Abstract
This work tests the mode-of-action (MOA) hypothesis that maternal and developmental triclosan (TCS) exposure decreases circulating thyroxine (T4) concentrations via up-regulation of hepatic catabolism and elimination of T4. Time-pregnant Long-Evans rats received TCS po (0–300 mg/kg/day) from gestational day (GD) 6 through postnatal day (PND) 21. Serum and liver were collected from dams (GD20, PND22) and offspring (GD20, PND4, PND14, PND21). Serum T4, triiodothyronine (T3), and thyroid stimulating hormone (TSH) concentrations were measured by radioimmunoassay. Ethoxy-O-deethylase (EROD), pentoxyresorufin-O-depentylase (PROD) and uridine diphosphate glucuronyltransferase (UGT) enzyme activities were measured in liver microsomes. Custom Taqman® qPCR arrays were employed to measure hepatic mRNA expression of select cytochrome P450s, UGTs, sulfotransferases, transporters, and thyroid-hormone responsive genes. TCS was quantified by LC/MS/MS in serum and liver. Serum T4 decreased approximately 30% in GD20 dams and fetuses, PND4 pups and PND22 dams (300 mg/kg/day). Hepatic PROD activity increased 2- to 3-fold in PND4 pups and PND22 dams, and UGT activity was 1.5-fold higher in PND22 dams only (300 mg/kg/day). Minor up-regulation of Cyp2b and Cyp3a expression in dams was consistent with hypothesized activation of the constitutive androstane and/or pregnane X receptor. T4 reductions of 30% for dams and GD20 and PND4 offspring with concomitant increases in PROD (PND4 neonates and PND22 dams) and UGT activity (PND22 dams) suggest that up-regulated hepatic catabolism may contribute to TCS–induced hypothyroxinemia during development. Serum and liver TCS concentrations demonstrated greater fetal than postnatal internal exposure, consistent with the lack of T4 changes in PND14 and PND21 offspring. These data support the MOA hypothesis that TCS exposure leads to hypothyroxinemia via increased hepatic catabolism; however, the minor effects on thyroid hormone metabolism may reflect the low efficacy of TCS as thyroid hormone disruptor or highlight the possibility that other MOAs may also contribute to the observed maternal and early neonatal hypothyroxinemia.
Keywords: triclosan, mode-of-action, thyroid disruption, developmental exposure
1. Introduction1
Triclosan (2,4,4′-trichloro-2′-hydroxydiphenylether) (TCS), a ubiquitous environmental contaminant, decreases thyroid hormones (THs) in rats (Crofton et al. 2007; Paul et al. 2010a, b; Zorrilla et al. 2009). This effect was hypothesized to result from the interaction of TCS with xenobiotic nuclear receptors, resulting in up-regulation of hepatic catabolism of THs and subsequently increased biliary excretion (Paul et al. 2010b). Previous work demonstrated modest maternal and early neonatal thyroxine (T4) decreases following oral administration to dams (Paul et al. 2010a), which is concerning because decreased maternal TH during gestation results in neurological deficits in rats (Axelstad et al. 2008; Comer and Norton 1982; Gilbert et al. 2007; Goldey and Crofton 1998; Iniguez et al. 1992; Morreale de Escobar et al. 1988), as well as irreversible decreases in neurodevelopment and motor function in human children (Haddow et al. 1999; Pop et al. 2003; Pop et al. 1999). The irreversible effects of moderate decreases in maternal T4 during gestation on neurological function in children makes the determination of whether TCS alters THs during pregnancy, and the mode-of-action (MOA) for these TH decreases in rats, essential to assess the potential for TCS-induced hypothyroxinemia in humans.
Measurements of TCS in serum (Allmyr et al. 2008; Allmyr et al. 2006a; Allmyr et al. 2009; Hovander et al. 2002), breast milk (Adolfsson-Erici et al. 2002; Allmyr et al. 2006b; Dayan 2007), and TCS metabolites in urine (Calafat et al. 2008; Wolff et al. 2007) demonstrate that humans are exposed to TCS, and highlight the potential for developmental exposure via maternal TCS exposures. A study of nursing mothers revealed that TCS concentrations in maternal serum was 0.010 μg/kg to 38 μg/kg plasma weight (Allmyr et al. 2006b); of particular interest is that the TCS partitioned into serum at a 4-fold higher concentration than in the breast milk of these volunteers, suggesting that use of maternal serum concentrations for calculation of the oral dose to infants, in the absence of a milk concentration, would overestimate infant exposure (Allmyr et al. 2006b). Several studies have confirmed widespread but low concentrations of TCS in human milk, from low μg/kg – lipid (generally the limit of detection) up to 2100 μg/kg-lipid, with the majority of samples containing 200 μg/kg-lipid or less (Adolfsson-Erici et al. 2002; Allmyr et al. 2006b; Dayan 2007). Based on these findings, and the standard volume of milk consumed by infants (Butte et al. 1984; Butte et al. 2002; EPA 1997), Dayan (2007) estimated the maximum infant exposure to TCS as approximately 7.4 μg/kg/d. These findings underscore the potential for human developmental exposure via breast milk.
Previous work in rats demonstrated that perinatal maternal TCS exposure resulted in maternal and early neonatal hypothyroxinemia (Paul et al. 2010a). While the mechanism(s) responsible for the observed hypothyroxinemia during development have not been determined, we hypothesized that the MOA would be similar to that found in weanling rats exposed to TCS (Paul et al. 2010b). In weanling female rats, TCS exposure for four days decreased serum T4 and up-regulated markers hepatic catabolism, including uridine diphosphate glucuronyltransferase (UGT) activity, and Ugt1a1 and Sult1c1 expression (Paul et al. 2010b). The UGT 1A subfamily, specifically Ugt1a1 and Ugt1a6, and SULTs 1b1 and 1c, conjugate T4 in rat liver (Kester et al. 2003; Vansell and Klaassen 2002), and increased activity of these enzymes previously has been demonstrated to correlate with decreased systemic T4 concentrations in rodents (Barter et al., 1994; Buckley et al., 2009; Cheng et al., 2005; Guo et al., 2002; Johnson et al., 2002b; Kohn, 2000; Lecureux et al., 2009; Schuur et al., 1997; Vansell et al., 2002; Vansell et al., 2001; Visser et al., 1993; Wong et al., 2005). In addition, TCS increased hepatic Cyp2b1 and Cyp3a1 expression, suggestive of a hepatic nuclear receptor-mediated increase in T4 catabolism (Kretschmer and Baldwin 2005).
The main hypothesis of the current work is that TCS decreases T4 in dams and offspring via up-regulation of hepatic catabolism, a demonstrated MOA for chemically-induced maternal and neonatal hypothyroxinemia (Capen 1994; Hood and Klaassen 2000; Miller et al. 2009; Zhou et al. 2002a). This work also tests the hypothesis that the observed recovery of T4 to control values in PND14 and PND21 offspring of perinatally exposed dams (Paul et al. 2010b) results from toxicokinetic rather than toxicodynamic differences; i.e., a lack of effect on T4 in PND14 and PND21 pups is due to reduced exposure resulting from a limited transfer of TCS via lactation in rats.
2. Materials and Methods
2.1 Animals
Time-pregnant Long-Evans female rats (n=155), approximately 80–90 days of age were obtained from Charles River Laboratories Inc. (Raleigh, NC) on gestation day (GD) 1 (defined as the day after vaginal plugs were observed), and were allowed five days of acclimation in an American Association for Accreditation of Laboratory Animal Care International (AALAC) approved animal facility prior to initiation of treatment on GD6. Animals were housed individually in plastic hanging cages (45 cm × 24 cm × 20 cm), with heat sterilized pine shavings bedding (Northeastern Products Corp., Warrenton, NC). Colony rooms were maintained at 21 ± 2°C with 50 ± 10 % humidity on a photo-period of 12L:12D. Food (Purina Rodent Chow #5001, Barnes Supply Co., Durham, NC) and water were provided ad libitum. Tap water (Durham, NC water) was filtered through sand, then activated charcoal, and finally re-chlorinated to 4–5 ppm Cl− before use in the animal facility. All animal procedures were approved in advance by the Institutional Animal Care and Use Committee of the National Health and Environmental Effects Research Laboratory of the US EPA.
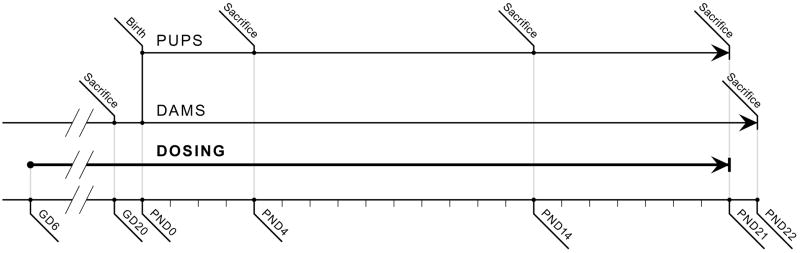
Dams were orally exposed via gavage each day between 0800 and 1000 hr, with the exception of GD21, when chemical was not administered to animals if there were signs of parturition. Figure 1 illustrates the dosing and tissue collection schedule. On GD21, dams were checked for the number of pups delivered at 0800, 1000, 1200, and 1500 hrs, and pups were aged as postnatal day (PND) 0 on the date of birth. All pups born from within a 24 hr period were considered to be the same age. On PND4, 14, and 21, offspring were counted, sexed, and group-weighed by sex. Average pup weight by sex was calculated by dividing the group weight by the number of pups. On PND4, litters were culled to 8 pups per litter, with the exception that litters comprised of less than 8 pups were not culled. When possible the sex ratio was balanced, with 4 male and 4 female pups maintained in each litter. Eye opening, determined as at least one eye open, was monitored once daily from PND11–17.
Figure 1.
Dosing and tissue collection schedule, from GD6 through PND22. Dams received TCS by oral gavage daily; no TCS was administered directly to offspring. Serum and liver were collected prenatally on GD20 from dams and fetuses and postnatally on PND4, PND14, and PND21 from pups and on PND22 from dams.
The current work includes some serum and liver samples from previous work (Experimental Block 1, Table 1) that used the same experimental design (Paul et al. 2010a); this previous short communication reported only reproductive toxicity parameters, body weights, and serum T4 for the postnatal period from Experimental Block 1. Two experimental blocks were completed with postnatal tissue collection from pups and dams (Table 1). Total combined sample numbers for T4 and hepatic microsomal assays were: 21, 12, 22, 22, 18 for 0, 10, 30, 100, and 300 mg/kg/day treatment groups. Due to limited serum volume particularly for early neonatal time points, TSH was measured in samples from Block 1 only (n=10 for 0, 30, 100 mg/kg/day and n=8 for 300 mg/kg/day), and T3 was measured in samples from Block 2 only (n=11 for vehicle control, n=12 for 10, 30, and 100 mg/kg/day, and n=10 for 300 mg/kg/day). For qPCR experiments, n=6/treatment group using liver tissue from experimental Block 2. For analytical determination of TCS in sera and liver tissue samples, n=6/treatment group from experimental Block 2. From experimental Blocks 1 and 2, two dams including one vehicle control and one in the 300 mg/kg/day treatment group, were never pregnant as determined by examination of the uterus; one dam in the 300 mg/kg/day group failed to deliver live pups; one other dam in the 300 mg/kg/day group died of unknown causes; and another was sacrificed early and excluded due to degenerative changes in one kidney and excessive urination.
