Abstract
Introduction
Electrical impedance myography (EIM) is a non-invasive technique used for assessment of muscle health in which a high-frequency, low-amplitude electric current is applied to the skin overlying a muscle, and the resulting surface voltage is measured. We have previously used adhesive electrodes, application of which is inconvenient. We present data using a handheld electrode array (HEA) that we devised to expedite the EIM procedure in a clinical setting.
Methods
Thirty-four healthy volunteers and 24 radiculopathy subjects underwent EIM testing using the HEA and adhesive electrodes.
Results
The HEA was shown to have good test-retest reproducibility, with intraclass correlation coefficients as high as 0.99. HEA data correlated strongly with data from adhesive electrodes, ρ = 0.85 in healthy volunteers (p < 0.001) and ρ = 0.75 in radiculopathy subjects (p < 0.001).
Discussion
These data support the potential use of a handheld array for performing rapid localized surface impedance measurements.
Keywords: electrical impedance, radiculopathy, subcutaneous fat, muscle, reproducibility
INTRODUCTION
Electrical impedance myography (EIM) is a tetrapolar technique for non-invasive evaluation of muscle in which a high-frequency, low-amplitude electric current is applied via surface electrodes over a muscle of interest and the resulting surface voltages are measured.1,2 The technique is under study as a quantitative measure of muscle health and can provide useful information in a variety of neuromuscular disorders, including amyotrophic lateral sclerosis (ALS), inflammatory myopathy and radiculopathy.3-5,1 EIM can be performed using commercially available bioimpedance devices.6 Each measurement takes just a few seconds to complete once the electrodes are placed. Initial studies of EIM demonstrated a very high test-retest reproducibility for the spatially averaged phase at a single-frequency current of 50 kHz.7
In nearly all of our previous work, adhesive electrodes have been utilized to obtain measurements. Typically, a series of four strip electrodes are placed 2.5 cm apart over the muscle of interest. This is done by measuring and marking the electrode distances on the muscle(s), aligning the four adhesive electrodes to those markings and securing them with tape to ensure good contact.8,9 This is time-consuming, especially since several muscles usually need to be studied in order to fully evaluate the disorder under question, whether it is a localized problem such as radiculopathy or a generalized disease such as ALS. A typical study to evaluate 8 upper limb muscles, for example, can take more than 30 minutes, as multiple distance measurements are necessary and electrodes need to be repeatedly applied and removed. The actual impedance measurement time makes up just a small fraction of that total period. A faster, more convenient method of electrode placement and application would be highly desirable in a clinical setting, as it would reduce the length of time it takes for the procedure to be completed and eliminate the taxing process of applying and reapplying adhesive electrodes. We therefore developed a simple, fixed handheld electrode array (HEA) that can be moved easily from muscle to muscle and requires only a dampening of the skin with saline in order to obtain impedance measurements of muscle. In this study, we compare the EIM parameters resistance (R), reactance (X), and phase (θ) obtained using the HEA with those obtained with conventional adhesive electrodes in groups of healthy volunteers and patients with radiculopathy. We also evaluate the test-retest reproducibility of the HEA and the impact of skin-subcutaneous fat layer (SFL) thickness on EIM measurements using the HEA.
MATERIALS AND METHODS
Subject Recruitment
After approval by the Committee for Clinical Investigations at the Beth Israel Deaconess Medical Center (BIDMC), healthy volunteers and patients with cervical or lumbosacral radiculopathy were recruited through word-of-mouth and advertisements. After signing an approved informed consent form, all subjects underwent a history and neurological examination prior to inclusion. Subjects were 18-85 years of age. A clinical “gold standard” was utilized for inclusion of radiculopathy subjects, defined as two or more of the following: neck or low back pain, radicular pain and/or sensory symptoms (including a positive straight leg raise test or Spurling maneuver), radicular sensory loss, myotomal weakness and myotomal reflex change. Subjects were excluded if they were morbidly obese (body mass index > 40 kg/m2), if there was an implanted electrical device such as a cardiac pacemaker, or there was a known history or clinical evidence of a neuromuscular disorder in the limbs being evaluated.
