Abstract
Twelve thiorhodamine derivatives have been examined for their ability to stimulate the ATPase activity of purified human P-glycoprotein (P-gp)-His10, to promote uptake of calcein AM and vinblastine into multidrug-resistant, P-gp-overexpressing MDCKII-MDR1 cells, and for their rates of transport in monolayers of multidrug-resistant, P-gp-overexpressing MDCKII-MDR1 cells. The thiorhodamine derivatives have structural diversity from amide and thioamide functionality (N,N-diethyl and N-piperidyl) at the 5-position of a 2-thienyl substituent on the thiorhodamine core and from diversity at the 3-amino substituent with N,N-dimethylamino, fused azadecalin (julolidyl), and fused N-methylcyclohexylamine (half-julolidyl) substituents. The julolidyl and half-julolidyl derivatives were more effective inhibitors of P-gp than the dimethylamino analogues. Amide-containing derivatives were transported much more rapidly than thioamide-containing derivatives.
Keywords: Multidrug resistance, P-glycoprotein, thiorhodamine, transport inhibition, ATPase stimulation
1. Introduction
Multidrug resistance (MDR) often emerges in the treatment of cancer following exposure of the patient to chemotherapeutic agents. While MDR appears from a variety of mechanisms, efflux proteins that are members of the ATP-Binding Cassette (ABC) super-family are most commonly associated with the emergence of MDR.1-3 P-glycoprotein (P-gp, also known as MDR1, encoded by ABCB1)1-5 is one member of the ABC superfamily and was the first efflux protein identified and associated with MDR in cancer chemotherapy. Many approaches to the development of inhibitors/modulators of P-gp have been examined. While a large number of compounds, possessing diverse chemical structures and biological activities, are able to reverse MDR, there are currently no approved reversal agents available in the clinic.3,6,7
Rhodamine and rosamine dyes with small structural changes have been used to assay P-gp-mediated transport giving large changes (>100-fold) in rates of transport.8-10 Efflux of rhodamine 123 from cells was used to define P-gp transport substrates/antagonists in a cross-correlation of drug resistance patterns in the NCI 60 set of cells with the NCI Drug Screen Database of compounds.11,12 Interaction of rhodamine and rosamine dyes with P-gp can also be assayed for their ability to stimulate P-gp ATPase activity. Our work with rhodamine/rosamine-related structures has demonstrated that small structural changes give >1000-fold range of P-gp affinities (as measured by KM) with some rhodamine/rosamine molecules being highly stimulating for ATPase activity. Some of the rhodamine/rosamine-related compounds were found to be potent inhibitors of P-gp drug transport.13,14
In more recent work, it was demonstrated that essentially single-atom changes – i.e., interchanging amide and thioamide functionality – in a small series of rosamine/rhodamine structures gave either molecules with high affinity for P-gp and high stimulation of ATPase activity or molecules with high affinity but low stimulation of ATPase activity.15 Specifically, tertiary amide groups on the 9-aryl or heteroaryl substituent gave high P-gp ATPase stimulation while tertiary thioamide groups on the 9-aryl or heteroaryl substituent gave low stimulation of ATPase activity. The single-atom change of amide to thioamide also slows the rate of P-gp-mediated transport of the rhodamine derivatives in both absorptive and secretory directions in the cell. In addition, the substitution of a fused azadecalin for a dimethylamino substituent in the xanthylium core also gave higher affinity for P-gp in both isolated protein and in whole cells.
The chalcogenorhodamines are also photosensitizers for treatment of multidrug-resistant cancer cells in vitro with photodynamic therapy (PDT) via the generation of singlet oxygen from a biopolymer-bound chalcogenorhodamine.16-18 Understanding the parameters that impact ATPase activity and binding affinity in rhodamine-like modulators of P-gp and the factors that influence the drug availability to the pump in the native membrane environment are critical for the development of more potent rhodamine-like inhibitors of P-gp that may have a dual application as photosensitizers for treatment of multidrug-resistant cells by PDT.
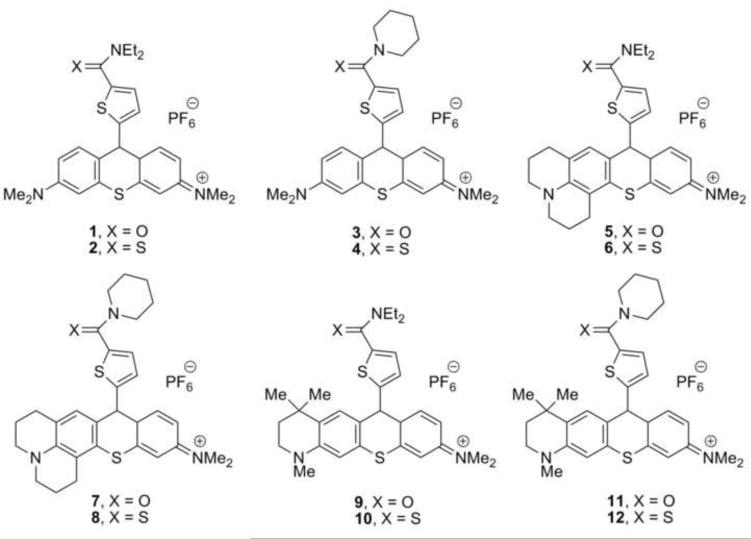
To examine further the roles of the amide/thioamide “switch” and the lipophilicity/hydrophobicity of one amino substituent on P-gp ATPase stimulation, inhibition of P-gp-mediated efflux in whole cells, and transport of the chalcogenorhodamines in whole cells, the series of amide- and thioamide-containing thiorhodamines 1-12 (Chart 1) were prepared. The thiorhodamine core was selected 1) to avoid any ambiguities associated with the chalcogen atom and 2) because a thiorhodamine was the best inhibitor in earlier work.15 The thiorhodamines were evaluated for their ability to stimulate ATPase activity in isolated, wild-type human P-gp, their ability to be transported in multidrug-resistant, P-gp over-expressing cells, and their ability to inhibit P-gp drug transport in multidrug-resistant cells.
Chart 1.
Thiorhodamine analogues 1-12 examined in this study.
2. Results and Discussion
2.1. Synthesis of thiorhodamine analogues 1-12
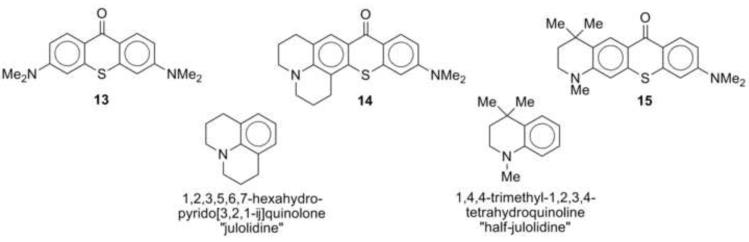
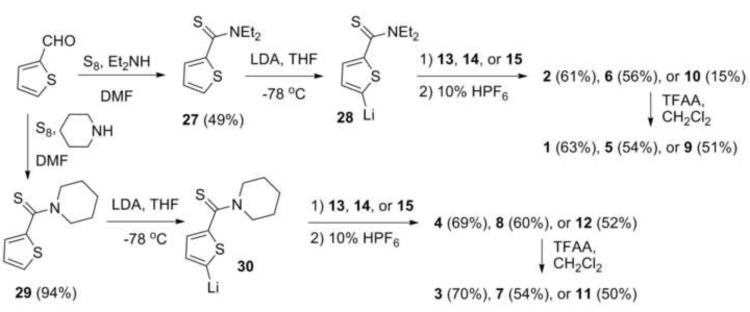
Thiorhodamines 1-12 were prepared from thioxanthones 13-15 (Chart 2), which were prepared by reported procedures.19,20 Compound 14 replaces a dimethylamino substituent with an azadecalin substituent. The fused aniline equivalent of this substitution is known as julolidine (Chart 2) and compounds derived from 14 are referred to as “julolidyl” rhodamines in the remainder of the manuscript. Thioxanthone 15 incorporates a trimethyltetrahydroquinoline group (Chart 2) and compounds derived from 15 are referred to as “half-julolidyl” rhodamines in the remainder of the manuscript. The thioxanthone 15 has the same number of carbon atoms as 14, but the gem-dimethyl group adds the potential for steric interactions absent in 14.
Chart 2.
Thioxanthone precursors to thiorhodamines 1-12.
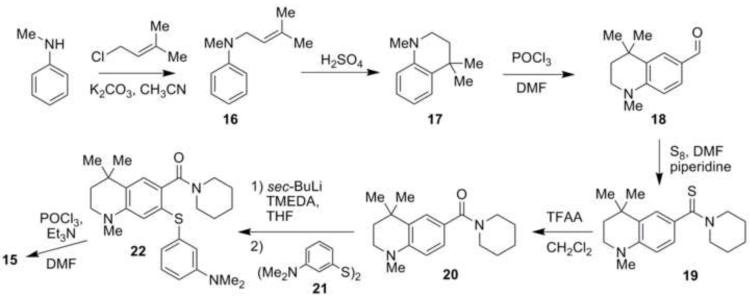
Thioxanthone 15 is a previously unknown structure and was prepared as outlined in Scheme 1. N-Methylaniline was first substituted with 1-chloro-3-methyl-2-butene in the presence of K2CO3, and the aniline product 16 was obtained in 78-87% isolated yields. Cyclization of 16 with concentrated sulfuric acid gave 17 in isolated yields of 70-80%. Subsequent Vilsmeier-Haack reaction with POCl3 and dimethylformamide (DMF) converted 17 to aldehyde 18 in yields of >96%. Oxidation of 18 to a carboxylic acid oxidation state was problematic as was observed for the oxidation of 9-formyljulolidine.20 A modified Willgerodt-Kindler18 reaction oxidized 18 to the thioamide 19 in 75-83% isolated yields using elemental sulfur and piperidine in refluxing DMF.20 Amide 20 was formed from 19 using trifluoroacetic anhydride (TFAA) in isolated yields of > 90%.19
Scheme 1.
Synthesis of thioxanthone 15.
Directed ortho-lithiation of 20 in THF at −78 °C with sec-butyllithium and N,N,N’,N’-tetramethylethylenediamine (TMEDA) was followed immediately by the addition of 3-dimethylaminophenyl disulfide (21)19,23 at −78 °C to minimize the amount of self-condensed side product formation that has been seen in similar reactions. The isolated yield of diaryl sulfide 22 was 35-45%. Subsequent cyclization of 22 with POCl3 in acetonitrile19 gave the desired thioxanthone 15 in 90-91% isolated yield, putting the overall yield for the synthesis of the half-julolidyl thioxanthone 15 at 20%.
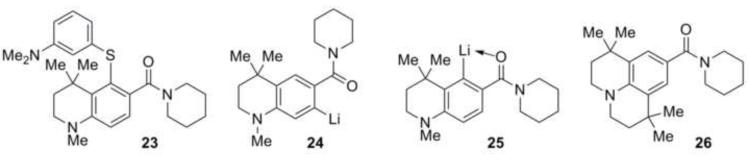
The reaction is regioselective for ortho-lithiation at the desired position. None of the isomeric diaryl sulfide 23 (Chart 3) was observed in the product mixture suggesting either that only ortho-lithiated intermediate 24 was formed in the reaction or that ortho-lithiated intermediate 25, if formed, then hindered sterically approach of the electrophile and formation of 23 was prevented. We have been unable to ortho-lithiate the tetramethyljulolidyl derivative 2620 (Chart 3) under a variety of conditions suggesting that kinetic deprotonation next to the gem-dimethyl group is extremely slow and that anion 24 is likely the only lithiated species formed.
Chart 3.
Possible directed lithiation intermediates, products and substrates.
With thioxanthones 13-15 readily available, the syntheses of dyes 1-12 followed the pathways outlined in Scheme 2. Willgerodt-Kindler oxidation of thiophene-2-carboxaldehyde with elemental sulfur and diethylamine gave thioamide 27 in 49% isolated yield.24 Deprotonation of 27 with sterically bulky lithium diisopropylamide (LDA) gave the 2-thienyl anion 28, which was then added to solutions of the thioxanthones 13-15. Workup with aqueous HPF6 gave the diethyl thioamide-containing dyes 2, 6, and 10 in 61, 56, and 15% isolated yields, respectively.
Scheme 2.
Syntheses of thiorhodamines 1-12 from thioxanthones 13-15.
