Abstract
Objective
Insulin degludec/insulin aspart (IDegAsp) is a soluble co-formulation of insulin degludec (70%) and insulin aspart (IAsp: 30%). Here, we compare the efficacy and safety of IDegAsp, an alternative IDegAsp formulation (AF: containing 45% IAsp), and biphasic IAsp 30 (BIAsp 30).
Design
Sixteen-week, open-label, randomised, treat-to-target trial.
Methods
Insulin-naive subjects with type 2 diabetes (18–75 years) and a HbA1c of 7–11% were randomised to twice-daily IDegAsp (n=61), AF (n=59) or BIAsp 30 (n=62), all in combination with metformin. Insulin was administered pre-breakfast and dinner (main evening meal) and titrated to pre-breakfast and pre-dinner plasma glucose (PG) targets of 4.0–6.0 mmol/l.
Results
Mean HbA1c after 16 weeks was comparable for IDegAsp, AF and BIAsp 30 (6.7, 6.6 and 6.7% respectively). With IDegAsp, 67% of subjects achieved HbA1c <7.0% without confirmed hypoglycaemia in the last 4 weeks of treatment compared with 53% (AF) and 40% (BIAsp 30). Mean fasting PG was significantly lower for IDegAsp vs BIAsp 30 (treatment difference (TD): −0.99 mmol/l (95% confidence interval: −1.68; 0.29)) and AF vs BIAsp 30 (TD: −0.88 mmol/l (−1.58; −0.18)). A significant, 58% lower rate of confirmed hypoglycaemia was found for IDegAsp vs BIAsp 30 (rate ratio (RR): 0.42 (0.23; 0.75)); rates were similar for AF vs BIAsp 30 (RR: 0.92 (0.54; 1.57)). IDegAsp and AF had numerically lower rates of nocturnal confirmed hypoglycaemia vs BIAsp 30 (RR: 0.33 (0.09; 1.14) and 0.66 (0.22; 1.93) respectively).
Conclusions
IDegAsp provided comparable overall glycaemic control to BIAsp 30 with a significantly lower rate of hypoglycaemia.
Introduction
As pancreatic β-cell function declines in people with type 2 diabetes, insulin treatment may be required, in combination with oral anti-diabetic drugs (OADs), to achieve recommended levels of glycaemic control (1). Insulin treatment is commonly initiated as a once-daily injection of a basal insulin analogue, or a once- or twice-daily injection of a premixed (biphasic) insulin analogue suspension (containing fixed proportions of a rapid-acting soluble insulin analogue and an intermediate-acting, insoluble, protamine-bound form) that provides both basal and meal-related insulin. Given the substantial contribution of postprandial hyperglycaemia to overall glycaemia, initiating insulin therapy with a combination of rapid- and long-acting insulin in one injection may, for patients where basal insulin alone is inadequate, be a simpler and more convenient approach to achieving and sustaining optimal glycaemic control compared with basal–bolus therapy (2). In a recent study, ∼20% of insulin-naive patients needed to add prandial insulin within 6 months of initiating basal insulin because of unacceptable hyperglycaemia (3). Moreover, studies have shown that greater and more sustainable reductions in HbA1c can be obtained when insulin treatment is commenced with a premixed rather than basal-only insulin regimen – especially a twice-daily premixed insulin regimen – although the price for the lower average glycaemic level is commonly greater weight gain and higher rates of hypoglycaemia (2, 3, 4, 5, 6, 7, 8, 9). In particular, increased risk and fear of hypoglycaemia is a barrier to the achievement of reduced HbA1c with insulin (10).
Insulin degludec (IDeg) is an ultra-long-acting basal insulin analogue that forms soluble multi-hexamers upon s.c. injection (11), resulting in a prolonged, stable and consistent glucose-lowering effect at steady state (12, 13, 14). These attributes are thought to contribute to the lower rates of hypoglycaemia, especially nocturnal hypoglycaemia, observed for IDeg compared with other basal insulin analogues (15, 16, 17). IDeg can be co-formulated with the rapid-acting analogue insulin aspart (IAsp) (18), resulting in the first soluble combination (i.e. not requiring resuspension) of two different insulin analogues (insulin degludec/insulin aspart (IDegAsp): 70% v/v IDeg as basal insulin and 30% v/v IAsp as prandial insulin).
Here, we report the results of a clinical proof-of-concept trial that compared the efficacy and safety of IDegAsp with biphasic IAsp 30 (BIAsp 30: 30% v/v soluble IAsp and 70% v/v protamine-crystallised IAsp), both given twice daily in combination with metformin, in insulin-naive subjects with type 2 diabetes inadequately controlled on OADs. To determine the optimal ratio of IDeg to IAsp, an alternative formulation of IDegAsp (AF) containing a higher percentage of IAsp (45% v/v) was also investigated.
