Abstract
Despite its significant role in oocyte generation and hormone production in adulthood, the ovary, with regard to its formation, has received little attention compared to its male counterpart, the testis. With the exception of germ cells, which undergo a female-specific pattern of meiosis, morphological changes in the fetal ovary are subtle. Over the past 40 years, a number of hypotheses have been proposed for the organogenesis of the mammalian ovary. It was not until the turn of the millennium, thanks to the advancement of genetic and genomic approaches, that pathways for ovary organogenesis that consist of positive and negative regulators have started to emerge. Through the action of secreted factors (R-spondin1, WNT4, and follistatin) and transcription regulators (β-catenin and FOXL2), the developmental fate of the somatic cells is directed toward ovarian, while testicular components are suppressed. In this chapter, we review the history of studying ovary organogenesis in mammals and present the most recent discoveries using the mouse as the model organism.
1. Evolution of the Hypotheses for Ovary Organogenesis in Mammals
The importance of the ovary in mammalian reproduction was not recognized until von Baer’s discovery that ovarian follicles are the source of mammalian eggs (von Baer, 1827). Known as “the testicle of the female” before the 16th century, the ovary was considered merely a structure insignificant to generation of species (Harvey, 1653) or a gland that produced female “semen” (Descartes, 1664; Le Grand, 1672; Wharton, 1656). Revelation of the egg-producing ability and endocrine capability of the ovary transformed scientists’ view on its role in reproduction. Since then, the ovary has become the centerpiece of the female reproductive system.
The structural and functional foundation of the ovary is established during embryonic development in most eutherian or placental mammals. At the time of fertilization, the sex of the embryo is determined when the sperm carrying either an X or Y chromosome fertilizes the oocyte, which contains one X chromosome. The Y chromosome and its testis-determining element play an indisputable role in testis formation (see Chapter 2 on testis development). However, the number of X chromosomes is irrelevant to the establishment of the ovary, as humans and mice with XO aneuploidy still develop ovaries (Morris, 1968; Ohno and Cattanach, 1962; Singh and Carr, 1966, 1967; Welshons and Russell, 1959).
Alfred Jost, in his groundbreaking work in the early 1950s, revealed the relationship between the sexes of the gonads and phenotypic sexual characteristics. Jost discovered that when the gonads of either sex were removed before the onset of sexual differentiation, female internal and external sexual characteristics arose regardless of the chromosomal sex of the embryo. As a result it was concluded that the female sexual phenotypes appear by default, independent of the presence of gonads (Jost et al., 1953). Jost later extended this paradigm to gonadal differentiation and proposed that a putative “male organizer” prevents the gonadal primordium from developing into an ovary and forces it to become a testis (Jost, 1972) (Fig. 7.1).
Figure 7.1.
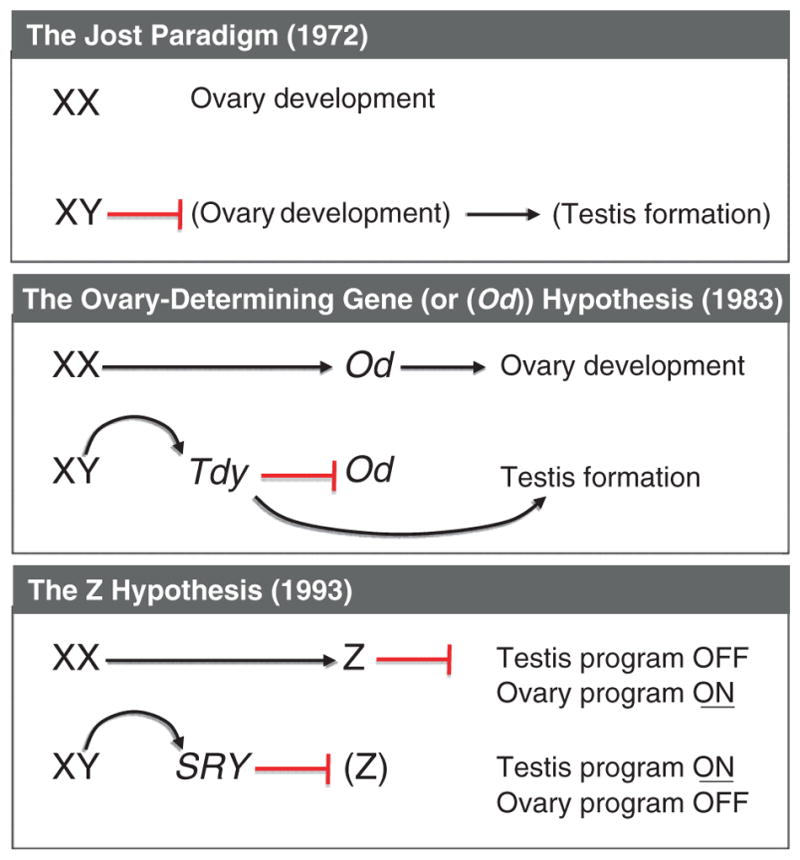
Evolution of the hypotheses for sex determination in eutherian mammals. In 1972, Jost extended his paradigm on sexually dimorphic development of the reproductive tracts to gonad differentiation and proposed that a putative “male organizer” prevents the gonadal primordium from developing into an ovary and forces it to become a testis. In 1983, Eicher and Washburn proposed that an ovary-determining gene (Od) initiates ovary differentiation in the XX individual. The testis-determining gene on the Y chromosome (or Tdy), which becomes active earlier than the Od gene, suppresses the Od gene in the XY individuals. The Z theory was proposed in 1993 by McElreavey et al. after the discovery of SRY gene. It was stated that the Z gene in the XX gonad inhibits the testis pathway, therefore leading to the progression of the ovary pathway. In the XY gonad, the SRY acts as an inhibitor of the Z gene, allowing the development of the testis.
The mechanism of ovary organogenesis was explored further by Eicher and Washburn in 1983. Based on the observation of a strain of XY mice where testes were sex-reversed to ovaries (Eicher and Washburn, 1983; Washburn and Eicher, 1983), they proposed that an ovary-determining gene (or Od), located on an autosome or the X chromosome, initiates ovary differentiation. In the male embryo, the Od gene and subsequent ovary differentiation are inhibited by the testis-determining gene on the Y chromosome (or Tdy), which presumably gains its functions preceding the Od gene (Fig. 7.1). The discovery of the SRY gene (Sex-determining region on the Y chromosome) in the early 1990s confirmed the identity of Tdy and revealed its dominant role in testis determination. However, the puzzling cases of XX male in humans and the polled intersex syndrome (PIS) XX goats, where testes develop in females without SRY or any Y chromosome fragments, kindled a rethinking of the mechanism for ovary differentiation. To explain these cases, McElreavey and colleagues proposed the Z hypothesis that in normal XX gonads the Z gene suppresses the emergence of testis program (Fig. 7.1). Loss-of-function of the Z gene in the XX gonad, therefore, results in formation of the testis (McElreavey et al., 1993). In contrast in normal XY gonads, the SRY gene antagonizes the functions of the Z gene, allowing the progression of the testis program (Fig. 7.1).
At the turn of the 21st century, mouse genetic models and human clinical cases have made the case that the mechanism for ovary differentiation is beyond X and Y chromosomes and the Z factor. Complicated antagonism and synergism at the levels of cell–cell interaction and transcriptional regulation have already occurred in the undifferentiated ovary. New findings also provide insights into sexually dimorphic regulation of germ cell meiosis and prompt a paradigm shift in our views on how the somatic environment and female germ cells interact. In this chapter, we review the current knowledge of ovary organogenesis using mouse models as the core and make comparisons to humans.
2. Building the Foundation: Morphogenesis of the Ovary
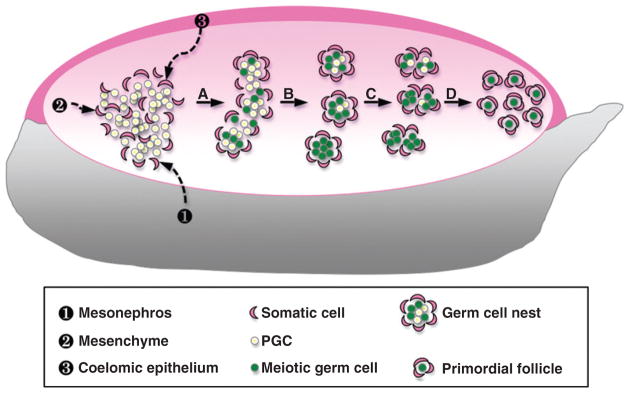
The genital ridge (or gonadal primordium), the structural precursor of both the testis and the ovary, emerges as a thickening of the coelomic epithelium that overlays the ventral aspect of the mesonephros at 10 days post coitum (dpc) in the mouse embryo. In both sexes, primordial germ cells (PGCs), which originate from the proximal epiblast, migrate through the wall of the hindgut at ~9 dpc and into the genital ridge at 10.5–11.5 dpc (Anderson et al., 2000; Molyneaux et al., 2001; Tam and Snow, 1981). Once the PGCs colonize the genital ridges, clusters of PGCs coalesce with somatic cells and form the ovigerous cords, which are delineated by the deposition of the basal lamina (Merchant, 1975; Pepling and Spradling, 1998; Ruby et al., 1969). In the developing testis, ovigerous cords differentiate into well-defined tubule structures known as testis cords as a result of the action of Sertoli cells (see Chapter 2 on testis development). In contrast in the fetal ovary, ovigerous cords remain as clusters of female germ cells (or germ cell nests) that are surrounded loosely by somatic cells (Fig. 7.2). Around the time of birth, somatic cells start to break down the germ cell nests by enclosing individual female germ cells or oocytes, leading to the formation of primordial follicles (Merchant-Larios and Chimal-Monroy, 1989; Pepling and Spradling, 1998). Germ cell nests and the forming primordial follicles populate predominantly the outer zone or cortex of the fetal ovary.
Figure 7.2.
Morphogenesis of the mouse fetal ovary. Once the PGCs migrate into and colonize the genital ridge, they coalesce with somatic cells which could be derived from three potential sources: ❶ the neighboring mesonephros, ❷ the existing mesenchyme, and/or ❸ the surrounding coelomic epithelium. (A) Somatic cells and PGCs form ovigerous cords. Female germ cells start to enter meiosis around 13.5 dpc. (B) Germ cell nests that are surrounded loosely by somatic cells begin to form. (C) Around the time of birth, somatic cells start to break down the germ cell nests by enclosing individual oocytes. (D) Breakdown of the germ cell cysts leads to the formation of the primordial follicles.
