Abstract
OBJECTIVE:
High fructose consumption contributes to the incidence of metabolic syndrome and, consequently, to cardiovascular outcomes. We investigated whether exercise training prevents high fructose diet-induced metabolic and cardiac morphofunctional alterations.
METHODS:
Wistar rats receiving fructose overload (F) in drinking water (100 g/l) were concomitantly trained on a treadmill (FT) for 10 weeks or kept sedentary. These rats were compared with a control group (C). Obesity was evaluated by the Lee index, and glycemia and insulin tolerance tests constituted the metabolic evaluation. Blood pressure was measured directly (Windaq, 2 kHz), and echocardiography was performed to determine left ventricular morphology and function. Statistical significance was determined by one-way ANOVA, with significance set at p<0.05.
RESULTS:
Fructose overload induced a metabolic syndrome state, as confirmed by insulin resistance (F: 3.6±0.2 vs. C: 4.5±0.2 mg/dl/min), hypertension (mean blood pressure, F: 118±3 vs. C: 104±4 mmHg) and obesity (F: 0.31±0.001 vs. C: 0.29±0.001 g/mm). Interestingly, fructose overload rats also exhibited diastolic dysfunction. Exercise training performed during the period of high fructose intake eliminated all of these derangements. The improvements in metabolic parameters were correlated with the maintenance of diastolic function.
CONCLUSION:
The role of exercise training in the prevention of metabolic and hemodynamic parameter alterations is of great importance in decreasing the cardiac morbidity and mortality related to metabolic syndrome.
Keywords: Metabolic Syndrome, Diastolic function, Exercise Training, Insulin resistance, Cardiac hypertrophy
INTRODUCTION
Metabolic syndrome (MS) has proven to be a major clinical challenge in this century. The presence of this syndrome increases mortality, especially due to cardiovascular disease (1). It is worth noting that the association of MS with cardiovascular disease increases overall and cardiovascular mortalities by 1.5- to 2.5-fold (2,3).
One of the factors that contributes to the increase in MS incidence is poor eating habits, which are mainly characterized by a large increase in fructose consumption (4,5). Several changes are observed after chronic unhealthy eating habits, including increased blood pressure, hyperlipidemia, obesity, glucose intolerance, insulin resistance and hyperinsulinemia.
Insulin resistance may precede and is a pathogenic factor of diabetes. Furthermore, insulin resistance is associated with left ventricular diastolic dysfunction in pre-diabetic adults, independent of blood pressure levels (6). Studies have shown that MS patients also present with left ventricular remodeling and diastolic dysfunction.
Experimentally, fructose overload in drinking water or chow has been used to study MS and promote similar metabolic and autonomic derangements. Our research group has shown that, in female rats, eight weeks of fructose overload promoted impairment in metabolic parameters and cardiac autonomic control by reducing vagal tone and increasing sympathetic modulation with additional insulin resistance in the absence of diabetes (7). However, morphometric and left ventricular diastolic functions in this model are seldom reported.
As a non-pharmacological treatment, aerobic physical training at moderate intensity can effectively mitigate fructose-induced hypertension in rats (8), and it has been considered a significant component in the treatment and prevention of human cardiovascular disease (9). However, little is known about the mechanisms by which exercise acts in models of high-fructose diets. Moreover, it is not known whether physical exercise of moderate intensity, performed concomitantly with a high-fructose diet, can minimize the metabolic changes, left ventricular hypertrophy and diastolic dysfunction that occur due to MS.
Therefore, the aim of this study was to investigate whether moderate physical exercise performed during 10 weeks of a high-fructose diet can prevent metabolic changes and morphometric and left ventricular diastolic dysfunction in Wistar rats.
MATERIALS AND METHODS
Animals
Experiments were performed on 21 male Wistar rats (251±10 g) from the Animal Shelter at the University of Sao Paulo, Sao Paulo, Brazil. The rats received standard laboratory chow and water ad libitum. The animals were housed in individual cages in a temperature-controlled room (22°C) with a 12-h dark-light cycle. All surgical procedures and protocols used were in accordance with the Guidelines for Ethical Care of Experimental Animals from the International Animal Care and Use Committee. This study was approved by the University of Sao Paulo Medical School Ethical Committee (360/11). The rats were randomly assigned to one of three groups: control, kept sedentary (C, n = 7); sedentary, receiving a high-fructose diet (F, n = 7); and trained, receiving a high-fructose diet (FT, n = 7).