Table 1.
Experimental blocks and n for each parameter measured. Experimental Blocks 1 and 2 included postnatal time points and experimental Block 3 included a single prenatal time point. The n reported here is based on the litter, i.e. for each endpoint the n is the same for dam and offspring samples.
| Experimental Block | Time Points | T4 | T3 | TSH | EROD/PROD UGT-T4 | qPCR | Analytical measurement |
|---|---|---|---|---|---|---|---|
| 1 | Offspring: PND4, 14, 21; Dams: PND22 | n = 21, 12, 22, 22, 18 for 0, 10, 30, 100, 300 mg/kg/d | Not measured | n = 10, 10, 10, 8 for 0, 30, 100, 300 mg/kg/d | n = 21, 12, 22, 22, 18 for 0, 10, 30, 100, 300 mg/kg/d | Not measured | Not measured |
| 2 | n = 11, 12, 12, 12, 10 for 0, 10, 30, 100, 300 mg/kg/d | Not measured | n = 6/treatment group | n = 6/treatment group | |||
| 3 | Fetuses and Dams: GD20 | n = 11, 11, 11, 11, 10 for 0, 10, 30, 100, 300 mg/kg/d | n = 6/treatment group | n = 6/treatment group | |||
Fetal and dam tissues at GD20 were collected from an additional experimental block, Block 3 (n=55, with 11 animals/treatment group). Dams were dosed GD6 through GD19 only. The GD20 sample size for all measurements is as follows: n=11 for 0, 10, 30, and 100 mg/kg/day treatment groups, and n=10 for the 300 mg/kg/day treatment group, except for analytical determinations of TCS in sera and liver tissue samples, which used n=6/treatment group (Table 1). One dam in the 300 mg/kg/day group was euthanized on GD15 due to morbity likely caused by abdominal torsion.
2.2 Chemicals and treatment
TCS (CAS#3380-34-5; 98+ % pure) was obtained from Sigma-Aldrich Chemical Company (St Louis, MO, LOT#06415CD, Cat#524190-10G) and Ciba Grenzach GmbH (Germany, Lot#60023CL7). Mass spectrometry analysis revealed that the TCS used was greater than 98.2% pure; the sample also contained 0.05% iso-TCS, 0.12% 2,8-dichlorodibenzodioxin, and 0.1% 2,4,8-trichlorodibenzodioxin, but was free of biologically active dioxin compounds. The dosing solutions (0, 30, 100, and 300 mg/mL) were prepared in corn oil (Sigma, Lot#117K0127), sonicated for 30 minutes, and stored in sealed amber vials at room temperature. Solutions were prepared every 5–7 days. The 300 mg/kg/day dose partially precipitated within 24 hours and was therefore sonicated daily before use. All doses were mixed on a stir plate during the dosing period each morning. Dams were semi-randomly assigned to treatment groups by counter-balancing body weights to obtain equivalent group bodyweight means. Administered volume was 1.0 ml corn oil/kg body weight; daily body weights were recorded, and administered volumes were adjusted daily by weight. Prior to the sacrifice of dams and fetuses on GD20, pups on PND4 (only culled pups), 14 and 21 (one pup per sex per litter), animals were moved to a holding room, dams were weighed and dosed, pups were weighed, and all animals were acclimated for a minimum of 30 min. Tissue collection was conducted between 0800 and 1200 hrs in an adjacent room with a separate air supply. The time of necropsy within the 4-hr period was balanced among dose groups to control for time-of day effects on thyroxine levels (Dohler et al. 1979; Jordan et al. 1980). Trunk blood was collected from GD20 fetuses, PND4 culled pups, and one male and one female pup per litter on PND14 and PND21, and each sample was then pooled into one tube per litter. Blood from dams was collected after decapitation on GD20 and PND22, 24 hr after the final dose. Blood was collected into serum separator tubes (Beckton Dickinson, 36-6154). Serum was obtained after clotting whole blood for 30 min on ice, followed by centrifugation at 1278× g at 4°C for 30 min. Serum samples were stored at −80°C until analysis. Liver was obtained from all offspring and dams, divided into sections, and immediately frozen in liquid nitrogen and stored at −80°C until analysis.
2.3 Thyroid hormone assays
Serum total T4 and total T3 was measured in duplicate by standard solid-phase Coat-A-Count radioimmunoassay (RIA) kits (Siemens Medical Solutions Diagnostics, Los Angeles, CA) for GD20 dams, PND22 dams, and PND4, 14, and 21 offspring. T3 was not measured for GD20 fetuses nor PND4 neonates due to limited serum volumes and a lack of effect on any other time point. Serum TSH concentrations were analyzed in duplicates with a double antibody RIA method (Greenwood et al. 1963) with some modification (Zorrilla et al. 2009). The TSH radioimmunoassays were performed using materials supplied by the National Hormone and Peptide Program (Harbor-UCLA Medical Center): iodination preparation (I-9-TSH); reference preparation (RP-3); and antisera (S-6-TSH). Iodination material was radiolabeled with 125I (Perkin Elmer, Shelton, CT). Assay variation was assessed using the multivalent control module (Siemens Medical Solutions Diagnostics, Los Angeles, CA; Lot021) to measure low, medium, and high total T4, T3, and TSH values before and after measuring the experimental samples. Intra-assay coefficients of variance for all assays were below 15%, and the inter-assay coefficients of variance for T4, T3, and TSH were 5.8, 10.8, and 13.1% for assays conducted over a two year period. Total serum T4 and TSH were calculated as ng T4/ml serum, and total serum T3 was calculated as ng T3/dL serum. Spiking of human serum calibration samples (included in RIA kits) and control rat serum samples with 8 ug/ml (average concentration found in PND4 neonates) confirmed that TCS itself did not interfere with the assay.
Total serum T4 for GD20 fetuses was measured in duplicate as described elsewhere (Bansal et al. 2005; Gauger et al. 2004). Briefly, each assay contained: 5 μl of rat serum, 100 μl barbital buffer (0.11 M barbital pH 8.6, 0.1% w/v 8-anilino-1-napthalene-sulfonic acid ammonium salt (ANS), 15% bovine γ-globulin Cohn fraction II, 0.1% gelatin), 100 μl anti-T4 (rabbit, Sigma) diluted to provide a final concentration of 1:21,000, and 100 μl 125I-T4 (diluted to yield a total of 12,000–15,000 cpm; Perkin Elmer/NEN). Triplicate standards ranging from 2 ng/mL to 64 ng/mL were prepared from T4 (Sigma). Following a 60 min incubation at 37°C, the tubes were chilled on wet ice for 30 min. Antibody-bound radiolabeled T4 was precipitated by addition of 300 μl ice-cold polyethylene glycol 8000 (20% w/w; Sigma). Tubes were then centrifuged at 1800 × g for 20 min at 4 °C, and the supernatant was aspirated and counted on a gamma counter (Packard CobraII). The lower limit of detection for this method was 2.0 ng/mL.
2.4 Microsome preparation and EROD and PROD assays
Liver microsomal fractions were prepared as described previously (DeVito et al. 1993) and standardized using total protein (Bio-Rad, Richmond, CA). Hepatic microsomal EROD and PROD activities were assayed using a method described previously (Paul et al. 2010b). Both EROD and PROD activity values were calculated as picomoles (pmol) resorufin per milligram protein per minute. Two positive controls were used to facilitate inter-assay and mechanistic comparison: pooled microsomes from rats acutely exposed to 10 μg/kg 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) or 300 mg/kg Aroclor (A1254, AccuStandard, New Haven, CT, Lot 124-191), and pooled microsomes from rats that received 4-day intraperitoneal exposure to phenobarbital (81 mg/kg/d; Sigma P5178, CAS#57-30-7, lot105K2614) or 5-pregnen-3 beta-ol-20-one-16 alpha-carbonitrile (PCN) (50 mg/kg; Sigma P0543, 97%, CAS#1434-54-4, lot#018K1093). The Aroclor/TCDD and PB/PCN pooled microsomes were employed to demonstrate prototypical AhR/CAR and CAR/PXR activator responses in microsomal assays.
2.5 UGT activity assay
UGT activity for T4 was measured by the method of (Beetstra et al. 1991) as modified by (Zhou et al. 2001). Detergent such as Brijj 56 was not included due to the potential for increased basal T4 glucuronidation (Craft et al. 2002). Two positive controls were used to facilitate inter-assay and mechanistic comparison: pooled microsomes from rats acutely exposed to 10 μg/kg 2,3,7,8-tetrachlorodibenzo-p-dioxin or 300 mg/kg Aroclor, and pooled microsomes from rats that received 4-day intraperitoneal exposure to phenobarbital (81 mg/kg/d) or PCN (50 mg/kg) (as described above).
2.6 mRNA preparation and analysis
For each of the six time points included in this study, mRNA was extracted from an n=6/treatment group, with all treatment groups represented (0, 10, 30, 100, 300 mg/kg/day). Extraction of mRNA from frozen tissue was performed using Qiagen RNeasy Mini Kits (Qiagen, Valencia, CA). Approximately 60 mg of frozen tissue was homogenized and split between two Qiagen QIAShredder columns to assure complete homogenization. Contaminating DNA was removed on the Qiagen Mini-Kit columns using the Qiagen DNase kit. RNA content and purity was assessed using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc.; Wilmington, DE) at absorbances of 230, 260, and 280 nm for the duplicate RNA samples. Samples used in experiments were selected from duplicates based on optimizing the 260/230 and 260/280 ratios to 2.0 (range was 1.80 – 2.14 for 260/230 and 1.95 – 2.11 for 260/280). The average 260/230 ratio for samples used was 2.02, and the average 260/280 ratio for samples used was 2.06.
RNA was converted to cDNA using the High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Applied Biosystems, Foster City, CA), as per the manufacturer directions. Two micrograms of RNA were added to each 20 μL reverse transcription reaction.
qPCR gene expression assays were performed using Custom Taqman ® Low Density Arrays (Applied Biosystems, Foster City, CA; Cat.#: 4309169), which are 384-well microfluidic cards preloaded with 23 unique gene targets, including four potential endogenous controls, each performed in duplicate for each sample. Eight samples were loaded per card, in a semi-random order, where samples for each developmental time point were randomized and then run as separate cohorts by developmental time point. Following sample loading, plates were centrifuged at 1200 rpm, 24°C for1 min two times before qPCR on a ABI Prizm 7900HT (Applied Biosystems).
qPCR data sets were analyzed using a relative quantification method (2−ΔΔCT) to describe the change in expression of the target experimental gene relative to an endogenous reference gene (Livak and Schmittgen 2001). Choice of an endogenous reference gene was based on constant gene expression across all of the dosing groups for each developmental time point (Dunn and Klaassen 1998; Livak and Schmittgen 2001). The candidate endogenous reference genes Actb, GAPDH, Rps18, and Rpl13a were run for each sample (Pohjanvirta et al. 2006). Since there can be differential expression of common endogenous control genes at different developmental ages, choice of the endogenous control gene was performed by picking the gene for which the standard deviation from the mean of treatment group expression values was the smallest, as per the a priori criteria. Rps18 was chosen for GD20 and PND22 dams, GD20 fetuses, and PND21 offspring. Actb was selected for PND4 and PND14 offspring. However, using Rps18 for all time points did not significantly alter the results (data not shown). Choice of the genes included on the low density array was contingent upon three criteria: involvement of the isoform in TH metabolism and transport in rats, regulation of the isoform by nuclear receptors AhR, CAR, PXR, and/or PPAR, and sensitivity to tissue levels of thyroid hormones. A list of the genes and Taqman identifiers for these is listed in Table 2.