Unlike the tertiary amide group,25 which is highly directing, the thioamide functionality does not direct lithiation in thiophenes. Only the more acidic α-proton was removed and none of the corresponding 2,3-disubstituted thiophenes were detected in the product mixtures.26
The synthesis of 8 and 12 followed an earlier procedure that was developed for the preparation of thioamide-containing thiorhodamine dye 4.15 Willgerodt-Kindler oxidation of thiophene-2-carboxaldehyde with elemental sulfur and piperidine gave thioamide 29 in 94% isolated yield (Scheme 2).27 Deprotonation of 29 with LDA gave anion 30, which was added to thioxanthone 13 to give thioamide-containing dye 4 in 69% isolated yield following workup with aqueous HPF6.15 Similarly, the addition of 30 to thioxanthones 14 and 15 followed by workup with aqueous HPF6 gave piperidyl thioamide-containing dyes 8 and 12 in 60% and 52% isolated yields, respectively.
The amide-containing thiorhodamines 1, 3, 5, 7, 9 and 11 were prepared from their thioamide counterpart via hydrolysis of the thioamide. Trifluoroacetic anhydride was added to CH2Cl2 solutions of thioamides 2, 4, 6, 8, 10 and 12 to give the corresponding amides in isolated yields of 51-70%.19
2.2. Measurement of n-octanol/water partition coefficients
Experimental values of the n-octanol/water partition coefficient (log P) were measured using the “shake flask” method.28 A saturated n-octanol solution of dye 1-12 was shaken with an equal volume of phosphate buffered saline (PBS) at pH 7.4 and the concentrations in the two layers were determined spectrophotometrically. Values of log P are compiled in Table 1 and covered a range from log P = 1.2 for dyes 1 and 2 to log P ~ 2.6-2.7 for dyes 8, 10 and 12. Based on these values of log P, thiorhodamine compounds 1-12 would have access to both aqueous and hydrophobic environments in the studies with whole cells described below.
Table 1.
Stimulation of ATPase activity, inhibition of verapamil-induced ATPase activity, IC50’s for calcein AM (CAM) uptake and tritiated vinblastine (VIN) efflux by thiorhodamine amide and thioamide analogues, and n-octanol/water partition coefficients (log P) for thiorhodamines 112 and VER.
| Isolated | Human | P-gp-His10 | MDCKII-MDR1 | Cells | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Compd |
Vmaxa, Fold stimulation |
KMb 10−6 M |
IC50,c 10−6 M |
IC50 CAM uptake, 10− 6 Md |
IC50 VIN efflux, 10−6 Md |
log P |
| VER | 17.9 ± 2.0 | 24 ± 2.6 | -- | -- | -- | |
| 1 | 12.1 ± 1.6 | 5.7 ± 1.0 | -- | >100 | -- | 1.2 |
| 2 | 12.1 ± 1.9 | 5.8 ± 0.6 | -- | 14 ± 1 | -- | 1.2 |
| 3 | 8.6 ± 2.3 | 6.4 ± 0.4 | -- | >100 | -- | 1.5 |
| 4 | 6.6 ± 0.7 | 5.5 ± 1.3 | -- | NDf (19 ± 2)g |
-- | (1.7)g |
| 5 | 5.6 ± 0.6 | 3.6 ± 0.4 | -- | 10.0 ± 1.6 | -- | 1.9 |
| 6 | 4.6 ± 1.6 | 2.3 ± 0.4 | -- | 9.6 ± 1.2 | -- | 1.7 |
| 7 | 4.1 ± 0.6 | 1.9 ± 0.4 | -- | 56 ± 1 | -- | 1.4 |
| 8 | 3.5 ± 0.9 | 2.5 ± 0.4 | -- | 12.0 ± 1.1 (2.1 ± 1.2)g |
-- | (2.7)g |
| 9 | 4.7 ± 0.3 | 2.2 ± 1.0 | -- | 7.1 ± 1.2 | 8.0 ± 1.2 | 1.4 |
| 10 | 4.3 ± 0.5 | 3.1 ± 0.7 | -- | 8.8 ± 1.1 | 8.7 ± 1.1 | 2.7 |
| 11 | < 2 | NDe | 1.1 ± 0.2 | 13.0 ± 1.1 | 5.6 ± 1.1 | 1.7 |
| 12 | < 2 | NDe | 1.7 ± 0.3 | 9.3 ± 1.1 | 8.9 ± 1.1 | 2.6 |
Vmax is the ratio of maximum stimulation in the presence of compound to that without compound (basal activity).
KM is the concentration of compound required to achieve 50% maximal stimulation.
IC50 is the concentration of compound required to inhibit 50% of verapamil-stimulated ATPase activity.
Details for methods are provided in Experimental Procedures. Error limits represent ± standard deviation.
ND, not determined because stimulation of ATPase activity too low.
ND, not determined because of limited solubility in BSA-buffer.
Values in parentheses from reference 15.
2.3. P-gp ATPase activity with isolated human P-gp-His10
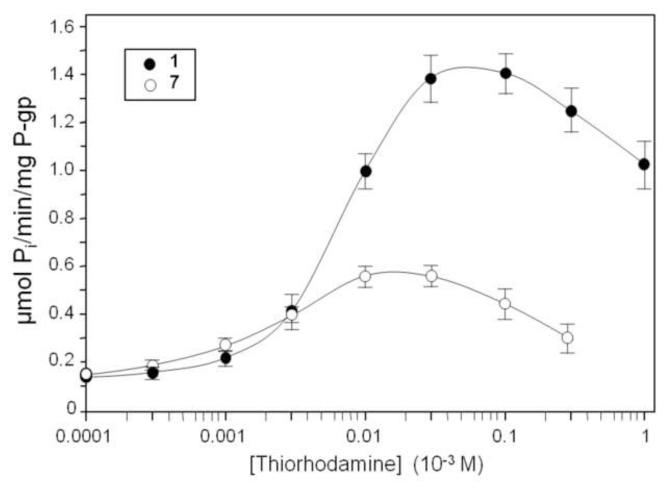
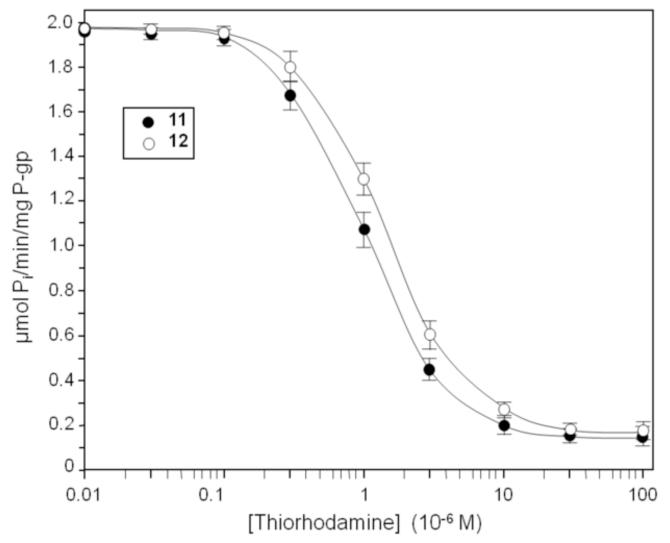
The ATPase activity of thiorhodamine compounds 1-12 (Chart 1) was examined using isolated human P-gp-His10, which was activated with sheep brain phosphatidylethanolamine.29-31 The apparent Michaelis-Menten constant (KM) for ATPase-stimulating compounds was determined as well as the drug-induced stimulation of maximal ATPase activity (Vmax) using isolated human P-gp-His10 (Table 1). Verapamil (VER) was included as a control compound (generally considered relatively robust and one of the most stimulating drugs known for P-gp).32,33 ATPase activity was determined from the release of inorganic phosphate.33 Representative curves are shown in Figure 1 for compound 1, which was the most stimulating rhodamine for ATPase activity, and for compound 7, which had the lowest value of KM. For compounds 11 and 12, which were too weakly stimulating for P-gp ATPase activity to permit determination of KM, the concentration of compound required for 50% inhibition of VER-stimulated (4 × 10−4 M) P-gp ATPase activity (IC50) was determined (Figure 2 and Table 1).
Figure 1.
Stimulation of human P-gp ATPase activity by compounds 1 and 7. Histidine-tagged P-glycoprotein was expressed in BHK cells, isolated by nickel-chelate chromatography and mixed with lipid. P-glycoprotein ATPase activity was then measured in the presence of various concentrations of thiorhodamine derivative 1 or 7. Error bars represent one standard deviation from the mean.
Figure 2.
Inhibition of human P-gp VER-stimulated ATPase activity. Histidine-tagged P-glycoprotein was expressed in BHK cells, isolated by nickel-chelate chromatography and mixed with lipid. P-glycoprotein ATPase activity was then measured in the presence of 4 × 10−4 M VER with various concentrations of thiorhodamines 11 and 12. Data represent the average for triplicate measurements and error bars represent the standard deviation.
Several trends emerged from the data shown in Table 1. With respect to Vmax, values were significantly larger (p < 0.05, Student t-test) for dimethylamino analogues 1-4 (6.6-12.1-fold stimulation) relative to their corresponding julolidyl analogues 5-8 (3.5-5.6-fold stimulation) and half-julolidyl analogues 9-12 (≤4.7-fold stimulation). The latter two groups did not show significant differences. There were no statistically significant differences in values of Vmax for any specific amide/thioamide pair. With respect to KM, values were significantly higher (p < 0.03 for amides, p < 0.01 for thioamides) for the dimethylamino analogues 1-4 [(5.5-6.4) × 10−6 M] relative to either their corresponding julolidyl analogues 5-8 [(1.9-3.6) × 10−6 M] or the corresponding half-julolidyl analogues 9 and 10 (2.2 × 10−6 M and 3.1 × 10−6 M], respectively). For 11 and 12 where values of KM could not be determined, values of IC50 for 50% inhibition of VER-stimulated (4 × 10−4 M) P-gp ATPase activity were 1.1 × 10−6 M and 1.7 × 10−6 M, respectively. It should be noted that compound 8 showed a lower KM (8.7 × 10−8 M) in a previous study15 because those assays were performed in the presence of 10-fold lower lipid (0.5 mg/ml) and detergent (2.0 × 10−4 M dodecyl-β-D-maltoside) concentrations.
2.4. Enhancement of CAM uptake into MDCKII-MDR1 transfected cells
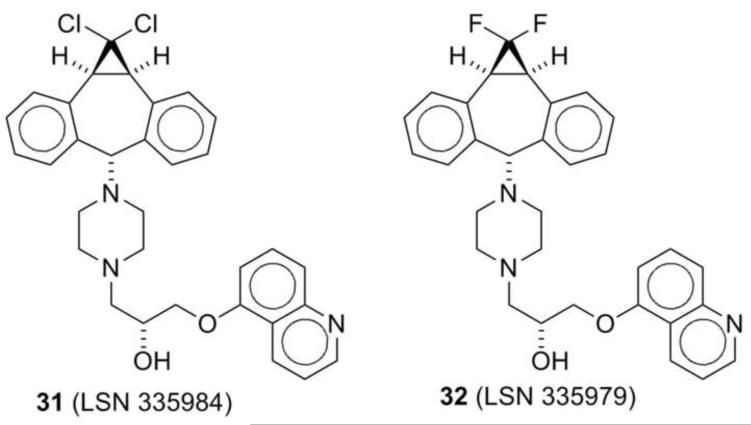
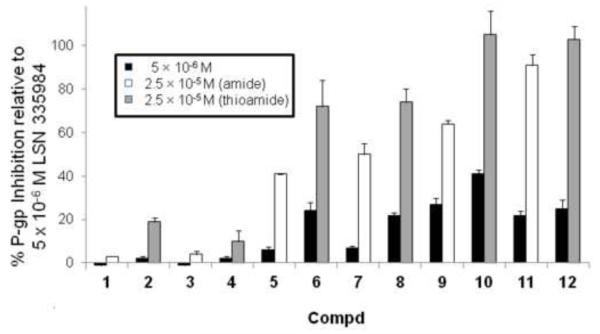
Thiorhodamine compounds 1-12 were also evaluated for their ability to facilitate the uptake of calcein AM (CAM) into MDCKII-MDR1 transfected cells, which over express P-gp.34 The CAM uptake in these cells was determined at concentrations of 5 × 10−6 M and 2.5 × 10−5 M in the thiorhodamine and the inhibition of P-gp was compared as a percentage of the inhibition observed with 5 × 10−6 M (R)-4-[(1a,6,10b)-1,1-dichloro-1,1a,6,10b-tetrahydrodibenzo[a,e]-cyclopropa[c]cyclohepten-6-yl]-[(5-quinolinyloxy)methyl]-1-piperazineethanol (31, LSN 335984, IC50 = 0.4 × 10−6 M, Chart 4), which completely inhibits P-gp. LSN 335984 is structurally related to the P-gp-specific inhibitor (R)-4-[(1a,6,10b)-1,1-difluro-1,1a,6,10b-tetrahydrodibenzo[a,e]cyclo-propa[c]cyclohepten-6-yl]-[(5-quin-olinyloxy)methyl]-1-piperazineethanol (32, LSN 335979 or zosuquidar, Chart 4).7,35 Values of the percentage inhibition at the two concentrations are shown in Figure 3.