Materials and methods
Design overview
This was a phase 2, open-label, three-arm, parallel-group, randomised, controlled, 16-week, treat-to-target trial carried out according to the Declaration of Helsinki (accessed at www.wma.net on 27 Aug 2008) and Good Clinical Practice (accessed at www.ich.org on 27 Aug 2008) and approved by ethics committees and health authorities according to local regulations. Informed consent was obtained from participants before enrolment.
Setting and participants
Twenty-seven sites in five European countries (Finland, France, Germany, Poland and Spain) participated in the trial. Adults with type 2 diabetes were enrolled if they were 18–75 years of age, had an HbA1c of 7–11% and had a body mass index of 25–37 kg/m2. Subjects had to be insulin-naive (no previous insulin treatment or insulin treatment for ≤14 days in the 3 months prior to trial) and had to be treated with one to two OADs in the 2 months before the trial at stable maximum doses or at least half-maximum-allowed doses. Subjects were excluded if they had been treated with thiazolidinediones in the 3 months preceding the trial (see Supplementary Tables 1 and 2, see section on supplementary data given at the end of this article for a list of inclusion and exclusion criteria).
Randomisation and interventions
Before randomisation, eligible subjects discontinued their pre-trial OAD treatment and underwent a 2-week forced metformin titration period (dose increased up to 2000 mg/day: 1000 mg at breakfast and evening meal) followed by a 1-week metformin maintenance period. Subjects taking metformin at enrolment could undergo a modified titration period or advance directly to the metformin maintenance period. Metformin could be decreased to a minimum of 1500 mg/day in the case of unacceptable hypoglycaemia or other adverse events. Subjects were eligible for randomisation, provided the maximum daily metformin dose (2000 mg) or maximum tolerated dose (1500 mg) remained unchanged in the maintenance period and the median pre-breakfast self-measured plasma glucose (SMPG) value (measured on the 3 days before randomisation) was ≥7.5 mmol/l.
Randomisation was carried out using a telephone- or web-based randomisation system. Eligible subjects were stratified according to pre-trial OAD treatment (Table 1) and randomly assigned (1:1:1) to twice-daily, s.c. injections of either IDegAsp (70% v/v IDeg and 30% v/v IAsp; Novo Nordisk A/S, Bagsværd, Denmark; 100 U/ml), AF (55% v/v IDeg and 45% v/v IAsp; Novo Nordisk A/S; 100 U/ml) or BIAsp 30 (NovoMix 30, Novo Nordisk A/S; 100 U/ml) for 16 weeks, all in combination with metformin.
Table 1.
Characteristics of randomised population. Data are mean (s.d.) unless otherwise indicated.
| IDegAsp | AF | BIAsp 30 | |
|---|---|---|---|
| na | 61 | 59 | 62 |
| Sex: male/female (%) | 48/52 | 49/51 | 63/37 |
| Race: W/B (n) | 60/1 | 58/1 | 61/1 |
| Age (years) | 58.7 (8.5) | 60.5 (8.9) | 59.7 (8.0) |
| Weight (kg) | 87.8 (16.3) | 84.9 (14.3) | 91.8 (13.5) |
| Height (m) | |||
| BMI (kg/m2) | 31.5 (3.6) | 30.8 (3.6) | 31.9 (3.5) |
| Duration of diabetes (years) | 9.0 (6.1) | 10.7 (6.4) | 8.6 (6.3) |
| HbA1c (%) | 8.5 (1.2) | 8.5 (0.9) | 8.6 (1.0) |
| FPG (mmol/l) | 11.4 (2.7) | 11.8 (2.9) | 11.7 (3.1) |
| Pre-study OAD treatment (n (%)) | |||
| Met and/or α-gluc | 14 (23) | 13 (22) | 14 (23) |
| SU and/or α-gluc | 2 (3) | 2 (3) | 2 (3) |
| Met and SU | 45 (74) | 45 (74) | 46 (74) |
W, white; B, black or African American; Met, metformin; SU, sulphonylurea; α-gluc, α-glucosidase inhibitor.
Full analysis set (all randomised subjects).
The insulin starting dose was 6 units (U) administered in the abdomen before both breakfast and dinner (main evening meal) using a 3 ml FlexPen device (Novo Nordisk A/S). Patients were to adjust their breakfast and dinner doses once a week throughout the trial (by clinic or telephone contacts), aiming at a pre-breakfast and pre-dinner PG level of 4.0–6.0 mmol/l (see Supplementary Table 3, see section on supplementary data given at the end of this article for titration algorithm). The breakfast dose was adjusted on the basis of pre-dinner SMPG values (lowest PG value from the preceding 3 days); the dinner dose was adjusted according to pre-breakfast SMPG values (lowest PG value from the preceding 3 days).