Table 2.
Taqman Gene Expression Assays included in the Taqman Custom Low Density Array. Rps18 was used as the endogenous control to standardize the gene expression assay results for GD20 and PND22 dams, GD20 fetuses, and PND21 offspring. PND4 and PND14 offspring were standardized to Actb.
| Summary of Taqman Gene Expression Assays included in the Taqman Custom Low Density Array | |||
|---|---|---|---|
| Function category | Gene | Gene abbreviation | Taqman Gene Expression Assay ID |
| Nuclear receptors | aryl-hydrocarbon receptor | AhR | Rn00565750_m1 |
| peroxisome proliferator activated receptor alpha | Pparα | Rn00566193_m1 | |
| Phase I metabolism | cytochrome P450, family 1, subfamily a, polypeptide 1 | Cyp1a1 | Rn00487218_m1 |
| cytochrome P450, family 2, subfamily b, polypeptide 2 | Cyp2b2 | Rn02786833_m1 | |
| cytochrome P450, family 3, subfamily a, polypeptide 23/polypeptide 1 | Cyp3a1/3a23 | Rn01640761_gH | |
| cytochrome P450, family 3, subfamily a, polypeptide 2 | Cyp3a2 | Rn01412889_mH | |
| cytochrome P450, family 3, subfamily a, polypeptide 9 | Cyp3a9 | Rn00595977_m1 | |
| cytochrome P450, family 4, subfamily a, polypeptide 2 | Cyp4a2 | Rn01417066_m1 | |
| Phase II metabolism | UDP glucuronosyltransferase 1 family, polypeptide A1 | Ugt1a1 | Rn00754947_m1 |
| UDP glucuronosyltransferase 1 family, polypeptide A6 | Ugt1a6 | Rn00756113_mH | |
| UDP glycosyltransferase 2 family, polypeptide B | Ugt2b | Rn02349652_m1 | |
| sulfotransferase family, cytosolic, 1B, member 1 | Sult1b1 | Rn00673872_m1 | |
| sulfotransferase family, cytosolic, 1C, member 3 | Sult1c3 | Rn00581955_m1 | |
| Hepatic transport | ATP-binding cassette, sub-family C (CFTR/MRP), member 2 | Abcc2/Mrp2 | Rn00563231_m1 |
| solute carrier organic anion transporter family, member 1a4 | Slco1a4/Oatp1a4 | Rn00756233_m1 | |
| solute carrier family 16 (monocarboxylic acid transporters), member 2 | Slc16a2/Mct8 | Mm00486202_m1 | |
| Thyroid hormone-responsive | deiodinase, iodothyronine, type I | Dio1 | Rn00572183_m1 |
| malic enzyme 1 NADP(+)-dependent, cytosolic | Me1 | Rn00561502_m1 | |
| thyroid hormone responsive | Thrsp | Rn01511034_m1 | |
| Candidate endogenous controls | ribosomal protein L13A | Rpl13a | Rn00821946_g1 |
| ribosomal protein S18 | Rps18 | Rn01428915_g1 | |
| glyceraldehyde-3-phosphate dehydrogenase | Gapdh | Rn99999916_s1 | |
| actin, beta | Actb | Rn00667869_m1 | |
2.7 Analytical measurement of TCS in sera and liver
Serum and liver homogenates were analyzed for TCS content by liquid chromatography-mass spectrometry (LC/MS/MS) utilizing isotope dilution (Agilent 1200 high performance liquid chromatography [HPLC] with API 4000 Triple Quadrapole MS), similar to the method previously reported for measuring parent TCS (Fort et al. 2010). The liver homogenates were prepared from frozen liver tissue by homogenization of 0.5 g of tissue in 2 mL of diH2O. Ionization was performed with TurboIon spray in negative mode for the TCS ion, with multiple reaction monitoring detection for parent ion (286.0 amu) and product ion (35.1 amu). The calibrated range for TCS determination was 1–1000 ng/mL with 13C6-TCS as an internal standard in blank serum. Serum and liver homogenate were analyzed undiluted unless preliminary data suggested out-of-range values for TCS.
Parent TCS was measured directly without a hydrolysis step. 100 μL serum or liver homogenate was spiked with 10 ng 13C6-TCS as internal standard. Acetonitrile (200 μL) was added to precipitate protein. Samples were vortexed (1 min), briefly centrifuged, and then analyzed by LC/MS/MS. The limit of detection for parent TCS was 1 ng/mL.
Conjugated and parent TCS, or total TCS, were measured following an enzymatic hydrolysis step with combined glucuronidase and sulfatase and precipitation with acetonitrile. Serum or liver homogenate (100 μL) was spiked with 10 ng 13C-TCS and a glucuronide/sulfate standard tracer. After addition of 50 μL of β-glucuronidase and sulfatase enzymes (0.2 mg of enzyme total per sample), a 4 hr incubation at 37°C was performed. Acetonitrile (400 μL) was added to stop the reaction and precipitate the protein. Samples were vortexed (1 min) and centrifuged. The supernatant containing total TCS was then analyzed by LC/MS/MS. The limit of detection for total TCS was 5 ng/mL serum or liver homogenate.
2.8 Data Analysis
Dam bodyweight data for prenatal and postnatal stages were analyzed separately using repeated measures ANOVAs (SAS 9.1, SAS Institute, Cary, NC), followed by mean contrast testing with Duncan’s New Multiple Range Test (p<0.05), with dose and animal as independent variables. All other data were analyzed by ANOVAs, with significant main effects followed by Duncan’s New Multiple Range Test. Gestation length was defined as the period from GD0 to the day of birth. The viability index was calculated as the number of pups alive on PND0 divided by the number alive on PND4 per litter prior to culling. Sex ratio was calculated as the number of female pups divided by the number of male pups prior to culling. Eye opening was calculated as mean percent of pups with at least one eye open in a litter for each treatment. Litter was used as the statistical unit in the analysis of all endpoints in offspring.
Benchmark dose (BMD) and lower-bound confidence limit (BMDL) estimates were determined using USEPA Benchmark Dose Software (BMDS Version 2.0beta) as previously described (Crofton et al. 2007; Zorrilla et al. 2009). The benchmark response (BMR) (EPA 2000) was set at a 20% decrease in T4, reflecting previous use of this BMR in the literature.
3. Results
Gestational maternal exposure to TCS did not affect the number of fetuses [F(4,48)=1.13, p<0.3549], and there were no effects of treatment on maintenance of pregnancy, as indicated by the lack of treatment-related difference in the number of implantation sites and fetuses on GD20 [F(4,48)=1.41, p<0.2452]. Perinatal maternal triclosan exposure did not affect any reproductive parameters including: gestation length [F(3, 93)=0.05, p<0.8155], litter size [F(3,93)=0.62, p<0.4314], viability index [F(3,92)=3.55, p=0.0627], or sex ratio [F(3,92)=2.93, p<0.0901]. Treatment did not elicit effects on the day of eye opening [F(1,90)=1.51, p<0.2216] (data not shown). Further, no gross terata were observed in any of the pups, and offspring viability was unaffected by treatment.
No treatment-related clinical signs of toxicity were observed in the dams or offspring at any time following TCS exposure. There was no main effect of treatment on dam body weight during gestation [F(4,91)=1.65, p<0.1681]. However, dam body weights for the 300 mg/kg/day treatment group decreased by approximately 10% throughout the postnatal period (p<0.05). This observation is supported by a main effect of treatment on the body weight of dams [F(4,83)=4.80, p<0.0016], though there was no postnatal-day and treatment interaction [F(80,1660)=1.76, p<0.7005]. There were no effects of treatment on pup body weight, male or female, at ages PND4, PND14, or PND21 (data not shown). There were no effects of treatment on dam liver weight [F(4,89)=1.31, p<0.2740] or liver-body weight ratio [F(4,85)=1.52, p<0.2025].
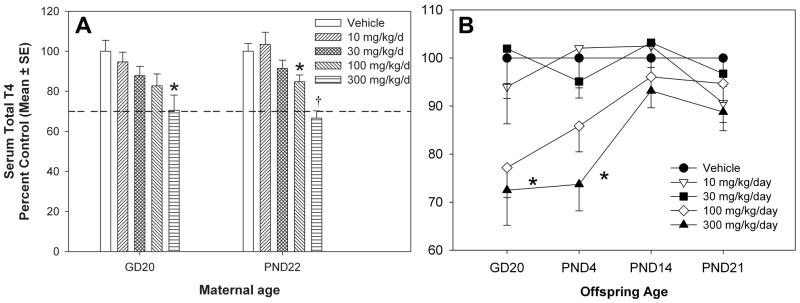
A main effect of TCS treatment on serum total T4 was observed, with a significant decrease of 15% for PND22 dams with 100 mg/kg/day of TCS [F(4,85)=12.77, p<0.0001], and 30% for both the GD20 dams [F(4,48)=3.78, p<0.0095] and the PND22 dams with 300 mg/kg/day of treatment (Figure 2). The no-observed-effect level (NOEL) for TCS on serum T4 was 100 mg/kg/day for GD20 dams and 30 mg/kg/day for PND22 dams. The BMDs for serum T4 concentrations were 124 and 115 mg/kg/day with 95% lower confidence limits of 25.2 and 62.1 mg/kg/day for GD20 and PND22 dams, respectively (Table 3). For offspring, there was a main effect of TCS treatment on serum total T4, except that this effect was limited to GD20 fetuses and PND4 neonates. Serum total T4 was decreased by 23% and 28% in GD20 fetuses from the 100 and 300 mg/kg/day treatment groups [F(4,48)=2.91, p<0.0312] and by 26% in PND4 pups from the 300 mg/kg/day treatment group [F(4,85)=5.02, p<0.0011]. The computed BMDs for serum T4 were 95.4 and 150 mg/kg/day with 95% lower confidence limits of 33.0 and 61.8 mg/kg/day for GD20 fetuses and PND4 neonates, respectively (Table 3). There were no effects of treatment on serum T4 for PND14 [F(4,89)=1.44, p<0.2261] or PND21 [F(4,89)=1.59, p<0.1831] neonates from any treatment group.
Figure 2.
Percent of control T4. A) GD20 and PND22 Dams, with vehicle control = 23.1 ± 1.3 and 51.8 ± 2.1 ng/mL, respectively. B) GD20 Fetuses and Pups aged PND4, PND14, and PND21, with vehicle control = 4.15 ± 0.22, 9.70 ± 0.43, 42.8 ± 1.9, 38.2 ± 1.3 ng/mL, by age, respectively. V=vehicle control; † = significantly different from control and all other treatment groups; * = significantly different from vehicle control.
Table 3.
No-observed-effect level (NOEL) and benchmark dose levels for T4 decreases. NOELs are reported as the mg/kg/day exposure group. BMD = benchmark dose US EPA Benchmark Dose Software (BMDS Version 2.0Beta) was used to determine the BMD and BMDL, with a 95% confidence limit, for the selected BMR = 20% reduction in serum T4.
| No-observed-effect level and benchmark dose levels for T4 decreases by age
| |||
|---|---|---|---|
| Age | NOEL | BMD | BMDL |
| GD20 Dams | 100 | 124 | 25.2 |
| PND22 Dams | 30 | 115 | 62.1 |
| GD20 Fetuses | 100 | 95.4 | 33.0 |
| PND4 Pups | 100 | 150 | 61.8 |
There were no effects of treatment on serum T3 on any dam or offspring timepoint (Supplementary Information, Part A). Only sera from GD20 dams [F(4,49)=0.82, p<0.5215], PND22 dams [F(4,51)=2.23, p<0.0783], PND21 pups [F(4,51)=0.37, p<0.8296], and PND14 pups [F(4,52)=0.52, p<0.7238] were analyzed. Offspring from GD20 and PND4 were not tested due to limited serum volumes. There were no effects on T3 for the GD20 or PND22 dams, both of which demonstrated T4 reductions.
