Abstract
Background
To date, there have been conflicting reports of the association of psychosocial stressors with prenatal corticotropin-releasing hormone (CRH) levels.
Methods
We examined whether racial discrimination, community violence, interpersonal violence (IPV), negative life events, considered independently, and as a composite measure of cumulative stress, were associated with prenatal CRH levels in the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project, a multiethnic pre-birth cohort in urban Boston. Blood was collected between 20 and 37 weeks gestation (Mean = 28.1, SD = 4.6 weeks gestation). During pregnancy, women were administered the Conflict Tactics Scale survey to assess IPV, the Crisis in Family Systems-Revised survey to assess negative life events, the My Exposure to Violence survey to assess community violence, and the Experiences of Discrimination survey. A cumulative stress measure was derived from these instruments to characterize exposure to high levels of multiple stressors.
Results
None of the individual stressors or cumulative stress was associated with CRH in combined analyses including Whites (n=20), Blacks (n=46), and Hispanics (n=110). In separate analyses of Blacks and Hispanics, racial discrimination, community violence, and cumulative stress were associated with CRH in Blacks, but were not associated with CRH in Hispanics.
Conclusions
Though these results require replication, they suggest that the effects of stress on prenatal CRH levels may be mediated by factors that differ between racial/ethnic groups. Further studies in larger samples are warranted to clarify whether associations of chronic stressors and prenatal CRH levels differ by race/ethnicity and to better understand underlying mechanisms.
INTRODUCTION
In the United States (U.S.), substantial racial disparities in preterm birth exist, with rates of 18.3% in Blacks, 12.3% in Hispanics, and 11.5% in Whites in 2007 (1). The causes of these disparities are multi-factorial and remain even after taking into account known risk factors for preterm birth (2), which has led to the hypothesis that chronic stressors prevalent in minority populations might, in part, account for these disparities (3–5).
It has been hypothesized that chronic stress might alter a woman’s HPA axis, either before she conceives or during pregnancy, thus changing the endocrine environment in which the placenta is established and predisposing a woman to delivering before term by impacting endocrine dynamics between the mother, fetus, and placenta (3). In particular, altered production of corticotropin-releasing hormone (CRH) levels in mid to later pregnancy in response to stress may lead to increases in risk of preterm birth (6). CRH, a key factor in the hypothalamic pituitary adrenal axis, is not detectable in peripheral blood in the non-pregnant state but is produced by the placenta, decidua, and fetal membranes during pregnancy and rises exponentially until delivery (7, 8). CRH has received particular attention because elevated levels during the second and third trimesters have been consistently associated with risk of preterm birth, though the reasons are unknown (9–11). Chronic psychosocial stress might increase the risk of preterm birth through increases in placental CRH which may be upregulated in response to maternal cortisol and catecholamine stress response alterations that predate conception (4, 6, 12). Though cortisol exhibits negative feedback on hypothalamic CRH, both maternal and fetal cortisol upregulate placental CRH in vitro (13, 14).
Studies examining the associations of prenatal CRH levels with a wide range of psychosocial measures, including abuse, pregnancy related anxiety, perceived stress, coping styles, and negative life events have reported mostly null associations (15–18). Others have reported associations of perceived stress and pregnancy anxiety with elevated mid to late pregnancy CRH (18–20). However, studies on the associations of prenatal CRH levels and more chronic stressors are scant and typically consider each stressor type independently. For example, Chen et al. recently reported finding no associations between the chronic stressor of childhood or adult abuse with prenatal CRH levels in a study which consisted of a large number of minority women (18).
Overlapping stress research suggests that individuals may be increasingly vulnerable to disrupted physiology and negative health outcomes when exposed to the cumulative effects of multiple stressors (21–23). Cumulative environmental stressors may contribute to accelerated bodily wear and tear more than each stressor considered independently (24). Such considerations may be particularly relevant in urban poor communities, where exposure to multiple stressors is more prevalent. For example, a recent large study of pregnant women demonstrated that women of lower SES are more likely to experience multiple stressors (25). Specifically, lower-income ethnic minority women are more likely to experience financial hardships (25), exposure to community violence (26), racism or discrimination (27), and interpersonal violence (28, 29). No prior study has examined the relationship between experiencing multiple stressors together (i.e. cumulative stress) in relation to CRH production during pregnancy. Our study further contributes to the literature by studying the impacts of cumulative effects of several chronic stressors, which may have even greater impacts on allostatic load (23).
We examined the cumulative effects of stressors from multiple domains including community violence, interpersonal violence, racial discrimination, and other negative life events on maternal prenatal CRH levels in a cohort of mostly minority women in urban Boston. We hypothesized that greater cumulative stress would be associated with elevated mid to later pregnancy CRH levels in these women. We also examined whether these associations differed by race/ethnicity given prior evidence in this cohort showing racial differences in stress-elicited changes in prenatal maternal cortisol levels (30)
METHODS
Study design and population
Study participants were enrolled in the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project, a prospective cohort of 997 mother-child pairs originally designed to examine the impact of prenatal and early life stress and environmental factors on childhood asthma risk in an urban setting. Details of the study design have been published elsewhere (31). In brief, participants were recruited from women receiving prenatal care at Brigham and Women’s Hospital, Boston Medical Center, and three community health centers and their associated WIC programs in metropolitan Boston. Trained research assistants approached women receiving prenatal care on selected clinic days between August 2002 and January 2007. Women who did not speak English or Spanish or who were younger than 18 years of age were not eligible to participate. At baseline, a questionnaire was administered to obtain information on medical history and demographics. In subsequent study visits conducted in the home and in the clinic, questionnaires were administered to obtain information on psychosocial stressors. Written informed consent was obtained, and the study was approved by human studies committees at Brigham and Women’s Hospital and Boston Medical Center.
