Abstract
Because of advances in our understanding of the hypereosinophilic syndrome (HES) and the availability of novel therapeutic agents, the original criteria defining these disorders are becoming increasingly problematic. Here, we discuss shortcomings with the current definition of HES and recent developments in the classification of these disorders. Despite significant progress in our understanding of the pathogenesis of some forms of HES, the current state of knowledge is still insufficient to formulate a new comprehensive etiologic definition of HESs. Nevertheless, we suggest a new working definition that overcomes some of the most obvious limitations with the original definition.
Keywords: Definition, eosinophilia, eosinophilic leukemia, hypereosinophilic syndromes
The hypereosinophilic syndrome (HES) is characterized by the presence of marked unexplained blood and tissue eosinophilia associated with a variety of clinical manifestations. Since 1975, 3 criteria have been used to define HES: (1) blood eosinophilia ≥1500/mm3 for longer than 6 months (or death before 6 months associated with signs and symptoms of hypereosinophilic disease), (2) lack of evidence for parasitic, allergic, or other known causes of eosinophilia, and (3) presumptive signs of organ involvement, such as heart failure, gastrointestinal dysfunction, central nervous system abnormalities, fever, or weight loss.1
LIMITATIONS OF THE CURRENT DEFINITION OF HES
As new and varied diagnostic and treatment modalities have become available, appropriate clinical management of eosinophilic disorders has become increasingly dependent on the accurate identification and classification of patients. In this context, the criteria established by Chusid et al1 in 1975 are becoming increasingly problematic. For instance, it is unlikely that a patient with symptomatic HES would remain untreated for 6 months given the availability of effective therapies that can reduce eosinophilia before irreversible damage occurs. Similarly, the accepted threshold for blood eosinophilia excludes patients with increased numbers of activated eosinophils in tissues unaccompanied by marked blood eosinophilia, although the pathophysiology in such patients is likely similar to those with peripheral eosinophilia ≥1500/mm3. For example, patients with a wide variety of disorders, including eosinophilic pneumonia, eosinophil-associated gastrointestinal disorders (EGID), Churg-Strauss syndrome (CSS), nasal polyposis with bronchial asthma, and eosinophilic dermatitis (Wells syndrome), may fulfill all HES criteria except for blood eosinophil levels, yet are not classified as HES. Conversely, some patients with blood eosinophilia ≥1500/mm3 do not exhibit signs of eosinophil-mediated organ damage and/or dysfunction at the time of presentation or present with signs and symptoms, such as asthma or allergic rhinitis, for which the relationship to the eosinophilia is unclear. Although such patients would not meet the criteria of Chusid et al,1 they warrant special attention with regard to the potential development of disease complications. Finally, pathogenic studies of patient subgroups fulfilling the diagnostic criteria of Chusid et al1 have led to the identification of several well characterized disease entities, such as Fip1-like-1 (FIP1L1)/platelet-derived growth factor receptor α (PDGFRA)–associated HES2 that no longer fit into the original definition of HES as a disease of unknown pathophysiology. In conclusion, adjustments to the original definition appear to be required to ensure optimal patient treatment.
CLINICAL CLASSIFICATION OF HESs
The Hypereosinophilic Diseases Working Group of the International Eosinophil Society proposed a classification algorithm that addressed some of the issues raised.3 The term “hypereosinophilic syndromes (HESs)” was introduced without the use of “idiopathic” in order to capture the broad range of disorders included in the original definition with 1 classification scheme.3 Moreover, organ-restricted hypereosinophilic disorders when accompanied by blood eosinophilia ≥1500/mm3 as well as CSS and isolated blood eosinophilia ≥1500/mm3 were included in the new classification scheme of HESs.3 Accordingly, it was concluded that all patients with blood eosinophilia ≥1500/mm3 without a discernable secondary cause (eg, allergic diseases, drug hypersensitivity, parasitic helminth infections, HIV infection, nonhematologic malignancies) should be considered to have HES or a disorder that overlaps in definition with HES, regardless of the number and nature of affected organs or potential pathogenic mechanisms.
