Abstract
Recently, we have noticed a series of patients presenting for cognitive complaints after gastric bypass, without any identifiable etiology. We set out to determine whether any focal brain atrophy could account for the complaints. A retrospective case series was performed to identify patients with cognitive complaints following gastric bypass that had a volumetric MRI. Voxel-based morphometry was used to assess patterns of grey matter loss in all 10 patients identified, compared to ten age and gender-matched controls. All patients had undergone Roux-en-Y gastric bypass at a median age of 54 (range: 46–64). Cognitive complaints began at a median age of 57 (52–69). Formal neuropsychometric testing revealed only minor deficits. No nutritional abnormalities were identified. Voxel-based morphometry demonstrated focal thalamic atrophy in the gastric bypass patients when compared to controls. Patients with cognitive complaints after gastric bypass surgery have focal thalamic brain atrophy that could account for the cognitive impairment.
Keywords: Gastric bypass, Voxel based morphometry, Thalamus, Cognitive impairment
INTRODUCTION
Obesity is increasingly prevalent with approximately a 25% lifetime risk in the United States.(1) Obesity is associated with increased mortality, hypertension and diabetes mellitus.(2) Due to these complications, gastric bypass surgery is increasingly used for weight loss. The number of surgical procedures for weight loss increased dramatically from 16,000 in the early 1990s to about 103,000 in 2003 and 344,221 in 2008.(3, 4) In addition to reducing comorbidities, gastric bypass also decreases mortality in obese patients.(5)
Recently, several papers have reported neurological complications related to gastric surgery. One review reported 96 patients with neurological symptoms after gastric surgery with peripheral neuropathy and encephalopathy, of which 90% were Wernicke’s encephalopathy, being the most common.(6) Other neurological complications include myelopathy associated with low copper or low vitamin B12 levels.(7) The overall incidence of neurological complications after gastric bypass ranges from 5–16%.(8, 9)
Over the past 2 years we have had a number of neurology referrals for cognitive complaints in patients after gastric bypass surgery. These patients were typically evaluated without a formal diagnosis and the majority was assumed to have subjective cognitive complaints that were non-organic. Given the increasing referrals, concern for potential misdiagnosis, and the fact that there is no literature on brain changes associated with gastric bypass and cognitive impairment outside of Wernicke’s, we aimed to determine whether there was any anatomic correlate for the cognitive complaint in these patients.
METHODS
Subject selection
To capture all appropriate patients we used the Mayo Clinic Medical Records Linkage system to identify all patients that had undergone surgery for obesity and were evaluated in our Department of Neurology between January 1st, 1996 and May 31st, 2009. The computer search strategy employed the text words “gastric bypass” or “laparoscopic adjustable gastric band” or “lap band” or “bariatric surgery”, crossed referenced with “Department of Neurology. Given this approach, any patient evaluated by a neurologist after undergoing bariatric surgery were captured (n=570). Many of these patients underwent gastric bypass at another institution. The medical records of these 570 patients were reviewed to determine whether the patients presented for cognitive impairment, either as the primary complaint or as a secondary complaint. We only selected patients in which the cognitive impairment occurred after the gastric bypass. Twenty two cases were found to have cognitive complaints after gastric bypass surgery. None of these 22 patients had any cognitive complaint prior to surgery. Clinical data abstracted for all patients included age at onset of cognitive complaint, age at surgery, type of surgery, age at onset of first neurological sign/symptom, gender, first and final clinical diagnosis, physical/neurological findings on examination, nutritional deficiency testing, and any treatment received.
Of these 22 patients with cognitive complaints, 10 had completed a volumetric head MRI scan using a standardized protocol appropriate for analysis. The other 12 patients either had a non-volumetric head MRI scan or a head CT scan that could not be used in the analysis and hence were not included in the main analysis of study. There were no significant differences in demographics or clinical features between the 10 patients that had a volumetric head MRI scan and the 12 patients without a volumetric head MRI (Table 1). The 10 gastric bypass patients were matched by age and gender to a cohort of 10 healthy control subjects. As a secondary analysis, in order to account for any potential confounding effects of obesity, we also matched the patients to a different group of 10 healthy control subjects matched as close as possible to the BMI measured before surgery. Unfortunately, it was not possible to also match this group by age to the gastric patients. All controls were recruited from the Mayo Clinic Alzheimer’s Disease Research Center. None of the control subjects had any cognitive complaints or had undergone gastric bypass surgery, and all performed within normal limits on the Short Test of Mental Status (STMS),(10) a test of cognitive severity. Subject demographics are shown in (Table 1). All patients consented to the use of their clinical records for the purpose of research and the study was approved by the Mayo Clinic IRB.