In the adult ovary, development of follicles occurs mainly in the ovarian cortex, whereas the medulla is the structure where the vasculature and nerves enter. Ovarian follicles in the cortex consist of oocytes and surrounding somatic cells including granulosa and theca cells. Granulosa cells, an epithelial cell type that forms connections with the oocytes during the fetal stage, support oocyte development and produce hormones responsible for the development and maintenance of the female reproductive system. Theca cells, a mesenchymal cell type that appear only postnatally, are the major source of androgens, which are ultimately converted to estrogens by the granulosa cells (Erickson et al., 1985; Quattropani, 1973).
Granulosa cells in the ovary and Sertoli cells in the testis are derived from common somatic precursor cells in the genital ridge, at least in mice (Albrecht and Eicher, 2001; McLaren, 1991, 2000). Based on morphological and histological observations, granulosa cell precursors could originate from three possible sources (Fig. 7.2): rete ovarii connecting to the neighboring mesonephros (Byskov, 1975, 1978; Byskov and Lintern-Moore, 1973; Byskov and Rasmussen, 1973; Zamboni et al., 1975), the existing mesenchymal cells in the genital ridge (Albrecht and Eicher, 2001; Pinkerton et al., 1961), or ovarian surface epithelium (Gondos, 1975; Motta and Makabe, 1982; Sawyer et al., 2002). Species variation seems evident in regard to the cellular origin(s) of granulosa cell precursors and no definitive sources have been identified in species other than mice. The possible contribution of multiple origins to the granulosa cell lineage cannot be excluded.
Theca cells, the ovarian counterpart of Leydig cells in the testis, begin to appear in the postnatal ovary. Theca cells are thought to be derived from fibroblast-like precursors in the ovarian stroma (Erickson et al., 1985; Hirshfield, 1991; Quattropani, 1973). As the ovarian follicles develop to the secondary stage with multiple layers of granulosa cells surrounding the oocyte, stromal cells adjacent to the basal lamina form a layer of elongated cells. This layer is known as the theca interna, which is a highly vascularized steroidogenic tissue. Outside of the theca interna, a loosely organized band of nonsteroidogenic cells or theca externa is formed. Theca cells are only found in the developing follicle and are adjacent to granulosa cells; therefore, their differentiation is considered to be under the control of granulosa cells (Kotsuji et al., 1990; Orisaka et al., 2006). This concept is supported by the findings that small-molecular-weight proteins enriched from secretions of developing follicles stimulate theca cell differentiation (Magoffin and Magarelli, 1995).
3. Making Eggs: Establishment of the Female Germline
Generation of the female germline (or oogenesis) is the one of the other key functions of the ovary in addition to hormone production. Between 1920 and 1950, the field of oogenesis was dominated by the doctrine that the germinal epithelium, or ovarian surface epithelium encapsulating the ovary, gave rise to oocytes during each estrous or menstrual cycle. This doctrine was later rejected based on evidence that a finite stock of meiotic oocytes is present in the ovary before birth and no new oocytes are generated during adult life (Zuckerman, 1951). However, the discovery of putative germline stem cells in postnatal ovaries led to the resurgence of the controversial idea of “neo-oogenesis” (Johnson et al., 2005, 2004; Zou et al., 2009). In this chapter, we focus only on the establishment of female germline during fetal life.
In the mouse fetal ovary, oocytes start to form around 13.5 dpc when female germ cells (or oogonia) stop proliferating and enter the first meiosis (McLaren, 2000). The oocytes progress through leptonema, zygonema, pachynema, and diplonema and eventually rest at the dictyate stage of meiotic prophase I around the time of birth (Borum, 1961; Speed, 1982). Oocytes do not resume meiosis until ovulation when the female reaches sexual maturity. Once ovulated from the ovary, oocytes complete the first meiotic division, enter the second meiotic division, and arrest again. The second meiotic division is completed after fertilization (Lewis et al., 2006).
Germ cells in the mouse testis behave very differently from their female counterparts. Instead of entering meiosis at 13.5 dpc, male germ cells arrest in mitosis in fetal life and resume mitosis immediately after birth (Hilscher et al., 1974). The male germ cells or spermatogonia then undergo the first meiotic and second meiotic divisions to generate spermatids. The process is repeated many times to constitute a renewing supply of mature sperm (Lewis et al., 2006). In contrast to the finite stock of oocytes at the time of birth, spermatogonia in the testis retain the ability to self-renew throughout the entire reproductive life.
How germ cells make the decision to follow the female or male path has been a central focus of study since 1970s. By creating an XX/XY chimeric embryo, researchers observed that XY germ cells in the ovarian tissue enter meiosis and become functional Y-bearing oocytes (Evans et al., 1977; McLaren et al., 1972; Mystkowska and Tarkowski, 1970). By contrast, XX germ cells avoid entering meiosis if they find themselves in a testicular environment (Palmer and Burgoyne, 1991). The conclusion was, therefore, drawn that all germ cells, regardless of their sex chromosome constitution, are programmed to follow male or female pattern of meiosis according to the surrounding somatic environment (McLaren, 1995, 2003).
Byskov and others proposed that germ cell meiosis is controlled by meiosis-inducing substance or/and a meiosis-preventing substance produced by the somatic cells in the gonads (Byskov, 1974; Byskov et al., 1995, 1998; Byskov et al., 1993; Byskov and Saxen, 1976; Gondos et al., 1996; Grinsted and Byskov, 1981). When an undifferentiated fetal testis is cultured with ovaries containing meiotic germ cells, the male germ cells in the testis are coaxed into entering meiosis (Byskov and Saxen, 1976). On the other hand, when ovaries containing germ cells in meiosis are cultured with fetal testes with well-formed testicular structure, the oocytes are prevented from reaching the diplotene stage of meiotic prophase. Thus it was hypothesized that the fetal ovary secretes a “meiosis-inducing substance”, which triggers the meiotic entry of germ cells. The fetal testis instead produces a “meiosis-inhibiting substance” that prevents germ cells from entering meiosis (Byskov and Saxen, 1976).
In contrast to the hypothesis that the ovarian somatic environment secretes factor(s) that induces germ cell meiosis, McLaren and others were in favor of the concept that germ cell entry into meiosis follows a cell-autonomous or intrinsic program (McLaren, 1984; McLaren and Southee, 1997). This concept was based on the observation that when male germ cells lose their way during migration and settle in nongonadal organs such as mesonephros and adrenal, these stray germ cells enter meiosis following the same developmental time frame as their female counterparts in the ovary (Chuma and Nakatsuji, 2001; McLaren, 1983; McLaren and Southee, 1997; Upadhyay and Zamboni, 1982; Zamboni and Upadhyay, 1983). Likewise, XY germ cells enter and progress through meiotic prophase after they are separated from Sertoli cells and then cultured with other nongonadal somatic cells such as embryonic lung cells (McLaren and Southee, 1997). These findings led to the hypothesis that germ cells in the fetal ovary enter meiosis spontaneously, whereas meiosis is inhibited in the testis by factors produced by the somatic cells, probably Sertoli cells (Donovan et al., 1986; McLaren and Southee, 1997).
The debate on how sexually dimorphic pattern of germ cell meiosis is established was resolved by the findings of Bowles et al. and Koubova et al. in 2006. A mechanism involving retinoic acid (RA) and its degrading enzyme CYP26B1 is in action, with both meiosis-inducing (RA) and meiosis-inhibiting (CYP26B1) properties (Fig. 7.3). The first clue that RA might play a role in meiotic entry of germ cells in the ovary came from an expression screen designed to identify sexually dimorphic genes in mouse fetal gonads. It was found that Cyp26b1, the gene encoding a P450 enzyme that degrades RA (Hernandez et al., 2007; Romand et al., 2006; White et al., 2000; Yashiro et al., 2004), shows a testis-specific expression pattern. Initially present in gonads of both sexes, Cyp26b1 becomes undetectable in female gonads after 11.5 dpc. However in the testis, Cyp26b1 expression is maintained and reaches its maximum at 13.5 dpc (Bowles et al., 2006). The presence of RA-degrading CYP26b1 in fetal testes is consistent with a significant lower level of RA in the testis compared to the ovary at 13.5 dpc. This evidence suggests that a low RA level is necessary for preventing germ cell meiosis in the testis (Bowles et al., 2006). In other words, a high RA level in the fetal ovary is responsible for inducing germ cell entry into meiosis. Indeed, when exogenous RA is given to fetal testes in culture, XY germ cells enter meiosis (Bowles et al., 2006; Koubova et al., 2006). In addition, treatment of fetal testes with CYP26 inhibitors in culture induces meiotic entry of XY germ cells and an upregulation of stimulated by retinoic acid gene 8 (Stra8), which is required for premeiotic DNA replication and the subsequent events of meiotic prophase in germ cells of embryonic ovaries (Baltus et al., 2006; Bowles et al., 2006). Finally, exposure of fetal ovaries to RA antagonists prevents XX germ cells from entering meiosis (Bowles et al., 2006). These in vitro results were later substantiated by examination of the Cyp26b1 knockout mice. In the absence of functional Cyp26b1 genes, RA levels are increased in embryonic testes and XY germ cells enter meiosis prophase at 13.5 dpc, similar to germ cells in a normal fetal ovary (Bowles et al., 2006; MacLean et al., 2007). Collectively, this evidence supports the concept that a high RA in the fetal ovary due to lack of CYP26B1 is responsible for inducing germ cell meiosis (Fig. 7.3) (Bowles and Koopman, 2007). Presence of CYP26B1 in the fetal testis prevents accumulation of RA and consequent germ cell meiosis.
