Abstract
The most important service that imaging provides to patients with ischemic stroke is to rapidly identify those patients who are most likely to benefit from immediate treatment. This group includes patients who have severe neurological symptoms due to an occlusion of a major artery, and who are candidates for recanalization using intravenous thrombolysis or intra-arterial intervention to remove the occlusion. Outcomes for these patients are determined by symptom severity, the artery that is occluded, the size of the infarct at the time of presentation, and the effect of treatment. MRI provides key physiological information through MR angiography and diffusion MRI that has been proven to be of high clinical value in identify patients who are in need of immediate treatment. Perfusion MRI provides information about the ischemic penumbra, but its clinical value is unproven. In current clinical practice the time since stroke onset is dominant over physiologic information provided by MRI in treatment decisions. This will change only when clinical trials prove that stroke physiology as revealed by MRI is superior to time from stroke onset in promoting good clinical outcomes.
Keywords: Ischemic stroke, MR angiography, diffusion MRI, perfusion MRI
Introduction: Time and Physiology in Acute Stroke
Ischemic stroke is the most common neurological cause of severe disability and death. MRI reveals stroke biology in real-time, and may benefit the clinical management of patients, especially in the most severe strokes. Yet when the time of stroke onset is known, it is time that is usually decisive in decisions to treat. This is because the clinical trials that led to current stroke treatment used time and noncontrast CT scans for patient inclusion,(1) and will change only after clinical trials demonstrate that MRI is useful to identify patients who will benefit from treatment.
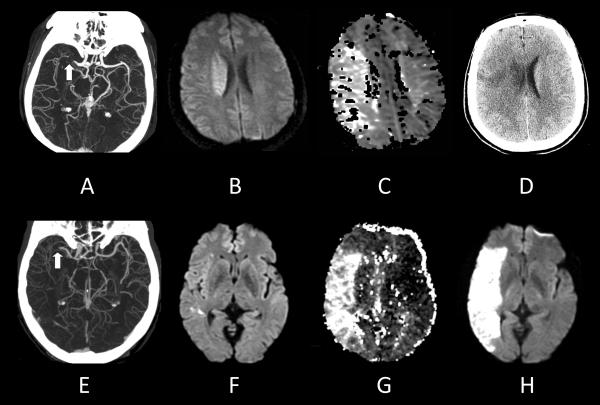
To illustrate the present circumstances in the use of MRI in acute ischemic stroke, consider the imaging data from 2 patients (Figure 1). In the first case, a man presented with severe neurological symptoms but with an unknown time of stroke onset. Imaging revealed an occlusion of a major cerebral artery, but that only a small part of the brain had undergone irreversible damage. Based on the physiology revealed by imaging, intra-arterial removal of the occlusion was executed, and the patient made a full recovery. In the second case, a man presented with moderate symptoms, but unlike the first case, the time of stroke onset was known. Once again, a major artery was occluded, but only a small potion of the territory at risk was irreversibly injured at the time of initial imaging. The decision not to intervene was made because the patient fell outside the established time windows for treatment. The infarction grew accompanied by substantial worsening of symptoms. These cases illustrate the current dominance of time over information provided by imaging in stroke management. This review presents evidence on how MRI-revealed stroke biology may lead to better outcomes in spite of time of stroke onset. But, routine use of MRI to guide treatment requires verification in clinical trials.
Figure 1. Right middle cerebral artery ischemic strokes in 2 patients.
(Top row) 38-year-old man with severe left-sided paralysis of unknown time of onset. A, CT angiography demonstrates occlusion of the right MCA (arrow). B, Diffusion MR imaging shows a small diffusion abnormal in the right hemisphere. C, Perfusion MR imaging displays abnormalities involving the entire right MCA territory. The time-to-minimum-perfusion map at the same level as the DWI image demonstrates a perfusion deficit that is much larger than the DWI abnormality. Because of the large diffusion/perfusion mismatch and the likely poor long-term outcome, the decision was made to proceed to intra-arterial thrombolysis, which was successful. D, A follow-up head CT scan shows infarction in the region of the right corona radiata and a small portion of cerebral cortex. The patient made a complete recovery.
(Bottom row). 61 year old male with mild left sided weakness that began 12.7 hours earlier. E, CTA demonstrates a proximal right MCA occlusion (arrow). F, Initial DWI reveals multiple punctate foci of diffusion abnormality in the right hemisphere. G, Mean transit time (MTT) MR perfusion map demonstrates a large volume of poor perfusion involving the majority of the right MCA territory. The patient did not undergo thrombolytic therapy because of the long time between stroke onset and imaging. H, The patient’s condition worsened to severe paralysis, and follow-up MRI showed a large area of infarction.
The purpose of imaging in acute ischemic stroke
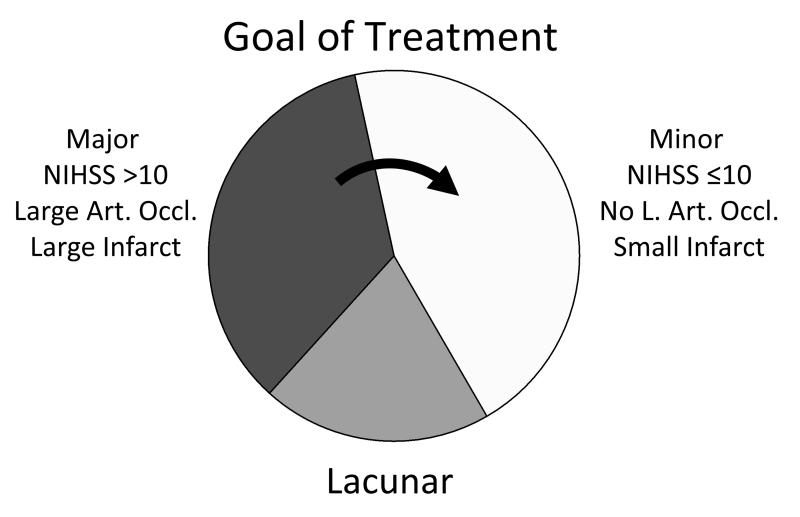
The role of any clinical tool is to improve patient care. To understand how MRI may be most useful in improving patient outcomes in ischemic stroke, it is important to appreciate that this disease is not a single entity, but a collection of maladies with distinct natural histories. It is useful to categorize ischemic stroke patients into the 3 groups shown in Figure 2. The most important group includes patients with severe strokes due to occlusions of large cerebral arteries including the distal internal carotid artery (ICA), proximal middle cerebral artery (MCA) and basilar artery (BA). Such occlusions nearly always produce severe neurological deficits, and poor outcomes are likely if not treated. While this group comprise between 25% and 35% of all stroke patients, they account for the large majority of deaths due to stroke, and the majority of patients with poor outcomes. (2,3) The mortality rates in patients with distal ICA occlusions are reported at 41.7-53% (4-6); in those with proximal MCA occlusion are reported at 27-78% (7,8); and those with basilar occlusions are 92% (9).
Figure 2. Frequency of stroke types and goal of treatment.
Between 25-35% of acute ischemic strokes are major, characterized by NIH stroke scale of 10 or greater and are due to occlusion of a major cerebral artery that produces a large infarction. Outcomes without treatment are usually poor with modified Rankin scale of 3 or greater. Minor strokes have mild symptoms of less than 10 and are is caused by an occlusion of a distal, small branch artery. This typically results in small infarcts and good outcomes (modified Rankin scale of 0 to 2) with or without treatment. Lacunar infarcts have similar outcomes to minor strokes. The major goal of current treatments is to convert major strokes into minor strokes (curved arrow).
Patients who have major artery occlusions may be most successfully treated (10) using catheter-based interventions (Table 1). Because the typical outcome for most of these patients is poor, it makes sense to target these patients: identify them rapidly, and intervene to convert as many as possible from major stroke patients into minor stroke patients. Thus, the single most important role for imaging in acute ischemic stroke is to identify these patients. The next most important role is to definitively determine that ischemia is the source of the neurological symptoms.
Table 1.
