Abstract
Mitochondrial complex I has previously been shown to release superoxide exclusively towards the mitochondrial matrix, whereas complex III releases superoxide to both the matrix and the cytosol. Superoxide produced at Complex III has been shown to exit the mitochondria through voltage dependent anion channels (VDAC). To test whether complex I-derived, mitochondrial matrix-directed superoxide can be released to the cytosol, we measured superoxide generation in mitochondria isolated from wild type and from mice genetically altered to be deficient in MnSOD activity (TnIFastCreSod2fl/fl). Under experimental conditions that produce superoxide primarily by complex I (glutamate/malate plus rotenone, GM+R), MnSOD-deficient mitochondria release ~4-fold more superoxide than mitochondria isolated from wild type mice. Exogenous CuZnSOD completely abolished the EPR-derived GM+R signal in mitochondria isolated from both genotypes, evidence that confirms mitochondrial superoxide release. Addition of the VDAC inhibitor DIDS significantly reduced mitochondrial superoxide release (~75%) in mitochondria from either genotype respiring on GM+R. Conversely, inhibition of potential inner membrane sites of superoxide exit, including the matrix face of the mitochondrial permeability transition pore and the inner membrane anion channel did not reduce mitochondrial superoxide release in the presence of GM+R in mitochondria isolated from either genotype. These data support the concept that complex I-derived mitochondrial superoxide release does indeed occur and that the majority of this release occurs through VDACs.
Keywords: mitochondria, superoxide, voltage dependent anion channels
1. Introduction
Electron transport chain respiratory complex I and complex III have been proposed to be the main sites of mitochondrial superoxide production [1]. At complex I, superoxide radicals have been shown to be directed exclusively toward the mitochondrial matrix [2,3], while at complex III superoxide is released to both the matrix and the intermembrane space (IMS) [1,3,4]. Because superoxide is negatively charged it is regarded to be essentially membrane impermeable and unlikely to cross the inner mitochondrial membrane into the IMS. Thus, superoxide in the IMS is thought to be exclusively derived from complex III. Furthermore, superoxide released towards the matrix is rapidly converted to hydrogen peroxide by MnSOD, and in the IMS by CuZnSOD [5,6,7]. Together these barriers make it unlikely that matrix-derived superoxide could escape these scavenging enzymes and exit from the mitochondria.
In support of this, we previously showed that CuZnSOD added to buffer containing mitochondria and GM+R does not increase the rate of mitochondrial H2O2 production [3]. This suggests that rotenone-inhibited complex I does not directly release superoxide towards the cytosol, as superoxide released from the mitochondria would be scavenged by the exogenously added CuZnSOD and converted to H2O2. In contrast, mitochondrial H2O2 production detected outside the mitochondria is 3-fold or more elevated in response to GM+R when compared to GM alone, suggesting that the elevated complex I-derived superoxide generated in response to rotenone is converted in the matrix by MnSOD to H2O2, which diffuses readily and is detected outside the mitochondria [3]. However, in situations where MnSOD is limited, the potential release of Complex I-derived superoxide from the mitochondria has not been investigated.
Superoxide produced by the Qo site of complex III has previously been shown to be released toward the cytosol through voltage dependent anion channels (VDAC) [8]. VDAC is localized at contact sites [9] that connect the outer and inner mitochondrial membranes [10] and, is strongly bound to the mitochondrial inner membrane [11]. VDAC functions as the major channel allowing passage of low molecular weight solutes and proteins between the intermembrane space and cytoplasm, and is more selective for the passage of anions (superoxide is negatively charged) than cations [12,13]. In addition, the combination of VDAC, cyclophilin D and the adenine nucleotide translocase and have been proposed to form the mitochondrial permeability transition pore (mPTP) [14] that spans across both the mitochondrial outer and inner mitochondrial membranes. Because VDAC is a component of the mPTP, the mPTP is a potential route for exit of superoxide from the mitochondrial matrix. Superoxide could also escape from the mitochondrial matrix through the peripheral benzodiazepine receptor (PBR) that is localized at contact junctions between the inner and outer mitochondrial membranes [15]. The PBR has been shown to copurify with VDAC [16]; furthermore, a functional interaction between the PBR and the mitochondrial inner membrane anion channel (IMAC) has been suggested [17]. In the current study, we asked whether complex I-derived mitochondrial matrix-directed superoxide is released toward the cytosol and, whether this release occurs through the VDACs, the mPTP, the PBR or IMAC.
