackinton J, Beilina A, van der Brug M, Sandebring A, Miller D, Maric D, Cedazo-Minguez A, Cookson MR (2011) DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum Mol Genet 20: 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofaris GK, Garcia Reitbock P, Humby T, Lambourne SL, O’Connell M, Ghetti B, Gossage H, Emson PC, Wilkinson LS, Goedert M, Spillantini MG (2006) Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1–120): implications for Lewy body disorders. J Neurosci 26: 3942–3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Hansson A, Wredenberg A, Rovio AT, Dufour E, Khvorostov I, Spelbrink JN, Wibom R, Jacobs HT, Larsson NG (2005) Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci USA 102: 17993–17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG (2004) Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423 [DOI] [PubMed] [Google Scholar]
- Tsika E, Moore DJ (2012) Mechanisms of LRRK2-mediated neurodegeneration. Curr Neurol Neurosci Rep 12: 251–260 [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW, Stichel-Gunkel C, Lubbert H, Lee G, Chung KC (2009) Molecular interaction between parkin and PINK1 in mammalian neuronal cells. Mol Cell Neurosci 40: 421–432 [DOI] [PubMed] [Google Scholar]
- Uversky VN (2010) Mysterious oligomerization of the amyloidogenic proteins. FEBS J 277: 2940–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B et al. (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304: 1158–1160 [DOI] [PubMed] [Google Scholar]
- Van Humbeeck C, Cornelissen T, Hofkens H, Mandemakers W, Gevaert K, De Strooper B, Vandenberghe W (2011) Parkin interacts with ambra1 to induce mitophagy. J Neurosci 31: 10249–10261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar VS, Arnold B, Cassady SJ, Chu CT, Burton EA, Berman SB (2011) Bioenergetics of neurons inhibit the translocation response of Parkin following rapid mitochondrial depolarization. Hum Mol Genet 20: 927–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar VS, Berman SB (2009) Mitochondrial dynamics in Parkinson’s disease. Exp Neurol 218: 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeriah S, Taylor BS, Meng S, Fang F, Yilmaz E, Vivanco I, Janakiraman M, Schultz N, Hanrahan AJ, Pao W, Ladanyi M, Sander C, Heguy A, Holland EC, Paty PB, Mischel PS, Liau L, Cloughesy TF, Mellinghoff IK, Solit DB et al. (2010) Somatic mutations of the Parkinson’s disease-associated gene PARK2 in glioblastoma and other human malignancies. Nat Genet 42: 77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L (2011) Pathological roles of alpha-synuclein in neurological disorders. Lancet Neurol 10: 1015–1025 [DOI] [PubMed] [Google Scholar]
- Venda LL, Cragg SJ, Buchman VL, Wade-Martins R (2010) alpha-Synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci 33: 559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila M, Ramonet D, Perier C (2008) Mitochondrial alterations in Parkinson’s disease: new clues. J Neurochem 107: 317–328 [DOI] [PubMed] [Google Scholar]
- Vilain S, Esposito G, Haddad D, Schaap O, Dobreva MP, Vos M, Van Meensel S, Morais VA, De Strooper B, Verstreken P (2012) The yeast complex I equivalent NADH dehydrogenase rescues pink1 mutants. PLoS Genet 8: e1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrane J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S (2010) PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA 107: 378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT Jr. (2003) Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry 42: 7871–7878 [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM (2011) Exogenous alpha-Synuclein Fibrils Induce Lewy Body Pathology Leading to Synaptic Dysfunction and Neuron Death. Neuron 72: 57–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos M, Esposito G, Edirisinghe JN, Vilain S, Haddad DM, Slabbaert JR, Van Meensel S, Schaap O, De Strooper B, Meganathan R, Morais VA, Verstreken P (2012) Vitamin K2 Is a mitochondrial electron carrier that rescues pink1 deficiency. Science 336: 1306–1310 [DOI] [PubMed] [Google Scholar]
- Wang C, Ko HS, Thomas B, Tsang F, Chew KC, Tay SP, Ho MW, Lim TM, Soong TW, Pletnikova O, Troncoso J, Dawson VL, Dawson TM, Lim KL (2005a) Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin’s protective function. Hum Mol Genet 14: 3885–3897 [DOI] [PubMed] [Google Scholar]
- Wang C, Tan JM, Ho MW, Zaiden N, Wong SH, Chew CL, Eng PW, Lim TM, Dawson TM, Lim KL (2005b) Alterations in the solubility and intracellular localization of parkin by several familial Parkinson’s disease-linked point mutations. J Neurochem 93: 422–431 [DOI] [PubMed] [Google Scholar]
