Abstract
Objective:
To investigate the relation between retinopathy and the risk of dementia.
Methods:
We investigated the associations between retinopathy and dementia and its subtypes Alzheimer disease (AD) and vascular dementia both cross-sectionally and prospectively in the Rotterdam Study, a large population-based cohort study. Digitized retinal images were available for 195 participants with prevalent dementia and 6,078 participants without dementia at baseline (1990–1993). Participants were reexamined in 1993–1994, 1997–1999, and 2002–2004 and were continuously monitored for development of dementia until January 1, 2007. Retinopathy was graded on fundus photographs and was defined as the presence of one or more dot/blot hemorrhages, microaneurysms, cotton wool spots, or evidence of laser treatment for retinopathy.
Results:
Retinopathy was associated with prevalent dementia (age and sex-adjusted odds ratio 2.04, 95% confidence interval [CI] 1.34–3.09). Results were similar for AD and vascular dementia. During a mean follow-up of 11.4 years, 735 participants developed incident dementia, of whom 583 had AD and 80 had vascular dementia. There was no association of retinopathy at baseline with the risk of incident dementia during follow-up (age- and sex-adjusted hazard ratio 1.15, 95% CI 0.88–1.48) or the risk of incident AD or vascular dementia.
Conclusions:
Retinopathy is more prevalent in persons with dementia but is not associated with an increased risk of dementia over time.
Dementia is a major cause of morbidity and mortality in elderly people. Many factors contribute to the development of dementia, and although the exact causes are still unclear, cerebrovascular disease is thought to be an important risk factor.1 To study the role of cerebral microvascular disease in the pathogenesis of dementia, there is much interest in retinal microvascular signs, because embryologic, anatomic, and physiologic characteristics of the retinal vasculature are similar to those of the cerebral circulation, and the retina is easy to visualize noninvasively.2,3 We have previously shown an association of larger retinal venular caliber and smaller arteriolar caliber with the risk of developing vascular dementia.4 Another interesting retinal microvascular sign that has been associated with cognition and dementia is retinopathy.5–9 In prospective MRI studies, retinopathy has also been associated with the development of silent brain infarcts10 and ventricular enlargement11 over a 10-year period; these features have also been associated with dementia12 or cognition.13 At present, studies investigating the association between retinopathy and the development of dementia in a prospective setting are lacking.
In this study, we investigated the associations of retinopathy with both prevalent and incident dementia and its subtypes Alzheimer disease (AD) and vascular dementia in the Rotterdam Study, a large population-based cohort study.
METHODS
Study population.
The Rotterdam Study is a prospective population-based cohort study that is conducted among all inhabitants aged 55 years and over of Ommoord, a district of Rotterdam, the Netherlands.14 Of 10,274 eligible subjects, 7,983 (78%) participated in the baseline examinations between 1990 and 1993. Because eye examinations became operational a few months after the baseline examinations had started, a smaller number (n = 6,787) participated in the ophthalmic part of the study. For technical reasons (mostly absence of technicians), fundus photographs were not available for 352 participants. Fundus photographs were available for 6,435 participants, and, of these, 3 were ungradable. We excluded participants with end-stage age-related macular degeneration (n = 106) or retinal arterial or vein occlusions (n = 51) and participants who were not screened for dementia (n = 2).
The final study population was 6,273 participants, of whom 195 had dementia at baseline. The cohort at risk of dementia at baseline thus comprised 6,078 participants. Follow-up examinations were conducted in 1993−1994, 1997−1999, and 2002−2004. In addition, through linkage with records of general practitioners, the total cohort was continuously monitored for morbidity and mortality. Follow-up for dementia was virtually complete until January 1, 2007.
Standard protocol approvals, registrations, and patient consents.
The medical ethics committee at Erasmus University of Rotterdam approved the study, and written informed consent was obtained from all participants.
Dementia diagnoses.
Participants were screened for dementia with a 3-step procedure, which was similar at baseline and follow-up examinations.15 Two brief tests of cognition (Mini-Mental State Examination [MMSE]16 and Geriatric Mental State schedule [GMS]17 organic level) were used to screen all subjects. Individual with positive screen results (MMSE score <26 or GMS organic level >0) underwent the Cambridge Examination for Mental Disorders of the Elderly.18 Persons who were suspected of having dementia were, if necessary, examined by a neuropsychologist. In addition, the total cohort was continuously monitored for incident dementia through computerized linkage between the study database and digitized medical records from general practitioners and the Regional Institute for Outpatient Mental Health Care.15 The diagnoses of dementia and AD were made in accordance with internationally accepted criteria for dementia (DSM-III-R),19AD (National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association),20 and vascular dementia (National Institute of Neurological and Communicative Disorders and Stroke−Association Internationale pour la Recherché et l'Enseignement en Neurosciences)21 by a panel of a neurologist, neuropsychologist, and research physician.