High-fructose diet
The F and FT groups received an overload of D-fructose (100 g/l) in drinking water for 10 weeks. Control animals received only water during this period (7).
Exercise training
Moderate-intensity exercise training (50-70% of the maximum running speed) was performed on a treadmill once per day, five days per week for 10 weeks, as described in detail elsewhere (10).
All animals were adapted to the procedure (10 min/day; 0.3 km/h) for one week before the beginning of the exercise training protocol. Sedentary and trained groups underwent a maximal treadmill test (10,11). The tests were performed in the first, fifth and tenth weeks of exercise training to determine aerobic capacity and adequate exercise training intensity.
Obesity parameter in rats
The Lee index for each animal was calculated after 10 weeks of fructose overload to obtain the obesity parameter. This index was calculated as the cube root of body weight (g)×10/naso-anal length (mm), and a value equal to or less than 0.300 was classified as normal at the third month of life. Rats with values higher than 0.300 were classified as obese (12).
Echocardiographic evaluation
One day after the last training session, echocardiography was performed by an observer blinded to the treatment group, according to the guidelines of the American Society of Echocardiography. Rats were anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg), and images were obtained using a Sequoia 512 ultrasound system (ACUSON, Mountain View, CA, USA) with a 10-14 MHz linear transducer for the measurement of morphometric parameters (left ventricular (LV) mass and relative wall thickness (RWT)), systolic function (ejection fraction (EF), fractional shortening (FS) and velocity of circumferential fiber shortening (VCF)) and diastolic function (absolute and normalized LV isovolumetric relaxation time; IVRT and nIVRT, respectively), and the ratio of maximal early diastolic peak velocity (E) and late peak velocity (A) of mitral flow (E/A ratio). The global index was quantified by the myocardial performance index (MPI). Echocardiographic parameters were measured as previously described (13,14).
Cardiovascular assessments
After the last training session, two catheters were implanted into the femoral artery and vein (PE-10) while the animals were anesthetized (ketamine 50 mg/kg+xylazine 10 mg/kg) for the direct measurement of arterial pressure (AP) and drug administration, respectively.
Rats were studied one day after catheter placement; the rats were conscious and allowed to move freely during the experiments. The arterial cannula was connected to a strain-gauge transducer (P23Db, Gould-Statham, Oxnard, CA, USA), and AP signals were recorded over a 30-minute period by a microcomputer equipped with an analog-to-digital converter board (Windaq, 2-kHz sampling frequency; Dataq Instruments, Inc., Akron, OH). The recorded data were analyzed on a beat-to-beat basis to quantify changes in mean AP and heart rate (HR).
The rate constant for blood glucose disappearance
One day after blood pressure recording, the blood glucose of all animals was measured after a 4-h fast with a glucosimeter (ACCUCHEK Advantage, Roche, Brazil). The rats were also submitted to an intravenous insulin tolerance test (ITT) after a 2-h fast. Animals were anesthetized with thiopental (40 mg/kg body weight, i.p.), and a drop of blood from the tail was collected to measure blood glucose. This procedure was performed at baseline and 4, 8, 12 and 16 min after insulin administration (0.75 U/kg). The rate constant for blood glucose disappearance (KITT) was calculated using the formula 0.693/t1/2, and the blood glucose half-time (t1/2) was calculated from the least-squares regression slope of the blood glucose concentration during the linear phase of decline 7.
Statistical analysis
Data are reported as the means ± SEM, and one-way ANOVA was used to compare the groups, followed by the Student-Newman-Keuls post-hoc test. The Pearson correlation was used to study the association between variables. Significance was established at p<0.05.