Although the new classification addressed some of the shortcomings of the criteria used in the original definition of HES, a number of issues remain. For instance, the current state of knowledge makes it difficult to classify patients on the basis of etiology. Consequently, the classification scheme proposed in 2006 was based predominantly on clinical phenotype and included the following categories: myeloproliferative, lymphocytic, familial, idiopathic, overlap (blood eosinophilia ≥1500/mm3 in the setting of single organ involvement), and associated (blood eosinophilia ≥1500/mm3 in association with a distinct second diagnosis, such as inflammatory bowel disease or autoimmune lymphoproliferative disorder, in which eosinophilia has been reported with increased frequency but rarely leads to end organ manifestations).3 Although this scheme has been helpful in guiding management and predicting prognosis for some patients, the majority of cases fall under the “idiopathic” heading, meaning that pathogenesis and disease course remain unknown and are likely heterogeneous. In addition, future investigations may reveal novel disease mechanisms that could be difficult to incorporate into the current scheme.
WORLD HEALTH ORGANIZATION CLASSIFICATION OF HESs
In the revised World Health Organization (WHO) classification, patients presenting with signs and symptoms of HES can be classified in 1 of 4 ways: (1) myeloproliferative neoplasm/HES, (2) myeloproliferative syndrome/chronic eosinophilic leukemia, not otherwise categorized, (3) myeloid neoplasm associated with eosinophilia and abnormalities of PDGFRA, platelet-derived growth factor receptor-beta (PDGFRB), or fibroblast growth factor receptor 1 (FGFR1), or (4) T-cell neoplasm/lymphoma, unclassifiable.4 Although this classification system has some utility when a clonal or neoplastic process is clearly defined, the majority of patients with HES have idiopathic disease that precludes definitive placement into 1 of the WHO categories. The artificial separation of patients with molecularly defined myeloproliferative disease from those with similar clinical features but no identifiable mutation is also problematic.5
PATHOGENESIS-DRIVEN CLASSIFICATION OF HESs
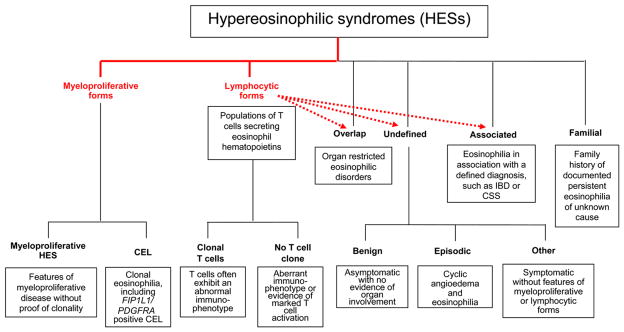
The division of HES into 2 subgroups, a “leukemic” form and a second form in which the underlying mechanism was related to “a hypersensitivity reaction of some type,” was first proposed in the landmark article by Chusid et al1 in 1975. Dramatic differences in clinical manifestations, prognosis, and response to treatment between the 2 groups were noted. Since that time, the identification of specific HES entities with defined etiologies has confirmed the existence of 2 “pathogenic” forms of HES, myeloproliferative and lymphocytic,6 each of which includes several clinically defined distinct HES disorders (Fig 1). Here we discuss the classification of HES using a pathogenesis-driven scheme, as well as the limitations of this approach.
FIG 1.
Revised classification of HESs. Changes from the previous classification3 are indicated in red. Dashed arrows identify HES forms for which at least some patients have T cell–driven disease. Classification of myeloproliferative forms has been simplified, and patients with HES and eosinophil hematopoietin–producing T cells in the absence of a T-cell clone are included in the lymphocytic forms of HES. IBD, Inflammatory bowel disease.
