Abstract
Glucocorticoids (GCs) provide a powerful and widely used anti-inflammatory and disease-modifying therapy for rheumatoid arthritis (RA). However, concerns about adverse effects are driving efforts to find ‘safer’ GC or GC analogues. One novel approach has been to change the timing of GC delivery, targeting the early hours of the morning to suppress the observed circadian peak in interleukin-6 (IL-6). The CAPRA-1 study has shown that this produces a clinically useful beneficial improvement in morning stiffness and mechanistic studies have shown that this correlates with a strong suppression of the IL-6 early morning peak. With no obvious additional adverse reactions, this improvement in the therapeutic ratio offers additional treatment options in RA, and perhaps in other inflammatory diseases that show circadian variation in symptoms.
Keywords: chronotherapy, glucocorticoids, modified-release prednisone, rheumatoid arthritis, safety
Introduction
Glucocorticoids (GCs) are one of the most commonly used treatments in rheumatoid arthritis (RA), but there are widespread concerns about adverse effects [van der Goes et al. 2010]. Alternative formulations have been developed to try to improve the benefits of GC treatment and a recent technological innovation in tablet formulation has allowed the introduction of GC chronotherapy, that is, medication delivered at specific, targeted times of the day, with the promise of greater safety and efficacy. Modified-release (MR) prednisone is one such formulation and consists of standard prednisone enclosed in a coating that allows time-targeted treatment. More specifically the medication is designed to be taken at bedtime. The outer coating slowly absorbs water from the gut and the active ingredient is rapidly released from the core and quickly absorbed through the gut approximately 4 h postingestion. This article reviews the rationale that led to the development of MR prednisone, why it works and why it might be better than routine GCs in clinical practice.
History of glucocorticoids in RA
The treatment of RA has progressed substantially in recent decades, and the advent of the biological treatment has revolutionized the way persistently active RA can be managed and the clinical outcomes that can be achieved. However, GCs have remained a valuable tool in the armoury of treatment options [Gorter et al. 2010], and continue to be extensively used today. Hench and colleagues were the first to recognize the therapeutic benefits in 1949 [Hench et al. 1949], although it was soon realized that the high doses then used to abolish inflammation resulted in significant and unacceptable adverse effects. Low-dose GCs continue to be used in combination with other disease-modifying antirheumatic drugs (DMARDs) and moderate and high doses help to manage acute flares of the disease, but adverse effects have remained a difficult problem.
These unwanted effects of GCs are wide ranging and include weight gain, bruising, immunosuppression, hypothalamic–pituitary–adrenal (HPA)-axis suppression, altered glycaemic control, glaucoma and hypertension. These are dose-related effects, therefore, although GCs are frequently used, the aim is always to use the lowest necessary dose for the shortest necessary time.
Disease-modifying properties
The ability of GCs to control inflammation is widely accepted. In RA they have an effect size that is substantially greater than other anti-inflammatory agents [Criswell et al. 1998; Gotzsche and Johansen, 1998]. However, more recently, and in addition to this, the disease-modifying properties of low-dose prednisolone in early disease have become apparent. First reported conclusively in 1995 [Kirwan, 1995], this has since been confirmed by several groups using different trial designs [Jacobs et al. 2006; Wassenberg et al. 2005; Svensson et al. 2005; Goekoop-Ruiterman et al. 2005; Landewé et al. 2002], and the evidence synthesized in a review by the Cochrane group [Kirwan et al. 2007]. The evidence in newly diagnosed patients suggests that the disease-modifying effect of combination DMARD therapy including GC might be as powerful as biological agents: a combination DMARD therapy including tapered high-dose prednisolone performed equally well as infliximab combined with methotrexate in slowing radiographic progression at 2 years [Goekoop-Ruiterman et al. 2007].