RESULTS
Hemodynamic and metabolic evaluations
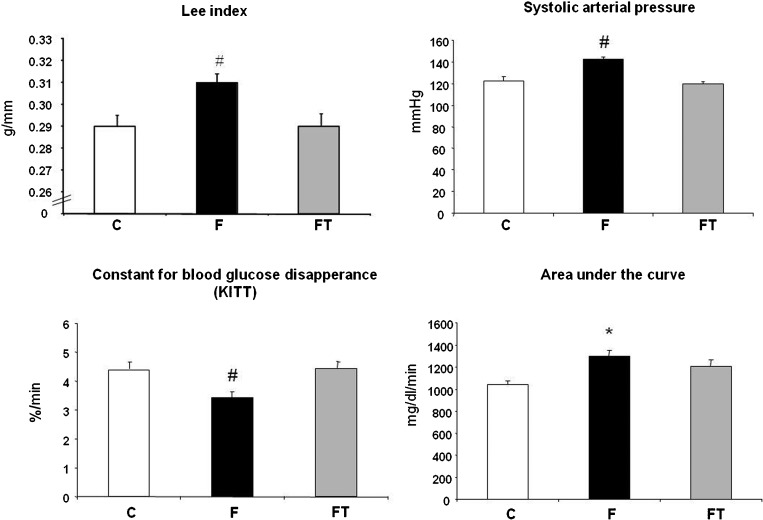
The body weight in the F group was higher than that in the C and FT groups (F = 515±8 vs. C = 412±14 and FT = 416±10 g). The naso-anal length was similar among the groups (F = 26.49±0.13, C = 26.19±0.21 and FT = 26.04±0.25 cm). Consequently, the Lee index was increased in the F group compared with the C and FT groups (F = 0.31±0.001 vs. C = 0.29±0.001 and FT = 0.29±0.001 g/mm). The Lee index was similar between the C and FT groups (Figure 1).
Figure 1.
Lee Index, systolic arterial pressure, the rate constant for blood glucose disappearance and area under the curve in control (C) high fructose-fed (F) and trained high fructose-fed (FT) groups. One-way ANOVA, *p<0.05 vs. C and # p<0.05 vs. C and FT.
Glycemia was also increased in the F group after fructose overload compared with the control rats, but there was no difference with respect to the F and FT groups (F = 100±4, FT 99±1 vs. C = 88±1 mg/dl).
In the intravenous insulin tolerance test, the rate constant for blood glucose disappearance (KITT) was reduced in F rats compared with C and FT rats (3.6±0.2 vs. 4.5±0.2 and 4.6±0.2 %/min, respectively). Additionally, when the area under the curve was calculated, a higher area was obtained in the F group compared with the C group (1300±48 vs. 1044±30 mg/dl/min). No difference was observed between the C and FT groups for this parameter (1044±30 vs. 1208±38 mg/dl/min) (Figure 1).
The systolic (SBP), diastolic (DBP) and mean (MBP) blood pressure were increased in the F group compared with the C and FT groups. These parameters were similar between the C and FT groups (SBP = 142±2 vs. 122±4 and 119±2 mmHg, DBP = 101±2 vs. 83±4 and 87±4 mmHg and MAP = 118±3 vs. 104±4 and 101±2 mmHg, respectively). No difference was observed in heart rate among the studied groups (C = 330±7, FT = 320±6 and F = 335±9 bpm).
Echocardiographic evaluation
The results of echocardiographic evaluations performed 10 weeks after fructose overload are shown in Table 1. The left ventricular morphometric evaluations indicated that the left ventricular mass was higher in the F and FT groups compared with the control group. Furthermore, the RWT was increased in the F group compared with the FT and control groups. The FT and C groups exhibited similar RWT values.
Table 1.
Table 1 - Cardiac morphometric and functional evaluations by echocardiography in control (C), fructose-fed (F) and trained fructose-fed (FT) groups.
| Variable | C (7) | F (7) | FT (7) |
| morphometric | |||
| LV mass (g) | 0.73±0.03 | 1.3±0.01* | 1.2±0.01* |
| RWT | 0.33±0.007 | 0.38±0.005* | 0.35±0.004 |
| systolic function | |||
| LVFS (%) | 40±1.5 | 36±1 | 40±1.6 |
| LVEF (%) | 77±1.6 | 72±1.1 | 76±1.5 |
| VCF (circ/sec) | 0.0053±0.0003 | 0.0046±0.0001 | 0.0052±0.0002 |
| diastolic function | |||
| IVRT (ms) | 22±0.4 | 27±0.9# | 0.22±0.6 |
| nIVRT (ms) | 1.6±0.03 | 2.05±0.07# | 1.73±0.03 |
| E/A ratio (ms) | 1.72±0.08 | 2.24±0.12# | 1.72±0.15 |
| global index | |||
| MPI | 0.32±0.02 | 0.41±0.02# | 0.33±0.01 |
Values are the mean ± SEM. The morphometric evaluation consisted of the assessment of left ventricular mass (LV mass) and relative wall thickness (RWT). Systolic function was evaluated by the left ventricular fractional shortening (LVFS), left ventricular ejection fraction (LVEF), and velocity of circumferential fiber shortening (VCF). Diastolic function was evaluated by the absolute and normalized LV isovolumetric relaxation time (IVRT and nIVRT, respectively) and the ratio of the maximal early diastolic peak velocity (E) and late peak velocity (A) of mitral flow (E/A ratio). Cardiac global function was evaluated by the myocardial performance index (MPI). * p<0.05 vs. C and # p<0.05 vs. C and FT (one-way ANOVA).