In the lymphocytic forms of HES, lymphocytes generate increased amounts of at least 1 eosinophil hematopoietin (IL-3 and/or IL-5) and are therefore believed to be the primary cause of the secondary polyclonal blood hypereosinophilia.7 Clear-cut involvement of dysregulated T cells in HES has been proven in studies showing marked IL-5 overexpression by immunophenotypically abnormal T cells, on a single-cell basis. The surface immunophenotype of these IL-5 (and/or IL-3)–secreting T cells is variable, suggesting different underlying mechanisms of T-cell dysregulation, and clonality can be demonstrated in many, but not all, patients by T-cell receptor rearrangement studies.8 However, the majority of patients with steroid responsiveness do not demonstrate a T-cell clone with an aberrant immunophenotype, but the eosinophilia is likely driven by T cell–derived cytokines, particularly when increased expression of eosinophil hematopoietins by T cells can be demonstrated or markers of T-cell activation, such as elevated serum thymus and activation-regulated chemokine (TARC), are present. The pathogenic events responsible for the generation of IL-5–producing lymphocytes in the lymphocytic forms of HES, both in the presence and absence of a T-cell clone, remain obscure.
Many patients classified as having undefined, overlapping, or associated HES forms likely have a lymphocytic form (Fig 1). This is exemplified by the case of episodic angioedema and eosinophilia, currently classified under undefined HES (Fig 1), in which cyclic elevations in IL-5 levels precede the episodic eosinophilia and clinical symptoms and appearance of a detectable IL-5–secreting clone has been described in a number of cases.9 Similarly, lymphocytic overexpression of IL-5 has been demonstrated in a number of organ-restricted eosinophilic disorders, including eosinophilic pneumonia, eosinophilic intrinsic asthma, CSS, eosinophilic sinus disease, eosinophilic dermatitis, and eosinophil-associated gastrointestinal disorder (EGID), suggesting that they may also represent T cell–driven HES (supporting literature is found in Simon and Simon10). The clinical efficacy of anti–IL-5 mAbs in patients with eosinophilic dermatitis11 and eosinophilic sinusitis12 provides further evidence that these disorders might be part of the spectrum of lymphocytic HES.
Patients are classified as having one of the myeloproliferative forms of HES if they have clinical (hepatomegaly, splenomegaly), laboratory (circulating myeloid precursors, increased serum vitamin B12 or tryptase, anemia, thrombocytopenia), hematologic (myeloid fibrosis, left shift in maturation of myeloid precursors), and/or cytogenetic abnormalities suggestive of myeloproliferative disease. The primary stimulation of the eosinophilia in these patients is a mutation in hematopoietic multipotent precursor cells rather than an increased production of eosinophil hematopoietins, although these may sometimes be detected at increased levels in the serum of such patients.13 As in the lymphocytic forms, several diseases can be distinguished on the basis of the mutation-related gain-of-function kinase specifically involved in the pathogenesis (eg, FIP1L1/PDGFRA-associated HES; information regarding other kinases can be found in Valent5 and Simon and Simon10). Moreover, it appears that a spectrum of diseases exists that may or may not present with blasts.
