Abstract
A field study was conducted under commercial feedlot conditions at 2 sites in western Canada to determine the relative effects of a univalent viral vaccine (MLV 1) program versus a multivalent viral vaccine (MLV 4) program on animal health; feedlot performance; and carcass characteristic variables of fall-placed, auction market derived, feedlot calves. Five thousand one hundred and sixty-three calves were processed and randomly allocated to 1 of 2 experimental groups as follows: MLV 1, which received a modified live infectious bovine rhinotracheitis (IBR) virus vaccine upon arrival at the feedlot and again at approximately 70 days on feed (DOF); or MLV 4, which received a modified live IBR virus, parainfluenza-3 virus, bovine viral diarrhea virus, and bovine respiratory syncytial virus vaccine upon arrival at the feedlot and again at approximately 70 DOF. A total of 20 pens (10 pens at the site located near High River, Alberta and 10 pens at the site located near Vegreville, Alberta) were allocated to the study.
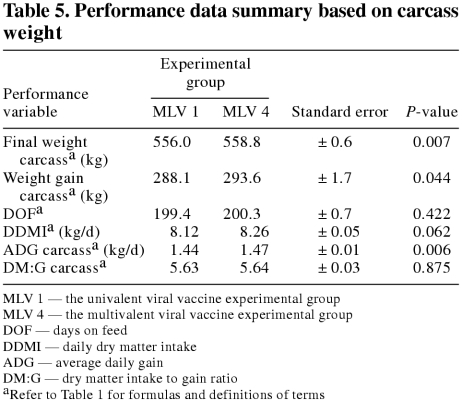
On both a live and carcass weight basis, final weight, weight gain, and average daily gain (ADG) were significantly (P < 0.05) improved in the MLV 4 group as compared with the MLV 1 group. However, there were no significant (P ≥ 0.05) differences in DOF, daily dry matter intake, dry matter intake to gain ratio (DM:G) live, or DM:G carcass between the experimental groups. In addition, there were no significant (P ≥ 0.05) differences between the experimental groups in any of the carcass characteristic variables measured.
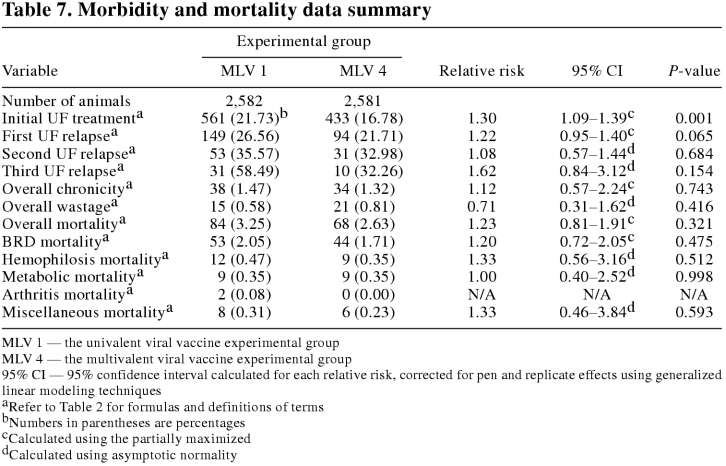
The initial undifferentiated fever (UF) treatment rate was significantly (P < 0.05) lower in the MLV 4 group as compared with the MLV 1 group. There were no significant (P ≥ 0.05) differences in the other measures of health between the experimental groups.
In the economic analysis, there was a net advantage of $0.74 CDN per animal in the MLV 4 group as compared with the MLV 1 group due to lower initial UF treatment and improved ADG, even though the cost of the vaccine program was higher in the MLV 4 group.
Introduction
Undifferentiated fever (UF), also referred to as bovine respiratory disease (BRD) complex or shipping fever, continues to be one of the most economically significant health problems in calves entering beef feedlots (1,2,3,4,5,6,7,8). Numerous bacterial and viral pathogens have been associated with the development of UF, including Mannheimia (formerly Pasteurella) haemolytica, Mycoplasma spp., Haemophilus somnus, infectious bovine rhinotracheitis (IBR) virus, bovine viral diarrhea (BVD) virus, and bovine respiratory syncytial (BRS) virus (9,10,11,12,13,14,15,16,17,18,19,20,21). In addition, several studies have demonstrated that higher antibody titers upon arrival at the feedlot to some of the aforementioned pathogens are protective and reduce the occurrence of subsequent UF (2,11,15,16,21,22,23,24,25,26,27). However, there is limited data available from properly designed, large-scale field studies to determine the relative effects of vaccinating feedlot calves with a multivalent viral vaccine containing IBR, parainfluenza type 3 (PI3), BVD, and BRS viruses to a univalent viral vaccine containing only IBR virus on animal health, feedlot performance, and carcass characteristic variables.