Cerebral Artery Recanalization Rates in Acute Ischemic Stroke
| Treatment | Recanalization Rate | Source |
|---|---|---|
| Spontaneous recanalization | 24.1% | 1985-2002 Meta-analysis |
| IV tPA | 46.2% | 1985-2002 Meta-analysis |
| IA Thrombolysis | 63.2% | 1985-2002 Meta-analysis |
| Combined IV/IA Thrombolysis | 67.5% | 1985-2002 Meta-analysis |
| Combined IV/IA Thrombolysis | 56-58% | IMS I & II |
| IA Mechanical | 83.6% | 1985-2002 Meta-analysis |
| IA Mechanical | 81.6% | Penumbra Pivotal |
| Mechanical & Thrombolysis | 60-68% | MERCI & Multi-MERCI |
Recanalization rates were obtained from the following sources: 1985-2002 Meta-analysis – Rha and Saver, “The impact of recanalization on ischemic stroke outcome: a meta-analysis.” (39) IMS I – “Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study.” (57) IMS II – “The Interventional Management of Stroke (IMS) II Study.” (58) Penumbra Pivotal - “The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease.” (59) MERCI – Smith et al.,“Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial.” (60) Multi-MERCI - Smith et al., “Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I.” (61)
In patients who have occlusions of branch arteries, small regions of brain are at risk, and symptoms are typically mild or moderate. They generally do well regardless of treatment. The final group includes patients that have ‘lacunar’ infarcts. These are due to occlusions of small penetrating vessels, usually in the brain stem, deep gray matter, internal capsule or corona radiata. Presenting symptoms are variable, but most will also have good outcomes.
Stroke physiology: Core and Penumbra
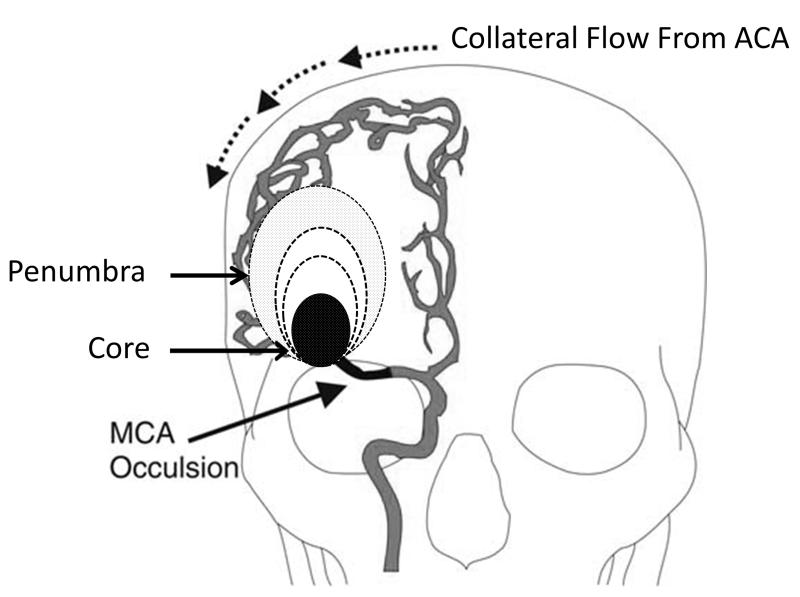
After the occlusion of a major cerebral artery two zones can be demarcated within the brain (Figure 3). Brain tissue immediately beyond the occlusion receives little or no flow and quickly undergoes infarction. This is known as the core of the infarct. Other parts of the brain, while still alive have abnormally low blood flow, and are in danger of proceeding to infarction. This region is known as the penumbra; it maintains viability through blood flow provided by the collateral circulation. (11) In major artery occlusions of the anterior circulation, the core and the penumbra are dependent parameters, and the relative size of each depends on the vigor of the collateral circulation. Arterial occlusion may be detected with MRA. The best current estimate of the infarct core is provided by diffusion MRI. The extent of the penumbra can be estimated using perfusion MRI. Subtle changes in the apparent diffusion coefficient of water may also provide an estimate of the penumbra. When there is an occlusion of a major anterior circulation artery, the core and penumbra are related by the collateral circulation. Therefore, knowledge of one permits a clinically relevant deduction of other. For example in a proximal MCA occlusion, if diffusion MRI reveals a small core, one can confidently deduce that the region of brain with poor blood flow is large, involving the remainder of the MCA territory. The neurological examination is also highly sensitive to abnormal blood flow to brain areas that produce symptoms. (12) However, the neurological exam cannot distinguish between the core and penumbra.
Figure 3. The dynamic ischemic penumbra.
An ischemic penumbra created by occlusion of the proximal right middle cerebral artery changes with time. Shortly after the MCA is occluded, two regions of brain become manifest. The smaller region is designated as the core that represents irreversibly injured. Surrounding this core is a region of reduced perfusion, the penumbra, which may give rise to significant neurological symptoms. The relative sizes of the core and the penumbra are determined by the quality of the collateral flow. With the passage of time there is shrinkage of the ischemic penumbra and a corresponding growth of the core. With good collaterals, the shrinkage of the penumbra and growth of the core is slow, while with poor collaterals very rapid growth of the core and shrinkage of the penumbra is observed.
Stroke physiology: Blood Flow and Infarction
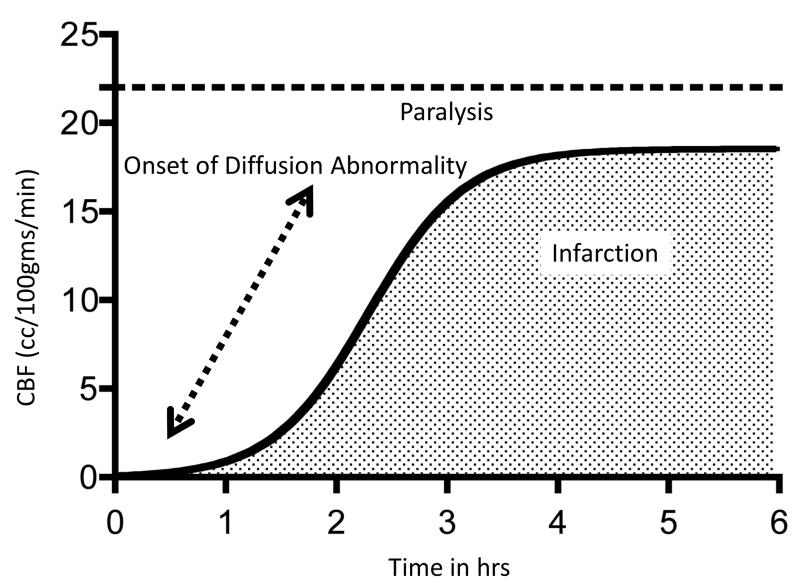
The relationships between cerebral blood flow, clinical symptoms and infarction have been elucidated in animal models and subsequently in human subjects. Figure 4 demonstrates the basic relationship between cerebral blood flow, time and infarction. This figure is derived from a study on awake rhesus macaques that had permanent or temporary MCA occlusions. (13) The major conclusions of this study was subsequently been reproduced in baboons and in humans using positron emission tomography (PET). A review of this PET literature may be found in reference 17 (11) The figure demonstrates that below a threshold level of cerebral blood flow (CBF), the animal develops paralysis. However, the presence of paralysis is not synonymous with infarction: if the CBF remains within the upper abnormal range, the brain remains viable and may, fully recover upon reperfusion. At lower rates of CBF, tissue will undergo infarction but the time required for irreversible injury is dependent on the specific CBF. At very low CBF levels, brain tissue of infarct within several minutes. Higher levels of CBF such as 15 mL/100 g tissue/minute infarction will not occur for two or more hours.
Figure 4. Blood flow and infarction.
Graph derived from experiments on awake monkeys in which an MCA was occluded. (13) Animals become paralyzed when the blood flow declines below a threshold level ( 20 –25 mL/100 g per minute). The time required for the tissue to become irreversibly injured (infarction) depends on the rate of blood flow and the time of reduced flow. The apparent diffusion coefficient of water becomes reduced and detectable by MRI before infarction ensues, but little time is available for tissue salvage.