Systolic function, expressed by fractional shortening, ejection fraction and VCF, was similar among the three groups. However, in the evaluation of diastolic function, lower ratios were found for IVRT, nIVRT and E/A in the F group than in the C and FT groups. The C and FT groups exhibited similar diastolic function. The myocardial performance index (MPI) was increased in the F group compared with the C and FT groups, whereas the FT and C groups exhibited similar values.
Correlation Analyses
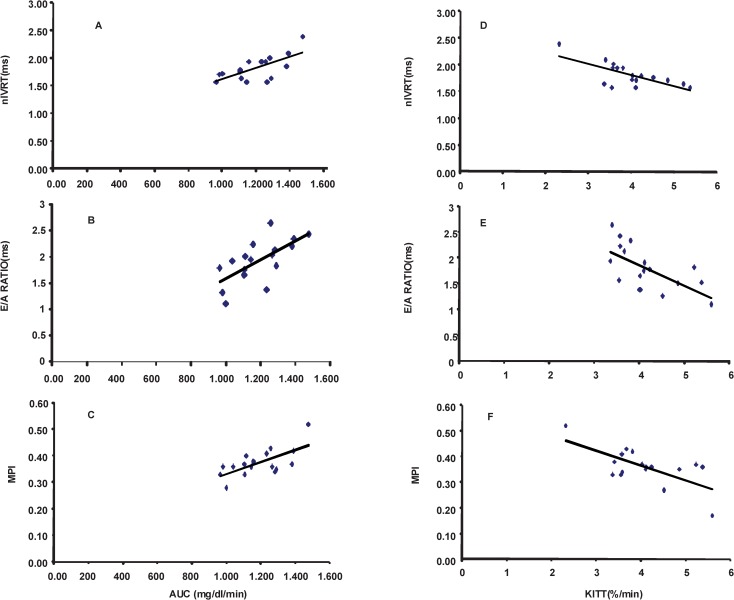
Correlation analyses were carried out to associate metabolic parameters with diastolic dysfunction variables in all three groups (Figure 2). A negative correlation was found between IVRT and KITT (r = -0.67; p = 0.002). Similarly, a negative correlation between nIVRT and KITT (r = -0.70; p = 0.0016) was demonstrated. We observed a negative correlation between the E/A ratio and KITT (r = -0.63; p = 0.004). A negative correlation was also found between the global index MPI and KITT (r = -0.74; p = 0.004).
Figure 2.
Correlation analyses between insulin resistance index, diastolic index and global index. A: normalized isovolumetric relaxation time (nIVRT), B: ratio of maximal early diastolic peak velocity (E) and late peak velocity (A) of mitral flow (E/A ratio), C: myocardial performance index (MPI) vs. area under the curve of the rate constant for blood glucose disappearance (AUC) and D: nIVRT, E: E/A ratio and F: MPI vs. the rate constant for blood glucose disappearance (KITT), involving control, fructose-fed and trained fructose-fed rats.
A positive correlation was found between IVRT, nIVRT and AUC (r = 0.67; p = 0.002 and r = 0.68; p = 0.0036, respectively). Furthermore, the E/A ratio and AUC were positively correlated (r = 0.66; p = 0.003), as were the MPI and AUC (r = 0.67; p = 0.003).
DISCUSSION
The purpose of this study was to investigate whether moderate physical exercise performed during 10 weeks of a high-fructose diet in Wistar rats can prevent metabolic changes, cardiac morphofunctional alterations and left ventricular diastolic dysfunction.