Clearly, there is a considerable overlap between myeloproliferative forms of HES and chronic eosinophilic leukemia (CEL), and many patients, including those with detectable FIP1L1/PDGFRA fusion genes, fulfill the current WHO criteria for CEL.4 On the other hand, not all patients with a myeloproliferative form of HES can currently be characterized at the molecular level. If the causative mutation leads to a concomitant clonal expansion of T cells, as has been described in some patients with detectable FIP1L1/PDGFRA fusion genes,14–16 and/or increased production of IL-5, such patients could be mistakenly diagnosed with lymphocytic HES. In addition, as in the case of myeloproliferative HES and CEL, the lymphocytic forms of HES clearly overlap with T-cell malignancies, including lymphoma, particularly in the setting of a demonstrable clonal T-cell population. This is further complicated by the fact that some patients with eosinophilic clonal T-cell disease develop cytogenetic abnormalities and clinical evidence of lymphoma over time.6,8,17,18
In addition to these issues, there are a number of technical issues that limit our ability to classify patients accurately on the basis of disease pathogenesis. The lack of robust, standardized assays that conclusively determine whether an eosinophilia is cytokine-driven hinders definitive diagnosis of lymphocytic forms of HES in patients without demonstrable T-cell clones secreting eosinophilopoietic cytokines. Cytokine analysis in T cells by flow cytometry or in culture supernatants often requires in vitro stimulation and may not reflect the in vivo situation. Moreover, normal levels have not been established, and these assays do not appear to be practical for routine use by clinical laboratories. Increased expression of eosinophil hematopoietins can be demonstrated locally in tissues, such as nasal polyps, eosinophilic pneumonia, and so forth; however, such analyses are currently performed exclusively in research laboratories.7,8,17 Similarly, the identification of causative mutations other than FIP1L1/PDGFRA in patients with myeloproliferative forms of HES has proven to be difficult even in research laboratories with considerable expertise. In spite of these limitations, the classification of the different forms of HESs according to their pathogenesis stimulates the development of an algorithm for the appropriate testing of the different subtypes.10,19
A WORKING DEFINITION OF HES
A modified or improved classification does not solve the problem that a new HES definition is required. Because blood eosinophil numbers result from a balance between eosinophilopoiesis, rate of entry into the vascular lumen, and rate of egress into tissue, the choice of 1500/mm3 as the threshold eosinophil count is, to a large extent, arbitrary. However, in patients in whom eosinophilic tissue infiltration is unproven, historical experience suggests that the risk of serious end organ involvement increases when the eosinophil count is ≥1500/mm3. Because patients with mild disease are often overtreated on the basis of an increased eosinophil count,20 lowering the threshold defining HES in patients with unproven eosinophilic tissue infiltration would likely enhance this tendency. How long should blood hypereosinophilia persist? In the absence of data, a definition of this criterion is difficult, but the 6-month period is no longer acceptable because therapeutic interventions, such as imatinib, should not be delayed in the face of progressive disease. As a practical criterion to define HES, we suggest that otherwise unexplained eosinophilia must be documented on more than 1 occasion, using clinical judgment about the interval and clearly excluding secondary etiologies.
As mentioned, the diagnosis of patients in whom eosinophilic infiltration of tissues is present but the peripheral blood eosinophil count is <1500/mm3 is currently problematic. Because it is unlikely that a patient with chronic eosinophilic pneumonia and an eosinophil count of 1400/mm3 is different from one with the same clinical findings and an eosinophil count of 1500/mm3, inclusion of patients with prominent tissue eosinophilia in the broad definition of HESs seems appropriate from a pathogenetic perspective. Prominent tissue eosinophilia here means that eosinophils are the predominant cell in the inflammatory infiltrate. Consequently, we recommend changing the first criterion of Chusid et al1 and suggest that, for diagnosis of HES in the absence of common triggers, a proven prominent tissue eosinophilia with associated symptoms and marked blood eosinophilia is sufficient, even when blood eosinophil levels may not reach the 1500/mm3 threshold level. We emphasize that allergic diseases, including eosinophilic esophagitis, in which a specific trigger is suspected, are not considered a form of HES.
The second criterion of Chusid et al1 excludes patients with eosinophilia of known cause. As discussed, new diagnostic approaches have led to the identification of causative mutations and/or clonal lymphocytic populations in some patients with HES. These patients are often indistinguishable clinically from subgroups of patients with HES in whom the etiology is unknown. Thus, we recommend changing the wording of the second criterion to exclude secondary causes of eosinophilia (eg, parasitic or viral infections, allergic diseases, drug-induced or chemical-induced eosinophilia, hypoadrenalism, and neoplasms). In contrast, patients with myeloproliferative or lymphoproliferative disorders, presenting with peripheral blood eosinophilia ≥1500/mm3 and evidence of eosinophilic end organ involvement, would be included even when the underlying etiology is known (eg, FIP1L1/PDGFRA mutation, clonal or aberrant T-cell population).