Retinopathy.
Ophthalmic history was taken from the study subjects before they underwent ophthalmic examination and fundus photography, covering a 35-degree field centered on the macula after pharmacologic mydriasis of both eyes at each visit (Topcon TRV-50VT fundus camera; Topcon Optical Co., Tokyo, Japan). Fundus photographs were checked for quality and the presence of age-related macular degeneration, using the International Classification and Grading System. Study subjects with ungradable photographs, end-stage age-related macular degeneration, and retinal arterial or vein occlusions were excluded from further analyses. The presence of cotton wool exudates and the presence and number of dot/blot hemorrhages were graded, without differentiation between microaneurysms and hemorrhages. In addition, the presence of laser coagulation scars were graded and the indication for laser therapy was categorized into either retinopathy or other diseases (most often retinal vein occlusion), using available clinical data. Retinopathy was defined as the presence of one or more dot/blot hemorrhages, microaneurysms, or cotton wool spots or evidence of laser treatment for retinopathy.
Other variables.
Smoking habits and use of antihypertensive medication were assessed during the baseline home interview. Blood pressure was measured twice with a random zero sphygmomanometer at the brachial artery with the subject in a sitting position, and the measurements were averaged. Hypertension was defined as blood pressure ≥140/90 mm Hg or use of antihypertensive medication, prescribed for the indication of hypertension. Nonfasting serum total cholesterol concentrations were determined by an automated enzymatic procedure. Serum levels of high-sensitivity C-reactive protein (CRP) were determined by the rate near infrared particle immunoassay method (Immage high-sensitivity CRP; Beckman Coulter, Fullerton, CA). Diabetes mellitus was defined as a self-reported history of diabetes or a random nonfasting or postload serum glucose level ≥11.1 mmol/L. History of stroke at baseline was assessed during the baseline interview and verified by reviewing medical records. After enrollment, participants were continuously monitored for incident stroke through automated linkage of the study database with files from general practitioners and the municipality. Additional information was obtained from hospital records. Coronary heart disease was defined as a previous myocardial infarction, percutaneous transluminal coronary angioplasty, or coronary bypass. APOE genotype was assessed on coded DNA samples using PCR without knowledge of the dementia diagnosis.22 APOE ϵ4 carriership was defined as the presence of at least one APOE ϵ4 allele. Missing values in covariates (6% or less) were imputed based on age and sex for continuous variables and as the mean for dichotomous variables.
Statistical analyses.
First, we investigated the cross-sectional associations between retinopathy and prevalent dementia, AD, and vascular dementia. We used logistic regression analyses adjusted for age and sex (model 1), with additional adjustments for stroke (model 2) and for systolic blood pressure, use of antihypertensive medication, educational level, APOE ϵ4 carriership, current cigarette smoking, diabetes, total cholesterol, CRP, and coronary heart disease.
Next, we used Cox proportional hazard models to investigate the associations between retinopathy at baseline and incident dementia, AD, or vascular dementia during follow-up. Adjustments were made for the above-mentioned covariates. Stroke before the end of follow-up was included in the model as a time-varying covariate. We tested the proportional hazard assumption by including the interaction of retinopathy with time as covariate in the models. Interaction terms of retinopathy with follow-up time were nonsignificant (p = 0.14 for all dementia, p = 0.08 for AD, and p = 0.21 for vascular dementia), indicating that the associations of retinopathy with dementia, AD, and vascular dementia did not significantly differ according to length of follow-up. Given the long duration of follow-up, we were, however, also interested in the associations of retinopathy with the short-term vs the longer-term risk of dementia, and we repeated the analyses in 3 different strata according to time to event (0–5, 5–10, and more than 10 years).
We repeated both the cross-sectional and the prospective analyses in strata of hypertension and diabetes and tested for interactions of diabetes or hypertension with retinopathy by adding an interaction term of diabetes and retinopathy, and an interaction term of hypertension and retinopathy to model 1. Furthermore, we tested whether there was a relation between severity of retinopathy and prevalent or incident dementia, by categorizing retinopathy into 4 categories (no retinopathy, 1 retinopathy lesion, 2 or more lesions, and laser coagulation scars).