Attempts to minimize adverse effects
As the mechanisms of action of GCs have become more clear [Buttgereit et al. 2011a], so have the approaches to maximizing beneficial effects (mediated mainly through genomic transrepression), whilst minimizing unwanted effects (mediated mainly through genomic transactivation). Various methods have been tested including selective GC-receptor agonists [Schacke et al. 2006, 2009], liposomal drug delivery systems [Metselaar et al. 2003], and combining GCs with other drugs [Zimmermann, 2009]. So far these have not yielded medications that have reached routine clinical practice. Efforts have also been made to investigate how standard GCs could be used differently to maximize clinical benefit, and one possibility has been the timing of treatment.
Developing time-targeted treatment
The inflammatory symptoms of RA in any one patient are highly variable. This variation occurs from day to day, and week to week, but also within a 24-h period. During active disease in particular, symptoms of pain and joint stiffness are at their worst early in the morning [Bellamy et al. 1991; Harkness et al. 1982], and this is often used as a marker of disease activity in the clinical setting. Indeed, duration of early morning joint stiffness (EMS) correlates strongly with overall functional disability [Yazici et al. 2004]. However, the cause of the circadian variation in RA symptoms and EMS is not fully understood. It is likely to be related to parallel variations in inflammatory cytokines such as interleukin-6 (IL-6) and hormones of the HPA axis. As first elucidated by Arvidson and colleagues systemic IL-6 is elevated in patients with RA compared with healthy controls [Arvidson et al. 1994]. It also shows circadian variation and morning concentrations are significantly decreased in parallel with clinical improvement in symptoms after a 2-week course of prednisolone [Arvidson et al. 1994]. In 1997 the circadian variation of IL-6 and cortisol in RA patients was documented for a full 24-h period and showed that peak IL-6 occurs early in the morning [Crofford et al. 1997], and this was confirmed recently [Perry et al. 2009].
Although a direct link has yet to be proven, it is likely that the circadian IL-6 is correlated with symptoms such as EMS, which are also at their peak first thing in the morning. An inherent failure or undersecretion of HPA axis hormones has been suspected in RA [Jessop and Harbuz, 2005], and it may be this relative undersecretion of endogenous GC that allows IL-6 secretion to increase unchecked. As yet it is not entirely clear if deficiencies in the HPA axis are a cause or effect of the disease process.
The potential relationship between symptoms, circulating inflammatory compounds and hormones of the HPA axis led to the hypothesis that time-targeted treatment might result in amplification of the therapeutic benefit of low-dose GCs. In 1984, De Silva and colleagues showed that dosing at 10 p.m. improved EMS in RA [De Silva et al. 1984], as had also been shown in a small study of 10 patients in 1964 [Deandrade et al. 1964]. In 1997, Arvidson and colleagues went on to demonstrate that 2 a.m. dosing resulted in greater relief of EMS symptoms compared with the standard morning dosing regimen [Arvidson et al. 1997]. However, waking patients overnight to take medication is impractical, likely to cause poor concordance, and will itself alter the circadian rhythm. There is also the possibility that treatment in the evening or during the night might result in more potent suppression of the HPA axis and therefore increase this unwanted effect [Nichols et al. 1965].
The problem of convenient time-targeted dosing of GCs has now been solved by the development of various MR formulations with physical characteristics that ensure a reliable timed release of the contained drug. Two different approaches have been developed for RA [Buttgereit et al. 2008], and for other medical conditions such as Addison’s disease [Debono et al. 2009]. In the formulation that has been used in RA, the outer coating slowly absorbs water from the gut and prednisone, the active ingredient, is released rapidly from the core approximately 4 h +/-15 min postingestion. Thus tablets taken at 10 p.m. release the active ingredient at 2 a.m., the time of greatest change in systemic IL-6 concentrations and also the time of the lowest concentrations of intrinsically produced systemic cortisol.
In an alternative approach that has been used in patients with Addison’s disease, MR hydrocortisone tablets have an insoluble coating protecting all but the upper surface of the tablet. A delaying layer on this surface slowly erodes in the small intestine to release the active ingredient [Debono et al. 2009]. (Hydrocortisone is metabolized more quickly than prednisone, is shorter acting and has greater mineralocorticoid action, hence its preferential use in Addison’s disease.)