The purpose of the study reported herein was to determine the relative effects of a multivalent viral vaccine (Pyramid MLV 4; Ayerst Veterinary Laboratories, Division of Wyeth-Ayerst Canada, Guelph, Ontario) program versus an univalent viral vaccine (Pyramid IBR; Fort Dodge Animal Health, Division of American Home Products Corporation, Fort Dodge, Iowa, USA) program on the animal health, feedlot performance, and carcass characteristic variables of auction market derived, fall-placed feedlot calves.
Materials and methods
Study facilities
The study was conducted at 2 commercial feedlots in western Canada. One feedlot is located near High River, Alberta (site 1), and the other feedlot is located near Vegreville, Alberta (site 2). Each feedlot has a capacity of approximately 30 000 animals. The basic designs of these feedlots are representative of standard designs used in western Canada. The animals were housed in open-air, dirt-floor pens that are arranged side by side with central feed alleys and 20% porosity wood-fence windbreaks. Each pen has a capacity of approximately 250 to 300 animals. There are 2 hospital facilities located at each feedlot. Each facility is equipped with a hydraulic chute, an individual animal scale, a chute-side computer for the collection of animal health data, and separation alleys to facilitate the return of animals to designated pens. Open-air hospital pens are located adjacent to each hospital. Also, there are several receiving pens at each feedlot that are located adjacent to an enclosed processing facility.
Study animals
The animals utilized in the study were crossbred beef steer and bull calves purchased from auction markets throughout western Canada. Animals were transported by truck to the feedlots after assembly at auction markets. The animals allocated to the study were approximately 8 to 10 mo of age and entered the feedlots between September 30, 1999, and October 8, 1999. The average initial weight of animals in pens allocated to the study was between 253 kg and 274 kg.
Upon arrival at the feedlot, the animals were moved through a hydraulic chute for a group of procedures known collectively as processing. All animals were ear-tagged (to provide unique, individual animal identification), implanted with a zeranol implant (Ralgro; Schering Canada, Pointe-Claire, Quebec), and administered a multivalent clostridial/Haemophilus somnus vaccine (Vision 7 Somnus with Spur; Agriculture Division-Animal Health, Bayer, Toronto, Ontario). In addition, each animal received prophylactic, IM long-acting oxytetracycline (Tetradure LA-300; Merial Canada, Baie D'Urfe, Quebec) at a rate of 30 mg per kg body weight (BW), topical doramectin (Dectomax Pour-On Solution; Animal Health Group, Pfizer Canada, London, Ontario) at a rate of 1 mL per 10 kg BW, and a Mannheimia haemolytica bacterial extract (Presponse; Ayerst Veterinary Laboratories, Division of Wyeth-Ayerst Canada). At both feedlots, all bulls were castrated.
At site 1, all animals were implanted with an estradiol/ trenbolone acetate combination implant (Synovex Plus; Ayerst Veterinary Laboratories, Division of Wyeth-Ayerst Canada) and vaccinated with the same viral vaccine, Pyramid IBR or Pyramid MLV 4, that they had received at processing, at an average days on feed (DOF) for each pen of approximately 70 d. At site 2, all animals were implanted with a zeranol implant (Ralgro) and vaccinated with the same viral vaccine, Pyramid IBR or Pyramid MLV 4, that they had received at processing, at an average DOF for each pen of approximately 70 d. In addition, all animals at site 2 were implanted with an estradiol/trenbolone acetate combination implant (Synovex Plus) at an average DOF for each pen of approximately 140 d.
Experimental design
During the processing procedures at the feedlot, individual animals from each processing group were randomly assigned, using a computer generated randomization table, to 1 of 2 experimental groups as follows: MLV 1, which received an IBR virus vaccine (Pyramid IBR) at processing and again at approximately 70 DOF; or MLV 4, which received a multivalent viral vaccine containing IBR, PI3, BVD, and BRS viruses (Pyramid MLV 4) at processing and again at approximately 70 DOF. Animals in each experimental group were assembled in designated pens until those pens contained up to 265 animals. Replicates (1 pen from each experimental group) were filled consecutively until there were 5 replicates with a total of 10 pens at each of the 2 feedlots. In total, 2582 animals were allocated to the MLV 1 group (10 pens) and 2581 animals were allocated to the MLV 4 group (10 pens).
Feeding program
At both feedlots, standard, mixed, complete feedlot diets, formulated to meet or exceed the nutritional requirements of feedlot cattle (Nutrient Requirements for Beef Cattle, National Research Council, 1996), were offered ad libitum. The diets were delivered to the pens once or twice daily. Daily feed allowances to each pen were recorded. Water was provided ad libitum.