Diffusion MRI heralds the imminence of brain infarction due to ischemia which was first demonstrated in experimental animal models. (14-16) With severe reduction of CBF, a reduction in tissue water diffusion may be detected with MRI within 30 minutes. The sensitivity of diffusion MRI at the early stages of severe ischemia is illustrated in Figure 5, which shows images of a patient who developed severe left sided paralysis in the presence of an emergency department physician. If there is reperfusion shortly after the diffusion abnormality appears, it may reverse, and infarction will not occur. Clinically, spontaneous reversal is extremely rare. (17) It is also unusual with treatment. (18) In most cases, the reduction in CBF persists and infarction ensues. Later reperfusion or normalization of CBF may result in temporary normalization of the diffusion abnormality; however neuronal injury will still occur. (19) Reduced diffusivity of brain tissue detected by MRI is the most reliable early indicator of brain infarction that is widely available. (20,21)
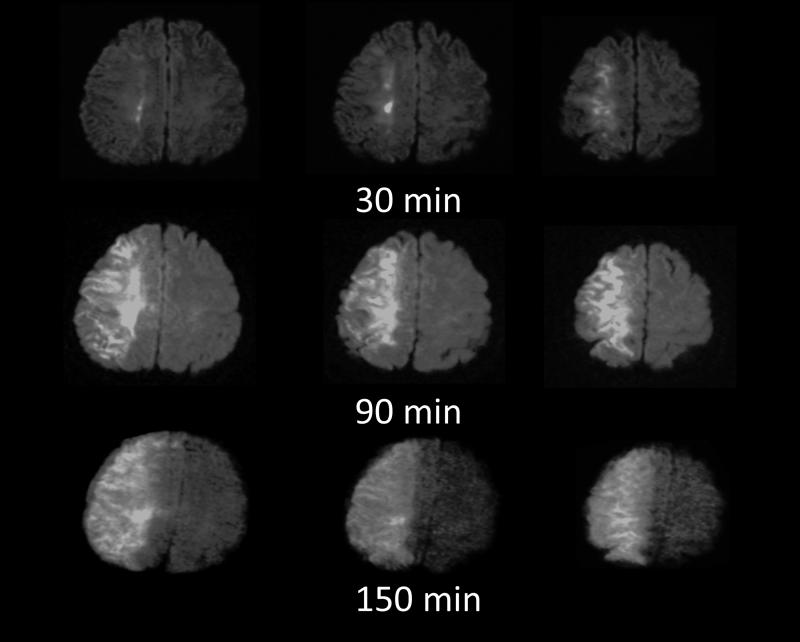
Figure 5. High rate of neuronal loss.
Diffusion MRI performed on a 22 year old woman who had a right internal carotid artery occlusion caused by traumatic dissection of that artery. The patient had inadequate collateral circulation and a hemodynamically isolated right hemisphere. DWI performed at 30 minutes after witnessed onset of paralysis demonstrated lesions that may have been reversible. Attempts at treatment failed. Repeat DWI at 90 and 150 minutes demonstrated expansion and severe reduction in the diffusion coefficient of water throughout the right cerebral hemisphere. The rate of neuronal loss in this individual is nearly 50 times higher than the Saver estimate.
Stroke physiology: Time is brain but every patient has their own time
The management of the acute ischemic stroke patient is driven by the concept of “time is brain”. In a celebrated paper (22) Saver quantified the rate of neuronal loss in stroke. By his calculations, an average of 1.8 million neurons is lost every minute that appropriate treatment is not given. However, the important issue of variability in the rate of neuronal loss amongst patients was not addressed. The rate of infarct growth may be very low as is illustrated in Figure 6. (23) In this individual, no significant expansion of the DWI lesion was observed over a four hour period.
Figure 6. Stability of large diffusion/perfusion mismatch.
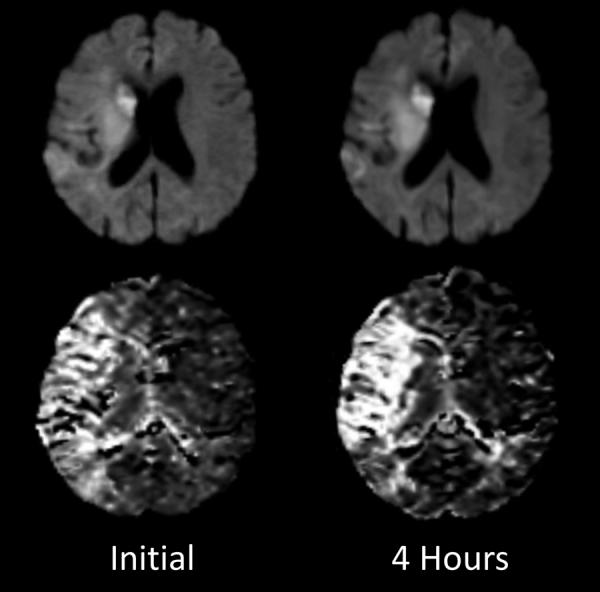
Imaging data are from a patient with a right MCA occlusion and paralysis on the left side. DWI (top row) and mean transit time (MTT, bottom row) maps obtained 4.2 hours after symptom onset. The sizes of the diffusion and MTT abnormalities are very similar 4 hours after the initial imaging study. Adapted from Gonzalez et al. (23)
Following the assumptions of Saver, an empirical assessment on the variability of neuronal loss amongst patients with occlusions of major arteries of the anterior circulation was performed by Hakimelahi et al. (11) The results derived from 68 consecutive patients are shown in Figure 7. The variability in the rate of neuronal loss was found to be very high, although interestingly, the average rate of neuronal loss derived empirically was similar to the theoretical average calculated by Saver. Since the occlusions in these patients involved similar, large territories at risk, the variability observed is best explained by differences in the adequacy of the collateral circulation. (24)
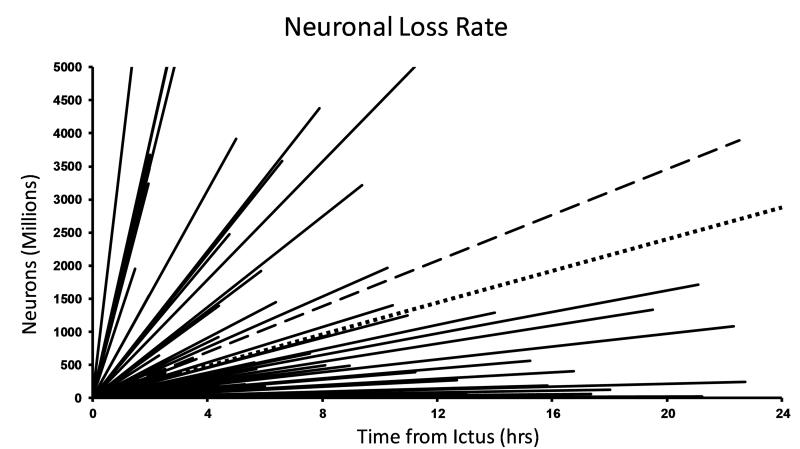
Figure 7. Time is brain, but each patient has his own time.
Neuronal loss estimates from 68 consecutive patients who presented to the emergency department within 24 hours of stroke onset. Each patient had a DWI abnormality volume that was quantified. Using the assumptions of Saver, the time course of neuronal loss for each patient was estimated assuming a linear increase from the time of stroke onset to the time of imaging. The wide variability between patients is evident. The Saver estimate is shown by the dotted line. The mean for all 68 patients is the dashed line, which is similar to the Saver estimate. (11,50)
Stroke physiology: Prevalence and stability of a significant clinical ischemic penumbra
If there is a large variability in the vigor of the collateral circulations in patients in patients with major anterior circulation occlusions, then significant penumbras should be observed in patients even many hours after the onset of occlusion. Such observations have been reported by several investigators (25-27) including a study by Ribo et al (28), who reported that 43 of 56 stroke patients who presented 3 to 6 hours after stroke onset had a DWI/PWI mismatch (a biomarker of infarct core and the clinical penumbra) of 50% or greater. The largest study to date involved 109 patients with anterior circulation (29). All had undergone diffusion/perfusion MRI within 24 hours of stroke onset, and more than 50 percent had DWI/MTT mismatch volumes of 160% or greater (Figure 8). This was most common among patients with occlusions involving the distal internal carotid artery and/or the proximal middle cerebral artery. There was no difference between patients who were scanned at different times after ictus. Persistence of mismatch after 9 hours was common in patients with proximal occlusions of the anterior circulation.