Table 1.
Subject demographics
| Age and gender matched controls | BMI matched controls | Patients with volumetric MRI | † Patients without volumetric MRI | |
|---|---|---|---|---|
| N | 10 | 10 | 10 | 12 |
| % Female | 80% | 80% | 80% | 67% |
| Age at bypass (yrs) | NA | NA | 54 (46–64) | 54 (28–74) |
| Age at Cognitive Complaints (yrs) | NA | NA | 57 (52–69) | 58 (33–75) |
| Age at MRI scan (yrs) | 59 (53–69)** | 73 (58–84) | 59 (53–69)** | N/A |
| Time from first cognitive symptom to MRI (yrs) | NA | NA | 0.7 (0–7) | N/A |
| Time from gastric bypass to MRI (yrs) | NA | NA | 4.9 (2–11) | N/A |
| STMS at the time of MRI | 38 (33–38) | 35 (35–38) | 34 (29–36)* | 35 (32–38)*χ |
| BMI before surgery (or at time of MRI for controls) | NA | 38 (32–50) | 44 (38–63) | 46 (36–79) |
Data are shown as median (range).
STMS= Short Test of Mental Status; BMI = Body Mass Index; NA = not applicable
These patients were excluded from the study because they did not have a volumetric MRI scan;
= time at evaluation
P<0.05 when compared to age and gender-matched controls;
p<0.05 when compared to BMI-matched controls
Formal Neuropsychological testing
Patients underwent formal neuropsychometric testing that included tests of executive function (Trail-Making Test A and B and Stroop), language function (Boston Naming Test and Category Fluency), memory (Auditory Verbal Learning Test) and visuospatial function (Rey-Osterrieth complex figure).(11) The raw scores from these neuropsychological tests were converted to MOANS values(12) that are age-adjusted to norms derived from Olmsted County population, and transformed to a standardized score with a mean of 10 and a standard deviation (SD) of 3. A MOANS score of less than 1.5 standard deviations (<5) is typically considered evidence of mild impairment.
MRI analysis
All patients had a T1-weighted volumetric MRI performed with a standardized protocol(13) and hence suitable for image analysis. All images underwent pre-processing correction for gradient non linearity(14) and intensity non-uniformity(15) as previously described(16.) Patterns of atrophy at the voxel level were assessed using the technique of VBM(17) and SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Images were normalized and segmented into grey matter, white matter and CSF using customized tissue probability maps created from all subjects in the study and the unified segmentation(18) routine followed by the Hidden Markov Random Field clean-up step. Grey matter images were modulated and smoothed with a Gaussian kernel of 8mm full-width at half maximum. Two-sided t-tests were used to compare grey matter volume between the gastric bypass patients and age and gender-matched controls, both looking for regions with smaller grey matter volume in the gastric bypass patients compared to controls, and for regions that showed smaller grey matter volume in controls compared to the gastric bypass patients. Age and gender were included in the models as covariates and the analyses were assessed corrected for multiple comparisons at the cluster level using the family wise error (FWE) correction at p<0.05.
An atlas-based parcellation technique was also employed using SPM5 and the automated anatomic labeling (AAL) atlas (19) in order to generate grey matter volumes of specific regions-of-interest for each subject. Based on the results of the VBM analysis, the thalamus was the primary region-of-interest. However, in order to help rule out the possibility that the cognitive impairment in the gastric bypass patients was associated with Alzheimer’s disease or frontotemporal dementia, which are the most common causes of organic cognitive impairment in this age group, we also assessed volumes of the hippocampus and the frontal and lateral temporal lobes; regions which are typically associated with these diseases, retrospectively. The processing steps have been described in detail previously(20). Briefly, the AAL atlas was normalized to a customized template and edited. Each subject MRI scan was spatially normalized to the custom template, and then for each subject, the inverse transformation was applied to the atlas in order to warp the atlas to the subject’s native anatomical space. All scans were segmented, and the native-space atlas was used to calculate grey matter volume for each ROI. All grey matter volumes were divided by total intracranial volume (TIV) to correct for differences in head size. Total intracranial volume was calculated by propagating a template-drawn TIV mask to the subject space and then performing an erosion step to remove border voxels.
