Abstract
To guide the implementation of a Hazard Analysis Critical Control Point (HACCP) system at a small abattoir, the microbiological conditions of pig carcasses at various stages of processing were assessed by enumerating total aerobes, coliforms, and Escherichia coli in samples collected from randomly selected sites on the carcasses. Those data indicated that carcasses were contaminated with bacteria mainly during dehairing and operations on the head. When carcasses were pasteurized after head removal, the numbers of total aerobes on dressed carcasses were reduced by about 1 order and the numbers of coliforms and E. coli were reduced by more than 2 orders of magnitude. Implementation of an HACCP system on the basis of the microbiological data gave cooled carcasses with mean numbers of total aerobes < 100/cm2, and mean numbers of coliforms and E. coli about 1/1000 cm2.
Introduction
Meat inspecting authorities around the world are requiring meat packing plants to implement Hazard Analysis Critical Control Point (HACCP) systems for the control of hazardous microbial contamination of meat (1). Although HACCP systems must take account of physical and chemical, as well as microbiological hazards, the latter hazards are the major concern in the production of raw meats at packing plants. The procedures recommended for implementing HACCP systems depend upon the subjective assessment of risks, with consequent uncertain identification of critical control points (CCPs) for the control of microbiological hazards. If the CCPs in a process are misidentified, the standard operating procedures (SOPs) prescribed for the control of hazards are likely to be ineffective (2). There is then good reason for supposing that some of the HACCP systems that are currently operated at meat packing plants give only variable and often inadequate control over the microbiological conditions of raw meats.
As subjective assessment of meat plant processes must always be uncertain, it has been suggested that HACCP systems at meat plants be based on microbiological data that allow estimation of the effects on the microbiological condition of the product of individual operations within any process (3). Then, operations can be modified and reevaluated, as necessary, to attain a final product that complies with stringent food safety objectives (FSOs). An FSO is a statement of a maximum level of microbiological contamination considered acceptable for consumer protection (4). For that purpose, it is necessary to refer to the mean numbers of indicator organisms on products, as pathogenic organisms are generally too few and are present too infrequently to be useful for the routine investigation and evaluation of process performance (5).
Methods that are practicable for recovering and enumerating indicator organisms from raw meat at most stages of product processing within packing plants, and methods for estimating the changes in numbers of bacteria on a product as a result of processing have been identified (6); also the use of those methods in procedures for improving the hygienic performances of some commercial processes has been demonstrated (7). However, as yet, few meat packing plants have implemented a full HACCP system on the basis of microbiological data to attain stringent FSOs. To address that deficiency, an HACCP system based on the objective evaluation of the hygiene of processing through microbial testing is being implemented at a meat packing plant that is operated at an Agriculture and Agri-Food Canada Research Centre. The development of such a system necessarily proceeds in stages, as the various processes at the plant are investigated and brought under control. Therefore, the objective of this study was to apply objective assessment procedures to arrive at a validated system for controlling the microbiological contamination of pig carcasses, as a first step towards assuring the microbiological safety of all meat produced at the plant.
Materials and methods
The meat packing plant
The plant is operated at the Agriculture and Agri-Food Canada Lacombe Research Centre and is inspected by the Canadian Food Inspection Agency (CFIA). Although some carcasses are subjected to experimental treatments, most are processed by using procedures that are usual in the meat industry. Much of the meat from those latter carcasses is dispatched for normal commercial uses. To comply with CFIA requirements, an HACCP system for the usual processes at the plant must be developed by the plant staff and be approved by the CFIA.
Typically, the abattoir is operated 2 d per week, with between 20 and 40 animals of a single species being slaughtered on each day. The carcasses are cooled overnight and are then processed into primal cuts or dispatched as hanging meat within 3 d after slaughter. The species slaughtered are mainly pigs and cattle, but sheep, exotic domestic animals, and farmed game animals are slaughtered occasionally.