EIM Technique
Impedance data were obtained using the ImpSFB7® bioimpedance spectroscopy device (Impedimed, Inc. San Diego, CA). This device collects data at multiple frequencies (range 3 kHz to 1 MHz). Resistance (R) and reactance (X) were collected at each frequency, and plots of R and X versus frequency were created. The phase, θ [θ = arctan (X/R)] was calculated and also plotted. In each subject, three muscles in either one upper limb or one lower limb were studied. In the upper limbs, the deltoid, biceps brachii and forearm flexor muscles were studied. In the lower limbs, the vastus medialis, tibialis anterior, and medial gastrocnemius muscles were studied.
Handheld electrode array
The HEA was constructed by Proxy Manufacturing, Inc (Methuen, MA) as follows: four stainless steel electrodes corresponding to the current-injecting and voltage-measuring electrodes were mounted to an acrylic bar which in turn was attached to a round acrylic handle (Figure 1). Each stainless steel electrode was 0.75 cm wide and 2.5 cm long (corresponding to the dimensions of the adhesive electrodes, described below) and was placed in a linear array with the outer electrodes 6 cm apart and the inner electrodes 3 cm apart. Connecting wires from these four electrodes were contained in the handle of the HEA device, with lead plugs from the impedance device at the proximal handle end. Prior to application of the HEA and EIM measurement, the skin over the muscle was lightly moistened with isotonic saline solution to ensure proper electrical contact.
Figure 1.

Handheld electrode array.
EIM procedure
EIM measurements were first obtained using the HEA. Either the upper (UL) or lower limb (LL) was studied in each subject, three muscles per limb. Twenty minutes were allowed to elapse before the measurements were repeated with the HEA. Immediately after this second set of measurements was completed, an ink mark was placed on the skin with an indelible marker to indicate the exact contact points of the four electrodes of the HEA. Four Ag/AgCl adhesive electrodes (part number 019-435500, Viasys Healthcare/Nicolet Biomedical, Madison, WI), 0.75 cm wide and cut to one quarter length, or 2.5 cm, were applied at these markings. The electrodes were secured with medical adhesive tape to ensure good contact. EIM measurements were then obtained using these four adhesive electrodes. Via this approach, we ensured that the footprint of the adhesive electrode array was virtually identical to that of the HEA.
Skin-Subcutaneous Fat Layer Thickness
Ultrasound images were obtained with a Terason 2000 Handheld Ultrasound System (Terason Ultrasound, Burlington, MA) as previously described.10 A 5 MHz probe set at a depth of 5-7 cm with a focus of 1.3 cm was utilized. The probe was held perpendicular to the direction of the muscle with minimal pressure at the center of the electrode array midway between the skin marks for the two inner electrodes, transverse to the long axis of the limb. Subjects were asked to gently contract the muscle in order to identify the interface between SFL and muscle. SFL thickness was measured to the nearest 0.01 cm using electronic calipers.
Data analysis
EIM parameters R, X, and θ in the frequency spectrum 50-500 kHz were utilized for this analysis. Single-frequency parameters were obtained by extrapolating the 50 kHz data for R, X, and θ from this frequency spectrum in the first and second trials with the HEA. In addition, three multi-frequency summary parameters were used, as previously described.11 These included: 1. The log-resistance slope, which is calculated by taking the resistance data from 50-500 kHz, performing a log transformation of both the measured values and the applied current frequencies, plotting the points, and taking the slope of a linear regression that fit those points; 2. the reactance-slope, which is obtained by performing a linear regression of the reactance values from 100-500 kHz and calculating its slope; and, 3. the phase-slope, by performing the same procedure as for the reactance slope using phase values (see Figure 2). Thus, six EIM parameters were available for each muscle from each of the two HEA trials: 50 kHz resistance, reactance and phase and multi-frequency log-resistance slope, reactance-slope, and phase-slope. Similarly, these six EIM parameters were also available for the adhesive electrode measurements from each muscle.
Figure 2.