Statistics
Statistical analyses were performed utilizing the JMP computer software (JMP Software, version 8.0; SAS Institute Inc, Cary, NC) with α set at 0.05. Regional volumes obtained from the anatomic labeling atlas were compared across the gastric bypass patients and controls using the non-parametric Mann-Whitney U test. Correlations between weights, weight loss and regional volumes were performed using Spearman rank correlations.
RESULTS
All 10 patients had undergone either open or laparoscopic Roux-en-Y gastric bypass at the Mayo Clinic, Rochester, MN. All patients were considered obese with a maximum medium weight during adulthood of 123kg (range: 101–172kg); BMI 44 (range: 38–64). The median age at the time of surgery was 54 years (range: 46–64 years). At the time of presentation all patients were taking multi-vitamin supplementation and nine of the 10 were receiving vitamin B12 injections. The cognitive complaints were relatively non-specific but generally involved the domains of memory and executive function (Table 2). The cognitive complaints began typically around in the 5th–6th decade, with a median interval of 4 years after gastric bypass surgery. The median weight at the time of surgery was 110kg (range: 94–172kg) and the median weight loss from time of surgery to the time of onset of the cognitive complaint was 53kg (range: 33–93kg). Four patients had diabetes mellitus type 2 prior to surgery. Three patients had had their postoperative course complicated by a small bowel obstruction, one patient had a stricture and one had post-operative diarrhea.
Table 2.
Cognitive complaints described by spouses of the 10 gastric bypass patients
| “The patient tells the same story several times a day. She also mixes up what her kids have told her.” |
| “She is slow to remember how to do something. She has had difficulty remembering, for example, how to “set up a tray.” Her supervisor had concerns that she is exhibiting multiple episodes of forgetfulness.” |
| “The patient has had trouble with names of acquaintances and grandchildren. He forgets casual conversations.” |
| “She seems to be slower at remembering to do things and to follow through on tasks. She has trouble multitasking.” |
| “She forgets things easily. For example, where she parks her car, what she went to the grocery story to purchase and her driving destination. Her co-workers have had to redirect her quite a bit, and she sometimes gets upset about that. She sometimes babbles and is somewhat tangentional.” |
| “She could not remember the names of her medications when filling out her medication list. She listens to conversations and then 15–20 minutes later, she is unable to recall them. She has trouble remembering protocols she has used at work for a long time.” |
| “He has problems coming up with words and names.” |
| “She has difficulty finding the right word. For example, she wanted to ask for a pen and said “spoon” instead. She has memory difficulties as well and forgets where she placed objects such as her jewelry. She forgets her computer password and occasionally has forgotten to take her medications. She uses a pill box, but still forgets.” |
| “Her family is frustrated by the fact that she may ask a question two or three times. She will start a task and then leave it and then start something else and then go back to the original one. She says that she cannot read and comprehend what she is reading without at least a couple of passes. She has some difficulty with spelling words.” |
| “She has difficulty remembering the content of conversations that she has during a specific day, things that occur in recent days, as well as appointments which she needs to write down.” |
Bedside tests of cognitive severity revealed evidence for minor cognitive impairment with median STMS score of 34 (range: 29–36 out of 38). In no subject was the STMS in the range consistent with moderate cognitive impairment and certainly not in the range consistent with dementia. Neurological examination was normal in all subjects. Routine blood studies at the time of cognitive impairment evaluations were all normal including erythrocyte sedimentation rate, complete blood count, electrolytes including calcium, phosphorus and magnesium, tests of thyroid, parathyroid, liver and renal function, alkaline phosphatase, iron studies, ferritin, vitamin-A, vitamin B-12, Vitamin-E, 25-hydroxy vitamin D2, D3 and total levels. Additional laboratory studies, not completed in all patients, included serum protein electrophoresis (9 patients), folate (7 patients), homocysteine (5 patients), amylase and lipase (4 patients), paraneoplastic antibodies (3 patients), and vitamin B1 (thiamine) and CSF study (protein, glucose, cell count and oligoclonal bands) in one patient each, were all normal or negative. Visual assessment of T2, fluid attenuated inversion recovery (FLAIR) and diffusion-weighted sequences on head MRI did not reveal any significant abnormalities.