Supplemental funding was obtained to measure CRH levels in a subsample of 223 of the 658 women who provided blood samples during pregnancy. Women were excluded if they had a non-singleton pregnancy or reported use of exogenous steroids or shift work, as these factors may disrupt the hormonal stress axes in pregnancy. Samples were analyzed on the basis of participants having information on depressive symptoms and sufficient serum. After further exclusions due to race/ethnicity other than White, Black, or Hispanic (n=14), blood sampling earlier than 20 weeks gestation (n=2), and missing information on at least one of the four psychosocial measures (n=29), 176 women remained for this analysis. Compared to mothers not included in the analysis, those who were included were similar in age, parity, and pre-pregnancy BMI distributions. Mothers who were included in the analysis were more likely to be U.S. born (44% vs. 35%, p=0.02).
CRH measurement
Maternal plasma was collected in EDTA tubes at a home visit scheduled for research purposes between 20.4 and 37.4 weeks gestation (Mean = 28.1, SD = 4.6 weeks gestation). Blood samples were collected between 1 pm and 5 pm at least 5 hours after morning awakening. The samples were cooled, centrifuged and stored at −70 C degrees. Briefly, either 150 ul (for <33 weeks since last menstrual period, LMP) or 40 ul (for ≥33 weeks since LMP) was extracted with a final concentration of 87.5% methanol and was assayed using previously described methods (10, 32). Assay sensitivity was 10.8 pg/ml. The samples less than 10.8 pg/ml (n=2) were assigned 10.8 pg/ml. Average intra-assay error at 70.8 pg/ml was 9.7%. Coefficient of variation was 4.6%.
Assessments of psychosocial stressors
Intimate partner violence
Intimate partner violence (IPV) was assessed using the revised Conflict Tactics Scale (CTS) short form, which has previously documented reliability and validity in both English and Spanish speaking samples (33, 34). Women were asked whether they had ever been pushed, grabbed, or shoved, kicked bit, or punched, hit with something that hurt their body, choked or burned, forced to have sexual activities, or physically attacked in some other way. Participants were asked if they had experienced these events during adulthood, including both prior to and during the current pregnancy. Of the few women who reported abuse during pregnancy (n=12), 33% also experienced abuse during adulthood before this pregnancy. Thus, both periods were combined to represent IPV exposure. A summary score for interpersonal violence was obtained by summing the number of events experienced. Exposure was categorized as none (81%), 1 or 2 (11%), and 3 or more (8%) types of adult IPV.
Community violence
Community violence was measured using the My Exposure to Violence (ETV) survey (35), which has previously described acceptable internal consistency, test-retest reliability, and validity. Women were asked whether they had either directly experienced or witnessed specific events in the last year in their neighborhood, including hearing gunshots, witnessing and/or experiencing shoving, hitting or punching, knife attacks, and shootings. In addition, participants were asked about other factors known to influence the impact of violence, including location, frequency, and identity of the victim and/or perpetrator. Because the CTS assesses interpersonal violence, we only included items from the ETV which occurred outside of the home.
To summarize responses to the multi-item community ETV questionnaire into a continuous variable, we implemented previously described Rasch modeling techniques to produce continuous scores while taking into account the severity and frequency with which each of the violent acts was experienced or witnessed (36). While Rasch scores do not have an absolute meaning, they have relative meaning; a lower score indicates a woman experiencing less severe violence (e.g. pushing/shoving as opposed to stabbings/shootings) and/or less frequent exposure compared to a woman with a higher score. We categorized women with the lowest possible score into low exposure and used the median of the remaining values as a cutoff, thus producing categories of low (49%), medium (26%) and high exposure (24%) to community violence.
Discrimination
Perceived racial discrimination was measured using the Experiences of Discrimination (EOD) Scale, which has previously documented high validity and reliability in working class African Americans and Hispanics (37). The EOD has also been shown to be sensitive to effects on preterm delivery and low birth weight (38). Women were asked whether they had experienced unfair or bad treatment because of their race or ethnicity in eight domains, including at school, getting hired or getting a job, at work, getting housing, getting medical care, getting service in a store or restaurant, on the street or in a public setting, or from the police or in the courts. Items were summed across each of the eight situations to produce a summary score for discrimination. Discrimination categories of 0 (57%), 1 or 2 (21%), and 3 or more (22%) types of discrimination were created, with 1 or 2 types as the reference category based on previous work that demonstrated elevated health risks in both the lowest and highest categories of racial discrimination in working class black populations (39).
Other negative life events
During the prenatal home visit, participants were administered The Crisis in Family Systems-Revised (CRYSIS-R), which assesses 63 potentially negative life events spanning several domains (financial, legal, career, stability in relationships, safety in the home, safety in the community, medical issues pertaining to the respondent, medical issues pertaining to others, housing problems, difficulty with authority, and prejudice) occurring in the past six months (40). Participants were also asked to rate each event as positive, negative, or neutral. This scale has been validated in both English and Spanish in low income participants from urban settings (40). For each woman, we summed up the number of domains for which she endorsed at least one event to produce a negative life event domains score. Negative life events were categorized as occurring in 0 domains (31%), 1 or 2 domains (40%), and 3 or more domains (29%) using tertile cut-points.
Cumulative stress
A summary score of cumulative stress was created by combining the four subscales of negative life events, racism, IPV, and community violence. We first created quartiles of each of the subscales and created a dummy variable that equaled 1 if they were in the upper quartile and the value of 0 if they were in the bottom three quartiles. The cumulative stress measure was then created by summing over the dichotomous variables. Categories of cumulative stress scores of 0 (46%), 1 (26%), and 2 or more (28%) were then created. Because very few women had cumulative stress scores of more than 2 (n=17), we collapsed women who had cumulative stress scores of 2 or above into one category.