Chart 4.
Structures of LSN 335984 (31) and LSN 335979 (32)
Figure 3.
% Inhibition of CAM uptake by 5 × 10−6 and 2.5 × 10−5 M thiorhodamines 1-12 relative to MDCKII-MDR1 cells fully-inhibited by LSN 335984. Values shown are the average of duplicate runs and error bars show the reproducibility of the two runs. White bars show values for amides 1, 3, 5, 7, 9 and 11 at 2.5 × 10−5 M while gray bars show values for thioamide 2, 4, 6, 8, 10 and 12 at 2.5 × 10−5 M. The black bars are for the adjacent thiorhodamine at 5 × 10−6 M.
As shown in Figure 3, all of the compounds 1-12 were weak inhibitors at 5 × 10−6 M (0-41%). At 2.5 × 10−5 M in this assay, several trends emerged. In pair-wise comparisons, the amides were weaker inhibitors than the corresponding thioamides. With a given amine functionalization (dimethylamino, julolidyl, half julolidyl), the piperidyl thioamides and diethyl thioamides displayed comparable inhibition. Within the different amine functionalization, the dimethylamino analogues 1-4 were weaker inhibitors (3-19% inhibition) than their corresponding julolidyl analogues 5-8 (41-74% inhibition), which in turn were weaker inhibitors than the corresponding half-julolidyl analogues 9-12 (64-105% inhibition).
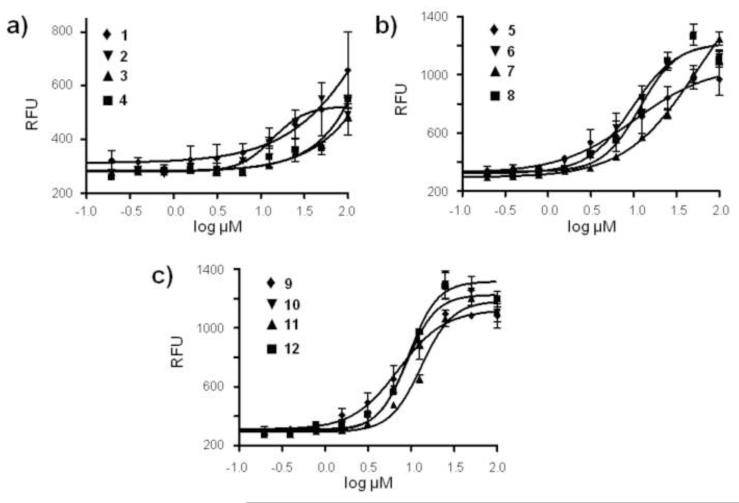
Values of IC50 for the enhancement of CAM uptake into MDCKII-MDR1 transfected cells by thiorhodamines 1-12 (Table 1) were determined by measuring relative fluorescence values obtained after 20-min incubation with CAM at 37 °C. Typical curves are shown in Figure 4 for CAM uptake in the presence of various concentrations of thiorhodamines 1-12. Among the dimethylamino analogues 1-4, the amides 1 and 3 were weaker inhibitors (IC50’s for CAM uptake >1 × 10−4 M) than thioamide analogues 2 and 4 (IC50’s of 1.4 × 10−5 M and 1.9 × 10−5 M,15 respectively). Among the julolidyl analogues 5-8, diethyl amide 5, diethyl thioamide 6, and piperidyl thioamide 8 all had comparable values of IC50 for CAM uptake [9.6-12.0) × 10−6 M], which were lower than the IC50 for piperidyl amide 11 (5.6 × 10−5 M). All of the half-julolidyl amides and thioamides 9-12 had comparable values of IC50 for CAM uptake [(7.1-13.0) × 10−6 M], which were comparable to those observed for 5, 6 and 8. With the exception of IC50 for CAM uptake for half-julolidyl amide 11 relative to julolidyl amide 7 where there was a > 4-fold difference in IC50, there were no significant differences in the other three amide/thioamide pairs for the julolidyl and half-julolidyl derivatives 5-12 with respect to IC50.
Figure 4.
Uptake of CAM into MDCKII-MDR1 cells as a function of concentration of thiorhodamines 1-4 (Panel a), 5-8 (Panel b) and 9-12 (Panel c). Values of IC50 (Table 1) were determined by a sigmoidal dose-response (variable slope) analysis. Data represent the average and standard error mean for triplicate measurements.
2.5. Inhibition of vinblastine efflux by thiorhodamines 9-12 in MDCKII-MDR1 cells
Vinblastine (VIN) is a clinical chemotherapeutic drug and inhibition of its transport by the thiorhodamine compounds is indicative of potential translational value. Inhibition of P-gp-mediated VIN efflux by the half-julolidyl series 9-12 was examined in MDCKII-MDR1 transfected cells. [3H]-Vinblastine, in an appropriate dilution series with 9-12 and BSA, was introduced to the basolateral chamber of a monolayer of MDCKII-MDR1 transfected cells. The appearance of [3H]-VIN in the apical chamber was monitored by scintillation counting and gave comparable values of IC50 of (5.6-8.9) × 10−6 M for 9-12 (Table 1) for inhibition of VIN efflux.
2.6. Transport across monolayers of MDCKII-MDR1 cells
The transport of thiorhodamine amide and thioamide derivatives 1-12 was measured in monolayers of MDCKII-MDR1 transfected cells (Table 2).34 Bovine serum albumin (BSA) addition to the buffer was required because a marked fraction of mass added to the donor equilibrated with the cell monolayer for some of the compounds and this resulted in gross underestimation of the permeability coefficient.36 The assay was repeated in the presence of 31 (LSN 335984),35 to measure transport when P-gp was fully inhibited.
Table 2.
Transport and cell association studies of thiorhodamine amide and thioamide analogues 1-12 with MDCK-MDR1 cells.a
| Compd |
PAB, 10−9 m s−1 |
PBA, 10−9 m s−1 |
PBA/(− inh)/ PBA(+ inh) |
PPassive,b 10−9 m s−1 |
% Cell Associatedc |
Ratio (+/− inh)d |
|---|---|---|---|---|---|---|
|
1 (+ inh) |
≤ 1 ≤ 1 |
280 ± 35 13 ± 2 |
22 | ~7 | 2.1 ± 0.4 12 ± 3.5 |
5.7 |
|
2 (+ inh) |
≤ 1 ≤ 1 |
210 ± 17 5.1 ± 0.1 |
41 | ~3 | 11 ± 3 56 ± 11 |
5.1 |
|
3 (+ inh) |
≤ 1 ≤ 1 |
290 ± 9 7.7 ± 0.1 |
38 | ~4 | 2.8 ± 1.0 11 ± 3 |
3.9 |
|
4 (+ inh) |
≤ 1 ≤ 1 |
110 ± 35 1.5 ± 0.1 |
73 | ~1 | 12 ± 1 58 ± 2 |
4.8 |
|
5 (+ inh) |
≤ 1 ≤ 1 |
210 ± 22 17.0 ± 0.4 |
12 | ~9 | 5.8 ± 0.2 28 ± 3 |
4.8 |
|
6 (+ inh) |
≤ 1 ≤ 1 |
65.0 ± 1.5 2.7 ± 0.1 |
24 | ~2 | 25.0 ± 0.2 45 ± 6 |
1.8 |
|
7 (+ inh) |
≤ 1 ≤ 1 |
220 ± 29 8.1 ± 0.1 |
27 | ~4 | 6.9 ± 0.1 42 ± 7 |
6.1 |
|
8 (+ inh) |
≤ 1 ≤ 1 |
83 ± 3 1.2 ± 0.1 |
69 | ~1 | 31 ± 3 64 ± 9 |
2.1 |
|
9 (+ inh) |
≤ 1 ≤ 1 |
370 ± 17 27.0 ± 0.3 |
14 | ~14 | 10 ± 2 33 ± 6 |
3.3 |
|
10 (+ inh) |
≤ 1 ≤ 1 |
69.0 ± 2.5 1.0 ± 0.1 |
69 | ~1 | 52 ± 6 64 ± 7 |
1.2 |
|
11 (+ inh) |
≤ 1 ≤ 1 |
230 ± 24 7.5 ± 0.1 |
31 | ~4 | 8.6 ± 0.1 45.0 ± 0.1 |
5.2 |
|
12 (+ inh) |
≤ 1 ≤ 1 |
34 ± 22 0.2 ± 0.1 |
170 | ≤1 | 34.0 ± 2.7 62.0 ± 0.2 |
1.8 |
Experiments were run with 5 × 10−6 M dye and 4.3 mg mL−1 BSA. Values of transport in the absorptive (PAB) and secretory (PBA) mode in the absence or presence of inhibitor, the ratio [PBA (no inhibitor)/PBA (with inhibitor)], the % cell associated rhodamine analogue in the absence or presence of inhibitor, the ratio of cell associated rhodamine in the presence or absence of inhibitor. Details for methods are provided in Experimental Procedures. Error limits represent ± standard deviation.
PPassive represents the mean of PAB and PBA in the fully inhibited system.
% Cell Associated is the fraction of mass extracted from the cell monolayer by methanol wash after 1 -h flux in the AB direction.
For % cell associated dye.
MDCKII-MDR1 monolayers display apical and basolateral polarized membranes and are considered to be a near-physiological model for studying P-gp drug transport. In the monolayer, P-gp is solely present at the apical membrane. For thiorhodamines 1-12, transport was measured in the absorptive (apical to basolateral or AB) and secretory (basolateral to apical or BA) transport direction of the cell monolayer. Values of transport in the absorptive (PAB) and secretory (PBA) mode in the absence of inhibitor, passive transport (PPassive) in the presence of inhibitor, and the % cell-associated dye in the AB direction in the absence or presence of inhibitor are compiled in Table 2.
Normally, the role of P-gp in the transport of various molecules is determined by comparison of transport in the absorptive (apical to basolateral or AB) and secretory (basolateral to apical or BA) direction of the cell monolayer, and then an efflux ratio (PBA/AB) is calculated.37 In the case of thiorhodamine compounds 1-12, transport in the absorptive direction was too slow (PAB ≤ 1 × 10−9 m s−1) for measurement and an accurate efflux ratio (PBA/AB) could not be calculated. Consequently, a ratio of transport in the secretory direction in the absence and presence of inhibitor [PBA(−inh)/PBA(+inh)] is included in Table 2 instead.
Large PBA/AB ratios are assumed to be due to efficient P-gp-mediated efflux of the compound and we assume that the large values of PBA(−inh)/PBA(+inh) observed for 1-12 (14-170, Table 2) are also consistent with these compounds being actively transported by P-gp. In every amide/thioamide pair, PBA for the amide is greater than the thioamide. For the amides, PBA is in the range (2.1-3.7) × 10−7 m s−1 and, for the thioamides, PBA is in the range (0.34-2.1) × 10−7 m s−1. In the fully inhibited system, PPassive is slow for all of the compounds (from <1 × 10−9 m s−1 to 1.4 × 10−8 m s−1) and values of PPassive are slower for the thioamide in every amide/thioamide pair.
The % cell-associated dye for thiorhodamines 1-12 was higher in the inhibited system (12-64%) relative to the uninhibited system where the pump was active (2-52%). These differences were 2-fold or greater for all compounds except for diethyl thioamide 10 for which the inhibited and uninhibited systems were quite similar (64% and 52%, respectively). In every amide/thioamide pair, the % cell-associated dye was higher for the thioamide relative to the amide in both the inhibited and uninhibited system. The ratio for % cell-associated dye between inhibited and uninhibited pump was comparable for both amides and thioamides for the dimethylamino analogues 1-4 (3.9-5.7). For the julolidyl analogues 5-8 and the half-julolidyl analogues 9-12, the ratio for % cell-associated dye between inhibited and uninhibited pump was much greater for the amide analogues 5, 7, 9 and 11 (3.3-6.1) than the thioamide analogues 6, 8, 10 and 12 (1.2-2.1).
2.7. Discussion of Biological Results
In earlier work, we saw differences among a group of amide- and thioamide-containing chalcogenorhodamine compounds with respect to rates of transport across monolayers of MDCKII-MDR1 transfected cells and with respect to ATPase stimulation/inhibition in isolated protein.15 The amide and thioamide functionality were located on aryl or heteroaryl substituents at the 9-position of the chalcogenorhodamine core. The compound with the highest affinity for P-gp in isolated protein and with the lowest values of IC50 for CAM uptake was thiorhodamine 8 with the piperidyl thioamide at the 5-position of a 2-thienyl group. Compound 8 was transported extremely slowly in both secretory and absorptive directions in monolayers of MDCKII-MDR1 transfected cells while amide analogues related in structure to 8 gave much faster transport in the secretory direction (PBA). One can ask whether these observations are general for amide- and thioamide-substituted groups at the 9-position of chalcogenorhodamine analogues or whether the observations are specific for the particular compounds examined.