Clinical trials of MR prednisone
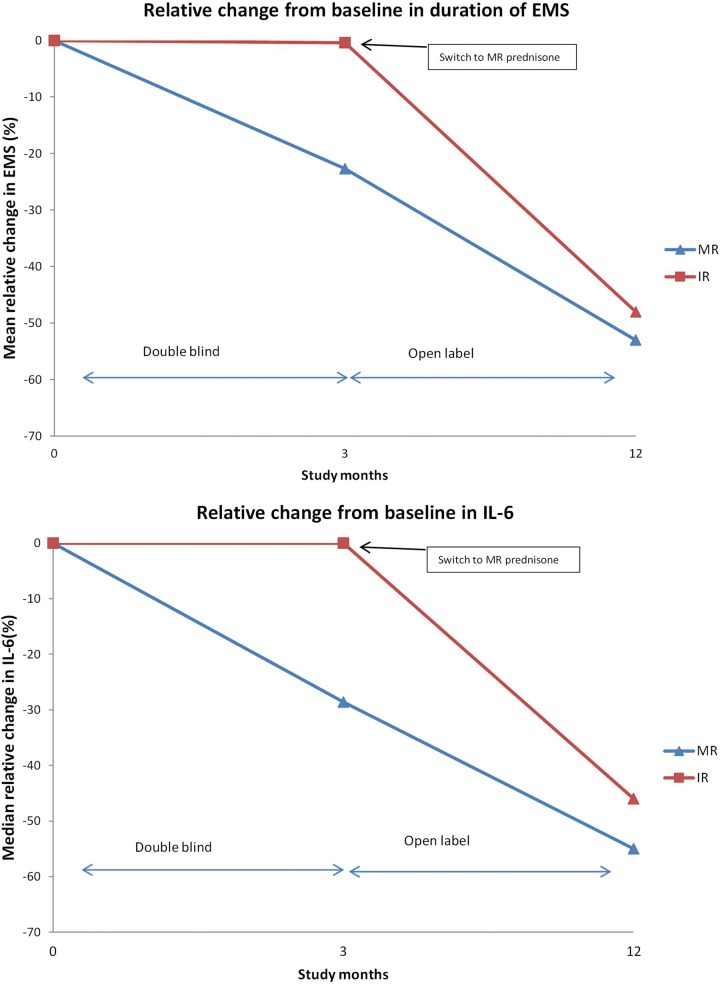
The clinical efficacy of this medication was first tested in a double-blind randomized controlled trial, CAPRA-1, published in 2008 [Buttgereit et al. 2008]. The primary aim of the study was to compare MR prednisone with standard immediate release (IR) prednisone in controlling symptoms of EMS in 288 patients with active RA. Patients were already established on prednisone at a variety of doses (2.5–10 mg daily) for at least 1 month prior to taking part in the study. Half of the patients were randomized to their usual dose of IR prednisone taken in the morning with a placebo taken at 10 p.m. The other half took a placebo in the morning and MR prednisone at their usual dose at 10 p.m. Thus the total dose of prednisone was unaltered for each patient, only the timing of its absorption into the bloodstream (soon after the morning dose, or 4 h after the evening dose at approximately 2 a.m.). The study lasted for 12 weeks in the blind phase and another 36 weeks in an open follow-up phase in which almost all patients took the night-time treatment (Figure 1) [Buttgereit et al. 2010].
Figure 1.
Illustration of EMS and IL-6 following IR or MR prednisone in the CAPRA-1 trial (n = 288) and the open-label extension (n = 249). (Adapted from Buttgereit et al. [2008, 2010].)
EMS, early morning joint stiffness; IL-6, interleukin-6; IR, immediate release; MR, modified release.
There was a significant mean (standard deviation) reduction in duration of EMS in the MR arm, -44 min (137), compared with the IR arm, -23 min (138) (p = 0.045) at 12 weeks with a difference in duration of EMS between the two groups evident from 2 weeks (Figure 1 and Table 1). There was also a significant relative reduction in IL-6 at 12 weeks (p = 0.0322), although other laboratory and clinical measures did not show significant changes.
