Abstract
MicroRNAs have been implicated in tumor progression. Recent studies have shown that miR-200 family regulates Epithelial-Mesenchymal Transition (EMT) by targeting zinc-finger E-box binding homeobox 1 (ZEB1) and ZEB2. Emerging evidence from our laboratory and others suggest that the processes of EMT can be triggered by various growth factors such as Transforming Growth Factor-beta (TGF-β) and Platelet-Derived Growth Factor-D (PDGF-D). Moreover, we have recently reported that over-expression of PDGF-D in prostate cancer cells (PC3 PDGF-D cells) leads to the acquisition of EMT phenotype, and this model offers an opportunity for investigating the molecular interplay between PDGF-D signaling and EMT. Here we report, for the first time, significant down-regulation of miR-200 family in PC3 PDGF-D cells as well as in PC3 cells exposed to purified active PDGF-D protein, resulting in the up-regulation of ZEB1, ZEB2 and snail2 expression. Interestingly, re-expression of miR-200b in PC3 PDGF-D cells led to the reversal of EMT phenotype, which was associated with the down-regulation of ZEB1, ZEB2 and snail2 expression and these results were consistent with increased gene expressions of epithelial markers. Moreover, transfection of PC3 PDGF-D cells with miR-200b inhibited cell migration and invasion with concomitant repression of cell adhesion to culture surface and cell detachment. From these results, we conclude that PDGF-D induced acquisition of EMT phenotype of PC3 cells is in part due to repression of miR-200 and that any novel strategies by which miR-200 could be up-regulated would become a promising approach for the treatment of invasive prostate cancer.
Keywords: Platelet-Derived Growth Factor-D (PDGF-D), Epithelial–Mesenchymal Transition (EMT), miR-200, zinc-finger E-box binding homeobox 1 (ZEB1), snail2
Introduction
Epithelial-Mesenchymal Transition (EMT) is a process that is reminiscent of “cancer stem-like cell” characteristics whereby epithelial cells with cobblestone phenotype acquire mesenchymal cell characteristics with a spindle-shaped fibroblast-like morphology. This process involves a disassembly of cell-cell junction including down-regulation and relocation of E-cadherin and zonula occludens-1 (ZO-1) as well as down-regulation and translocation of β-catenin from the cell membrane to the nucleus, actin cytoskeleton reorganization and up-regulation of mesenchymal molecular markers such as vimentin, fibronectin and N-cadherin (1). Typically, epithelial cells form clusters mediated through the regulation of cell-cell junction and adhesion such as tight junction, adherens junctions, desmosomes and gap junction, and thereby inhibit cell movement of individual cells. In contrast, mesenchymal cells have less adhesion between cells compared to their epithelial counterpart, allowing for more motile and invasive characteristics that contribute to cancer cell invasion and metastasis (2).
During the acquisition of EMT, loss of epithelial markers is a critical process, which is regulated by important transcription repressors. In the last few years, several transcription repressors have been identified, including zinc-finger E-box binding homeobox 1 (ZEB1), ZEB2/SIP1, a member of the δEF-1 family of two-handed zinc finger nuclear factors, snail1, snail2/slug, Twist and E47. The ZEB1 has been shown to be a critical mediator of EMT induced by various inducers in different cell lines (3–6). Transcription factor ZEB1 has been shown to regulate expression of genes by binding to ZEB-type E-boxes (CACCTG) within the promoter region of target genes, resulting in the chromatin condensation and gene silencing (7). The expression of E-cadherin is negatively regulated by ZEB1, which is fundamental for the processes of EMT (8). Recent studies have also demonstrated that ZEB1 promotes cell migration and tumor metastasis by repressing the expressions of cell polarity factors (9, 10). ZEB2/SIP1 has been shown to down-regulate the expression of many genes coding for crucial proteins of epithelial phenotype including E-cadherin (11) and up-regulate the expression of vimentin (12). Snail2/slug has been shown to play a central role in the induction of EMT by growth factors (13, 14), suggesting that ZEB1, ZEB2 and snail2 are important regulators in the induction of EMT.
The processes of EMT could be triggered by many growth factors including transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF) A, B and D (15–19). It is also known that PDGF-D could regulate many cellular processes, including invasion and angiogenesis by activating its cognate receptor PDGFR-β (20, 21). A recent study has shown the increased expression of PDGF-D in human prostate carcinoma samples, suggesting that PDGF-D could play an important role in the progression of human prostate cancer (22). We have recently shown that stable transfection of PC3 cells with PDGF-D cDNA led to the acquisition of EMT phenotype and this process was consistent with increased invasiveness and in vivo tumor growth rate of PC3 PDGF-D cells (23). This model offers an opportunity for further investigation of the molecular interplay between PDGF-D signaling and EMT, and the precise mechanism by which PC3 PDGF-D cells acquire EMT phenotype.
Emerging evidence suggest that miR-200 family could regulate the processes of EMT by targeting E-box binding protein ZEB1 and ZEB2 (4, 5, 24). The microRNAs (miRNAs) are small (19–24 nucleotides) non-coding RNA molecules that down-regulate gene expression by interacting with sequences located in the 3'UTR of multiple target mRNAs, resulting in either translational repression or degradation of mRNAs (25). It is known that miRNAs are involved in embryonic development and in cancer progression, a process that is known to be associated with the acquisition of EMT phenotype of epithelial tumor cells (26). Moreover, recent studies showed that miR-200 could repress ZEB1-SIP1 expression through binding to sequence of 3'-UTR of ZEB1-SIP1 mRNA and that ZEB1 and ZEB2 could directly bind to the promoter of the miR-200 gene cluster resulting in the repression of miR-200 expression, establishing a double negative feedback loop controlling expressions of ZEB1-SIP1 and miR-200 family during EMT (4, 24, 27). Under this condition, discovery of factors which regulate the expression of miR-200 or ZEB1-SIP1/ZEB2 could be very important for controlling EMT and such knowledge could be useful for designing strategies for the treatment of aggressive prostate cancer. Therefore, in the current study, we sought to test our hypothesis whether the loss of miR-200 could contribute to the acquisition of EMT phenotype observed in PC3 PDGF-D cells and whether re-expression of miR-200 could lead to the reversal of EMT phenotype of PC3 PDGF-D cells. We further hypothesized that the above mentioned processes could be mediated by the deregulated expression of ZEB1, ZEB2 and snail2 transcription factors.
We found that miR-200 expression was significantly reduced in PC3 PDGF-D cells as well as in PC3 cells exposed to purified active PDGF-D protein compared to parental PC3 cells, which was associated with the over-expression of ZEB1, ZEB2 and snail2 and that the transfection of PC3 PDGF-D cells with miR-200b led to the down-regulation of ZEB1, ZEB2 and snail2 with corresponding up-regulation of epithelial markers. Overexpression of PDGF-D in LNCaP cells resulted in the decreased expression of miR-200 and induced EMT. From these results, we conclude that the loss of miR-200 plays an important role in the acquisition of EMT phenotype of LNCaP and PC3 cells induced by PDGF-D and that the re-expression of miR-200 could cause reversal of the EMT phenotype to mesenchymal-epithelial transition (MET) phenotype. These results provide mechanistic role of miR-200 in the processes of EMT/MET in PDGF-D over-expressing prostate cancer cells.