The carcass dressing process
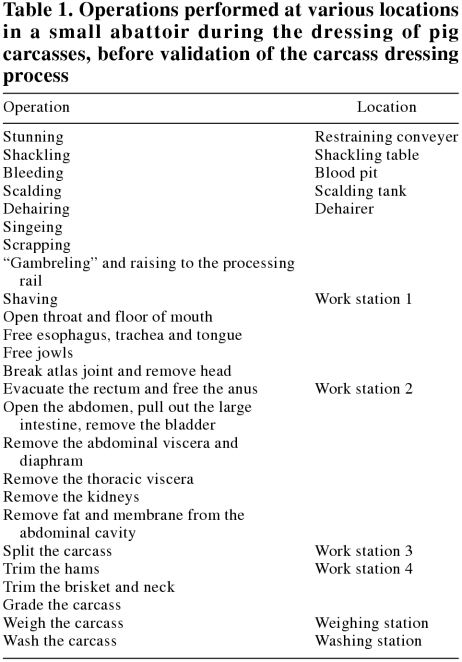
Pig carcasses are processed at a rate of 8 carcasses per hour. Before the development of the validated HACCP system, the pig carcass dressing process was comprised of 25 operations performed by 4 workers (Table 1). Each pig entered the slaughtering floor and was stunned in a restraining conveyor. After shackling and bleeding, the carcass was immersed in a scalding tank before being placed on the cradle of a dehairing machine. After dehairing, the carcass was singed and scraped while on the cradle, before being raised to the processing rail. At the first work stand, the carcass was shaved of any remaining hair. Then operations on the head and throat were performed, after which the head was removed.
Table 1.
The carcass was then moved to a 2nd stand for eviscerating operations, to a 3rd for splitting, and to a 4th stand for trimming. The sides were then graded, weighed, and washed with water from a hand-held hose before being transferred to a chiller. The carcasses were cooled in a chiller in which the refrigeration equipment was operated to deliver air from the coils at a temperature of 0°C and a speed of 4 m/s. Carcasses were well spaced and were not sprayed during overnight cooling for between 18 and 22 h.
Carcass sampling
At each time of sampling carcasses at any stage of the process, the first 25 carcasses that reached the specified stage during processing on one day were sampled. A single sample was collected from a carcass at any stage of processing, but on most days, a sample was collected from each carcass at 2 stages of the process. Each sample was collected by swabbing an undefined limit of approximately 100 cm2 with moistened medical gauze, with the site to be sampled being selected at random from a grid that specifies 83 sites on one side of a carcass, as previously described (6).
Enumerations of bacteria
Each swab was pummeled for 2 min with 10 mL of 0.1% (w/v) peptone water. For the enumeration of total aerobic counts, 1 mL of the pummeled fluid was diluted tenfold in peptone water, then 0.1 mL of the tenfold dilution was diluted in 10 mL of peptone. A 1 mL portion of the tenfold dilution, the remaining 8.9 mL of the tenfold dilution, and the 10.1 mL volume of the highest dilution were each filtered through a separate hydrophobic grid membrane filter (QA Life Sciences, San Diego, California USA). Each filter was placed on a plate of tryptone soy fast green agar (TSFG; QA Life Sciences), which was incubated at 25°C for 3 d (8). Squares containing green or blue-green colonies were counted, and a most probable number (MPN) for the colonies on each filter was obtained by application of the formula MPN = N × logn (N/(N − X)) where N is the total number of squares and X is the count of squares containing colonies.
For the enumerations of coliforms and Escherichia coli, 0.1 mL of the pummeled fluid was diluted in 10 mL of peptone water. Then, the remaining stomacher fluid and the whole of the dilution were each filtered through a separate hydrophobic grid membrane filter. Each filter was placed on a plate of lactose monensin glucuronate agar (LMG; QA Life Sciences), which was incubated at 35°C for 24 h (9). Squares containing blue colonies were counted and an MPN value for coliforms was obtained as for the total aerobic counts described above. The filter was then transferred to a plate of buffered 4-methylumbelliferyl-β-D glucuronide agar (BMA; QA Life Sciences), which was incubated at 35°C for 3 h. The incubated filters were illuminated with long-wavelength ultraviolet light, squares containing blue-white fluorescent colonies were counted, and an MPN value for E. coli was obtained from that count as for the total aerobic counts.
Analysis of data
All bacterial counts were transformed to log10 values. When bacteria of a type were recovered from ≥ 20 of 25 samples, values for the mean log (¯x) and standard deviation (s) of the set of bacterial counts were calculated on the assumption of a log normal distribution of counts (10). In the calculation of ¯x and s, a log value of −0.5/cm2 or 100 cm2 was assumed for the count from each sample in which bacteria were not detected. Values for the log means (log A) for sets of counts were calculated from the formula log A = ¯x + logn 10, s2/2 (11). A value for the total number of bacteria recovered (N) was calculated for each set of counts by summing the counts in the set and obtaining the log of the sum. Those calculations were performed with software (Microsoft Excel Version 4, Statistical functions; Microsoft, Redmond, Washington USA).