Outcomes
The primary end point was HbA1c (%) after 16 weeks of treatment. Other pre-specified efficacy end points included laboratory-measured fasting plasma glucose (FPG), changes in insulin doses and nine-point SMPG profiles. The proportion of subjects achieving HbA1c <7.0 and ≤6.5% at the end of the trial and the proportion reaching these HbA1c targets without confirmed hypoglycaemia (confirmed by a PG measurement of <3.1 mmol/l or if classified as ‘severe’) in the last 4 weeks of treatment (subjects treated for ≥8 weeks) were also determined.
Safety variables included hypoglycaemic episodes, adverse events, body weight, vital signs, physical examination, fundoscopic examination, electrocardiogram (ECG), standard biochemical and haematology measures, and serum insulin antibodies (IDeg- and IAsp-specific antibodies and antibodies cross-reacting between IDeg and IAsp and between IDeg and human insulin). Hypoglycaemia was classified as ‘severe’ (assistance from another person required) or ‘confirmed’ (if confirmed by a PG measurement of <3.1 mmol/l irrespective of symptoms or if classified as ‘severe’). Hypoglycaemia was considered ‘nocturnal’ if the time of onset was between 2300 and 0559 h.
Laboratory analyses were performed by Quintiles Central Laboratories (Edinburgh, Scotland). HbA1c was assayed using a validated HPLC method certified by the National Glycohemoglobin Standardization Program. FPG was measured using the Gluco-quant system (Roche). Insulin antibodies were analysed by Celerion (Fehraltorf, Switzerland) using a subtraction RIA method (19) validated by standard procedures (20). Subjects used glucose meters (Abbott Diabetes Care, Alameda, CA, USA) to determine SMPG and recorded values in their diaries.
Statistical analysis
The statistical evaluation of HbA1c, FPG and hypoglycaemic episodes was based on all randomised participants following the intention-to-treat principle. Missing values for HbA1c and FPG were imputed using last observation carried forward. Treatment differences (TD) in HbA1c and FPG values after 16 weeks of treatment were estimated by ANOVA, adjusted by country, sex, age and baseline values. The rate of hypoglycaemic episodes during the exposure to trial insulin was estimated by a negative binomial regression model in which the number of episodes per patient year of exposure (episodes/patient year) was adjusted by country, sex, age and HbA1c at randomisation (21). Statistical testing of differences between IDegAsp and BIAsp 30 in the proportion of patients achieving end-of-trial HbA1c levels of <7.0 and ≤6.5% was carried out using Fisher's exact test (post hoc analysis).
This proof-of-concept trial did not aim to test superiority or non-inferiority of treatments. Instead, the aim was to estimate a TD (in HbA1c) with sufficient precision. A 95% confidence interval (CI) for the TD with a total width of 0.8% (absolute) was considered sufficient for this trial and could be obtained with 50 completed participants per group. Given the chosen precision for HbA1c and an expected dropout of 15–20%, 59 participants were to be randomised to each treatment group.
Values are presented as mean (s.d.) for descriptive statistics, estimated TDs (95% CI) for inferential statistics from the ANOVA and estimated rate ratios (RR; 95% CI) from the negative binomial model. Statistical analyses were performed using SAS Version 9.13 software (Cary, NC, USA) on a UNIX platform.
Results
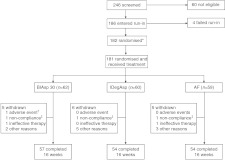
A total of 246 people with type 2 diabetes were screened for the trial, of whom 60 failed screening criteria and four were run-in failures. The remaining 182 subjects were randomly assigned to treatment following the metformin run-in period (Fig. 1); one patient in the IDegAsp group was excluded before receiving insulin treatment due to a serious adverse event (respiratory insufficiency). Baseline characteristics at randomisation were comparable across treatment groups, with the exception of a higher male-to-female ratio in the BIAsp 30 group and a longer mean duration of diabetes for the AF group (Table 1). A similar proportion (8–12%) of subjects withdrew from each treatment group during the trial (Fig. 1).
Figure 1.
Trial flow diagram. *One participant randomised to IDegAsp was excluded from the trial before receiving insulin treatment because of a serious adverse event (respiratory insufficiency); †fatal serious adverse event (cardiac failure); ‡non-compliance with protocol-specified dosing of study drug.
Glycaemic control
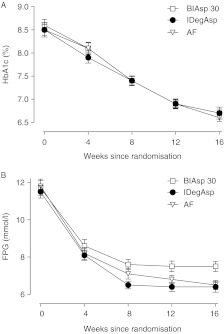
Mean HbA1c values decreased over the course of the 16-week trial (Fig. 2A); mean reductions from baseline were similar for all treatment groups (∼1.8% points), as were mean end-of-trial values (∼6.7%; Table 2). Estimated mean TDs were −0.02% points (−0.27; 0.24) (IDegAsp – BIAsp 30), −0.10% points (−0.35; 0.16) (AF – BIAsp 30) and −0.08% points (−0.33; 0.17) (AF – IDegAsp).