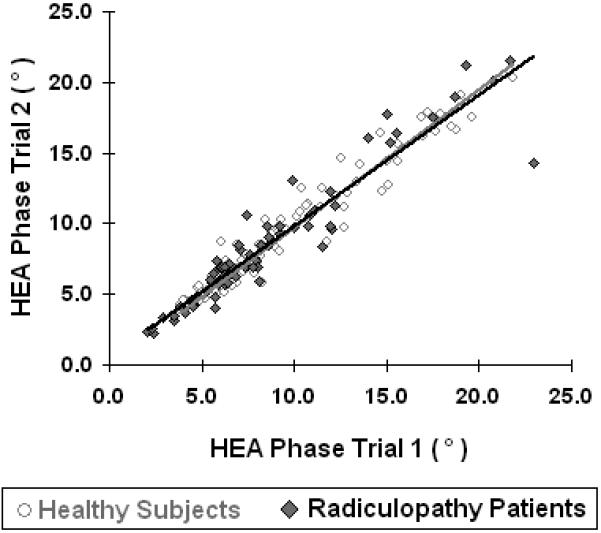
Test-retest reproducibility of 50 kHz phase with HEA in all healthy subjects (gray trend line) and radiculopathy patients (black trend line).
In order to assess test-retest reproducibility of the HEA, the absolute percentage difference was computed between the first and second trials for each of the single-frequency parameters for each muscle. Data obtained from the first trial were also compared to data obtained from the second trial using the intraclass correlation coefficient (ICC).
Next, the absolute percentage difference was computed for the same three parameters, for each muscle, between the data obtained from the HEA first trial and the data obtained from the adhesive electrodes. To measure how well the two methods correspond to one another, the six parameters were also compared using the Spearman rank correlation coefficient (ρ). To compare the distribution of the data obtained between the two methods, the coefficient of variation (CoV) was calculated for each data set, per muscle, per parameter, and compared.
Lastly, the differences between the EIM parameters obtained by each method were calculated and correlated with SFL thickness. The F-test was used for significance. For all analyses, results were considered significant at p < 0.05, two-tailed.
RESULTS
Subject demographics
(Table 1) Thirty-four healthy volunteers and 24 subjects with radiculopathy were included in the study. Of the 10 subjects with cervical radiculopathy, 1 was at the C5 level, 4 at C6, 6 at C7 and 2 at C8. The 14 lumbosacral radiculopathies included 2 at L2, 2 at L3, 2 at L4, 10 at L5 and 9 at S1. Four cervical and 8 lumbosacral radiculopathy patients had multiple levels of radiculopathy.
Table 1.
Demographic Data
| Healthy | Radiculopathy | |||
|---|---|---|---|---|
| Upper Limb | Lower Limb | Cervical | Lumbosacral | |
| Total Subjects | 19 | 15 | 10 | 14 |
| Male/Female | 8/11 | 8/7 | 8/2 | 8/6 |
| Age Range and Median (years) | 31-85 (70.9) | 20-83 (65.6) | 29-71 (52) | 28-74 (58) |
HEA test-retest reproducibility
Figure 2 illustrates the test-retest reproducibility of 50 kHz phase with HEA in all muscles. As noted in Table 2, the absolute percentage difference for the single-frequency resistance, reactance, and phase between the two trials for both controls and radiculopathy subjects is only 4-12%, 4-14% and 4-15%, respectively. Test-retest reproducibility for all three 50 kHz EIM parameters was very high as determined by the ICC between the two trials, as shown in Table 3. The mean ICC for all healthy muscles for resistance, reactance and phase were r = 0.97, r = 0.96, and r = 0.98, respectively; p < 0.001 for all correlations. The corresponding ICC in radiculopathy subjects for resistance, reactance and phase were 0.87, 0.93 and 0.91 respectively. The multi-frequency parameters log-resistance slope, reactance-slope and phase-slope also revealed good test-retest reproducibility in both healthy volunteers (r = 0.95, 0.95 and 0.95 respectively) and radiculopathy subjects (r = 0.90, r = 0.86, and r = 0.87)
Table 2.
Test-retest reproducibility dataa for 50 kHz R, X, and θ using HEA.