Table 1.
Early morning stiffness duration and interleukin-6 data for the CAPRA-1 trial and open-label extension.
|
EMS (min) |
IL-6 (IU/L) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MR prednisone | IR prednisone | MR prednisone | IR prednisone | ||||||||
| Baseline | Mean (SD) | 164.1 (101.4) n = 125 | 182.5 (125.0) n = 129 | ||||||||
| Median (range) | 146.6 (13.6–659.3) n = 125 | 152.9 (32.1–720) n = 129 | 860 (200–23,000) n = 142 | 1110 (200–20,800) n = 142 | |||||||
| Absolute change at 3 months (end of double-blind phase) | Mean (SD) | −43.96*(136.6) n = 125 | −22.68 (138.1) n = 129 | ||||||||
| Median (range) | −39.3 (−537.6–600) n = 125 | −21.5 (−586.3–618.6) n = 129 | −160* (−13,460.0–9080.0) n = 135 | 0 (−16,190.0– 18,100.0) n = 132 | |||||||
| MR/MR group | IR/MR group | MR/MR group | IR/MR group | ||||||||
| Absolute change at 12 months (end of open phase) | Mean (SD) | 83 (83.7) n =103 | 88 (128.3) n = 114 | ||||||||
| Median (range) | −390* (200–18,300) n = 103 | −595* (200–8100) n = 114 | |||||||||
In the open-label extension almost all patients took part and either continued or transferred to the MR prednisone treatment. Those continuing on MR prednisone showed a sustained benefit, including a further (though uncontrolled) reduction in EMS, and 17% of patients no longer had EMS at 12 months [Buttgereit et al. 2010]. The additional reduction in EMS in the open-label phase could at least be partly a placebo effect [Jacobs and Bijlsma, 2010]. The control patients who switched to night-time MR prednisone showed a substantial reduction in EMS, mirroring the treated group during the blinded phase of the study, and adding support to the main study finding.
Underlying mechanisms
A separate study sought to investigate the possible mechanisms of time-targeted treatment by investigating the influence of the MR medication on circadian variations in IL-6 and cortisol in patients with active RA [Clarke et al. 2011]. Nine patients with active RA who were not taking GCs and had not received GCs in the previous 3 months were recruited. Blood samples were taken over a 24-h period before and after a 2-week course of 5 mg MR prednisone. The circadian variation in IL-6 was again confirmed in the pretreatment measurements, rising to a peak of 42 ng/L (42 pg/ml) at 8:05 a.m. Following only 2 weeks of treatment with this relatively low dose of prednisone at 2 a.m., IL-6 concentrations were reduced throughout the night, peak levels only reaching 21 ng/L (21 pg/ml) and at a much earlier time (01:21 a.m.), with concentrations being close to afternoon values at the time of waking. The difference between the circadian patterns was highly significant (p < 0.005). Importantly, cortisol concentrations were not significantly suppressed posttreatment [Clarke et al. 2011].
Safety
In the CAPRA-1 trial there were no significant differences in the rates of adverse events reported in the two arms of the study [Buttgereit et al. 2008], with equal rates of any adverse event between the two arms. Treatment-related event rates were 13% in the MR arm versus 11% in the IR arm. The short-term safety of 5 mg of MR prednisone plus standard DMARD therapy compared with placebo plus DMARD therapy was compared in the CAPRA-2 trial with very little difference between the two arms (43% versus 49%) [Buttgereit et al. 2011b].
In an open-label extension of the CAPRA-1 trial the authors reported that adverse events for MR prednisone did not differ from the known profile of standard prednisone although there was no formal comparison arm [Buttgereit et al. 2010]. Therefore, the balance of evidence suggests both a relatively low and an equal rate of adverse effects from morning and evening low-dose GCs. However, there are limitations to the interpretation of this adverse event data and more detailed studies are needed to assess long-term adverse event rates.