There were no effects of treatment on serum TSH on any age tested, including GD20 dams [F(4,49)=0.64, p<0.6382], PND22 dams[F(3,34)=0.27, p<0.8478], PND21 pups [F(3,34)=0.42, p<0.7406], PND14 pups [F(3,33)=0.52, p<0.6733], or PND4 pups [F(3,27)=2.01, p<0.1363] from any treatment group (Supplementary Information, Part B). Serum obtained from GD20 fetuses was not tested due to limited serum volumes.
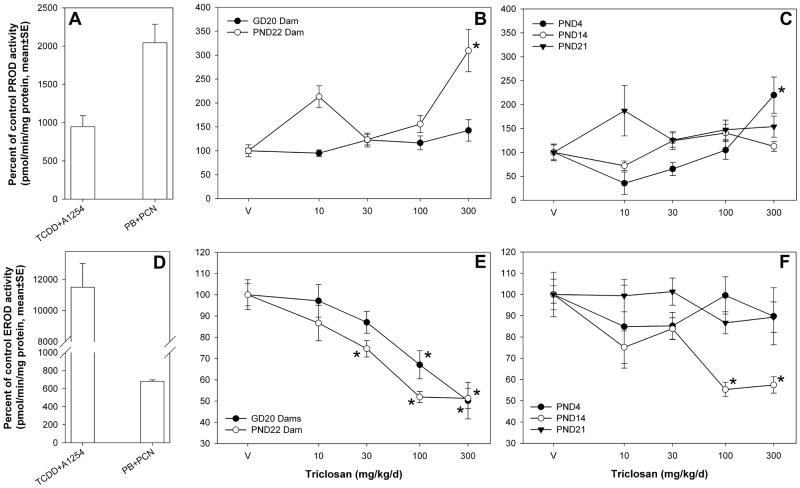
TCS increased PROD activity in an age-dependent manner for the groups associated with T4 decreases in this study. Increased hepatic microsomal PROD activity was observed for PND4 neonates and PND22 dams only (Figure 3). PROD activity increased to 220 ± 38 percent of vehicle control for PND4 pups [F(4,51)=8.19, p<0.0001] and to 309 ± 44 percent of vehicle control for PND22 dams [F(4,85)=9.55, p<0.0001]. No significant effects were observed in GD20 dams [F(4,48)=1.89, p<0.1272] despite an effect of TCS on T4 for animals at this time point. No effects of TCS were observed on PROD activity induction for PND14 [F(4,85)=2.34, p<0.0616) or PND21 [F(4,85)=1.64, p<0.1705] neonates. PROD activity was not tested in GD20 as Cyp2b expression is non-detectable at this age (Borlakoglu et al. 1993). TCS decreased EROD activity for GD20 dams [F(4,48)=9.04, p<0.0001], PND22 dams [F(4,85)=20.03, p<0.0001], and PND14 neonates [F(4,85)=11.82, p<0.0001] by approximately 40–50% for the 100 and 300 mg/kg/day treatment groups (Figure 4). No effects of TCS were seen on EROD activity for PND4 [F(4,51)=0.44, p<0.7797] or PND21 [F(4,85)=1.34, p<0.2616] neonates, and GD20 fetuses were not tested. Positive controls (Figure 3) representing prototypical microsomal enzyme inducers have been included for comparison. Positive control #1 is a pool of microsomes obtained from animals treated once by gavage with either TCDD (10 μg/kg) or Aroclor 1254 (300 mg/kg); this pool induced PROD and EROD activities to 946 ± 140 and 11500 ± 1500 percent of control, respectively. Positive control #2 is a pool of microsomes obtained from animals treated for four days by intraperitoneal injection with either phenobarbital (81 mg/kg/d) or PCN (50 mg/kg); this pool induced PROD and EROD activities to 2040 ± 243 and 681 ± 17.5 percent of control, respectively.
Figure 3.
Percent of control EROD and PROD activity for dams and offspring compared to controls. A) PROD activity for control microsome pools. The controls are presented as the mean percent of vehicle control for all of the age groups tested. TCDD+ A1254 is a microsome pool from rats exposed to a single gavage dose of TCDD (10 μg/kg) or Aroclor 1254 (300 mg/kg); PB+PCN is a microsome pool from rats that received a 4-day intraperitoneal exposure to phenobarbital (81 mg/kg/d) or PCN (50 mg/kg). B) PROD activity for dams at GD20 and PND22. C) PROD activity for pups aged PND4, PND14, and PND21. D) EROD activity for controls. E) EROD activity for dams at GD20 and PND22. F) EROD activity for pups aged PND4, PND14, and PND21.V=vehicle control; * = significantly different from vehicle control.
Figure 4.
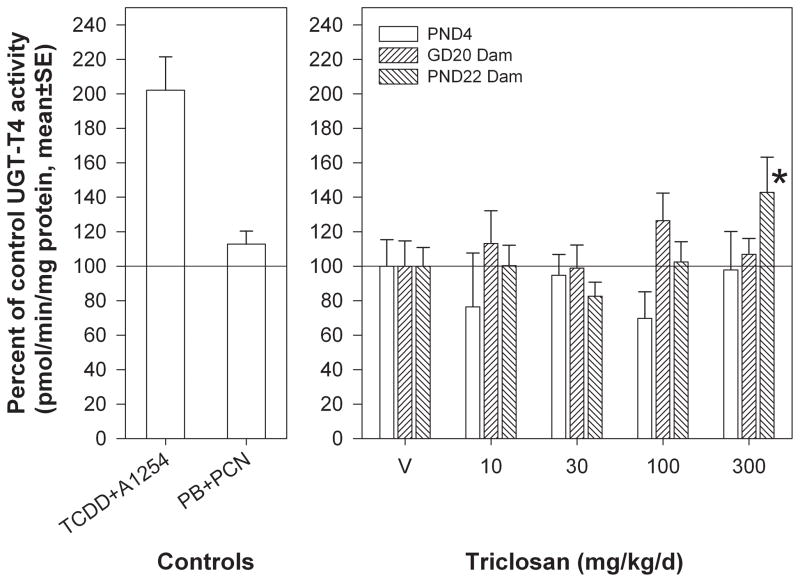
Hepatic microsomal UGT-T4 activity. A) Positive controls. TCDD+ A1254 is a microsome pool from rats exposed to a single gavage dose of TCDD (10 μg/kg) or Aroclor 1254 (300 mg/kg); PB+PCN is a microsome pool from rats that received a 4-day intraperitoneal exposure to phenobarbital (81 mg/kg/d) or PCN (50 mg/kg). B) PND4 neonates, GD20 dams, and PND22 dams. V=vehicle control; * = significantly different from vehicle control.
Hepatic microsomal UGT-T4 activity was induced by TCS only for PND22 dams [F(4,84)=3.00, p<0.0228] to approximately 150% of vehicle control with no effect of block (Figure 4). UGT-T4 activity for GD20 dams [F(4,48)=0.57, p<0.6849] and PND4 neonates [F(4,49)=0.70, p<0.5954] was not affected by treatment, and UGT-T4 activity was not measured for ages which did not have a treatment-related effect on T4 (PND14, PND21 neonates) or GD20 fetuses, due to limited tissue. Positive control #1 induced UGT activity 202 ± 19 percent of control, and positive control #2 failed to induce UGT-T4 activity in this assay (113 ± 7.6 percent of control).
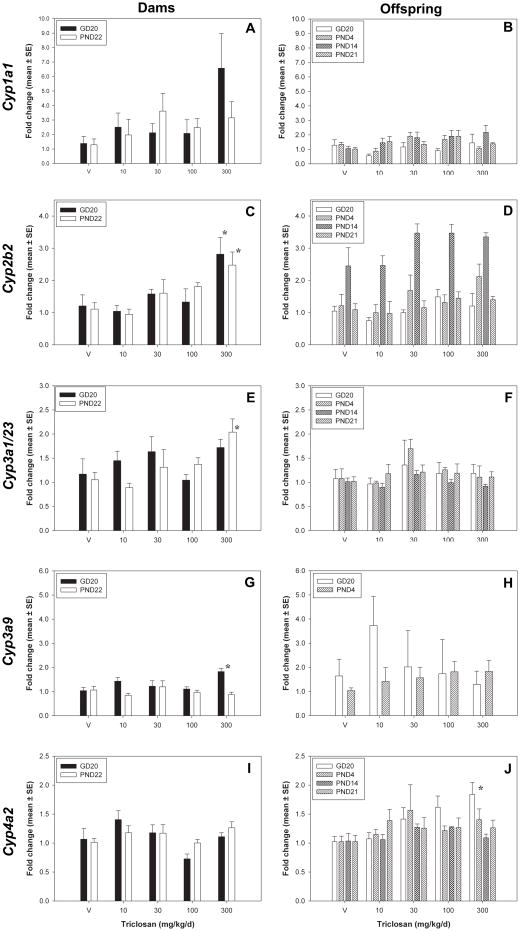
TCS exposure produced minor effects on CYP gene expression, producing few and only modest significant effects at any time point included in this study (Figure 5). Cyp2b2 expression was increased to 2.81 ± 0.52 and 2.48 ± 0.41 fold-control at 300 mg/kg/d for GD20 dams [F(4,25)= 4.12, p<0.0107] and PND22 dams[F(4,25)=4.35, p<0.0083], respectively. Cyp3a1 was induced 2.04 ± 0.27 fold-control for PND22 dams only at 300 mg/kg/day [F(4,25)=3.55, p<0.0200], and there was also a strong increasing trend for Cyp3a2 in PND22 dams as well (2.6 fold-control at 300 mg/kg/day, p<0.0529) (data not shown). Cyp3a9 expression was induced to 1.83 ± 0.14 fold-control for GD20 dams [F(4,25)=4.17, p<0.0100], and values for Cyp3a9 expression in PND14 and PND21 pups could not be reported due to low expression. Cyp4a2 was induced in GD20 fetuses to 1.84 ± 0.20 fold-control [F(4,25)=4.46, p<0.0074].
Figure 5.
Hepatic CYP gene expression changes. A,C,E, and G) Dams: GD20 and PND22 dams for Cyp1a1, Cyp2b2, Cyp3a1/23, Cyp3a2, Cyp3a9, and Cyp4a2. B, D, F, and I) Offspring: GD20 fetuses, PND4, PND14, and PND21 pups for Cyp1a1, Cyp2b2, Cyp3a1/23, Cyp3a2, Cyp3a9, and Cyp4a2. V=vehicle control.* = significantly different from vehicle control.