Materials and methods
Cell lines and culture condition
Generation of stable cell lines over-expressing PDGF-D was accomplished by transfection of PC3 and LNCaP cells with the corresponding empty vector pcDNA3 Neo or pcDNA3-PDGF-D:His as previously described (22) and referred to as PC3 Neo, or PC3 PDGF-D cells and LNCaP Neo or LNCaP PDGF-D cells, respectively. The PC3, LNCaP and resultant transfected cell lines were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA, www.invitrogen.com) supplemented with 5% or 10% fetal bovine serum (FBS), 2 mmol/L glutamine, 10 μmol/L Hepes, 50 units/ml Penicillin, and 50 μg/ml Streptomycin. All cells were maintained in a 5% CO2-humidified atmosphere at 37°C.
Research reagents and antibodies
Recombinant human PDGF-D (rhPDGF-D) was purchased from R&D systems (Minneapolis, MN, www.rndsystems.com). Antibodies against snail2, N-cadherin and vimentin were purchased from Cell Signaling Technology (Beverly, MA, www.cellsignal.com), BD Biosciences (Bedford, MA, www.bdbiosciences.com) and Abcam (Cambridge MA, www.abcam.com), respectively. Antibodies against ZEB1, ZEB2, Twist, snail1, fibronectin, retinoblastoma protein (RB) and E-cadherin were obtained from Santa Cruz (Santa Cruz, CA, www.scbt.com). Antibodies against PDGF-D, ZO-1, Alex Fluro 594 goat anti-rabbit IgG or Alex Fluro 594 goat anti-mouse IgG and Alexa Fluor 594 phalloidin for F-actin staining were purchased from Invitrogen. Goat anti-rabbit IgG (H + L)-HRP conjugate and goat anti-mouse IgG (H + L)-HRP conjugate were obtained from Bio-Rad (Reinach, BL, www.bio-rad.com). Antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was purchased from Affinity BioReagents (Golden, CO, www.bioreagents.com).
siRNA and transfection
PC3 PDGF-D cells were transfected with 100 nmol/L of ZEB1 siRNA or control siRNA (Santa Cruz) using DharmaFECT3 siRNA transfection reagent (DHARMACON, Lafayette, CO, www.dharmacon.com). The media were removed after 24 h transfection and then the cells were incubated in media containing 5% FBS for another 24 h. The cell lysates were prepared for Western blot analysis and the total RNA was extracted for real time RT-PCR assay.
Western blot analysis
Western blot analysis was performed using cytoplasmic, nuclear extract or total cell lysates. Total cell lysates from different experiments were obtained by lysing the cells in RIPA buffer containing 50 mM Tris–HCl, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 2 mM sodium fluoride, 2 mM Na3VO42, 1 mM EDTA, 1 mM EGTA, and 1 × protease inhibitor cocktail. Nuclear and cytoplasmic extracts were prepared according to the method described by our laboratory previously (28) and Western blotting was performed as previously described (28).
Transfection of microRNA precursors (pre-miRNA) and specific anti-miRNA (inhibitors)
PC3 PDGF-D cells were seeded at 3 × 105 cells per well in 6 well plates and transfected with pre-miR-200b or miRNA negative control #1 (Ambion, Austin, TX, www.ambion.com) at a final concentration of 20 nM using DharmaFECT3 transfection reagent (DHARMACON). LNCaP cells were transfected with specific anti-miRNA (miRNA inhibitors) or anti-miRNA control at a final concentration of 600 nM [200 nM each of miR-200a, miR-200b and miR-200c anti-miRNAs (Ambion)] using DharmaFECT3 transfection reagent (DHARMACON). After 3 days of transfection, cells were split and transfected repeatedly with pre-miR-200b, miRNA inhibitors or control every 3–4 days for indicated times.
Real-time RT-PCR
The total RNA was isolated using the Trizol reagent. One microgram of RNA was reverse transcribed using a reverse transcription system (Invitrogen) according to the manufacturer's instruction. Real time PCR was used to quantify mRNA expression. Sequences of primers for ZEB1, ZEB2, snail2, vimentin, epithelial cell adhesion molecule (EpCAM), sciellin, stratifin, crumbs homologue 3 (CRB3), connexin 26, F11 receptor (F11R, junctional adhesion molecule 1) and GAPDH were shown in supplementary information table S. The primer sequence used for E-cadherin in this study was described earlier (4) and the relative amount of RNA was normalized to the expression of GAPDH. For miRNA analysis, the total RNA was isolated using mirVana miRNA isolation kit (Ambion) according to the manufacturer's instruction. The miRNA and RNU6B-specific cDNA was produced from 10 ng of total RNA samples using the Taqman MicroRNA Reverse Transcription Kit (Applied Biosystems, Fostor, CA, www.appliedbiosystems.com) with miRNA-specific RT primer from the Taqman MicroRNA Assay (Applied Biosystems). The levels of miRNAs were determined by using the miRNA-specific Taqman MGB probes from the Taqman MicroRNA Assay (Applied Biosystems). The relative amount of miRNA was normalized to RNU6B.
Cell invasion and migration assay
Cell invasion and migration assay was performed using 24 well Transwell Permeable Supports with 8 μm pores (Corning, Lowell, MA, www.corning.com) and shown in supplementary section.
Immunofluorscence microscopy
Immunofluorscence staining was performed as described previously by our laboratory (23). Briefly, cells were fixed with 4% paraformaldehyde and permeabilized in 0.5% Triton x-100, then blocked with 10% goat serum. The cells were incubated for 1 h with antibodies against E-cadherin (1:20), ZO-1 (1:50), or vimentin (pre-diluted) in 5% goat serum, and were stained for 1 h with Alex Fluro 594 conjugated secondary antibody (1: 250). For F-actin staining, cells were incubated with 0.33 μM of Alexa Fluor 594 phalloidin for 1 h at 4°C. The slides were mounted with mounting medium containing anti-fade reagent and DAPI. Cells were viewed by fluorescence microscopy and images were analyzed using Advanced Sport software (Diagnostic Instruments, Sterling Heights, MI).
Cell attachment and detachment assay
Cell attachment and detachment assay was shown in supplementary section.
Data analysis
Experiments presented in this study are representative of three or more repetitions. The data are shown as the mean values ± SE. A two-tailed student's t test was used for comparisons between groups. Values of p < 0.05 were considered to be statistically significant.