Assessment of covariates
Information on sociodemographic factors, health behaviors, self-reported last menstrual period, and pre-pregnancy weight and height was obtained in a baseline questionnaire. Because maternal CRH levels rise from the second trimester onwards, we adjusted for gestational age at time of blood draw, which was calculated using self-reported last menstrual period in the baseline questionnaire. Race/ethnicity information was obtained using the question, “Are you Hispanic or Latino?”, followed by “What is your race? (White/Caucasian, Black/African American, Native Hawaiian or other Pacific Islander, Asian, American Indian/Alaskan native or other)”. Women who responded “Yes” to identifying as Hispanic/Latino were classified as Hispanic/Latino with one exception; women who identified themselves as Hispanic/Latino and subsequently answered “Black/African” to the race question were classified as Black. Women who responded “No” to identifying as Hispanic/Latino were assigned into race/ethnicity categories based upon their answers to the race question.
We also adjusted for other potential confounders identified as predictors of pregnancy CRH in the literature, including parity, race/ethnicity, education, and pre-pregnancy BMI (36), which was calculated as weight in kilograms divided by height in meters squared. We also adjusted for nativity because of the documented difference in rates of poor birth outcomes in U.S. versus foreign born populations in both Blacks and Hispanics.
ANALYSIS
Due to non-normality, all CRH values were log-transformed. Prior to examining our associations of interest, we estimated mean levels of log CRH adjusted for gestational age at blood draw by descriptive factors using ANCOVA. Linear regression was then employed to compare mean levels of log CRH across categories of discrimination, interpersonal violence, community violence, negative life events, and cumulative stress while adjusting for gestational age at blood draw, education, parity, pre-pregnancy BMI, age, and nativity, and race/ethnicity. Gestational age was added to regression models as a linear term because the likelihood ratio test comparing models with and without a squared term for gestational age was not statistically significant. We also considered whether the relationships may differ by race/ethnicity in stratified analyses. To formally test for effect modification by race/ethnicity, we entered cross-product terms between race and stress in subsequent models. The test for effect modification by race/ethnicity was conducted using the type III sum of squares test for the cross-product terms.
RESULTS
Descriptive Statistics
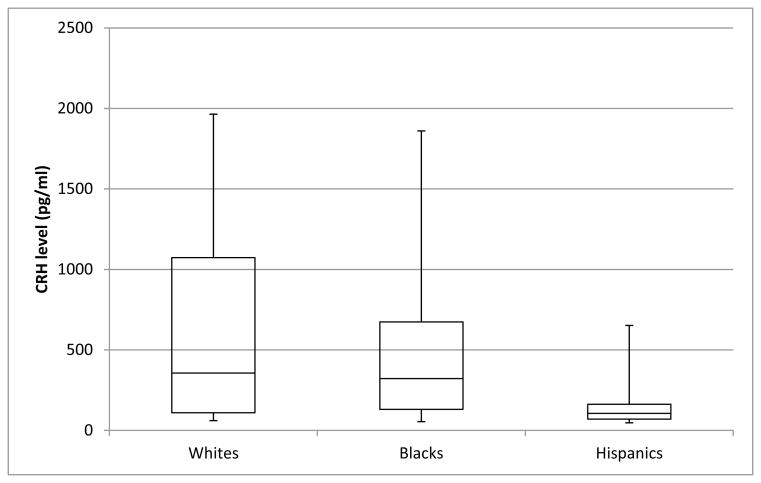
The sample consisted primarily of women of Hispanic (63%) and Black (26%) race/ethnicity (Table 1). The majority of these women had a high school education or less (70.2%) and were foreign born (65.3%). Overall mean log CRH (SD) was 5.56 (1.22) pg/mL. Mean log CRH (SD) was 5.99 (1.35) pg/mL in Whites, 5.44 (1.36) pg/mL in Blacks and 5.53 (1.13) pg/mL in Hispanics. Although there was substantial variation within each racial/ethnic group (Figure 1), there were no significant differences in mean log CRH levels across racial groups (Table 1). A marginally significant inverse association was evident between pre-pregnancy BMI and pregnancy CRH levels (p=0.06). Women in Project ACCESS experienced high prevalences of each of the chronic stress domains, with 19% reporting interpersonal violence, 43% reporting experiences of racial discrimination in at least one category, and 51% reporting intermediate levels or above of community violence (Table 2). Blacks were more likely than Hispanics to report the highest levels of negative life events, interpersonal violence, and community violence. Spearman correlations between individual stressor domains ranged from r=0.13 to 0.47 (Table 3). These moderate correlations suggest that while there is some overlap, each subscale measures a distinct domain of psychosocial stress.
Table 1.
Mean (SE) log prenatal CRH levels and descriptive characteristics in Project ACCESS
| N (%) | Mean log CRH level in pg/ml (SE)* | p-value | |
|---|---|---|---|
| Race/ethnicity | 0.46 | ||
| White | 20 (11.4) | 5.69 (0.18) | |
| Black | 46 (26.1) | 5.45 (0.12) | |
| Hispanic | 110 (62.5) | 5.58 (0.08) | |
| Nativity | 0.88 | ||
| U.S. born | 61 (34.7) | 5.57 (0.10) | |
| Foreign born | 115 (65.3) | 5.55 (0.08) | |
| Education | 0.76 | ||
| Less than 12 years | 68 (39.8) | 5.60 (0.10) | |
| High school or GED | 52 (30.4) | 5.62 (0.11) | |
| Some college or more | 51 (29.8) | 5.51 (0.11) | |
| Age | 0.71 | ||
| Less than 20 | 26 (14.8) | 5.60 (0.16) | |
| 20 to less than 30 | 96 (54.6) | 5.59 (0.08) | |
| 30 or older | 54 (30.7) | 5.48 (0.11) | |
| Parity | 0.66 | ||
| 0 | 52 (29.7) | 5.65 (0.11) | |
| 1 | 44 (25.1) | 5.51 (0.12) | |
| 2 or more | 79 (45.1) | 5.54 (0.09) | |
| Pre-pregnancy BMI | 0.06 | ||
| Less than 20 | 4 (2.9) | 6.39 (0.40) | |
| 20 to less than 25 | 45 (32.4) | 5.71 (0.12) | |
| 25 to less than 30 | 47 (33.8) | 5.46 (0.12) | |
| 30 or more | 43 (30.9) | 5.44 (0.12) |
Adjusted for gestational length at blood draw
Figure 1.