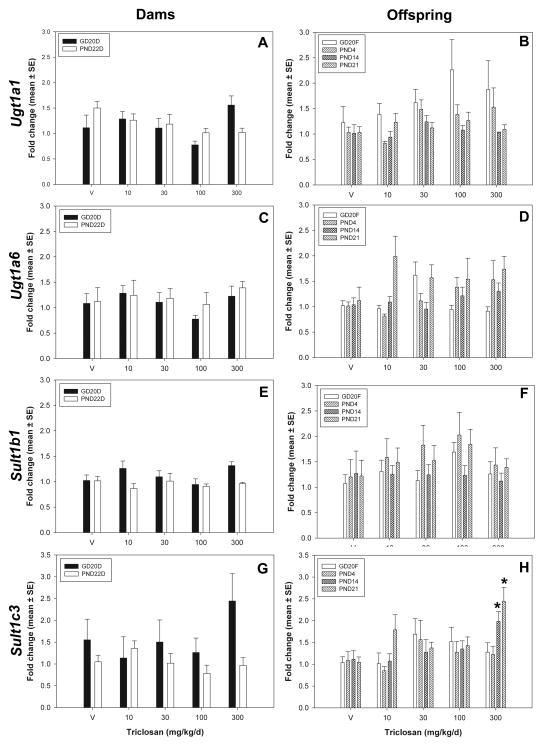
There were no statistically significant treatment related effects on hepatic gene expression of Ugt1a1 or Ugt1a6 for any of the developmental time points included in this study (Figure 6). Hepatic sulfotransferase expression of Sult1b1 was not affected by treatment for any of the time points included. However, expression of Sult1c3 was induced for PND14 and PND21 neonates to 1.98 ± 0.23 fold-control [F(4,25)=2.86, p<0.0444] and 2.44 ± 0.32 fold-control [F(4,25=4.81, p<0.0051], respectively. There were no significant changes in gene expression in GD20 dams, PND22 dams, GD20 fetuses, or PND4 neonates, the groups which experienced decreased serum T4 at 300 mg/kg/day.
Figure 6.
Hepatic Phase II gene expression. A,C,E, and G) Dams: GD20 and PND22 dams for Ugt1a1, Ugt1a6, Sult1b1, Sult1c3. B, D, F, and I) Offspring: GD20 fetuses, PND4, PND14, and PND21 pups for Ugt1a1, Ugt1a6, Sult1b1, Sult1c3. V=vehicle control.
There were no significant dose-dependent effects of TCS treatment on transporter gene expression for the isoforms chosen: Mct8, Mrp2, and Oatp1a4 (data not shown). Oatp1a4 basal expression was too low in control GD20 fetuses to be included in our relative quantitative analysis, and so values for GD20 fetuses are were not calculated.
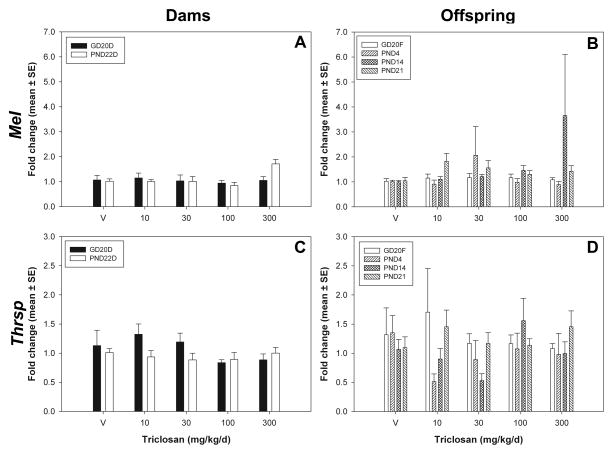
TCS did not significantly affect the gene expression of any of the TH-responsive genes measured, i.e. DioI, Me1, and Thsrp (also known as Spot14) at any time point included in this study (Figure 7).
Figure 7.
Hepatic TH-responsive gene expression. A and C) Dams: GD20 and PND22 dams for MeI and Thrsp. B and D) Offspring: GD20 fetuses, PND4, PND14, and PND21 pups for MeI and Thrsp. V=vehicle control.
As no statistically significant effects were observed for TCS exposure and expression of hepatic transporters or TH-responsive genes, comparison to positive control data was particularly important (Supplementary Information, Part C). With respect to hepatic transport, prototypical inducers increased the expression of Mrp2 and Oatp1a4, but produced no effects on Mct8 expression. Prototypical inducers increased the expression of MeI and Thrsp, but did not appear to induce or significantly change expression of DioI. Refer to Supplementary Information for qPCR data from the prototypical inducer set and expanded results and discussion.
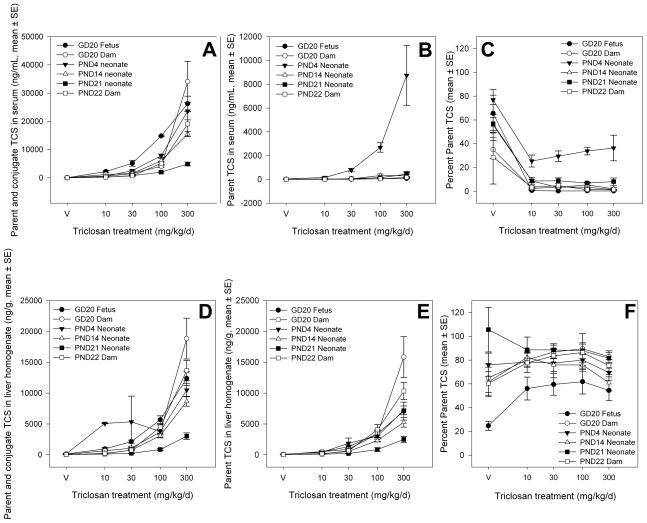
Analytical measurement of total TCS in sera, defined here as the parent TCS and conjugated TCS, demonstrated similar serum TCS concentrations for GD20 dams, PND22 dams, GD20 fetuses, and PND4 neonates (Figure 8). Total TCS in serum was markedly lower in PND14 and PND21 offspring; PND14 and PND21 neonates had roughly 50% and 5%, respectively, of the serum concentrations found in dams and younger offspring. TCS was predominantly found in its conjugated forms in the systemic circulation of rats, with the exception of PND4 neonates, which had markedly increased parent TCS in their sera. This is further illustrated by examination of the percent parent (Figure 8C) in PND4 neonates, which is consistently measured as 30–40% of the total TCS found in their circulation. This is in contrast to all of the other age groups, which all contained approximately 10% parent TCS in their sera.
Figure 8.
Mean total, parent, and percent parent TCS content in sera and liver (ng/mL, ± SE). A) Total TCS in sera, comprised of parent and glucuronide and sulfate conjugates (ng/mL); B) Parent TCS only in sera (ng/mL); C) Percent parent out of the total TCS in sera; D) Total TCS in liver, comprised of parent and glucuronide and sulfate conjugates (ng/g); E) Parent TCS only in liver (ng/g); F) Percent parent out of the total TCS in liver.
Measurement of total TCS in liver homogenate illustrated a similar relationship found with the sera data set. GD20 dams and PND22 dams demonstrated the highest liver concentrations of total TCS, followed by GD20 fetuses and PND4 neonates with similar amounts of total TCS present in liver (Figure 8). Total TCS appeared in liver per the following relationship: GD20 dams ≈ PND22 dams > GD20 fetuses ≈ PND4 neonates > PND14 neonates ≫ PND21 neonates, indicating that as expected, the animals that were directly gavaged with TCS, i.e. the dams, had the highest target tissue dose of total TCS. The relative concentrations of total TCS in liver appear to have been consistent with the concentration of parent TCS in liver. In contrast to the concentrations of parent TCS in sera, parent TCS appears to have comprised a larger fraction of the TCS present in the livers of these animals. For PND21 neonates, which demonstrated the lowest target tissue doses, nearly all of the TCS was parent (80–100%), with the amount of conjugate present increasing with the amount of total TCS exposure. For GD20 fetuses, approximately half of the TCS present in their livers appears to have been parent, except for the vehicle control group, which appeared to have about 20% parent TCS. However, the amounts of TCS present in the vehicle control group are close to zero. For all of the other time points, including GD20 dams, PND22 dams, PND4 neonates, and PND14 neonates, the amount of parent TCS in the liver was about 70–80%, except for the vehicle group, which again had trace amounts of TCS that were about 60% parent TCS.
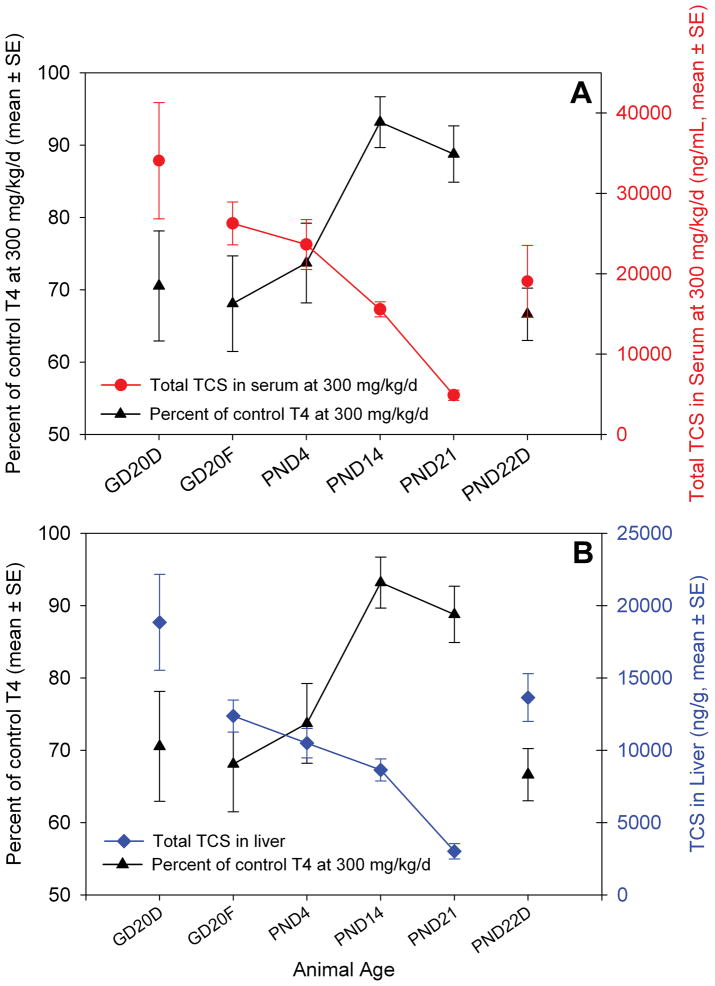
Figure 9 illustrates the amount of total TCS in sera (A) and liver (B) at 300 mg/kg/day, the dose that elicited T4 effects in GD20 dams and fetuses, PND4 neonates, and PND22 dams. A decreasing internal exposure in offspring throughout lactation is evident, with an apparent relationship that is consistent for both sera and liver samples: GD20 fetuses ≈ PND4 neonates > PND14 neonates ≫ PND21 neonates. For total TCS in sera and liver, it appears that for TCS to induce equivalent hypothyroxinemia in GD20 and PND22 dams, a 30% reduction in T4, GD20 dams maintain a higher internal concentration of TCS. Similarly, GD20 fetuses and PND4 neonates also experienced an approximate 30% decrease in T4 in the 300 mg/kg/day treatment group, and their sera and liver concentrations appear to be nearly the same as the two groups of dams.
Figure 9.
TCS content in sera and liver versus serum T4 concentrations at 300 mg/kg/day. A) TCS content in sera (ng/mL). B) TCS content in liver (ng/g tissue).