One might assume that molecules with closely related structures would bind to a common site in P-gp, but this assumption is challenged by several observations. The “H” and “R” sites for Hoechst 33342 and rhodamine 123, respectively, on P-gp have been described by Shapiro and Ling indicating that there are at least two binding sites on the protein.29,38 Furthermore, the binding sites may be fluid. The Clarke laboratory described an “induced fit” model for binding of small molecules to P-gp in which the shape of the drug-binding site changes upon to accommodate the shape of the small molecule.39 Modeling studies of P-gp have also indicated a variety of different conformations for binding of small molecules to P-gp.40-42 It is possible that the structurally related amides and thioamides of this study could have different binding to P-gp. Recent studies have shown that thiourea functionality imparted greater inhibitory activity toward MRP1 in a series of drug derivatives relative to P-gp or BCRP, which is consistent with different types of binding.43
The thiorhodamines 1-12 of this study examined a very small structure-activity space: compounds 1-7 and 9-12 were designed as related structures to 8 to compare amide/thioamide-substituted rhodamines with the tertiary amides/thioamides bearing diethylamino or piperidyl groups and to examine the systematic changes of one amino substituent at the 3-position of the thiorhodamine core. Because the structure-activity space is limited, one might not expect great differences among the compounds, but hopefully trends are identified. In isolated protein, the series 1-12 gave a range of values for Vmax (maximum stimulation of ATPase activity) from < 2-fold stimulation for 11 and 12 to 12.1-fold stimulation for 1 and 2 and for KM from 1.9 × 10−6 M for 7 to 6.4 × 10−6 M for 3. All twelve compounds entered MDCKII-MDR1 cells as indicated by their active transport by P-gp [PBA(−inh)/PBA(+inh) > 12, Table 2] and by their facilitation of CAM uptake (Table 1). Thus, all of the compounds 1-12 bind to transport sites including the half-julolidyl amide 11 and thioamide 12 that have Vmax values at or near basal levels.
Compounds 11 and 12, while showing no apparent ATPase stimulation in isolated P-gp, inhibited VER-induced ATPase activity with IC50’s of (1.1 ± 0.2) × 10−6 and (1.7 ± 0.3) × 10−6 M, respectively. Compounds 11 and 12 act as inhibitors in the isolated protein.
In the series of compounds 1-12, certain structural features appear critical for high affinity and impact on ATPase activity. Thiorhodamine compounds 5-12 bearing the more hydrophobic julolidyl and half-julolidyl fragments displayed significantly higher affinity for P-gp as indicated by lower values of KM (p < 0.03, Student t-test) relative to the corresponding thiorhodamine compounds 1-4 bearing dimethylamino fragments (Table 1). Furthermore, the increased affinity for compounds 5-12 bearing the julolidyl and half-julolidyl fragments gave decreased P-gp ATPase stimulation that was statistically significant (p < 0.05, Student t-test) relative to the corresponding thiorhodamine compounds 1-4 bearing dimethylamino fragments. The presence of a thioamide or an amide group in thiorhodamines 1-12 gave no predictable trend in either Vmax or KM in isolated protein.
In whole cell experiments, values of IC50 for CAM uptake were 5-fold higher for amides 1, 3, and 7 relative to their corresponding thioamides 2, 4, and 8 (Table 1). For the remaining six compounds (5, 6, and 9-12), IC50’s for CAM uptake were similar for both amides and thioamides and were similar to IC50 values for 2, 4 and 8. Values of IC50 for VIN efflux were also quite similar for both compounds in the amide/thioamide pairs among compounds 9-12 (Table 1). One would anticipate that the performance of thiorhodamines 1-12 as inhibitors would be similar in isolated protein or in whole cells. These compounds effectively interact with P-gp in a native membrane environment and facilitate the uptake of CAM or inhibit the P-gp-mediated efflux of VIN. If one compares the magnitude of KM and the IC50 for inhibition of VER-induced ATPase activity in isolated protein with the magnitude of the values of IC50 for CAM uptake (via inhibition of P-gp efflux of CAM) and P-gp-mediated VIN efflux in whole cells, values for the whole-cell data are 3- to 30-fold higher (i.e., are less effective in cells than in isolated protein) than values in isolated protein for the julolidyl and half-julolidyl derivatives 5-12.
There are numerous reasons why effective concentrations in the cell may be different than effective concentrations in isolated protein. Differences between the local environment of P-gp as well as lipid composition and fluidity can impact drug interactions with P-gp.44,45 These differences are not a simple function of lipophilicity among the thiorhodamines 1-12 since values of log P, the n-octanol/water partition coefficient, fall in the range of 1.2-2.7 (Table 1). All compounds should have access to aqueous and lipophilic environments.
Alternatively, these data suggest that the thiorhodamines may have limited availability to the pump in the native environment especially when one considers that rates of transport in the absorptive direction (PAB) were all ≤ 1 nm s−1 and that values of PPassive were all ≤ 14 nm s−1 (Table 2). This is consistent with both active and passive replenishing of the membrane concentration in the native environment lagging behind active removal mediated by the pump [PBA in the uninhibited system in the range (0.34-3.7) × 10−7 m s−1]. If the available membrane concentrations are indeed low, then more drug will be required in the whole cell system to raise membrane concentrations effectively to levels that result in pump inhibition.
The rate of secretory transport (PBA) of thiorhodamines 1-12 across monolayers of MDCKII-MDR1 transfected cells was sensitive to both the amine fragment (dimethylamino, julolidyl, half-julolidyl) and the amide/thioamide functionality. In every example, transport of the thioamide was slower than transport of the amide both in the uninhibited and fully inhibited systems (Table 2). In the fully inhibited system, PBA(+inh) was ≤ 5 × 10−9 m s−1for all of the thioamide derivatives while PBA(+inh) for the amide derivatives were in the range (7.5-27) × 10−9 m s−1. In the uninhibited system, transport of the amide/thioamide pair was most similar for thiorhodamines 1 and 2 with the dimethylamino fragment where the ratio of PBA (amide)/PBA (thioamide) was 1.3. For the remaining amide/thioamide pairs, the ratio of PBA (amide)/PBA (thioamide) was in the range 2.6-6.8 and PBA (amide)/PBA (thioamide) ratios were larger for the half-julolidyl derivatives (5.5 for 9/10 and 6.8 for 11/12) relative to the julolidyl derivatives (3.2 for 5/6 and 2.7 for 7/8).
All of the thiorhodamines in the series 1-12 were substrates for P-gp as indicated by the large values of PBA(−inh)/PBA(+inh) (> 12, Table 2). An interesting correlation can be found with the % cell-associated dye in the absence of the added inhibitor 31 (LSN 335984). The % cell-associated dye was higher for the thioamide derivatives relative to the amide derivatives in the uninhibited system (Table 2). An active pump should limit the % cell-associated dye and the results are consistent with increased inhibition with the thioamides. More hydrophobic compounds would be expected to have a higher local concentration in the membrane (% cell-associated dye) and have a greater potential both to inhibit and to invoke recognition by P-gp. This is reflected in the higher values of % cell-associated dye for the more hydrophobic thioamides 6, 8, 10, and 12 relative to 2 and 4, suggesting more inhibition in the former set of compounds relative to the latter.
An active pump should limit the % cell-associated dye relative to the % cell-associated dye in the presence of inhibitor. The amide systems 5, 7, 9 and 11 have ratios for % cell-associated dye between inhibited and uninhibited pump that are between 3.3 and 6.1. Clearly, the active pump [absence of added 31 (LSN 335984)] limits the amount of cell-associated dye. For the thioamide analogues 6, 8, 10 and 12, ratios for % cell-associated dye between inhibited and uninhibited pump were much closer to a value of 1 (1.2-2.1, Table 2), which suggests that the pump is inhibited even in the absence of added 31.
3. Conclusions
An initial lead comparing amide and thioamide functionality in two thiorhodamine derivatives suggested that thioamides might be better inhibitors of P-gp than amide derivatives.15 We have examined a small segment of structure-activity space surrounding this observation through a series of thiorhodamine derivatives 1-12 varying in amide vs. thioamide functionality on a 2-thienyl substituent at the 9-position of the thiorhodamine core and varying in an amine fragment (dimethylamino, julolidyl, half-julolidyl) at the 3-position of the thiorhodamine core. While overall differences are small within this series, certain trends emerge. The net effect of all the compounds 1-12 is to function as an inhibitor of P-gp in the native environment of whole cells as measured by IC50 values for the uptake of CAM. Compounds 9-12 also have the net effect of functioning as inhibitors of P-gp as measured for IC50 values for inhibition of P-gp-mediated efflux of tritiated VIN. Derivatives with julolidyl and half-julolidyl fragments have lower values of IC50 for CAM uptake in whole cells than derivatives with a dimethylamino fragment suggesting that these derivatives have higher affinity for P-gp. Values of KM for the julolidyl thiorhodamines 5-8 and half-julolidyl thiorhodamines 9-12 are lower than values of KM for the dimethylamino-derivatives 1-4, which is consistent with a higher affinity
The thioamide derivatives are transported quite slowly in both absorptive and secretory directions in monolayers of MDCKII-MDR1 transfected cells suggesting that the thioamide derivatives have limited availability to the pump in the native membrane environment in this model system. Understanding the factors that determine this availability in the amide/thioamide pairs may help in the development of more potent inhibitors of P-gp that may function also as photosensitizers for PDT of multidrug-resistant cells. The slow transport of the thioamide analogues from P-gp-expressing cells suggests that heavy-atom analogues of 6, 8, 10, and 12 should be efficient photosensitizers for the treatment of multidrug-resistant cells with PDT. The corresponding selenorhodamines should be available via similar synthetic chemistry.
4. Experimental
4.1. General Methods
Thiorhodamines 415 and 815 and thioxanthones 1319 and 1420 were prepared by literature methods. Reactions were run under argon. Tetrahydrofuran (THF) was distilled from sodium benzophenone ketyl prior to use. Reactions were concentrated in vacuo on a Büchi rotary evaporator. NMR spectra were recorded on an Inova 500 instrument (500 MHz for 1H, 125 MHz for 13C) with residual solvent signal as internal standard. Infrared spectra were recorded on a Perkin-Elmer FTIR instrument. UV-vis near-IR spectra were recorded on a Perkin-Elmer Lambda 12 spectrophotometer or on a Shimadzu UV-3600 spectrophotometer in quartz cuvettes with a 1-cm path length. Melting points were determined with a Buchi capillary melting point apparatus and are uncorrected. All compounds tested have a purity of at least 95%, which was determined by an analysis on a C18 reverse-phase HPLC column [Protein RP] using 75% CH3CN/H2O with a flow rate of 0.4 mL/min and monitoring by a UV-visible detector operating at 254 or 442 nm.
4.1.1. Preparation of 3,6-bis(dimethylamino)-9-(5-(diethylcarbamothioyl)thiophen-2-yl)thioxanthylium hexafluorophosphate (2)
n-Butyllithium (0.890 M in hexanes, 5.86 mL, 5.23 mmol, 3.9 eq) was added to a solution of N,N-diisopropylamine (0.889 mL, 6.30 mmol, 4.7 eq) in THF (10 mL) at −78° C. The resulting mixture was stirred for 0.5 h before it was added to a solution of N,N-diethylthiophene-2-carbothioamide (1.07 g, 5.36 mmol, 4 eq) in THF (40 mL) at −78° C. The resulting solution was stirred for 2 min before it was transferred via cannula into a solution of 3,6-bis-(dimethylamino)-9-thioxanthen-9-one19 (13, 400 mg, 1.34 mmol) in THF (20 mL) at ambient temperature with stirring. The reaction mixture was heated to 40 °C for 0.5 h, and then cooled to ambient temperature. Glacial acetic acid (2 mL) was added and the mixture was poured into a 10% v/v aqueous HPF6 solution at 0 °C. The resulting precipitate was collected via vacuum filtration after 3 h of stirring and washed with water (30 mL) and diethyl ether (50 mL). The product was purified via column chromatography (SiO2, 1:9 Et2O:CH2Cl2, Rf = 0.40), followed by recrystallization from CH2Cl2/Et2O to give 514 mg (61.3%) of thioamide 2 as a purple solid, mp 248-250 °C. 1H NMR (500 MHz, CD2Cl2) δ 7.78 (d, 2 H, J = 10 Hz), 7.25 (d, 1 H, J = 3.5 Hz), 7.11 (d, 1 H, J = 3.5 Hz), 7.09 (d, 2 H, J = 2.5 Hz), 7.05 (dd, 2 H, J = 2.5, 10 Hz), 4.12 (br s, 2 H), 3.87 (br s, 2 H), 3.30 (s, 12 H), 1.41 (t, 6H, J = 2.0 Hz); 13C NMR (75.5 MHz, CD2Cl2) δ 188.5, 153.9, 151.9, 149.6, 144.4, 137.9, 136.4, 130.5, 124.7, 120.1, 116.0, 105.8, 45.2 (br), 44.5 (br), 37.2, 10.6 (br), 7.4 (br); IR (film on NaCl) 2933, 1594, 1498, 1446, 1391, 1364, 1343, 1252 cm−1; λmax in CH2Cl2 (log ε) 308 (4.76), 339 (sh), 568 (sh), 610 nm (5.02); HRMS (ESI) m/z 480.1613 (calcd for C26H30N3S3+: 480.1596).