RESULTS
Baseline characteristics of participants with prevalent dementia and the cohort at risk for incident dementia are shown in table 1. Of the 195 participants with prevalent dementia, 149 had AD, 29 had vascular dementia, and 17 had other subtypes of dementia. In the cohort at risk for dementia, 438 participants (7%) had retinopathy at baseline and of those with prevalent dementia, 35 (18%) had retinopathy. In both groups, the majority only had dot/blot hemorrhages or microaneurysms (table 2). Associations of retinopathy with prevalent dementia are shown in table 3. Retinopathy was significantly associated with dementia (odds ratio [OR] 2.04, 95% confidence interval [CI] 1.34–3.09, adjusted for age and sex). This association remained the same after adjustment for stroke and other possible confounders. For AD, the associations were similar. The association of retinopathy with vascular dementia seemed stronger than the associations with all-cause dementia and AD in the first model (OR 3.01, 95% CI 1.26–7.21), but after adjustment for stroke the effect size became similar, although nonsignificant, probably because of the low number of cases, to the effect sizes observed for overall dementia and AD (OR 2.12, 95% CI 0.80–5.62). Analyses stratified for hypertension or diabetes showed no differences between strata (p values of interaction terms for dementia and AD ≥ 0.36, data not shown). For vascular dementia, there was no interaction of diabetes with retinopathy (p = 0.48), but the association with retinopathy seemed stronger in persons without hypertension (p = 0.059). This difference disappeared after adjustment for stroke (p = 0.31). There was no clear relation between severity of retinopathy and prevalent dementia, AD, or vascular dementia (data not shown).
Table 1.
Baseline characteristics
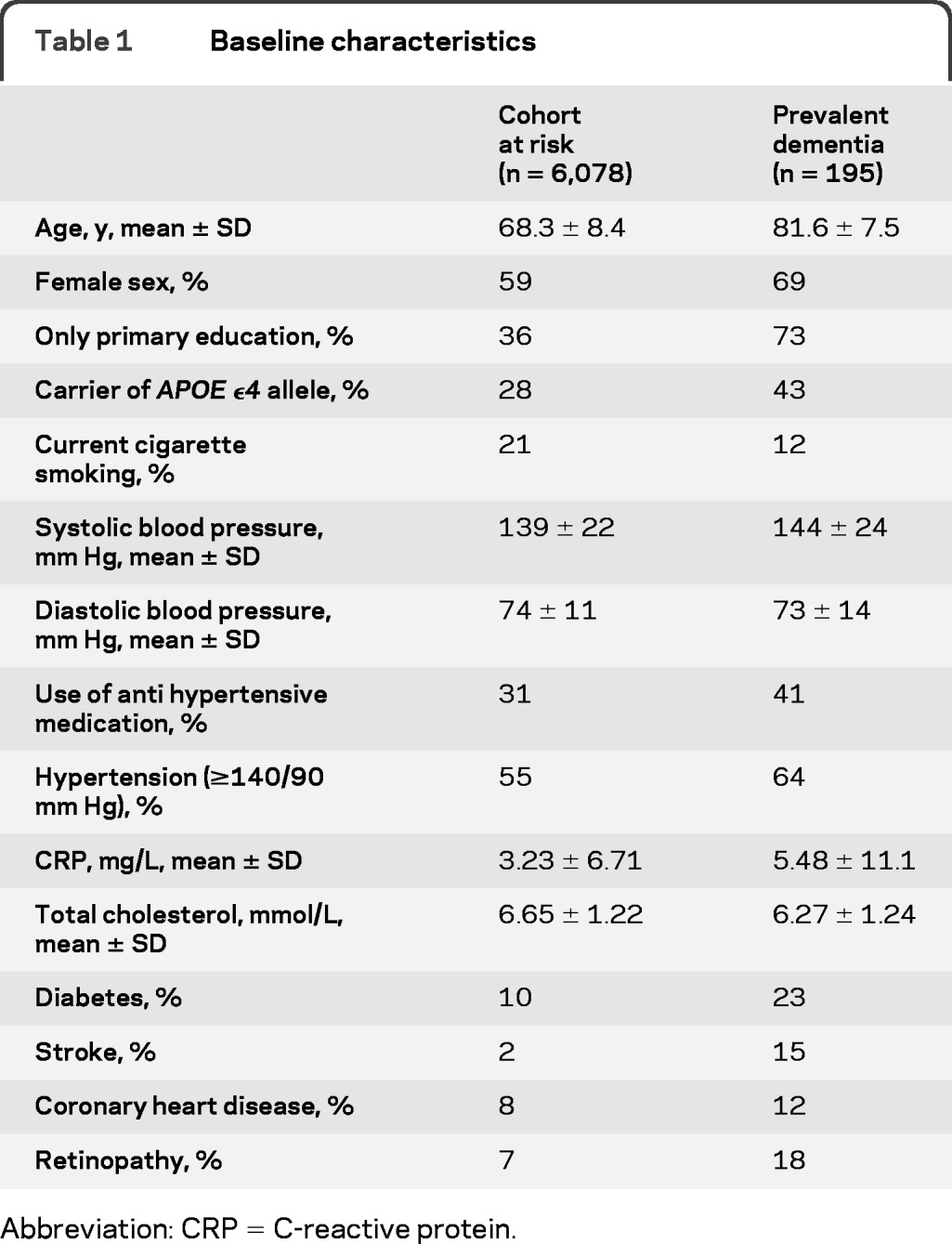
Abbreviation: CRP = C-reactive protein.