4. Discussion
This work provides the first report of several key results following developmental TCS exposure. First, dams, fetuses, and early neonates are susceptible to TCS-induced hypothyroxinemia, with no effects on T3 or TSH concentrations at any time point. Serum total T4 concentrations were reduced in GD20 fetuses and PND4 neonates, but recovered to control values for PND14 and PND21 neonates. This confirms the unique pattern of T4 effects in offspring previously reported (Paul et al. 2010a) and extends the serum T4 findings to late gestation for fetuses and dams. Second, we observed modest increases in markers of hepatic microsomal enzyme induction that were generally consistent with moderate decreases in serum T4 and our previously hypothesized MOA. Third, analysis of serum and liver TCS content revealed that PND14 and PND21 neonates had lower internal concentrations of TCS, which suggested that toxicokinetics may account for the recovery of serum T4 to control values in these animals. Overall these data demonstrate that TCS does not cause the fetal and neonatal rat to experience more exposure or greater effects compared to the perinatally-exposed dam. The data indicate that in the rat, TCS is a low-potency and low-efficacy thyroid hormone disruptor. Developmental exposures of up to 300 mg/kg/day resulted in moderate decreases in T4 and minor up-regulation of hepatic catabolism, which supports our hypothesis that TCS may increase catabolism and elimination of T4, potentially as one of several possible MOAs contributing to the observed systemic T4 decreases. The current work also confirms previous findings of no reproductive toxicity, with no effects of treatment on gestation length, viability, eye opening, sex ratio, or pup body weights, with minor decreases in dam bodyweight observed during the postnatal period for the 300 mg/kg/d group (Paul et al. 2010a).
Perinatal maternal exposure to TCS resulted in mild hypothyroxinemia in GD20 dams and PND22 dams, as well as in GD20 fetuses and PND4 neonates. The observed hypothyroxinemia in dams, fetuses, and young offspring demonstrates and confirms the previous findings of TCS-induced hypothyroxinemia following developmental and juvenile exposures (Crofton et al. 2007; Paul et al. 2010a, b; Zorrilla et al. 2009). The approximate 30% reductions in T4 observed in dams at GD20 and PND22, and GD20 fetuses and PND4 is consistent with our previous studies in rats (Crofton et al. 2007; Paul et al. 2010a, b). However, different rat strains, gender, chemical source, and exposure duration may impact the relative potency and efficacy of TCS exposure and limit the ability to compare studies across laboratories. A pubertal assay, which employed a 31-day exposure with juvenile male Wistar rats, demonstrated 50% decreases in serum total T4 with 30–100 mg/kg/day TCS (Zorrilla et al. 2009), suggesting greater potency in the pubertal Wistar model. Currently there is no evidence to inform a hypothesis about the differences in potency between the study in juvenile weanling male Wistar rats and studies in Long-Evans female rats, particularly pregnant rats, but a plausible hypothesis is that there are strain differences in the toxicodynamic and/or toxicokinetic effects of TCS.
The unique pattern of effects on serum T4 in offspring in response to maternal TCS exposure, i.e. T4 was decreased at early postnatal ages and then recovered to control levels during the second and third week of lactation, confirmed our previous preliminary findings (Paul et al. 2010a). The lack of effect on T4 concentrations in PND14 and PND21 offspring during the lactation period generated two hypotheses: (1) TCS elicits a different toxicodynamic response in perinatally-exposed PND14 and PND21 neonates with no subsequent effects on T4 concentrations; and, (2) the toxicokinetics of TCS during lactation in the rat limit exposure during middle and late lactation. We pursued both of these hypotheses in this work by assessing hepatic catabolic expression and activity across developmental stage, and via measurement of internal TCS concentrations in sera and liver as a surrogate for exposure.
Previous studies on polybrominated diphenyl ethers, polychlorinated biphenyls, propylthiouracil, and 2,3,7,8-tetrachlorodibenzodioxin (TCDD) have demonstrated that postnatal thyroid hormones are affected by perinatal (i.e., during gestation and lactation) maternal xenobiotic exposure during the entirety of the postnatal lactation period (Crofton et al. 2000; Goldey et al. 1995a; Goldey et al. 1995b; Lau et al. 2003; Morse et al. 1993; York et al. 2004; Zhou et al. 2002a). In a cross-fostering study, perinatal maternal exposure to Aroclor 1254 caused extensive hypothyroxinemia in neonates throughout lactation, whereas exposure confined to the prenatal period only caused marginal hypothyroxinemia that resolved to control levels by the end of the lactation period (Crofton et al., 2000). This pattern of effects was consistent with the decreased transfer of PCBs via lactation to neonates from prenatally-exposed dams (Crofton et al. 2000). Perinatal TCS exposure demonstrated a similar pattern of T4 effects in offspring, i.e. T4 decreases were observed at the end of gestation in GD20 fetuses, and in young offspring at PND4, but not for offspring later in the lactation period (PND14 and PND21), suggesting that TCS exposure to offspring via dams during gestation may be greater than during lactation.
Using serum and liver concentrations as biomarkers of exposure, analysis of TCS concentrations demonstrated two key findings: (1) oral perinatal maternal treatment results in fetal and neonatal exposure; and, (2) neonatal exposure declines as age increases even though internal dose in dams is similar between gestation and the end of lactation (Figure 8). The striking finding of the analysis of TCS concentrations in serum and liver is that the internal exposure appears inversely proportional to the effects on serum T4 concentration, as demonstrated by juxtaposition of the percent decline in serum T4 with serum and liver concentrations of TCS (Figure 9). The serum and liver concentrations of TCS in GD20 fetuses and PND4 neonates were similar and correspond to 30% decreases in serum T4, whereas the serum and liver concentrations for PND14 and PND21 offspring were much lower, and corresponded to no changes in serum T4. The total TCS dose received via transplacental exposure appeared to be slightly higher, but the magnitude of the effect on T4 concentrations in GD20 fetuses was still comparable to dams and PND4 neonates, indicating that GD20 fetuses are similarly or slightly less sensitive to TCS-induced T4 perturbation than other life-stages exposed in this study.
A secondary finding from analysis of tissue concentrations of TCS is that TCS is found predominately in a conjugated form in rat serum and liver, as TCS-sulfate and/or TCS-glucuronide. This is consistent with previous findings in human sera that demonstrated that 65–70% (Sandborgh-Englund et al. 2006) to nearly all (DeSalva et al. 1989) of the TCS in circulation was conjugated, depending on the exposure and sampling times employed. The exception to this in the current work was observed in PND4 neonates, where there was increased parent TCS present in serum compared to other ages (Figure 8). Further experimentation would be necessary to characterize the potential distribution and metabolic contributions to this observation, but one explanation may be that PND4 neonates do not metabolize TCS as rapidly as dams or PND14 or PND21 offspring, as the total TCS measured in PND4 neonates is still consistent with the total TCS measured in GD20 and PND22 dams and GD20 fetuses. The possibility that differences in metabolism of TCS between PND4 neonates and older offspring contribute to the pattern of effects on serum T4 cannot be excluded. The finding that nearly all of the TCS present in the GD20 fetus is conjugated suggests that either the late gestation age fetal liver can conjugate TCS or that the TCS-conjugate is transported across the placenta. There are reports of transplacental transport of conjugates and limited fetal capacity to conjugate xenobiotics (Tzimas et al. 1995; Wang et al. 1986). However, based on the available data for TCS no conclusions can be made about the source of the conjugates in fetal serum or liver.
Previous reports on TCS measurements in human plasma allow a comparison of human internal exposures to the serum concentrations obtained in the present study. The lowest internal serum concentration of TCS that elicited a statistically significant effect on T4 was found in PND22 dams, which experienced a 15% decrease in serum T4 with a total serum TCS concentration of 5120 ng/mL. Allmyr et al. (2009) reported a median total TCS (parent and conjugate) serum concentration of 54 ng/mL after a 14-day exposure to TCS-containing toothpaste using a normal dental hygiene routine. This yields a margin of exposure of approximately 95. Importantly, the entire range of human exposures in the study failed to result in statistically significant changes in CYP3A4 activity or THs for the study group as a whole (Allmyr et al. 2009).
An additional major finding of this work is that markers of hepatic catabolism expression and activity are up-regulated in animals that experienced TCS-induced hypothyroxinemia. The up-regulation of biomarkers of Phase I and Phase II hepatic metabolism further supports the MOA that TCS interacts with the constitutive androstane and/or pregnane X receptor, resulting in subsequent increases in hepatic catabolism of T4 (Paul et al. 2010b). In this work, indicators of Phase I metabolism were slightly increased in response to TCS exposures that produced mild hypothyroxinemia. Increased expression of Cyp2b1/2 and Cyp3a1 is consistent with our previous observations from a short-term exposure, which demonstrated that a 4-day TCS-exposure resulted in up-regulated expression of both Cyp2b1/2 and Cyp3a1/23 (Paul et al. 2010b). In addition, PROD activity, a marker of Cyp2b activity in the rat, was increased 2–3-fold control for PND22 dams and PND4 neonates, consistent with previous reports (Paul et al. 2010b; Zorrilla et al. 2009). The lack of effect on PROD activity for GD20 dams was unexpected and may be due to a combination of the mild hypothyroxinemia and their unique metabolic status during pregnancy; several CYPs including Cyp2b2 protein are known to be decreased in rat liver during pregnancy and return to pre-pregnancy levels during lactation (He et al. 2005a, b). No increases in PROD activity were observed for PND14 or PND21 pups. However, these CYPs are functional at these ages, with assessment of the ontogeny of Cyp2b1/2 activity demonstrating high activity during the mid- to late-lactation period (de Zwart et al. 2008); further, PROD is inducible at these postnatal ages as demonstrated by perinatal exposure to DE-71 (Szabo et al. 2009; Zhou et al. 2002a) Based on analytical measurement of TCS in the sera and livers of these PND14 and PND21 offspring, we propose that the lack of effect on PROD activity is consistent with a reduced TCS exposure rather than a toxicodynamic difference between PND14 and PND21 offspring and PND4 offspring and dams. Importantly, these markers of Phase I metabolism suggest that triclosan may interact with one or more nuclear receptors upstream, i.e. CAR and/or PXR, resulting in subsequent increases in the overlapping set of genes regulated by these receptors, to include UGTs, SULTs, and transporters known to catabolize or transport THs for excretion (Baldwin and Roling 2008; Pascussi et al. 2008; Qatanani et al. 2005; Wong et al. 2005). These findings are also consistent with a previous literature report that TCS activated human PXR in an in vitro receptor-reporter model (Jacobs et al. 2005).
Concordant with the small observed reduction in serum T4, the observed effects of TCS on markers of hepatic catabolism were also minor. UGT-T4 activity was induced to 150% of control in PND22 dams only; failure to observe an increase in UGT-T4 activity in PND4 offspring and GD20 dams may be the result of a failure to greatly perturb UGT-T4 activity, as the observed T4 decreases were small. Previous reports with the UGT-T4 activity assay demonstrated significant increases in UGT activity when T4 concentrations decreased by 40% or more (Craft et al. 2002; Paul et al. 2010b; Zhou et al. 2002b). In the current work, T4 concentrations, regardless of life-stage, were only decreased 20–30%, and so measurement of significant changes in UGT-T4 activity is at or below the limit of detection for this assay; this may explain why a small statistically significant effect was observed in PND22 dams and not GD20 dams or PND4 neonates, though non-significant increases were noted in these groups. Failure of TCS to significantly increase the expression of Ugt1a1 and Sult1c3 is inconsistent with our previously published increases in these isoforms following non-developmental 4-day exposure to weanling rats (Paul et al. 2010b). Thus it would seem probable that TCS is a weak activator of Phase II hepatic catabolism, consistent with the small decreases in circulating T4 concentrations in vivo. Another confounding factor may be that UGT-T4 activity does not have a clear linear relationship with T4 concentrations (Craft et al. 2002; Hood and Klaassen 2000; Richardson and Klaassen 2010).