Figure 2.
Mean HbA1c (A) and mean FPG (B) over time. Error bars show s.e.m.
Table 2.
Observed mean changes from baseline HbA1c, FPG and body weight. Data are observed as mean (s.d.) for all randomised subjects (full analysis set).
| n | Baselinea | Week 16b | Change from baseline | |
|---|---|---|---|---|
| HbA1c (%) | ||||
| IDegAsp | 62 | 8.5 (1.2) | 6.7 (1.0) | −1.8 (1.1)c |
| AF | 60 | 8.5 (0.9) | 6.6 (0.6) | −1.9 (1.1)c |
| BIAsp 30 | 61 | 8.6 (1.0) | 6.7 (0.7) | −1.8 (0.9)c |
| FPG (mmol/l) | ||||
| IDegAsp | 62 | 11.5 (2.6) | 6.4 (2.2) | −5.1 (2.9) |
| AF | 60 | 11.8 (2.9) | 6.5 (1.9) | −5.3 (3.0) |
| BIAsp 30 | 61 | 11.7 (3.1) | 7.5 (2.1) | −4.3 (3.0) |
| Body Weight (kg) | ||||
| IDegAsp | 62 | 87.5 (16.3) | 88.6 (16.9) | 1.1 (2.8) |
| AF | 60 | 84.9 (14.3) | 85.6 (14.9) | 0.7 (2.5) |
| BIAsp 30 | 61 | 91.8 (13.5) | 93.2 (13.1) | 1.4 (3.2) |
Values at randomisation.
Last observation carried forward.
% points.
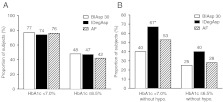
For all treatments, similar proportions of patients reached HbA1c targets of <7.0 and ≤6.5% by trial end (Fig. 3A). However, compared with BIAsp 30, a significantly higher proportion of subjects in the IDegAsp group achieved an HbA1c target of <7.0% in the absence of confirmed hypoglycaemia in the last 4 weeks of treatment (Fig. 3B).
Figure 3.
(A) Percentage of subjects achieving HbA1c targets of <7.0 and ≤6.5% at the end of the trial. (B) Percentage of subjects treated for at least 8 weeks achieving HbA1c targets of <7.0 and ≤6.5% at the end of the trial in the absence of confirmed hypoglycaemia in the last 4 weeks of treatment. Without hypo = no confirmed hypoglycaemia (PG <3.1 mmol/l) in the last 4 weeks of treatment; *significant difference (IDegAsp vs BIAsp 30).
Mean laboratory-measured FPG values decreased during the trial, with the greatest reductions observed for all groups in the first 8 weeks of treatment (Fig. 2B). After 16 weeks of treatment, mean FPG values were significantly lower for IDegAsp (6.4 mmol/l) and AF (6.5 mmol/l) compared with BIAsp 30 (7.5 mmol/l) (Table 2); estimated mean TDs were −0.99 mmol/l (−1.68; −0.29) (IDegAsp – BIAsp 30), −0.88 mmol/l (−1.58; −0.18) (AF – BIAsp 30) and 0.11 mmol/l (−0.59; 0.81) (AF – IDegAsp).
All treatment groups had improvements from baseline in mean nine-point SMPG profile (Supplementary Figure 1, see section on supplementary data given at the end of this article). After 16 weeks, mean PG levels (based on nine-point SMPG measurements) had decreased to a similar extent with BIAsp 30 (12.0 to 7.6 mmol/l), IDegAsp (11.8 to 7.6 mmol/l) and AF (12.2 to 7.5 mmol/l).
Pre-breakfast and pre-dinner SMPG titration targets of 4.0–6.0 mmol/l were reached in the last 4 weeks of treatment (subjects exposed for at least 8 weeks) in the absence of confirmed hypoglycaemia by 53, 33 and 18% of participants on IDegAsp, AF and BIAsp 30 respectively. Post-breakfast and post-dinner PG targets (<8.0 mmol/l) were reached in the last 4 weeks of treatment (subjects exposed for at least 8 weeks) in the absence of confirmed hypoglycaemia by 81, 64 and 43% of IDegAsp-, AF- and BIAsp 30-treated subjects respectively. The median time taken for subjects to reach pre-breakfast and pre-dinner PG targets for the first time was shorter for IDegAsp (8 weeks) and AF (11 weeks) than BIAsp 30 (13 weeks).