Results
PDGF-D regulates the expression of transcription factors, ZEB1, ZEB2 and snail2, in PC3 cells
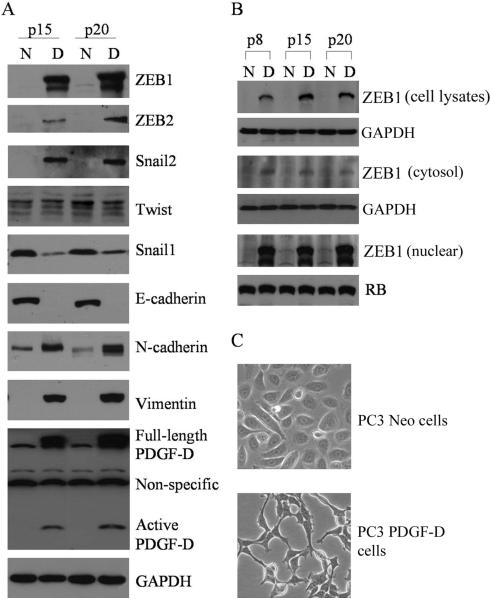
Since it is known that transcriptional repressors such as ZEB1, ZEB2, snail1 and snail2 are the key regulators in inducing the processes of EMT, we first determined the expression status of these transcription repressors in our cell culture model. The results showed that the expression of ZEB1, ZEB2 and snail2 were dramatically up-regulated in the PC3 PDGF-D cells concomitant with the loss of E-cadherin, and gain of vimemtin and N-cadherin expression. Moreover, we found that the expression of snail1 was significantly decreased and the level of Twist was not significant changed in PC3 PDGF-D cells compared to PC3 Neo cells (Fig. 1A). To further determine sub-cellular localization of ZEB1 expression, we tested ZEB1 levels in whole cell lysates, cytoplasmic and nuclear extracts. We found that the levels of ZEB1 were significantly higher in whole cell lysates and in the nuclei from PC3 PDGF-D cells compared to PC3 Neo cells (Fig. 1B). These results suggest that ZEB1 is mainly located in the nuclear compartment. Most importantly, PC3 PDGF-D cells displayed elongated/irregular fibroblastoid morphology compared to PC3 Neo cells which showed epithelial cobblestone appearance (Fig. 1C). These results are consistent with the expression status of PDGF-D in PC3 PDGF-D and PC3 Neo cells (Fig. 1A).
Fig. 1.
Over-expression of PDGF-D induces EMT in PC3 cells. (A) Western blot analysis showed the expression of PDGF-D, transcription repressors and other mesenchymal as well as epithelial markers in PC3 Neo and PC3 PDGF-D cells, passage 15 and 20. (GAPDH protein was used as protein loading control). (B) Total cell lysates, cytosol and nuclear extracts were prepared from PC3 Neo and PC3 PDGF-D cells, passage 8, 15 and 20. The result from Western blot showed the expression of ZEB1, which mainly localized in the nuclear. GAPDH was used for protein loading control for cell lysates and cytosolic extracts, whereas retinoblastoma (RB) protein was used for protein loading control for nuclear extracts. (C) Photomicrographs of cells are shown: PC3 Neo cells display rounded epithelial cell shape (upper panel) and PC3 PDGF-D cells exhibit a fibroblastic-type phenotype (lower panel); original magnification, 200×. (N: PC3 Neo cells, D: PC3 PDGF-D cells, p8: passage 8).
Over-expression of PDGF-D represses expressions of epithelial-specific genes
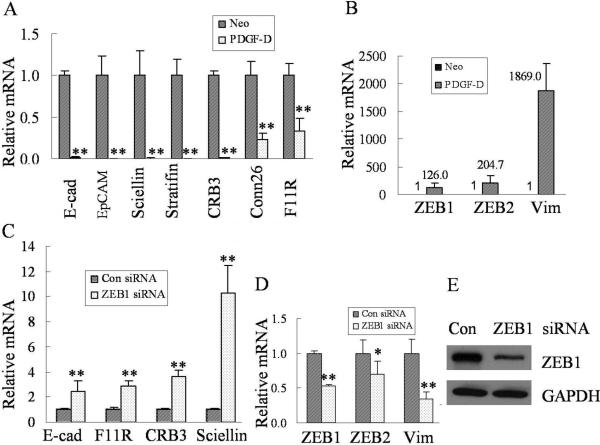
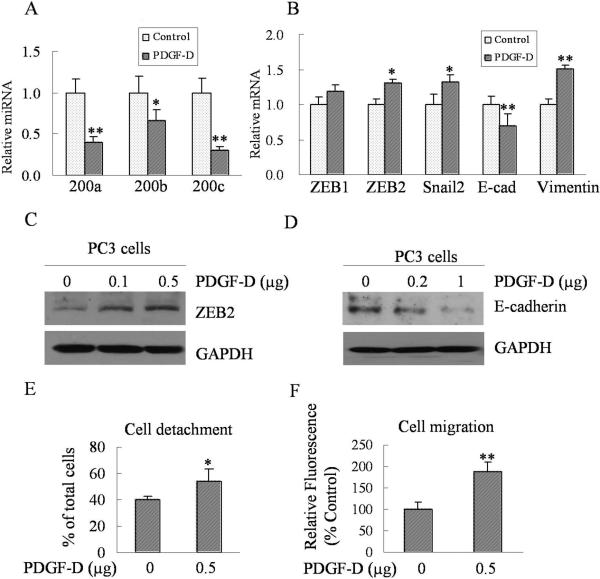
In this study, we found that over-expression of PDGF-D significantly down-regulated expressions of epithelial-specific genes such as E-cadherin, EpCAM, sciellin, stratifin, CRB3, connexin 26 and F11R (Fig. 2A) concomitant with increased mRNA levels of ZEB1 and ZEB2 (Fig. 2B).
Fig. 2.
Over-expression of PDGF-D in PC3 cells up-regulates the expression of transcription factors and down-regulates expressions of epithelial specific genes. (A) Real time RT-PCR was used to determine mRNA levels of epithelial markers in PC3 Neo and PC3 PDGF-D cells. Relative mRNA levels were normalized to GAPDH. (B) Real time RT-PCR was used to quantify the expression of ZEB1, ZEB2 and vimentin mRNA in PC3 Neo and PC3 PDGF-D cells. GAPDH was used for internal control to correct for the potential variation in RNA loading. (C) The results from real time RT-PCR showed the increased mRNA levels of E-cadherin, F11R, CRB3, and sciellin in PC3 PDGF-D transfected with ZEB1 siRNA compared to cells transfeced with control siRNA. Relative mRNA levels were normalized to GAPDH. (D) The decreased mRNA levels of ZEB1, ZEB2 and vimentin were observed in PC3 PDGF-D transfected with ZEB1 siRNA compared to control siRNA. Relative mRNA levels were normalized to GAPDH. (E) PC3 PDGF-D cells were transfected with ZEB1 or control siRNA and incubated for 72 h. Western blot analysis was performed using primary antibodies against ZEB1 and GAPDH. GAPDH was used for protein loading control. *, p < 0.05, **, p < 0.01 compared to control cells. (Neo: PC3 Neo cells, PDGF-D: PC3 PDGF-D cells, Con: control, E-cad: E-cadherin, Vim: vimentin).