2. Materials and Methods
2.1 Isolation of skeletal muscle mitochondria
Young adult (less than 15 months) TnIFastCreSod2fl/fl and wild-type (Sod2fl/fl) mice were used for all experiments. All procedures were approved by the IACUC at the University of Texas Health Science Center San Antonio and the Division of Research at Audie L. Murphy VA Hospital. Mitochondria from combined white gastrocnemius and quadricep muscles (with red portions of these muscles removed) were isolated from TnIFastCreSod2fl/fl and wild-type mice based on the method of King et al. [18] and as described by Lustgarten et al. [19]. MnSOD activity has previously been shown to be reduced by greater than 80% in mitochondrial preparations isolated from TnIFastCreSod2fl/fl, relative to mitochondria isolated from wild type mice [19].
2.2 Measurement of mitochondrial superoxide release
The direct measurement of mitochondrial superoxide release was obtained via electron paramagnetic resonance (EPR) with use of the spin trap, 5-diisopropoxyphosphoryl-5-methyl-1-pyrroline-N-oxide, (DIPPMPO). EPR measurements were performed using an X-band MS200 spectrometer (Magnetech, Berlin, Germany), following the methodology previously described by Lustgarten et al. [19]. Glutamate plus malate, (GM, 24 mM) was used to drive respiration starting at complex I; succinate in the presence of rotenone (succinate, 24mM; rotenone, 2.4 µM, SR) was used to drive respiration starting at complex II. The electron transport chain inhibitors stigmatellin (1.8 µM) and diphenyleneiodonium (DPI, 25 µM) were added as indicated. DIDS (final concentration, 1mM) cyclosporine A (100 µM), PK11195 (45 µM), 5’ chlorodiazepam (45 µM) and CuZnSOD (1 U/µL) were added as indicated. EPR data are expressed as RIU (Relative Intensity Units)/20 µg mitochondrial protein.
2.3 Statistics
Results were analyzed by one-way ANOVA with Newman Keul’s post-hoc test and expressed as mean ± SEM. Significance was determined as p<0.05.
3. RESULTS
3.1 Complex I-derived, mitochondrial matrix-directed superoxide is released towards the cytosol by both wild type and MnSOD-deficient mitochondria
Mitochondria isolated from skeletal muscle in wild type mice release superoxide that can be detected by EPR when respiring on the complex I substrate, GM (Figure 1A, left). To test whether superoxide generated at Complex I can be released from the mitochondria, we added the complex I-specific inhibitor rotenone (R) [1] to wild-type mitochondria that respired on GM. The EPR-detectable signal obtained from wild-type mitochondria utilizing GM+R was not higher than the EPR signal found in the presence of GM without rotenone (Figure 1B, left compared with Figure 1A, left). These data suggest that the presence of MnSOD in wild type mitochondria is sufficient to scavenge the increase in superoxide production when complex I is inhibited by rotenone.
Figure 1.
Complex I-derived, mitochondrial matrix-directed superoxide is released towards the cytosol by both wild type and MnSOD-deficient mitochondria. (A) Representative EPR spectra (n = 5) indicating mitochondrial superoxide release towards the cytosol when wild type (Left) and MnSOD-deficient mitochondria (TNI, Right) mice utilize GM. (B) Mitochondrial superoxide release with GM and the complex I specific inhibitor, rotenone (GM+R, n = 7). (C) Mitochondrial superoxide release with GM+R and CuZnSOD (n = 4). (D) Mitochondrial superoxide release with GM+R and an inhibitor specific for the flavin binding site of complex I, DPI (n = 4). E) Mitochondrial superoxide release with GM+R and stigmatellin (n = 4). (F) Quantification of mitochondrial superoxide release using the various substrate plus inhibitor combinations shown in Figure 1A–1E. Values shown represent the EPR intensity per 20 µg of mitochondrial protein, and are means ± SEM. aSignificant difference when compared with WT, for any given substrate plus inhibitor combination; bSignificant difference compared with TNI GM; cSignificant difference compared with TNI GM+R.
We have previously shown that MnSOD-deficient mitochondria (TnIFastCreSod2fl/fl) have decreased aconitase activity (suggesting increased matrix superoxide) and release greater than 2-fold superoxide when respiring with either complex I or complex II-linked substrate, compared to values measured in wild type [19]. In the current study, we asked whether MnSOD-deficient mitochondria would release a greater amount of complex I-derived superoxide towards the cytosol than mitochondria containing wild type levels of MnSOD. MnSOD-deficient mitochondria respiring on GM+R release approximately 4.4-fold more superoxide than wild type mitochondria that utilized GM+R (Figure 1B). In addition, superoxide release in the presence of GM+R by MnSOD-deficient mitochondria was significantly elevated by 78%, relative to the value obtained with GM (Figure 1B, right, compared with Figure 1A, right).