Insulin and metformin dose
Mean daily insulin doses at initiation were comparable across treatment arms (0.14–0.16 U/kg), with doses increasing for all groups during the trial. At the end of the trial, mean (s.d.) daily insulin doses were ∼13% lower for IDegAsp (0.57 (0.23) U/kg) compared with AF (0.65 (0.31) U/kg) and BIAsp 30 (0.66 (0.30) U/kg). The majority of subjects (99%) received 2000 mg metformin throughout the randomised treatment period.
Body weight
Small and similar increases in mean body weight were observed from baseline to week 16 for all treatment groups (IDegAsp: 1.1 kg; AF: 0.7 kg; BIAsp 30: 1.4 kg; Table 2).
Hypoglycaemic events
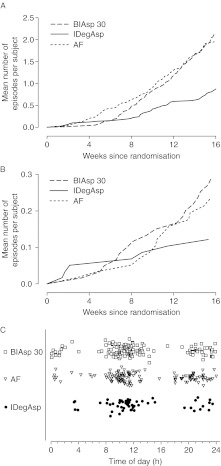
No severe hypoglycaemia was reported. Confirmed hypoglycaemia (PG <3.1 mmol/l) was reported for 36, 56 and 60% of subjects in the IDegAsp, AF and BIAsp 30 groups respectively (Supplementary Table 4, see section on supplementary data given at the end of this article). IDegAsp was associated with a 58% lower rate of overall confirmed hypoglycaemia than BIAsp 30 (2.9 vs 7.3 episodes/patient year; RR IDegAsp/BIAsp 30: 0.42 (0.23; 0.75)); rates were similar for AF vs BIAsp 30 (7.3 vs 6.8 episodes/patient year; RR AF/BIAsp 30: 0.92 (0.54; 1.57)). Numerically lower rates of nocturnal confirmed hypoglycaemia were observed for IDegAsp compared with BIAsp 30 (0.4 vs 1.1 episodes/patient year; RR: 0.33 (0.09; 1.14)) and for AF compared with BIAsp 30 (0.8 vs 1.1 episodes/patient year; 0.66 (0.22; 1.93)). Figure 4A shows the cumulative number of confirmed hypoglycaemic episodes during the course of the trial. For all treatment groups, the majority of hypoglycaemic events occurred between 0800 and 1200 h and between 2000 and 2400 h (Fig. 4B).
Figure 4.
Cumulative number of confirmed hypoglycaemic episodes (A), cumulative number of confirmed nocturnal hypoglycaemic episodes (B) and scatter plot of the time of occurrence of confirmed hypoglycaemic episodes (C).
Adverse events and other safety measures
The incidence of adverse events was similar across treatment arms: 45, 54 and 55% of participants in the IDegAsp, AF and BIAsp 30 groups, respectively, reported at least one adverse event, of which the majority (>99%) were mild or moderate in severity. Two serious adverse events were reported, both for BIAsp 30 (two subjects; two events: fatal cardiac failure, mild transient ischaemic attack), and neither was considered by the investigator to be related to the trial product. Adverse events judged to have possible or probable relation to insulin were reported for three subjects in the IDegAsp group (hunger, increased appetite, headache and acquired lipodystrophy), two subjects in the AF group (hunger, increased appetite and headache) and one subject in the BIAsp 30 group (peripheral oedema).
Overall, levels of IAsp- and IDeg-specific antibodies remained low (median of ≤0.7% bound/total (B/T)) or undetectable during the trial for all treatment groups. Levels of antibodies cross-reacting between IDeg and IAsp (and IDeg and human insulin) remained low throughout the trial in the IDegAsp and AF groups (median of ≤0.1% B/T). For the BIAsp 30 group, levels of cross-reacting antibodies increased from baseline to the end of the trial, both for antibodies cross-reacting between IDeg and IAsp (to a median of 7.0% B/T at the end of the trial) and between IDeg and human insulin (to a median of 6.8% B/T at the end of the trial). No clinically relevant differences were observed between treatments in physical examination findings, vital signs, standard laboratory analyses (haematology and biochemistry), fundoscopic examination or ECG.
Discussion
This clinical proof-of-concept, treat-to-target trial investigated the efficacy and safety of insulin initiation with twice-daily administration of IDegAsp, the first soluble combination of distinct rapid-acting and basal insulin analogues, as add-on therapy to metformin in patients with type 2 diabetes inadequately controlled with OAD therapy. IDegAsp was compared with BIAsp 30, a widely used premixed insulin analogue suspension that is often used in a twice-daily treatment regimen when commencing insulin therapy.