To further verify whether ZEB1 could repress the expression of these epithelial markers, we have knocked down the ZEB1 by transfection of PC3 PDGF-D cells with ZEB1-specific siRNA. Our results showed that knockdown of ZEB1 significantly increased the mRNA levels of E-cadherin, F11R, CRB3, and sciellin (Fig 2C), suggesting that ZEB1 is responsible for the regulation of these genes that are associated with the acquisition of EMT phenotype in PC3 PDGF-D cells. Moreover, Fig. 2D and E showed that the transfection of cells with ZEB1 siRNA was effective in reducing the expression of ZEB1 both at the mRNA and protein levels; however for reasons that are presently unknown, we found that the knockdown of ZEB1 decreased the mRNA expression of ZEB2, although this finding is consistent with the results shown by other investigator (29).
The miR-200 contributes to the regulation of epithelial marker genes in PC3 PDGF-D cells, partly by regulation of ZEB1, ZEB2 and snail2
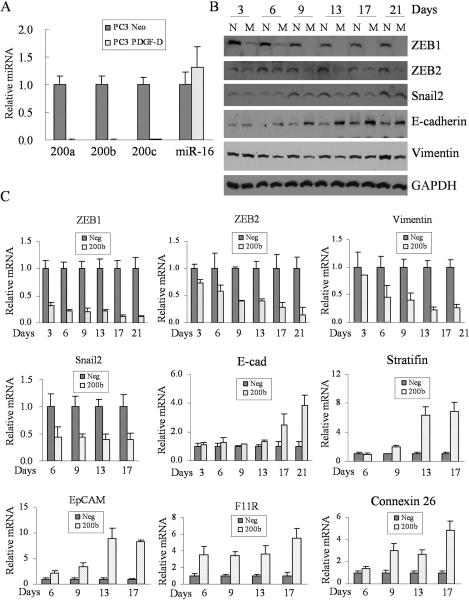
To test whether the miR-200 contributes to the regulation of epithelial marker genes in PC3 PDGF-D cells, we first determined the expression levels of miR-200 in PC3 Neo and PC3 PDGF-D cells. We found that the expressions of miR-200a, miR-200b, and miR-200c were significantly down-regulated in PC3 PDGF-D cells compared to PC3 Neo cells, while there was no significant change in the expression of miR-16 level (Fig. 3A).
Fig. 3.
miR-200 regulates the expressions of transcription factors and protein expressions associated with cell polarity, tight junctions, desmosomes, gap junctions and cell surface receptor. (A) Levels of miR-200a, miR-200b, miR-200c and miR-16 from PC3 Neo and PC3 PDGF-D cells were determined by using the miRNA-specific Taqman MGB probes and primers. The relative amount of miRNA was normalized to RNU6B. (B) The results from Western blot showed that expressions of ZEB1, ZEB2, snail2 and vimentin were significantly inhibited, while expression of E-cadherin was increased in PC3 PDGF-D cells transfected with miR-200b compared to PC3 PDGF-D cells transfected with control miRNA. GAPDH was used for protein loading control. (C) Real time RT-PCR was used to quantify the mRNA levels of ZEB1, ZEB2, snail2 and vimentin as well as mRNA levels of E-cadherin, stratifin, EpCAM, F11R and connexin 26 in PC3 PDGF-D cells transfected with miR-200b compared to transfection with negative control miRNA. Relative mRNA levels were normalized to GAPDH. (N: negative control miRNA, M: miR-200b, Neg: negative control miRNA, 200a, 200b and 200c: miR-200a, miR-200b, miR-200c, respectively).
In order to mechanistically investigate the role of miR-200 in the regulation of ZEB1 and ZEB2 during the processes of EMT, we transfected PC3 PDGF-D cells with miR-200b and subsequently determined the expression status of transcriptional repressors and epithelial marker genes. We found that not only the expression of ZEB1 and ZEB2 also snail2 were markedly reduced in PC3 PDGF-D cells transfected with miR-200b compared to cells transfected with control miRNA, which is consistent with the enhanced expression of epithelial marker genes, such as E-cadherin, stratifin, EpCAM, F11R and connexin 26 (Fig. 3B and C). These results clearly suggest that the loss of miR-200b contributes to the induction of ZEB1, ZEB2 and snail2; resulting in the negative regulation of epithelial marker factors during the acquisition of EMT phenotype in PDGF-D over-expressing PC3 cells.
Re-expression of miR-200b induces mesenchymal-epithelial transition (MET) in PC3 PDGF-D cells
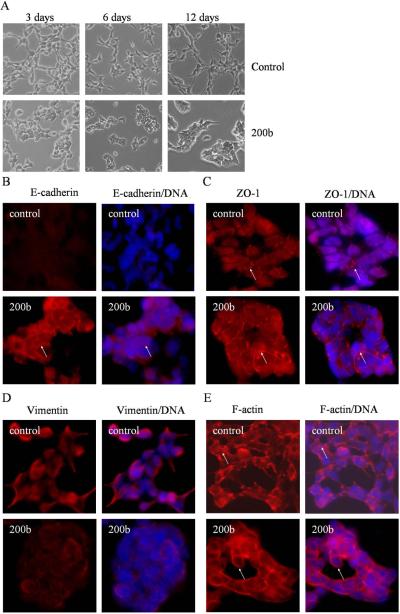
Since transfection of miR-200b into PC3 PDGF-D cells resulted in the up-regulation in the expression of epithelial marker genes, we sought to assess whether EMT phenotype was reversed in these cells. As expected, we found that PC3 PDGF-D cells transfected with miR-200b displayed round-like morphology and adhered together after 3 days of transfection (Fig. 4A). The results from immunofluorescence staining showed that the expression of E-cadherin was significantly increased and mainly located on the cell membrane at the cell-cell junction (Fig. 4B). Concomitantly, we found that ZO-1 protein was localized on the cell membrane at tight junction in miR-200b transfected PC3 PDGF-D cells. In contrast, ZO-1 was disrupted from the tight junction in PC3 PDGF-D cells transfected with control miRNA as shown in Fig. 4C. In addition, the pattern of expression and distribution of mesenchymal markers were changed in PC3 PDGF-D cells transfected with miR-200b. We found a dramatically reduced expression of vimentin in PC3 PDGF-D cells transfected with miR-200b (Fig. 4D). We also found changes in the expression patterns of F-actin. Cortical actin pattern, characteristic of epithelial phenotype, was observed in PC3 PDGF-D cells transfected with miR-200b, whereas actin stress fiber was consistent with the characteristics of mesenchymal phenotype in PC3 PDGF-D cells transfected with control miRNA (Fig. 4E). These results suggest that the loss of miR-200 expression found in PC3 PDGF-D cells contributes to the up-regulation of ZEB1, ZEB2 and snail2, resulting in the down-regulated expressions of epithelial marker genes and the acquisition of EMT phenotype and that this process could be reversed (acquisition of MET phenotype) by the re-expression of miR-200b in PC3 PDGF-D cells.