Table 2.
Type of lesion in participants with retinopathy
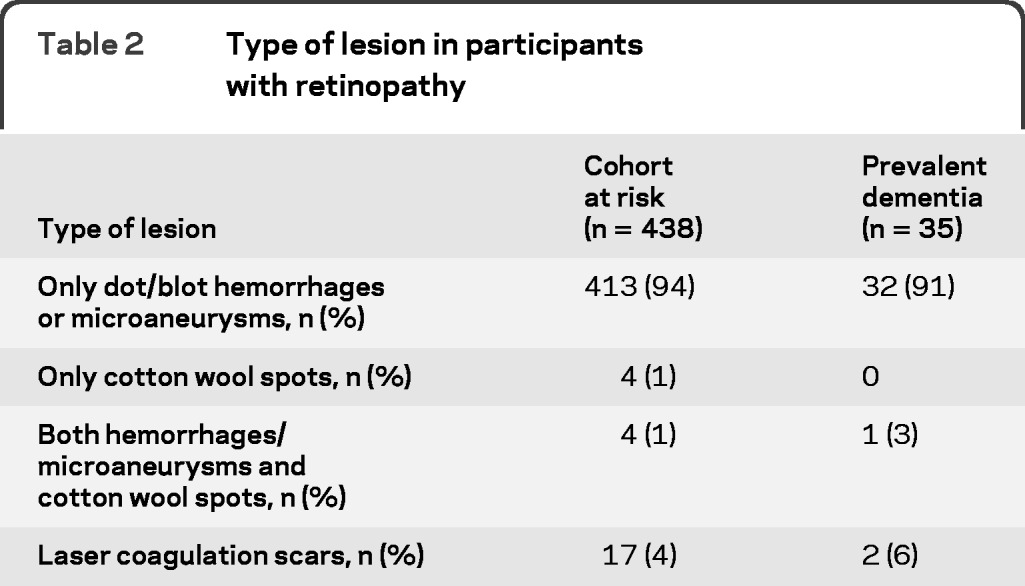
Table 3.
ORs of retinopathy and prevalent dementiaa
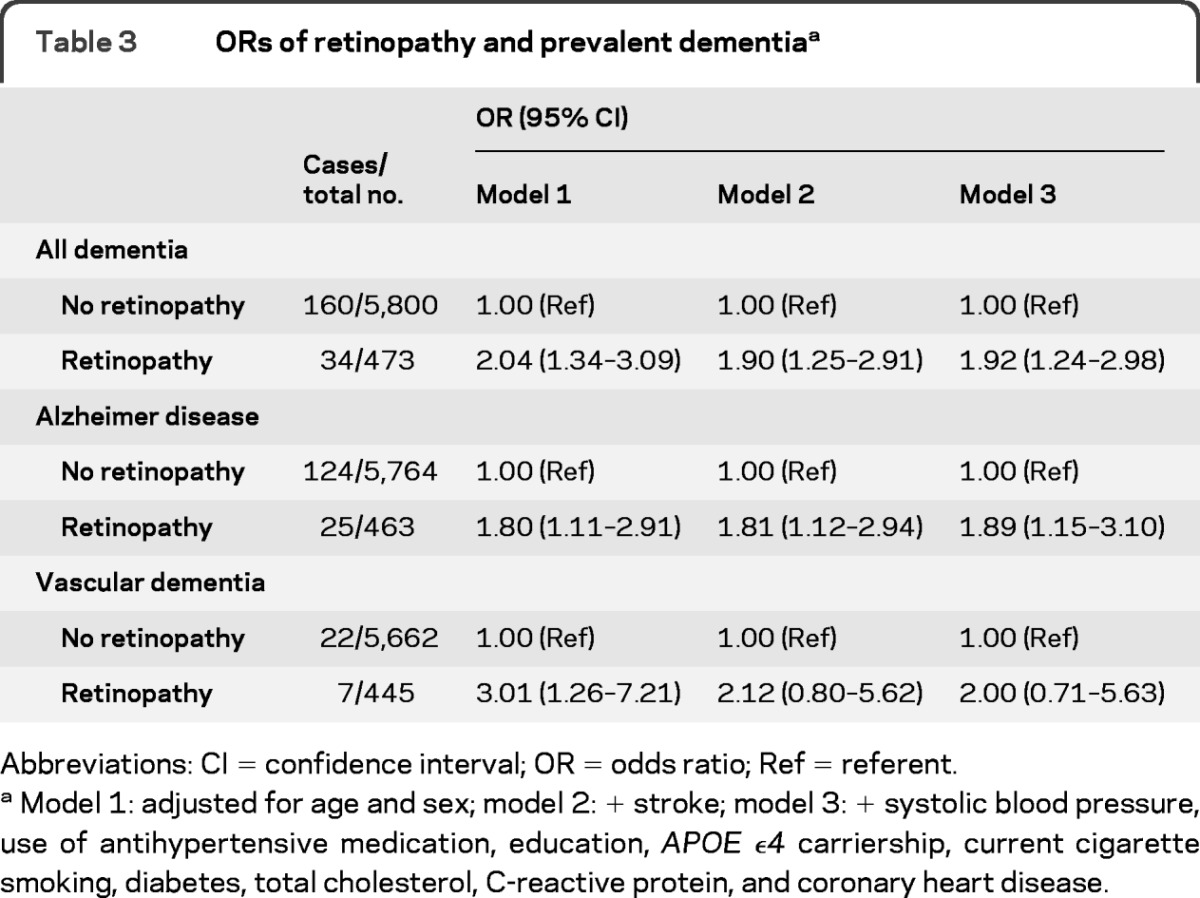
Abbreviations: CI = confidence interval; OR = odds ratio; Ref = referent.