Both IDegAsp and BIAsp 30 achieved clinically meaningful improvements in HbA1c of ∼1.8% points after 16 weeks of treatment. The magnitude of the HbA1c reduction was in line with other studies with twice-daily BIAsp 30 in insulin-naive type 2 patients (22, 23, 24). In general, IDegAsp and BIAsp 30 had similar safety profiles; no clinically relevant differences were found with respect to standard safety assessments, and there were no apparent group-specific patterns or clustering of adverse events. Notably, IDegAsp was associated with a significant, 58% lower rate of confirmed hypoglycaemia than BIAsp 30, which was reflected in a substantially higher proportion of subjects reaching HbA1c targets (<7.0 and ≤6.5%) in the absence of hypoglycaemia in the last 4 weeks of treatment. The reduction in absolute numbers of confirmed hypoglycaemic events (PG <3.1 mmol/l) amounts to a difference of approximately three hypoglycaemic events per patient per year between groups (i.e. on average, patients treated with IDegAsp will experience three less hypoglycaemic episodes per year compared with those on BIAsp 30). We believe that such a reduction will be considered beneficial by both the patient and healthcare professional. By contrast, the AF of IDegAsp (AF: containing a higher percentage (45%) of IAsp) had an approximately twofold higher rate of confirmed hypoglycaemia compared with IDegAsp, and consequently, fewer subjects achieved HbA1c targets without hypoglycaemia. The higher rate of hypoglycaemia associated with AF indicated that the percentage (amount) of rapid-acting IAsp in this formulation exceeded typical mealtime requirements; in view of these findings and the lack of any clinical advantage over IDegAsp, clinical development of AF has been discontinued.
IDegAsp produced a significantly greater reduction in FPG than BIAsp 30, which was not achieved at the expense of a higher rate of nocturnal hypoglycaemia. Indeed, IDegAsp was associated with a numerically 67% lower rate of nocturnal confirmed hypoglycaemia compared with BIAsp 30. The lower frequency and shorter period of hypoglycaemia with IDegAsp after the evening injection – relatively few hypoglycaemic episodes were reported for IDegAsp between midnight and 0400 h compared with BIAsp 30 – suggest that hypoglycaemia occurring with IDegAsp is primarily driven by the IAsp component of this co-formulation.
As would be expected from the treat-to-target trial design, similar improvements in HbA1c were observed with IDegAsp and BIAsp 30: at the end of the trial, HbA1c had decreased to a mean of 6.7% in both treatment groups, although it was noteworthy that comparable glycaemic control was achieved with ∼13% lower doses of IDegAsp. In a similar-sized exploratory trial of the same length and patient population, once-daily IDegAsp provided comparable overall glycaemic control (mean HbA1c) to once-daily insulin glargine, with the additional benefit of greater post-dinner glucose control in the IDegAsp group (25). In line with the current study, similar glycaemic control was achieved with lower doses of IDegAsp compared with the comparator. However, as in both the studies IDegAsp was titrated using the same algorithm as the comparator, this finding has no clinical implications in these studies. The significance, if any, of this in clinical practice remains to be established.
It is possible that a more ambitious titration algorithm may have led to greater improvements in HbA1c with IDegAsp (the open-label design of the trial may have influenced how aggressively insulin was dose-titrated by the subject or clinician), although it is likely that larger, longer-term trials will be necessary to show any differences between treatments. It should be noted, however, that there is an on going discussion of the benefits vs risks of reducing HbA1c beyond the levels achieved in this study (10, 26).
In summary, despite the limitations of this proof-of-concept study (small sample size, short treatment duration and open-label design), IDegAsp was shown to be a promising new treatment option for initiating insulin therapy in subjects with type 2 diabetes inadequately controlled with OADs. Twice-daily IDegAsp (in combination with metformin) was safe and well tolerated and provided overall glycaemic control similar to BIAsp 30 at a significantly lower rate of confirmed hypoglycaemia.
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/EJE-12-0293.
Author contribution statement
All authors accept public responsibility for the content of the paper and confirm that they meet the ICJME conditions for authorship, namely that they conceived and planned the work that led to the article or played an important role in interpreting the results (or both), wrote the paper and/or made substantive suggestions for revision and approved the final version of the manuscript.
Acknowledgements
The patients and all trial staff are thanked for their participation (see Supplementary Table 5, see section on supplementary data given at the end of this article for a list of principal investigators). The authors thank Paul G Drake, PhD (Novo Nordisk), for assistance with manuscript preparation and Mark Nelson (Watermeadow Medical; funded by Novo Nordisk) for submission assistance.
Declaration of interest
J Weng, M Muñoz-Torres and J-P Donnet have no conflicts to declare.
