Over the last years, a number of potent drugs have been developed that inhibit HIV-1 replication in vivo. Treatment regimes involving a combination of three or more different drugs can induce a decline in virus load by several orders of magnitude, as well as a significant rise in the CD4 cell count. The drugs currently in use mainly fall into two classes: reverse transcriptase inhibitors prevent infection of new cells, whereas protease inhibitors interfere with the production of new infectious virions by infected cells. Recent pharmaceutical research has focused on the development of another class of drugs, the integrase inhibitors, preventing HIV from integrating into the genome of its host cells. Although research is finding more drugs to combat HIV infection, the virus is continuously catching up by evolving resistance against these drugs (1–5). Although combination therapy can result in sustained suppression of virus load in many patients, it is not effective in all patients and fails after the emergence of multidrug-resistant strains (6). Hence, although finding new drugs to fight HIV is important for improving our chances for success, it is equally important to devise therapy regimes that minimize the chance of drug resistance emerging. To do this, we need more detailed information about the evolutionary dynamics that lead to the emergence of drug-resistant strains.
Imperfect adherence to a prescribed regimen is one of the critical obstacles to successful drug therapy (7–13). Maintaining adherence may be particularly difficult when the drug regimen is complex and the side effects are severe, as is often the case with combination therapy. If only a subset of the prescribed drugs is taken for certain periods of time, the virus can successively evolve resistance to each of the drugs used while the patient is on therapy.
However, drug resistance also has been observed in patients who do adhere to their prescribed drug regimen. In these cases, the strategy required to minimize the chances of treatment failure depends on the mechanism by which drug-resistant strains evolve. In a recent issue of PNAS, Ribeiro and Bonhoeffer (14) use mathematical models to examine this question. There are two basic possibilities. Either drug-resistant strains evolve during therapy, or drug-resistant strains exist in the virus population before the onset of therapy. They are maintained by a mutation-selection balance and are selected to grow when the drugs are applied. If drug-resistant strains evolve during therapy, then the dosage of treatment should be increased so that the residual replication of the sensitive virus during treatment is minimized. On the other hand, if resistant strains exist before therapy, the effect of the drug on wild-type virus does not matter in the long run. A potent drug will lead to a fast decline of wild-type virus and a fast rise of resistant mutants. A weak drug will lead to a slow decline of wild-type virus and a slow rise of resistant mutants. The average virus load remains constant (15). If strains pre-exist that are resistant to one or several of the drugs, the strategy should be to combine more drugs with different resistance profiles to minimize the chance that any virus strain will be resistant to all drugs (14).
Although it has not been possible to distinguish between these two mechanisms experimentally, mathematical models suggest that it is more likely that treatment failure is caused by resistant strains existing before therapy than by resistant strains evolving during therapy. This result has been derived by a variety of modeling approaches, both deterministic (14, 15) and stochastic (14). During therapy the resistant mutants are most likely to be produced from the sensitive virus population while it declines to low levels. This event is significantly less likely than the generation of resistant strains before the onset of therapy (14, 15).
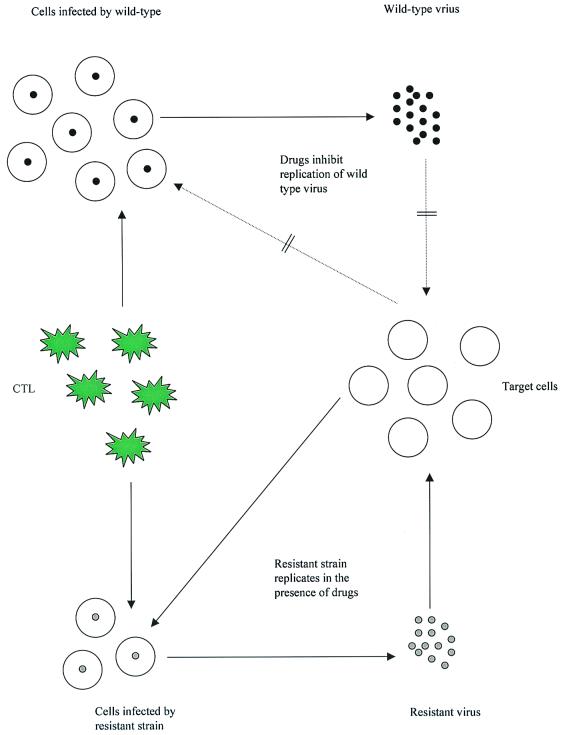
This result implies that it is not only important to use the right combination of drugs with different resistance profiles, but also that it is important to treat as early as possible to minimize the chances that strains resistant to all drugs exist before the onset of treatment. The findings by Ribeiro and Bonhoeffer (14) not only have practical implications, but also pose new questions regarding our understanding of HIV dynamics during highly active antiretroviral therapy. Treatment failure can be manifested in the viral dynamics in a variety of ways (16). In some individuals already the initial response to treatment is suboptimal, and the virus population converges to an equilibrium characterized by relatively high loads. In other patients, the initial response to treatment is good and virus load is reduced below the levels of detection. However, after a given period, the virus re-emerges. The time span until the virus re-emerges can vary between patients and can be of the order of months (16). With the above discussion in mind, such dynamics are likely to be caused by two mechanisms: either the patients are noncompliant and HIV evolves resistance to all drugs used during therapy, or the mutant strains exist before treatment, but initially cannot grow. They can emerge only later when conditions are favorable. Two dynamical factors could potentially influence the ability of mutants to grow during therapy (Fig. 1): (i) the level of susceptible target cells, and (ii) the level of antiviral immune responses.