Model 1: adjusted for age and sex; model 2: + stroke; model 3: + systolic blood pressure, use of antihypertensive medication, education, APOE ϵ4 carriership, current cigarette smoking, diabetes, total cholesterol, C-reactive protein, and coronary heart disease.
In the cohort at risk, 735 participants had developed dementia after a mean follow-up of 11.4 years (maximum 17.3 years), of whom 583 had AD, 80 had vascular dementia, and 72 had other types of dementia. There was no association of retinopathy at baseline with incident dementia, AD, or vascular dementia (table 4). In the analyses according to follow-up time, there was also no clear association between retinopathy and incident dementia, AD, or vascular dementia in the different strata (table 5). The effect sizes of the risks within 5 years of baseline were, however, in between the effect sizes of the associations with prevalent dementia and incident dementia during the total follow-up time. In strata of hypertension and diabetes, there was also no association of retinopathy with incident dementia, AD, or vascular dementia (data not shown). Interaction terms of diabetes and hypertension with retinopathy were all nonsignificant (p > 0.12). There was also no relation between severity of retinopathy and risk of incident dementia, AD, or vascular dementia.
Table 4.
HRs of retinopathy and risk of incident dementia during follow-up
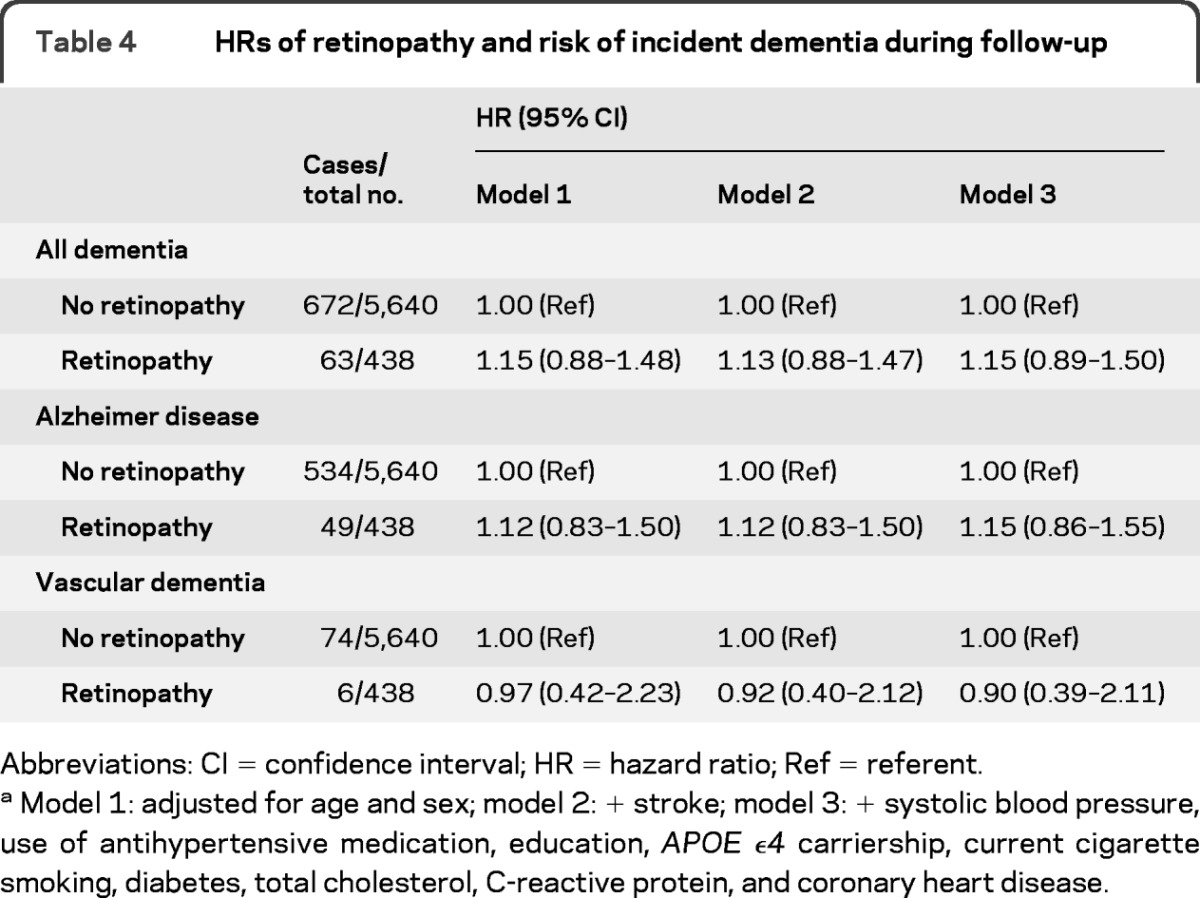
Abbreviations: CI = confidence interval; HR = hazard ratio; Ref = referent.