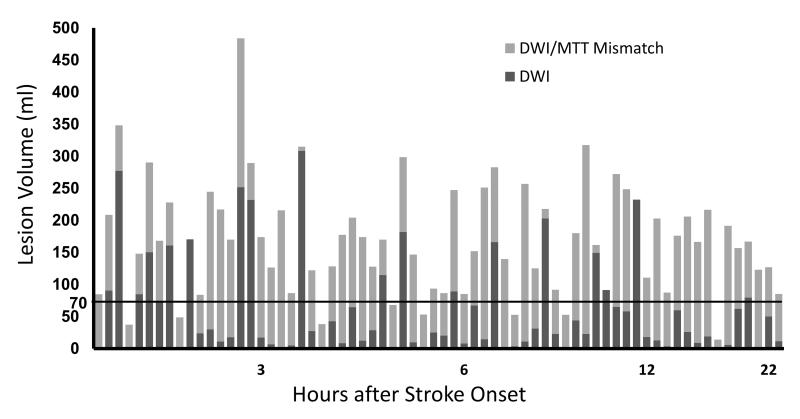
Figure 8. DWI and mismatch volumes in patients imaged within 24 hours after stroke onset.
The data is from the 68 consecutive patients shown in figure 7. The abnormal DWI volume for each patient is depicted in dark gray, and light gray bars represent DWI/MTT mismatch. The horizontal line delineates the DWI threshold of 70 ml. All patients with DWI lesion volume ≤70 ml had at least a 100% mismatch. There was no correlation between time from stroke onset and DWI lesion volume or between time and mismatch volume was found. Adapted from Copen et al. (29)
The observation of a large penumbra in patients scanned many hours after stroke onset suggests that for a significant number of patients the ischemic penumbra, while dynamic, changes at a very slow rate and may be stable for a long period of time. For practical reasons, few longitudinal studies have been performed to evaluate the rate of change of the penumbra over first few hours and days. But as demonstrated in a study (30) in which patients underwent a diffusion-perfusion imaging at study entry, at 4 hours and at 24 hours after the initial study, as well as a follow one week later suggests that the clinical ischemic penumbra may be stable for a subset of patients. Fourteen patients with ICA and/or proximal MCA occlusion and a diffusion/perfusion mismatch at presentation were evaluated. When baseline and 4 hour scans were compared, there was no significant interval change in the mean abnormal DWI volume. Figure 9 is a graph of the mean abnormal DWI volumes for the 3 time points and mean final infarct volumes.
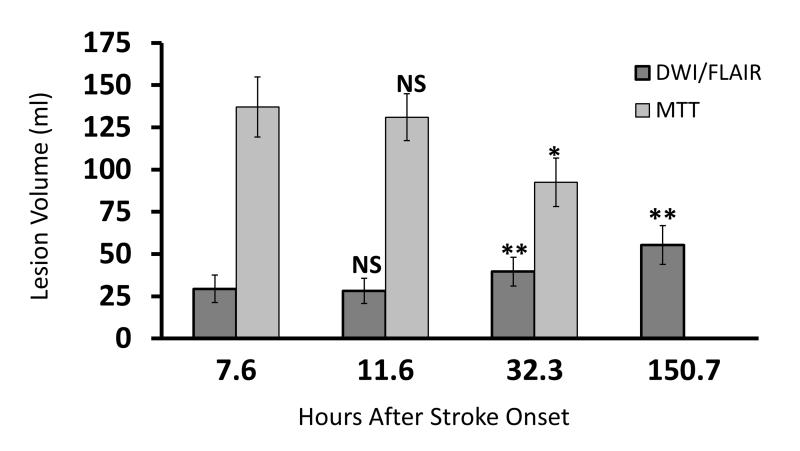
Figure 9. Stability of large diffusion/perfusion mismatch.
The mean abnormal DWI and MTT volumes for 14 patients with MCA occlusions who presented too late for thrombolytic therapy, and who were found to have a large diffusion/perfusion mismatch. The mean abnormal DWI/FLAIR volumes at baseline, 4 hour and 24 hour time points are shown in dark gray. The light gray bars represent the mean abnormal MTT volume. The mean time in hours that the images were acquired after stroke onset is shown on the horizontal axis. No differences in these measures were found between the baseline and 4 hour scans. There were significant differences between the baseline and 24 hour DWI volumes (**, P < 0.001), between the baseline abnormal DWI and 1 week abnormal FLAIR volumes (***, P < .0001), and the baseline and 24 hour MTT volumes (*, P = 0.015). The mean MTT abnormality volumes remained substantially larger than the mean DWI abnormal volumes at the baseline, 4 and 24 hour time point. (23)
Taken together, the observation of a large proportion of patients with a significant clinical ischemic penumbra many hours post stroke onset, the stability of infarcts in patients with excellent collaterals, and the large variability in neuronal loss reinforces the arguments put forth by Baron and others (31) that strict reliance on time alone in the management of patients with ischemic stroke is ill considered. The evidence also suggests that attention to physiology may allow a significant number of patients to undergo treatment of a major artery occlusion even many hours after the onset of symptoms.
Determinants of clinical outcomes: neurological symptom severity
The most important factors that determine the outcomes of patients with acute ischemic stroke include the severity of neurological symptoms, the artery that is occluded, the size of the infarct, and whether treatment successfully keeps the infarct small. Reliable and validated instruments to assess the severity of neurological symptoms have been developed. The most widely employed and validated is the NIH stroke scale (NIHSS). (12) Using this instrument, the trained examiner rapidly evaluates the neurological status of 11 areas that involve motor function, sensory deficits and cognition. (32)The more severe the symptoms, the higher NIHSS score. Patients with an NIHSS of between 0 and 5 have mild symptoms. These patients typically recover fully, and in most centers they are not considered thrombolytic therapy such as tPA (tissue plasminogen activator). Patients with an NIHSS of 10 or greater have severe neurological deficits. These patients have a high likelihood of poor outcomes such as death or dependency if they are not successfully treated.
Determinants of clinical outcomes: site of arterial occlusion
The specific artery that is occluded is the cause of symptom severity and is a major determinant outcome in stroke. In the absence of treatment, the worst outcomes occur in patients who have occlusions of the distal internal carotid artery, proximal middle cerebral artery and the basilar artery. The Boston Acute Stroke Imaging Scale (BASIS) is a simple dichotomized classification instrument that is based on whether or not one of these vessels are occluded or there is infarction in the vascular distribution of these vessels at the time of presentation. (2,3) Studies using this classification instrument in which MRI and/or CT were employed for classification purposes have demonstrated that between 25-35% of patients have occlusions or evidence of occlusions involving one of these major arteries. Such patients, classified as BASIS+, account for nearly all of the in-hospital deaths. In addition over 70% of these patients are discharged to rehabilitation centers. On the other hand, over 70% of BASIS− patients are discharged directly to home. In a multicenter, prospective study in which CTA was employed as part of the routine imaging evaluation of acute stroke patients, basilar and internal carotid terminus occlusions, in addition to National Institutes of Health Stroke Scale and age, independently predicted outcome. (33)The finding that major artery occlusion is independent of the NIH stroke scale in predicting outcomes indicates that the clinical examination and arterial occlusion provide complementary information, and that both are useful in predicting the clinical course of the patient.