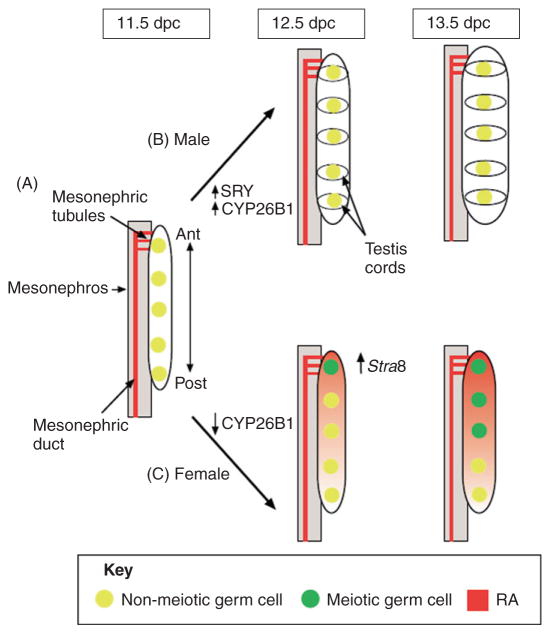
Figure 7.3.
Regulation of germ cell entry into meiosis in the developing gonads. (A) At 11.5 dpc, PGCs are present in the genital ridge and RA is produced in the neighboring mesonephric duct and tubules. Cyp26b1 is expressed at low levels in the gonad of both sexes. The mesonephric tubules, which produce RA, are physically connected with the anterior (Ant) end of the gonad during this time. (B) In the male gonad, once Sry is expressed (~11.5 dpc), the RA-degrading enzyme Cyp26b1 expression is upregulated. The testis cords, which form around germ cell clusters around 12.5 dpc, might concentrate the enzyme in these regions, thereby protecting germ cells from the actions of RA. Germ cells in the male gonad therefore do not enter meiosis at 13.5 dpc. (C) In the female gonad, Cyp26b1 expression is detectable at 11.5 dpc, but disappears by 12.5 dpc. Germ cells at the anterior end of the gonad begin to express Stra8 at 12.5 dpc. By 13.5 dpc, female germ cells enter meiosis in an anterior-to-posterior (Post) wave. Germ cells at the anterior end of the gonad might be exposed to RA earlier than those at the posterior end, or the RA concentration might be greater at the anterior end than the posterior end [this figure is modified from Figure 2 in Bowles and Koopman (2007)].
Intriguingly, fetal testes and ovaries are not the source of RA. Gonads apparently lack the ability to synthesize RA because they do not express Aldh1a2, the gene encoding the major enzyme for RA synthesis (Bowles et al., 2006). Instead, RA is secreted by mesonephroi, the mesoderm-derived tissues immediately adjacent to the gonads (Bowles et al., 2006). In 1970s, Byskov proposed that rete ovarii, the extending mesonephric derivative that connects to the ovarian medulla, may be the source of “meiosis-inducing factor” (Byskov, 1974, 1975; Byskov and Lintern-Moore, 1973). In the mouse gonads, mesonephric tubules connect to the anterior portion of the gonads. If indeed the meiosis-inducing factor (or RA) comes from the mesonephric tubules, one would expect that the RA level would be higher at the anterior end than at the posterior end of the gonad. This anterior-to-posterior gradient of RA in the gonads was later confirmed (Fig. 7.3) (Bowles et al., 2006). This phenomenon is also supported by the fact that female germ cells in the anterior part of the fetal ovary enter meiosis earlier than those in the posterior end of the fetal ovary (Bullejos and Koopman, 2004; Menke and Page, 2002; Yao et al., 2003).
Germ cell meiosis in the fetal ovary is controlled by not only the availability of the meiosis-inducing RA, but also the competence of germ cells to respond to RA. Germline specific RNA-binding protein DAZL (deleted in azoospermia-like gene) and NANOS2 have emerged as intrinsic factors in germ cells that define their ability to enter meiosis in response to RA. When Dazl became nonfunctional, germ cells in the fetal ovary fail to enter meiosis. In addition, male germ cells in the Dazl knockout testis lose their ability to enter meiosis in response to exogenous RA (Lin et al., 2008). This evidence implies that the presence of Dazl is a prerequisite for germ cells to gain the ability to respond to RA, therefore, becoming meiosis-competent. Nanos2, on the other hand, plays a role in suppressing germ cell meiosis. Nanos2 is expressed exclusively in germ cells in the fetal testis, whereas it is absent in female germ cells (Tsuda et al., 2003). Loss of Nanos2 in the fetal testis results in upregulation of Stra8 and meiosis of male germ cells. Ectopic expression of Nanos2 in the female germ cells decreases Stra8 expression and inhibits germ cell meiosis (Suzuki and Saga, 2008). NANOS2 probably inhibits germ cell meiosis by decreasing Stra8 expression, the downstream target of RA (Suzuki et al., 2010).
In summary, establishment of the female germline, characterized by entry into meiosis in fetal life, requires a synchronized action of both extracellular and intracellular factors. Extrinsic RA, derived from the mesonephros, serves as a meiosis-inducing agent in the fetal ovary. Female germ cells become competent to enter meiosis in response to RA only after they are primed by the presence of intrinsic factor DAZL. The fetal ovary also suppresses production of the meiosis-inhibiting factors including Cyp26b1 and Nanos2, clearing the path for female germ cells to differentiate into oocytes. The observation of putative female germline stem cells in adult ovaries (Johnson et al., 2004, 2005; Zou et al., 2009) raises the question of how these putative germline stem cells escape from the meiosis-inducing RA during fetal development and re-enter the meiosis path later in life. Knowledge of sexually dimorphic regulation of the germline could have implications for solving the controversy around the existence of female germline stem cells in adult ovaries.
4. Determination of the Ovarian Identity: Differentiation of Granulosa Cells
Lessons from regulation of germ cell meiosis highlight the importance of the somatic cell environment in ovary differentiation. In contrast to female germ cells, somatic cells in the fetal ovary have received little attention because their differentiation lacks dramatic elements compared to their counterparts in the fetal testis. The Sry-expressing Sertoli cells in the testis orchestrate morphological transformation of the testis (see Chapter 2 on testis development). Granulosa cells, the main somatic cells in the developing ovary, are derived from the same progenitor cells as the Sertoli cells. It was originally thought that the progenitor cells differentiate into granulosa cells by default when the Sry is absent, as in XX animals (Fig. 7.1). Influenced by the hypotheses of ovary-determining genes and the Z factor (Fig. 7.1), researchers began to search for genes exclusively expressed in the somatic cells in the ovary. Functional genetic analyses have identified many ovary-specific and somatic cell-derived genes that can be classified into two categories: intracellular factors such as transcription factors (DAX1, FOXL2, and β-catenin) and extracellular factors with paracrine and/or autocrine properties (R-spondin1 and WNT4).
Dax1 (dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1) and Foxl2 were once the prime candidates for ovary organogenesis and establishment of granulosa cell lineage. DAX1 was initially considered as the ovary-determining gene because of its X-linked nature and function as a transcriptional regulator (Swain et al., 1998). However, null mutation of Dax1 in female mouse embryos did not affect formation and development of the ovary (Meeks et al., 2003a–c; Yu et al., 1998). Instead of being an ovary-determining gene, Dax1 is found to have the anti-testis or Z property in the fetal testis. Duplication of a small piece of the X chromosome that contains DAX1 in XY humans leads to testis-to-ovary sex reversal (Zanaria et al., 1994). Transgenic mice carrying multiple copies of Dax1 genes also have testis-to-ovary sex reversal (Swain et al., 1998), suggesting a dose-dependent, anti-testis role of DAX1.
FOXL2, a member of the forkhead transcription factor family, gained attention because of its potential link to the ovary-to-testis sex reversal in the PIS XX goats (Pailhoux et al., 2001, 2002). FOXL2, an autosomal gene, shows a granulosa cell-specific pattern conserved among vertebrate species (Loffler et al., 2003; Wang et al., 2004). Originally thought to be the candidate Z factor, FOXL2 was later found not to be involved in early ovary organogenesis at least in humans and mice. In the absence of functional FOXL2 genes, human and mouse females develop granulosa cell defects and signs of premature ovarian failure postnatally; however, no signs of ovary-to-testis sex reversal are observed as predicted by the Z hypothesis (Ottolenghi et al., 2005; Schmidt et al., 2004). FOXL2 and probably DAX1 may not be critical for early ovary formation, but the possibility of synergy or compensation by other factors cannot be excluded, as discussed below.
In contrast to the uncertain roles of DAX1 and FOXL2, secreted factor R-spondin1 (RSPO1) and WNT4 are doubtlessly critical for establishment of the somatic cell environment in the fetal ovary. Initially expressed in somatic cells in gonads of both sexes, Rspo1 and Wnt4 expression become ovary-specific after the time of sex determination (Chassot et al., 2008b; Parma et al., 2006; Tomizuka et al., 2008; Vainio et al., 1999). Female mouse embryos lacking functional Rspo1 or Wnt4 develop similar ovarian defects including formation of ectopic testis vasculature, appearance of androgen-producing cells, loss of female germ cells, and appearance of testicular structure at birth (Biason-Lauber et al., 2004; Chassot et al., 2008b; Jeays-Ward et al., 2003; Parma et al., 2006; Tomizuka et al., 2008; Vainio et al., 1999; Yao et al., 2004). The shared phenotypes of the Rspo1 and Wnt4 knockout ovaries indicate that these two factors are components of a common pathway in the fetal ovary (see below). Genetic analyses further revealed that RSPO1 is responsible for stimulating the expression of Wnt4 (Fig. 7.4) (Chassot et al., 2008b; Trautmann et al., 2008; Yao et al., 2004). The RSPO1/WNT4 pathway apparently operates independent of FOXL2 as the expression of these genes is not affected when either one of these genes become inactive (Chassot et al., 2008b; Ottolenghi et al., 2007).
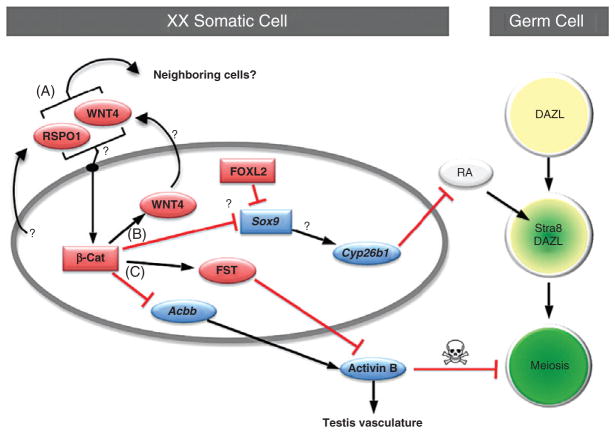
Figure 7.4.