- Wang F, Denison S, Lai JP, Philips LA, Montoya D, Kock N, Schule B, Klein C, Shridhar V, Roberts LR, Smith DI (2004) Parkin gene alterations in hepatocellular carcinoma. Genes Chromosomes Cancer 40: 85–96 [DOI] [PubMed] [Google Scholar]
- Wang H, Song P, Du L, Tian W, Yue W, Liu M, Li D, Wang B, Zhu Y, Cao C, Zhou J, Chen Q (2011a) Parkin ubiquitinates Drp1 for proteasome-dependent degradation: implication of dysregulated mitochondrial dynamics in Parkinson disease. J Biol Chem 286: 11649–11658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Chou AH, Wu AS, Chen SY, Weng YH, Kao YC, Yeh TH, Chu PJ, Lu CS (2011b) PARK6 PINK1 mutants are defective in maintaining mitochondrial membrane potential and inhibiting ROS formation of substantia nigra dopaminergic neurons. Biochim Biophys Acta 1812: 674–684 [DOI] [PubMed] [Google Scholar]
- Wang J, Silva JP, Gustafsson CM, Rustin P, Larsson NG (2001) Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc Natl Acad Sci USA 98: 4038–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, Liao J, Auclair JR, Johnson D, Landeru A, Simorellis AK, Ju S, Cookson MR, Asturias FJ, Agar JN, Webb BN, Kang C, Ringe D, Petsko GA, Pochapsky TC, Hoang QQ (2011c) A soluble alpha-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA 108: 17797–17802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL (2011d) PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147: 893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yan MH, Fujioka H, Liu J, Wilson-Delfosse A, Chen SG, Perry G, Casadesus G, Zhu X (2012) LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet 21: 1931–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Giasson BI (2009) Molecular mechanisms of alpha-synuclein neurodegeneration. Biochim Biophys Acta 1792: 616–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber PJ, Smith AD, Sen S, Renfrow MB, Mobley JA, West AB (2011) Autophosphorylation in the leucine-rich repeat kinase 2 (LRRK2) GTPase domain modifies kinase and GTP-binding activities. J Mol Biol 412: 94–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ (2009) Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry 48: 2045–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel DM, Lissounov A, Brzovic PS, Klevit RE (2011) UBCH7 reactivity profile reveals parkin and HHARI to be RING/HECT hybrids. Nature 474: 105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Kapatos G, O’Farrell C, Gonzalez-de-Chavez F, Chiu K, Farrer MJ, Maidment NT (2004) N-myc regulates parkin expression. J Biol Chem 279: 28896–28902 [DOI] [PubMed] [Google Scholar]
- West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM (2005) Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA 102: 16842–16847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Moore DJ, Choi C, Andrabi SA, Li X, Dikeman D, Biskup S, Zhang Z, Lim KL, Dawson VL, Dawson TM (2007) Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet 16: 223–232 [DOI] [PubMed] [Google Scholar]
- Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11: 872–884 [DOI] [PubMed] [Google Scholar]
- Whitworth AJ, Lee JR, Ho VM, Flick R, Chowdhury R, McQuibban GA (2008) Rhomboid-7 and HtrA2/Omi act in a common pathway with the Parkinson’s disease factors Pink1 and Parkin. Dis Model Mech 1: 168–174discussion 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Collins JL, Hod Y, Ringe D, Petsko GA (2003) The 1.1-A resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early onset Parkinson’s disease. Proc Natl Acad Sci USA 100: 9256–9261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklhofer KF, Haass C (2010) Mitochondrial dysfunction in Parkinson’s disease. Biochim Biophys Acta 1802: 29–44 [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Henn IH, Kay-Jackson PC, Heller U, Tatzelt J (2003) Inactivation of parkin by oxidative stress and C-terminal truncations: a protective role of molecular chaperones. J Biol Chem 278: 47199–47208 [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Tatzelt J, Haass C (2008) The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J 27: 336–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, Brown S, O’Kane CJ, Rubinsztein DC (2010) alpha-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol 190: 1023–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ES, Tan JM, Wang C, Zhang Z, Tay SP, Zaiden N, Ko HS, Dawson VL, Dawson TM, Lim KL (2007) Relative sensitivity of parkin and other cysteine-containing enzymes to stress-induced solubility alterations. J Biol Chem 282: 12310–12318 [DOI] [PubMed] [Google Scholar]