To confirm the superoxide-specificity of the EPR-detectable signal, we added the membrane impermeant CuZnSOD to mitochondria that respired in the presence of GM+R and found complete abolition of the EPR-derived signal in mitochondria isolated from both genotypes (Figure 1C). These data provide evidence that confirms complex I-derived, mitochondrial matrix directed superoxide is released from the mitochondria towards the cytosol.
To address the potential contribution of the flavin binding site within complex I to the EPR-detectable signal found in the presence of GM+R, we added a flavin-specific inhibitor, DPI [20,21]. The EPR-detectable signal in wild-type mitochondria is reduced by 19% in the presence of GM+R+DPI when compared with GM+R; however, this value is not statistically significant (Figure 1D, left compared with Figure 1B, left). In contrast, addition of DPI to MnSOD-deficient mitochondria that utilized GM+R significantly reduced superoxide release by 24%, compared to the value obtained with GM+R (Figure 1D, right compared with Figure 1B, right). In addition, MnSOD-deficient mitochondria release 4.2-fold more superoxide than wild type mitochondria in the presence of GM+R+DPI (Figure 1D). Furthermore, we find that the combination of GM+R plus stigmatellin in either wild type or MnSOD-deficient mitochondria did not produce an amount of superoxide that was significantly different from the value obtained in the absence of stigmatellin (Figure 1E compared with Figure 1B), suggesting that stigmatellin does not inhibit superoxide production derived from rotenone-inhibited complex I, in contrast to that previously reported [22].
Collectively we find that under a variety of experimental conditions (Figure 1F), mitochondria isolated from wild-type mice release complex I-produced, mitochondria matrix-directed superoxide towards the cytosol and, this effect is greater in mitochondria deficient in MnSOD.
3.2 Complex I-derived superoxide is released towards the cytosol under conditions previously thought to produce superoxide exclusively by complex III
Superoxide production by mitochondria in the presence of SR is thought to occur exclusively at complex III. However, during the enzymatic conversion of succinate to fumarate, fumarate to malate, and malate to oxaloacetate, NADH is produced when malate is converted to oxaloacetate, via malate dehydrogenase. It is possible that oxidation of this NADH would increase production of superoxide at Complex I in the presence of SR. To test the hypothesis that complex I produces and releases superoxide towards the cytosol when mitochondria utilize SR, we added DPI. Addition of DPI to wild-type mitochondria that utilized SR reduced mitochondrial superoxide release by 17%, but this value was not statistically significant (Figure 2B, left compared with Figure 2A, left). In contrast, addition of DPI to MnSOD-deficient mitochondria that utilized SR significantly reduced mitochondrial superoxide release by 32% when compared with the value obtained with SR (Figure 2B, right compared with Figure 2A, right). These data indicate that this amount of superoxide was formed at the flavin binding site of complex I in the presence of SR, and confirms the ability of complex I to produce and release superoxide towards the cytosol under experimental conditions previously thought to drive electron flow exclusively through complex II, III and IV. Relative quantification of superoxide release in the presence of SR or SR+DPI is shown for mitochondria isolated from both genotypes in Figure 2C.
Figure 2.
Complex I-derived superoxide is released towards the cytosol under conditions previously thought to produce superoxide exclusively by complex III. (A) Representative EPR spectra are shown when wild type (Left) and MnSOD-deficient mitochondria (TNI, Right) utilize SR (n = 7). (B) Mitochondrial superoxide release with SR and DPI (n = 4). (C) Quantification of mitochondrial superoxide release in the presence/absence of DPI (A–B).Values shown represent the EPR intensity per 20 µg of mitochondrial protein, and are means ± SEM. aSignificant difference when compared with WT, for any given substrate plus inhibitor combination; bSignificant difference compared with TNI SR.