Model 1: adjusted for age and sex; model 2: + stroke; model 3: + systolic blood pressure, use of antihypertensive medication, education, APOE ϵ4 carriership, current cigarette smoking, diabetes, total cholesterol, C-reactive protein, and coronary heart disease.
Table 5.
HRs of retinopathy and risk of incident dementia according to follow-up time
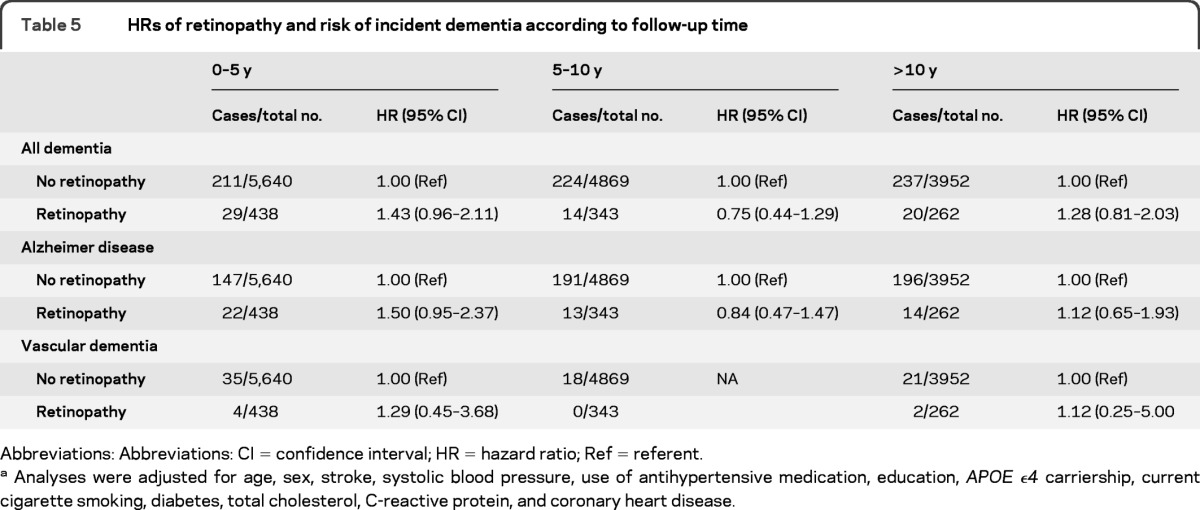
Abbreviations: Abbreviations: CI = confidence interval; HR = hazard ratio; Ref = referent.
Analyses were adjusted for age, sex, stroke, systolic blood pressure, use of antihypertensive medication, education, APOE ϵ4 carriership, current cigarette smoking, diabetes, total cholesterol, C-reactive protein, and coronary heart disease.
DISCUSSION
Our data suggest that retinopathy is more prevalent in persons with dementia but does not precede the development of incident dementia over time. Although the presence of retinopathy might be used as a general marker for microvascular pathology, it will not be useful as an early marker or risk predictor for dementia.