Determinants of clinical outcomes: the infarct core volume
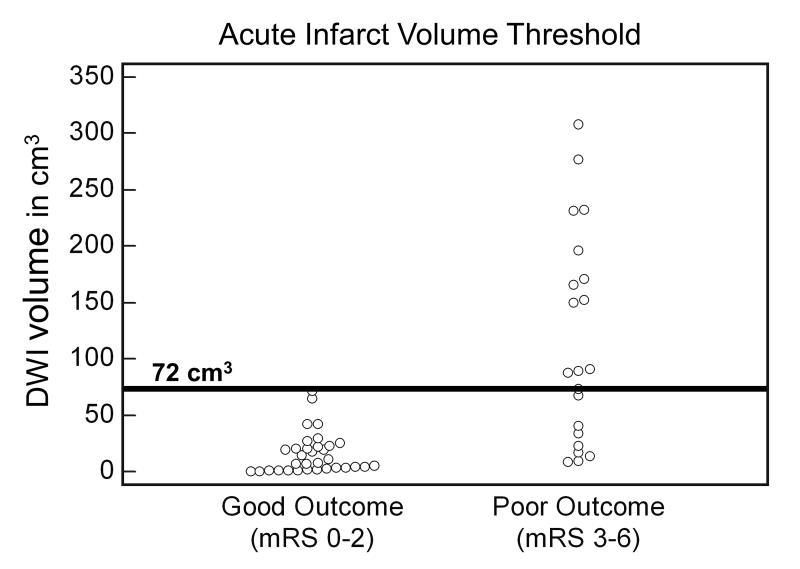
The amount of brain tissue that has undergone a reversible injury at the time of presentation is a major determinant of clinical outcomes in acute stroke. It is now well-established that diffusion MRI the best method for identifying the core of the infarct at the early stages of ischemic stroke. (21,34-36) There has been research in identifying the volume of infarct produced by an anterior circulation stroke that predicts a poor outcome regardless of intervention. Clinical tradition holds that infarcts involving approximately 1/3 of the MCA territory (~100 mL) or greater have high likelihood of poor outcomes. More recently, data is accumulating that the volume that is specific for a poor outcome is likely lower than this. Figure 10 was derived from a study of a series of patients who underwent diffusion MRI the time of admission. (37) Many of the patients received therapy including intravenous tPA or/and catheter-based intervention to open the occluded artery. It was found that all patients who had diffusion abnormality of greater than 72 mL had a poor outcome regardless of treatment. A separate study by a different group found a similar volume threshold. (38) Of course, more research is needed because, for example, it is likely that there will be different thresholds for the right and left hemispheres.
Figure 10. Acute Infarct Volume Threshold.
54 acute stroke patients imaged within 9 hours of stroke onset. Scatterplot of DWI volume versus good (mRS 0 to 2) and poor (mRS 3 to 6) outcomes. The minimum DWI volume above which no patient had a good outcome was 72 mL (100% specific [95% CI: 89.4% to 100%], 61.9% sensitive [95% CI: 38.4% to 81.9%] for poor outcome). All 13 patients (5 received IV tPA) with DWI volume >72 mL had poor outcomes. (37)
Determinants of clinical outcomes: treatment
The final major factor that determines the outcomes in patients with ischemic stroke is whether or not a patient is successfully treated. The only proven treatments in ischemic stroke are agents that reopen the occluded artery (Table 1). There is a strong correlation between recanalization and outcome, and recanalization is strongly associated with improved functional outcomes and reduced mortality. (39) The spontaneous recanalization rate is ~20%, and some individuals are fortunate if the collateral circulation maintains tissue viability until the artery reopens spontaneously. Treatments for the removal of the occlusion include intravenous and intra-arterial administration of thrombolytic agents, and mechanical recanalization.
It is logical to assume that a higher proportion of good outcomes should accompany a higher rate of recanalization. This is not borne out in published clinical trials because of an important additional factor: the amount of brain already irreversibly injured at the time of recanalization. This was demonstrated by Yoo et al. (40)who showed that patients undergoing intra-arterial therapy had poor outcomes even with successful treatment if the volume of abnormal brain on DWI exceeded 70 ml. The variable dynamics of infarct growth due to differences in collateral circulation renders time from stroke onset an unreliable guide for stroke treatment aimed at alleviation of arterial occlusion. Using DWI to identify the volume of irreversibly injured brain to guide treatment is a viable alternative, but clinical trials are necessary.
Role of perfusion MRI in the clinical management of patients
The importance of identifying a major artery occlusion, which may be done with MRA,(41) and an estimate of the infarct core with DWI in acute ischemic stroke are strongly supported by the available evidence. There is much weaker evidence to support the value of perfusion imaging with MRI (or with CT) in the management of acute stroke patients. (21) The most widely employed MRI-based measure of potential for infarct growth is the diffusion/perfusion mismatch. Perfusion measurements by MRI (also CT) have many problems, most notably the lack of standards for collecting and analyzing the perfusion data. There is also measurement error that may be very large. (42) The reasons for the inadequacy of diffusion/perfusion mismatch for patient selection in ischemic stroke treatment trials were reviewed by Schaebitz.(43) The potential benefits and limitations of perfusion MRI in specific clinical circumstances are considered next.
Perfusion MRI: Extending the time window
The diffusion/perfusion mismatch has been used to identify patients that are likely to benefit from thrombolytic therapy, but are outside the traditional time window for such treatment. (44-48) Based on positive outcomes in preliminary studies, a series of clinical trials were undertaken to use this method to select patients for treatments using intravenously administered tPA or desmoteplase, a new thrombolytic agent. A meta-analysis of the DEDAS, DIAS, DIAS II, DEFUSE and EPITHET trials included 502 patients who were selected based on a diffusion/perfusion and treated with intravenous thrombolysis or placebo beyond 3 hours. (49). The meta-analysis found no significant improvement in favorable clinical outcome by thrombolytic therapy in these trials.
Important factors that were not considered for inclusion/exclusion criteria in these clinical trials were the presence of a therapeutic target (a vascular occlusion documented by MRA or CTA), and the volumes of the diffusion and perfusion abnormalities. Not screening out patients with DWI volumes of greater than 70ml would have included patients that even if treated successfully would not have a good outcome. (40) Not screening out patients with small abnormal perfusion volumes would have included patients that would have a good outcome regardless of treatment. (37) The value of identifying a target occlusion has been recognized by investigators pursuing clinical trials with desmoteplase, and is implemented in an ongoing phase III clinical trial of this thrombolytic agent (Efficacy and Safety Study of Desmoteplase to Treat Acute Ischemic Stroke (DIAS-4); ClinicalTrials.gov identifier NCT00856661). In this randomized, double-blind, parallel-group placebo-controlled study, treatment can be initiated within 3 to 9 hours after the onset of stroke symptoms in patients with NIHSS Score of 4 to 24 and vessel occlusion or high-grade stenosis on MRI or CTA in proximal cerebral arteries.
Perfusion MRI: major strokes of the anterior circulation
As emphasized previously (Figure 2) the most important role for imaging in acute ischemic stroke is to rapidly identify patients with severe stroke syndromes that are most likely to benefit from intervention (Table 1). However, these procedures require significant resources in people and equipment, and should be limited to those who are most likely to benefit. The evidence supports proceeding to endovascular intervention when there is a significant neurological deficit, the presence of an occlusion of the major cerebral artery and a small core infarct (less than approximately 70 to 100 ml).
There is little evidence to indicate that perfusion MRI provides significant additional information in this clinical scenario. The most important reason is that the core and remaining tissue that has low blood flow are not independent parameters. The relative sizes of the core and region of abnormal perfusion are determined by the adequacy of the collateral circulation. In the presence of robust collateral circulation, the diffusion abnormality will be small and the volume of poor perfusion will be large. Conversely, if the diffusion abnormality is large, the perfusion abnormality will be small, and it is unlikely that such patients will benefit from recanalization. (24)
The linkage between the diffusion and the perfusion abnormalities by the collateral circulation suggests a simple way to identify patients who have a large mismatch without performing a perfusion MRI study. This is demonstrated in Figure 7. All patients with a diffusion abnormality volume of less than 70 ml had perfusion deficit of at least 100% greater than the diffusion lesion volume. (11,50)Physiological understanding of cerebral blood flow suggests, and the evidence supports the principle that in occlusions of major anterior circulation arteries knowledge of the diffusion abnormality volume is sufficient to deduce the approximate size of perfusion deficit. Another approach to estimate the penumbra is to further analyze the diffusion data. Reduction of blood flow will subtly decrease the apparent diffusion coefficient of water in the region surrounding the core infarct. This may be used to estimate the penumbra. (Figure 11) (51)
Figure 11. Using Diffusion MRI to estimate clinical ischemic penumbra.