3.3 Wild type and MnSOD-deficient mitochondria both release complex I-derived superoxide through VDAC
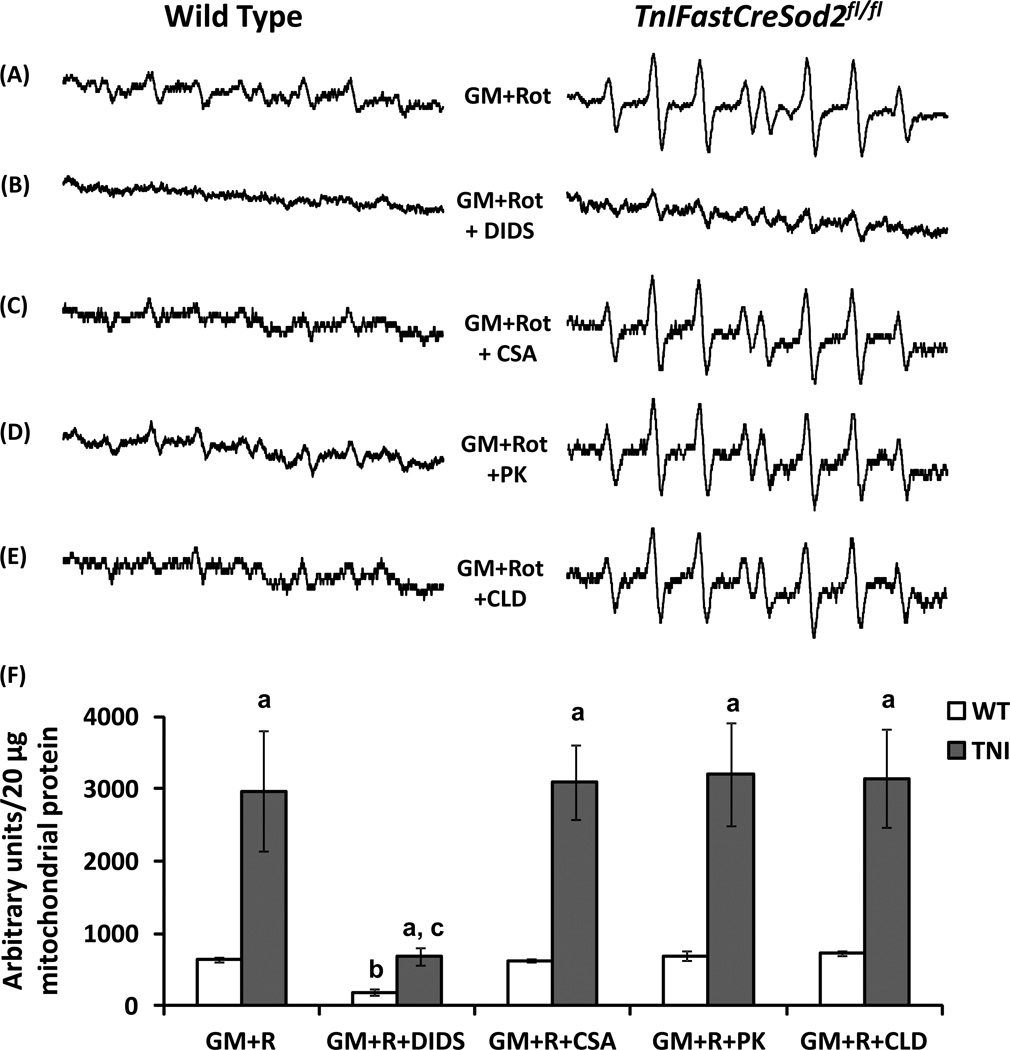
The mitochondrial release of complex III-produced superoxide towards the cytosol has previously been shown by Han et al. [8] to occur through VDAC, which can be inhibited by DIDS [23]. To determine if complex I produced, mitochondrial matrix-directed superoxide exits the mitochondria through VDAC, we added DIDS to mitochondria that utilized GM+R. DIDS addition under these experimental conditions significantly reduced the EPR-derived superoxide signal by 73% and 77% in wild type and MnSOD-deficient mitochondria, respectively (Figure 3B compared with Figure 3A), providing evidence that complex I-produced, mitochondrial matrix-directed superoxide is released through VDAC’s. It is also important to note that superoxide release by MnSOD-deficient mitochondria in the presence of GM+R and DIDS was ~3.7-fold higher than the corresponding value found in wild type mitochondria (Figure 3B), suggesting non-VDAC sources of exit for superoxide when the MnSOD concentration is reduced.
Figure 3.
Wild type and MnSOD-deficient mitochondria both release complex I-derived superoxide through VDAC. (A) Representative EPR spectra are shown when wild type (Left) and MnSOD-deficient mitochondria (TNI, Right) mice utilize GM+R. (B) Mitochondrial superoxide release with GM+R and the VDAC inhibitor DIDS (n = 4). (C) Superoxide release with GM+R and the mPTP inhibitor, CSA (n = 4). (D) Superoxide release with GM+R and the IMAC/PBR inhibitor, PK11195 (PK, n = 4). (E) Superoxide release with GM+R and the IMAC/PBR inhibitor, 4’-chlorodiazepam (CLD, n = 4). (F) Quantification of superoxide release using the various substrate plus inhibitor combinations shown in Figure 3A–3E. Values shown represent the EPR intensity per 20 µg of mitochondrial protein, and are means ± SEM. aSignificant difference when compared with WT, for any substrate plus inhibitor combination; bSignificant difference compared with WT GM+R; cSignificant difference compared with TNI GM+R.