Fig. 4.
MiR-200b reverses EMT phenotype of PC3 PDGF-D cells. (A) Photographs of cells are shown: PC3 PDGF-D cells transfected with negative control miRNA exhibit a fibroblastic-type phenotype (upper panel), PC3 PDGF-D cells transfected with miR-200b display round-like epithelial cell shape and cells form a cluster (lower panel). Original magnification, 200 ×. After 21 days of transfection, PC3 PDGF-D cells transfected with negative control miRNA or miR-200b were immunostained for the expressions of E-cadherin (B), ZO-1 (C), vimentin (D), or stained with Alexa Fluor 594 phalloidin for F-actin (E) with DAPI for DNA to show cell nucleus, as described under method section. Arrows indicate changes in the expression or location of epithelial and mesenchymal markers. Original magnification, 200 ×.
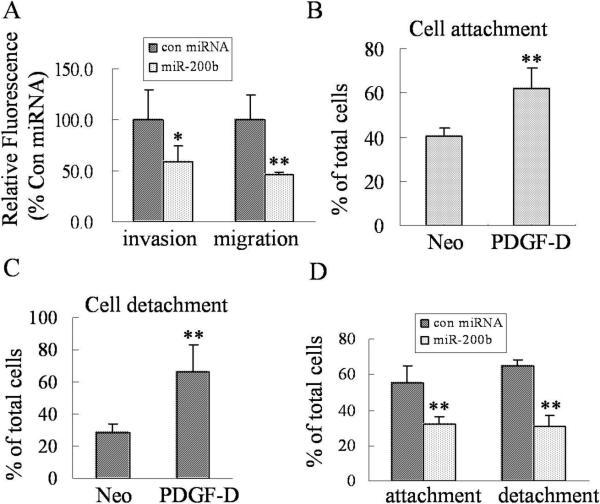
The miR-200b inhibits the adhesion and invasion of PC3 PDGF-D cells
In order to demonstrate whether miR-200b could affect behaviors of PC3 PDGF-D cells, we tested cell migration and invasion, and we found that transfection of PC3 PDGF-D cells with miR-200b significantly inhibited the migration and invasion of PC3 PDGF-D cells (Fig. 5A). It is well known that cell detachment from the matrix in the growth environment, and attachment to the secondary site is the “hallmark” of cell migration and invasion during metastatic process. Interestingly, we found that PC3 PDGF-D cells displayed enhanced detachment and attachment (Fig. 5B and C). More importantly, transfection of PC3 PDGF-D cells with miR-200b markedly reduced the cell detachment and attachment compared to transfection with control miRNA (Fig. 5D). These results clearly suggest that re-expression of miR-200b inhibits migration and invasion of PC3 PDGF-D cells through the reversal of EMT to MET phenotype. Thus we believe that novel therapeutic strategies by which miR-200b could be re-expressed in invasive human prostate cancer would become a useful approach for the treatment of invasive and metastatic prostate cancer in the future. However, the question remains whether PDGF-D in fact is responsible for the acquisition of EMT characteristics in PC3 cells and whether the repression of miR-200 is mechanistically linked with this process or not. Therefore, we assessed the effect of purified active PDGF-D protein treatment on PC3 cells.
Fig. 5.
MiR-200b inhibits the migration and invasion, and reduces attachment and detachment of PC3 PDGF-D cells. (A) The effects of miR-200b on cell migration and invasion of PC3 PDGF-D cells were determined by using 24 well Transwell Permeable Supports with 8 μM pores. For the invasion assay, PC3 PDGF-D cells transfected with miR-200b or control miRNA were seeded into the Transwell inserts coated with growth factor reduced Matrigel. For the migration assay, PC3 PDGF-D cells transfected with miR-200b or control miRNA were seeded into the Transwell inserts not coated with Matrigel. After 24 h incubation, the cells were stained with 4 μg/ml Calcein AM in PBS at 37°C for 1 h. The fluorescence of the cells from lower sides of inserts was read in ULTRA Multifunctional Microplate Reader at excitation/emission wavelengths of 485/530 nm. Values of relative fluorescence are shown. The values represent the comparative amount of invaded or migrated cells. Panel (B) and (C) showed that over-expression of PDGF-D significantly increased cell attachment and detachment of PC3 cells, respectively. (D) Transfection of PC3 PDGF-D cells with miR-200b dramatically inhibited the attachment and detachment of PC3 PDGF-D cells after 15 days of transfection. n = 4. *, p < 0.05, **, p < 0.01 compared to control cells.
PDGF-D protein treatment represses the expression of miR-200 family and induces EMT characteristics
In order to further verify whether PDGF-D could be indeed responsible for the induction of EMT phenotype of PC3 cells through regulating the expression of miR-200 family, we have determined the expression levels of miR-200 family and assessed the expression of ZEB1, ZEB2, snail2, vimentin and E-cadherin in PC3 cells chronically treated with purified active form of PDGF-D. The treatment of cells with purified PDGF-D protein resulted in a significant repression in the expression of miR-200a, miR-200b and miR-200c (Fig. 6A), which is consistent with findings that were obtained from PC3 PDGF-D cells. Moreover, we also found that PDGF-D protein treatment significantly increased the expression of ZEB2, Snail2 and vimentin at the mRNA level with concomitantly decreased expression of E-cadherin both at the mRNA and protein levels (Fig. 6B, C and D). Most importantly, PDGF-D treatment dramatically enhanced cell detachment and migration of PC3 cells (Fig 6E and F), suggesting that PDGF-D is indeed responsible for the induction of EMT phenotype in PC3 cells, which is in part mediated via down-regulation of miR-200 expression and the regulation of its target genes.
Fig. 6.
Purified active PDGF-D treatment down-regulates the expression of miR-200 family and induces EMT. (A) The total RNA was isolated using Trizol reagent. miRNA levels were determined by using the miRNA-specific Taqman MGB probes and primers. The relative amount of miRNA was normalized to RNU6B. (B) Real time RT-PCR was used to quantify the expressions of ZEB1, ZEB2, snail2, E-cadherin and vimentin mRNA in PC3 cells treated with purified active PDGF-D protein for 4 weeks compared to PC3 cells treated with control. Relative mRNA levels were normalized to GAPDH. Panel (C) and (D) PC3 cells were treated with purified active PDGF-D for 10 or 20 days and then cell lysates were prepared. Western blot analysis showed that the expression of ZEB2 was increased and the expression of E-cadherin was decreased in a dose-dependent manner. (E) The effects of purified active PDGF-D treatment on cell detachment of PC3 cells were determined and results showed that cell detachment significantly increased in PC3 cells treated with PDGF-D for 4 weeks. (F) The effects of purified active PDGF-D treatment on migration of PC3 cells were determined by using 24 well Transwell Permeable Supports with 8 μM pores. Values of relative fluorescence are shown. The values represent the comparative amount of migrated cells. *, p < 0.05, **, p < 0.01 compared to control.