| Deltoid | Biceps | Forearm Flexor | ||||
|---|---|---|---|---|---|---|
| Parameter % Difference | H | RA | H | RA | H | RA |
| R Mean | 6.6 (1.0) | 4.5 (1.2) | 6.4 (1.5) | 9.7 (3.8) | 6.7 (1.1) | 9.1 (6.6) |
| X Mean | 7.7 (1.4) | 6.4 (1.2) | 7.1 (1.6) | 8.8 (2.3) | 4.4 (0.87) | 5.5 (2.8) |
| θ Mean | 7.9 (1.4) | 10.1 (1.8) | 10.1 (2.3) | 12.1 (3.2) | 8.8 (1.4) | 4.3 (2.3) |
| Vastus Medialis | Medial Gastroc. | Tibialis Anterior | ||||
|---|---|---|---|---|---|---|
| Parameter % Difference | H | RA | H | RA | H | RA |
| R Mean | 4.1 (1.1) | 6.3 (1.3) | 5.2 (1.0) | 7.4 (2.0) | 4.2 (1.3) | 11.9 (5.7) |
| X Mean | 5.2 (1.9) | 10.2 (3.1) | 9.8 (2.4) | 14.2 (2.3) | 6.6 (1.7) | 6.4 (1.2) |
| θ Mean | 6.2 (1.7) | 9.3 (2.1) | 9.3 (1.5) | 14.6 (3.0) | 6.6 (2.2) | 9.1 (2.5) |
Data are expressed as mean (standard error)
H – Healthy participants
RA – Radiculopathy patients
Table 3.
Test-retest reproducibility ICCa values for single- and multi-frequency EIM.
| Healthy |
Radiculopathy |
|||
|---|---|---|---|---|
| UL | LL | Cervical | Lumbosacral | |
| R | 0.98 | 0.96 | 0.83 | 0.91 |
| X | 0.95 | 0.98 | 0.95 | 0.91 |
| θ | 0.98 | 0.98 | 0.97 | 0.91 |
| Log-R slope | 0.95 | 0.95 | 0.91 | 0.81 |
| X-slope | 0.94 | 0.95 | 0.88 | 0.86 |
| θ-slope | 0.95 | 0.96 | 0.90 | 0.86 |
All p-values < 0.001.
LL – Lower limbs
UL – Upper limbs
HEA vs. adhesive electrodes
Figure 3 illustrates the relation between the data obtained with the HEA and the adhesive electrodes in all subjects; the absolute percentage differences in the 50 kHz data between the HEA and adhesive electrodes in all subjects is summarized in Table 4. The mean resistance was consistently lower with the HEA as compared to the adhesive electrodes with mean percentage difference in healthy volunteers ranging from 10-21%; in contrast, the mean reactance was consistently higher in the HEA as compared to the adhesive electrodes with mean percentage difference ranging from 11-26%. Consequently, the HEA showed a higher mean phase for all six muscles with the HEA as compared to the adhesive electrode data with mean percentage difference ranging from 12-37%. Similar differences were noted in radiculopathy subjects, with resistance being 8-17% lower with the HEA, reactance being 12-22% higher, and phase being 15-25% higher. Figure 4, comparing 50 kHz phase between HEA and adhesive electrodes in the deltoid and medial gastrocnemius reveals that the HEA 50 kHz phase is higher in nearly all subjects, both the healthy participants and the radiculopathy patients. Table 5 summarizes the Spearman correlation coefficients for single- and multi-frequency parameters in each muscle. The overall Spearman correlation coefficient for comparison between the two methods was ρ = 0.85 for healthy subjects and ρ = 0.75 for radiculopathy patients. The CoVs within the two methods are also extremely close, with the highest differential (0.13) in the upper limb reactance, and the lowest differential (0.003) in the lower limb resistance for healthy subjects. In radiculopathy patients the CoV within the 2 methods was even lower with highest differential (0.049) again in upper limb reactance and lowest differential (0.0020) in lower limb reactance. Thus, although there are consistent differences in the data obtained between the two methods of EIM, those differences are quite minor, and we can expect the correspondence between the values obtained to be very high and the distribution of data obtained by each method to be approximately the same. Therefore, although percentage differences in EIM parameters are markedly higher in some muscles between methods (e.g. upper limb reactance and phase,) the absolute variation in measurements is probably acceptable.
Figure 3.
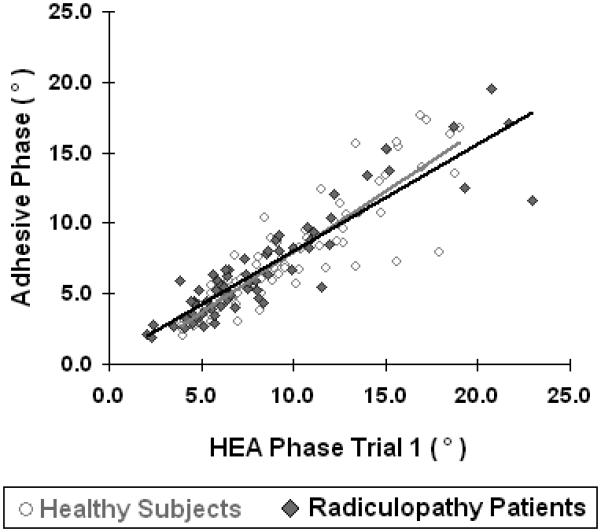
Comparison between HEA and adhesive electrodes 50 kHz phase in healthy subjects (gray trend line) and radiculopathy patients (black trend line).