The marginal effects of TCS on UGT-T4 activity and hepatic mRNA were not due to the methods used. Positive control microsome and mRNA pools from rats treated with TCDD/Aroclor1254 or rats treated with PB/PCN demonstrated that AhR agonists, namely TCDD and Aroclor 1254, significantly increased UGT-T4 EROD and PROD activity, as well as mRNA expression using the PCR arrays. These positive control results clearly demonstrate the inducibility of hepatic catabolic gene expression using this methodology.
Hepatic gene expression of markers of Phase III hepatic transport activity were also unchanged by TCS exposure for any time point, in accordance with our previous in vivo assessment of hepatic transporter expression following a 4-day exposure (Paul et al. 2010b). Positive controls demonstrated that the low density arrays were capable of detecting increased hepatic transporter mRNA expression (Supplemental Information, Part C). Prototypical inducers, TCDD-, Aroclor1254, PB, and PCN all increased Mrp2 mRNA expression, and PCN, a PXR agonist, also increased the expression of Oatp1a4 mRNA, consistent with previous reports (Guo et al. 2002; Johnson and Klaassen 2002). These results with positive control compounds suggest that activation of AhR, CAR, and in particular PXR, can up-regulate expression of these hepatic transporter targets and be detected using low density arrays.
Hepatic gene expression of markers of liver-specific TH-responsive genes demonstrated no effects of TCS treatment. Genes defined as TH-responsive for this work were deiodinase I (DioI), malic enzyme I (MeI), and Spot14 (Thrsp). Hepatic expression of MeI has been shown to increase with TH-disrupting chemicals, including perfluorooctanesulfonate (PFOS) (Chang et al. 2008) and polychlorinated biphenyls (PCBs) (Gauger et al. 2007; Giera et al. 2011) with concomitant serum T4 decreases. Spot 14 (Thrsp) is transcriptionally regulated by the thyroid receptor, and PCBs and Aroclor 1254 have been reported to increase its expression (Giera et al. 2011). Deiodinase I is a peripheral deiodinase primarily responsible for the hepatic conversion of T4 to T3, and is thought to be transcriptionally regulated by both CAR (Tien et al. 2007) and the thyroid receptor (Schmutzler et al. 2007); however, a previous report demonstrated that Aroclor 1254 and PCBs may decrease the expression of DioI (Giera et al. 2011). We hypothesize that the lack of change in expression of genes in liver thought to be TH-sensitive was due to the mild hypothyroxinemia observed with TCS, which likely failed to significantly alter tissue T3 levels.
5. Conclusions
This study confirms previous observations of moderate hypothyroxinemia in dams and young neonates induced by TCS exposure. This study provides the first report demonstrating the importance of transplacental exposure and effects on GD20 T4 in dams and offspring. Further, this work establishes that exposure conveyed from dams to offspring diminishes over the lactation period, such that PND14 and PND21 neonatal T4 concentrations return to control levels, likely due to a lack of significant TCS exposure. This study provides some evidence that TCS up-regulated hepatic catabolism in GD20 and PND22 dams and PND4 offspring, though the effects were minor, and UGT-T4 activity was increased for PND22 dams only. The minor hypothyroxinemia observed was consistent with the minor effects on markers of hepatic catabolism, but the lack of consistent expression and activity changes in markers of hepatic catabolism leaves the possibility that other MOAs also contribute to the observed hypothyroxinemia. Continuing work will evaluate the putative initiating key event, interaction with CAR and/or PXR, in both rat and human models, as this is a highly species-dependent event.
Supplementary Material
Acknowledgments
The authors would like to thank the following for technical assistance: W. Anderson, K. Grant, A. Buckalew, A. Murr, E.,Boykin, and Dr. G.Knapp. Triclosan was a generous gift from Drs. James Plautz and Lisa Navarro of Ciba Specialty Chemicals.
7. Funding
K.B. Paul was funded by a PhRMA Foundation Predoctoral Pharmacology/Toxicology Fellowship, the EPA/UNC Toxicology Research Program Training Agreement (CR833237), and the National Institute of Environmental Health Science Training Grant (T32-ES07126) during this work. This work was partially supported by a cooperative research and development agreement (CRADA) between the U.S. EPA and BASF Corporation (CRADA No. 546-09).
The information in this document has been funded in part by the U.S. Environmental Protection Agency. It has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names of commercial products constitute endorsement or recommendation for use.
Footnotes
Abbreviations: TCS, triclosan; TH, thyroid hormones; T4, thyroxine; T3, triiodothyronine; MOA, mode-of-action; GD, gestational day; PND, postnatal day.
Triiodothyronine (T3) and thyroid-stimulating hormone (TSH) concentration data is available in Supplemental Information Parts A and B respectively. Positive control data from the qPCR studies is available as Supplemental Information Part C. Raw data files can be obtained by contacting the corresponding author.
9. Conflict of Interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Crofton KM, Paul KB, Hedge JM, DeVito MJ. Short-term in vivo exposure to the water contaminant triclosan: Evidence for disruption of thyroxine. Environ Toxicol Pharmacol. 2007;24:194–197. doi: 10.1016/j.etap.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Devito MJ, Crofton KM. Developmental triclosan exposure decreases maternal and neonatal thyroxine in rats. Environmental toxicology and chemistry/SETAC. 2010a;29:2840–2844. doi: 10.1002/etc.339. [DOI] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, DeVito MJ, Crofton KM. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in Young Long-Evans rats. Toxicol Sci. 2010b;113:367–379. doi: 10.1093/toxsci/kfp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, Stoker TE. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol Sci. 2009;107:56–64. doi: 10.1093/toxsci/kfn225. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Hansen PR, Boberg J, Bonnichsen M, Nellemann C, Lund SP, Hougaard KS, Hass U. Developmental neurotoxicity of propylthiouracil (PTU) in rats: relationship between transient hypothyroxinemia during development and long-lasting behavioural and functional changes. Toxicology and applied pharmacology. 2008;232:1–13. doi: 10.1016/j.taap.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Comer CP, Norton S. Effects of perinatal methimazole exposure on a developmental test battery for neurobehavioral toxicity in rats. Toxicol Appl Pharmacol. 1982;63:133–141. doi: 10.1016/0041-008x(82)90032-1. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Sui L, Walker MJ, Anderson W, Thomas S, Smoller SN, Schon JP, Phani S, Goodman JH. Thyroid hormone insufficiency during brain development reduces parvalbumin immunoreactivity and inhibitory function in the hippocampus. Endocrinology. 2007;148:92–102. doi: 10.1210/en.2006-0164. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Crofton KM. Thyroxine replacement attenuates hypothyroxinemia, hearing loss, and motor deficits following developmental exposure to Aroclor 1254 in rats. Toxicol Sci. 1998;45:94–105. doi: 10.1006/toxs.1998.2495. [DOI] [PubMed] [Google Scholar]
- Iniguez MA, Rodriguez-Pena A, Ibarrola N, Morreale de Escobar G, Bernal J. Adult rat brain is sensitive to thyroid hormone. Regulation of RC3/neurogranin mRNA. J Clin Invest. 1992;90:554–558. doi: 10.1172/JCI115894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Maternal-fetal thyroid hormone relationships and the fetal brain. Acta Med Austriaca. 1988;15(Suppl 1):66–70. [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. The New England journal of medicine. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clinical endocrinology. 2003;59:282–288. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clinical endocrinology. 1999;50:149–155. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- Allmyr M, Harden F, Toms LM, Mueller JF, McLachlan MS, Adolfsson-Erici M, Sandborgh-Englund G. The influence of age and gender on triclosan concentrations in Australian human blood serum. The Science of the total environment. 2008;393:162–167. doi: 10.1016/j.scitotenv.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Allmyr M, McLachlan MS, Sandborgh-Englund G, Adolfsson-Erici M. Determination of triclosan as its pentafluorobenzoyl ester in human plasma and milk using electron capture negative ionization mass spectrometry. Analytical chemistry. 2006a;78:6542–6546. doi: 10.1021/ac060666x. [DOI] [PubMed] [Google Scholar]
- Allmyr M, Panagiotidis G, Sparve E, Diczfalusy U, Sandborgh-Englund G. Human exposure to triclosan via toothpaste does not change CYP3A4 activity or plasma concentrations of thyroid hormones. Basic Clin Pharmacol Toxicol. 2009;105:339–344. doi: 10.1111/j.1742-7843.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- Hovander L, Malmberg T, Athanasiadou M, Athanassiadis I, Rahm S, Bergman A, Wehler EK. Identification of hydroxylated PCB metabolites and other phenolic halogenated pollutants in human blood plasma. Archives of environmental contamination and toxicology. 2002;42:105–117. doi: 10.1007/s002440010298. [DOI] [PubMed] [Google Scholar]
- Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere. 2002;46:1485–1489. doi: 10.1016/s0045-6535(01)00255-7. [DOI] [PubMed] [Google Scholar]
- Allmyr M, Adolfsson-Erici M, McLachlan MS, Sandborgh-Englund G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. The Science of the total environment. 2006b;372:87–93. doi: 10.1016/j.scitotenv.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Dayan AD. Risk assessment of triclosan [Irgasan((R))] in human breast milk. Food Chem Toxicol. 2007;45:125–129. doi: 10.1016/j.fct.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environmental health perspectives. 2008;116:303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, Godbold J, Biro F, Kushi LH, Pfeiffer CM, Calafat AM. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environmental health perspectives. 2007;115:116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte NF, Garza C, Smith EO, Nichols BL. Human milk intake and growth in exclusively breast-fed infants. J Pediatr. 1984;104:187–195. doi: 10.1016/s0022-3476(84)80990-7. [DOI] [PubMed] [Google Scholar]
- Butte NF, Lopez-Alarcon MG, Garza C. Nutrient adequacy of exclusive breast feeding for the term infant during the first 6 months of life. WHO; Geneva: 2002. pp. 1–49. [Google Scholar]
- EPA, U.S. Exposure Factors Handbook Vol II Food Ingestion Factors. US Government Printing Office EPA; 1997. pp. 14.11–14.15. 600/P-002Fb. [Google Scholar]
- Kester MH, Kaptein E, Roest TJ, van Dijk CH, Tibboel D, Meinl W, Glatt H, Coughtrie MW, Visser TJ. Characterization of rat iodothyronine sulfotransferases. American journal of physiology. 2003;285:E592–598. doi: 10.1152/ajpendo.00046.2003. [DOI] [PubMed] [Google Scholar]
- Vansell NR, Klaassen CD. Increase in rat liver UDP-glucuronosyltransferase mRNA by microsomal enzyme inducers that enhance thyroid hormone glucuronidation. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:240–246. doi: 10.1124/dmd.30.3.240. [DOI] [PubMed] [Google Scholar]