Putative pathways for ovary organogenesis in the mouse embryo. (A) Two somatic cell-derived factors, R-spondin1 (RSPO1) and WNT4, activate synergistically or independently the canonical β-catenin (β-cat) pathway in XX somatic cells in an autocrine or paracrine manner. (B) β-catenin then induces expression of Wnt4 while suppresses expression of Sox9 (probably through the action of FOXL2) and its putative downstream target Cyp26b1. Without the presence of Cyp26b1, RA is not degraded and therefore induces Stra8 expression and meiosis in DAZL-positive germ cells. (C) β-catenin also induces expression of follistatin (Fst) and at the same time maintains a low expression of activin βB (Acbb). FST antagonizes the action of activin B, which is the protein product of Acbb. Lack of activin B ensures that no testis-specific vasculature forms and the survival of female germ cells is maintained. See text for more details. (See Color Insert.)
RSPO1 and WNT4 elicit their actions in ovarian somatic cells via β-catenin, the intracellular regulator of the canonical WNT pathway. Inactivation of β-catenin specifically in the steroidogenic factor 1 (SF1)-positive ovarian somatic cells (putative precursors of granulosa cells) produces ovarian defects similar to those found in Rspo1 and Wnt4 knockouts (Liu et al., 2009; Manuylov et al., 2008). The involvement of β-catenin is further confirmed by the gain-of-function experiments where ectopic activation of β-catenin in the absence of Rspo1 or Wnt4 restores normal ovarian development (Chassot et al., 2008b; Liu et al., 2010). These experiments also reveal a molecular connection between RSPO1 and WNT4 (Fig. 7.4). In the absence of β-catenin, Rspo1 expression in the ovary remains unchanged, whereas expression of Wnt4 is lost, indicating the requirement of RSPO1 and β-catenin for Wnt4 expression (Liu et al., 2009). RSPO1 is able to induce β-catenin either directly by itself or synergistically in the presence of WNT ligands in vitro (Binnerts et al., 2007; Kim et al., 2008, 2006; Lu et al., 2008; Wei et al., 2007). It remains to be determined whether RSPO1 and WNT4 act in a linear fashion or synergistically in activating β-catenin in the somatic cells of the ovary.
Despite their different modes of action (secreted factor versus transcription factor), RSPO1, WNT4, and FOXL2 eventually converge for a common purpose: maintenance of the identity of granulosa cells. In the absence of Rspo1, Wnt4, or Foxl2, the XX gonadal primoridia develop into ovaries initially but testis components (Sertoli cells and testis cords) start to appear amongst ovarian structure after birth (Chassot et al., 2008b; Ottolenghi et al., 2005; Tomizuka et al., 2008; Vainio et al., 1999). However, when Wnt4 and Foxl2 are inactivated together, Sox9, the testis-determing gene downstream of SRY (see Chapter 2 on testis development), is significantly upregulated, leading to ovary-to-testis sex reversal (Ottolenghi et al., 2007). These observations support the model that extracellular (RSPO1/WNT4) and intracellular (FOXL2) factors synergistically direct the gonadal somatic cells to follow the ovarian path by antagonizing Sox9 expression (Figs. 7.4 and 7.5). Sox9 is expressed in gonads of both sexes before the onset of sex determination. SRY in the testis maintains/stimulates Sox9 expression, whereas in the ovary Sox9 expression is lost (see Chapter 2 on testis development). Lack of Sry and its downstream effectors in the ovary is thought to be responsible for the absence of Sox9 expression. However, the rise of Sox9 in the Wnt4/Foxl2 double knockout ovary without the presence of Sry gene argues against this notion. We hypothesize that if neither Sry nor RSPO1/WNT4/FOXL2 is present, the default status of gonads is testis, as a result of gradual increase of Sox9 expression (Fig. 7.5). In the XY individual, SRY in the testis jump-starts Sox9 expression, which subsequently suppresses the pro-ovary functions of RSPO1/WNT4/FOXL2. On the other hand, in the absence of Sry as in the XX individual, RSPO1/WNT4/FOXL2 prevents the rise of Sox9 and its ability to induce testis differentiation, therefore, allowing the somatic progenitors to differentiate into granulosa cells and subsequent ovary organogenesis.
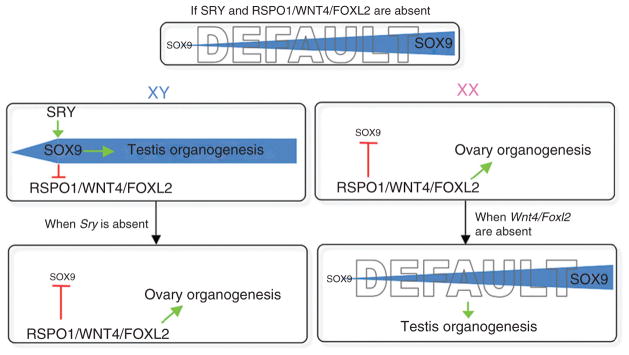
Figure 7.5.
The 2010 version of sex determination hypothesis based on mouse genetic models. We propose that if both SRY and RSPO1/WNT4/FOXL2 are absent, the default status of gonads is testis, as a result of gradual increase of Sox9 expression. When both SRY and RSPO1/WNT4/FOXL2 are present as in the XY individual, SRY in the testis jumpstarts Sox9 expression, which subsequently suppresses the pro-ovary functions of RSPO1/WNT4/FOXL2. On the other hand, in the absence of Sry as in the XX individual, RSPO1/WNT4/FOXL2 prevent the rise of Sox9 and its ability to induce testis differentiation, therefore allowing the gonads to follow the ovarian path. When Sry is nonfunctional or lost, RSPO1/WNT4/FOXL2 synergistically suppresses Sox9 expression and facilitates ovary organogenesis. If the action of RSPO1/WNT4/FOXL2 is silenced in the XX individual, the default testis pathway arises despite the absence of Sry.
5. Emerging Pathways for the Establishment of Female Somatic Environment and Maintenance of Female Germ Cells
In addition to their involvement in ovarian somatic cell differentiation, RSPO1 and WNT4 have unique functions in maintaining proper ovarian environment and survival of female germ cells. One of the earliest morphological differences between fetal testis and ovary is the establishment of an organized vascular network in the testis and the lack of a similar structure in the ovary (Brennan et al., 2002). When either Rspo1 or Wnt4 or follistatin (Fst) is inactivated, the testis-specific vasculature appears in the fetal ovary at 12.5 dpc (Jeays-Ward et al., 2003; Tomizuka et al., 2008; Yao et al., 2004). In addition to this phenotype, female germ cells undergo apoptosis starting at 15.5 dpc and are lost at the time of birth (Tomizuka et al., 2008a; Vainio et al., 1999; Yao et al., 2004). Similar vasculature and germ cell loss phenotypes are also observed in the fetal ovary that lacks β-catenin in the SF1-positive somatic cells (Liu et al., 2009; Manuylov et al., 2008).
The connection between Rspo1, Wnt4, β-catenin, and Fst is further confirmed by the genetic models in which the constitutively active form of β-catenin is introduced to the somatic cells of Rspo1 or Wnt4 knockout ovary. In the presence of the active β-catenin, normal ovarian characteristics are restored despite a lack of Rspo1 or Wnt4 (Chassot et al., 2008b; Liu et al., 2010). In the Wnt4 knockout ovary, where Fst expression is lost, active β-catenin is able to maintain Fst expression, placing β-catenin downstream of Wnt4 (Fig. 7.4). β-catenin probably stimulates Fst expression directly via the TCF/LEF consensus sequence in the promoter region of Fst (de Groot et al., 2000; Willert et al., 2002).
Fst, a component in the RSPO1/WNT4/β-catenin pathway, encodes a secreted protein that antagonizes the activity of activins. Binding of FST to activins inhibits or limits the ability of activins to interact with their receptors, therefore, silencing the functions of activins (Muttukrishna et al., 2004). Activins consist of either homodimers or heterodimers of activin βA and βB subunits. mRNA for activin βB (Acbb), but not activin βA, is present in the fetal mouse ovary although its expression is low (Yao et al., 2006). Acbb mRNA expression is suppressed by RSPO1, WNT4, and β-catenin, and when any of these three genes is inactivated, expression of Acbb is significantly elevated (Yao et al., 2006). The inhibitory effects of RSPO1/ WNT4/β-catenin on Acbb expression are further confirmed by the finding that addition of active β-catenin to the Wnt4 knockout ovary suppresses Acbb expression to the low levels seen in the normal ovary (Liu et al., 2010).
The elevated Acbb in the Rspo1, Wnt4, and β-catenin knockout ovary leads to the hypothesis that Acbb could be responsible for appearance of testis-specific vasculature and loss of female germ cells. Two observations support this hypothesis: first, presence of exogenous activin B (protein product of Acbb) induces formation of ectopic testis vasculature in normal fetal ovaries in culture (Yao et al., 2006) and second, when the Acbb gene is inactivated in the Wnt4 knockout ovary, normal ovarian development is restored (lack of testis-specific vasculature and maintenance of female germ cells) (Liu et al., 2010; Yao et al., 2006). Intriguingly, inactivation of Acbb in the Fst knockout ovary also restores normal ovarian development (Yao et al., 2006). These results support the model that in the fetal ovary, Wnt4 and Fst antagonize functions of Acbb. WNT4, via the action of β-catenin, suppresses but does not completely abolish the expression of Acbb. The function of FST is to antagonize and inhibit the action of the residual activin B to prevent it from inducing testicular vasculature and demise of female germ cells (Fig. 7.4).
When the ovary-specific functions of Wnt4 was first discovered in 1999, Wnt4 was labeled as the inhibitor that prevents the appearance of Leydig cells, the androgen-producing cells present only in the testis (Vainio et al., 1999). In the Wnt4 knockout ovary, androgen-producing cells appeared ectopically, leading to masculinization of the female. However, the identity of these ectopic androgen-producing cells was later found to be adrenal origin instead of Leydig cells (Heikkila et al., 2002; Jeays-Ward et al., 2003). In addition, these adrenal cells appeared in not only the fetal ovary, but also fetal testis of the Wnt4 knockout embryos (Heikkila et al., 2002). The appearance of these androgen-producing adrenal cells are also observed in the Rspo1 and β-catenin knockout ovaries (Chassot et al., 2008a; b; Liu et al., 2009; Manuylov et al., 2008; Tomizuka et al., 2008; Vainio et al., 1999). Adrenals and gonads are derived from a common adrenogonadal primordium, which later separate into two identities. Presence of Rspo1 and Wnt4 in the adrenogonadal primordium before the separation of adrenal and gonads suggests that these two genes play a role in proper allocation of the adrenal cell lineage (Heikkila et al., 2002; Jeays-Ward et al., 2003). Rspo1 and Wnt4 probably are not involved in suppressing the appearance of Leydig cells in the fetal ovary.