Funding
Novo Nordisk sponsored the study, participated in protocol development and provided logistical support. The sponsor monitored the trial, collected and analysed data, was involved in data interpretation and drafted the initial manuscript from an outline agreed by all authors. The authors then modified the manuscript as appropriate; the final decision on content was made by all authors. This trial is registered at www.clinicaltrials.gov ID number: NCT00613951. L Niskanen has received funding for clinical research to unit from Astra-Zeneca, Eli Lilly, Boehringer Ingelheim, Pfizer, Ipsen, GlaxoSmithKline, Novartis, Janssen-Cilag, Novo Nordisk and Sanofi. During the last 24 months, he has participated in scientific congresses supported by Sanofi, Novartis, Novo Nordisk, Ipsen and Bristol-Myers Squibb. He has also received lecture fees in educational symposia organised or supported by Amgen, Novo Nordisk, Merck Sharp and Dohme, Novartis, Bayer and Orion. L A Leiter has received research funding from, provided CME on behalf of and/or has acted as a consultant to Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Servier. E Franek has participated in advisory boards for Boehringer-Ingelheim, Novo Nordisk and Polpharma and received speaker's fees from Boehringer-Ingelheim, Bioton, Eli-Lilly, Merck, Sharp and Dohme, Novo Nordisk, Polpharma and Sanofi. T Damci has received honoraria from Novo Nordisk, Sanofi, MSD, GlaxoSmithKline, Bristol Myers Squibb, AstraZeneca, Bilim Ilac, Sanovel and Takeda. L Endahl and T V Skjøth are employees and shareholders of Novo Nordisk (sponsor of the trial). A Vaag was, until February 28th 2011, an employee of Steno Diabetes Center, owned by Novo Nordisk.
References
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaag A, Lund S. Insulin initiation in patients with type 2 diabetes mellitus: treatment guidelines, clinical evidence and patterns of use of basal vs premixed insulin analogues. European Journal of Endocrinology. 2012;166:159–170. doi: 10.1530/EJE-11-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S, Levy JC. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. New England Journal of Medicine. 2007;357:1716–1730. doi: 10.1056/NEJMoa075392. [DOI] [PubMed] [Google Scholar]
- Stehouwer MH, DeVries JH, Lumeij JA, Ader HJ, Engbers AM, Iperen AA, Snoek FJ, Heine RJ. Combined bedtime insulin – daytime sulphonylurea regimen compared with two different daily insulin regimens in type 2 diabetes: effects on HbA1c and hypoglycaemia rate – a randomised trial. Diabetes/Metabolism Research and Reviews. 2003;19:148–152. doi: 10.1002/dmrr.356. [DOI] [PubMed] [Google Scholar]
- Malone JK, Kerr LF, Campaigne BN, Sachson RA, Holcombe JH. Combined therapy with insulin lispro Mix 75/25 plus metformin or insulin glargine plus metformin: a 16-week, randomized, open-label, crossover study in patients with type 2 diabetes beginning insulin therapy. Clinical Therapeutics. 2004;26:2034–2044. doi: 10.1016/j.clinthera.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Raskin P, Allen E, Hollander P, Lewin A, Gabbay RA, Hu P, Bode B, Garber A. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260–265. doi: 10.2337/diacare.28.2.260. [DOI] [PubMed] [Google Scholar]
- Kazda C, Hulstrunk H, Helsberg K, Langer F, Forst T, Hanefeld M. Prandial insulin substitution with insulin lispro or insulin lispro mid mixture vs. basal therapy with insulin glargine: a randomized controlled trial in patients with type 2 diabetes beginning insulin therapy. Journal of Diabetes and its Complications. 2006;20:145–152. doi: 10.1016/j.jdiacomp.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Wolffenbuttel BH, Klaff LJ, Bhushan R, Fahrbach JL, Jiang H, Martin S. Initiating insulin therapy in elderly patients with type 2 diabetes: efficacy and safety of lispro mix 25 vs. basal insulin combined with oral glucose-lowering agents. Diabetic Medicine. 2009;26:1147–1155. doi: 10.1111/j.1464-5491.2009.02824.x. [DOI] [PubMed] [Google Scholar]
- Strojek K, Bebakar WM, Khutsoane DT, Pesic M, Smahelova A, Thomsen HF, Kalra S. Once-daily initiation with biphasic insulin aspart 30 versus insulin glargine in patients with type 2 diabetes inadequately controlled with oral drugs: an open-label, multinational RCT. Current Medical Research and Opinion. 2009;25:2887–2894. doi: 10.1185/03007990903354674. [DOI] [PubMed] [Google Scholar]
- Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, Wetterslev J. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ. 2011;343:d6898. doi: 10.1136/bmj.d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzhals P, Heise T, Strauss HM, Bøttcher SG, Granhall C, Haahr H, Jonassen I. Multi-hexamer formation is the underlying basis for the ultra-long glucose-lowering effect of insulin degludec. Diabetologia. 2011;54:S426. doi: 10.1007/s00125-011-2276-4. [DOI] [Google Scholar]