Neuropsychology results
Neuropsychological test scores were average-normal in all cognitive domains with the median MOANS scores ranging from 7–12 for all domains. Only one patient had a MOANS score below 5 on executive tests. On tests of executive function the median MOAN scores for Trails A = 7 (range: 5–13), Trails B = 7 (range: 2–12) and Stroop = 10 (range: 2–13). Performance on tests of memory and visuospatial function were slightly better with median MOANS score of 8 in both domains (range: 5–12 for memory and 7–12 for visuospatial). The patients scored highest in tests of language function, with median MOANS scores of 12 (range: 8–16) on the Boston Naming test and 10 (range: 7–17) on the Category fluency tests.
MRI analysis results
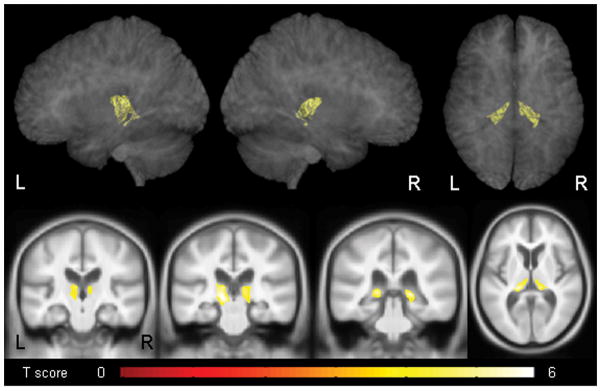
The VBM analysis showed that the gastric bypass patients had volume loss in the bilateral thalamus compared to age and gender-matched controls (Figure 1). The loss was predominantly located in posterior medial regions of the thalamus. In the reverse comparison, no regions showed reduced grey matter volume in the control subjects compared to the gastric bypass patients.
Figure 1.
Regions that showed significant grey matter volume loss in the gastric bypass patients compared to controls. Results are corrected for multiple comparisons at the cluster level using the family wise error (FWE) correction at p<0.05, and shown both on transparent renders of the brain and representative coronal and axial slices.
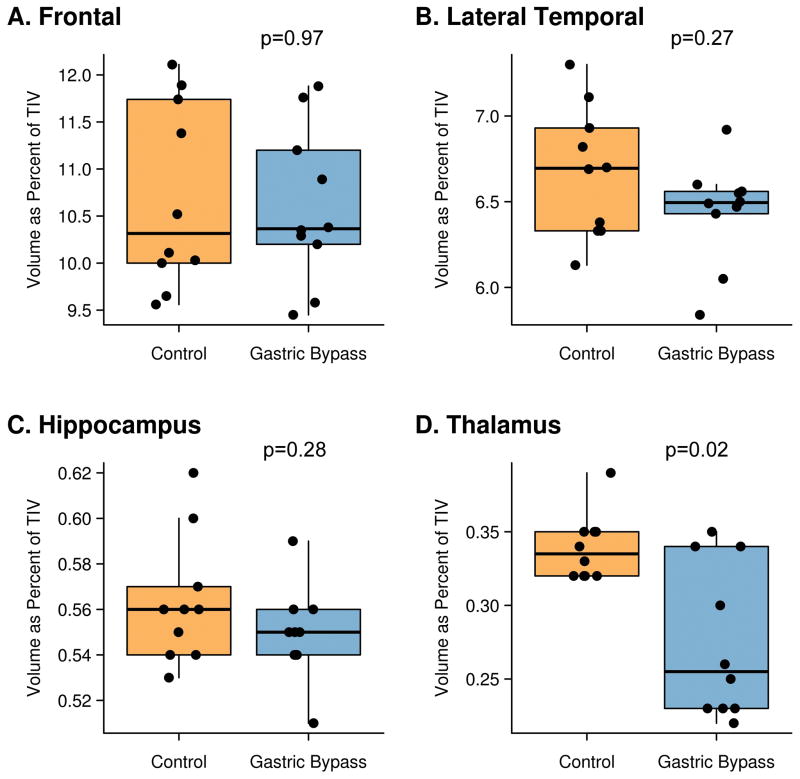
Volumes of the thalamus, as well as the hippocampus, frontal lobe and lateral temporal lobe in each subject are shown in Figure 2. The gastric bypass patients had significantly smaller thalamic volumes compared to age and gender-matched controls (p=0.003). In fact, seven of the gastric bypass patients had thalamic volumes smaller than the smallest control subject, with only three gastric patient volumes overlapping with the control range. No significant differences were observed between the gastric bypass patients and controls in the hippocampus, frontal lobe or lateral temporal lobe. The gastric bypass patients also had significantly smaller thalamic volumes compared to the BMI-matched control subjects (p=0.013), with no significant difference observed in the hippocampus, frontal lobe or lateral temporal lobe. There was no correlation between thalamic volumes and maximum weight (r=−0.4; p=0.33), weight at the time of surgery (r= 0.0; p= 0.96), BMI before surgery (r=−0.1; p=0.87), or between thalamic volumes and weight loss from the time of surgery to the time of cognitive complaint (r=−0.1, p= 0.87).