At site 1, the feedlot diets were blended by combining tempered-rolled barley, barley silage, tallow, medicated premix, and supplement in truck-mounted mixer boxes (Harshmixer; Harsh International, Eaton, Colorado, USA) equipped with electronic load cells. The medicated premix contained chlortetracycline and sulphamethazine (Aureo S-700 G; Hoffmann-La Roche, Cambridge, Ontario) and was formulated into the mixed, complete, feedlot diets to provide 350 mg/animal/d of each antimicrobial. The supplement contained an ionophore (Rumensin; Elanco Animal Health, Division Eli Lilly Canada, Guelph, Ontario) at a rate of 25 mg/kg diet dry matter and an antimicrobial for the control of liver abscesses (Terramycin; Animal Health Group, Pfizer Canada, London, Ontario, or Tylan; Elanco Animal Health, Division Eli Lilly Canada, depending on the days to slaughter) at a rate of 11 mg/kg diet dry matter. A commercial feed mill (Landmark Feeds, Strathmore, Alberta) manufactured the granular supplement and the medicated premix. The animals were adapted to a finisher diet over a 34- to 39-day period by increasing the proportion of tempered-rolled barley and decreasing the proportion of barley silage at approximately 6-day intervals.
At site 2, the feedlot diets were blended by combining dry-rolled barley, barley silage, medicated pellets, and supplement in truck-mounted mixer boxes (Harshmixer) equipped with electronic load cells. The medicated pellets contained chlortetracycline and sulphamethazine (Aureo S-700 G) and were formulated into the mixed, complete, feedlot diets to provide 350 mg/animal/d of each antimicrobial. The supplement contained an ionophore (Rumensin) at a rate of 25 mg/kg diet dry matter and an antimicrobial for the control of liver abscesses (Terramycin or Tylan depending on the days to slaughter) at a rate of 11 mg/kg diet dry matter. A commercial feed mill (Landmark Feeds, Strathmore, Alberta) manufactured the granular supplement and the medicated pellets. The animals were adapted to a finisher diet over a 45- to 49-day period by increasing the proportion of dry-rolled barley and decreasing the proportion of barley silage at approximately 7-day intervals.
At each feedlot, silage was sampled weekly and the dry matter content was determined. From these data, the weekly average dry matter content of each diet was calculated and used to determine the weekly dry matter intake for each pen.
Sampling
The finishing diets were sampled at approximately 1-month intervals. The samples were analyzed for crude protein (CP), acid detergent fiber (ADF), calcium (Ca), phosphorus (P), potassium (K), magnesium (Mg), sodium (Na), and salt (NaCl) (Norwest Labs, Lethbridge, Alberta).
Animal health
Experienced animal health personnel observed the study animals once or twice daily. The animal health personnel were blinded as to the experimental status of each pen. Animals deemed to be “sick” by the animal health personnel were moved to the hospital facility, diagnosed, and treated as per the written treatment protocols provided by the consulting veterinarians. In this study, the case definition for UF was a lack of abnormal clinical signs referable to body systems other than the respiratory system and an elevated rectal temperature of > 40.5°C. All animal health events, including treatment date, presumptive diagnosis, rectal temperature, BW, drug(s) used, and dose(s) used, were recorded on the chute-side computer systems (Feedlot Health Animal Record Management, Feedlot Health Management Services, Okotoks, Alberta).
At site 1, all animals that died during the study were weighed by feedlot personnel and necropsied by the attending feedlot veterinarian. The attending veterinarian diagnosed the cause of death, based on the findings of the gross postmortem examination. At site 2, trained feedlot personnel weighed and prosected all animals that died by using a standardized method and appropriate digital images, as outlined in the written necropsy protocol provided by the study investigators. Subsequently, these images were electronically transferred to the study investigators, where a veterinarian diagnosed the cause of death for each animal (29).
Marketing
The cattle were sold under normal marketing procedures, whereby the feedlot manager, based on visual appraisal, determined that a replicate, or a portion of a replicate, was ready for sale. It should be noted that when animals were sold, approximately the same numbers of animals were shipped from each experimental group within a replicate to the same packing plant (Cargill Foods, High River, Alberta) on the same day.
Data collection and management
At both feedlots, initial weight, frame score (a scale of 1–7), and sex (bull or steer) were recorded for each animal at processing. These data were subsequently imported into a spreadsheet program (Microsoft Excel 97; Microsoft Corporation, Redmond, Washington, USA), where the average initial weight, average frame score, and average proportion of steers were calculated for each pen. These baseline variables were used to assess the homogeneity of the animals in each experimental group.