4.1.2. Preparation of 3,6-bis(dimethylamino)-9-(5-(diethylcarbamoyl)thiophen-2-yl)thioxan-thylium hexafluorophosphate (1)15
Trifluoroacetic anhydride (0.449 mL, 3.20 mmol, 10 eq) was added dropwise to a solution of thioamide 2 (200 mg, 0.320 mmol) in CH2Cl2 (30 mL). The mixture was heated at reflux for 2 h, and then cooled to ambient temperature. A solution of 10% Na2CO3 (20 mL) was added, and the resulting mixture was extracted with CH2Cl2 (3 × 15 mL). The combined organic extracts were concentrated. The crude product was purified via column chromatography (SiO2, 1:9 Et2O:CH2Cl2, Rf = 0.20), followed by recrystallization from CH2Cl2/Et2O, to give 123 mg (63.0 %) of thiorhodamine 1 as a purple solid, mp 264-266 °C (lit.15 mp: 264-266 °C): 1H NMR (500 MHz, CD2Cl2) δ 7.72 (d, 2 H, J = 10 Hz), 7.48 (d, 1 H, J = 3.5 Hz), 7.18 (d, 1 H, J = 3.5 Hz), 7.10 (d, 2 H, J = 2.5 Hz), 7.01 (dd, 2 H, J = 2.5, 10 Hz), 3.61 (br s, 4 H), 3.30 (s, 12 H), 1.31 (br s, 6 H); 13C NMR (75.5 MHz, CD2Cl2) δ 162.4, 153.8, 151.8, 144.2, 142.7, 137.7, 136.2, 130.9, 128.0, 120.0, 115.9, 105.8, 43.3 (br), 40.9, 13.5 (br); λmax in CH2Cl2 (log ε) 313 (4.85), 343 (sh), 574 (sh), 615 nm (5.13)
4.1.3. Preparation of 3,6-bis(dimethylamino)-9-(5-(piperidine-1-carbonyl)thiophen-2-yl)thio-xanthylium hexafluorophosphate (3)
Trifluoroacetic anhydride (0.272 mL, 1.96 mmol, 5 eq) and thiorhodamine 415 (250 mg, 0.392 mmol, 1 eq) in CH2Cl2 (30 mL) were treated as described for the preparation of 1 from 2. The crude product was purified via column chromatography (SiO2, 1:9 Et2O:CH2Cl2, Rf = 0.25), followed by recrystallization from CH2Cl2/Et2O, to give 171 mg (70.3 %) of thiorhodamine 3 as a purple solid, mp 242-244 °C. 1H NMR (500 MHz, CD2Cl2) δ 7.72 (d, 2 H, J = 9.5 Hz), 7.43 (d, 1 H, J = 4.0 Hz), 7.18 (d, 1 H, J = 4.0 Hz), 7.10 (d, 2 H, J = 2.5 Hz), 7.02 (d × d, 2 H, J = 9.5, 2.5 Hz), 3.74 (t, 4 H, J = 5.0 Hz), 3.30 (s, 12 H), 1.79-1.72 (m, 2 H), 1.71-1.66 (m, 4 H); 13C NMR (75.5 MHz, CD2Cl2) δ 162.0, 153.9, 152.0, 144.4, 141.9, 137.4, 136.3, 130.7, 128.5, 120.1, 116.0, 105.8, 47.1 (br), 41.0, 26.5 (br), 24.9; IR (film on NaCl) 2933, 2850, 1593 cm−1; λmax in CH2Cl2 (log ε) 308 (4.68), 338 (sh), 565 (sh), 609 nm (4.98); HRMS (ESI) m/z 476.1833 (calcd for C26H30N3S +3: 476.1825).
4.1.4. Preparation of 12-(Dimethylamino)-2,3,6,7-tetrahydro-9-(5-(diethylcarbamothioyl)-thiophen-2-yl)-1H,5H-thioxantheno[2,3,4-ij]quinolizin-14-ium hexafluorophosphate (6)
n-Butyllithium (0.920 M in hexanes, 4.24 mL, 3.90 mmol), N,N-diisopropylamine (0.663 mL, 4.70 mmol) in THF (10 mL) and N,N-diethylthiophene-2-carbothioamide (797 mg, 4.00 mmol) in THF (80 mL) at −78° C were treated as described in the preparation of thiorhodamine 2. A solution of thioxanthone 1420 (350 mg, 0.999 mmol) in THF (30 mL) was treated with the thienyl anion as described for the preparation of thioamide 2. The crude product was purified via column chromatography (SiO2 gel, 1:9 Et2O:CH2Cl2, Rf = 0.50), followed by recrystallization from CH2Cl2/Et2O to give 0.381 g (56.2 %) of thiorhodamine 6 as a purple solid, mp 153-155 °C: 1H NMR (500 MHz, CD2Cl2) δ 7.65 (d, 1 H, J = 10 Hz), 7.39 (s, 1 H), 7.24 (d, 1 H, J = 4.0 Hz), 7.09 (d, 1 H, J = 2.5 Hz), 7.07 (d, 1 H, J = 4.0 Hz), 6.99 (dd, 1 H, J = 2.5, 10 Hz), 4.12 (br s, 2 H), 3.89 (br s, 2 H), 3.56 (t, 4 H, J = 6.0 Hz), 3.25 (s, 6 H), 2.86 (t, 2 H, J = 6.0 Hz), 2.78 (t, 2 H, J = 6.0 Hz), 2.18 (quintet, 2 H, J = 6.0 Hz), 2.01 (quintet, 2 H, J = 6.0 Hz), 1.41 (t, 6 H, J = 7.0 Hz); 13C NMR (75.5 MHz, CD2Cl2) δ 188.6, 153.1, 149.7, 149.4, 149.1, 142.3, 140.0, 139.0, 135.3, 132.9, 130.3, 126.5, 120.3, 119.1, 115.2, 114.4, 105.9, 51.9, 50.9, 48.9 (br), 48.3 (br), 40.7, 28.2, 24.3, 20.6, 20.1, 14.3 (br), 11.1 (br); IR (film on NaCl) 2932, 1593, 1456, 1387, 1364, 1315, 1180 cm−1; λmax in CH2Cl2 (log ε) 310 (4.74), 339 (sh), 578 (sh), 621 nm (5.04); HRMS (ESI) m/z 532.1917 (calcd for C30H34N3S3+: 532.1909).
4.1.5. Preparation of 12-(dimethylamino)-2,3,6,7-tetrahydro-9-(5-(diethylcarbamoyl)thiophen-2-yl)-1H,5H-thioxantheno[2,3,4-ij]quinolizin-14-ium hexafluorophosphate (5)
Trifluoroacetic anhydride (0.307 mL, 2.21 mmol, 7.5 eq) and thiorhodamine 6 (200 mg, 0.295 mmol, 1 eq) in CH2Cl2 (30 mL) were treated as described for the preparation of 1. The crude product was purified via column chromatography (SiO2, 1:9 Et2O:CH2Cl2, Rf = 0.25), followed by recrystallization from CH2Cl2/Et2O, to give 105 mg (54.0 %) of thiorhodamine 5 as a purple solid, mp 159-161 °C. 1H NMR (500 MHz, CD2Cl2) δ 7.58 (d, 1 H, J = 9.5 Hz), 7.48 (d, 1 H, J = 3.5 Hz), 7.32 (s, 1 H), 7.14 (d, 1 H, J = 3.5 Hz), 7.10 (d, 1 H, J = 2.5 Hz), 6.96 (dd, 1 H, J = 2.5, 9.5 Hz), 3.62 (br s, 4 H), 3.56 (t, 4 H, J = 6.0 Hz), 3.25 (s, 6 H), 2.86 (t, 2 H, J = 6.0 Hz), 2.76 (t, 2 H, J = 6.0 Hz), 2.18 (quintet, 2 H, J = 6.0 Hz), 2.00 (quintet, 2 H, J = 6.0 Hz), 1.38-1.23 (m, 6 H); IR (film on NaCl) 2934, 1593 cm−1; λmax in CH2Cl2 (log ε) 310 (4.65), 341 (sh), 577 (sh), 621 nm (5.01); HRMS (ESI) m/z 516.2144 (calcd for C30H34N3OS2+: 516.2138).
4.1.6. Preparation of 12-(dimethylamino)-2,3,6,7-tetrahydro-9-(N-piperidyl-2-thienyl-5-carboxamido)-1H,5H-thioxantheno[2,3,4-ij]quinolizin-14-ium hexafluorophosphate (7)
Trifluoroacetic anhydride (0.754 mL, 5.43 mmol) and thiorhodamine 815 (250 mg, 0.362 mmol) in CH2Cl2 (30 mL) were treated as described for the preparation of 1. The crude product was purified via column chromatography (SiO2, 1:9 Et2O:CH2Cl2, Rf = 0.25), followed by recrystallization from CH2Cl2/Et2O, to give 132 mg (54.2 % yield) of thiorhodamine 7 as a purple solid, mp 183-185 °C. 1H NMR (500 MHz, CD2Cl2) δ 7.59 (d, 1 H, J = 10 Hz), 7.43 (d, 1 H, J = 3.5 Hz), 7.34 (s, 1 H), 7.13 (d, 1 H, J = 3.5 Hz), 7.10 (d, 1 H, J = 2.5 Hz), 6.96 (dd, 1 H, J = 2.5, 10 Hz), 3.74 (t, 4 H, J = 5.0 Hz), 3.56 (t, 4 H, J = 6.0 Hz), 3.24 (s, 6 H), 2.86 (t, 2 H, J = 6.0 Hz), 2.76 (t, 2 H, J = 6.0 Hz), 2.18 (quintet, 2 H, J = 6.0 Hz), 2.01 (quintet, 2 H, J = 6.0 Hz), 1.79-1.72 (m, 2 H), 1.72-1.66 (m, 4 H); 13C NMR (75.5 MHz, CD2Cl2) δ 162.1, 153.0, 149.7, 149.5, 142.2, 141.5, 140.0, 138.3, 135.2, 132.9, 130.4, 128.5, 126.5, 120.4, 119.2, 115.2, 114.4, 105.9, 51.9, 50.9, 47.7 (br), 40.6, 28.1, 26.5 (br), 24.8, 24.3, 20.6, 20.1; IR (film on NaCl) 2937, 2854, 1593 cm−1; λmax in CH2Cl2 (log ε) 310 (4.65), 341 (sh), 578 (sh), 622 nm (5.00); HRMS (ESI) m/z 528.2153 (calcd for C31H34N3OS2+: 528.2138).
4.1.7. Preparation of 6-(5-(diethylcarbamothioyl)thiophen-2-yl)-9-(dimethylamino)-1,4,4-trimethyl-2,3,4,6-tetrahydro-1H-thiochromeno[3,2-g]quinolin-6-ylium hexafluorophosphate (10)
n-Butyllithium (0.891 M, 2.79 mL, 2.48 mmol), diisopropylamine (0.370 mL, 2.62 mmol), and piperidin-1-yl(thiophen-2-yl)methanethione (495 mg, 2.48 mmol) in THF (20 mL) were treated as described for the preparation of thioamide 2. A solution of 9-(dimethylamino)-1,4,4-trimethyl-3,4-dihydro-1H-thiochromeno[3,2-g]quinolin-6(2H)-one (15, 250 mg, 0.709 mmol) in THF (10 mL) was treated with the thienyl anion as described for the preparation of 2. The crude product was purified via column chromatography (SiO2, 4% MeOH/CH2Cl2) followed by recrystallization from MeOH/ether to give 28.4 mg (15 %) of 10 as dark blue crystals, mp 130-132 °C. 1H NMR (500 MHz, CD2Cl2) δ 7.79 (d, 1 H, J = 9.5 Hz), 7.59 (s, 1 H), 7.26 (d, 1 H, J = 3.5 Hz), 7.10 (d, 1 H, J = 3.5 Hz), 7.06 (d, 1 H, J = 2.0 Hz), 7.02 (s, 1 H), 7.01 (d × d, 1 H, J = 3.0 Hz, 10 Hz), 4.12 (br s, 2 H), 3.87 (br s, 2 H), 3.65 (t, 2 H, J = 6.0 Hz), 3.27 (s, 9 H), 1.82 (t, 2 H, 6 Hz), 1.20 (s, 6 H); 13C NMR (75.6 MHz, CD2Cl2) δ 188.8, 153.5, 151.2, 150.8, 149.3, 143.7, 143.4, 137.9, 136.0, 135.9, 130.3, 130.2, 124.7, 120.5, 119.7, 115.6, 105.7, 105.0, 48.9, 48.1 (br), 40.8, 40.4, 34.5, 32.3, 28.6, 14.7 (br); IR (film, CCl4) vmax: 2937, 1594, 1480, 1446, 1389, 1330, 1251 cm−1; λmax in CH2Cl2 (log ε) 312 (4.83), 346 (sh), 576 (sh), 619 nm (5.04). HRMS (ESI) m/z 534.2060 (calcd for C30H36N3S3+: 534.2066).