- Kretschmer XC, Baldwin WS. CAR and PXR: xenosensors of endocrine disrupters? Chemico-biological interactions. 2005;155:111–128. doi: 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Capen CC. Mechanisms of chemical injury of thyroid gland. Progress in clinical and biological research. 1994;387:173–191. [PubMed] [Google Scholar]
- Hood A, Klaassen CD. Differential effects of microsomal enzyme inducers on in vitro thyroxine (T(4)) and triiodothyronine (T(3)) glucuronidation. Toxicol Sci. 2000;55:78–84. doi: 10.1093/toxsci/55.1.78. [DOI] [PubMed] [Google Scholar]
- Miller MD, Crofton KM, Rice DC, Zoeller RT. Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environmental health perspectives. 2009;117:1033–1041. doi: 10.1289/ehp.0800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002a;66:105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Wong CC, von zur Muhlen A. The rat as model for the study of drug effects on thyroid function: consideration of methodological problems. Pharmacol Ther B. 1979;5:305–318. doi: 10.1016/0163-7258(79)90099-8. [DOI] [PubMed] [Google Scholar]
- Jordan D, Rousset B, Perrin F, Fournier M, Orgiazzi J. Evidence for circadian variations in serum thyrotropin, 3,5,3′-triiodothyronine, and thyroxine in the rat. Endocrinology. 1980;107:1245–1248. doi: 10.1210/endo-107-4-1245. [DOI] [PubMed] [Google Scholar]
- Greenwood FC, Hunter WM, Glover JS. The Preparation of I-131-Labelled Human Growth Hormone of High Specific Radioactivity. Biochem J. 1963;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, You SH, Herzig CT, Zoeller RT. Maternal thyroid hormone increases HES expression in the fetal rat brain: an effect mimicked by exposure to a mixture of polychlorinated biphenyls (PCBs) Brain Res Dev Brain Res. 2005;156:13–22. doi: 10.1016/j.devbrainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Gauger KJ, Kato Y, Haraguchi K, Lehmler HJ, Robertson LW, Bansal R, Zoeller RT. Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environmental health perspectives. 2004;112:516–523. doi: 10.1289/ehp.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito MJ, Maier WE, Diliberto JJ, Birnbaum LS. Comparative Ability of Various PCBs, PCDFs, and TCDD to INduce Cytochrome P450 1A1 and 1a2 Activity Following 4 Weeks of Treatment. Fundamental and Applied Toxicology. 1993;20:125–130. [PubMed] [Google Scholar]
- Beetstra JB, van Engelen JG, Karels P, van der Hoek HJ, de Jong M, Docter R, Krenning EP, Hennemann G, Brouwer A, Visser TJ. Thyroxine and 3,3′,5-triiodothyronine are glucuronidated in rat liver by different uridine diphosphate-glucuronyltransferases. Endocrinology. 1991;128:741–746. doi: 10.1210/endo-128-2-741. [DOI] [PubMed] [Google Scholar]
- Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001;61:76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
- Craft ES, DeVito MJ, Crofton KM. Comparative responsiveness of hypothyroxinemia and hepatic enzyme induction in Long-Evans rats versus C57BL/6J mice exposed to TCDD-like and phenobarbital-like polychlorinated biphenyl congeners. Toxicol Sci. 2002;68:372–380. doi: 10.1093/toxsci/68.2.372. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Dunn RT, 2nd, Klaassen CD. Tissue-specific expression of rat sulfotransferase messenger RNAs. Drug metabolism and disposition: the biological fate of chemicals. 1998;26:598–604. [PubMed] [Google Scholar]
- Pohjanvirta R, Niittynen M, Linden J, Boutros PC, Moffat ID, Okey AB. Evaluation of various housekeeping genes for their applicability for normalization of mRNA expression in dioxin-treated rats. Chemico-biological interactions. 2006;160:134–149. doi: 10.1016/j.cbi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Fort DJ, Rogers RL, Gorsuch JW, Navarro LT, Peter R, Plautz JR. Triclosan and anuran metamorphosis: no effect on thyroid-mediated metamorphosis in Xenopus laevis. Toxicol Sci. 2010;113:392–400. doi: 10.1093/toxsci/kfp280. [DOI] [PubMed] [Google Scholar]
- EPA, U.S. Benchmark Dose Technical Guidance Document External Review Draft. Risk Assessment Forum. 2000 http://www.epa.gov/ncea/pdfs/bmds/BMD-External_10_13_2000.pdf.
- Borlakoglu JT, Scott A, Henderson CJ, Wolf CR. Expression of P450 isoenzymes during rat liver organogenesis. Int J Biochem. 1993;25:1659–1668. doi: 10.1016/0020-711x(93)90525-j. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Kodavanti PR, Derr-Yellin EC, Casey AC, Kehn LS. PCBs, thyroid hormones, and ototoxicity in rats: cross-fostering experiments demonstrate the impact of postnatal lactation exposure. Toxicol Sci. 2000;57:131–140. doi: 10.1093/toxsci/57.1.131. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol. 1995a;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Rehnberg GL, Crofton KM. Effects of developmental hypothyroidism on auditory and motor function in the rat. Toxicol Appl Pharmacol. 1995b;135:67–76. doi: 10.1006/taap.1995.1209. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci. 2003;74:382–392. doi: 10.1093/toxsci/kfg122. [DOI] [PubMed] [Google Scholar]
- Morse DC, Groen D, Veerman M, van Amerongen CJ, Koeter HB, Smits van Prooije AE, Visser TJ, Koeman JH, Brouwer A. Interference of polychlorinated biphenyls in hepatic and brain thyroid hormone metabolism in fetal and neonatal rats. Toxicology and applied pharmacology. 1993;122:27–33. doi: 10.1006/taap.1993.1168. [DOI] [PubMed] [Google Scholar]
- York RG, Barnett J, Jr, Brown WR, Garman RH, Mattie DR, Dodd D. A rat neurodevelopmental evaluation of offspring, including evaluation of adult and neonatal thyroid, from mothers treated with ammonium perchlorate in drinking water. Int J Toxicol. 2004;23:191–214. doi: 10.1080/10915810490475835. [DOI] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. Journal of toxicology and environmental health. 2006;69:1861–1873. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- DeSalva SJ, Kong BM, Lin YJ. Triclosan: a safety profile. American journal of dentistry. 1989;2(Spec No):185–196. [PubMed] [Google Scholar]
- Tzimas G, Collins MD, Nau H. Developmental stage-associated differences in the transplacental distribution of 13-cis- and all-trans-retinoic acid as well as their glucuronides in rats and mice. Toxicol Appl Pharmacol. 1995;133:91–101. doi: 10.1006/taap.1995.1130. [DOI] [PubMed] [Google Scholar]
- Wang LH, Rudolph AM, Benet LZ. Pharmacokinetic studies of the disposition of acetaminophen in the sheep maternal-placental-fetal unit. The Journal of pharmacology and experimental therapeutics. 1986;238:198–205. [PubMed] [Google Scholar]
- He XJ, Ejiri N, Nakayama H, Doi K. Changes in cytochrome P450 isozymes (CYPs) protein levels during lactation in rat liver. Exp Mol Pathol. 2005a;79:224–228. doi: 10.1016/j.yexmp.2005.08.009. [DOI] [PubMed] [Google Scholar]
- He XJ, Ejiri N, Nakayama H, Doi K. Effects of pregnancy on CYPs protein expression in rat liver. Exp Mol Pathol. 2005b;78:64–70. doi: 10.1016/j.yexmp.2004.08.011. [DOI] [PubMed] [Google Scholar]
- de Zwart L, Scholten M, Monbaliu JG, Annaert PP, Van Houdt JM, Van den Wyngaert I, De Schaepdrijver LM, Bailey GP, Coogan TP, Coussement WC, Mannens GS. The ontogeny of drug metabolizing enzymes and transporters in the rat. Reprod Toxicol. 2008;26:220–230. doi: 10.1016/j.reprotox.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PR, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107:27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin WS, Roling JA. A Concentration Addition Model for the Activation of the Constitutive Androstane Receptor by Xenobiotic Mixtures. Toxicol Sci. 2008 doi: 10.1093/toxsci/kfn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascussi JM, Gerbal-Chaloin S, Duret C, Daujat-Chavanieu M, Vilarem MJ, Maurel P. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu Rev Pharmacol Toxicol. 2008;48:1–32. doi: 10.1146/annurev.pharmtox.47.120505.105349. [DOI] [PubMed] [Google Scholar]
- Qatanani M, Zhang J, Moore DD. Role of the constitutive androstane receptor in xenobiotic-induced thyroid hormone metabolism. Endocrinology. 2005;146:995–1002. doi: 10.1210/en.2004-1350. [DOI] [PubMed] [Google Scholar]
- Wong H, Lehman-McKeeman LD, Grubb MF, Grossman SJ, Bhaskaran VM, Solon EG, Shen HS, Gerson RJ, Car BD, Zhao B, Gemzik B. Increased hepatobiliary clearance of unconjugated thyroxine determines DMP 904-induced alterations in thyroid hormone homeostasis in rats. Toxicol Sci. 2005;84:232–242. doi: 10.1093/toxsci/kfi094. [DOI] [PubMed] [Google Scholar]
- Jacobs MN, Nolan GT, Hood SR. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR) Toxicology and applied pharmacology. 2005;209:123–133. doi: 10.1016/j.taap.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002b;66:105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
- Richardson TA, Klaassen CD. Disruption of thyroid hormone homeostasis in Ugt1a-deficient Gunn rats by microsomal enzyme inducers is not due to enhanced thyroxine glucuronidation. Toxicology and applied pharmacology. 2010;248:38–44. doi: 10.1016/j.taap.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo GL, Staudinger J, Ogura K, Klaassen CD. Induction of rat organic anion transporting polypeptide 2 by pregnenolone-16alpha-carbonitrile is via interaction with pregnane X receptor. Molecular pharmacology. 2002;61:832–839. doi: 10.1124/mol.61.4.832. [DOI] [PubMed] [Google Scholar]
- Johnson DR, Klaassen CD. Regulation of rat multidrug resistance protein 2 by classes of prototypical microsomal enzyme inducers that activate distinct transcription pathways. Toxicol Sci. 2002;67:182–189. doi: 10.1093/toxsci/67.2.182. [DOI] [PubMed] [Google Scholar]
- Chang SC, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork JA, Froehlich JW, Lau C, Singh RJ, Wallace KB, Butenhoff JL. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS) Toxicology. 2008;243:330–339. doi: 10.1016/j.tox.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Gauger KJ, Giera S, Sharlin DS, Bansal R, Iannacone E, Zoeller RT. Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists after cytochrome P4501A1 activation in rat pituitary GH3 cells. Environmental health perspectives. 2007;115:1623–1630. doi: 10.1289/ehp.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT. Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development. Endocrinology. 2011;152:2909–2919. doi: 10.1210/en.2010-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien ES, Matsui K, Moore R, Negishi M. The nuclear receptor constitutively active/androstane receptor regulates type 1 deiodinase and thyroid hormone activity in the regenerating mouse liver. The Journal of pharmacology and experimental therapeutics. 2007;320:307–313. doi: 10.1124/jpet.106.112706. [DOI] [PubMed] [Google Scholar]
- Schmutzler C, Gotthardt I, Hofmann PJ, Radovic B, Kovacs G, Stemmler L, Nobis I, Bacinski A, Mentrup B, Ambrugger P, Gruters A, Malendowicz LK, Christoffel J, Jarry H, Seidlova-Wuttke D, Wuttke W, Kohrle J. Endocrine disruptors and the thyroid gland--a combined in vitro and in vivo analysis of potential new biomarkers. Environmental health perspectives. 2007;115(Suppl 1):77–83. doi: 10.1289/ehp.9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.