Interestingly, we found that metabolic and cardiac morphofunctional alterations promoted by a high-fructose diet can be attenuated by physical exercise when both occur in the same time period. This is the first study to demonstrate the preventive effect of physical training on the diastolic dysfunction induced by fructose overload in rats.
Metabolic and echocardiographic changes induced by fructose overload
In our study, although high fructose consumption promoted an increase in glycemia compared with control rats, the values were still within the normal range. Other studies, even those utilizing high-fructose diets over longer periods, did not find significant alterations in glycemia (7). However, we observed several changes that were characteristic of metabolic syndrome in the rats that received a high-fructose diet. For instance, obesity was defined as an increased Lee index and was associated with insulin resistance, as demonstrated by the KITT and the area under the curve. In fact, this model characterizes an increase in insulin resistance and not hyperglycemia (7). In fact, this model is characterized by an increase in systolic and diastolic blood pressure.
In humans with normal beta cell function, the secretion of insulin may be altered to accommodate different degrees of insulin sensitivity and, thus, to maintain blood glucose within the normal range. Likewise, clinical studies have also demonstrated that the consumption of fructose can induce weight gain, dyslipidemia, reduced insulin sensitivity and high blood pressure (15,16).
Interestingly, fructose overload promoted changes in left ventricular morphometry, diastolic dysfunction and increased cardiac effort, as evidenced by the increase in the myocardial performance index (MPI). Other authors found similar results after submitting Wistar rats to 10% fructose overload for eight weeks (17).
Left ventricular diastolic dysfunction and morphometric alterations can result from several factors, such as increased collagen concentration and fibrosis and decreased compliance, which are influenced by the autonomic nervous system and the renin-angiotensin system (17). In fact, several authors showed increased blood pressure, decreased vagal tonus and increased angiotensin II in a high fructose-fed rat model (7,18,19). Furthermore, insulin resistance is a pathogenic factor and has been associated with abnormal myocardial performance in pre-diabetic adults. Additionally, as demonstrated in Figure 1, this relationship between resistance and myocardial performance reinforces the fact that the heart is a target organ in metabolic syndrome, and its evaluation should not be neglected in clinical practice.
Preventive effect of exercise training during high fructose intake
Physical exercise has been shown to be an effective, non-pharmacological treatment for cardiovascular disease (20-27). However, in the present study, it was used as a preventive tool when performed during the same period in which the rats received fructose overload in drinking water. Interestingly, the FT group presented similar values to those of the C group for several parameters, such as the Lee index, insulin resistance, and systolic and diastolic blood pressure, which confirms that exercise training was effective in the prevention of these changes induced by a high-fructose diet. The caloric expenditure due to the exercise training during the high-fructose diet period probably enhanced the energetic demand and prevented changes such as weight and fat gain, which contributed to improved insulin sensitivity and the maintenance of blood pressure in the normal range. In fact, the reduction of weight, intra-abdominal adiposity and insulin resistance by exercise training may interfere with other mechanisms of action in the treatment of hypertension, as hypertension has been associated with all of these variables (28). Indeed, regular physical exercise can promote chronic metabolic and cardiovascular adaptations. It is known that regular exercise improves glycemia, the lipid profile (29) and insulin sensitivity; promotes weight reduction; and improves muscle glucose uptake.
Additionally, the morphometric and functional alterations demonstrated by echocardiography in the F rats were not observed in the C and FT groups. In diabetic and hypertensive rats, physical training has been important in the treatment of metabolic and autonomic disorders by contributing to increased baroreflex sensitivity and decreased blood pressure (10,30,31). Moreover, exercise training can modulate the renin-angiotensin system and prevent the development of cardiac hypertrophy in SHR rats (32). Exercise training can increase the antioxidant capacity, increase vascular compliance and promote atheroprotective effects (33).
Altogether, these improvements in several systems resulting from exercise training were important in the prevention of cardiovascular derangements promoted by MS. It is notable that the cardiac changes observed in the F rats and absent in the FT rats due to exercise training were correlated with the indexes of insulin resistance, suggesting that these alterations play a significant role in the process of cardiac damage due to MS or diabetes.
Among the derangements caused by metabolic syndrome, cardiac morphofunctional alterations were also observed. Diastolic function seemed to be the cardiac parameter most influenced by the metabolic alterations, especially the insulin resistance.