Finally, because it is impossible to determine at presentation if an asymptomatic patient will develop clinical signs and symptoms in the future, we suggest including this group of patients as a special subform of HESs (benign HES). Therefore, we suggest excluding the last criterion of the definition of Chusid et al.1 This change also eliminates the need to determine whether a given patient’s clinical manifestations are directly attributable to the eosinophilia, a task that is particularly challenging because of the heterogeneity of signs and symptoms in HES. A summary of the old and newly proposed criteria for the definition of HES is provided in Table I.
TABLE I.
Diagnostic criteria of HESs
| Old definition: idiopathic hypereosinophilic syndrome | Proposed new definition: HESs |
|---|---|
|
|
RESEARCH PRIORITIES TO OVERCOME THE CURRENT LIMITATIONS
The primary goal of clinical research is to provide information that contributes to our understanding of the epidemiology, pathogenesis, and prognosis of the disease being studied and that leads to rational therapeutic interventions that reduce morbidity and mortality. The identification of the FIP1L1/PDGFRA mutation in a subset of patients meeting diagnostic criteria for the myeloproliferative form of HES is a perfect example of this paradigm, providing a diagnostic marker that predicts poor prognosis with a high prevalence of tissue fibrosis and a therapeutic response to tyrosine kinase inhibitors with activity against PDGFRA. Similarly, the recognition that patients with the lymphocytic form and a detectable T-cell clone are more likely to develop T-cell lymphoma has altered the approach to monitoring and treatment in this subgroup of patients. Thus, the identification of biomarkers that correlate with disease etiology, eosinophil activation, clinical manifestations and/or disease activity, and response to therapy is clearly a priority.
The development of a standardized panel of clinical and laboratory tests for evaluation in large cohorts of well characterized patients is crucial in this regard. To this end, normal values must be established for tissue eosinophilia in different organs and for a variety of genetic, molecular, cytokine, and cellular markers associated with eosinophilia and/or eosinophil activation. Furthermore, because of the rarity of these disorders, clinical networks committed to the collection of data and samples for prospective evaluation should be formed. Some promising markers have already been identified, including serum levels of eosinophil granule proteins as a marker for tissue eosinophilia,21 thymus and activation-regulated chemokine (TARC) for the identification of lymphocytic forms of HES,22,23 and the eosinophil to serum tryptase ratio as a surrogate for the FIP1L1/PDGFRA mutation.24 However, prospective validation in a large and diverse cohort of patients with HES is lacking in all cases.
Finally, clinical trials of targeted agents, including mAbs20 and small molecule inhibitors,2 are important not only for the identification of new therapeutic modalities but also for help in ascertaining the pathways involved in disease pathogenesis. In some cases, as has been reported with imatinib therapy, the response to a specific therapy may lead to the identification of a novel mutation or HES subgroup.2
CONCLUSIONS
Advances in diagnostic approaches and therapeutic options for HES have prompted reevaluation of the definition and classification of HESs. Although gaps in our knowledge preclude a definitive definition at this time, we propose some adjustments to the definition of HESs that we believe overcome some of the obvious limitations of the diagnostic criteria that have been in use since 1975.1 Whereas classification of HESs into clinical disease entities, including myeloproliferative, lymphocytic, overlapping, undefined, associated, and familial forms, is useful in guiding clinical evaluation and therapeutic decisions, a pathogenesis-driven approach to classification may prove more useful in the future once diagnostic techniques are sufficiently advanced to permit classification of most patients into myeloproliferative, lymphocytic, or other forms. These recommendations should be viewed as a work in progress, and we expect that they will be modified in the near future because of the intense research ongoing in the field.