Table 4.
Comparisona of 50 kHz R, X, and θ between HEA and adhesive electrodes.
| Deltoid | Biceps | Forearm Flexor | ||||
|---|---|---|---|---|---|---|
| Parameter % Difference | H | RA | H | RA | H | RA |
| R Mean | 16.9 (3.2) | 7.8 (2.4) | 20.8 (4.6) | 9.2 (2.2) | 13.0 (2.5) | 7.8 (2.0) |
| X Mean | 19.4 (3.1) | 15.6 (4.9) | 26.3 (2.8) | 17.7 (4.3) | 19.1 (2.8) | 11.5 (3.0) |
| θ Mean | 25.4 (2.3) | 17.6 (5.0) | 36.5 (2.9) | 16.2 (4.1) | 22.4 (2.9) | 15.2 (3.3) |
| Vastus Medialis | Medial Gastroc. | Tibialis Anterior | ||||
|---|---|---|---|---|---|---|
| Parameter % Difference | H | RA | H | RA | H | RA |
| R Mean | 13.4 (2.5) | 12.6 (2.5) | 9.8 (2.5) | 16.6 (5.0) | 12.0 (1.9) | 14.8 (6.5) |
| X Mean | 25.0 (3.2) | 21.7 (3.4) | 14.8 (2.6) | 18.9 (5.4) | 11.0 (1.9) | 13.0 (2.6) |
| θ Mean | 23.5 (3.6) | 24.8 (3.3) | 19.3 (2.7) | 24.0 (4.6) | 11.9 (2.3) | 18.3 (4.0) |
Data are expressed as mean (standard error)
H – Healthy participants
RA – Radiculopathy patients
Figure 4.
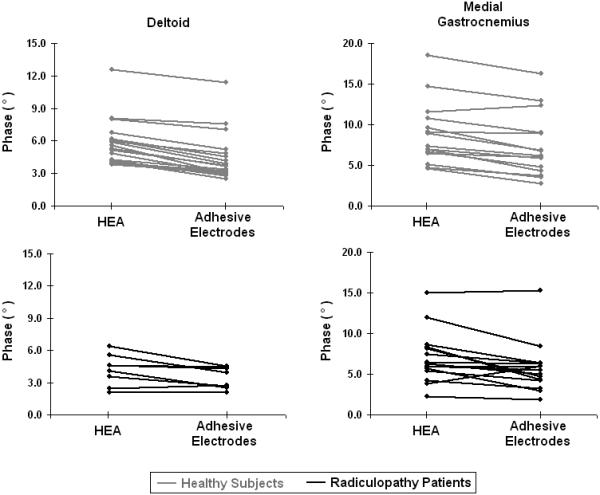
Method comparison between HEA and adhesive electrodes in deltoid and medial gastrocnemius 50 kHz phase.
Table 5.
Spearman correlation coefficientsa between HEA data and adhesive electrode data
| Healthy |
Radiculopathy |
|||
|---|---|---|---|---|
| UL | LL | Cervical | Lumbosacral | |
| R | 0.86 | 0.88 | 0.80 | 0.86 |
| X | 0.75 | 0.84 | 0.93 | 0.78 |
| θ | 0.89 | 0.94 | 0.88 | 0.70 |
| Log-R slope | 0.86 | 0.90 | 0.76 | 0.65 |
| X-slope | 0.74 | 0.89 | 0.74 | 0.64 |
| θ-slope | 0.72 | 0.89 | 0.68 | 0.63 |
All p-values < 0.001.