- Wood-Kaczmar A, Gandhi S, Yao Z, Abramov AY, Miljan EA, Keen G, Stanyer L, Hargreaves I, Klupsch K, Deas E, Downward J, Mansfield L, Jat P, Taylor J, Heales S, Duchen MR, Latchman D, Tabrizi SJ, Wood NW (2008) PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS One 3: e2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Wang D, Chen L, Choo YS, Ma H, Tang C, Xia K, Jiang W, Ronai Z, Zhuang X, Zhang Z (2009) Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J Clin Invest 119: 650–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Yuan C, Chen R, Dawson TM, Dawson VL (2012) ArfGAP1 Is a GTPase activating protein for LRRK2: reciprocal regulation of ArfGAP1 by LRRK2. J Neurosci 32: 3877–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadava N, Nicholls DG (2007) Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci 27: 7310–7317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S, Takahashi R, Lu B (2005) Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci USA 102: 13670–13675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B (2006) Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA 103: 10793–10798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Nishimura I, Imai Y, Takahashi R, Lu B (2003) Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron 37: 911–924 [DOI] [PubMed] [Google Scholar]
- Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B (2008) Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA 105: 7070–7075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, Palmer LA, Rockenstein EM, Zhang Z, Masliah E, Uehara T, Lipton SA (2004) Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci USA 101: 10810–10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Miyachi S, Kitagawa R, Wada K, Nihira T, Ren YR, Hirai Y, Ageyama N, Terao K, Shimada T, Takada M, Mizuno Y, Mochizuki H (2007) Neuronal specificity of alpha-synuclein toxicity and effect of Parkin co-expression in primates. Neuroscience 144: 743–753 [DOI] [PubMed] [Google Scholar]
- Yavich L, Tanila H, Vepsalainen S, Jakala P (2004) Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci 24: 11165–11170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo CW, Ng FS, Chai C, Tan J, Koh GR, Yuk Kien C, Koh LW, Foong CS, Sandanaraj E, Holbrook JD, Ang BT, Takahashi R, Tang C, Lim KL (2012) Parkin pathway activation mitigates glioma cell proliferation and predicts patient survival. Cancer Res 72: 2543–2553 [DOI] [PubMed] [Google Scholar]
- Yoshii SR, Kishi C, Ishihara N, Mizushima N (2011) Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J Biol Chem 286: 19630–19640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan XL, Guo JF, Shi ZH, Xiao ZQ, Yan XX, Zhao BL, Tang BS (2010) R492X mutation in PTEN-induced putative kinase 1 induced cellular mitochondrial dysfunction and oxidative stress. Brain Res 1351: 229–237 [DOI] [PubMed] [Google Scholar]
- Zach S, Felk S, Gillardon F (2010) Signal transduction protein array analysis links LRRK2 to Ste20 kinases and PKC zeta that modulate neuronal plasticity. PLoS One 5: e13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55: 164–173 [DOI] [PubMed] [Google Scholar]
- Zhang C, Lin M, Wu R, Wang X, Yang B, Levine AJ, Hu W, Feng Z (2011) Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci USA 108: 16259–16264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, Torp R, Torgner IA, Ottersen OP, Dawson TM, Dawson VL (2005) Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet 14: 2063–2073 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang C, Zhu Y, Cai Q, Chan P, Ueda K, Yu S, Yang H (2008) Semi-quantitative analysis of alpha-synuclein in subcellular pools of rat brain neurons: an immunogold electron microscopic study using a C-terminal specific monoclonal antibody. Brain Res 1244: 40–52 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM (2000) Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci USA 97: 13354–13359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44: 601–607 [DOI] [PubMed] [Google Scholar]
- Ziviani E, Tao RN, Whitworth AJ (2010) Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci USA 107: 5018–5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, Parman Y, Evgrafov O, Jonghe PD, Takahashi Y, Tsuji S, Pericak-Vance MA, Quattrone A, Battaloglu E, Polyakov AV, Timmerman V et al. (2004) Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet 36: 449–451 [DOI] [PubMed] [Google Scholar]