6. Conclusion and Perspectives
Default or not, the pathways that lead to organogenesis of the ovary are far more complicated than what was originally hypothesized (Figs. 7.1 and 7.5). Components of the pathway possess properties of both ovary-determining gene and anti-testis Z factor, which operate at levels of cell-to-cell interaction and transcriptional regulation. Through the action of the secreted factors RSPO1 and WNT4, somatic cells in the fetal mouse ovary are instructed to follow the program for granulosa cell differentiation. Signals from RSPO1 and WNT4 are interpreted intracellularly via β-catenin, which with a synergistic action of FOXL2, silences the SOX9-induced testis differentiation (Figs. 7.4 and 7.5). This antagonism is a critical connection between ovarian somatic cells and germ cells. Lack of SOX9-induced testis differentiation results in absence of CYP26b1 (a putative target of SOX9) that degrades the meiosis-inducing RA. RA, therefore, becomes available only in the fetal ovary and induces meiosis of female germ cells. Once female germ cells have entered meiosis, they can only survive in the ovarian somatic environment that is set up by RSPO1, WNT4, and other components of the pathway (Fig. 7.4).
Despite this progress in our understanding of ovary organogenesis and its regulation, many questions remain. First, in addition to regulating somatic cell development, do Rspo1 and Wnt4 have a direct role on female germ cells? Female germ cells are almost completely lost in Rspo1, Wnt4, and β-catenin knockout ovaries at the time of birth. However, it is unclear whether the affected germ cells enter meiosis before their demise. Some reports show meiotic defects in female germ cells in the Rspo1 and Wnt4 knockout ovaries (Chassot et al., 2008b; Naillat et al., 2010). However, others find proper progression of germ cell meiosis in the absence of Rspo1, Wnt4, and β-catenin (Chassot et al., 2008b; Tomizuka et al., 2008; Liu et al., 2009; Liu et al., 2010). It is worth noting that the meiosis-inducing system (RA and Stra8) is not significantly altered in the Rspo1 knockout ovary, but is compromised in the Wnt4 knockout ovary (Chassot et al., 2008b; Naillat et al., 2010). More experiments are needed to reconcile these discrepancies and clarify whether RSPO1 and WNT4 intersect with RA in regulating germ cell meiosis.
Second, do fetal and adult ovaries utilize different mechanisms to maintain the differentiated state? Whereas loss of Foxl2 does not affect formation of the fetal mouse ovary, Foxl2 is essential for the maintenance of somatic cell identity in the adult ovary. Inactivation of Foxl2 in the adult mouse ovary leads to upregulation of Sox9, transdifferentiation of granulosa cells into Sertoli cells, and appearance of testis structure and cell types (Uhlenhaut et al., 2009). Granulosa-to-Sertoli transdifferentiation is also observed in adult ovaries of estrogen receptor alpha and beta double knockout (ERαβKO) and aromatase knockout mice (Britt et al., 2001; Britt and Findlay, 2003; Britt et al., 2004a, b; Dupont et al., 2003, 2000). FOXL2 is known to stimulate expression of aromatase, the enzyme responsible for estrogen synthesis (Pannetier et al., 2006; Uhlenhaut et al., 2009; Wang et al., 2007). It is therefore proposed that in the adult ovary FOXL2 serves to maintain granulosa cell identity by promoting the synthesis of estrogen, which is the conserved mechanism responsible for ovary formation in species such as reptiles, birds, fish, and even marsupials (Bruggeman et al., 2002; Elf, 2003; Kobayashi and Nagahama, 2009; Mittwoch, 1998; Nakamura, 2009; Pask and Renfree, 2001; Yao, 2005; Yao and Capel, 2005). The maintenance of granulosa cell differentiation apparently shifts from estrogen-independent at the fetal stage to estrogen-dependent in adulthood (Fig. 7.6). Female mammals, particularly eutherian mammals, are constantly exposed to maternal estrogens. The estrogen-insensitive mechanisms for ovary organogenesis make physiological sense; otherwise all the male embryos would be sex-reversed by estrogens. Finding out what roles the RSPO1/WNT4 pathway plays in other vertebrate species will shed light onto the evolution of the mechanism for ovary organogenesis.
Figure 7.6.
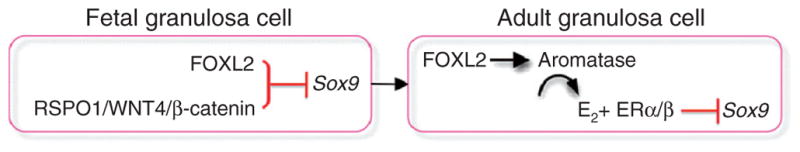
Maintenance of granulosa cell identity in fetal and adult ovaries. In the fetal ovary, granulosa cell identity is maintained in an estrogen-independent manner via the RSPO1/WNT4/β-catenin pathway and FOXL2, which together repress the expression of Sox9. This mechanism becomes estrogen-dependent after birth and in the adult ovary FOXL2 stimulates estrogen-producing enzyme aromatase and through the action of estrogen (E2) and their receptors (ERα/β) suppresses Sox9 and maintain the identity of granulosa cells.
Third, where do the theca cell lineage originate from and how is their identity established? Theca cells, the female counterparts of testis Leydig cells, are essential for steroidogenesis and formation of the follicles. At present, no lineage markers have been identified for theca cells. Based on their similar functions and mesenchymal nature to Leydig cells, we propose that the mechanisms for their specification could also share similarities with Leydig cells. Specification of fetal Leydig cells is under the control of Sertoli cell-derived Desert hedgehog (Barsoum et al., 2009; Barsoum and Yao, 2010; Huang and Yao, 2010; Yao et al., 2002). We are currently investigating whether hedgehog ligands derived from the granulosa cells instruct mesenchymal precursor cells to differentiate into the theca cell lineage in the fetal ovary.
A final, important question is whether knowledge gained from mouse models is applicable to other mammalian species such as human. It is naïve to think that animals utilize identical regulatory pathways for one biological process such as ovary organogenesis. Components of the pathways may be conserved but species variations are expected as the organisms adapt to their unique developmental environment for survival. Comparing the mouse genetic models with human clinical cases collected so far (Table 7.1), RSPO1 and WNT4 both are involved in ovary organogenesis with species differences. Loss of function in human RSPO1 gene leads to complete female-to-male sex reversal (Parma et al., 2006), a phenotype much more severe than that in the mouse Rspo1 knockout model (Chassot et al., 2008b; Tomizuka et al., 2008). Human patients with defective function of WNT4 develop various degrees of female-to-male sex reversal (Biason-Lauber et al., 2004; Mandel et al., 2008). In addition, humans seem to be more sensitive to gene dosage than the mouse (see gain-of-function cases in Table 7.1).
Table 7.1.
Genes involved in fetal ovary development in mice and humans
| Gene | Mutation | Sex | Phenotypes in mice | Phenotypes in humans | Reference |
|---|---|---|---|---|---|
| Ctnnb1* | LOF* | F | Appearance of testis-specific vasculature, loss of female germ cells, and appearance of androgen-producing adrenal cells | None reported | Liu et al., 2009; Manuylov et al., 2008. |
| Ctnnb1 | GOF* | M | Testis-to-ovary sex reversal | None reported | Maatouk et al., 2008. |
| Dax1 | LOF | F | No phenotypes in the fetal ovary | X-linked syndrome with adrenal hypoplasia congenital and hypogonadtropic hypogonadism | Meek et al., 2003a; Yu et al., 1998; Zanaria et al.,1994. |
| Dax1 | GOF | M | Insertion of multiple copies of Dax1 gene in the XY mouse delays testis development and causes male-to-female sex reversal in the YPos background | Duplication of a portion of the X chromosome containing DAX1 gene cause male-to-female sex reversal | Barbaro, 2008; Sanlaville et al., 2004; Swain et al., 1998. |
| Foxl2 | LOF | F | Defects in folliculogenesis postnatally and Blepharophimosis Ptosis Epicanthus inversus Syndrome (BPES)-like symptoms | Premature ovarian failure and Blepharophimosis Ptosis Epicanthus inversus Syndrome (BPES) | Crisponi et al., 2001; Ottolenghi et al., 2005; Schmidt et al., 2004. |
| Fst | LOF | F | Appearance of testis-specific vasculature and loss of female germ cells | None reported | Yao et al., 2004. |
| Rspo1 | LOF | F | Appearance of testis-specific vasculature, loss of female germ cells, and appearance of androgen-producing adrenal cells | Female-to-male sex reversal; Palmoplantar hyperkeratosis (PPK) and predisposition to squamous, cell carcinoma of the skin (SCC) | Chassot et al., 2008a; Parma et al., 2006; Tomizuka et al., 2008. |
| Wnt4 | LOF | F | Appearance of testis-specific vasculature, loss of female germ cells, and appearance of androgen-producing adrenal cells | Various degree of female-to- male sex reversal and SERKAL syndrome (sex reversal; dysgenesis of kidneys, adrenals, and lungs) | Biason-Lauber et al., 2004; Jeays-Ward et al., 2003; Mandel et al., 2008; Philibert et al., 2008; Sultan et al., 2009; Vainio et al., 1999. |
| Wnt4 | GOF | M | Transgenic mouse carrying human WNT4 gene disrupts testicular vasculature and decreases testosterone synthesis | Duplication of a DNA fragment containing WNT4 gene leads to XY female phenotypes | Jordan et al., 2001, 2003. |
Ctnnb1 = β-catenin; LOF = loss of function; GOF = gain of function
Despite these species variations, research using mouse models has identified or confirmed the involvement of components in the pathways toward organogenesis of the ovary. New candidate genes continue to be discovered by mRNA array experiments (Beverdam and Koopman, 2006; Bouma et al., 2009; Cederroth et al., 2007; Coveney et al., 2008; Houmard et al., 2009; Jorgensen and Gao, 2005; Nef et al., 2005), protein screening (Ewen et al., 2009), or regulatory sequence comparison (Lee et al., 2009). It will not be a surprise in the near future if components of ovary organogenesis expand from X, Y, and Z to the entire alphabet.