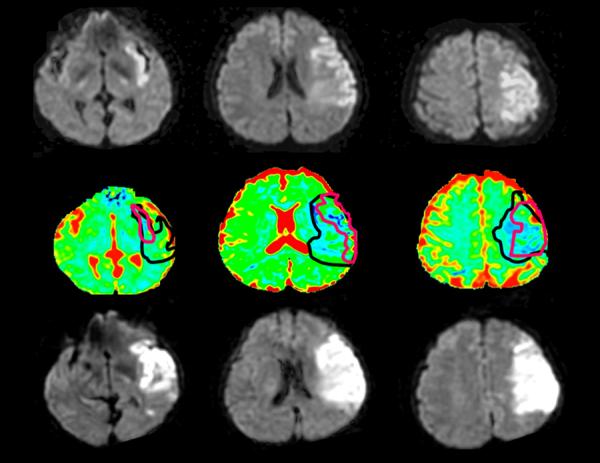
Top row, DWI obtained ~3 hours after stroke onset. Middle row, pseudocolor images with outlines of initial DWI lesion (red line) and prediction of final infarct (black line). (51) Bottom row, follow-up DWI obtained ~24 hours after stroke in this patient who was not recanalized. (Images provided by Y. Samson).
Perfusion MRI: Minor Strokes
There is one scenario in which perfusion MRI may be of clinical value: patients that have minor neurological deficits despite a major artery occlusion. These patients will have small DWI abnormalities nearly without exception. The mild symptoms indicate that there is excellent blood flow to areas of brain beyond the occlusion because of a robust collateral circulation. Perfusion MRI demonstrating a small volume of brain with low perfusion would confirm the presence of a vigorous collateral circulation. This concept is supported by a study in which all patients with an MTT volume of <47 mL (16 of 54) and an NIHSS score <8 (17 of 54) had good outcomes. (37)
Perfusion MRI may be also be of value in patients with anterior circulation ischemic strokes due to occlusions of minor branch arteries. While this specific question has not been directly tested directly, the logic is compelling. These patients commonly present with minor neurological symptoms with NIH stroke scale values of less than 10. The major cerebral arteries are patent, and a small branch occlusion may be not be easily identified by MR or CT angiography. The value of perfusion MRI would be to confirm ischemia as the cause of the symptoms, rather than a stroke mimic such as seizure. (17,52,53) The certain identification of ischemia as the cause of a neurological abnormality has major implications for the management of such a patient, most importantly in the institution of treatment to prevent another, possibly major stroke. Additionally, if the perfusion abnormality is significantly larger than any diffusion abnormality that is identified, IV thrombolytic therapy is potentially useful, but this will require further research.
Perfusion MRI: Strokes of the posterior circulation
There is little evidence that perfusion MRI is of value in assessing patients with posterior circulation strokes. The most devastating of these involve occlusion of the basilar artery, which may produce infarctions of the brainstem, cerebellum and thalamus. Even if they are small in size, symptoms may be major with a high likelihood of poor outcomes. (9) Occlusion of the basilar artery is such a devastating process that an attempt at opening this artery is often made regardless of the time stroke onset. Such procedures can be successful because the collateral circulation may be particularly permissive, and may maintain tissue viability for long periods despite severe symptoms. Data on the value of perfusion MRI in basilar artery occlusions are rare. One likely reason is that it is difficult to acquire good quality images of the posterior fossa due to magnetic susceptibility artifacts.
The role of CT in acute ischemic stroke
MRI provides physiologic information in acute ischemic stroke that is clearly superior to computed tomography (CT). Nonetheless, CT remains the most commonly employed neuroimaging method in the rapid evaluation of the ischemic stroke patient. One reason is the convenient availability of CT scanners for patients being evaluated for a sudden neurological problem. But the most important reason is that CT is recommended for patients being considered for treatment with IV tPA, (54) a recommendation based on clinical trials including the pivotal NINDS IV tPA clinical trial. (1) The use of MRI in place of CT for decisions regarding the use of IV thrombolytics requires validation in clinical trials. Moreover, CT also has proven clinical efficacy in identifying hemorrhage, which is an important possible cause of the presenting symptoms. While MRI has been shown to be more sensitive than CT in the detection of small hemorrhages, (55) this has not been shown to be of clinical relevance in acute stroke. Additionally, MRI may not detect early subarachnoid hemorrhage because of the high oxygen tension of the CSF. (55)
There have been significant efforts in developing CT perfusion methods to substitute for DWI in the detection of early infarct core. (56) While high correlations between lesions observed on diffusion MRI and perfusion CT have been reported, there are important theoretical and methodological limitations that make perfusion CT methods unreliable for determination of the core. A detailed review of this issue is beyond the scope of the present review, but it is considered in some detail in reference 11. (11) With respect to the ischemic penumbra, CT perfusion methods are similar to MR perfusion, with the exception that coverage of the head is usually more limited. However, as discussed previously, the role of perfusion MRI in the assessment of patients with major anterior circulation stroke is limited. The same limitations also hold for CT perfusion.
Recommended Acute Stroke Imaging Algorithm
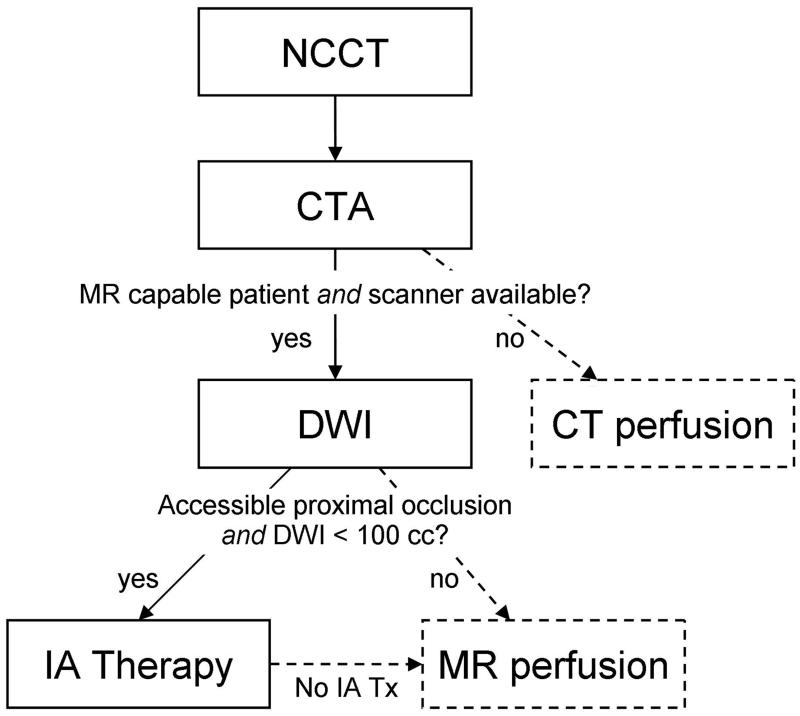
The Neuroradiology Division at our hospital has adopted the recommended acute stroke imaging algorithm shown in Figure 12. This algorithm was adopted after a formal review of the data collected from our acute stroke patients in the past 15 years as well as a critical review of the literature. Much of the reasoning that led to this algorithm has been presented here. The emergency department at our hospital is fortunate to have available CT and MRI at all times, and we employ both in acute stroke patients. The algorithm is explained in the figure legend, and reflects the ready availability of both technologies. It is practical and optimized for the rapid assessment of the large number of patients with neurological symptoms due to a variety of causes. How this algorithm might be applied to other stroke care programs will depend on local conditions.
Figure 12. Recommendeed acute ischemic stroke recommended imaging algorithm.