LL – Lower limbs
UL – Upper limbs
Skin-subcutaneous fat layer thickness
In a previous study, we showed that skin-subcutaneous fat layer thickness (SFL) does not significantly influence phase at 50 kHz.10 In that study, current electrodes were placed on the hands or feet, and an array of voltage sensing electrodes were placed over the muscle to be studied. In this study, as expected, the proximal muscles (deltoid in the upper limb and vastus medialis in the lower limb) have greater SFL thickness than the distal muscles in those limbs. In general, the differences are not significant, with the exception of reactance in healthy subjects (r = -0.20, p = 0.0498) and phase in radiculopathy patients (r = 0.26, p = 0.043), which just reached statistical significance. Although this is a statistically significant correlation, the correlation coefficient is not one of practical significance in this context, and thus SFL thickness variation is unlikely to sufficiently account for differences in EIM parameters with the two methods.
DISCUSSION
In this study we have attempted to refine the EIM procedure to make it more efficient and easy to apply in routine clinical practice. Since a major part of the time taken to perform EIM is in electrode application, we have developed a handheld array which can be used with commercially available equipment and be quickly moved from muscle to muscle to obtain measurements. We have demonstrated that this handheld electrode array provides reproducible EIM measurements in healthy and radiculopathy subjects in the upper and lower limb muscles; the ICCs in healthy subjects for phase at 50 kHz using the HEA are very similar to those obtained with the adhesive electrodes in an earlier study (biceps adhesive 0.97, HEA 0.97 and tibialis anterior adhesive 0.94, HEA 0.98),7 although in that earlier study repeated measurements were made days or weeks apart, not just after 20 minutes. In addition to 50 kHz EIM parameters, the multi-frequency measurements obtained with the HEA also revealed very high test-retest reproducibility.
Importantly, the intent of this report was not to address the differences in the diagnostic signature of EIM in radiculopathy subjects versus healthy volunteers,. It also was not to compare EMG results with EIM data, although all patients had EMG performed as part of the study; as stated in the methods, a clinical gold standard was used for the diagnosis of radiculopathy. Rather, the goal of this study was to validate the approach of using a handheld array to collect the EIM data. The comparison between EIM and EMG in the diagnosis of radiculopathy will be the subject of a future report.
In much of our previous work, fixed landmarks measured from bony prominences have been used to guide electrode placements to help reduce variation in electrode position.7 In contrast, in this study the HEA was simply placed over the bulk of the muscle based on visual inspection and the physician's knowledge of anatomy. This somewhat less precise approach to electrode placement may be offset to some extent by the fact that the array provides electrodes that are entirely fixed in position relative to one another, thus reducing intra-array differences in electrode spacing or orientation. If it were deemed useful, the reproducibility of the electrode array placement could be further improved by marking the skin with an indelible marker or a pinpoint tattoo to assist in accurate placement during the second measurement period. Other potential sources of error in using the HEA include variability in the amount of saline used and the pressure applied to hold the HEA over the muscle being examined. Despite these potential variables, the test-retest reproducibility of the HEA is very high, implying that that it can be used to obtain quick, reliable EIM measurements.
When compared to the adhesive electrodes, the phase values are 12-37% higher with the HEA than with the adhesive electrodes. Thus, it is unlikely that the two methods are truly interchangeable in a clinical setting. Several reasons for this discrepancy are possible. Our first consideration was that the pressure of the device on the surface of the skin may bring the electrodes closer to the muscle by compressing fat as it is applied. If that were truly the case, we would have anticipated a correlation between the difference observed between the two measurement methods and the thickness of subcutaneous fat, but only a relatively weak correlation was identified. Another possible explanation is that the adhesive electrodes are flexible and conform to the shape of the limb and do not distort the shape of the fat or the underlying muscle. In contrast, the rigid array deforms the shape of the muscle, fat and the limb as a whole, at least slightly. It is possible that such distortions could alter the impedance data; however, to understand why would require detailed studies using the finite element method,12 a task beyond the scope of this preliminary study. Finally, it is also possible that the use of saline solution for contact with the HEA in comparison to the Ag/AgCl gel of the adhesive electrodes could play some role in causing this discrepancy. However, this too is difficult to determine without sophisticated modeling. Future improvements to this very basic technique could include a method of ensuring that constant force is being applied with each measurement and a process for confirming that all 4 electrodes are making good contact prior to the measurement actually being made.