4.1.8. Preparation of 6-(5-(diethylcarbamoyl)thiophen-2-yl)-9-(dimethylamino)-1,4,4-trimethyl-2,3,4,6-tetrahydro-1H-thiochromeno[3,2-g]quinolin-6-ylium hexafluorophosphate (9)
Trifluoroacetic anhydride (0.082 mL, 0.59 mmol) and thiorhodamine 10 (200.0 mg, 0.294 mmol) in anhydrous CH2Cl2 (10 mL) were treated as described for the preparation of 1. The crude product was purified via column chromatography (SiO2, 10 % Et2O/CH2Cl2) to give 101 mg (51 %) of 9 as dark blue crystals, mp 155-160 °C. 1H NMR (500 MHz, CD2Cl2) δ 7.69 (d, 1 H, J = 9.5 Hz), 7.54 (s, 1 H), 7.50 (d, 1 H, J = 3.5 Hz), 7.18 (d, 1 H, J = 4.0 Hz), 7.07 (d, 1 H, J = 2.0 Hz), 7.03 (s, 1 H), 6.98 (dd, 1 H, J = 9.5 Hz, 2.0 Hz), 3.65 (t, 2 H, J = 6.5 Hz), 3.61 (br s, 4 H,), 3.29 (s, 3 H), 3.27 (s, 6 H), 1.81 (t, 2 H, J = 6.5 Hz), 1.32 (t, 6 H, J = 6.5 Hz), 1.18 (s, 6 H); 13C NMR (75.6 MHz, CD2Cl2) δ 162.6, 153.5, 151.3, 150.9, 143.7, 143.4, 142.8, 138.1, 136.1, 135.9, 130.7, 130.3, 128.1, 120.5, 119.8, 115.6, 105.7, 105.1, 49.0, 42.8 (br), 40.9, 40.4, 34.5, 32.3, 28.6, 13.9 (br); IR (film, CCl4) vmax: 2935, 1593, 1477, 1448,1390, 1330, 1254 cm−1; λmax in CH2Cl2 (log ε) 313 (4.81), 346 (sh), 575 (sh), 618 nm (5.08); HRMS (ESI) m/z 518.2299 (calcd for C30H36O1N3S2+: 518.2294).
4.1.9. Preparation of 9-(dimethylamino)-1,4,4-trimethyl-6-(5-(piperidine-1-carbonothioyl)thiophen-2-yl)-2,3,4,6-tetrahydro-1H-thiochromeno[3,2-g]quinolin-6-ylium hexafluorophosphate (12)
A solution of n-butyllithium (0.891 M in hexanes, 5.58 mL, 4.97 mmol) and N,N- diisopropylamine (0.740 mL, 5.25 mmol) was stirred 0.5 h at −78 °C and was then was added slowly to a solution of piperidin-1-yl(thiophen-2-yl)methanethione (1.05 g, 4.97 mmol) in THF (20 mL) at −78 °C. The resulting mixture was stirred 5 min before it was transferred via cannula into a solution of thioxanthone 15 (500 mg, 1.42 mmol) in THF (10 mL) at ambient temperature. The resulting mixture was stirred for 0.5 h at 35 °C. After cooling to ambient temperature, glacial acetic acid (2 mL) was added, and the reaction mixture was poured into a 10% v/v solution of stirring, cold aqueous HPF6. The resulting precipitate was collected via filtration after 1 h of stirring and washed with water (10 mL) and diethyl ether (10 mL). The product was purified via column chromatography (SiO2, 4% MeOH/CH2Cl2) followed by recrystallization from MeOH/ether to give 511 mg (52.1 %) of 12 as dark blue crystals, mp 168-170 °C. 1H NMR (500 MHz, CD3CN) δ 7.72 (d, 1 H, J = 9.5 Hz), 7.50 (s, 1 H), 7.25 (d, 1 H, J = 3.6 Hz), 7.18 (d, 1 H, J = 4.0 Hz), 7.13 (d, 2 H, J = 2.8 Hz), 7.07 (d × d, 1 H, J = 2.8, 9.5 Hz), 4.31 (br s, 2 H), 3.99 (br s, 2 H), 3.60 (t, 2 H, J = 6.0 Hz), 3.22 (s, 9 H), 1.78 (br s, 6 H), 1.75 (t, 2 H, 6.0 Hz), 1.13 (s, 6 H); 13C NMR (75.6 MHz, CD2Cl2) δ 188.9, 153.5, 151.2, 150.8, 149.1, 143.7, 143.4, 138.1, 136.0, 130.4, 125.2, 120.5, 119.7, 115.6, 105.8, 105.0, 52.4 (br), 49.0, 40.9, 40.4, 34.5, 32.4, 28.6, 27.2 (br), 24.5; IR (film, CCl4) vmax: 2936, 1594, 1479, 1447, 1389, 1330, 1252 cm−1; λmax in CH2Cl2 (log ε) 312 (4.78), 345 (sh), 579 (sh), 620 nm (4.99); HRMS (ESI) m/z 546.2071 (calcd for C31H36N3S3+: 546.2066).
4.1.10. Preparation of 9-(dimethylamino)-1,4,4-trimethyl-6-(5-(piperidine-1-carbonyl)thiophen-2-yl)-3,4-dihydro-2H-thiochromeno[3,2-g]quinolin-1-ium hexafluorophosphate (11)
Trifluoroacetic anhydride (0.080 mL, 0.58 mmol) and thiorhodamine 12 (200 mg, 0.289 mmol) in anhydrous CH2Cl2 (10 mL) were treated as described for the preparation of amide 1. The product was purified via column chromatography (SiO2, 10 % Et2O/CH2Cl2) to give 98.0 mg (50.1 %) of 11 as dark blue crystals, mp 142-147 °C. 1H NMR (500 MHz, CD2Cl2) δ 7.72 (d, 1 H, J = 9.5 Hz), 7.53 (s, 1 H), 7.44 (d, 1 H, J = 3.5 Hz), 7.17 (d, 1 H, J = 3.5 Hz), 7.06 (d, 1 H, J = 3.0 Hz), 7.02 (s, 1 H), 6.99 (dd, 1 H, J = 2.5, 9.5 Hz), 3.74 (t, 4 H, J = 6.0 Hz), 3.65 (t, 2 H, J = 6.5 Hz), 3.28 (s, 3 H), 3.27 (s, 6 H), 1.81 (t, 2 H, J = 6.5 Hz), 1.74-1.77 (m, 2 H), 1.66-1.72 (m 4 H), 1.18 (s, 6 H); 13C NMR (75.6 MHz, CD2Cl2) δ 162.2, 153.5, 151.2, 150.7, 143.7, 143.5, 141.7, 137.9, 136.0, 135.9, 130.6, 130.2, 128.4, 120.5, 119.8, 115.6, 105.8, 105.2, 48.9, 47.1 (br) 40.9, 40.4, 34.5, 32.3, 28.5, 26.5 (br), 24.9; IR (film, CCl4) vmax: 2936, 1594, 1478, 1448, 1389, 1330, 1253 cm−1; λmax in CH2Cl2 (log ε) 313 (4.81), 344 (sh), 576 (sh), 619 nm (5.08); HRMS (ESI) m/z 530.2288 (calcd for C31H36O1N3S2+: 530.2294).
4.1.11. Preparation of N-methyl-N-(3-methylbut-2-enyl)aniline (16)46
N-Methylaniline (7.13 g, 66.6 mmol) and potassium carbonate (9.20 g, 66.6 mmol) were stirred for 10 min at ambient temperature in CH3CN (100 mL). 1-Chloro-3-methyl-2-butene (4.64 g, 88.7 mmol) was added slowly over 10 min. The resulting mixture was heated at 40 °C for 20 h. After cooling to ambient temperature, water (100 mL) was added and products were extracted with CH2Cl2 (5 × 50 mL). The organic fractions were dried over anhydrous MgSO4 and concentrated. The resulting red-brown oil was purified via column chromatography (SiO2, 30:70 CH2Cl2/hexanes) to give 10.1 g (86.7 %) of 16. 1H NMR (500 MHz, CDCl3) δ 7.25-7.20 (m, 2 H), 6.74 (d, 2 H, J = 8.0 Hz), 6.71 (t, 1 H, J = 7.0 Hz), 5.22 (t, 1 H, J = 5.0 Hz), 3.89 (d, 2 H, J = 6.0 Hz), 2.89 (s, 3 H), 1.72 (d, 6 H, J = 1.0 Hz). HRMS (EI) m/z 175.1359 (calcd for C12H17N1+: 175.1356).
4.1.12. Preparation of 1,4,4-trimethyl-1,2,3,4-tetrahydroquinoline (17)47
Concentrated sulfuric acid (1.5 mL) was slowly added to 16 (0.500 g, 2.85 mmol) at 0 °C. After 1 h at 0 °C, ice water (150 mL) was added. The product was extracted with CH2Cl2 (5 × 50 mL). The combined organic extracts were dried over anhydrous MgSO4 and concentrated. The resulting brown oil was purified via chromatography (SiO2, 1:9 Et2O/CH2Cl2) to give 380 mg (76.0 %) of 17. 1H NMR (500 MHz, CDCl3) δ 7.20 (dd, 1 H, J = 2.0, 7.2 Hz), 7.09 (td, 1 H, J = 1.5, 8.4 Hz), 6.67 (td, 1 H, J = 1.5, 7.2 Hz), 6.60 (dd, 1 H, J = 1.5, 8.0 Hz), 3.23 (t, 2 H, J = 6.0 Hz), 2.90 (s, 3 H), 1.79 (t, 2 H, J = 6.0 Hz), 1.28 (s, 6 H). HRMS (EI) m/z 175.1357 (calcd for C12H17N1+: 175.1356).
4.1.13. Preparation of 1,4,4-trimethyl-1,2,3,4-tetrahydroquinoline-6-carbaldehyde (18)48
Phosphorus oxychloride (481 mg, 3.14 mmol) was added slowly to N,N-dimethylformamide (DMF) (2.5 mL) at 0 °C. Compound 17 (500 mg, 2.85 mmol) in dry DMF (2.5 mL) was added and the resulting mixture was heated at reflux for 1 h. After cooling to ambient temperature, cold NaOH (100 mL, 1 M) was added, and the product was extracted diethyl ether (5 × 50 mL). The combined organic fractions were washed with water (4 × 50 mL) and brine (5 × 50 mL), were dried over anhydrous MgSO4 and concentrated. The product was purified via chromatography (SiO2, 1:9 Et2O/CH2Cl2) to give 560 mg (96.6 %) of 18 as a yellow solid, mp 48-50 °C. 1H NMR (500 MHz, CDCl3) δ 9.69 (s, 1 H), 7.71 (s, 1 H), 7.57 (d, 1 H, J = 8.5 Hz), 6.58 (d, 1 H, J = 8.5 Hz), 3.42 (t, 2 H, J = 6.0 Hz), 3.03 (s, 3 H), 1.76 (t, 2 H, J = 6.0 Hz), 1.30 (s, 6 H); 13C NMR (75.5 MHz, CDCl3) δ 190.9, 150.6, 131.9, 131.3, 127.2, 125.5, 110.2, 48.1, 39.6, 36.5, 32.4, 30.3.
4.1.14. Preparation of piperidin-1-yl(1,4,4-trimethyl-1,2,3,4-tetrahydroquinolin-6-yl)methanethione (19)
A mixture of 18 (3.19 g, 15.7 mmol), elemental sulfur (1.26 g, 39.2 mmol), piperidine (314 mg, 47.1 mmol) and DMF (5 mL) were heated at reflux for 0.5 h. Cold water (100 mL) was added to the resulting mixture and the orange solid that formed was collected by filtration, dissolved in CH2Cl2, and the resulting solution was dried over anhydrous MgSO4 and concentrated. The product was purified via column chromatography (SiO2, CH2Cl2) yielding 3.85 g (81.0 %) of 19 as a yellow crystalline solid, mp 150-152 °C. 1H NMR (500 MHz, CDCl3) δ 7.18 (m, 1 H), 7.16 (d, 1 H, J = 2.0 Hz), 6.49 (d, 1 H, J = 9.0 Hz), 4.32 (s, 2 H), 3.67 (s, 2 H), 3.28 (t, 2 H, J = 6.0 Hz), 2.93 (s, 3 H), 1.81 (s, 2 H), 1.76 (t, 4 H, J = 6.0 Hz), 1.60 (s, 2 H), 1.26 (s, 6 H); 13C NMR (75.5 MHz, CDCl3) δ 201.5, 146.1, 130.45, 130.0, 126.6, 124.7, 109.7, 53.6, 51.6, 47.5, 39.1, 36.8, 32.0, 30.6, 27.0, 25.7, 24.4; IR (film, CCl4) vmax: 2934, 2853, 2363, 1603, 1516, 1473, 1440, 1410, 1330, 1310, 1264, 1238, 1209, 1115, 1017, 786 cm−1; HRMS (EI) m/z 303.1890 (calcd for C18H27N2S1+: 303.1889).