All patients who present with a new onset of a significant neurological deficit receive a non contrast CT (NCCT) scan that is followed immediately by CT angiography (CTA). If no hemorrhage is identified, head and neck CTA is performed. While the patient is undergoing CT examination, it is determined whether the patient is able to undergo an MRI including that there are no contraindications. If the patient is able and the scanner is available, a diffusion MRI scan (DWI) is acquired. The next step is dependent on the findings on vessel imaging and DWI. If there is an occlusion of a major artery (ICA, proximal MCA or basilar artery), and there is a small diffusion abnormality (defined as less than 70ml in an anterior circulation stroke), then the patient proceeds to endovascular therapy if the patient meets all other criteria for such treatment. If endovascular therapy is not indicated, if the DWI abnormality is large or there is no large artery occlusion, then the patient will proceed to MRI perfusion. CT perfusion (CTP) is provided to patients who are not able to undergo MRI. Perfusion imaging information obtained by CT or MRI may help to more fully delineate the patient’s physiology that may be useful in the consideration of other therapies. Patients that are eligible and are within the 3 or 4.5 hour time limit for IV fPA will receive the treatment in the CT scanner suite before proceeding to MRI, if that is the next step.
Summary
Acute ischemic stroke is a heterogeneous disease, with major stroke caused by proximal artery occlusions representing the stroke subtype with the most devastating outcomes. Because there are effective treatments for major strokes, the primary role of imaging is to identify these patients rapidly and accurately. MRI is particularly powerful in depicting the most important relevant physiology in acute ischemic stroke: the occlusion site and the size of the infarct core. Patient outcomes are related to the severity of the symptoms, as measured by the NIH stroke scale, the site of occlusion, the size of the core of the infarct at the time of presentation, and the success of the treatment. In present clinical practice, time is dominant over physiology for decisions related to implementing therapy. There is evidence that suggests that the physiology revealed by MRI may be an improvement over time in clinical decisions for treatment of patients with major strokes. However, this will require clinical trials to demonstrate the value of using MRI in improving outcomes when it is used to select patients for treatment.
Acknowledgments
Supported in part by: NIH grants NS050041, NS051343
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Cipriano LE, Steinberg ML, Gazelle GS, Gonzalez RG. Comparing and predicting the costs and outcomes of patients with major and minor stroke using the Boston Acute Stroke Imaging Scale neuroimaging classification system. AJNR American journal of neuroradiology. 2009;30(4):703–709. doi: 10.3174/ajnr.A1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres-Mozqueda F, He J, Yeh IB, et al. An acute ischemic stroke classification instrument that includes CT or MR angiography: the Boston Acute Stroke Imaging Scale. AJNR Am J Neuroradiol. 2008;29(6):1111–1117. doi: 10.3174/ajnr.A1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold M, Nedeltchev K, Mattle HP, et al. Intra-arterial thrombolysis in 24 consecutive patients with internal carotid artery T occlusions. J Neurol Neurosurg Psychiatry. 2003;74(6):739–742. doi: 10.1136/jnnp.74.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen O, von Kummer R, Forsting M, Hacke W, Sartor K. Thrombolytic therapy in acute occlusion of the intracranial internal carotid artery bifurcation. AJNR American journal of neuroradiology. 1995;16(10):1977–1986. [PMC free article] [PubMed] [Google Scholar]
- 6.Zaidat OO, Suarez JI, Santillan C, et al. Response to intra-arterial and combined intravenous and intra-arterial thrombolytic therapy in patients with distal internal carotid artery occlusion. Stroke. 2002;33(7):1821–1826. doi: 10.1161/01.str.0000020363.23725.67. [DOI] [PubMed] [Google Scholar]
- 7.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53(4):309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 8.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. Jama. 1999;282(21):2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 9.Brandt T, von Kummer R, Muller-Kuppers M, Hacke W. Thrombolytic therapy of acute basilar artery occlusion. Variables affecting recanalization and outcome. Stroke; a journal of cerebral circulation. 1996;27(5):875–881. doi: 10.1161/01.str.27.5.875. [DOI] [PubMed] [Google Scholar]
- 10.Yoo A, Nogueira R, Hakimelahi R, Gonzalez RG, Hirsch JA. Imaging for Endovascular Stroke Therapy. In: Gonzalez R, Hirsch J, Lev M, Schaefer P, Schwamm L, editors. Acute Ischemic Stroke: Imaging and Intervention Second Edition. Springer-Verlag; Berlin: 2010. pp. 245–265. [Google Scholar]
- 11.Hakimelahi R, Gonzalez R. The Clinical Ischemic Penumbra. In: Gonzalez R, Hirsch JA, Lev MH, Schaefer PW, Schwamm LH, editors. Acute Ischemic Stroke: Imaging and Intervention Second Edition. Springer-Verlag; Berlin: 2010. pp. 197–209. [Google Scholar]
- 12.Adams HP, Jr., Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Neurology. 1999;53(1):126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 13.Jones TH, Morawetz RB, Crowell RM, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981;54(6):773–782. doi: 10.3171/jns.1981.54.6.0773. [DOI] [PubMed] [Google Scholar]
- 14.Kucharczyk J, Mintorovitch J, Asgari HS, Moseley M. Diffusion/perfusion MR imaging of acute cerebral ischemia. Magn Reson Med. 1991;19(2):311–315. doi: 10.1002/mrm.1910190220. [DOI] [PubMed] [Google Scholar]
- 15.Moonen CT, Pekar J, de Vleeschouwer MH, van Gelderen P, van Zijl PC, DesPres D. Restricted and anisotropic displacement of water in healthy cat brain and in stroke studied by NMR diffusion imaging. Magn Reson Med. 1991;19(2):327–332. doi: 10.1002/mrm.1910190223. [DOI] [PubMed] [Google Scholar]
- 16.Minematsu K, Li L, Fisher M, Sotak CH, Davis MA, Fiandaca MS. Diffusion-weighted magnetic resonance imaging: rapid and quantitative detection of focal brain ischemia. Neurology. 1992;42(1):235–240. doi: 10.1212/wnl.42.1.235. [DOI] [PubMed] [Google Scholar]
- 17.Grant PE, He J, Halpern EF, et al. Frequency and clinical context of decreased apparent diffusion coefficient reversal in the human brain. Radiology. 2001;221(1):43–50. doi: 10.1148/radiol.2211001523. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer PW, Hassankhani A, Putman C, et al. Characterization and evolution of diffusion MR imaging abnormalities in stroke patients undergoing intra-arterial thrombolysis. AJNR Am J Neuroradiol. 2004;25(6):951–957. [PMC free article] [PubMed] [Google Scholar]
- 19.Li F, Liu KF, Silva MD, et al. Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats : correlation with histopathology. Stroke; a journal of cerebral circulation. 2000;31(4):946–954. doi: 10.1161/01.str.31.4.946. [DOI] [PubMed] [Google Scholar]
- 20.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000;217(2):331–345. doi: 10.1148/radiology.217.2.r00nv24331. [DOI] [PubMed] [Google Scholar]
- 21.Schellinger PD, Bryan RN, Caplan LR, et al. Evidence-based guideline: The role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75(2):177–185. doi: 10.1212/WNL.0b013e3181e7c9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saver JL. Time is brain--quantified. Stroke. 2006;37(1):263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez RG, Hakimelahi R, Schaefer PW, Roccatagliata L, Sorensen AG, Singhal AB. Stability of large diffusion/perfusion mismatch in anterior circulation strokes for 4 or more hours. BMC neurology. 2010;10:13. doi: 10.1186/1471-2377-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bang OY, Saver JL, Buck BH, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. Journal of neurology, neurosurgery, and psychiatry. 2008;79(6):625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorensen AG, Copen WA, Ostergaard L, et al. Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology. 1999;210(2):519–527. doi: 10.1148/radiology.210.2.r99fe06519. [DOI] [PubMed] [Google Scholar]