Results and discussion
When the carcasses of various species dressed at the abattoir were examined in a previous study, those of pigs were found to be in the poorest microbiological condition (12). As meat from pig carcasses produced at the abattoir would then pose the greatest microbiological risk to consumers, it was appropriate to commence an HACCP implementation by seeking enhanced control over microbiological contamination during the pig carcass dressing process (3).
When seeking enhanced control over the microbiological condition of a product, it is necessary to formulate an attainable FSO against which to judge the success or otherwise of the actions taken to improve the condition of the product. Previous studies had suggested that an appropriate FSO for cooled red meat carcasses could be that log mean numbers of total aerobic counts would be < 2 log cfu/cm2 and that log total numbers of both coliforms and E. coli would be < 0.5 log cfu/2500 cm2 (13). That FSO was adopted for all cooled carcasses produced at the abattoir, including those of pigs.
It is economically desirable that an FSO be achieved with minimum modification of an existing process. Therefore, the possibility of achieving the FSO by modifying operating procedures, without modification of the plant or the installation of new equipment, was considered.
The stated FSO will generally be attainable for pig carcasses only if an effective decontaminating treatment is employed at some point in the dressing process, because scalded carcasses, which carry few bacteria, are recontaminated during mechanical dehairing operations (14). At some plants, the singeing of dehaired carcasses provides a decontaminating treatment (13). Therefore, the effects on carcasses of the singeing operation were examined. As was expected, the dehaired carcasses were contaminated with relatively large numbers of aerobes, coliforms, and E. coli (Table 2). Singeing of the carcasses did not obviously reduce the numbers of any of the organisms.
Table 2.
As singeing was ineffective for reducing bacterial numbers, an apparatus for pasteurizing carcasses with sheets of hot water (15) was installed on the dressing floor. Although pasteurizing carcasses at the end of the dressing process is possible, the appearances of cut muscle surfaces are commonly degraded by the treatment (16). Consequently, it is preferable to apply the treatment to pig carcasses at an early stage of dressing, when little or no muscle has been cut, provided that an acceptable microbiological condition can be preserved throughout the subsequent dressing operations. Therefore, a pasteurizing treatment was applied to carcasses after the shaving operation. That treatment reduced the numbers of aerobes, coliforms, and E. coli by 3 orders of magnitude, and gave carcasses a microbiological condition compatible with the FSO (Table 3). However, the operations for exposing the tongue and removing the head resulted in the carcasses being recontaminated, with the numbers of all 3 groups of bacteria being 2 orders of magnitude more than on the pasteurized carcasses.
Table 3.
The contamination of carcasses during head removal was expected, as such contamination had previously been observed at a large packing plant (17). To counter the undesirable microbiological effects of operations on the head, the operations were modified to reduce the contacting of other parts of the carcass by hands or equipment that had previously contacted the mouth and throat, and by pasteurizing the carcass after, instead of before, removal of the head. That gave carcasses with log total numbers of aerobes about 2 log cfu/25 cm2, and log total numbers of coliforms and E. coli both about 1 log cfu/2500 cm2 (Table 4). After evisceration, splitting, and washing, the numbers of aerobes on carcasses were the same as after pasteurizing, but the log total numbers of coliforms and E. coli had both increased to about 1.5 log cfu/2500 cm2.
Table 4.
The numbers of coliforms and E. coli on the washed carcasses exceeded the FSO. Those numbers could have been reduced by applying a 2nd pasteurizing treatment to the carcasses. However, such a treatment was considered unnecessary, because at the abattoir carcasses are routinely cooled without spraying, and the surface drying that occurs during cooling can result in the numbers of coliforms and E. coli on carcasses being reduced by an order of magnitude or more (18). Reductions in the numbers of coliform and E. coli during the cooling of carcasses evidently occurred, as the numbers of those organisms, as well as the numbers of total aerobes, on cooled carcasses that had been through the modified dressing process generally met with the FSO (Table 5).
Table 5.
The routine attainment of a stringent FSO for cooled pig carcasses demonstrates that it is practical to greatly improve the microbiological condition of pig carcasses, when the operations and processes are adjusted by referring to appropriate microbiological data. Adjustments to improve processing are undoubtedly easier to introduce at small, rather than at larger, plants, but improvement of processing hygiene at large plants is by no means impossible, though the SOPs adopted at the small abattoir to attain stringent FSOs may not be appropriate for improving actions at all pork packaging plants. However, the general approach to the development of SOPs for the effective control of microbial contamination should be applicable to all pig carcass dressing processes. The SOPs developed during this study are detailed in the HACCP manual for carcass dressing and cooling at the small abattoir, which is available on the website (19).