References
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- Anderson R, Copeland TK, Scholer H, Heasman J, Wylie C. The onset of germ cell migration in the mouse embryo. Mech Dev. 2000;91:61–68. doi: 10.1016/s0925-4773(99)00271-3. [DOI] [PubMed] [Google Scholar]
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–1434. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- Barbaro M, Cicognani A, Balsamo A, Lofgren A, Baldazzi L, Wedell A, Oscarson M. Gene dosage imbalances in patients with 46, XY gonadal DSD detected by an in-house-designed synthetic probe set for multiplex ligation-dependent probe amplification analysis. Clin Genet. 2008;73:453–464. doi: 10.1111/j.1399-0004.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- Barsoum IB, Bingham NC, Parker KL, Jorgensen JS, Yao HHC. Activation of the hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal Leydig cells and female pseudohermaphroditism. Dev Biol. 2009;329:96–103. doi: 10.1016/j.ydbio.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum IB, Yao HHC. Fetal Leydig cells: Progenitor cell maintenance and differentiation. J Androl. 2010;31:11–15. doi: 10.2164/jandrol.109.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverdam A, Koopman P. Expression profiling of purified mouse gonadal somatic cells during the critical time window of sex determination reveals novel candidate genes for human sexual dysgenesis syndromes. Hum Mol Genet. 2006;15:471–431. doi: 10.1093/hmg/ddi463. [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A, Konrad D, Navratil F, Schoenle EJ. A WNT4 mutation associated with mullerian-duct regression and virilization in a 46, XX woman. N Engl J Med. 2004;351:792–798. doi: 10.1056/NEJMoa040533. [DOI] [PubMed] [Google Scholar]
- Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas WE, 3rd, Dixon M, Hazell SA, Wagle M, et al. R-spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci USA. 2007;104:14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borum K. Oogenesis in the mouse. A study of the meiotic prophase. Exp Cell Res. 1961;24:495–507. doi: 10.1016/0014-4827(61)90449-9. [DOI] [PubMed] [Google Scholar]
- Bouma GJ, Hudson QJ, Washburn LL, Eicher EM. New candidate genes identified for controlling mouse gonadal sex determination and the early stages of granulosa and Sertoli cell differentiation. Biol Reprod. 2009;82:380–389. doi: 10.1095/biolreprod.109.079822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Sciences (New York) 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- Bowles J, Koopman P. Retinoic acid, meiosis and germ cell fate in mammals. Development (Cambridge, England) 2007;134:3401–3411. doi: 10.1242/dev.001107. [DOI] [PubMed] [Google Scholar]
- Brennan J, Karl J, Capel B. Divergent vascular mechanisms downstream of Sry establish the arterial system in the XY gonad. Dev Biol. 2002;244:418–428. doi: 10.1006/dbio.2002.0578. [DOI] [PubMed] [Google Scholar]
- Britt KL, Drummond AE, Dyson M, Wreford NG, Jones ME, Simpson ER, Findlay JK. The ovarian phenotype of the aromatase knockout (ArKO) mouse. J Steroid Biochem Mol Biol. 2001;79:181–185. doi: 10.1016/s0960-0760(01)00158-3. [DOI] [PubMed] [Google Scholar]
- Britt KL, Findlay JK. Regulation of the phenotype of ovarian somatic cells by estrogen. Mol Cell Endocrinol. 2003;202:11–17. doi: 10.1016/s0303-7207(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Britt KL, Saunders PK, McPherson SJ, Misso ML, Simpson ER, Findlay JK. Estrogen actions on follicle formation and early follicle development. Biol Reprod. 2004a;71:1712–1723. doi: 10.1095/biolreprod.104.028175. [DOI] [PubMed] [Google Scholar]
- Britt KL, Stanton PG, Misso M, Simpson ER, Findlay JK. The effects of estrogen on the expression of genes underlying the differentiation of somatic cells in the murine gonad. Endocrinology. 2004b;145:3950–3960. doi: 10.1210/en.2003-1628. [DOI] [PubMed] [Google Scholar]
- Bruggeman V, Van As P, Decuypere E. Developmental endocrinology of the reproductive axis in the chicken embryo. Comp Biochem Physiol A Mol Integr Physiol. 2002;131:839–846. doi: 10.1016/s1095-6433(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol Reprod Dev. 2004;68:422–428. doi: 10.1002/mrd.20105. [DOI] [PubMed] [Google Scholar]
- Byskov AG. Does the rete ovarii act as a trigger for the onset of meiosis? Nature. 1974;252:396–397. doi: 10.1038/252396a0. [DOI] [PubMed] [Google Scholar]
- Byskov AG. The role of the rete ovarii in meiosis and follicle formation in the cat, mink and ferret. J Reprod Fertil. 1975;45:201–209. doi: 10.1530/jrf.0.0450201. [DOI] [PubMed] [Google Scholar]
- Byskov AG. The anatomy and ultrastructure of the rete system in the fetal mouse ovary. Biol Reprod. 1978;19:720–735. doi: 10.1095/biolreprod19.4.720. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Andersen CY, Nordholm L, Thogersen H, Xia G, Wassmann O, Andersen JV, Guddal E, Roed T. Chemical structure of sterols that activate oocyte meiosis. Nature. 1995;374:559–562. doi: 10.1038/374559a0. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Baltsen M, Andersen CY. Meiosis-activating sterols: Background, discovery, and possible use. J Mol Med. 1998;76:818–823. doi: 10.1007/s001090050286. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Fenger M, Westergaard L, Andersen CY. Forskolin and the meiosis inducing substance synergistically initiate meiosis in fetal male germ cells. Mol Hum Reprod. 1993;34:47–52. doi: 10.1002/mrd.1080340108. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Lintern-Moore S. Follicle formation in the immature mouse ovary: The role of the rete ovarii. J Anat. 1973;116:207–217. [PMC free article] [PubMed] [Google Scholar]
- Byskov AG, Rasmussen G. Ultrastructural studies of the developing follicle. In the development and maturation of the ovary and its functions. Excerpta Medica Int Congr Ser. 1973;267:55–62. [Google Scholar]
- Byskov AG, Saxen L. Induction of meiosis in fetal mouse testis in vitro. Dev Biol. 1976;52:193–200. doi: 10.1016/0012-1606(76)90239-6. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Pitetti JL, Papaioannou MD, Nef S. Genetic programs that regulate testicular and ovarian development. Mol Cell Endocrinol. 2007;265–266:3–9. doi: 10.1016/j.mce.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Chassot AA, Gregoire EP, Magliano M, Lavery R, Chaboissier MC. Genetics of ovarian differentiation: Rspo1, a major player. Sex Dev. 2008a;2:219–227. doi: 10.1159/000152038. [DOI] [PubMed] [Google Scholar]
- Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008b;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- Chuma S, Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev Biol. 2001;229:468–479. doi: 10.1006/dbio.2000.9989. [DOI] [PubMed] [Google Scholar]
- Coveney D, Ross AJ, Slone JD, Capel B. A microarray analysis of the XX Wnt4 mutant gonad targeted at the identification of genes involved in testis vascular differentiation. Gene Expr Patterns. 2008;8:529–537. doi: 10.1016/j.gep.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- de Groot E, Veltmaat J, Caricasole A, Defize L, van den Eijnden-van Raaij A. Cloning and analysis of the mouse follistatin promoter. Mol Biol Rep. 2000;27:129–139. doi: 10.1023/a:1007159031000. [DOI] [PubMed] [Google Scholar]
- Descartes R. L’Homme, et un traitt’e la formation du foetus du mesme autheur. C. Angot; Paris: 1664. [Google Scholar]
- Donovan PJ, Stott D, Cairns LA, Heasman J, Wylie CC. Migratory and postmigratory mouse primordial germ cells behave differently in culture. Cell. 1986;44:831–838. doi: 10.1016/0092-8674(86)90005-x. [DOI] [PubMed] [Google Scholar]
- Dupont S, Dennefeld C, Krust A, Chambon P, Mark M. Expression of Sox9 in granulosa cells lacking the estrogen receptors, ERalpha and ERbeta. Dev Dyn. 2003;226:103–106. doi: 10.1002/dvdy.10202. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL. Inherited sex reversal in mice: Identification of a new primary sex-determining gene. J Exp Zool. 1983;228:297–304. doi: 10.1002/jez.1402280213. [DOI] [PubMed] [Google Scholar]
- Elf PK. Yolk steroid hormones and sex determination in reptiles with TSD. Gen Comp Endocrinol. 2003;132:349–355. doi: 10.1016/s0016-6480(03)00098-4. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Magoffin DA, Dyer CA, Hofeditz C. The ovarian androgen producing cells: A review of structure/function relationships. Endocr Rev. 1985;6:371–399. doi: 10.1210/edrv-6-3-371. [DOI] [PubMed] [Google Scholar]
- Evans EP, Ford CE, Lyon MF. Direct evidence of the capacity of the XY germ cell in the mouse to become an oocyte. Nature. 1977;267:430–431. doi: 10.1038/267430a0. [DOI] [PubMed] [Google Scholar]
- Ewen K, Baker M, Wilhelm D, Aitken RJ, Koopman P. Global survey of protein expression during gonadal sex determination in mice. Mol Cell Proteomics. 2009;8:2624–2641. doi: 10.1074/mcp.M900108-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondos B. Surface epithelium of the developing ovary. Possible correlation with ovarian neoplasia. Am J Pathol. 1975;81:303–321. [PMC free article] [PubMed] [Google Scholar]
- Gondos B, Byskov AG, Hansen JL. Regulation of the onset of meiosis in the developing testis. Ann Clin Lab Sci. 1996;26:421–425. [PubMed] [Google Scholar]
- Grinsted J, Byskov AG. Meiosis-inducing and meiosis-preventing substances in human male reproductive organs. Fertil Steril. 1981;35:199–204. [PubMed] [Google Scholar]
- Harvey W. Anatomical Exercitations Concerning the Generation of Living Creatures. London: Printed by J. Young for O. Pulleyn; 1653. [Google Scholar]
- Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- Hernandez RE, Putzke AP, Myers JP, Margaretha L, Moens CB. Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development. 2007;134:177–187. doi: 10.1242/dev.02706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilscher B, Hilscher W, Bulthoff-Ohnolz B, Kramer U, Birke A, Pelzer H, Gauss G. Kinetics of gametogenesis. I Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell Tissue Res. 1974;154:443–470. doi: 10.1007/BF00219667. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Theca cells may be present at the outset of follicular growth. Biol Reprod. 1991;44:1157–1162. doi: 10.1095/biolreprod44.6.1157. [DOI] [PubMed] [Google Scholar]
- Houmard B, Small C, Yang L, Naluai-Cecchini T, Cheng E, Hassold T, Griswold M. Global gene expression in the human fetal testis and ovary. Biol Reprod. 2009;81:438–443. doi: 10.1095/biolreprod.108.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Yao H-C, HHC Diverse functions of the hedgehog signaling in formation and physiology of steroidogenic organs. Mol Reprod Dev. 2010;77:489–496. doi: 10.1002/mrd.21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, Tschudy KS, Tilly JC, Cortes ML, Forkert R, Spitzer T, Iacomini J, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- Jordan BK, Mohammed M, Ching ST, Delot E, Chen XN, Dewing P, Swain A, Rao PN, Elejalde BR, Vilain E. Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet. 2001;68:1102–1109. doi: 10.1086/320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BK, Shen JH, Olaso R, Ingraham HA, Vilain E. Wnt4 overexpression disrupts normal testicular vasculature and inhibits testosterone synthesis by repressing steroidogenic factor 1/beta-catenin synergy. Proc Natl Acad Sci USA. 2003;100:10866–10871. doi: 10.1073/pnas.1834480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JS, Gao L. Irx3 is differentially up-regulated in female gonads during sex determination. Gene Expr Patterns. 2005;5:756–762. doi: 10.1016/j.modgep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Jost A. A new look at the mechanisms controlling sex differentiation in mammals. Johns Hopkins Med J. 1972;130:38–53. [PubMed] [Google Scholar]
- Jost A, Gonse-Danysz P, Jacquot R. Studies on physiology of fetal hypophysis in rabbits and its relation to testicular function. J Physiol (Paris) 1953;45:134–136. [PubMed] [Google Scholar]
- Kim KA, Wagle M, Tran K, Zhan X, Dixon MA, Liu S, Gros D, Korver W, Yonkovich S, Tomasevic N, Binnerts M, Abo A. R-spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell. 2008;19:2588–2596. doi: 10.1091/mbc.E08-02-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, Abo A, Tomizuka K, Funk WD. R-spondin proteins: A novel link to beta-catenin activation. Cell Cycle. 2006;5:23–26. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Nagahama Y. Molecular aspects of gonadal differentiation in a teleost fish, the nile tilapia. Sex Dev. 2009;3:108–117. doi: 10.1159/000223076. [DOI] [PubMed] [Google Scholar]
- Kotsuji F, Kamitani N, Goto K, Tominaga T. Bovine theca and granulosa cell interactions modulate their growth, morphology, and function. Biol Reprod. 1990;43:726–732. doi: 10.1095/biolreprod43.5.726. [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand A. Institutio Philosophiae, Secundum Principia Renati Descartes Londini. 1672. [Google Scholar]
- Lee TL, Li Y, Cheung HH, Claus J, Singh S, Sastry C, Rennert OM, Lau YF, Chan WY. GonadSAGE: A comprehensive SAGE database for transcript discovery on male embryonic gonad development. Bioinformatics. 2009;26(4):585–586. doi: 10.1093/bioinformatics/btp695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W, Rosa B, Jeremy B, Thomas J. Principles of Development. Oxford University Press; USA: 2006. [Google Scholar]
- Lin Y, Gill ME, Koubova J, Page DC. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science (New York) 2008;322:1685–1687. doi: 10.1126/science.1166340. [DOI] [PubMed] [Google Scholar]
- Liu CF, Bingham N, Parker K, Yao HH. Sex-specific roles of beta-catenin in mouse gonadal development. Hum Mol Genet. 2009;18:405–417. doi: 10.1093/hmg/ddn362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Parker K, Yao HHC. Wnt4/beta-catenin pathway maintains female germ cell survival by inhibiting activin beta B in the mouse fetal ovary. PLoS One. 2010;5:e10382. doi: 10.1371/journal.pone.0010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler KA, Zarkower D, Koopman P. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology. 2003;144:3237–3243. doi: 10.1210/en.2002-0095. [DOI] [PubMed] [Google Scholar]
- Lu W, Kim KA, Liu J, Abo A, Feng X, Cao X, Li Y. R-spondin1 synergizes with Wnt3a in inducing osteoblast differentiation and osteoprotegerin expression. FEBS Lett. 2008;582:643–650. doi: 10.1016/j.febslet.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean G, Li H, Metzger D, Chambon P, Petkovich M. Apoptotic extinction of germ cells in testes of cyp26b1 knockout mice. Endocrinology. 2007;148:4560–4567. doi: 10.1210/en.2007-0492. [DOI] [PubMed] [Google Scholar]
- Magoffin DA, Magarelli PC. Preantral follicles stimulate luteinizing hormone independent differentiation of ovarian theca-interstitial cells by an intrafollicular paracrine mechanism. Endocrine. 1995;3:107–112. doi: 10.1007/BF02990061. [DOI] [PubMed] [Google Scholar]
- Mandel H, Shemer R, Borochowitz ZU, Okopnik M, Knopf C, Indelman M, Drugan A, Tiosano D, Gershoni-Baruch R, Choder M, Sprecher E. SERKAL syndrome: An autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am J Hum Genet. 2008;82:39–47. doi: 10.1016/j.ajhg.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuylov NL, Smagulova FO, Leach L, Tevosian SG. Ovarian development in mice requires the GATA4-FOG2 transcription complex. Development. 2008;135:3731–3743. doi: 10.1242/dev.024653. [DOI] [PubMed] [Google Scholar]
- McElreavey K, Vilain E, Abbas N, Herskowitz I, Fellous M. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc Natl Acad Sci USA. 1993;90:3368–3372. doi: 10.1073/pnas.90.8.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A. Studies on mouse germ cells inside and outside the gonad. J Exp Zool. 1983;228:167–171. doi: 10.1002/jez.1402280203. [DOI] [PubMed] [Google Scholar]
- McLaren A. Meiosis and differentiation of mouse germ cells. Symp Soc Exp Biol. 1984;38:7–23. [PubMed] [Google Scholar]
- McLaren A. Development of the mammalian gonad: The fate of the supporting cell lineage. BioEssays. 1991;13:151–156. doi: 10.1002/bies.950130402. [DOI] [PubMed] [Google Scholar]
- McLaren A. Germ cells and germ cell sex. Philos Trans R Soc Lond, Ser B Biol Sci. 1995;350:229–233. doi: 10.1098/rstb.1995.0156. [DOI] [PubMed] [Google Scholar]
- McLaren A. Germ and somatic cell lineages in the developing gonad. Mol Cell Endocrinol. 2000;163:3–9. doi: 10.1016/s0303-7207(99)00234-8. [DOI] [PubMed] [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- McLaren A, Chandley AC, Kofman-Alfaro S. A study of meiotic germ cells in the gonads of foetal mouse chimaeras. J Embryol Exp Morphol. 1972;27:515–524. [PubMed] [Google Scholar]
- McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol. 1997;187:107–113. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- Meeks JJ, Crawford SE, Russell TA, Morohashi K, Weiss J, Jameson JL. Dax1 regulates testis cord organization during gonadal differentiation. Development. 2003a;130:1029–1036. doi: 10.1242/dev.00316. [DOI] [PubMed] [Google Scholar]
- Meeks JJ, Russell TA, Jeffs B, Huhtaniemi I, Weiss J, Jameson JL. Leydig cell-specific expression of DAX1 improves fertility of the dax1-deficient mouse. Biol Reprod. 2003b;69:154–160. doi: 10.1095/biolreprod.102.011429. [DOI] [PubMed] [Google Scholar]
- Meeks JJ, Weiss J, Jameson JL. Dax1 is required for testis determination. Nat Genet. 2003c;34:32–33. doi: 10.1038/ng1141. [DOI] [PubMed] [Google Scholar]
- Menke DB, Page DC. Sexually dimorphic gene expression in the developing mouse gonad. Gene Expr Patterns. 2002;2:359–367. doi: 10.1016/s1567-133x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Merchant H. Rat gonadal and ovarioan organogenesis with and without germ cells. An ultrastructural study. Dev Biol. 1975;44:1–21. doi: 10.1016/0012-1606(75)90372-3. [DOI] [PubMed] [Google Scholar]
- Merchant-Larios H, Chimal-Monroy J. The ontogeny of primordial follicles in the mouse ovary. Prog Clin Biol Res. 1989;296:55–63. [PubMed] [Google Scholar]
- Mittwoch U. Phenotypic manifestations during the development of the dominant and default gonads in mammals and birds. J Exp Zool. 1998;281:466–471. [PubMed] [Google Scholar]
- Molyneaux KA, Stallock J, Schaible K, Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240:488–498. doi: 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
- Morris T. The XO and OY chromosome constitutions in the mouse. Genet Res. 1968;12:125–137. doi: 10.1017/s0016672300011745. [DOI] [PubMed] [Google Scholar]
- Motta PM, Makabe S. Development of the ovarian surface and associated germ cells in the human fetus. A correlated study by scanning and transmission electron microscopy. Cell Tissue Res. 1982;226:493–510. doi: 10.1007/BF00214779. [DOI] [PubMed] [Google Scholar]
- Muttukrishna S, Tannetta D, Groome N, Sargent I. Activin and follistatin in female reproduction. Mol Cell Endocrinol. 2004;225:45–56. doi: 10.1016/j.mce.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Mystkowska ET, Tarkowski AK. Behaviour of germ cells and sexual differentiation in late embryonic and early postnatal mouse chimeras. J Embryol Exp Morphol. 1970;23:395–405. [PubMed] [Google Scholar]
- Naillat F, Prunskaite-Hyyrylainen R, Pietila I, Sormunen R, Jokela T, Shan J, Vainio SJ. Wnt4/5a signalling coordinates cell adhesion and entry into meiosis during presumptive ovarian follicle development. Hum Mol Genet. 2010;19(8):1539–1550. doi: 10.1093/hmg/ddq027. [DOI] [PubMed] [Google Scholar]