Although the side effects of high-dose GCs are widely recognized, the adverse events associated with low-dose GCs (prednisolone 7.5 mg and below) have probably been overestimated. A systematic review of studies using low-dose prednisolone suggested that adverse event rates are probably not significantly different from placebo [Da Silva et al. 2006]. Others have, however, found significantly increased adverse events even at low doses [Huscher et al. 2009]. The main limitation to interpreting adverse event rates is the wide difference in recording methods for the reporting of GC-induced adverse events in different studies, which may account for some of the confusion [Hoes et al. 2009]. The adverse events recorded for the MR prednisone studies have relied on patient reporting rather than predefined standardized data collection, which may be a limitation. Standardization of recording of GC-induced adverse events has been suggested for future trials to elucidate fully the safety of low-dose GCs [van der Goes et al. 2010]. Such standardization will then allow comparisons of the safety of standard low-dose prednisone compared with MR prednisone and other novel formulations.
To answer the specific concern of more potent HPA-axis suppression with MR prednisone, the same group has investigated corticotropin-releasing hormone (CRH) tests on 28 patients from the original CAPRA-1 trial. In comparing standard to MR prednisone there was no difference in adrenocortical function between the two groups [Alten et al. 2010], which is reassuring in this respect. Also when the CRH test was performed on those patients who had taken 12 months of MR prednisone, there was no change from baseline (mean increase in plasma cortisol of 151.7 mmol/L at baseline on IR prednisone versus 146.2 mmol/L after 12 months MR prednisone). A separate within-patient analysis of 17 patients within the CAPRA-1 study who had CRF tests at baseline on IR prednisone and then after 3 or 9 months of MR prednisone also showed no additional suppression of the HPA axis after MR prednisone. There was even some evidence of an improvement in function (p < 0.05, paired t-test) [Kirwan, 2011].
Further implications
Studies so far suggest that MR prednisone is superior to standard prednisone in controlling clinical symptoms of EMS. The other beneficial effects of low-dose prednisone in early RA include increased remission and reduced radiological progression. It will be interesting, therefore, to see if MR prednisone also has disease-modifying properties that might even be amplified as a result of the timed application. If markers of inflammation, such as IL-6 and CRP, show a circadian variation then perhaps joint destruction is also occurring in a time-dependent fashion, which could be better targeted by MR treatment. However, further work is needed to investigate this possibility.
MR prednisone use in standard practice may negate the need for escalation to (much more expensive) biological therapy in some patients, which has clear cost implications. It may also reduce the adverse events associated with escalating doses or frequent pulses of high-dose GCs. EMS remains a persistent symptom for many RA patients with a high impact on early retirement [Westhoff et al. 2008], therefore this group of patients may also benefit.
Polymyalgia rheumatica is an inflammatory condition in which virtually the only treatment option is GCs and the doses required, usually for around 2 years, almost always cause adverse effects [Hernandez-Rodriguez et al. 2009]. The circadian nature of symptoms in this disease are highly pronounced [Salvarani et al. 2002], and if this coincides with a circadian variation in inflammatory cytokines, then the rationale for time-targeted treatment might also apply. This is another condition in which underactivity of the HPA axis is thought to play a role in pathogenesis [Cutolo and Straub, 2000; Cutolo et al. 2002; Demir et al. 2006], and elevated circulating cytokines, such as IL-6, have been found [Alvarez-Rodriguez et al. 2010; Roche et al. 1993]. If lower doses of GCs could be used with similar clinical efficacy then the treatment of this condition would become much safer with fewer dose-related GC-induced adverse effects [Kirwan and Zakout, 2011].
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
J. Kirwan and L. Clarke have received an unconditional educational grant from NitecPharmaAG (now Horizon Pharma Inc).
Contributor Information
Lynsey Clarke, Academic Rheumatology Unit, Bristol Royal Infirmary, Bristol, UK.
John Kirwan, Academic Rheumatology Unit, The Courtyard, Bristol Royal Infirmary, Bristol BS2 8HW, UK.