Distribution of CRH levels adjusted for gestational age at blood draw by racial/ethnic group. Bars in box represent median and inter-quartile range.
Table 2.
Distribution of stress domains by race/ethnicity in Project ACCESS
| All races (N=176) | White (N=20) | Black (N=46) | Hispanic (N=110) | p-value* | |
|---|---|---|---|---|---|
| Negative life event domains c# | 0.01 | ||||
| None | 55 (31.3%) | 4 (20.0%) | 8 (17.4%) | 43 (39.1%) | |
| Medium | 70 (39.8%) | 10 (50.0%) | 17 (37.0%) | 43 (39.1%) | |
| High | 51 (29.0%) | 6 (30.0%) | 21 (45.7%) | 24 (21.8%) | |
| Racial discrimination a b¶ | 0.002 | ||||
| None | 101 (57.4%) | 18 (90.0%) | 27 (58.7%) | 56 (50.9%) | |
| Medium | 37 (21.0%) | 2 (10.0%) | 5 (10.9%) | 30 (27.3%) | |
| High | 38 (21.6%) | 0 (0%) | 14 (30.4%) | 24 (21.8%) | |
| Interpersonal violence § | 0.16 | ||||
| None | 143 (81.3%) | 14 (70.0%) | 35 (76.1%) | 94 (85.5%) | |
| Medium | 19 (10.8%) | 3 (15.0%) | 5 (10.9%) | 11 (10.0%) | |
| High | 14 (8.0%) | 3 (15.0%) | 6 (13.0%) | 5 (4.6%) | |
| Community violence bc §§ | <0.0001 | ||||
| Low | 87 (49.4%) | 5 (25.0%) | 11 (23.9%) | 71 (64.6%) | |
| Medium | 46 (26.1%) | 8 (40.0%) | 11 (23.9%) | 27 (24.6%) | |
| High | 43 (24.4%) | 7 (35.0%) | 24 (52.2%) | 12 (10.9%) | |
| Cumulative stress c | 0.0001 | ||||
| Low | 80 (45.5%) | 9 (45.0%) | 11 (23.9%) | 60 (54.6%) | |
| Medium | 46 (26.1%) | 5 (25.0%) | 12 (26.1%) | 29 (26.4%) | |
| High | 50 (28.4%) | 6 (30.0%) | 23 (50.0%) | 21 (19.1%) |
P-values are for differences between multi-group comparisons using a chi-squared distribution. Post-hoc tests between pairs of race/ethnicity groups indicate significant difference between
Whites and Blacks,
Whites and Hispanics,
Blacks and Hispanics where indicated.
Assessed by the Crisis in Family Systems-Revised (CRISYS-R).
Assessed by the Experiences of Discrimination Scale.
Assessed using the Revised Conflict Tactics Scale.
Assessed using a modified version of the My Exposure to Violence survey.
Composite measure combining negative life events, racial discrimination, interpersonal violence, and community violence.
Table 3.
Spearman correlations (p-values) between individual stressor scores in Project ACCESS
| community violence | IPV | discrimination | |
|---|---|---|---|
| negative life event domains | 0.47 (<0.0001) | 0.23 (0.002) | 0.22 (0.003) |
| racial discrimination | 0.13 (0.08) | 0.16 (0.03) | |
| IPV | 0.25 (0.0008) |
Stress and CRH: Main Analysis
Table 4 shows the mean difference in log CRH related to increasing stress across the different stressor domains considered in the study. In analyses using the sample as a whole, we found no significant relationship between the individual stress measures (i.e. interpersonal violence, community violence, racial discrimination, and other negative life events) and CRH levels (Table 4, first column). Associations between increased levels of the cumulative stress index and CRH were also not significant.
Table 4.
Mean difference in log CRH (95% CI) by levels of psychosocial stressors stratified by race/ethnicity in Project ACCESS*
| All races (N=176) | Blacks (N=46) | Hispanics (N=110) | |
|---|---|---|---|
|
| |||
| Log CRH (pg/ml) mean difference (95% CI) | Log CRH (pg/ml) mean difference (95% CI) | Log CRH (pg/ml) mean difference (95% CI) | |
|
| |||
| Negative life event domains | |||
| None | Referent (0) | Referent (0) | Referent (0) |
| Medium (1 or 2 domains) | 0.11 (−0.19, 0.41) | −0.76 (−1.70, 0.17) | 0.15 (−0.18, 0.48) |
| High (3 or more domains) | 0.14 (−0.20, 0.49) | 0.004 (−0.81, 0.82) | 0.15 (−0.26, 0.57) |
| Racial discrimination | |||
| None | −0.06 (−0.38, 0.26) | 1.24 (0.34, 2.13) | −0.23 (−0.57, 0.11) |
| Medium (1 or 2 types) | Referent (0) | Referent (0) | Referent (0) |
| High (3 or more types) | 0.12 (−0.27, 0.51) | 1.43 (0.45, 2.42) | −0.12 (−0.54, 0.29) |
| Interpersonal violence | |||
| None | Referent (0) | Referent (0) | Referent (0) |
| Medium (1 or 2 types) | −0.05 (−0.46, 0.35) | 0.71 (−0.31, 1.74) | −0.22 (−0.70, 0.25) |
| High (3 or more types) | 0.01 (−0.47, 0.50) | 0.45 (−0.53, 1.43) | 0.001 (−0.72, 0.73) |
| Community violence | |||
| Low (less than −0.55) | Referent (0) | Referent (0) | Referent (0) |
| Medium (−0.55 to less than 0.93) | 0.13 (−0.20, 0.45) | 0.49 (−0.41, 1.40) | 0.05 (−0.32, 0.42) |
| High (0.93 or more) | 0.15 (−0.22, 0.51) | 0.68 (−0.14, 1.50) | −0.04 (−0.55, 0.47) |
| Cumulative stress | |||
| Low | Referent (0) | Referent (0) | Referent (0) |
| Medium | 0.05 (−0.26, 0.37) | 0.20 (−0.81, 1.20) | 0.03 (−0.33, 0.39) |
| High | 0.12 (−0.21, 0.45) | 0.89 (0.12, 1.66) | −0.14 (−0.55, 0.27) |
| Per unit cumulative stress score | 0.06 (−0.08, 0.19) | 0.32 (0.05, 0.60) | −0.02 (−0.19, 0.16) |
Adjusted for gestational age at blood draw, age, parity, race/ethnicity (in combined analysis of all races), education, nativity, and pre-pregnancy BMI
Stress and CRH: Race Stratified Analyses