4.1.14. Preparation of piperidin-1-yl(1,4,4-trimethyl-1,2,3,4-tetrahydroquinolin-6-yl)methanone (20)
Trifluoroacetic anhydride (4.06 g, 19.3 mmol) was slowly added to a solution of 19 (5.84 g, 19.3 mmol) in dry CH2Cl2 (5 mL). The resulting mixture was stirred 10 min at room temperature. A solution of 10% aqueous Na2CO3 (100 mL) was added with stirring. The products were extracted with CH2Cl2 (7 × 50 mL), and the combined organic extracts were washed with brine (5 × 50 mL), dried over MgSO4 and concentrated. The product was purified via column chromatography (SiO2, 1:9 Et2O/CH2Cl2) to give 5.00 g (90.4 %) of 20 as red-orange crystals, mp 66-68 °C; 1H NMR (500 MHz, CDCl3) δ 7.30 (d, 1 H, J = 2.0 Hz), 7.18 (dd, 1 H, J = 2.0, 2.5 Hz), 6.52 (d, 1 H, J = 8.5 Hz), 3.56 (s, 4 H), 3.29 (t, 2 H, J = 6.0 Hz), 2.93 (s, 3 H), 1.77 (t, 2 H, J = 6.0 Hz), 1.68-1.64 (m, 2 H), 1.59 (br s, 4H), 1.27 (s, 6 H); 13C NMR (75.5 MHz, CDCl3) δ 172.01, 146.94, 130.97, 127.3, 126.3, 123.3, 110.2, 48.0, 47.0, 39.6, 37.3, 32.5, 31.1, 26.7, 25.3. IR (film, CCl4) vmax: 2932, 2852, 2360, 2341, 1607, 1518, 1468, 1428, 1406, 1331, 1265, 1210, 1115, 1008, 786, 764 cm−1; HRMS (EI) m/z 287.2119 (calcd for C18H27O1N2+: 287.2118).
4.1.15. Preparation of (7-(3-(dimethylamino)phenylthio)-1,4,4-trimethyl-1,2,3,4-tetrahydroquinolin-6-yl)(piperidin-1-yl)methanone (22)
sec-Butyllithium (1.0 M in cyclohexane, 1.05 mL, 1.05 mmol) was added dropwise to a stirred solution of N,N,N’,N’-tetramethylethylenediamine (150 mg, 1.14 mmol) and 21 (250 mg, 0.873 mmol) in 90 mL of THF at −78 °C. A solution of bis-3-(N,N-dimethylamino)phenyldisulfide (1.38 g, 4.54 mmol) in tetrahydrofuran at −78 °C was immediately added, and the resulting mixture was stirred at −78 °C for 1 h and was then stirred at room temperature for 1 h. Aqueous saturated ammonium chloride (15 mL) was added to the resulting mixture and the products were extracted with CH2Cl2 (3 × 50 mL). The organic extracts were dried over anhydrous MgSO4 and concentrated. The resulting yellow oil was purified via column chromatography (SiO2, 1:9 Et2O/CH2Cl2) to give 55.5 mg (37.4 %) of 22 as a yellow-brown oil. 1H NMR (500 MHz, CDCl3) δ 7.12 (t, 1 H, J = 8.0 Hz), 7.02 (s, 1 H), 6.80 (t, 1 H, J = 2.0), 6.71 (d, 1 H, J = 7.6 Hz), 6.59 (d × d, 1 H, J = 2.8, 8.4 Hz), 6.50 (s, 1 H), 3.67 (br s, 2 H), 3.21 (t, 4 H, J = 6.0 Hz), 2.91 (s, 6 H), 2.73 (s, 3 H), 1.72 (t, 2 H, J = 6.0 Hz), 1.60 (br s, 4 H), 1.46 (br s, 2 H), 1.24 (s, 6 H); 13C NMR (75.5 MHz, CDCl3) δ 169.4, 151.0, 145.8, 136.3, 130.9, 130.5, 129.4, 126.2, 124.5, 119.6, 115.5, 113.7, 111.2, 48.2, 47.5, 42.9, 40.5, 39.0, 36.8, 31.9, 30.6, 26.0, 24.7; HRMS (EI) m/z 438.2575 (calcd for C26H36O1N3S +1: 438.2574).
4.1.16. Preparation of 9-(dimethylamino)-1,4,4-trimethyl-3,4-dihydro-1H-thiochromeno[3,2-g]quinolin-6(2H)-one (15)
Phosphorus oxychloride (496 mg, 3.24 mmol) was added dropwise to a mixture of triethylamine (328 mg, 3.24 mmol) and 22 (118 mg, 0.270 mmol) in acetonitrile (25 mL). The resulting red mixture was stirred at ambient temperature for 5 min before stirring for 2 h at 80 °C. After cooling to ambient temperature, the resulting mixture was added to stirring, cold aqueous NaOH (200 mL, 1 M). The products were extracted with dichloromethane (5 × 50 mL), and the combined organic extracts were dried over MgSO4 and concentrated. The product was then purified via column chromatography (SiO2, 1:9 Et2/CH2Cl2) to give 80.0 mg (90.3 %) of desired product, mp 204-206 °C. 1H NMR (500 MHz, CDCl3) δ 8.44 (d, 1 H, J = 9.0 Hz), 8.39 (s, 1 H), 6.79 (dd, 1 H, J = 2.5, 9.0 Hz), 6.57 (s, 1 H), 6.45 (s, 1 H), 3.41 (t, 2 H, J = 6.0 Hz), 3.07 (s, 6 H), 3.02 (s, 3 H), 1.78 (t, 2 H, J = 6.5 Hz), 1.35 (s, 6 H); 13C NMR (75.5 MHz, CDCl3) δ 179.8, 178.5, 165.5, 152.2, 148.3, 139.5, 137.5, 131.4, 126.9, 119.7, 119.1, 111.7, 105.7, 104.3, 48.0, 40.6, 39.6, 36.7, 32.6, 30.4; IR (film, CCl4) vmax: 2956, 1594, 1514, 1320 cm−1; HRMS (EI) m/z 353.1687 (calcd for C21H25O1N2S +1: 353.1682).
4.1.17. Preparation of N,N-diethylthiophene-2-carbothioamide (27)
Thiophene-2-carbaldehyde (1.00 g, 8.92 mmol), elemental sulfur (713 mg, 22.3 mmol), and diethylamine (2.76 mL, 26.8 mmol) and DMF (5 mL) were combined in a three-neck flask fitted with reflux condenser. The resulting mixture was allowed to stir 3 h at 110°C. Ice cold water (100 mL) was added with stirring, and the mixture was extracted with diethyl ether (8 × 50 mL) and washed with brine (5 × 50 mL). The organic fractions were combined, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The product was purified via chromatography (silica, 10 % ethyl acetate/hexanes) to give 0.87 g (49 %) of the desired product as an orange oil. 1H NMR (500 MHz, CDCl3) δ 7.35 (dd, 1 H, J = 1.0, 5.5 Hz), 7.08 (dd, 1 H, J = 1.0, 3.5 Hz), 6.96 (dd, 1 H, J = 3.5, 5.5 Hz), 4.10 (br s, 2 H), 3.70 (br s, 2 H), 1.34 (br s, 6 H); 13C NMR (75.5 MHz, CDCl3) δ 190.9, 145.0, 127.9, 126.3, 14.9, 48.0 (br), 14.1 (br), 11.1 (br); HRMS: m/z 199.0477 (calcd for C9H13NS2: 199.0484); IR (film) vmax: 3070, 2969, 2931, 2869, 1520, 1489, 1418, 1380, 1354, 1305, 1276, 1245, 1210, 1175, 1131, 1095, 1074 cm−1.
4.2. Determination of partition coefficients
The octanol/water partition coefficients were all measured at pH 7.4 (PBS) using UV-visible spectrophotometry. The measurements were done using a shake flask direct measurement.28 Mixing for 3-5 min was followed by 1 h of settling time. Equilibration and measurements were made at 23 °C using a Perkin-Elmer Lambda 12 spectrophotometer. High-performance liquid chromatography grade 1-octanol was obtained from Sigma-Aldrich.
4.3. Expression of P-gp, purification and measurement of ATPase activity
To facilitate purification by metal-chelate chromatography31, the cDNA for human P-glycoprotein (P-gp)30 was modified to contain a 10-histidine tag at the COOH-terminal end (P-gp-His10). Histidine-tagged P-gp was then stably expressed in Baby hamster kidney (BHK) cells by co-transfecting with P-gp-His10 and pWL-neo (Stratagene, Cedar Creek, TX). The transfected cells were then treated with the cytotoxic agent G418 as described previously.49 Clones overexpressing P-gp were identified by subjecting whole cell extracts of G418-resistant colonies to immunoblot analysis with a rabbit polyclonal antibody to P-gp.50 Histidine-tagged P-gp was then isolated by nickel-chelate chromatography as described previously.31 A sample of the isolated histidine-tagged P-gp was mixed with an equal volume of 10 mg/ml sheep brain phosphatidylethanolamine (Type II-S, Sigma) and ATPase activity was determined in the absence or presence of various concentrations of thiorhodamine compounds. The samples were incubated for 30 min at 37 °C and the reactions were stopped by addition of SDS. The amount of inorganic phosphate released was then determined.33
To test for inhibition of P-gp VER-stimulated ATPase activity, samples of P-gp-His10 in lipid were pre-incubated with various concentrations of thiorhodamine compounds for 15 min at 20 °C. Verapamil was used as a substrate to test for inhibition because it is one of the most potent activators of P-gp ATPase activity.32 The reactions were started by addition of ATPase reaction mix containing VER (final concentration of 4 × 10−4 M) and ATPase activity determined as described above.
4.4. Enhancement of calcein-AM uptake into MDCKII-MDR1 cells by thiorhodamine analogues
MDCK cells transfected with wild-type MDR1 (ABCB1) were obtained at passage number 12 from Dr. Piet Borst at The Netherlands Cancer Institute. Cell growth was maintained in Dulbecco’s Modified Eagle’s Medium (Gibco) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin and 100 μg/ml streptomycin in 75-cm2 flasks. Cultures were passaged by trypsinization 1:10 twice a week and used at passage number 16-42. Cells were seeded at 40,000 cells/well in 96-well flat bottom plates (Falcon) using a medium volume of 2 × 10−4 L, which was replaced on day 3 prior to their use on day 4.
Cells were washed once with Dulbecco’s phosphate-buffered saline containing 10−2 M Hepes buffer at pH 7.4 (DPBSH) (Gibco) and incubated with solutions of the thiorhodamine analogue or control compound in DPBSH containing 4.3 mg mL−1 bovine serum albumin (BSA) at 37 °C in room atmosphere. IC50 values were calculated from 1:1 serial dilution series. After 30 min, the test compound was replaced to include 0.5μg mL−1 CAM and incubated an additional 20 min. Calcein fluorescence was read on a Cytofluor series 4000 Multi Well Plate Reader (PerSeptive Biosystems) with λEX and λEM set at 485 nm and 530 nm, respectively. Negative (0.25% DMSO in DPBSH), and positive [5 × 10−6 M LSN 335984] controls were included in each plate. IC50 values were calculated from the serial dilution curves using GraphPad PRISM version 4.03 software. Briefly, compound concentration was plotted as log μM concentration versus relative fluorescence units (rfu) and a sigmoidal dose-response (variable slope) analysis with no weighting or restrictions was applied.