References
- Alten R., Doring G., Cutolo M., Gromnica-Ihle E., Witte S., Straub R., Buttgereit F. (2010) Hypothalamus-pituitary-adrenal axis function in patients with rheumatoid arthritis treated with nighttime-release prednisone. J Rheumatol 37: 2025–2031 [DOI] [PubMed] [Google Scholar]
- Alvarez-Rodriguez L., Lopez-Hoyos M., Mata C., Jose Marin M., Calvo-Alen J., Blanco R., et al. (2010) Circulating cytokines in active polymyalgia rheumatica. Ann Rheum Dis 69: 263–269 [DOI] [PubMed] [Google Scholar]
- Arvidson N.G., Gudbjornsson B., Elfman L., Ryden A., Totterman T.H., Hallgren R. (1994) Circadian rhythm of serum interleukin-6 in rheumatoid arthritis. Ann Rheum Dis 53: 521–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidson N.G., Gudbjornssen B., Larsson A., Hallgren R. (1997) The timing of glucocorticoid administration in rheumatoid arthritis. Ann Rheum Dis 56: 27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy N., Sothern R.B., Campbell J., Buchanan W.W. (1991) Circadian rhythm in pain, stiffness, and manual dexterity in rheumatoid arthritis: relation between discomfort and disability. Ann Rheum Dis 50: 243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit F., Burmester G., Straub R., Seibel M., Zhou H. (2011a) Exogenous and endogenous glucocorticoids in rheumatic diseases. Arthritis Rheum 63: 1–9 [DOI] [PubMed] [Google Scholar]
- Buttgereit F., Doering G., Schaeffler A., Witte S., Sierakowski S., Gromnica-Ihle E., et al. (2008) Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet 371: 205–214 [DOI] [PubMed] [Google Scholar]
- Buttgereit F., Doering G., Schaeffler A., Witte S., Sierakowski S., Gromnica-Ihle E., et al. (2010) Targeting pathophysiological rhythms: prednisone chronotherapy shows sustained efficacy in rheumatoid arthritis. Ann Rheum Dis 69: 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit F., Mehta D., Kirwan J., Szechinski J., Boers M., Alten R., et al. (2011b) Low-dose glucocorticoid chronotherapy of rheumatoid arthritis: 12 week efficacy and safety of modified-release (MR) prednisone. Arthritis Rheum 62 (Suppl 10): 392 [Google Scholar]
- Clarke L., Jessop D., Hunt L., Straub R., Perry M., Kirwan J. (2011) Alleviation of morning joint stiffness by low-dose prednisone in rheumatoid arthritis is associated with circadian changes in IL-6 and cortisol. Int J Clin Rheumatol 6: 241–249 [Google Scholar]
- Criswell L., Saag K., Sems K., Welch V., Shea B., Wells G., et al. (1998) Moderate term, low-dose corticosteroids for rheumatoid arthritis. Cochrane Database Syst Rev: CD001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofford L., Kalogeras K., Mastorakos G., Magiakou M., Wells J., Kanik K., et al. (1997) Circadian relationships between interleukin (IL)-6 and hypothalamic-pituitary-adrenal axis hormones: failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. J Clin Endocrinol Metab 82: 1279–1283 [DOI] [PubMed] [Google Scholar]
- Cutolo M., Straub R. (2000) Polymyalgia rheumatica: evidence for a hypothalamic-pituitary-adrenal axis-driven disease. Clin Exp Rheumatol 18: 655–658 [PubMed] [Google Scholar]
- Cutolo M., Straub R., Foppiani L., Prete C., Pulsatelli L, Sulli A., et al. (2002) Adrenal gland hypofunction in active polymyalgia rheumatica. Effect of glucocorticoid treatment on adrenal hormones and interleukin 6. J Rheumatol 29: 748–756 [PubMed] [Google Scholar]