3.4 Complex I-derived, mitochondrial matrix-directed superoxide does not exit the mitochondria through mPTP, PBR, or IMAC
The presence of VDAC as a component of the mPTP allows for the possibility that mitochondrial matrix-localized superoxide exits from the matrix towards the cytosol through this pore. To determine if the mitochondrial release of superoxide occurs through the mPTP, we added cyclosporine A (CSA), an inhibitor that has been shown to be specific for cyclophilin D [24]. No significant difference in superoxide release was found when using GM+R+CSA, relative to the value found with GM+R in either MnSOD-deficient or wild type mitochondria (Figure 3C compared with Figure 3A).
To test the hypothesis that mitochondrial matrix localized superoxide exits the mitochondria by passing through IMAC or PBR, we added either 4’-chlorodiazepam (CLD) or PK11195 in combination with GM+R. PBR and IMAC can be inhibited by CLD [25,26] or PK11195 [16,25]. No significant difference in superoxide release was found in the presence of GM+R and either PK11195 or CLD in mitochondria isolated from either genotype, relative to the corresponding values found in the absence of these inhibitors (Figure 3D and Figure 3E compared with Figure 3A). Relative quantification of superoxide release in the presence of GM+R and DIDS, CSA, CLD or PK11195 is shown for mitochondria isolated from both genotypes in Figure 3F.
3.5 Complex III-linked mitochondrial superoxide release is elevated in MnSOD-deficient mitochondria, but exits the mitochondria through mPTP only in wild type
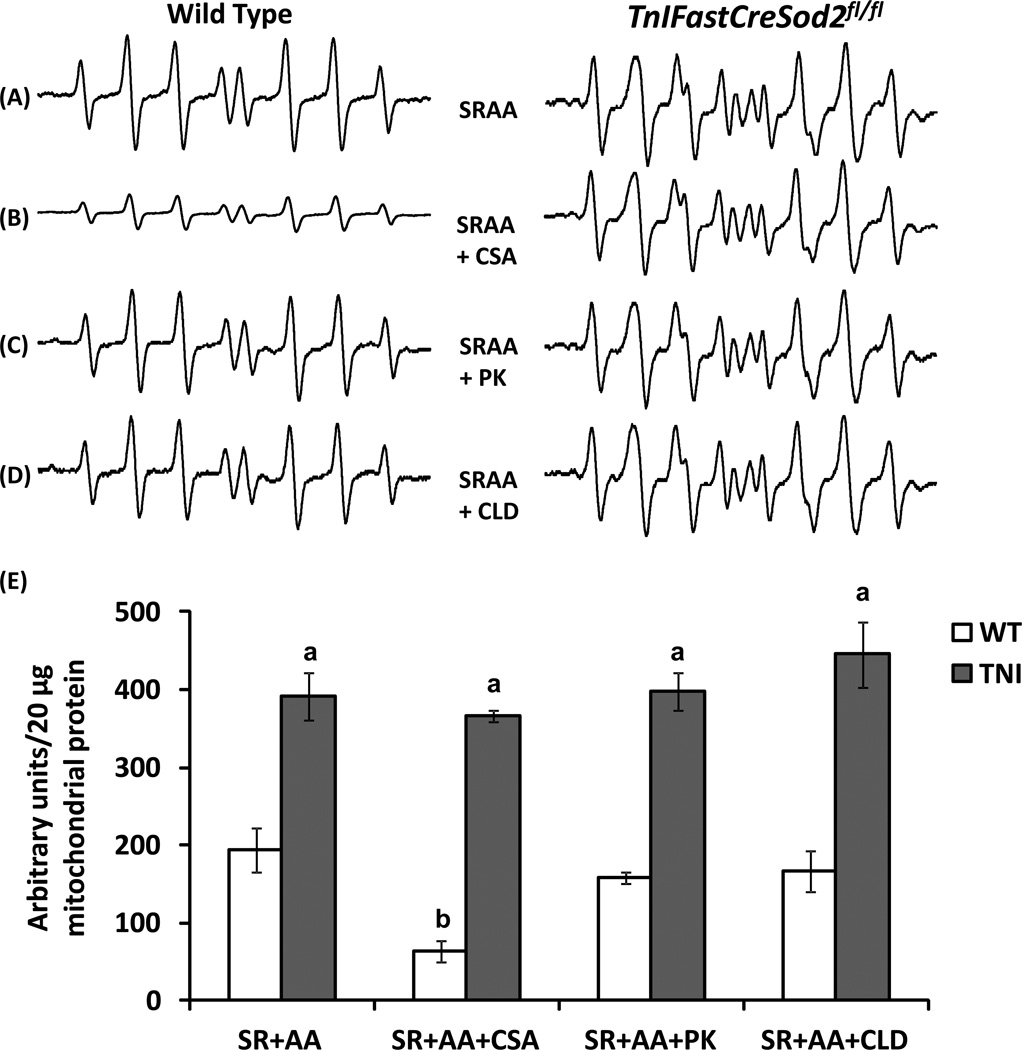
In a previous report we found that the rate of H2O2 production by MnSOD-deficient mitochondria is elevated ~5-fold in the presence of SR plus an inhibitor specific for the Qi site of complex III, antimycin A (SR+AA), when compared with the value found in wild type mitochondria [19]. To determine whether MnSOD-deficient mitochondria release a greater amount of superoxide relative to wild type when respiring with SR+AA, we measured mitochondrial superoxide release. Mitochondrial superoxide release by MnSOD-deficient mitochondria was found to be ~2-fold greater than the corresponding value found in wild type under these experimental conditions (Figure 4A).