Over-expression of PDGF-D in LNCaP cells induces EMT
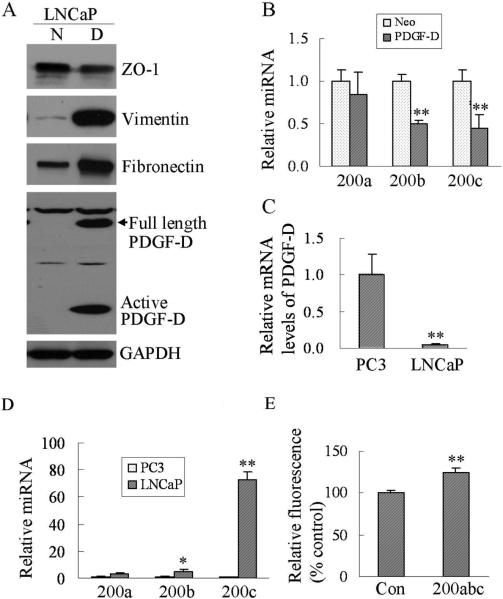
To demonstrate whether PDGF-D could induce EMT in different prostate cancer cell lines, we have established LNCaP stable cell lines over-expressing PDGF-D though transfection of LNCaP cells with PDGF-D plasmid. We found that over-expression of PDGF-D in LNCaP cells resulted in a significant decrease in the expression of ZO-1 (an epithelial marker) and enhanced expression of vimentin and fibronectin (Fig. 7A) with concomitantly decreased levels of miRNA miR-200b and miR-200c (Fig. 7B). More interestingly, we found that mRNA levels of PDGF-D in LNCaP cells were significantly lower than those in PC3 cells (Fig. 7C), whereas levels of miR-200, especially miR-200c, were dramatically higher in LNCaP cells than that in PC3 cells (Fig.7D). It is well known that LNCaP cells are less invasive than PC3 cells. To investigate whether miR-200 could contribute to the regulation of invasion of LNCaP cells, we transfected LNCaP cells with anti-miR-200a, miR-200b and miR-200c and performed invasion assay. The results showed that LNCaP cells transfected with anti-miR-200a, miR-200b and miR-200c could significantly increase cell invasion compared to cells transfected with anti-miRNA control (Fig. 7E). These results clearly suggest that over-expression of PDGF-D could induce EMT mediated in part by regulating the expression of miR-200 family not only in PC3 cells but also in LNCaP cells.
Fig. 7.
Over-expression of PDGF-D in LNCaP cells induces EMT characteristics. (A) Western blot analysis showed the expressions of ZO-1, vimentin, fibronectin and the levels of PDGF-D in LNCaP PDGF-D cells (PDGF-D over-expressing cells) compared to LNCaP Neo cells (GAPDH protein was used as protein loading control). (B) Levels of miR-200a, miR-200b, and miR-200c in LNCaP PDGF-D cells (PDGF-D) and LNCaP Neo cells (Neo) were determined by using the miRNA-specific Taqman MGB probes and primers. The relative amount of miRNA was normalized to RNU6B. (C) Real time RT-PCR was used to determine mRNA levels of PDGF-D in PC3 and LNCaP cells. Relative mRNA levels were normalized to GAPDH. (D) Levels of miR-200a, miR-200b and miR-200c from PC3 and LNCaP cells were determined by using the miRNA-specific Taqman MGB probes and primers. The relative amount of miRNA was normalized to RNU6B. (E) For the invasion assay, LNCaP cells transfected with anti-miR-200a, miR-200b and miR-200c (inhibitors) or anti-miRNA control were seeded into the Transwell inserts coated with growth factor reduced Matrigel. After 24 h incubation, the cells were stained with 4 μg/ml Calcein AM in PBS at 37°C for 1 h. The fluorescence of the cells at the bottom sides of the inserts was read in ULTRA Multifunctional Microplate Reader at excitation/emission wavelengths of 485/530 nm. Values of relative fluorescence are shown. The values represent the comparative values of invaded cells. [*, p < 0.05, **, p < 0.01 compared to control. N: LNCaP Neo cells, D: LNCaP PDGF-D cells, con: anti-miRNA control, 200abc: ant-miR-200a, miR-200b and miR-200c (miRNA inhibitors)].
Discussion
PDGF-D has been demonstrated to be expressed in many tumor cell lines (30, 31) and in prostate tumor tissues (21, 22), suggesting that PDGF-D could play an important role in the development and progression of prostate cancer. In our previous studies, we reported that the sustained over-expression of PDGF-D was responsible for the acquisition of EMT phenotype in PC3 cells with greater invasive characteristics and increased tumor growth rate in xenograft model (23, 32). However, the mechanistic role of PDGF-D in the greater context of tumorigenesis and in prostate cancer progression remains to be elucidated. The acquisition of EMT phenotype of epithelial tumor cells is believed to play critical roles in increased invasion and metastatic behavior of tumor cells during tumor progression, and this process is reminiscent of “cancer stem-like cells” characteristics. The transcription repressors including ZEB and snail family are strongly linked with the induction of EMT phenotype induced by various factors. In our current study, we found that the expressions of ZEB1, ZEB2 and snail2/slug were significantly increased in PC3 PDGF-D cells, which was consistent with the EMT characteristics of these cells concomitant with decreased expression of epithelial markers, such as E-cadherin, EpCAM, sciellin, stratifin, CRB3, connexin 26 and F11R.
To gain further insight whether ZEB1 could repress the expression of the above mentioned epithelial marker genes, we knocked down the expression of ZEB1 by transfection of PC3 PDGF-D cells with ZEB1-specific siRNA. Transfection of PC3 PDGF-D cells with ZEB1 siRNA led to an increase in the expression of E-cadherin, CRB3, sciellin and F11R, all of which are markers of epithelial cells. However, knockdown of ZEB1 by specific siRNA not only reduced the expression of ZEB1 but also down-regulated the mRNA levels of ZEB2, which is consistent with findings reported by other investigators (24, 29). Taken together, these results suggest that transcription factors, ZEB1, ZEB2 and snail2/slug, could mediate the induction of EMT phenotype observed in PC3 PDGF-D cells and that the down-regulation of specific transcription factors may prove to be useful for the reversal of EMT phenotype.
It is also tempting to speculate that these transcription factors could be regulated in cells by novel mechanisms. Indeed, recent findings suggested that microRNA, especially miR-200 family, could regulate the processes of EMT by repressing the expression of ZEB1 and ZEB2 through targeting the 3' UTR of ZEB1 and ZEB2 mRNA (4, 5, 24, 33). The levels of mature miRNAs within the cells are regulated by many complex steps where p68 RNA helicase is required for processing primary microRNAs into microRNA precursors (34). Interestingly, the phosphorylation of p68 RNA helicase at Y593 by PDGF-B has been shown to mediate the induction of EMT (19), suggesting that PDGF-B could induce EMT through regulating the expression of miRNAs by phosphorylating p68 RNA helicase.