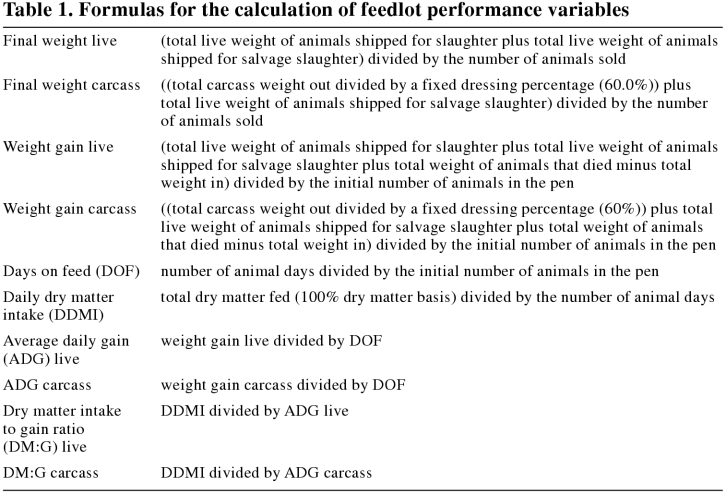
The outcome variables measured to assess feedlot performance were final weight live, final weight carcass, weight gain live, weight gain carcass, DOF, daily dry matter intake (DDMI), average daily gain (ADG) live, ADG carcass, the dry matter intake to gain ratio (DM:G) live, and DM:G carcass. The outcome variables used to assess feedlot performance were calculated for each pen (Table 1).
Table 1.
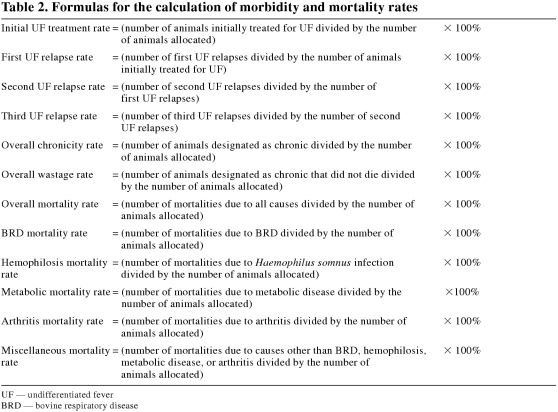
The Canadian quality grade (CQG) and Canadian yield grade (CYG) of each carcass were collected at slaughter. With respect to CQG, the proportions of animals grading Canada AAA (including Canada Prime), Canada AA, Canada A, B1, B2, and B4 were calculated for each pen. Also, for CYG, the proportions of Canada AAA, Canada AA, and Canada A carcasses within each pen that graded Canada 1, Canada 2, or Canada 3 were calculated. For each pen, dressing percentage was calculated by dividing the total carcass weight of slaughter animals by the total shrunk live weight of the same animals. The computerized animal health data were verified and summarized. From these data, risk rates for the various health variables were calculated for each pen (Table 2).
Table 2.
Statistical analysis
The data were analyzed using an analytical software program (The SAS System for Windows, Release 6.12; SAS Institute, Cary, North Carolina, USA). In all analyses, the data from both sites were combined when there was no significant (P ≥ 0.05) interaction between site and experimental group. The chemical analyses of the mixed complete diets were compared between the experimental groups by using least squares analysis of variance with the pen as the unit of analysis (29). The baseline, feedlot performance, and carcass characteristic variables were compared between the experimental groups by using least squares analysis of variance for replicate and treatment effects (29). The baseline variables were tested as covariates of the feedlot performance variables and included in the final model used for comparison of each variable between the experimental groups when significant (P < 0.05) effects were detected (30). The animal health variables were compared between the experimental groups by using generalized linear modeling techniques with individual animals as the unit of analysis, controlling for within-pen clustering of disease, as described by McDermott, Schukken, and Shoukri (31,32). Calculation of confidence intervals was done using the partially maximized likelihood function (LRCI), whenever possible; however, in some cases, LRCI could not be properly calculated due to errors in calculating the deviance function and/or failure of convergence to occur for one side of the confidence interval. In cases where LRCI could not be properly calculated, confidence intervals based on asymptotic normality of the parameter estimates (WALDCI) were calculated instead.
Economic analysis
The relative cost-effectiveness of the experimental groups was calculated using a proprietary computer spreadsheet program (Microsoft Excel 97) that simulates all economic aspects of feedlot production, as previously described (3,4,33). In the economic model, the initial weight, final weight, feeder price, slaughter price, ration cost, base processing cost, yardage rate, and interest rate were fixed for both groups. The actual values of outcome variables describing the feedlot performance (ADG carcass and DM:G carcass), carcass grading, and animal health of each experimental group were incorporated into the model when significant (P < 0.05) differences existed between the experimental groups. When no significant (P ≥ 0.05) difference existed between the experimental groups for an outcome variable, the value of that variable for the MLV 1 group was used for both experimental groups. The vaccine program costs used in the economic model were $0.60 CDN/animal and $2.26 CDN/ animal for MLV 1 and MLV 4 groups, respectively. The costs of each initial UF treatment regime, first UF relapse treatment regime, second UF relapse treatment regime, and third UF relapse treatment regime were $21.03 CDN, $11.75 CDN, $12.52 CDN, and $25.93 CDN, respectively. These costs were based on treating a 259 kg animal and included chute charges of $1.00 CDN per chute handling and hospital charges of $2.00 CDN/d occupation in the hospital. The yardage rate and interest rate used in the economic analysis were $0.17 CDN/ animal/d and 7.0%/annum.