- Da Silva J., Jacobs J., Kirwan J., Boers M., Saag K., Ines L., et al. (2006) Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data. Ann Rheum Dis 65: 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deandrade J.R., McCormick J.N.A., Hill A.G.S. (1964) Small doses of prednisolone in management of rheumatoid arthritis. Ann Rheum Dis 23: 158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debono M., Ghobadi C., Rostami-Hodjegan A., Huatan H., Campbell M., Newell-Price J., et al. (2009) Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab 94: 1548–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir H., Tanriverdi F., Ozogul N., Calis M., Kirnip M., Durak A., et al. (2006) Evaluation of the hypothalamic-pituitary-adrenal axis in untreated patients with polymyalgia rheumatica and healthy control. Scand J Rheumatol 35: 217–223 [DOI] [PubMed] [Google Scholar]
- De Silva M., Binder A., Hazleman B. (1984) The timing of prednisolone dosage and its effect on morning stiffness in rheumatoid arthritis. Ann Rheum Dis 43: 790–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goekoop-Ruiterman Y., de Vries-Bouwstra J., Allaart C., van Zeben D., Kerstens P., Hazes J., et al. (2005) Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 52: 3381–3390 [DOI] [PubMed] [Google Scholar]
- Goekoop-Ruiterman Y., de Vries-Bouwstra J., Allaart C., van Zeben D., Kerstens P., Hazes M., et al. (2007) Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med 146: 406–415 [DOI] [PubMed] [Google Scholar]
- Gorter S., Bijlsma J., Cutolo M., Gomez-Reino J., Kouloumas M., Smolen J., et al. (2010) Current evidence for the management of rheumatoid arthritis with glucocorticoids: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 69: 1010–1014 [DOI] [PubMed] [Google Scholar]
- Gotzsche P.C., Johansen H.K. (1998) Meta-analysis of short term low dose prednisolone versus placebo and non-steroidal anti-inflammatory drugs in rheumatoid arthritis. BMJ 316: 811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness J., Rickter M., Panayi G., van de, Pette K., Unger A., Pownall R., et al. (1982) Circadian variation in disease activity in rheumatoid arthritis. BMJ 284: 551–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hench P., Kendall E., Slocumb C., Polley H. (1949) The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin 24: 181–197 [PubMed] [Google Scholar]
- Hernandez-Rodriguez J., Sid M., Lopez-Soto A., Espigol-Figole G., Bosch X. (2009) Treatment of polymyalgia rheumatica. Arch Intern Med 169: 1839–1850 [DOI] [PubMed] [Google Scholar]
- Hoes J., Jacobs J., Verstappen S., Bijlsma J., Van der Heijden G. (2009) Adverse events of low- to medium-dose oral glucocorticoids in inflammatory diseases: a meta-analysis. Ann Rheum Dis 68: 1833–1838 [DOI] [PubMed] [Google Scholar]
- Huscher D., Thiele K., Gromnica-Ihle E., Hein G., Demary W., Dreher R., et al. (2009) Dose-related patterns of glucorticoid-induced side efffects. Ann Rheum Dis 68: 1119–1124 [DOI] [PubMed] [Google Scholar]
- Jacobs J., Bijlsma J. (2010) Modified release prednisone in patients with rheumatoid arthritis. Ann Rheum Dis 69: 1257–1259 [DOI] [PubMed] [Google Scholar]
- Jacobs J., van Everdingen A., Verstappen S., Bijlsma J. (2006) Followup radiographic data on patients with rheumatoid arthritis who participated in a two-year trial of prednisone therapy or placebo. Arthritis Rheum 54: 1422–1428 [DOI] [PubMed] [Google Scholar]
- Jessop D.S., Harbuz M.S. (2005) A defect in cortisol production in rheumatoid arthritis: why are we still looking? Rheumatology 44: 1097–1100 [DOI] [PubMed] [Google Scholar]
- Kirwan J. (1995) The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The Arthritis and Rheumatism Council Low-Dose Glucocorticoid Study Group. N Engl J Med 333: 142–146 [DOI] [PubMed] [Google Scholar]
- Kirwan J. (2011) Targeting the time of day for glucocorticoid delivery in rheumatoid arthritis. Int J Clin Rheum 6: 273–279 [Google Scholar]