Chronic high fructose intake favored the development of metabolic syndrome in rats. When performed concomitantly with a high-fructose diet, exercise training proved to be a vital tool for the prevention of not only metabolic but also cardiac changes promoted by fructose overload in rats. Additionally, it seems that the prevention of diastolic dysfunction was related to improved insulin resistance. Considering our results, we speculate that cardiac morphofunctional evaluations should also be performed in patients with metabolic syndrome or in patients at risk for metabolic syndrome development. Nevertheless, more clinical studies that assess cardiac function in these patients should be performed.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.First Brazilian Guideline for Diagnosis and Treatment of Metabolic Syndrome. 2005 [Google Scholar]
- 2.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 3.Girman CJ, Rhodes T, Mercuri M, Pyorala K, Kjekshus J, Pedersen TR, et al. The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) Am J Cardiol. 2004;93(2):136–41. doi: 10.1016/j.amjcard.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 5.Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2005;2(1):5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sliem H, Nasr G. Left ventricular structure and function in prediabetic adults: Relationship with insulin resistance. J Cardiovasc Dis Res. 2011;2(1):23–8. doi: 10.4103/0975-3583.78583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brito JO, Ponciano K, Figueroa D, Bernardes N, Sanches IC, Irigoyen MC, et al. Parasympathetic dysfunction is associated with insulin resistance in fructose-fed female rats. Braz J Med Biol Res. 2008;41(9):804–8. doi: 10.1590/s0100-879x2008005000030. [DOI] [PubMed] [Google Scholar]
- 8.Reaven GM, Ho H, Hoffman BB. Attenuation of fructose-induced hypertension in rats by exercise training. Hypertension. 1988;12(2):129–32. doi: 10.1161/01.hyp.12.2.129. [DOI] [PubMed] [Google Scholar]
- 9.Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities?A review of the metabolic syndrome. Sports Med. 2004;34(6):371–418. doi: 10.2165/00007256-200434060-00004. [DOI] [PubMed] [Google Scholar]
- 10.Moraes-Silva IC, De La Fuente RN, Mostarda C, Rosa K, Flues K, Damaceno-Rodrigues NR, et al. Baroreflex deficit blunts exercise training-induced cardiovascular and autonomic adaptations in hypertensive rats. :e114–20. doi: 10.1111/j.1440-1681.2009.05333.x. Clin Exp Pharmacol Physiol. 2010,37(3) [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues B, Figueroa DM, Mostarda CT, Heeren MV, Irigoyen MC, De Angelis K. Maximal exercise test is a useful method for physical capacity and oxygen consumption determination in streptozotocin-diabetic rats. Cardiovasc Diabetol. 2007;6:38. doi: 10.1186/1475-2840-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernardis LL, Patterson BD. Correlation between 'Lee index' and carcass fat content in weanling and adult female rats with hypothalamic lesions. J Endocrinol. 1968;40(4):527–8. doi: 10.1677/joe.0.0400527. [DOI] [PubMed] [Google Scholar]
- 13.Mostarda C, Rodrigues B, Vane M, Moreira ED, Rosa KT, Moraes-Silva IC, et al. Autonomic impairment after myocardial infarction: role in cardiac remodelling and mortality. Clin Exp Pharmacol Physiol. 2010;37(4):447–52. doi: 10.1111/j.1440-1681.2009.05327.x. [DOI] [PubMed] [Google Scholar]
- 14.Wichi R, Malfitano C, Rosa K, De Souza SB, Salemi V, Mostarda C, et al. Noninvasive and invasive evaluation of cardiac dysfunction in experimental diabetes in rodents. Cardiovasc Diabetol. 2007;6:14. doi: 10.1186/1475-2840-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76(5):911–22. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 16.Ferder L, Ferder MD, Inserra F. The role of high-fructose corn syrup in metabolic syndrome and hypertension. Curr Hypertens Rep. 2010;12(2):105–12. doi: 10.1007/s11906-010-0097-3. [DOI] [PubMed] [Google Scholar]