Abbreviations used
- CEL
Chronic eosinophilic leukemia
- CSS
Churg-Strauss syndrome
- FIP1L1
Fip1-like-1
- HES
Hypereosinophilic syndrome
- PDGFRA
Platelet-derived growth factor receptor α
- WHO
World Health Organization
Footnotes
Disclosure of potential conflict of interest: H.-U. Simon has received research support from GlaxoSmithKline, AstraZeneca, Roche, CSL Behring, and Nycomed, and has provided legal consultation or expert witness testimony for Pfizer on the topic of general pharmacology. M. E. Rothenberg has consulted for and given talks for Merck; has consulted for Centocor, Ception Therapeutics, Nycomed, Array BioPharma, Biocrystal Pharmaceuticals, and Endo Pharmaceuticals; has received research support from the National Institutes of Health, the Food Allergy and Anaphylaxis Network, and the Dana Foundation; is on the medical advisory board of the American Partnership for Eosinophilic Diseases; and is on the executive council of the International Eosinophil Society. B. S. Bochner has consulted for Sanofi-Aventis and GlaxoSmithKline and has received research support from Sanofi-Aventis. P. F. Weller has received research support from Merck and has provided legal consultation services or expert witness testimony on the topic of eosinophilic diseases. A. J. Wardlaw is on advisory boards for GlaxoSmithKline and has received research support from GlaxoSmithKline, Pfizer, and AstraZeneca. M. E. Wechsler has consulted for and given talks for Genentech, Merck, and Novartis; has consulted for MedImmune, Medicinova, and GlaxoSmithKline; is on the data safety monitoring board for MAP Pharmaceuticals; and has received research support from the National Institutes of Health and GlaxoSmithKline. L. J. Rosenwasser has received research support from Novartis and Genentech and has consulted for Alcon, Sanofi-Aventis, GlaxoSmithKline, and AstraZeneca. F. Roufosse has consulted for GlaxoSmithKline. G. J. Gleich has provided legal consultation or expert witness testimony on the topic of heparin contamination and is on the board of directors for the American Partnership for Eosinophilic Disorders. A. D. Klion declares that she has no relevant conflicts of interest to disclose.
References
- 1.Chusid MJ, Dale CD, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore) 1975;54:1–27. [PubMed] [Google Scholar]
- 2.Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–14. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 3.Klion AD, Bochner BS, Gleich GJ, Nutman TB, Rothenberg ME, Simon HU, et al. Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol. 2006;117:1292–302. doi: 10.1016/j.jaci.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 4.Bain B, Pierre R, Imbert M, Vardiman JW, Brunning RD, Fladrin G. Chronic eosinophilic leukaemia and the hypereosinophilic syndrome. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization classification of tumours: pathology and genetics of tumours of hematopoietic and lymphoid tissue. Lyon: IARC Press; 2001. pp. 29–31. [Google Scholar]
- 5.Valent P. Pathogenesis, classification, and therapy of eosinophilia and eosinophilic disorders. Blood Rev. 2009;23:157–65. doi: 10.1016/j.blre.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Roufosse F, Cogan E, Goldman M. The hypereosinophilic syndrome revisited. Annu Rev Med. 2003;54:169–84. doi: 10.1146/annurev.med.54.101601.152431. [DOI] [PubMed] [Google Scholar]
- 7.Vassina EM, Yousefi S, Simon D, Zwicky C, Conus S, Simon HU. cIAP2 and survivin contribute to cytokine-mediated delayed eosinophil apoptosis. Eur J Immunol. 2006;36:1975–84. doi: 10.1002/eji.200635943. [DOI] [PubMed] [Google Scholar]
- 8.Simon HU, Plotz SG, Dummer R, Blaser K. Abnormal clones of T cells producing interleukin-5 in idiopathic eosinophilia. N Engl J Med. 1999;341:1112–20. doi: 10.1056/NEJM199910073411503. [DOI] [PubMed] [Google Scholar]