These tantalizing and emerging evidence linking the regulation of mRNAs by miRNAs in the processes of EMT prompted us to investigate whether miR-200 family could play a role in PDGF-D induced EMT of prostate cancer cells and further assess whether the processes of EMT in our cell culture model system could also be regulated by ZEB1, ZEB2 and snail2 transcription factors. We found that the expressions of miR-200a, miR-200b and miR-200c were significantly reduced in LNCaP PDGF-D and PC3 PDGF-D cells as well as in PC3 cells exposed to PDGF-D protein compared to control cells, suggesting that miR-200 family may regulate the EMT process in our model. ZEB1 contains three putative binding sites for miR-200a, five for miR-200b/ miR-200c (4, 33), and ZEB2 harbors three putative binding sites for miR-200a and six for miR-200b (4). In addition, we found that the levels of miR-200b was 8 fold higher than miR-200c in PC3 cells, suggesting that miR-200b could play a central role in the negative regulation of ZEB1 and ZEB2 expression, and thus the loss of miR-200b leads to the up-regulation of ZEB1 and ZEB2, resulting in the acquisition of EMT induced by PDGF-D. Therefore, we elected to re-express the miR-200b in PC3 PDGF-D cells by transfection studies in order to gain further mechanistic insight as to the regulation of ZEB1 and ZEB2 and the consequence of re-expression of miR-200b on EMT phenotype and its biological consequence. The transfection of PC3 PDGF-D cells with miR-200b significantly reduced the expression of ZEB1, ZEB2 and snail2 both at the mRNA and protein levels with concomitantly increased expression of epithelial markers, such as E-cadherin, stratifin, CRB3, EpCAM, F11R and connexin 26. In addition, PC3 PDGF-D cells transfected with miR-200b displayed epithelial phenotype, suggesting that the loss of miR-200b contributes to the acquisition of EMT phenotype and that the re-expression of miR-200b could result in the reversal of EMT to MET phenotype.
The processes of EMT have been linked with cell migration and invasion, and the re-expression of miR-200b in PC3 PDGF-D cells led to the reversal of EMT phenotype. In this study, we found that the transfection of PC3 PDGF-D cells with miR-200b significantly inhibited cell migration and invasion of PC3 PDGF-D cells. It is well known that the metastatic process of cancer cells requires cell detachment from the site of origin, intravasation, translocation through blood and lymphatic vessels, extravasation, and attachment to the secondary site and colonization. Moreover, cell detachment from basement membrane (BM) and re-attachment play critical roles during cell migration and invasion as well as tumor cell metastasis. Interestingly, we found that PC3 PDGF-D cells exhibited a significant enhancement in cell detachment from culture surface and attachment to the culture surface. More importantly, transfection of PC3 PDGF-D cells with miR-200b remarkably reduced the ability of PC3 PDGF-D cells to attach to and detach from culture surface and our results are consistent with the role of miR-200b re-expression in PC3 PDGF-D cells with the reversal of EMT to MET characteristics with less invasive phenotype. More interestingly, LNCaP cells with less invasive capacity possess lower expression of endogenous PDGF-D and higher levels of miR-200 and that the over-expression of PDGF-D in LNCaP cells resulted in the down-regulation of miR-200 expression, which was associated with EMT characteristics.
Our earlier results showed that mTOR and NF-κB pathways play critical roles in the induction of EMT induced by over-expression of PDGF-D in PC3 cells (23). In the current study, we showed that the loss of miR-200, especially miR-200b is in part responsible for the induction of EMT in PC3 PDGF-D cells. It is well known that NF-κB plays an important role in mediating the processes of EMT induced by different factors through up-regulation of transcription repressor function of ZEB1 and ZEB2 (29, 35), which in turn repress the expression of miR-200 family by binding to E-box sequence of miR-200 promoter (27, 33). More interestingly, miR-200 could down-regulate the expressions of ZEB1 and ZEB2 by interacting with 3'-UTR of ZEB1 and ZEB2 mRNA (4, 27). Moreover, mTOR pathway could indirectly regulate NF-κB activity by regulating GSK-3β phosphorylation (23). These results also signifies a double-negative feedback loop between miR-200 and ZEB1/ZEB2 and that it allows the maintenance of EMT phenotype, even after cessation of the inducing signal which could become a critical target for the reversal of EMT. Together, our results are consistent with the findings that the re-expression of miR-200b could reverse the EMT phenotype induced by PDGF-D and in this process mTOR pathway could indirectly regulate NF-κB activity by regulating GSK-3β phosphorylation, thereby establishing a mechanistic link in the regulatory function of miR-200 although further mechanistic studies are warranted.
In order to further demonstrate whether PDGF-D could indeed be responsible for the induction of EMT phenotype of PC3 cells by regulating the expression of miR-200 family, we have analyzed the expression levels of miR-200 family and transcription repressors as well as EMT molecular markers in PC3 cells chronically treated with purified active form of PDGF-D. We found that the treatment of cells with purified PDGF-D protein resulted in a significant repression in the expression of miR-200a, miR-200b and miR-200c, and significantly increased the expression of ZEB2, Snail2 and vimentin at the mRNA level with concomitant decreased expression of E-cadherin. Most importantly, PDGF-D treatment dramatically enhanced cell detachment and migration of PC3 cells, suggesting that PDGF-D is indeed responsible for the induction of EMT phenotype in PC3 cells, which is in part mediated via the down-regulation of miR-200 expression and the regulation of target genes. However, we found that chronic exposure of PC3 cells to PDGF-D protein was not sufficient to induce significant morphological changes compared to those seen in PDGF-D transfected PC3 cells. This observation is consistent with our previous findings showing that PDGF-D transfected cells in early passages retained epithelial characteristics whereas the EMT characteristics could be seen only in later passages (greater than passage 7), suggesting that chronic exposure to high concentration of PDGF-D is required for the induction and maintenance of EMT phenotype in PC3 cells. Another explanation could be that an intracrine mechanism existed for PDGF-D action, which may not function when cells are exogenously treated with purified PDGF-D in contrast to intracellularly synthesized PDGF-D in PC3 PDGF-D cells. Interestingly, recent studies have shown that many growth factors such as VEGF, FGF, EGF and PDGF have intracrine mechanism of signaling (36–39), which could be one of the mechanism by which PDGF-D over-expression contributes to EMT as seen in our study.
Summary
In conclusion, we have developed a cell culture model for understanding the molecular regulation of EMT and its reversal to MET phenotype. More importantly, we found that PDGF-D could induce EMT thought regulating the expression of miR-200 family, which was associated with deregulation of ZEB1, ZEB2 and snail2. Based on our novel findings, we believe that the re-expression of miR-200b in human prostate cancer by innovative approaches could be useful for designing strategies for the treatment of invasive and metastatic prostate cancer.