Results
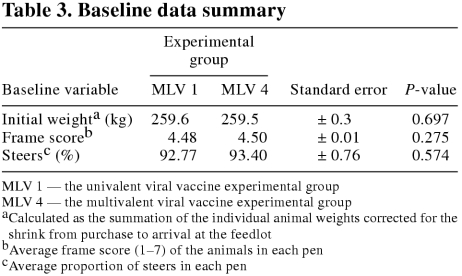
There were no significant (P ≥ 0.05) differences in the levels of CP, ADF, Ca, P, K, Mg, Na, or NaCl between the experimental groups. The baseline data summary is presented in Table 3. The groups were considered homogeneous (P ≥ 0.05) with respect to average initial weight, average frame score, and the proportion of steers within each pen.
Table 3.
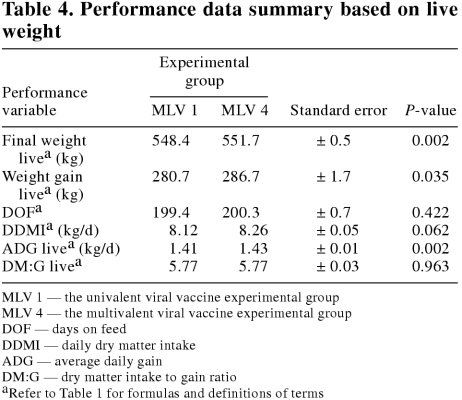
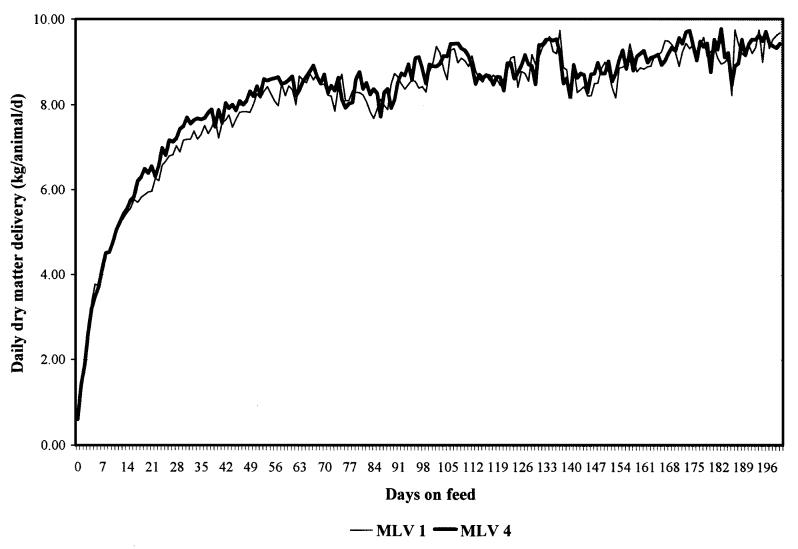
The feedlot performance data summaries are presented on a live weight basis and a carcass weight basis in Tables 4 and 5, respectively. The distribution of daily dry matter delivered by days on feed for each experimental group is presented in Figure 1. On both a live and carcass weight basis, final weight, weight gain, and ADG were significantly (P < 0.05) improved in the MLV 4 group as compared with the MLV 1 group. However, there were no significant (P ≥ 0.05) differences in DOF, DDMI, DM:G live, or DM:G carcass between the experimental groups. The carcass characteristic data summary is presented in Table 6. There were no significant (P ≥ 0.05) differences between the experimental groups in any of the carcass characteristic variables measured.
Table 4.
Table 5.
Figure 1. Distribution of daily dry matter delivery by experimental group.
Table 6.