Figure 2.
Box-plots showing frontal, lateral temporal, hippocampal and thalamic volumes for the gastric bypass patients and controls. The boxes indicate the median and interquartile range (IQR) of the distributions while the horizontal lines extending from the boxes stop at the most extreme data points within 1.5 IQRs. All individual points are shown and points have been shifted randomly in the horizontal direction to avoid overlap. TIV = total intracranial volume, NS = not significant
COMMENT
This study suggests that subjective cognitive impairment developing after Roux-en-Y gastric bypass surgery may have a uniform and unique neuroanatomic substrate: bilateral posterior thalamic atrophy. Remarkably, there was little overlap in thalamic volumes between the normal and gastric bypass patients, and the thalamus was the only region identified in the unbiased VBM analysis. This finding suggests an organic cause for the cognitive impairment in these patients and importantly suggests that these findings may be occurring as a result of these patients undergoing this type of surgery.
The thalamus is a subcortical structure and a relay nucleus for both motor and sensory systems. It receives input from cortical and other subcortical nuclei and projects to all areas of cortex. The thalamus has been previously associated with cognitive impairment in different neurological diseases. In patients with multiple sclerosis, cognitive performance in all domains has been shown to be related to thalamic volume(21), and in patients with unilateral temporal lobe epilepsy, thalamic volumes have been shown to correlate with performance on both memory and non-memory cognitive domains.(22) Focal thalamic lesions from strokes have also directly resulted in cognitive and behavioral impairment(23). The patients in our cohort showed slight objective evidence for cognitive impairment on bedside testing, with some minor deficits on formal neuropsychometric testing identified. This cohort’s poorest neuropsychological performance occurred with tests of executive function. Interestingly, injury to the dorsomedial nucleus has been described as a cause of executive dysfunction.(24) It is therefore plausible that the cognitive complaints of these patients were related to the diminutive thalami.
Cognitive impairment in patients of this age could however be related to a neurodegenerative disease, the most common of which are Alzheimer’s disease and frontotemporal dementia(25). However, the lack of hippocampal, temporal and frontal atrophy in our patients, and the lack of typical neuropsychometric findings for these two diseases, argues against these neurodegenerative etiologies. Furthermore, the unusual isolated thalamic atrophy suggests a unique neuroanatomic signature.
Diseases associated with focal or prominent thalamic pathology are not very common. Wernicke’s encephalopathy secondary to thiamine deficiency is associated with thalamic pathology and our gastric bypass patients are obviously at increased risk for thiamine deficiency(6). However, our cases had neither the typical imaging nor clinical picture of thiamine deficiency. Patients with Wernicke’s encephalopathy typically have characteristic signal changes seen on MRI affecting the thalamus, the periventricular region of the third ventricle, periaqueductal grey area and the mamillary bodies, which were absent in our patients. Classic Wernicke’s is associated with the clinical triad of confusion, ataxia, and ophthalmoplegia, symptoms that were not present in any of our patients. Finally, cases of Wernicke’s encephalopathy post-gastric bypass have typically present with malnutrition, emesis, and mechanical obstruction(6), usually 2 weeks to 18 months after surgery; this was not the clinical picture among our patients. Unfortunately, only one patient had thiamine testing suggesting that Wernicke’s encephalopathy was not considered a likely diagnosis at the time of evaluation of these patients. In this one patient, thiamine level was normal.
Gastric bypass is also notoriously associated with vitamin B12 deficiency; 30% of patients were deficient in one series, despite adequate intake and vitamin B12 supplementation(26). However, vitamin B-12 levels were normal in all patients and all but one was receiving vitamin B-12 injections at the time of presentation. Furthermore, the typical clinical picture of peripheral neuropathy, dorsal column and corticospinal tract symptoms/signs and ataxia were absent.