- 9.Banerji A, Weller PF, Sheikh J. Cytokine-associated angioedema syndromes including episodic angioedema with eosinophilia (Gleich’s syndrome) Immunol Allergy Clin North Am. 2006;26:769–81. doi: 10.1016/j.iac.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Simon D, Simon HU. Eosinophilic disorders. J Allergy Clin Immunol. 2007;119:1291–300. doi: 10.1016/j.jaci.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Plotz SG, Simon HU, Darsow U, Simon D, Vassina E, Yousefi S, et al. Use of an anti-interleukin-5 antibody in the hypereosinophilic syndrome with eosinophilic dermatitis. N Engl J Med. 2003;349:2334–9. doi: 10.1056/NEJMoa031261. [DOI] [PubMed] [Google Scholar]
- 12.Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–41. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Simon D, Salemi S, Yousefi S, Simon HU. Primary resistance to imatinib in Fip1-like 1-platelet-derived growth factor receptor α-positive eosinophilic leukemia. J Allergy Clin Immunol. 2008;121:1054–6. doi: 10.1016/j.jaci.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Metzgeroth G, Walz C, Score J, Siebert R, Schnittger S, Haferlach C, et al. Recurrent finding of the FIP1L1-PDGFRA fusion gene in eosinophilia-associated acute myeloid leukemia and lymphoblastic T-cell lymphoma. Leukemia. 2007;21:1183–8. doi: 10.1038/sj.leu.2404662. [DOI] [PubMed] [Google Scholar]
- 15.Capovilla M, Cayuela JM, Bilhou-Nabera C, Gardin C, Letestu R, Baran-Marzak F, et al. Synchronous FIP1L1-PDGFRA-positive chronic eosinophilic leukemia and T-cell lymphoblastic lymphoma: a bilineal clonal malignancy. Eur J Haematol. 2007;80:81–6. doi: 10.1111/j.1600-0609.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 16.Helbig G, Wieczorkiewicz A, Dziaczkowska-Suszek J, Majewski M, Kyrcz-Krzemien S. T-cell abnormalities are present at high frequencies in patients with hypereosinophilic syndrome. Haematologica. 2009;94:1236–41. doi: 10.3324/haematol.2008.005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roufosse F, Schandené L, Sibille C, Willard-Gallo K, Kennes B, Efira A, et al. Clonal Th2 lymphocytes in patients with the idiopathic hypereosinophilic syndrome. Br J Haematol. 2000;109:540–8. doi: 10.1046/j.1365-2141.2000.02097.x. [DOI] [PubMed] [Google Scholar]
- 18.Simon HU, Plotz SG, Simon D, Dummer R, Blaser K. Clinical and immunological features of patients with interleukin-5-producing T cell clones and eosinophilia. Int Arch Allergy Immunol. 2001;124:242–5. doi: 10.1159/000053723. [DOI] [PubMed] [Google Scholar]
- 19.Roufosse F, Weller PF. Practical approach to the patient with hypereosinophilia. J Allergy Clin Immunol. 2010;126:39–44. doi: 10.1016/j.jaci.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–28. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 21.Plager DA, Loegering DA, Checkel JL, Tang J, Kephart GM, Caffes PL, et al. Major basic protein homolog (MBP2): a specific human eosinophil marker. J Immunol. 2006;177:7340–5. doi: 10.4049/jimmunol.177.10.7340. [DOI] [PubMed] [Google Scholar]
- 22.De Lavareille A, Roufosse F, Schmid-Grendelmeier P, Roumier AS, Schandené L, Cogan E, et al. High serum thymus and activation-regulated chemokine levels in the lymphocytic variant of the hypereosinophilic syndrome. J Allergy Clin Immunol. 2002;110:476–9. doi: 10.1067/mai.2002.127003. [DOI] [PubMed] [Google Scholar]
- 23.Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124:1319–25. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maric I, Robyn J, Metcalfe DD, Fay MP, Carter M, Wilson T, et al. KIT D816V-associated systemic mastocytosis with eosinophilia and FIP1L1/PDGFRA-associated chronic eosinophilic leukemia are distinct entities. JAllergy Clin Immunol. 2007;120:680–7. doi: 10.1016/j.jaci.2007.05.024. [DOI] [PubMed] [Google Scholar]