Figure 4.
Complex III-derived mitochondrial superoxide release proceeds through mPTP in wild type mitochondria, but not in mitochondria that are MnSOD-deficient.(A) Representative EPR spectra are shown when wild type (Left, n = 9) and MnSOD-deficient mitochondria (TNI, Right, n = 7) mice utilize SR+AA. (B) Superoxide release with SR+AA and CSA (n = 4). (C) Superoxide release with SR+AA and the IMAC/PBR inhibitor, PK11195 (PK, n = 4). (D) Superoxide release with SR+AA and the IMAC/PBR inhibitor, 4’-chlorodiazepam (CLD, n = 4). (E) Quantification of superoxide release using the various substrate plus inhibitor combinations shown in Figure 4A–4D. Values shown represent the EPR intensity per 20 µg of mitochondrial protein, and are means ± SEM. aSignificant difference when compared with WT, for any given substrate plus inhibitor combination; bSignificant difference compared with WT SR+AA.
DIDS has been previously been shown to reduce mitochondrial superoxide release when wild type mitochondria are exposed to antimycin A [8]. However, the role of the mPTP during complex III-mediated superoxide production by isolated mitochondria had yet to be studied. To test the hypothesis that complex III-linked mitochondrial superoxide exits the mitochondria through the mPTP, we added CSA to mitochondria that utilized SR+AA. Superoxide release was significantly decreased by ~68% in wild type mitochondria in the presence of CSA and SR+AA, relative to the value obtained with SR+AA (Figure 4B, left compared with Figure 4A, left). However, addition of CSA to MnSOD-deficient mitochondria that utilized SR+AA did not reduce superoxide release, relative to the value obtained in the absence of CSA (Figure 4B, right compared with Figure 4A, right). Furthermore, addition of inhibitors specific for IMAC and PBR did not reduce superoxide release in the presence of SR+AA in mitochondria isolated from either genotype (Figure 4C and Figure 4D compared with Figure A). Relative quantification of superoxide release in the presence of SR+AA and CSA, CLD or PK11195 is shown for mitochondria isolated from both genotypes in Figure 4E.
4. DISCUSSION
The primary goal of this study was to test the hypothesis that complex I-derived, mitochondrial matrix-directed superoxide could be released from the mitochondria towards the cytosol. Because of the double membrane structure of the mitochondria and the fact that superoxide is negatively charged, it has been generally thought that superoxide directed toward the mitochondrial matrix would not be released from the mitochondria [27]. However, our data show for the first time that both wild-type and MnSOD-deficient mitochondria release matrix directed superoxide towards the cytosol. In addition, we found that mitochondrial superoxide release is increased by more than 4-fold in the presence versus the absence of rotenone in MnSOD-deficient mitochondria respiring on GM. In contrast, mitochondrial superoxide release is not increased in response to rotenone in wild-type mitochondria that respire with GM. This finding suggests that under normal conditions MnSOD in the matrix is sufficient to control the increase in superoxide that is formed as a result of rotenone inhibition of complex I.