Stratified analyses suggested effect modification by race/ethnicity (Table 4, columns 2 and 3). Among Blacks, discrimination was associated with CRH levels in a U-shaped pattern. Compared to Black women reporting 1 or 2 types of discrimination, Black women reporting no discrimination had CRH levels that were 1.24 log pg/mL greater (95% CI: 0.34, 2.13), while Black women reporting 3 or more types of discrimination had CRH levels that were 1.43 log pg/mL higher (95% CI: 0.45, 2.42). In addition among Blacks, a relation between increased exposure to community violence and higher CRH levels was suggested, with the highest level of community violence associated with 0.68 greater log pg/mL (95% CI: −0.14, 1.50) compared to low exposure to community violence, a difference that was borderline significant (p=0.10). Finally, in Black mothers, the composite measure of cumulative stress was associated with CRH levels in a dose response relation. In contrast, among Hispanic women, we saw no associations of the individual stressors or cumulative stress index with pregnancy CRH levels (Table 4, final column). Significant interactions were found between Black race and racial discrimination (p for interaction = 0.006) and higher cumulative stress (p for interaction = 0.002) associated with increased pregnancy CRH levels. The interaction between Black race and increased community violence exposure was also associated with higher levels of CRH though this interaction did not quite reach statistical significance (p for interaction 0.06).
DISCUSSION
In this cohort composed of mostly low income minority women, we found no associations of a number of chronic stressors considered independently or as a composite measure of cumulative stress with mid-pregnancy CRH when considered in the sample as a whole. However, racism, community violence, and higher levels of cumulative stress were associated with elevated CRH in Blacks, but not Hispanics in stratified analyses.
Our finding of no associations of a comprehensive range of chronic stressors with pregnancy CRH in analyses including all racial/ethnic groups is consistent with published studies on psychosocial stress and pregnancy CRH, which have been mostly null or moderate in effect size (15–18). Of the few studies that have reported associations of psychosocial stressors with pregnancy CRH, The Pregnancy Outcomes and Community Health (POUCH) Study in Michigan found that the highest versus the lowest quartile of perceived stress was associated with elevated second trimester CRH in women delivering at term but found no associations with other measures including coping, hostility, mastery, anomie, and abuse (18). Another group in Los Angeles reported that a higher composite score on anxiety and perceived stress was associated with elevated CRH at 18–20 weeks gestation in preterm cases; this group subsequently reported a positive association between pregnancy anxiety and CRH measured later in pregnancy (28–30 weeks gestation) (19, 20). Few studies have focused on more chronic stressors. Kramer et al. measured a number of chronic stressors including marital strain, lack of money, and crowding and found no associations (16). Consistent with Project ACCESS and POUCH study findings, Kramer et al. also found no associations with intimate partner violence (16). To our knowledge, no prior studies have examined associations of community violence and discrimination with pregnancy CRH levels nor considered a more comprehensive cumulative measure of multiple stressors.
Adjusted for gestational age at blood draw, we saw the highest CRH levels in Whites, followed by Hispanics, then Blacks, though these differences were not significant. Other studies have reported higher CRH in whites than Hispanics (32) and three studies have reported higher CRH levels in whites than Blacks (10, 18, 41). One study also reported lower levels of pregnancy CRH in Hispanics than Blacks (41). The lower CRH levels among Black women, who have higher rates of preterm birth and psychosocial stressors may seem paradoxical. However, Holzman et al. observed that the association between CRH and preterm birth was stronger in Blacks than Whites, suggesting that Blacks are more susceptible to elevated CRH than Whites (42–44).
Our study is the first to examine whether associations of psychosocial stress and pregnancy CRH differ by race/ethnicity. We observed that the associations of psychosocial stressors were stronger among Black mothers as compared with Hispanic mothers. Notably, previous work in Project ACCESS demonstrated that the relation between cumulative stress and pregnancy cortisol levels (another marker of the pregnancy stress axis) also differed between Blacks and Hispanics (30). In overlapping research, a study conducted among higher SES women found that perceived racism was significantly associated with birth weight in African Americans but not non-Hispanic Whites (45). Because birth weight has been associated with products of the maternal fetal HPA axis (46), these findings provide indirect support for racial differences in the effects of chronic stressors, including racial discrimination, on maternal fetal HPA axis responses
Our findings of associations of community violence and discrimination with pregnancy CRH in Black but not Hispanic women lends further support for racial/ethnic differences of the associations between chronic stressors and hormones of the fetal-placental HPA axis. Glynn et al. previously reported that the correlation between cortisol at 18–20 weeks with CRH later in pregnancy was stronger in Blacks (r=0.39) and Hispanics (r=0.35) than in Whites (r=0.09) (41). Because of the similar correlations between pregnancy CRH and cortisol levels in Blacks and Hispanics in Glynn et al., their results may seem contradictory to our findings of stronger associations of psychosocial stressors with pregnancy CRH in Blacks than Hispanics. However, because Glynn did not examine the relation between products of the HPA axis and psychosocial stressors in that particular study, results cannot be directly compared.