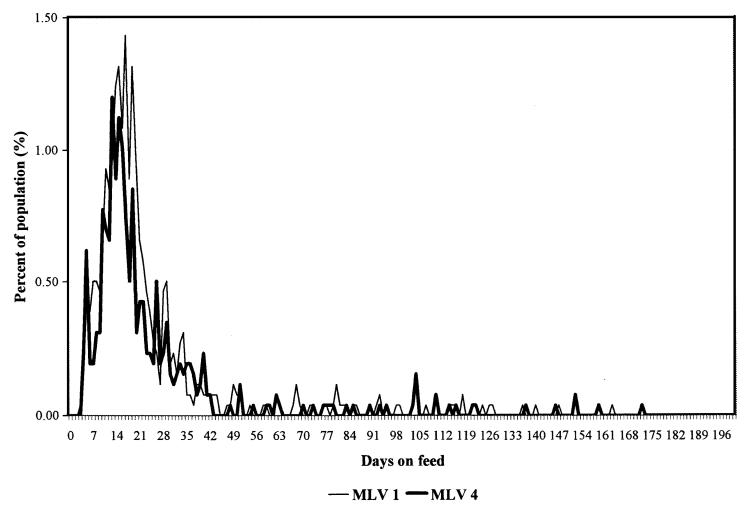
The morbidity and mortality data summary is presented in Table 7. The distribution of initial UF cases by DOF for each experimental group is presented in Figure 2. The initial UF treatment rate was significantly (P < 0.05) lower in the MLV 4 group as compared with the MLV 1 group. There were no significant (P ≥ 0.05) differences in first UF relapse, second UF relapse, third UF relapse, overall chronicity, or overall wastage rates between the experimental groups. In addition, there were no significant (P ≥ 0.05) differences in the overall or cause specific mortality rates between the experimental groups.
Table 7.
Figure 2. Distribution of new undifferentiated fever cases by days on feed.
In the economic analysis, there was a net advantage of $0.74 CDN/animal in the MLV 4 group as compared with the MLV 1 group due to lower initial UF treatment and improved ADG, even though the cost of the vaccine program was higher in the MLV 4 group.
Discussion
The results of this study demonstrate that it is more cost-effective to vaccinate auction market derived, fall-placed, feedlot calves with a multivalent viral vaccine containing IBR, PI3, BVD, and BRS viruses than with a univalent viral vaccine containing IBR virus only. However, it is not possible to determine which viral component(s) of the multivalent vaccine is/are responsible for the reduction in UF morbidity and increase in the rate of gain observed in this study. Numerous studies have demonstrated associations between the development of BRD/UF and changes in serological titers to the 4 viral pathogens listed above (10,11,12,16,21,28). However, in a recent review of the literature, Perino and Hunsaker (1997) concluded that there is very limited published information from properly designed, replicated, large-scale field studies that demonstrate the benefit of vaccinating feedlot calves against IBR, PI3, BVD, or BRS viruses (34). This assessment was based on the facts that there were no reliable reports of field trials examining the clinical efficacy of some of the viral pathogens listed above; the studies were conducted many years ago and may not apply to the current cattle feeding practices; and/or the results of the studies were equivocal.
A major limitation of the vaccine efficacy studies reported in the literature is that the vaccine groups have always been commingled in the same pens. Commingling of animals in vaccine or therapeutic studies has the potential of minimizing differences in outcome between the experimental groups and may lead to the conclusion that the vaccine or treatment of interest is not effective. In theory, this phenomenon occurs when the portion of the herd that is vaccinated or treated (test group) reduces the transmission of disease or the disease pressure on the unvaccinated or untreated portion of the herd (control group). In addition, this phenomenon occurs when the unvaccinated or untreated control group increases the disease pressure on the vaccinated or treated group. In reality, it is likely that both scenarios occur simultaneously; however, in either scenario, there is a potential bias toward accepting the null hypothesis of no treatment effect (35). To the best of the authors' knowledge, the current study is the first large scale, replicated field study conducted in a commercial feedlot environment where the herd effect has been removed by housing the experimental groups separately. In addition to minimizing the impact of the “herd effect” on the dynamics of disease, noncommingled studies also allow the researchers to measure the full impact of an experimental procedure in terms of its effect on feedlot performance variables (including feed conversion, which is measured at the pen level) and carcass characteristic variables, as well as animal health variables. Furthermore, the experimental design used in this study permits the effect of an experimental procedure on feed conversion in the commercial feedlot production system to be measured, facilitates the practical collection of carcass data from commercial packing plants, and optimizes the power of the study to detect differences in the health variables by using the animal as the unit of analysis.
In the economic analyses performed in the current study, ADG and initial UF treatment rate were the only outcome variables incorporated into the model, because these variables were significantly different between the experimental groups. In fixed final weight economic models, ADG affects the fixed day costs of feedlot production, which are yardage and interest. In the economic analysis, the yardage rate used was $0.17 CDN/animal/d. However, every $0.05 CDN/ animal/d change in yardage was associated with a $0.16 CDN/animal change in the cost-effectiveness of the MLV 4 group. The interest rate used in the economic analysis was 7%/annum. For every 1%/annum change in the interest rate, there was a $0.08 CDN/animal change in the cost-effectiveness of the MLV 4 group. In the economic analysis, the cost of the initial UF treatment regime was $21.03 CDN. However, every $2.00 CDN change in the cost of the initial UF treatment was associated with a $0.10 CDN/animal change in the cost-effectiveness of the MLV 4 group. These values demonstrate that the use of a MLV 4 program would be expected to result in a relative economic advantage across a wide spectrum of production scenarios. Note that other benefits, such as improved animal welfare and reduced antimicrobial usage associated with a lower UF treatment rate, were not included in the economic analysis.