Superoxide has previously been shown to cross membranes through anion channels. For example, erythrocyte membranes contain a DIDS sensitive anion channel through which superoxide radicals can diffuse [28]. In mitochondria, Han et al. [8] showed that complex III-derived superoxide was released from the mitochondria towards the cytosol through VDAC. In the present study we show for the first time that complex I produced, mitochondrial matrix-directed superoxide is also released from the mitochondria towards the cytosol through VDAC. VDACs are localized in the outer mitochondrial membrane, and have also been found at contact junctions at the inner mitochondrial membrane. Therefore, we looked for other potential sites of exit for superoxide, but did not find a significant difference in superoxide release in response to rotenone after inhibiting IMAC, PBR or the matrix side (cyclophilin D) of the mPTP in either wild-type or MnSOD-deficient mitochondria. However, we found that the mPTP is indeed an exit route for mitochondria matrix localized superoxide, but only in mitochondria isolated from wild type mice and under experimental conditions that are thought to drive superoxide primarily through complex III (SR+AA).
We were also interested in determining the sites within complex I responsible for forming the superoxide that was released towards the cytosol in the presence of rotenone. We determined that the DPI-inhibitable flavin binding site within complex I contributes approximately 20% of the total superoxide released from either wild type or MnSOD-deficient mitochondria that utilized either GM+R or SR. We also show for the first time that complex I is capable of releasing superoxide under experimental conditions that are thought to exclusively produce superoxide through complex III (i.e., in the presence of SR).
The pathological implications of an increase in superoxide radicals released from the mitochondria remains to be explored. Although this would not be expected to occur under normal conditions, the mitochondrial release of superoxide could become significant under conditions leading to reduced MnSOD or during pathological increases in superoxide generation. Elevated mitochondrial superoxide production has been shown to contribute to nuclear DNA damage, mutagenesis, and ultimately, tumorigenesis [29]. For example, Nakada et al. [30] reported an inverse association between decreased MnSOD activity and the presence of malignant pheochromocytoma, a type of neuroendocrine tumor located in the chromaffin cells of the adrenal medulla The inverse association found between pheochromocytoma and MnSOD activity reported by Nakada et al. [30] suggests that deficits in MnSOD, and potentially, elevated superoxide, may be related to the pathobiology of this disease, and supports a potential role for increased superoxide release from mitochondria in disease pathogenesis.
Highlights.
Complex I-generated, matrix-directed superoxide is released through VDACs.
MnSOD-deficient mitochondria release more superoxide than wild type mitochondria.
Complex I-linked superoxide release does not proceed through mPTP, PBR or IMAC.
ACKNOWLEDGEMENTS
This work was funded by funded by NIH grant P01AG020591 (to H.V.R.) and NIA Training Grant 5T3-AG021890-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembr. 1999;31:347–366. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- 2.Votyakova TV, Reynolds IJ. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 3.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 4.Lambert AJ, Brand MD. Reactive oxygen species production by mitochondria. Methods Mol Biol. 2009;554:165–181. doi: 10.1007/978-1-59745-521-3_11. [DOI] [PubMed] [Google Scholar]
- 5.Missirlis F, Hu J, Kirby K, Hilliker AJ, Rouault TA, Phillips JP. Compartment-specific protection of iron-sulfur proteins by superoxide dismutase. J Biol Chem. 2003;278:47365–47369. doi: 10.1074/jbc.M307700200. [DOI] [PubMed] [Google Scholar]
- 6.Korshunov SS, Imlay JA. A potential role for periplasmic superoxide dismutase in blocking the penetration of external superoxide into the cytosol of Gram-negative bacteria. Mol Microbiol. 2002;43:95–106. doi: 10.1046/j.1365-2958.2002.02719.x. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi MA, Asada K. Superoxide anion permeability of phospholipid membranes and chloroplast thylakoids. Arch Biochem Biophys. 1983;226:558–566. doi: 10.1016/0003-9861(83)90325-9. [DOI] [PubMed] [Google Scholar]