4.5. P-gp-transport studies across MDCKII-MDR1 monolayers
MDCKII-MDR1 cells that were seeded at 50,000 cells cm−2 onto 12-well (1.13 cm2 surface area) Transwell polycarbonate filters (Costar) were fed on days 3 and 5, and used on day 6. The upper and lower chamber volumes were 0.5 mL and 1.0 mL, respectively. Cells were rinsed 10 min in DPBSH at 37 °C with mixing on a nutator (Clay Adams). Cells were pre-incubated with 4.3 mg mL−1 BSA in DPBSH alone or containing 5 × 10−6 M LSN 335984. After 30 min, 5 μM test compound (1-12) in BSA/DPBSH with or without inhibitor was added to the donor chamber (0.5 mL upper or apical, 1.0 mL lower or basolateral). Initial donor samples were taken at t = 0. For apical-to-basolateral (AB) flux, D0 was taken from the mixing tube before addition to the cell monolayer. For basolateral-to-apical (BA) flux this sample was taken from the 12-well plate 10 min after transfer, but before cell wells were added. Samples were taken from both the donor and receiver chambers following a 1-h incubation at 37 °C with constant mixing by nutation. Cell monolayers were rinsed briefly two times using cold DPBS and extracted with 5 × 10−4 L methanol for 3 min. Samples (5 × 10−5 L) were combined into n = 3 cassettes in a 96-deep well assay plate and protein was precipitated by adding 4.5 × 10−4 L acetonitrile, shaken to mix. Plates were centrifuged 5 min at 5000 rpm. Compound concentrations were determined with an LC-MS/MS assay. Chromatography was performed using a Betasil C18 2×20 mm 5 micron Javelin column; (Thermo Scientific, Waltham, Massachusetts) and 1 of 2 mobile phase systems. System 1 consisted of 5 × 10−3 M ammonium bicarbonate in water (Mobile Phase A), and, 5 × 10−3 M ammonium bicarbonate in methanol (Mobile Phase B), with elution accomplished by a methanol gradient at 1.5 mL/min. System 2 consisted of 0.4% trifluoroacetic acid (TFA), 1 × 10−3 M ammonium bicarbonate in water (Mobile Phase A), and, 0.4% TFA/1 × 10−3 M ammonium bicarbonate in acetonitrile (Mobile Phase B), with elution accomplished by an acetonitrile gradient at 1.5 mL/min. Mass spectrometric detection was performed with an API4000 mass spectrometer (Applied Biosystems, Foster City, California) equipped with a turbo ion spray source, using selected reaction monitoring in positive ion mode with precursor and product ion transitions specific to each analyte.
4.6. Inhibition of vinblastine efflux in MDCKII-MDR1 cells by thiorhodamine compounds 9-12
MDCKII-MDR1 cells were seeded onto Costar Transwell polycarbonate membranes and maintained as described above. On day 6, cells were rinsed 1 × 10 min in DPBSH with nutation at 37 °C, and then conditioned in 1 × 10−10, 1 × 10−8, 5 × 10−8, 1 × 10−7, 5 × 10−7, 1 × 10−6 or 1 × 10−5 M 9-12 in BSA/DPBSH. After 30 min at 37 °C, 1 × 10−3 L [3H]-VIN (0.25 μCi mL−1 from 0.1 mCi mL−1 EtOH stock) in appropriate solution of 9-12 was introduced to the basolateral chamber and 5 × 10−4 M fresh thiorhodamine compound was added to the apical chamber. Initial donor samples were taken from the basolateral chamber at t = 0. The apical solution was replaced every 10 min with fresh buffer and appearance of [3H]-VIN in the apical chamber was measured by scintillation counting. An IC50 was calculated using XLfit software.
Supplementary Material
Acknowledgments
We thank Dr. Piet Borst at The Netherlands Cancer Institute for supplying the MDCKII-MDR1 cells. This research was supported in part by the NIH (GM-94367) to M.R.D. and grants from the Canadian Cancer Society (19074) and the Canadian Institutes for Health Research (25043) to D.M.C. D.M.C. is the recipient of the Canada Research Chair in Membrane Biology. We also acknowledge Ms. Michelle Linder, Ms. Kellie Davies, and Ms. Jackie Hill for their technical assistance in the synthesis of starting materials. We also acknowledge Mr. Codi Vinci for his assistance with analytical HPLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data (1H and 13C NMR spectra and HPLC data for compounds 2, 3, 5-7, and 9-12 and 1H and 13C NMR spectra for thioxanthone 15) can be found in the online version.
References and notes
- 1.Gottesman MM, Fojo T, Bates SE. Nat. Rev. Cancer. 2002;2:48. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 2.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Nat. Rev. Drug Discovery. 2006;5:219. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 3.Sharom FJ. Pharmacogenomics. 2008;9:105. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- 4.Dean M, Rzhetsky A, Allikmets R. Genome Res. 2001;11:1156. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y, Sadée W. Cancer Lett. 2006;239:168. doi: 10.1016/j.canlet.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Eckford PDW, Sharom FJ. Chem. Rev. 2009;109:2989. doi: 10.1021/cr9000226. [DOI] [PubMed] [Google Scholar]
- 7.Raub TJ. Mol. Pharmaceutics. 2006;3:3. doi: 10.1021/mp0500871. [DOI] [PubMed] [Google Scholar]
- 8.Eytan GD, Regev R, Hurwitz CD, Assaraf YG. Eur. J. Biochem. 1997;248:104. doi: 10.1111/j.1432-1033.1997.00104.x. [DOI] [PubMed] [Google Scholar]
- 9.Lu P, Liu R, Sharom FJ. Eur. J. Biochem. 2001;268:1687. [PubMed] [Google Scholar]
- 10.Loetchutinat C, Saengkhae C, Marbeuf-Gueye C, Garnier-Suillerot A. Eur. J. Biochem. 2003;270:476. doi: 10.1046/j.1432-1033.2003.03403.x. [DOI] [PubMed] [Google Scholar]
- 11.Scala S, Akhmed N, Rao US, Paull K, Lan L-B, Dickstein B, Lee J-S, Elgemeie GH, Stein WD, Bates SE. Mol. Pharmacol. 1997;51:1024. doi: 10.1124/mol.51.6.1024. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Paull K, Alvarez M, Hose C, Monks A, Grever M, Fojo AT, Bates SE. Mol. Pharmacol. 1994;46:627. [PubMed] [Google Scholar]
- 13.Tombline G, Donnelly DJ, Holt JJ, You Y, Ye M, Gannon MK, Nygren CL, Detty MR. Biochemistry. 2006;45:8034. doi: 10.1021/bi0603470. [DOI] [PubMed] [Google Scholar]
- 14.Tombline G, Holt JJ, Gannon MK, Donnelly DJ, Wetzel B, Sawada GA, Raub TJ, Detty MR. Biochemistry. 2008;47:3294. doi: 10.1021/bi7021393. [DOI] [PubMed] [Google Scholar]
- 15.Gannon MK, Holt JJ, Bennett SM, Wetzel BR, Loo TW, Bartlett MC, Clarke DM, Sawada GA, Higgins JW, Tombline G, Raub TJ, Detty MR. J. Med. Chem. 2009;52:3328. doi: 10.1021/jm900253g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson SL, Hilf R, Donnelly DJ, Detty MR. Bioorg. Med. Chem. 2004;12:4625. doi: 10.1016/j.bmc.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Gibson SL, Holt JJ, Ye M, Donnelly DJ, Ohulchanskyy TY, You Y, Detty MR. Biorg. Med. Chem. 2005;13:6394. doi: 10.1016/j.bmc.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 18.Holt JJ, Gannon MK, Tombline G, McCarty TA, Page PM, Bright FV, Detty MR. Biorg. Med. Chem. 2006;14:8635. doi: 10.1016/j.bmc.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Del Valle DJ, Donnelly DJ, Holt JJ, Detty MR. Organometallics. 2005;24:3807. [Google Scholar]
- 20.Holt JJ, Calitree BD, Vincek J, Gannon MK, II, Detty MR. J. Org. Chem. 2007;72:2690. doi: 10.1021/jo070086f. [DOI] [PubMed] [Google Scholar]
- 21.Kindler K. Justus Liebigs Annalen der Chemie. 1923;431:187. [Google Scholar]
- 22.Masuda R, Hojo M, Ichi T, Sasano S, Kobayashi T, Kuroda C. Tetrahedron Lett. 1991;32:1195. [Google Scholar]
- 23.Brennan NK, Donnelly DJ, Detty MR. J. Org. Chem. 2003;68:3344. doi: 10.1021/jo026635t. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Wei-min, Li Zhi-fang, Zhang Yong-min. Hecheng Huaxue. 2005;13:471. [Google Scholar]
- 25.Beak P, Brown RA. J. Org. Chem. 1982;47:34. [Google Scholar]
- 26.Carpenter AJ, Chadwick DJ. Tetrahedron Lett. 1985;26:1777. [Google Scholar]
- 27.Katritzky AR, Witek RM, Rodriguez-Garcia V, Mohapatra PP, Rogers JW, Cusido J, Abdel-Fattah AAA, Steel PJ. J. Org. Chem. 2005;70:7866. doi: 10.1021/jo050670t. [DOI] [PubMed] [Google Scholar]
- 28.Partition Coefficients. Sangster J. In: Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry; Wiley Series in Solution Chemistry. Fogg PGT, editor. John Wiley and Sons; New York: 1997. [Google Scholar]
- 29.Shapiro AB, Ling V. Eur. J. Biochem. 1998;254:189. doi: 10.1046/j.1432-1327.1998.2540189.x. [DOI] [PubMed] [Google Scholar]
- 30.Loo TW, Clarke DM. J. Biol. Chem. 1993;268:3143. [PubMed] [Google Scholar]
- 31.Loo TW, Clarke DM. J. Biol. Chem. 1995;270:21449. doi: 10.1074/jbc.270.37.21449. [DOI] [PubMed] [Google Scholar]
- 32.Loo TW, Clarke DM. J. Biol. Chem. 1999;274:35388. doi: 10.1074/jbc.274.50.35388. [DOI] [PubMed] [Google Scholar]
- 33.Chifflet S, Torriglia A, Chiesa R, Tolosa S. Anal. Biochem. 1988;168:1. doi: 10.1016/0003-2697(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 34.Evers R, Kool M, Smith AJ, van Deemter L, de Haas M, Borst P. Br. J. Cancer. 2000;83:366. doi: 10.1054/bjoc.2000.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dantzig AH, Shepard RL, Law KL, Tabas L, Pratt S, Gillespie JS, Binkley SN, Kuhfeld MT, Starling JJ, Wrighton SA. JPET. 1999;290:854. [PubMed] [Google Scholar]
- 36.Sawada GA, Barsuhn CL, Lutzke BS, Houghton ME, Padbury GE, Ho NFH, Raub TJ. J. Pharm. Exper. Therap. 1999;288:1317. [PubMed] [Google Scholar]
- 37.Troutman MD, Thakker DR. Pharm. Res. 2003;20:1192. doi: 10.1023/a:1025096930604. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro AB, Ling V. Eur. J. Biochem. 1997;250:130. doi: 10.1111/j.1432-1033.1997.00130.x. [DOI] [PubMed] [Google Scholar]
- 39.Loo TW, Bartlett MC, Clarke DM. J. Biol. Chem. 2003;278:13603. doi: 10.1074/jbc.C300073200. [DOI] [PubMed] [Google Scholar]
- 40.Pajeva IK, Wiese M. Quant. Struct.-Act. Relat. 2001;20:130. [Google Scholar]
- 41.Pajeva IK, Wiese M. J. Med. Chem. 2002;45:5671. doi: 10.1021/jm020941h. [DOI] [PubMed] [Google Scholar]
- 42.Pajeva IK, Globisch C, Wiese M. J. Med. Chem. 2004;47:2523. doi: 10.1021/jm031009p. [DOI] [PubMed] [Google Scholar]
- 43.Haecker H-G, Leyers S, Wiendlocha J, Guetschow M, Wiese M. J. Med. Chem. 2009;52:4586. doi: 10.1021/jm900688v. [DOI] [PubMed] [Google Scholar]
- 44.Omote H, Al-Shawi MK. Biophys. J. 2006;90:4046. doi: 10.1529/biophysj.105.077743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seelig A, Gatlik-Landwojtowicz E. Mini-Rev. Med. Chem. 2005;5:135. doi: 10.2174/1389557053402693. [DOI] [PubMed] [Google Scholar]
- 46.Babayan AT, Indzhikyan MG, Gegelyan ZG, Grigoryan AA. Doklady Akademii Nauk Armyanskoi SSR. 1962;35:67. [Google Scholar]
- 47.Thompson MD, Berlin KD. Proceed. Oklahoma Acad. Sci. 1985;65:39. [Google Scholar]
- 48.Hibi S, Kikuchi K, Yoshimura H, Nagai M, Tagami K, Abe S, Hishinuma I, Nagakawa J, Miyamoto N, Takayuki H, Aichi O, Seiko H, Kenji T, Takashi Y, Makoto A. 1995-JP2231
- 49.Loo TW, Bartlett MC, Clarke DM. Mol. Pharmaceutics. 2005;2:407. doi: 10.1021/mp0500521. [DOI] [PubMed] [Google Scholar]
- 50.Loo TW, Clarke DM. J. Biol. Chem. 1995;270:21839. doi: 10.1074/jbc.270.37.21839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.