A selective nutritional deficiency would seem the most likely substrate for the thalamic atrophy documented in our patients. However, the combination of mild cognitive impairment and selective thalamic atrophy fails to bring to mind any specific nutritional deficiency. Furthermore, laboratory studies on vitamins A, D and E were normal and additional testing, although not completed in all patients, did not reveal any abnormalities either.
Non-nutritional features do not seem to explain these findings. The gastric bypass patients showed greater thalamic atrophy than a group of controls matched for BMI measured before surgery, suggesting that thalamic atrophy is not a general feature of obesity. This finding was observed despite the fact that the BMI-matched controls were older in age and hence more likely to have age related atrophy. Previous VBM studies have similarly not found any evidence that obesity is associated with thalamic volume loss.(27, 28) It is conceivable that thalamic atrophy could be related to the precipitous weight loss from bariatric surgery, but we found no correlation between weight loss and thalamic volumes. A direct post-operative complication seems unlikely given the median interval of four years from surgery to the onset of cognitive impairment. Thus, this slow development raises the question of a heretofore unrecognized nutritional deficiency.
While our series is unique, our study has several strengths and limitations that should be acknowledged. One of the main strengths of the study is the application of the unbiased technique of VBM to assess for atrophy. This technique is performed without any a priori assumptions and assesses for atrophy over the entire brain, at the voxel level. Secondly, all patients in this study were evaluated by a board certified neurologist and had completed comprehensive clinical, neuropsychological, laboratory and head imaging studies. Limitations of the study included the retrospective design, and the lack of cognitive longitudinal evaluations due to the clinical uncertainty of a bona fide neurologic problem at the time of the initial evaluations. As a result of the latter, more extensive testing, including for example, assessment of trace nutrients, was not done. In addition, it would be ideal to compare these subjects to post-operative bypass patients without a complaint of cognitive impairment but the retrospective nature of this study and lack of MRI in gastric bypass patients without cognitive complaints were limiting.
These findings suggest that a small percentage of obese patients undergoing Roux-en-Y gastric bypass develop a mild cognitive disorder after several years. This is an important observation, since the individual evaluations at the time did not generate sufficient objective findings to convince the clinicians that a definite organic neurologic disorder was present. However with the uniform discovery of this selective thalamic atrophy, subsequent patients should now be closely scrutinized, especially for isolated deficiencies of nutritional factors. Also unresolved is whether there are any additional problems that surface in these patients, long-term.
Acknowledgments
Dr. Whitwell receives research support from NIH grants R21-AG38736 (PI), R01-DC010367 (Co-I), and R01-AG037491 (Co-I), and the Dana Foundation (Co-I).
Dr. Ahlskog receivedthe Fred Springer Award from the American Parkinson’s Disease Association; receives royalties from publishing The Parkinson’sDisease Treatment Book (Oxford University Press, 2005) and Parkinson’sDisease Treatment Guide for Physicians (Oxford University Press,2009), Parkinson’s Disease and Movement Disorders (HumanaPress, 2000), and Surgical Treatment of Parkinson’s Diseaseand Other Movement Disorders (Humana Press, 2003); has receivedhonoraria for lectures or educational activities not fundedby industry; and receives research support from NIH/NINDS [P50NS 40256-R (Co-I)].
Dr. Jack servesas a consultant for Elan Corporation; and receives researchsupport from Pfizer, Inc., the NIA [R01-AG11378 (PI), P50-AG16574(Co-I), R21-AG38736 (Co-I), and U01 AG024904-01 (Co-I)], and the Alexander Family Alzheimer’s Disease Research Professorshipof the Mayo Foundation.
Dr. Josephs is funded by NIH R01-DC010367 (PI), R01-AG037491 (PI), R21-AG38736 (Co-I), and the Dana Foundation (PI).