Supplementary Material
Acknowledgments
Grant support: This work was partly funded by grants from the National Cancer Institute, NIH (5R01CA108535 to FHS). This work was also partly funded by the Puschelberg foundation.
Footnotes
Dejuan Kong: Conception and design, execution, collection of data and data analysis, manuscript writing
Yiwei Li: Experimental design, data analysis, manuscript writing
Zhiwei Wang: Experimental design and execution, and data analysis
Sanjeev Banerjee: Experimental design, execution, and data analysis
Aamir Ahmad: Experimental execution and collection of data
Hyeong-Reh Choi Kim: Development of LNCaP PDGF-D and PC3 PDGF-D cell lines and experimental design
Fazlul H. Sarkar: Principal investigator, conception and design, laboratory facility and financial support, experimental design, data collection and interpretation, manuscript writing and final approval of the manuscript.
REFERENCES
- 1.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 2.Hugo H, Ackland ML, Blick T, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 3.Graham TR, Zhau HE, Odero-Marah VA, et al. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68:2479–2488. doi: 10.1158/0008-5472.CAN-07-2559. [DOI] [PubMed] [Google Scholar]
- 4.Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 5.Korpal M, Lee ES, Hu G, et al. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shirakihara T, Saitoh M, Miyazono K. Differential regulation of epithelial and mesenchymal markers by deltaEF1 proteins in epithelial mesenchymal transition induced by TGF-beta. Mol Biol Cell. 2007;18:3533–3544. doi: 10.1091/mbc.E07-03-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 8.Eger A, Aigner K, Sonderegger S, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 9.Aigner K, Dampier B, Descovich L, et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaderna S, Schmalhofer O, Wahlbuhl M, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- 11.Vandewalle C, Comijn J, De CB, et al. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res. 2005;33:6566–6578. doi: 10.1093/nar/gki965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bindels S, Mestdagt M, Vandewalle C, et al. Regulation of vimentin by SIP1 in human epithelial breast tumor cells. Oncogene. 2006;25:4975–4985. doi: 10.1038/sj.onc.1209511. [DOI] [PubMed] [Google Scholar]
- 13.Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelialmesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SH, Cheung LW, Wong AS, et al. Estrogen Regulates Snail and Slug in the Down-Regulation of E-Cadherin and Induces Metastatic Potential of Ovarian Cancer Cells through Estrogen Receptor {alpha} Mol Endocrinol. 2008;22:2085–2098. doi: 10.1210/me.2007-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed N, Maines-Bandiera S, Quinn MA, et al. Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am J Physiol Cell Physiol. 2006;290:C1532–C1542. doi: 10.1152/ajpcell.00478.2005. [DOI] [PubMed] [Google Scholar]
- 16.Fischer AN, Fuchs E, Mikula M, et al. PDGF essentially links TGF-beta signaling to nuclear beta-catenin accumulation in hepatocellular carcinoma progression. Oncogene. 2007;26:3395–3405. doi: 10.1038/sj.onc.1210121. [DOI] [PubMed] [Google Scholar]
- 17.Gotzmann J, Fischer AN, Zojer M, et al. A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene. 2006;25:3170–3185. doi: 10.1038/sj.onc.1209083. [DOI] [PubMed] [Google Scholar]
- 18.Strutz F, Zeisberg M, Ziyadeh FN, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Lin C, Liu ZR. P68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing Axin from beta-catenin. Cell. 2006;127:139–155. doi: 10.1016/j.cell.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Fredriksson L, Li X, et al. PDGF-D is a potent transforming and angiogenic growth factor. Oncogene. 2003;22:1501–1510. doi: 10.1038/sj.onc.1206223. [DOI] [PubMed] [Google Scholar]
- 21.Ustach CV, Taube ME, Hurst NJ, Jr., et al. A potential oncogenic activity of platelet-derived growth factor d in prostate cancer progression. Cancer Res. 2004;64:1722–1729. doi: 10.1158/0008-5472.can-03-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ustach CV, Kim HR. Platelet-derived growth factor D is activated by urokinase plasminogen activator in prostate carcinoma cells. Mol Cell Biol. 2005;25:6279–6288. doi: 10.1128/MCB.25.14.6279-6288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong D, Wang Z, Sarkar SH, et al. Platelet-derived growth factor-D overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells. 2008;26:1425–1435. doi: 10.1634/stemcells.2007-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SM, Gaur AB, Lengyel E, et al. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garzon R, Fabbri M, Cimmino A, et al. MicroRNA expression and function in cancer. Trends Mol Med. 2006;12:580–587. doi: 10.1016/j.molmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Cano A, Nieto MA. Non-coding RNAs take centre stage in epithelial-to-mesenchymal transition. Trends Cell Biol. 2008;18:357–359. doi: 10.1016/j.tcb.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Bracken CP, Gregory PA, Kolesnikoff N, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68:7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 28.Kong D, Li Y, Wang Z, et al. Inhibition of angiogenesis and invasion by 3,3'-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007;67:3310–3319. doi: 10.1158/0008-5472.CAN-06-4277. [DOI] [PubMed] [Google Scholar]
- 29.Chua HL, Bhat-Nakshatri P, Clare SE, et al. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 30.Reigstad LJ, Varhaug JE, Lillehaug JR. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J. 2005;272:5723–5741. doi: 10.1111/j.1742-4658.2005.04989.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Kong D, Banerjee S, et al. Down-regulation of platelet-derived growth factor-D inhibits cell growth and angiogenesis through inactivation of Notch-1 and nuclear factor-kappaB signaling. Cancer Res. 2007;67:11377–11385. doi: 10.1158/0008-5472.CAN-07-2803. [DOI] [PubMed] [Google Scholar]
- 32.Kong D, Banerjee S, Huang W, et al. Mammalian target of rapamycin repression by 3,3'-diindolylmethane inhibits invasion and angiogenesis in platelet-derived growth factor-D-overexpressing PC3 cells. Cancer Res. 2008;68:1927–1934. doi: 10.1158/0008-5472.CAN-07-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burk U, Schubert J, Wellner U, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuda T, Yamagata K, Fujiyama S, et al. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nat Cell Biol. 2007;9:604–611. doi: 10.1038/ncb1577. [DOI] [PubMed] [Google Scholar]
- 35.Min C, Eddy SF, Sherr DH, et al. NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem. 2008;104:733–744. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- 36.Lee TH, Seng S, Sekine M, et al. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Re RN, Cook JL. An intracrine view of angiogenesis. Bioessays. 2006;28:943–953. doi: 10.1002/bies.20459. [DOI] [PubMed] [Google Scholar]
- 38.Re RN, Cook JL. Potential therapeutic implications of intracrine angiogenesis. Med Hypotheses. 2007;69:414–421. doi: 10.1016/j.mehy.2006.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenmuller T, Rydh K, Nanberg E. Role of phosphoinositide 3OH-kinase in autocrine transformation by PDGF-BB. J Cell Physiol. 2001;188:369–382. doi: 10.1002/jcp.1126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.