In conclusion, we have shown that a fixed handheld electrode array provides a convenient, reproducible means for performing EIM measurements in health as well as disease. The resistance value is lower with the HEA than with adhesive electrodes; corresponding reactance and phase values obtained with the HEA are higher than those obtained with adhesive electrodes using the same footprint, yet there is strong concordance between the two methods, and the distribution of data between the methods is also quite similar. These differences do not appear to be influenced by subcutaneous fat thickness, suggesting that other factors are likely responsible for the discrepancy observed between the two methods. Regardless, these early results suggest that a fixed handheld electrode array is a promising tool for performing EIM quickly and reliably in the clinical setting. Future studies assessing the difference in EIM signature between radiculopathy and normal subjects, evaluating the utility of EIM in localizing the level of radiculopathy, and comparing EMG and EIM abnormalities in radiculopathy are planned.
Acknowledgements
This study was supported by: Grant K24NS060951, from the National Institutes of Health and the National Institute of Neurological Disorders and Stroke. We also thank Matt Gregas, PhD for reviewing the statistical analyses.
ABBREVIATIONS
- BIDMC
Beth Israel Deaconess Medical Center
- CoV
coefficient of variation
- EIM
electrical impedance myography
- HEA
handheld electrode array
- ICC
intraclass correlation coefficient
- LL
lower limb
- R
resistance
- SFL
skin-subcutaneous fat layer
- UL
upper limb
- X
reactance
- θ
phase
REFERENCES
- 1.Rutkove SB, Aaron R, Shiffman CA. Localized bioimpedance analysis in the evaluation of neuromuscular disease. Muscle Nerve. 2002;25:390–397. doi: 10.1002/mus.10048. [DOI] [PubMed] [Google Scholar]
- 2.Shiffman CA, Aaron R, Amoss V, Therrien J, Coomler K. Resistivity and phase in localized BIA. Phys Med Biol. 1999;44:2409–2429. doi: 10.1088/0031-9155/44/10/304. [DOI] [PubMed] [Google Scholar]
- 3.Rutkove SB, Esper GJ, Lee KS, Aaron R, Shiffman CA. Electrical impedance myography in the detection of radiculopathy. Muscle Nerve. 2005;32:335–341. doi: 10.1002/mus.20377. [DOI] [PubMed] [Google Scholar]
- 4.Rutkove SB, Zhang H, Schoenfeld DA, Raynor EM, Shefner JM, Cudkowicz ME, Chin AB, Aaron R, Shiffman CA. Electrical impedance myography to assess outcome in amyotrophic lateral sclerosis clinical trials. Clin Neurophysiol. 2007;118:2413–2418. doi: 10.1016/j.clinph.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarulli A, Esper GJ, Lee KS, Aaron R, Shiffman CA, Rutkove SB. Electrical impedance myography in the bedside assessment of inflammatory myopathy. Neurology. 2005;65:451–452. doi: 10.1212/01.wnl.0000172338.95064.cb. [DOI] [PubMed] [Google Scholar]
- 6.Rutkove SB. Electrical impedance myography: Background, current state, and future directions. Muscle Nerve. 2009;40:936–946. doi: 10.1002/mus.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutkove SB, Lee KS, Shiffman CA, Aaron R. Test-retest reproducibility of 50 kHz linear-electrical impedance myography. Clin Neurophysiol. 2006;117:1244–1248. doi: 10.1016/j.clinph.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Chin AB, Garmirian LP, Nie R, Rutkove SB. Optimizing measurement of the electrical anisotropy of muscle. Muscle Nerve. 2008;37:560–565. doi: 10.1002/mus.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garmirian LP, Chin AB, Rutkove SB. Discriminating neurogenic from myopathic disease via measurement of muscle anisotropy. Muscle Nerve. 2009;39:16–24. doi: 10.1002/mus.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarulli AW, Chin AB, Lee KS, Rutkove SB. Impact of skin-subcutaneous fat layer thickness on electrical impedance myography measurements: an initial assessment. Clin Neurophysiol. 2007;118:2393–2397. doi: 10.1016/j.clinph.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahad MA, Narayanaswami P, Kasselman LJ, Rutkove SB. The effect of subacute denervation on the electrical anisotropy of skeletal muscle: Implications for clinical diagnostic testing. Clin Neurophysiol. 2010 doi: 10.1016/j.clinph.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy JN. An Introduction to the Finite Element Method. McGraw-Hill Companies; 2005. The. [Google Scholar]