In summary, the results of this study indicate that it is more cost-effective to vaccinate auction market derived, fall placed, feedlot calves with a multivalent viral vaccine containing IBR, PI3, BVD, and BRS viruses than a univalent viral vaccine containing IBR virus only.
Footnotes
Acknowledgments
The authors thank the management and staff of Western Feedlots Limited, High River Site, High River, Alberta, and Highland Feeders Limited, Vegreville, Alberta for their assistance and cooperation in conducting this study. CVJ
This project was wholly supported by a research grant from Fort Dodge Animal Health, Overland Park, Kansas.
Address all correspondence and reprint requests to Dr. Oliver C. Schunicht.
References
- 1.Booker CW, Jim GK, Guichon PT, Schunicht OC, Thorlakson BE, Lockwood PW. Evaluation of florfenicol for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J 1997;38:555–560. [PMC free article] [PubMed]
- 2.Booker CW, Guichon PT, Jim GK, Schunicht OC, Harland RJ, Morley PS. Seroepidemiology of undifferentiated fever in feedlot calves in western Canada. Can Vet J 1999;40:40–48. [PMC free article] [PubMed]
- 3.Jim GK, Booker CW, Guichon PT, et al. A comparison of florfenicol and tilmicosin for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J 1999;40:179–184. [PMC free article] [PubMed]
- 4.Guichon PT, Jim GK, Booker CW, Schunicht OC, Wildman BK, Brown JR. Relative cost-effectiveness of treatment of feedlot calves with ivermectin versus treatment with a combination of fenbendazole, permethrin, and fenthion. J Am Vet Med Assoc 2000;216:1965–1969. [DOI] [PubMed]
- 5.National Agriculture Statistics Service. Cattle and Calves Death Loss. Washington, DC: United States Department of Agriculture, 1996.
- 6.National Agriculture Statistics Service. Cattle and Calves Death Loss. Washington, DC: United States Department of Agriculture, 1992.
- 7.Van Donkersgoed J, Janzen ED, Harland RJ. Epidemiological features of calf mortality due to hemophilosis in a large feedlot. Can Vet J 1990;31:821–825. [PMC free article] [PubMed]
- 8.Kelly AP, Janzen ED. A review of morbidity and mortality rates and disease occurrence in North American feedlot cattle. Can Vet J 1986;27:496–500. [PMC free article] [PubMed]
- 9.Rosendal S, Martin SW. The association between serological evidence of mycoplasma infection and respiratory disease in feedlot calves. Can J Vet Res 1986;50:179–183. [PMC free article] [PubMed]
- 10.Allen JW, Laurent V, Bateman KG, Nagy E, Rosendal S, Shewan PE. Serological titers to bovine herpesvirus 1, bovine viral diarrhea virus, parainfluenza-3 virus, bovine respiratory syncytial virus and Pasteurella haemolytica in feedlot calves with respiratory disease: associations with bacteriological and pulmonary cytological variables. Can J Vet Res 1992;56:281–288. [PMC free article] [PubMed]
- 11.Martin SW, Bateman KG, Shewan PE, Rosendal S, Bohac JG, Thorburn M. A group level analysis of the associations between antibodies to seven putative pathogens and respiratory disease and weight gain in Ontario feedlot calves. Can J Vet Res 1990;54: 337–342. [PMC free article] [PubMed]
- 12.Martin SW, Bateman KG, Shewen PE, Rosendal S, Bohac JE. The frequency, distribution and effects of antibodies, to seven putative respiratory pathogens, on respiratory disease and weight gain in feedlot calves in Ontario. Can J Vet Res 1989;53:355–362. [PMC free article] [PubMed]
- 13.Shewen PE, Wilkie BN. Antibody titers to Pastuerella haemolytica A1 in Ontario beef cattle. Can J Comp Med 1982;46:354–356. [PMC free article] [PubMed]
- 14.Shewen PE, Wilkie BN. Pasteurella haemolytica cytotoxin neutralizing activity in sera from Ontario beef cattle. Can J Comp Med 1983;47:497–498. [PMC free article] [PubMed]
- 15.Martin SW, Meek AH, Davis DG, et al. Factors associated with mortality in feedlot cattle: The Bruce County beef cattle project. Can J Comp Med 1980;44:1–10. [PMC free article] [PubMed]