Strengths of our study include the population-based design, the large number of participants, and the virtually complete follow-up for incident dementia. Furthermore, we were able to investigate the associations of retinopathy not only with prevalent dementia and its major subtypes AD and vascular dementia but also with incident dementia during follow-up, for which thus far data were lacking. Some further methodologic issues should be discussed. Only the central field (Early Treatment Diabetic Retinopathy Study Field 2) was taken and used for grading of retinopathy, and we did not perform fluorescein angiography, which is a more invasive but more sensitive method to assess retinopathy. Any possible underestimation of retinopathy due to these methods will, however, be unrelated to the clinical characteristics of our participants and will therefore be random. We were not able to take the development of retinopathy during follow-up into account. Although retinopathy was assessed at every follow-up visit at the research center, we lack data on retinopathy from the participants who did not come to the follow-up visits. We would therefore create a selection bias if we would use these follow-up examinations for retinopathy, because, on average, people who do not return for follow-up are older, less healthy, and probably more likely to have signs of retinopathy. Furthermore, although the participation rate of 78% in the Rotterdam Study is high for a cohort study, we can never totally rule out the possibility of selection bias. We have no data on the people who refused to participate and cannot rule out the possibility that the risk of retinopathy or dementia was different in these people. However, given the long duration and the completeness of follow-up in our study, we consider distortion of the reported associations by selection bias unlikely.
Our results are in line with cross-sectional studies that found an association between retinopathy and dementia5,8 or lower performance on cognitive tests7,9, although there are some differences. In the Cardiovascular Health Study retinopathy was associated with dementia only in persons with hypertension or without diabetes5, and in the Blue Mountain Eye Study there was an association between retinopathy and a low score on the MMSE (<23) only in persons with hypertension. We found an association of retinopathy and prevalent dementia in the whole population and found no differences between persons with or without hypertension and diabetes. In the AGES-Reykjavik Study, retinopathy was associated with vascular dementia but not with all-cause dementia or AD.8 Although this result is in line with our findings regarding the relation between retinal vascular caliber and incident dementia, for which we also found an association with vascular dementia only,4 we found no differences with retinopathy between subtypes of dementia. Interestingly, in the Atherosclerosis Risk in Communities (ARIC) cohort, the investigators found an association of retinopathy with worse performance on 3 cognitive tests measuring memory, psychomotor speed, and executive functioning in cross-sectional analyses,9 whereas prospectively they only found an association with psychomotor speed and executive functioning and not with memory.6 In their discussion, the authors hypothesized that retinal vascular changes are related to cognitive impairment, which is specifically microvascular in origin, rather than to memory, which is more related to AD.6 The lack of an association with incident vascular dementia in our current study does not support this hypothesis. The lack of an association in the ARIC cohort with memory prospectively is, however, consistent with our lack of an association between retinopathy and incident all-cause dementia and AD.
It is of interest that we did not find an association of retinopathy with incident dementia or vascular dementia. Because retinopathy is considered to reflect more severe retinal microvascular disease and a breakdown of the blood-retinal barrier3,5 and we had previously found an association of retinal vascular caliber with incident vascular dementia,4 we had expected to find an association of retinopathy with incident overall or vascular dementia. One possible explanation for us not finding such a relation may be that retinopathy is a dichotomous exposure and, compared with the continuous measures of retinal vascular caliber, lacks power to find an association with vascular dementia, for which numbers of cases are limited. The effect size, however, does not support any association with incident vascular dementia. Next, a competing risk effect of mortality could lead to an underestimation of the effect, although it is unlikely that this would completely obscure the effect. In addition, it could be that during follow-up participants had overall better diagnosis and treatment of vascular risk factors (especially hypertension and diabetes) than before the start of the study and that this better treatment slightly reduced the risk of developing dementia. However, we consider it unlikely that this would completely distort a positive association between retinopathy at baseline and incident dementia, especially because we have found no interaction effects of hypertension or diabetes in the analyses with either prevalent or incident dementia. Furthermore, although retinal vascular caliber and retinopathy are both considered retinal microvascular signs, they can reflect different pathologic conditions. Whereas our previous association between retinal venular caliber and the risk of incident vascular dementia might reflect cerebral hypoperfusion,4 retinopathy might be more a reflection of arteriolar damage from hypertension and diabetes.3 Finally, it is unlikely that retinal microvascular abnormalities are a causal factor in the development of dementia or cognitive decline. They are more likely markers of underlying microvascular disease, which might contribute to the development of dementia or cognitive decline. Perhaps because retinopathy reflects a more severe stage of the microvascular abnormalities, it is actually not implausible that we only see this end stage of vascular pathology occurring at about the same time as dementia, but not before the clinical symptoms of dementia start developing. This theory would also explain why we find no differences between prevalent all-cause dementia, AD, and vascular dementia, because vascular pathology often coexists with AD pathology.1
GLOSSARY
- AD
Alzheimer disease
- ARIC
Atherosclerosis Risk in Communities
- CI
confidence interval
- CRP
C-reactive protein
- DSM-III-TR
Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised
- GMS
Geriatric Mental State schedule
- MMSE
Mini-Mental State Examination
- OR
odds ratio
AUTHOR CONTRIBUTIONS
E.M.C.S. contributed to data collection, performed the data analysis, conducted the statistical analysis, and drafted the manuscript. G.H.S.B. contributed to data collection and drafting of the manuscript. M.K.I. contributed to data collection and interpretation of the data. P.J.K contributed to data collection and interpretation of the data. A.H. contributed to the design of the study and interpretation of the data. J.R.V. contributed to data collection, interpretation of the data and study supervision. M.M.B.B. contributed to the design of the study, data collection, drafting of the manuscript, and study supervision. All authors reviewed, edited, and approved the final version of the manuscript.