- 8.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 9.Hackenbrock CR. Chemical and physical fixation of isolated mitochondria in low-energy and high-energy states. Proc Natl Acad Sci U S A. 1968;61:598–605. doi: 10.1073/pnas.61.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brdiczka D. Contact sites between mitochondrial envelope membranes. Structure and function in energy- and protein-transfer. Biochim Biophys Acta. 1991;1071:291–312. doi: 10.1016/0304-4157(91)90018-r. [DOI] [PubMed] [Google Scholar]
- 11.Ono H, Tuboi S. Integration of porin synthesized in vitro into outer mitochondrial membranes. Eur J Biochem. 1987;168:509–514. doi: 10.1111/j.1432-1033.1987.tb13447.x. [DOI] [PubMed] [Google Scholar]
- 12.Sorgato MC, Moran O. Channels in mitochondrial membranes: knowns, unknowns, and prospects for the future. Crit Rev Biochem Mol Biol. 1993;28:127–171. doi: 10.3109/10409239309086793. [DOI] [PubMed] [Google Scholar]
- 13.Mannella CA. Conformational changes in the mitochondrial channel protein, VDAC, and their functional implications. J Struct Biol. 1998;121:207–218. doi: 10.1006/jsbi.1997.3954. [DOI] [PubMed] [Google Scholar]
- 14.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 15.Culty M, Li H, Boujrad N, Amri H, Vidic B, Bernassau JM, Reversat JL, Papadopoulos V. In vitro studies on the role of the peripheral-type benzodiazepine receptor in steroidogenesis. J Steroid Biochem Mol Biol. 1999;69:123–130. doi: 10.1016/s0960-0760(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 16.McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci U S A. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinnally KW, Zorov DB, Antonenko YN, Snyder SH, McEnery MW, Tedeschi H. Mitochondrial benzodiazepine receptor linked to inner membrane ion channels by nanomolar actions of ligands. Proc Natl Acad Sci U S A. 1993;90:1374–1378. doi: 10.1073/pnas.90.4.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King KL, Stanley WC, Rosca M, Kerner J, Hoppel CL, Febbraio M. Fatty acid oxidation in cardiac and skeletal muscle mitochondria is unaffected by deletion of CD36. Arch Biochem Biophys. 2007;467:234–238. doi: 10.1016/j.abb.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lustgarten MS, Jang YC, Liu Y, Muller FL, Qi W, Steinhelper M, Brooks SV, Larkin L, Shimizu T, Shirasawa T, McManus LM, Bhattacharya A, Richardson A, Van Remmen H. Conditional knockout of Mn-SOD targeted to type IIB skeletal muscle fibers increases oxidative stress and is sufficient to alter aerobic exercise capacity. Am J Physiol Cell Physiol. 2009;297:C1520–C1532. doi: 10.1152/ajpcell.00372.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ragan CI, Bloxham DP. Specific labelling of a constituent polypeptide of bovine heart mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone reductase by the inhibitor diphenyleneiodonium. Biochem J. 1977;163:605–615. doi: 10.1042/bj1630605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majander A, Finel M, Wikstrom M. Diphenyleneiodonium inhibits reduction of iron-sulfur clusters in the mitochondrial NADH-ubiquinone oxidoreductase (Complex I) J Biol Chem. 1994;269:21037–21042. [PubMed] [Google Scholar]
- 22.Fato R, Bergamini C, Bortolus M, Maniero AL, Leoni S, Ohnishi T, Lenaz G. Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species. Biochim Biophys Acta. 2009;1787:384–392. doi: 10.1016/j.bbabio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoshan-Barmatz V, Hadad N, Feng W, Shafir I, Orr I, Varsanyi M, Heilmeyer LM. VDAC/porin is present in sarcoplasmic reticulum from skeletal muscle. FEBS Lett. 1996;386:205–210. doi: 10.1016/0014-5793(96)00442-5. [DOI] [PubMed] [Google Scholar]
- 24.Bernardi P. The permeability transition pore. Control points of a cyclosporin A-sensitive mitochondrial channel involved in cell death. Biochim Biophys Acta. 1996;1275:5–9. doi: 10.1016/0005-2728(96)00041-2. [DOI] [PubMed] [Google Scholar]
- 25.Beavis AD. Properties of the inner membrane anion channel in intact mitochondria. J Bioenerg Biomembr. 1992;24:77–90. doi: 10.1007/BF00769534. [DOI] [PubMed] [Google Scholar]
- 26.Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 27.Gus'kova RA, Ivanov, Kol'tover VK, Akhobadze VV, Rubin AB. Permeability of bilayer lipid membranes for superoxide (O2-.) radicals. Biochim Biophys Acta. 1984;778:579–585. doi: 10.1016/0005-2736(84)90409-7. [DOI] [PubMed] [Google Scholar]
- 28.Lynch RE, Fridovich I. Effects of superoxide on the erythrocyte membrane. J Biol Chem. 1978;253:1838–1845. [PubMed] [Google Scholar]
- 29.Ishii N. Role of oxidative stress from mitochondria on aging and cancer. Cornea. 2007;26:S3–S9. doi: 10.1097/ICO.0b013e31812f6745. [DOI] [PubMed] [Google Scholar]
- 30.Nakada T, Kubota Y, Sasagawa I, Yagisawa T, Watanabe M, Ishigooka M. Remarkably suppressed manganese superoxide dismutase activity in malignant pheochromocytoma. J Urol. 1995;153:1787–1790. [PubMed] [Google Scholar]