- Kirwan J., Bijlsma J., Boers M., Shea B. (2007) Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Database Syst Rev: CD006356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan J., Zakout S. (2011) Polymyalgia rheumatica has a nocturnal rise in serum interleukin-6 which is almost completely suppressed by nighttime prednisone. Arthritis Rheum 63 (Suppl 27): 79 [Google Scholar]
- Landewé R., Boers M., Verhoeven A., Westhovens R., van de, Laar M., Markusse H., et al. (2002) COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum 46: 347–356 [DOI] [PubMed] [Google Scholar]
- Metselaar J., Wauben M., Wagenaar-Hilbers J., Boerman O., Storm G. (2003) Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum 48: 2059–2066 [DOI] [PubMed] [Google Scholar]
- Nichols T., Nugent C., Tyler F. (1965) Diurnal variation in suppression of adrenal function by glucocorticoids. J Clin Endocrinol Metab 25: 343. [DOI] [PubMed] [Google Scholar]
- Perry M., Kirwan J., Jessop D., Hunt L. (2009) Overnight variations in cortisol, interleukin 6, tumour necrosis factor alpha and other cytokines in people with rheumatoid arthritis. Ann Rheum Dis 68: 63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche N., Fulbright J., Wager A., Hunder G., Goronzy J., Weyand C. (1993) Correlation of interleukin-6 production and disease activity in polymyalgia rheumatica and giant cell arteritis. Arthritis Rheum 36: 1286–1294 [DOI] [PubMed] [Google Scholar]
- Salvarani C., Cantini F., Boiardi L., Hunder G. (2002) Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med 347: 261–271 [DOI] [PubMed] [Google Scholar]
- Schäcke H., Rehwinkel H., Asadullah K., Cato Al. (2006) Insight into the molecular mechanisms of glucocorticoid receptor action promotes identification of novel ligands with an improved therapeutic index. Exp Dermatol 15: 565–573 [DOI] [PubMed] [Google Scholar]
- Schäcke H., Zollner T., Docke W., Rehwinkel H., Jaroch S., Skuballa W., et al. (2009) Characterization of ZK 245186, a novel, selective glucocorticoid receptor agonist for the topical treatment of inflammatory skin diseases. Br J Pharmacol 158: 1088–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B., Boonen A., Albertsson K., van der Heijde D., Keller C., Hafstrom I. (2005) Low-dose prednisolone in addition to the initial disease-modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate – a two-year randomized trial. Arthritis Rheum 52: 3360–3370 [DOI] [PubMed] [Google Scholar]
- van der Goes M., Jacobs J., Boers M., Andrews T., Blom-Bakkers M., Buttgereit F., et al. (2010) Monitoring adverse events of low-dose glucocorticoid therapy: EULAR recommendations for clinical trials and daily practice. Ann Rheum Dis 69: 1913–1919 [DOI] [PubMed] [Google Scholar]
- Wassenberg S., Rau R., Steinfeld P., Zeidler H. (2005) Very low-dose prednisolone in early rheumatoid arthritis retards radiographic progression over two years: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 52: 3371–3380 [DOI] [PubMed] [Google Scholar]
- Westhoff G., Buttgereit F., Gromnica-Ihle E., Zink A. (2008) Morning stiffness and its influence on early retirement in patients with recent onset rheumatoid arthritis. Rheumatology 47: 980–984 [DOI] [PubMed] [Google Scholar]
- Yazici Y., Pincus T., Kautiainen H., Sokka T. (2004) Morning stiffness in patients with early rheumatoid arthritis is associated more strongly with functional disability than with joint swelling and erythrocyte sedimentation rate. J Rheumatol 31: 1723–1726 [PubMed] [Google Scholar]
- Zimmermann G., Avery W., Finelli A., Farwell M., Frase C., Borisy A. (2009) Selective amplification of glucocorticoid anti-inflammatory activity through synergistic multi-target action of a combination drug. Arthritis Res Ther 11: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]