Despite both Hispanics and Blacks having comparably low educational attainment, income levels, and access to healthcare, Blacks have twice the risk of preterm birth compared to Whites. Hispanics (mostly of Mexican origin), have comparable risk of preterm birth when compared to Whites, though it is important to note that the rates of poor birth outcomes vary within the heterogeneous group of Hispanics (1, 47–49). Racial and ethnic differences in physiologic stress responses may in part, help explain previously observed racial/ethnic disparities in birth outcomes. Though we can only speculate, our findings imply that race may be a marker for mediators of the fetal placental HPA response to chronic stress. Racial differences in CRH and CRH receptor polymorphisms in non-pregnant individuals have been described in the literature (50, 51), but the relevance of these studies to racial/ethnic differences in pregnancy CRH levels or levels of these hormones in response to stress is unknown. Differences in adaptive resources between racial/ethnic groups may also play a role in effect modification by race that we observed. Compared to Blacks, Hispanics may tend to have stronger and more extended social networks, though studies conflict and much heterogeneity exists within each of these racial groups (52). In addition, differences in the distribution of U.S. born versus foreign born women in Blacks and Hispanics may explain differences of physiologic response to stress. While a slight majority of Black women in Project ACCESS were U.S. born (59%), the vast majority of Hispanic women were foreign born (86%). In both Blacks and Hispanic populations, foreign born women tend to have better birth outcomes than women born in the U.S. (42–44) Better birth outcomes in foreign born women have been hypothesized be due to culturally based protections, including social support, religious beliefs, and positive pregnancy attitudes (53–55). Alternatively, better birth outcomes in immigrants may be due to a self selection process because healthier individuals are more likely to migrate (44). Further studies should examine whether associations of both acute and chronic stressors with pregnancy CRH differ by race/ethnicity in large samples of minority women as well as explore these potential pathways more explicitly.
Our study has notable strengths. Ours is the first study to examine racial differences in such a wide range of chronic stressors, including discrimination and community violence, in relation to CRH. This enabled us to consider a more comprehensive measure of cumulative stress in relation to prenatal CRH in this urban sample. We were also able to control for a number of potential confounders. We also note limitations. The relatively small sample size limited power to detect some associations (e.g. comparisons with whites in race-stratified analyses). Secondly, the use of self-report measures to ascertain exposure to stressors may have resulted in underreporting of exposures. We would expect underreporting to be random with respect to CRH levels, thus biasing results towards the null. We also did not collect information on illicit drug use or medications other than exogenous steroid use. Medications and illicit drug use may vary by race/ethnicity and could potentially influence HPA hormones, though data specifically in pregnant women are sparse. Finally, the assessment of CRH at one time point may be a limitation given evidence that the rise of CRH over the course of pregnancy (i.e., CRH trajectories) might be more relevant for distinguishing subtypes of preterm birth (56).
Though our results require replication in larger samples, they suggest that the effects of stress on pregnancy CRH differ between racial/ethnic groups. Further studies are warranted to clarify whether associations of chronic stressors considered more comprehensively with pregnancy CRH differ by race/ethnicity and whether such differences might help explain disparities in pregnancy and perinatal outcomes related to race/ethnicity. Future studies should also include more than a single time point of CRH measurement. For example, it would be of interest to examine the relationship between a comprehensive measure of chronic stress and the trajectory of CRH over the course of pregnancy, whether this too may differ by race/ethnicity, and how any altered trajectories may relate to preterm risk within subgroups.
Acknowledgments
*Role of the Funding Source
Funding for this study was provided by the NIEHS (grant no. R01ES010932) and NIMH (grant no. R01MH068596). The NIEHS and NIMH did not have any role in study design, the collection, analysis and interpretation of data, the writing of the report, or in the decision to submit the paper for publication.
The authors wish to thank Shakira Franco Suglia for providing important intellectual contributions and computer programming code to summarize stress measures. The authors would also like to thank Marina Jacobson Canner for assistance with data management and computer programming, Kathy Tseng for assistance with project management, and Brent Coull for assistance with creating the figure.
Footnotes
Contributors
Dr. Wright and Dr. Rich-Edwards designed and secured funding for the study. Dr. Tse conducted the statistical analysis, interpreted data, and drafted the manuscript. Dr. Wright, Dr. Rich-Edwards, and Dr. Koenen provided advice on analysis and data interpretation and edited the manuscript. All authors have contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010 Jan;125(1):4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 2.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010 Feb 11;362(6):529–35. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 3.Rich-Edwards J, Krieger N, Majzoub J, Zierler S, Lieberman E, Gillman M. Maternal experiences of racism and violence as predictors of preterm birth: rationale and study design. Paediatr Perinat Epidemiol. 2001 Jul;15( Suppl 2):124–35. doi: 10.1046/j.1365-3016.