- 26.Neumann-Haefelin T, Wittsack HJ, Wenserski F, et al. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke. 1999;30(8):1591–1597. doi: 10.1161/01.str.30.8.1591. [DOI] [PubMed] [Google Scholar]
- 27.Darby DG, Barber PA, Gerraty RP, et al. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke. 1999;30(10):2043–2052. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- 28.Ribo M, Molina CA, Rovira A, et al. Safety and efficacy of intravenous tissue plasminogen activator stroke treatment in the 3- to 6-hour window using multimodal transcranial Doppler/MRI selection protocol. Stroke. 2005;36(3):602–606. doi: 10.1161/01.STR.0000155737.43566.ad. [DOI] [PubMed] [Google Scholar]
- 29.Copen WA, Gharai L Rezai, Barak ER, et al. Existence of the diffusion-perfusion mismatch within 24 hours after onset of acute stroke: dependence on proximal arterial occlusion. Radiology. 2009;250(3):878–886. doi: 10.1148/radiol.2503080811. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez R, Hirsch J, Lev M, Schaefer P, Schwamm L. Acute Ischemic Stroke: Imaging and Intervention Second Edition. Springer-Verlag; Berlin: 2010. [Google Scholar]
- 31.Baron JC, von Kummer R, del Zoppo GJ. Treatment of acute ischemic stroke. Challenging the concept of a rigid and universal time window. Stroke; a journal of cerebral circulation. 1995;26(12):2219–2221. doi: 10.1161/01.str.26.12.2219. [DOI] [PubMed] [Google Scholar]
- 32.Rost N, Koroshetz WJ, Gonzalez RG, Schwamm LH. Clinical Management of Acute Stroke. In: Gonzalez R, Hirsch J, Lev M, Schaefer P, Schwamm L, editors. Acute Ischemic Stroke: Imaging and Intervention Second Edition. Springer-Verlag; Berlin: 2010. pp. 211–220. [Google Scholar]
- 33.Smith WS, Lev MH, English JD, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke; a journal of cerebral circulation. 2009;40(12):3834–3840. doi: 10.1161/STROKEAHA.109.561787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez RG, Schaefer PW, Buonanno FS, et al. Diffusion-weighted MR imaging: diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology. 1999;210(1):155–162. doi: 10.1148/radiology.210.1.r99ja02155. [DOI] [PubMed] [Google Scholar]
- 35.Kelly PJ, Hedley-Whyte ET, Primavera J, He J, Gonzalez RG. Diffusion MRI in ischemic stroke compared to pathologically verified infarction. Neurology. 2001;56(7):914–920. doi: 10.1212/wnl.56.7.914. [DOI] [PubMed] [Google Scholar]
- 36.Kim A, Kiruluta A, Gonzalez R, Schaefer P. Diffusion MR of Acute Stroke. In: Gonzalez R, Hirsch JA, Lev MH, Schaefer PW, Schwamm LH, editors. Acute Ischemic Stroke: Imaging and Intervention Second Edition. Springer-Verlag; Berlin: 2010. pp. 145–173. [Google Scholar]
- 37.Yoo AJ, Barak ER, Copen WA, et al. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with National Institutes of Health Stroke Scale Score improves the prediction of acute stroke outcome. Stroke. 2010;41(8):1728–1735. doi: 10.1161/STROKEAHA.110.582874. [DOI] [PubMed] [Google Scholar]
- 38.Sanak D, Nosal V, Horak D, et al. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology. 2006;48(9):632–639. doi: 10.1007/s00234-006-0105-0. [DOI] [PubMed] [Google Scholar]
- 39.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 40.Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40(6):2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim A, Vu D, Gonzalez RG, Schaefer PW. Conventional MRI and MR Angiography of Stroke. In: Gonzalez R, Hirsch JA, Lev MH, Schaefer PW, Schwamm LH, editors. Acute Ischemic Stroke: Imaging and Intervention Second Edition. Springer-Verlag; Berlin: 2010. pp. 123–144. [Google Scholar]
- 42.Kane I, Carpenter T, Chappell F, et al. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke; a journal of cerebral circulation. 2007;38(12):3158–3164. doi: 10.1161/STROKEAHA.107.483842. [DOI] [PubMed] [Google Scholar]
- 43.Schabitz WR. MR mismatch is useful for patient selection for thrombolysis: no. Stroke; a journal of cerebral circulation. 2009;40(8):2908–2909. doi: 10.1161/STROKEAHA.109.552885. [DOI] [PubMed] [Google Scholar]
- 44.Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology. 1996;199(2):391–401. doi: 10.1148/radiology.199.2.8668784. [DOI] [PubMed] [Google Scholar]
- 45.Schlaug G, Benfield A, Baird AE, et al. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology. 1999;53(7):1528–1537. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- 46.Sunshine JL, Tarr RW, Lanzieri CF, Landis DM, Selman WR, Lewin JS. Hyperacute stroke: ultrafast MR imaging to triage patients prior to therapy. Radiology. 1999;212(2):325–332. doi: 10.1148/radiology.212.2.r99au52325. [DOI] [PubMed] [Google Scholar]
- 47.Barber PA, Darby DG, Desmond PM, et al. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology. 1998;51(2):418–426. doi: 10.1212/wnl.51.2.418. [DOI] [PubMed] [Google Scholar]
- 48.Olivot JM, Mlynash M, Thijs VN, et al. Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE) Stroke. 2008;39(8):2257–2263. doi: 10.1161/STROKEAHA.107.511535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mishra NK, Albers GW, Davis SM, et al. Mismatch-based delayed thrombolysis: a meta-analysis. Stroke; a journal of cerebral circulation. 2010;41(1):e25–33. doi: 10.1161/STROKEAHA.109.566869. [DOI] [PubMed] [Google Scholar]
- 50.Hakimelahi R, Copen WA, Schaefer PW, Wu O, Yoo AJ, Schwamm LH, Gonzalez RG. Abstract 1695 A Small DWI Lesion in the Setting of an Anterior Circulation Proximal Artery Occlusion Predicts the Presence of a Large Diffusion-Perfusion Mismatch. American Society of Neuroradiology Annual Meeting; Vancouver, British Columbia. 2009. [Google Scholar]
- 51.Rosso C, Hevia-Montiel N, Deltour S, et al. Prediction of infarct growth based on apparent diffusion coefficients: penumbral assessment without intravenous contrast material. Radiology. 2009;250(1):184–192. doi: 10.1148/radiol.2493080107. [DOI] [PubMed] [Google Scholar]
- 52.Ay H, Buonanno FS, Rordorf G, et al. Normal diffusion-weighted MRI during stroke-like deficits. Neurology. 1999;52(9):1784–1792. doi: 10.1212/wnl.52.9.1784. [DOI] [PubMed] [Google Scholar]
- 53.Schaefer PW, Gonzalez RG, Hunter G, Wang B, Koroshetz WJ, Schwamm LH. Diagnostic value of apparent diffusion coefficient hyperintensity in selected patients with acute neurologic deficits. J Neuroimaging. 2001;11(4):369–380. doi: 10.1111/j.1552-6569.2001.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 54.Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):630S–669S. doi: 10.1378/chest.08-0720. [DOI] [PubMed] [Google Scholar]
- 55.Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for Detection of Acute Intracerebral Hemorrhage. JAMA: The Journal of the American Medical Association. 2004;292(15):1823–1830. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 56.Konstas A, Gonzalez R, Lev M. CT Perfusion. In: Gonzalez R, Hirsch JA, Lev MH, Schaefer PW, Schwamm LH, editors. Acute Ischemic Stroke: Imaging and Intervention Second Edition. Springer-Verlag; Berlin: 2010. pp. 83–121. [Google Scholar]
- 57.Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke; a journal of cerebral circulation. 2004;35(4):904–911. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]
- 58.The Interventional Management of Stroke (IMS) II Study. Stroke; a journal of cerebral circulation. 2007;38(7):2127–2135. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- 59.The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke; a journal of cerebral circulation. 2009;40(8):2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 60.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke; a journal of cerebral circulation. 2005;36(7):1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 61.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR American journal of neuroradiology. 2006;27(6):1177–1182. [PMC free article] [PubMed] [Google Scholar]