- 17.Xing SS, Bi XP, Tan HW, Zhang Y, Xing QC, Zhang W. Overexpression of interleukin-18 aggravates cardiac fibrosis and diastolic dysfunction in fructose-fed rats. Mol Med. 2010;16(11-12):465–70. doi: 10.2119/molmed.2010.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farah V, Elased KM, Chen Y, Key MP, Cunha TS, Irigoyen MC, et al. Nocturnal hypertension in mice consuming a high fructose diet. Auton Neurosci. 2006;130(1-2):41–50. doi: 10.1016/j.autneu.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Farah V, Elased KM, Morris M. Genetic and dietary interactions: role of angiotensin AT1a receptors in response to a high-fructose diet. Am J Physiol Heart Circ Physiol. 2007;293(2):H1083–9. doi: 10.1152/ajpheart.00106.2006. [DOI] [PubMed] [Google Scholar]
- 20.Bacchi E, Negri C, Zanolin ME, Milanese C, Faccioli N, Trombetta M, et al. Metabolic Effects of Aerobic Training and Resistance Training in Type 2 Diabetic Subjects: A randomized controlled trial (the RAED2 study) Diabetes Care. 2012;35(4):676–82. doi: 10.2337/dc11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieger EM, Da Silva GJ, Negrao CE. Effects of exercise training on baroreflex control of the cardiovascular system. Ann N Y Acad Sci. 2001;940:338–47. doi: 10.1111/j.1749-6632.2001.tb03689.x. [DOI] [PubMed] [Google Scholar]
- 22.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106(8):945–9. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]
- 23.Ignarro LJ, Balestrieri ML, Napoli C. Nutrition, physical activity, and cardiovascular disease: an update. Cardiovasc Res. 2007;73(2):326–40. doi: 10.1016/j.cardiores.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 24.Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, et al. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension. 2007;49(6):1298–306. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- 25.Legramante JM, Iellamo F, Massaro M, Sacco S, Galante A. Effects of residential exercise training on heart rate recovery in coronary artery patients. Am J Physiol Heart Circ Physiol. 2007;292(1):H510–5. doi: 10.1152/ajpheart.00748.2006. [DOI] [PubMed] [Google Scholar]
- 26.Senechal M, Bouchard DR, Dionne IJ, Brochu M. Lifestyle Habits and Physical Capacity in Patients with Moderate or Severe Metabolic Syndrome. Metab Syndr Relat Disord. 2012,[Epub ahead of print] doi: 10.1089/met.2011.0136. [DOI] [PubMed] [Google Scholar]
- 27.Alves AJ, Ribeiro F, Goldhammer E, Rivlin Y, Rosenschein U, Viana JL, et al. Exercise Training Improves Diastolic Function in Heart Failure Patients. Med Sci Sports Exerc. 2011, [Epub ahead of print] doi: 10.1249/MSS.0b013e31823cd16a. [DOI] [PubMed] [Google Scholar]
- 28.Jakicic JM, Clark K, Coleman E, Donnelly JE, Foreyt J, Melanson E, et al. American College of Sports Medicine position stand. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2001;33(12):2145–56. doi: 10.1097/00005768-200112000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Durstine JL, Haskell WL. Effects of exercise training on plasma lipids and lipoproteins. Exerc Sport Sci Rev. 1994;22:477–521. [PubMed] [Google Scholar]
- 30.Bertagnolli M, Schenkel PC, Campos C, Mostarda CT, Casarini DE, Bello-Klein A, et al. Exercise training reduces sympathetic modulation on cardiovascular system and cardiac oxidative stress in spontaneously hypertensive rats. Am J Hypertens. 2008;21(11):1188–93. doi: 10.1038/ajh.2008.270. [DOI] [PubMed] [Google Scholar]
- 31.Mostarda C, Rogow A, Silva IC, De La Fuente RN, Jorge L, Rodrigues B, et al. Benefits of exercise training in diabetic rats persist after three weeks of detraining. Auton Neurosci. 2009;28 145(1-2):11–6. doi: 10.1016/j.autneu.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Zamo FS, Barauna VG, Chiavegatto S, Irigoyen MC, Oliveira EM. The renin-angiotensin system is modulated by swimming training depending on the age of spontaneously hypertensive rats. Life Sci. 2011;18 89(3-4):93–9. doi: 10.1016/j.lfs.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Hagg U, Andersson I, Naylor AS, Gronros J, Jonsdottir IH, Bergstrom G, et al. Voluntary physical exercise-induced vascular effects in spontaneously hypertensive rats. Clin Sci (Lond) 2004;107(6):571–81. doi: 10.1042/CS20040171. [DOI] [PubMed] [Google Scholar]