- Heise T, Hermanski L, Nosek L, Feldmann A, Rasmussen S, Stryhn TK, Haahr H. Insulin degludec: less pharmacodynamic variability than insulin glargine under steady state conditions. Diabetologia. 2010;53:S387. doi: 10.1007/s00125-010-1872-z. [DOI] [PubMed] [Google Scholar]
- Heise T, Hermanski L, Nosek L, Feldmann A, Rasmussen S, Haahr H. The pharmacodynamic variability of insulin degludec is consistently lower than insulin glargine over 24 hours at steady state. Diabetes. 2011;60:A263. doi: 10.2337/db11-868-1281. [DOI] [PubMed] [Google Scholar]
- Nosek L, Heise T, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect. Diabetologia. 2011;54:S429. doi: 10.1007/s00125-011-2276-4. [DOI] [PubMed] [Google Scholar]
- Birkeland KI, Home PD, Wendisch U, Ratner RE, Johansen T, Endahl LA, Lyby K, Jendle JH, Roberts AP, DeVries JH, Meneghini LF. Insulin degludec in type 1 diabetes a randomized controlled trial of a new-generation ultra-long-acting insulin compared with insulin glargine. Diabetes Care. 2011;34:661–665. doi: 10.2337/dc10-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A, King AB, Del Prato S, Sreenan S, Rosenstock J, Endahl LA, Francisco AMO, Hollander P. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1498–1507. doi: 10.1016/S0140-6736(12)60205-0. [DOI] [PubMed] [Google Scholar]
- Heller S, Buse J, Fisher M, Garg S, Marre M, Merker L, Renard E, Russell-Jones D, Philotheou A, Francisco AMO, Pei H, Bode B. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1489–1497. doi: 10.1016/S0140-6736(12)60204-9. [DOI] [PubMed] [Google Scholar]
- Jonassen I, Hoeg-Jensen T, Havelund S, Ribel U. Ultra-long acting insulin degludec can be combined with rapid-acting insulin aspart in a soluble co-formulation. Journal of Peptide Science. 2010;16:32. doi: 10.1002/psc.1301. [DOI] [Google Scholar]
- Lindholm A, Jensen LB, Home PD, Raskin P, Boehm BO, Rastam J. Immune responses to insulin aspart and biphasic insulin aspart in people with type 1 and type 2 diabetes. Diabetes Care. 2002;25:876–882. doi: 10.2337/diacare.25.5.876. [DOI] [PubMed] [Google Scholar]
- Mire-Sluis AR, Barrett YC, Devanarayan V, Koren E, Liu H, Maia M, Parish T, Scott G, Shankar G, Shores E, Swanson SJ, Taniguchi G, Wierda D, Zuckerman LA. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. Journal of Immunological Methods. 2004;289:1–16. doi: 10.1016/j.jim.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Bulsara MK, Holman CD, Davis EA, Jones TW. Evaluating risk factors associated with severe hypoglycaemia in epidemiology studies – what method should we use? Diabetic Medicine. 2004;21:914–919. doi: 10.1111/j.1464-5491.2004.01250.x. [DOI] [PubMed] [Google Scholar]
- Kann PH, Wascher T, Zackova V, Moeller J, Medding J, Szocs A, Mokan M, Mrevlje F, Regulski M. Starting insulin therapy in type 2 diabetes: twice-daily biphasic insulin aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Experimental and Clinical Endocrinology & Diabetes. 2006;114:527–532. doi: 10.1055/s-2006-949655. [DOI] [PubMed] [Google Scholar]
- Khutsoane D, Sharma SK, Almustafa M, Jang HC, Azar ST, Danciulescu R, Shestakova M, Ayad NM, Guler S, Bech OM. Biphasic insulin aspart 30 treatment improves glycaemic control in patients with type 2 diabetes in a clinical practice setting: experience from the PRESENT study. Diabetes, Obesity & Metabolism. 2008;10:212–222. doi: 10.1111/j.1463-1326.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- Valensi P, Benroubi M, Borzi V, Gumprecht J, Kawamori R, Shaban J, Shah S, Shestakova M, Wenying Y. Initiating insulin therapy with, or switching existing insulin therapy to, biphasic insulin aspart 30/70 (NovoMix 30) in routine care: safety and effectiveness in patients with type 2 diabetes in the IMPROVE observational study. International Journal of Clinical Practice. 2009;63:522–531. doi: 10.1111/j.1742-1241.2009.02002.x. [DOI] [PubMed] [Google Scholar]
- Heise T, Tack CJ, Cuddihy R, Davidson J, Gouet D, Liebl A, Romero E, Mersebach H, Dykiel P, Jorde R. A new-generation ultra-long-acting basal insulin with a bolus boost compared with insulin glargine in insulin-naive people with type 2 diabetes a randomized, controlled trial. Diabetes Care. 2011;34:669–674. doi: 10.2337/dc10-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, Zagar T, Poole CD. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481–489. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]