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 4.Kramer MS, Goulet L, Lydon J, Seguin L, McNamara H, Dassa C, et al. Socio-economic disparities in preterm birth: causal pathways and mechanisms. Paediatr Perinat Epidemiol. 2001 Jul;15( Suppl 2):104–23. doi: 10.1046/j.1365-3016.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 5.Holzman C, Bullen B, Fisher R, Paneth N, Reuss L. Pregnancy outcomes and community health: the POUCH study of preterm delivery. Paediatr Perinat Epidemiol. 2001 Jul;15( Suppl 2):136–58. doi: 10.1046/j.1365-3016.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 6.Rich-Edwards JW, Grizzard TA. Psychosocial stress and neuroendocrine mechanisms in preterm delivery. Am J Obstet Gynecol. 2005 May;192(5 Suppl):S30–5. doi: 10.1016/j.ajog.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 7.Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003 Nov;997:136–49. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- 8.Smith R, Nicholson RC. Corticotrophin releasing hormone and the timing of birth. Front Biosci. 2007;12:912–8. doi: 10.2741/2113. [DOI] [PubMed] [Google Scholar]
- 9.Hobel CJ, Arora CP, Korst LM. Corticotrophin-releasing hormone and CRH-binding protein. Differences between patients at risk for preterm birth and hypertension. Ann N Y Acad Sci. 1999;897:54–65. doi: 10.1111/j.1749-6632.1999.tb07878.x. [DOI] [PubMed] [Google Scholar]
- 10.Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol. 2001 May;97(5 Pt 1):657–63. doi: 10.1016/s0029-7844(00)01209-6. [DOI] [PubMed] [Google Scholar]
- 11.Inder WJ, Prickett TC, Ellis MJ, Hull L, Reid R, Benny PS, et al. The utility of plasma CRH as a predictor of preterm delivery. J Clin Endocrinol Metab. 2001 Dec;86(12):5706–10. doi: 10.1210/jcem.86.12.8080. [DOI] [PubMed] [Google Scholar]
- 12.Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol. 2001 Jul;15( Suppl 2):17–29. doi: 10.1046/j.1365-3016.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 13.Robinson BG, Emanuel RL, Frim DM, Majzoub JA. Glucocorticoid stimulates expression of corticotropin-releasing hormone gene in human placenta. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5244–8. doi: 10.1073/pnas.85.14.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petraglia F, Aguzzoli L, Florio P, Baumann P, Genazzani AD, Di Carlo C, et al. Maternal plasma and placental immunoreactive corticotrophin-releasing factor concentrations in infection-associated term and pre-term delivery. Placenta. 1995 Mar;16(2):157–64. doi: 10.1016/0143-4004(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 15.Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM. Stress questionnaires and stress biomarkers during pregnancy. J Womens Health (Larchmt) 2009 Sep;18(9):1425–33. doi: 10.1089/jwh.2008.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer MS, Lydon J, Seguin L, Goulet L, Kahn SR, McNamara H, et al. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009 Jun 1;169(11):1319–26. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- 17.Petraglia F, Hatch MC, Lapinski R, Stomati M, Reis FM, Cobellis L, et al. Lack of effect of psychosocial stress on maternal corticotropin-releasing factor and catecholamine levels at 28 weeks’ gestation. J Soc Gynecol Investig. 2001 Mar-Apr;8(2):83–8. [PubMed] [Google Scholar]
- 18.Chen Y, Holzman C, Chung H, Senagore P, Talge NM, Siler-Khodr T. Levels of maternal serum corticotropin-releasing hormone (CRH) at midpregnancy in relation to maternal characteristics. Psychoneuroendocrinology. 2010 Jul;35(6):820–32. doi: 10.1016/j.psyneuen.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancuso RA, Schetter CD, Rini CM, Roesch SC, Hobel CJ. Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosom Med. 2004 Sep-Oct;66(5):762–9. doi: 10.1097/01.psy.0000138284.70670.d5. [DOI] [PubMed] [Google Scholar]
- 20.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999 Jan;180(1 Pt 3):S257–63. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993 Sep 27;153(18):2093–101. [PubMed] [Google Scholar]
- 22.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010 Feb;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers HF. Ethnicity- and socio-economic status-related stresses in context: an integrative review and conceptual model. J Behav Med. 2009 Feb;32(1):9–19. doi: 10.1007/s10865-008-9181-4. [DOI] [PubMed] [Google Scholar]
- 24.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation--allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med. 1997 Oct 27;157(19):2259–68. [PubMed] [Google Scholar]
- 25.Braveman P, Marchi K, Egerter S, Kim S, Metzler M, Stancil T, et al. Poverty, near-poverty, and hardship around the time of pregnancy. Matern Child Health J. Jan;14(1):20–35. doi: 10.1007/s10995-008-0427-0. [DOI] [PubMed] [Google Scholar]
- 26.Clark C, Ryan L, Kawachi I, Canner MJ, Berkman L, Wright RJ. Witnessing community violence in residential neighborhoods: a mental health hazard for urban women. J Urban Health. 2008 Jan;85(1):22–38. doi: 10.1007/s11524-007-9229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuru-Jeter A, Dominguez TP, Hammond WP, Leu J, Skaff M, Egerter S, et al. It’s the skin you’re in”: African-American women talk about their experiences of racism. an exploratory study to develop measures of racism for birth outcome studies. Matern Child Health J. 2009 Jan;13(1):29–39. doi: 10.1007/s10995-008-0357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hien D, Bukszpan C. Interpersonal violence in a “normal” low-income control group. Women Health. 1999;29(4):1–16. doi: 10.1300/J013v29n04_01. [DOI] [PubMed] [Google Scholar]
- 29.Holman EA, Silver RC, Waitzkin H. Traumatic life events in primary care patients: a study in an ethnically diverse sample. Arch Fam Med. 2000 Sep-Oct;9(9):802–10. doi: 10.1001/archfami.9.9.802. [DOI] [PubMed] [Google Scholar]