Footnotes
Acknowledgments
The authors thank Mr. C. Pimm for his assistance with the development of hygienic carcass dressing procedures, and Ms. C. Landers for her assistance with the collecting and processing of microbiological samples. CVJ
Address all correspondence and reprint requests to Mr. J. Bryant; e-mail address: bryantj@em.agr.ca
References
- 1.U.S. Department of Agriculture. Pathogen reduction: hazard analysis and critical control point (HACCP) system: final rule. Fed Register 1996;61:38805–38989.
- 2.Brown, MH. Implementing HACCP in a meat plant. In: Brown MH, ed. HACCP in the Meat Industry. Boca Raton: CRC Pr, 2000:177–201.
- 3.Gill CO. HACCP in primary processing: red meat. In: Brown MH, ed. HACCP in the Meat Industry. Boca Raton: CRC Pr, 2000: 81–122.
- 4.Lee JA, Hathaway SC. The challenge of designing valid HACCP plans for raw food commodities. Food Control 1998;9:111–117.
- 5.Brown MH, Gill CO, Hollingsworth J, et al. The role of microbiological testing in systems for assuring the safety of beef. Int J Food Microbiol 2000;62:7–16. [DOI] [PubMed]
- 6.Gill CO, Badoni M, Jones T. Hygienic effects of trimming and washing operations in a beef-carcass-dressing process. J Food Protect 1996;59:666–669. [DOI] [PubMed]
- 7.Gill CO, McGinnis JC. Improvement of the hygienic performance of the hindquarters skinning operations at a beef packing plant. Int J Food Microbiol 1999;51:123–132. [DOI] [PubMed]
- 8.Gill CO, Jones T. Microbiological sampling of carcasses by excision or swabbing. J. Food Protect 2000;63:167–173. [DOI] [PubMed]
- 9.Entis P, Boleszczuk P. Direct enumeration of coliforms and Escherichia coli by hydrophobic grid membrane filter in 24 h using MUG. J Food Protect 1990;53:948–952. [DOI] [PubMed]
- 10.Gill CO, Deslandes B, Rahn K, Houde A, Bryant J. Evaluation of the hygienic performances of the processes for beef carcass dressing at 10 packing plants. J Appl Microbiol 1998;84:1050–1058. [DOI] [PubMed]
- 11.Kilsby DC, Pugh ME. The relevance of the distribution of micro-organisms within batches of food to the control of microbiological hazards from foods. J Appl Bacteriol 1981;51:345–354. [DOI] [PubMed]
- 12.Gill CO, Jones T, Bryant J, Brereton DA. The microbiological conditions of the carcasses of six species after dressing at a small abattoir. Food Microbiol 2000;17:233–239.
- 13.Gill CO, Dussault F, Holley RA, et al. Evaluation of the hygienic performances of the processes for dressing and cooling pig carcasses at eight packing plants. Int J Food Microbiol 2000;58:65–72. [DOI] [PubMed]
- 14.Gill CO, Bryant J. The contamination of pork with spoilage bacteria during commercial dressing, chilling and cutting of pig carcasses. Int J Food Microbiol 1992;16:51–62. [DOI] [PubMed]
- 15.Gill CO, Bryant J, Bedard D. The effects of hot water pasteurizing treatments on the appearances and microbiological conditions of beef carcass sides. Food Microbiol 1999;16:281–289.
- 16.Gill CO, Jones T, Badoni M. The effects of hot water pasteurizing treatment on the microbiological conditions and appearances of pig and sheep carcasses. Food Res Int 1998;31:273–278.
- 17.Gill CO, Jones T. Control of the contamination of pig carcasses with Escherichia coli from their mouths. Int J Food Microbiol 1998;44:43–48. [DOI] [PubMed]
- 18.Gill CO, Jones T. Assessment of the hygienic performances of an air cooling process for lamb carcasses and a spray-cooling process for pig carcasses. Int J Food Microbiol 1997;38:85–93. [DOI] [PubMed]
- 19.Bryant, J. 2002. Lacombe Research Centre: Standard Operating Procedures for Pig Carcass Dressing. http:res2.agr.ca/lacombe/