We would like to acknowledge all internists, endocrinologists, family medicine doctors, diabetic specialists, general surgeons and neurologists that helped with the care of these patients. We acknowledge the Mayo Clinic Alzheimer’s Disease Research Center for use of the control subjects
References
- 1.Vasan RS, Pencina MJ, Cobain M, Freiberg MS, D’Agostino RB. Estimated risks for developing obesity in the Framingham Heart Study. Ann Intern Med. 2005 Oct 4;143(7):473–80. doi: 10.7326/0003-4819-143-7-200510040-00005. [DOI] [PubMed] [Google Scholar]
- 2.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009 Mar 28;373(9669):1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004 Mar 11;350(11):1075–9. doi: 10.1056/NEJMp048029. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009 Dec;19(12):1605–11. doi: 10.1007/s11695-009-0014-5. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 5.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007 Aug 23;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 6.Koffman BM, Greenfield LJ, Ali II, Pirzada NA. Neurologic complications after surgery for obesity. Muscle Nerve. 2006 Feb;33(2):166–76. doi: 10.1002/mus.20394. [DOI] [PubMed] [Google Scholar]
- 7.Juhasz-Pocsine K, Rudnicki SA, Archer RL, Harik SI. Neurologic complications of gastric bypass surgery for morbid obesity. Neurology. 2007 May 22;68(21):1843–50. doi: 10.1212/01.wnl.0000262768.40174.33. [DOI] [PubMed] [Google Scholar]
- 8.Abarbanel JM, Berginer VM, Osimani A, Solomon H, Charuzi I. Neurologic complications after gastric restriction surgery for morbid obesity. Neurology. 1987 Feb;37(2):196–200. doi: 10.1212/wnl.37.2.196. [DOI] [PubMed] [Google Scholar]
- 9.Thaisetthawatkul P, Collazo-Clavell ML, Sarr MG, Norell JE, Dyck PJ. A controlled study of peripheral neuropathy after bariatric surgery. Neurology. 2004 Oct 26;63(8):1462–70. doi: 10.1212/01.wnl.0000142038.43946.06. [DOI] [PubMed] [Google Scholar]
- 10.Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc. 1987 Apr;62(4):281–8. doi: 10.1016/s0025-6196(12)61905-3. [DOI] [PubMed] [Google Scholar]
- 11.Lezak M, editor. Neuropsychological assessment. 4. Oxford, New York: Oxford University Press; 2004. [Google Scholar]
- 12.Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Graff-Radford NR, et al. Mayo’s older Americans normative studies: category fluency norms. J Clin Exp Neuropsychol. 1998 Apr;20(2):194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- 13.Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008 Mar;131(Pt 3):665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006 Apr 1;30(2):436–43. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998 Feb;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 16.Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008 Apr;27(4):685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000 Jun;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 18.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005 Jul 1;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002 Jan;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 20.Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009 Nov;132(Pt 11):2932–46. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houtchens MK, Benedict RH, Killiany R, Sharma J, Jaisani Z, Singh B, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007 Sep 18;69(12):1213–23. doi: 10.1212/01.wnl.0000276992.17011.b5. [DOI] [PubMed] [Google Scholar]
- 22.Seidenberg M, Hermann B, Pulsipher D, Morton J, Parrish J, Geary E, et al. Thalamic atrophy and cognition in unilateral temporal lobe epilepsy. Journal of the International Neuropsychological Society. 2008 May;14(3):384–93. doi: 10.1017/S1355617708080399. [DOI] [PubMed] [Google Scholar]
- 23.Graff-Radford NR, Eslinger PJ, Damasio AR, Yamada T. Nonhemorrhagic infarction of the thalamus: behavioral, anatomic, and physiologic correlates. Neurology. 1984 Jan;34(1):14–23. doi: 10.1212/wnl.34.1.14. [DOI] [PubMed] [Google Scholar]
- 24.Sandson TA, Daffner KR, Carvalho PA, Mesulam MM. Frontal lobe dysfunction following infarction of the left-sided medial thalamus. Arch Neurol. 1991 Dec;48(12):1300–3. doi: 10.1001/archneur.1991.00530240106031. [DOI] [PubMed] [Google Scholar]
- 25.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002 Jun 11;58(11):1615–21. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 26.Provenzale D, Reinhold RB, Golner B, Irwin V, Dallal GE, Papathanasopoulos N, et al. Evidence for diminished B12 absorption after gastric bypass: oral supplementation does not prevent low plasma B12 levels in bypass patients. J Am Coll Nutr. 1992 Feb;11(1):29–35. doi: 10.1080/07315724.1992.10718193. [DOI] [PubMed] [Google Scholar]
- 27.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006 Jul 15;31(4):1419–25. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 28.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010 Jul;31(7):1052–64. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]