DISCLOSURE
E. Schrijvers, G. Buitendijk, and M.K. Ikram report no disclosures. P. Koudstaal has received travel and national coordinator fees for the Perform study from Servier and is funded by grants from the Neurovascular Research Fund Rotterdam. A. Hofman is Editor-in-Chief of the European Journal of Epidemiology; received LpPLA2 summit travel grants; and is funded by grants from the Netherlands Genomics Initiative for the Rotterdam Study and from the Ministry of Health for the Generation R study. J. Vingerling reports no disclosures. M. Breteler is funded by the Netherlands Organization for Scientific Research grant 948–00-010 and grant 918–46-615 and received research support from NIH subaward to grant 1-R01-AG033193–01, the Dutch Cancer Society, the Netherlands Heart Foundation, and the Netherlands Brain Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Schneider JA, Bennett DA. Where vascular meets neurodegenerative disease. Stroke 2010;41:S144–S146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwa VI. Our eyes: windows to our souls or crystal balls? Lancet Neurol 2006;5:108–110 [DOI] [PubMed] [Google Scholar]
- 3. Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol 2001;46:59–80 [DOI] [PubMed] [Google Scholar]
- 4. de Jong FJ, Schrijvers EM, Ikram MK, et al. Retinal vascular caliber and risk of dementia: The Rotterdam Study. Neurology 2011;76:816–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker ML, Marino Larsen EK, Kuller LH, et al. Retinal microvascular signs, cognitive function, and dementia in older persons: the Cardiovascular Health Study. Stroke 2007;38:2041–2047 [DOI] [PubMed] [Google Scholar]
- 6. Lesage SR, Mosley TH, Wong TY, et al. Retinal microvascular abnormalities and cognitive decline: the ARIC 14-year follow-up study. Neurology 2009;73:862–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liew G, Mitchell P, Wong TY, et al. Retinal microvascular signs and cognitive impairment. J Am Geriatr Soc 2009;57:1892–1896 [DOI] [PubMed] [Google Scholar]
- 8. Qiu C, Cotch MF, Sigurdsson S, et al. Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology 2010;75:2221–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong TY, Klein R, Sharrett AR, et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: the Atherosclerosis Risk in Communities Study. Stroke 2002;33:1487–1492 [DOI] [PubMed] [Google Scholar]
- 10. Cheung N, Mosley T, Islam A, et al. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: a prospective study. Brain 2010;133:1987–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawasaki R, Cheung N, Mosley T, et al. Retinal microvascular signs and 10-year risk of cerebral atrophy: the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2010;41:1826–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222 [DOI] [PubMed] [Google Scholar]
- 13. Breteler MM, van Amerongen NM, van Swieten JC, et al. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging: the Rotterdam Study. Stroke 1994;25:1109–1115 [DOI] [PubMed] [Google Scholar]
- 14. Hofman A, van Duijn CM, Franco OH, et al. The Rotterdam Study: 2012 objectives and design update. Eur J Epidemiol 2011;26:657–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ott A, Breteler MM, van Harskamp F, Stijnen T, Hofman A. Incidence and risk of dementia: the Rotterdam Study. Am J Epidemiol 1998;147:574–580 [DOI] [PubMed] [Google Scholar]
- 16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 17. Copeland JR, Kelleher MJ, Kellett JM, et al. A semi-structured clinical interview for the assessment of diagnosis and mental state in the elderly: the Geriatric Mental State Schedule. I. Development and reliability. Psychol Med 1976;6:439–449 [DOI] [PubMed] [Google Scholar]
- 18. Roth M, Tym E, Mountjoy CQ, et al. CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986;149:698–709 [DOI] [PubMed] [Google Scholar]
- 19. American Psychiatry Association Diagnostic and Statistical Manual of Mental Disorders, 3rd ed, revised. Washington DC: American Psychiatry Association; 1987 [Google Scholar]
- 20. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 21. Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop Neurology 1993;43:250–260 [DOI] [PubMed] [Google Scholar]
- 22. Wenham PR, Price WH, Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet 1991;337:1158–1159 [DOI] [PubMed] [Google Scholar]