- 30.Suglia S, Staudenmayer J, Cohen S, Enlow MB, Rich-Edwards J, Wright R. Cumulative stress and cortisol disruption among Black and Hispanic pregnant women in an urban cohort. Psychol Trauma: Theory, Research, Practice and Policy. 2(4):326–34. doi: 10.1037/a0018953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright RJ, Suglia SF, Levy J, Fortun K, Shields A, Subramanian S, et al. Transdisciplinary research strategies for understanding socially patterned disease: the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project as a case study. Cien Saude Colet. 2008 Nov-Dec;13(6):1729–42. doi: 10.1590/s1413-81232008000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siler-Khodr TM, Forthman G, Khodr C, Matyszczyk S, Khodr Z, Khodr G. Maternal serum corticotropin-releasing hormone at midgestation in Hispanic and white women. Obstet Gynecol. 2003 Mar;101(3):557–64. doi: 10.1016/s0029-7844(02)03072-7. [DOI] [PubMed] [Google Scholar]
- 33.Straus MA, Douglas EM. A short form of the Revised Conflict Tactics Scales, and typologies for severity and mutuality. Violence Vict. 2004 Oct;19(5):507–20. doi: 10.1891/vivi.19.5.507.63686. [DOI] [PubMed] [Google Scholar]
- 34.Connelly CD, Newton RR, Aarons GA. A psychometric examination of English and Spanish versions of the Revised Conflict Tactics Scales. J Interpers Violence. 2005 Dec;20(12):1560–79. doi: 10.1177/0886260505280341. [DOI] [PubMed] [Google Scholar]
- 35.Selner-O’Hagan MB, Kindlon DJ, Buka SL, Raudenbush SW, Earls FJ. Assessing exposure to violence in urban youth. J Child Psychol Psychiatry. 1998 Feb;39(2):215–24. [PubMed] [Google Scholar]
- 36.Suglia SF, Ryan L, Wright RJ. Creation of a community violence exposure scale: accounting for what, who, where, and how often. J Trauma Stress. 2008 Oct;21(5):479–86. doi: 10.1002/jts.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. 2005 Oct;61(7):1576–96. doi: 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Mustillo S, Krieger N, Gunderson EP, Sidney S, McCreath H, Kiefe CI. Self-reported experiences of racial discrimination and Black-White differences in preterm and low-birthweight deliveries: the CARDIA Study. Am J Public Health. 2004 Dec;94(12):2125–31. doi: 10.2105/ajph.94.12.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krieger N, Sidney S. Racial discrimination and blood pressure: the CARDIA Study of young black and white adults. Am J Public Health. 1996 Oct;86(10):1370–8. doi: 10.2105/ajph.86.10.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Serv Res. 1998 Dec;33(5 Pt 1):1381–402. [PMC free article] [PubMed] [Google Scholar]
- 41.Glynn LM, Schetter CD, Chicz-DeMet A, Hobel CJ, Sandman CA. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides. 2007 Jun;28(6):1155–61. doi: 10.1016/j.peptides.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Acevedo-Garcia D, Soobader MJ, Berkman LF. Low birthweight among US Hispanic/Latino subgroups: the effect of maternal foreign-born status and education. Soc Sci Med. 2007 Dec;65(12):2503–16. doi: 10.1016/j.socscimed.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 43.Pallotto EK, Collins JW, Jr, David RJ. Enigma of maternal race and infant birth weight: a population-based study of US-born Black and Caribbean-born Black women. Am J Epidemiol. 2000 Jun 1;151(11):1080–5. doi: 10.1093/oxfordjournals.aje.a010151. [DOI] [PubMed] [Google Scholar]
- 44.David RJ, Collins JW., Jr Differing birth weight among infants of U.S.-born blacks, African-born blacks, and U.S.-born whites. N Engl J Med. 1997 Oct 23;337(17):1209–14. doi: 10.1056/NEJM199710233371706. [DOI] [PubMed] [Google Scholar]
- 45.Dominguez TP, Dunkel-Schetter C, Glynn LM, Hobel C, Sandman CA. Racial differences in birth outcomes: the role of general, pregnancy, and racism stress. Health Psychol. 2008 Mar;27(2):194–203. doi: 10.1037/0278-6133.27.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Field T, Diego M, Hernandez-Reif M, Figueiredo B, Deeds O, Ascencio A, et al. Comorbid depression and anxiety effects on pregnancy and neonatal outcome. Infant Behav Dev. Feb;33(1):23–9. doi: 10.1016/j.infbeh.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hessol NA, Fuentes-Afflick E. The perinatal advantage of Mexican-origin Latina women. Ann Epidemiol. 2000 Nov;10(8):516–23. doi: 10.1016/s1047-2797(00)00073-9. [DOI] [PubMed] [Google Scholar]
- 48.Gould JB, Madan A, Qin C, Chavez G. Perinatal outcomes in two dissimilar immigrant populations in the United States: a dual epidemiologic paradox. Pediatrics. 2003 Jun;111(6 Pt 1):e676–82. doi: 10.1542/peds.111.6.e676. [DOI] [PubMed] [Google Scholar]
- 49.Becerra JE, Hogue CJ, Atrash HK, Perez N. Infant mortality among Hispanics. A portrait of heterogeneity. JAMA. 1991 Jan 9;265(2):217–21. [PubMed] [Google Scholar]
- 50.Ryckman KK, Simhan HN, Krohn MA, Williams SM. Predicting risk of bacterial vaginosis: the role of race, smoking and corticotropin-releasing hormone-related genes. Mol Hum Reprod. 2009 Feb;15(2):131–7. doi: 10.1093/molehr/gan081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimmin LC, Natarajan S, Ibarguen H, Montasser M, Kim DK, Hanis CL, et al. Corticotropin releasing hormone (CRH) gene variation: comprehensive resequencing for variant and molecular haplotype discovery in monosomic hybrid cell lines. DNA Seq. 2007 Dec;18(6):434–44. doi: 10.1080/10425170701388719. [DOI] [PubMed] [Google Scholar]
- 52.Kim HK, McKenry PC. Social networks and support: A comparison of African Americans, Asian Americans, Caucasians, and Hispanics. Journal of Comparative Family Studies. 1998;29(2):313–34. [Google Scholar]
- 53.Sherraden MS, Barrera RE. Maternal support and cultural influences among Mexican immigrant mothers. Families in Society. 1996;77(5):298–313. [Google Scholar]
- 54.Landale NS, Oropesa RS, Llanes D, Gorman BK. Does Americanization have adverse effects on health?: Stress, health habits, and infant health outcomes among Puerto Ricans. Social Forces. 1999;78(2):613–41. [Google Scholar]
- 55.Magaña A, Clark NM. Examining a paradox: Does religiosity contribute to positive birth outcomes in Mexican American populations? Health Education Quarterly. 1995;22(1):96–109. doi: 10.1177/109019819502200109. [DOI] [PubMed] [Google Scholar]
- 56.McGrath S, McLean M, Smith D, Bisits A, Giles W, Smith R. Maternal plasma corticotropin-releasing hormone trajectories vary depending on the cause of preterm delivery. Am J Obstet Gynecol. 2002 Feb;186(2):257–60. doi: 10.1067/mob.2002.119635. [DOI] [PubMed] [Google Scholar]