- Nakamura M. Sex determination in amphibians. Semin Cell Dev Biol. 2009;20:271–282. doi: 10.1016/j.semcdb.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, Parker KL, Vassalli JD. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Ohno S, Cattanach BM. Cytological study of an X-autosome translocation in mus musculus. Cytogenetics. 1962;1:129–140. [PubMed] [Google Scholar]
- Orisaka M, Tajima K, Mizutani T, Miyamoto K, Tsang BK, Fukuda S, Yoshida Y, Kotsuji F. Granulosa cells promote differentiation of cortical stromal cells into theca cells in the bovine ovary. Biol Reprod. 2006;75:734–740. doi: 10.1095/biolreprod.105.050344. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D. Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet. 2005;14:2053–2062. doi: 10.1093/hmg/ddi210. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, Cao A, Forabosco A, Schlessinger D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet. 2007;16:2795–2804. doi: 10.1093/hmg/ddm235. [DOI] [PubMed] [Google Scholar]
- Pailhoux E, Vigier B, Chaffaux S, Servel N, Taourit S, Furet JP, Fellous M, Grosclaude F, Cribiu EP, Cotinot C, Vaiman D. A 11.7-Kb deletion triggers intersexuality and polledness in goats. Nat Genet. 2001;29:453–458. doi: 10.1038/ng769. [DOI] [PubMed] [Google Scholar]
- Pailhoux E, Vigier B, Vaiman D, Servel N, Chaffaux S, Cribiu EP, Cotinot C. Ontogenesis of female-to-male sex-reversal in XX polled goats. Dev Dyn. 2002;224:39–50. doi: 10.1002/dvdy.10083. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Burgoyne PS. In situ analysis of fetal, prepuberal and adult XX–XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development (Cambridge, England) 1991;112:265–268. doi: 10.1242/dev.112.1.265. [DOI] [PubMed] [Google Scholar]
- Pannetier M, Fabre S, Batista F, Kocer A, Renault L, Jolivet G, Mandon-Pepin B, Cotinot C, Veitia R, Pailhoux E. FOXL2 activates P450 aromatase gene transcription: Towards a better characterization of the early steps of mammalian ovarian development. J Mol Genet Med. 2006;36:399–413. doi: 10.1677/jme.1.01947. [DOI] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- Pask A, Renfree MB. Sex determining genes and sexual differentiation in a marsupial. J Exp Zool. 2001;290:586–596. doi: 10.1002/jez.1109. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- Philibert P, Biason-Lauber A, Rouzier R, Pienkowski C, Paris F, Konrad D, Schoenle E, Sultan C. Identification and functional analysis of a new WNT4 gene mutation among 28 adolescent girls with primary amenorrhea and müllerian duct abnormalities: a French collaborative study. J Clin Endocrinol Metab. 2008;93:895–900. doi: 10.1210/jc.2007-2023. [DOI] [PubMed] [Google Scholar]
- Pinkerton JH, Mc KD, Adams EC, Hertig AT. Development of the human ovary—a study using histochemical technics. Obstet Gynecol. 1961;18:152–181. [PubMed] [Google Scholar]
- Quattropani SL. Morphogenesis of the ovarian interstitial tissue in the neonatal mouse. Anat Rec. 1973;177:569–583. doi: 10.1002/ar.1091770410. [DOI] [PubMed] [Google Scholar]
- Romand R, Dolle P, Hashino E. Retinoid signaling in inner ear development. J Neuroendocrinol. 2006;66:687–704. doi: 10.1002/neu.20244. [DOI] [PubMed] [Google Scholar]
- Ruby JR, Dyer RF, Skalko RG. The occurrence of intercellular bridges during oogenesis in the mouse. J Morphol. 1969;127:307–339. doi: 10.1002/jmor.1051270304. [DOI] [PubMed] [Google Scholar]
- Sanlaville D, Vialard F, Thepot F, Vue-Droy L, Ardalan A, Nizard P, Corre A, Devauchelle B, Martin-Denavit T, Nouchy M, Malan V, Taillemite JL, Portnoi MF. Functional disomy of Xp including duplication of DAX1 gene with sex reversal due to t(X;Y)(p21.2;p11.3) Am J Med Genet A. 2004;128A:325–330. doi: 10.1002/ajmg.a.30115. [DOI] [PubMed] [Google Scholar]
- Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002;66:1134–1150. doi: 10.1095/biolreprod66.4.1134. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- Singh RP, Carr DH. The anatomy and histology of XO human embryos and fetuses. Anat Rec. 1966;155:369–383. doi: 10.1002/ar.1091550309. [DOI] [PubMed] [Google Scholar]
- Singh RP, Carr DH. Anatomic findings in human abortions of known chromosomal constitution. Obstet Gynecol. 1967;29:806–818. [PubMed] [Google Scholar]
- Speed RM. Meiosis in the foetal mouse ovary. I An analysis at the light microscope level using surface-spreading. Chromosoma. 1982;85:427–437. doi: 10.1007/BF00330366. [DOI] [PubMed] [Google Scholar]
- Sultan C, Biason-Lauber A, Philibert P. Mayer-Rokitansky-Kuster-Hauser syndrome: recent clinical and genetic findings. Gynecol Endocrinol. 2009;25:8–11. doi: 10.1080/09513590802288291. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Igarashi K, Aisaki K, Kanno J, Saga Y. NANOS2 interacts with the CCR4-NOT deadenylation complex and leads to suppression of specific RNAs. Proc Natl Acad Sci USA. 2010;107:3594–3599. doi: 10.1073/pnas.0908664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Saga Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 2008;22:430–435. doi: 10.1101/gad.1612708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R. Dax1 antagonizes Sry action in mammalian sex determination. Nature. 1998;391:761–767. doi: 10.1038/35799. [DOI] [PubMed] [Google Scholar]
- Tam PP, Snow MH. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J Embryol Exp Morphol. 1981;64:133–147. [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008;17:1278–1291. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- Trautmann E, Guerquin MJ, Duquenne C, Lahaye JB, Habert R, Livera G. Retinoic acid prevents germ cell mitotic arrest in mouse fetal testes. Cell Cycle (Georgetown Tex) 2008;7:656–664. doi: 10.4161/cc.7.5.5482. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, Saga Y. Conserved role of nanos proteins in germ cell development. Sciences (New York) 2003;301:1239–1241. doi: 10.1126/science.1085222. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schutz G, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Upadhyay S, Zamboni L. Ectopic germ cells: Natural model for the study of germ cell sexual differentiation. Proc Natl Acad Sci USA. 1982;79:6584–6588. doi: 10.1073/pnas.79.21.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- vonBaer . Epistola de Ovo Mammalium et Hominis Genesi (On the Origin of the Mammalian and Human Ovum) 1827. [Google Scholar]
- Wang D, Kobayashi T, Zhou L, Nagahama Y. Molecular cloning and gene expression of Foxl2 in the nile tilapia, oreochromis niloticus. Biochem Biophys Res Commun. 2004;320:83–89. doi: 10.1016/j.bbrc.2004.05.133. [DOI] [PubMed] [Google Scholar]
- Wang DS, Kobayashi T, Zhou LY, Paul-Prasanth B, Ijiri S, Sakai F, Okubo K, Morohashi K, Nagahama Y. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol Endocrinol. 2007;21:712–725. doi: 10.1210/me.2006-0248. [DOI] [PubMed] [Google Scholar]
- Washburn LL, Eicher EM. Sex reversal in XY mice caused by dominant mutation on chromosome 17. Nature. 1983;303:338–340. doi: 10.1038/303338a0. [DOI] [PubMed] [Google Scholar]
- Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J Biol Chem. 2007;282:15903–15911. doi: 10.1074/jbc.M701927200. [DOI] [PubMed] [Google Scholar]
- Welshons WJ, Russell LB. The Y-chromosome as the bearer of male determining factors in the mouse. Proc Natl Acad Sci USA. 1959;45:560–566. doi: 10.1073/pnas.45.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton T. Adenographia: Sive glandularum totius corporis descriptio. Londini: typ. J. G. Impens; 1656. [Google Scholar]
- White JA, Ramshaw H, Taimi M, Stangle W, Zhang A, Everingham S, Creighton S, Tam SP, Jones G, Petkovich M. Identification of the human cytochrome P450, P450RAI-2, which is predominantly expressed in the adult cerebellum and is responsible for all-trans-retinoic acid metabolism. Proc Natl Acad Sci USA. 2000;97:6403–6408. doi: 10.1073/pnas.120161397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH. The pathway to femaleness: Current knowledge on embryonic development of the ovary. Mol Cell Endocrinol. 2005;230:87–93. doi: 10.1016/j.mce.2004.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, Aardema J, Holthusen K. Sexually dimorphic regulation of inhibin beta B in establishing gonadal vasculature in mice. Biol Reprod. 2006;74:978–983. doi: 10.1095/biolreprod.105.050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, Capel B. Temperature, genes, and sex: A comparative view of sex determination in trachemys scripta and mus musculus. J Biochem. 2005;138:5–12. doi: 10.1093/jb/mvi097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, DiNapoli L, Capel B. Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads. Development. 2003;130:5895–5902. doi: 10.1242/dev.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HHC, Whoriskey W, Capel B. Desert hedgehog/patched-1 signaling specifies early Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, Saijoh Y, Sakai Y, Hamada H. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev Cell. 2004;6:411–422. doi: 10.1016/s1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]
- Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL. Role of Ahch in gonadal development and gametogenesis. Nat Genet. 1998;20:353–357. doi: 10.1038/3822. [DOI] [PubMed] [Google Scholar]
- Zamboni L, Bezard J, Mauleon P. The role of the mesonephros in the development of the sheep fetal ovary. Ann Biol Anim Biochem Biophys. 1975;19:1153–1178. [Google Scholar]
- Zamboni L, Upadhyay S. Germ cell differentiation in mouse adrenal glands. J Exp Zool. 1983;228:173–193. doi: 10.1002/jez.1402280204. [DOI] [PubMed] [Google Scholar]
- Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ER, et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372:635–641. doi: 10.1038/372635a0. [DOI] [PubMed] [Google Scholar]
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, Hou R, Wu J. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. Rec Prog Horm Res. 1951;6:63–108. [Google Scholar]