- 16.Martin SW, Bohac JG. The association between serological titers in infectious bovine rhinotracheitis virus, bovine viral diarrhea virus, parainfluenza-3 virus, respiratory syncytial virus and treatment for respiratory disease in Ontario feedlot calves. Can J Vet Res 1986;50:351–358. [PMC free article] [PubMed]
- 17.Thomson RG, Chandler S, Savan M, Fox ML. Investigation of factors of probable significance in the pathogenesis of pneumonic pasteurellosis in cattle. Can J Comp Med 1975;39:194–207. [PMC free article] [PubMed]
- 18.Adegboye DS, Halbur PG, Nutsch RG, Kadlec RG, Rosenbusch RF. Mycoplasma bovis associated pneumonia and arthritis complicated with pyogranulomatous tenosynovitis in calves. J Am Vet Med Assoc 1996;209:647–649. [PubMed]
- 19.Van Donkersgoed J, Janzen ED, Potter AA, Harland RJ. The occurrence of Haemophilus somnus in feedlot calves and its control by post-arrival prophylactic mass medication. Can Vet J 1994;35:573–580. [PMC free article] [PubMed]
- 20.Guichon PT, Jim GK. Mortality due to Haemophilus somnus in calves. Can Vet J 1989;30:435. [PMC free article] [PubMed]
- 21.Durham PJK, Hassard LE, Van Donkersgoed J. Serological studies of infectious bovine rhinotracheitis, parainfluenza 3, bovine viral diarrhea, and bovine respiratory syncytial viruses in calves following entry to a bull test station. Can Vet J 1991;32:427–429. [PMC free article] [PubMed]
- 22.Harland RJ, Potter AA, Van Drunen-Littel-Van den Hurk S, et al. The effect of subunit or modified live bovine herpesvirus-1 vaccines on the efficacy of a recombinant Pasteurella haemolytica vaccine for the prevention of respiratory disease in feedlot calves. Can Vet J 1992;33:734–741. [PMC free article] [PubMed]
- 23.Van Donkersgoed J, Van den Hurk JV, McCartney D, Harland RJ. Comparative serological responses in calves to eight commercial vaccines against infectious bovine rhinotracheitis, parainfluenza-3, bovine respiratory syncytial, and bovine viral diarrhea viruses. Can Vet J 1991;32:727–733. [PMC free article] [PubMed]
- 24.Shewen PE, Wilkie BN. Vaccination of calves with leukotoxic culture supernatant from Pasteurella haemolytica. Can J Vet Res 1988;52:30–36. [PMC free article] [PubMed]
- 25.Jim K, Guichon T, Shaw G. Protecting feedlot calves from pneumonic pasteurellosis. Vet Med 1988;83:1084–1087.
- 26.Rice Conlon JA, Gallo GF, Shewen PE, Adlam C. Comparison of protection of experimentally challenged cattle vaccinated once or twice with a Pasteurella haemolytica bacterial extract vaccine. Can J Vet Res 1995;59:179–182. [PMC free article] [PubMed]
- 27.Ribble CS, Jim GK, Janzen ED. Efficacy of immunization of feedlot calves with a commercial Haemophilus somnus bacterin. Can J Vet Res 1988;52:191–198. [PMC free article] [PubMed]
- 28.Wildman BK, Schunicht OC, Jim GK, Guichon PT, Booker CW, Tollens RA. The use of computer imaging technology to facilitate the capture of feedlot necropsy information. Can Vet J 2000;41: 124–125. [PMC free article] [PubMed]
- 29.Snedecor GW, Cochran WG. Statistical Methods. 7th ed. Ames, Iowa: Iowa State Univ Pr, 1987:175–191.
- 30.SAS Institute Inc. SAS/STAT User's Guide, Version 6, 4th ed, Vol 1 and 2. Cary, North Carolina: SAS Institute Inc., 1989;1686 pp.
- 31.McDermott JJ, Schukken YH. A review of methods used to adjust for cluster effects in explanatory epidemiological studies of animal populations. Prev Vet Med 1994;18:155–173.
- 32.McDermott JJ, Schukken YH, Shoukri MM. Study design and analytic methods for data collected from clusters of animals. Prev Vet Med 1994;18:175–191.
- 33.Schunicht OC, Guichon PT, Booker CW, et al. Comparative cost-effectiveness of ivermectin versus topical organophosphate in feedlot yearlings. Can Vet J 2000;41:220–224. [PMC free article] [PubMed]
- 34.Perino LJ, Hunsaker BD. A review of bovine respiratory disease vaccine field efficacy. The Bovine Pract 1997;31:59–66.
- 35.Thurber ET, Bass EP, Beckenhauer WH. Field trial evaluation of a reo-coronavirus calf diarrhea vaccine. Can J Comp Med 1977; 41:131–136. [PMC free article] [PubMed]