Figure 1.
Dynamics of wild-type and resistant strains during therapy. Drug treatment inhibits replication of the wild-type virus, resulting in a significant decline of virus load. Drug-resistant mutants can replicate in the presence of the drugs. The ability of the mutant virus to grow depends both on the availability of target cells and on the number of virus-specific CTL during therapy.
It has been argued that an increase in the number of susceptible target cells (in particular CD4+ T cells) during treatment can enable virus mutants to grow after a given period (17, 18). Initially, when drug treatment is started, the number of susceptible cells is too low for the resistant strains to emerge. Growth of the virus is only possible once the number of susceptible cells has risen above a given threshold level.
In addition to target cell availability, the level of antiviral immune responses could significantly influence the ability of resistant strains to grow during therapy. Recently, an increasing amount of experimental observation has become available concerning the role and dynamics of immune responses against HIV. Cytotoxic T lymphocyte (CTL) responses have been shown to be of particular importance for limiting HIV load (19, 20), and both experimental and theoretical studies have accumulated examining the dynamics of CTLs during drug treatment. Data from HIV-infected patients (21, 22) indicate that CTL dynamics at the start of therapy can be divided into two phases. First, a temporary increase is observed in the number of HIV-specific CTLs, followed by a decline of these CTLs to very low levels. The initial temporary rise in CTLs at the start of therapy is likely to be brought about by a reduction of HIV-induced immune impairment while virus load is still high enough to provide sufficient levels of antigenic stimulation. The subsequent decline of CTL indicates that the response cannot be maintained at low levels of antigen and that it needs continuous exposure to high levels of antigen to persist. Mathematical models suggest that a short lifespan of CTLs at low levels of antigen could be the reason for HIV persistence and pathogenesis (23, 24). It also could contribute to a delayed emergence of drug-resistant strains that have existed before the onset of treatment. If the patient does have a persistent CTL response during the asymptomatic phase of the infection, basic mathematical models of infection dynamics indicate that the level of CTLs before treatment is significantly determined by the replication rate of the wild type. Mutations conferring drug resistance are thought to bear a certain cost for the virus, and hence the replication rate of the resistant strain in the presence of the drug is likely to be somewhat lower than the replication rate of the wild type in the absence of the drugs. In this case, the drug-resistant strains will not be able to grow if the level of CTLs is still around the pretherapy equilibrium. The resistant strains will be able to grow only once the level of CTLs has declined below a given threshold level, and this condition could be met only after a certain time span of therapy in some patients. Hence, even if resistant strains exist before therapy, it might be possible that their outgrowth is inhibited by maintaining the CTLs during treatment. The CTL response could be maintained or even increased by combining drug therapy with vaccination. Multiple vaccinations might increase the chances of success. This would maintain an effector CTL response while virus load is reduced to very low levels after drug therapy. In addition, structured therapy interruptions could lead to the generation of CTL responses that can be maintained at low levels of antigen (24–26).
With the knowledge that treatment failure is most likely to be caused by the pre-existence of drug-resistant strains, together with our knowledge regarding the dynamics of CTLs during drug therapy, it might be possible to create a pharmaco-immunological treatment regime under which viral resistance is futile. A sufficient number of drugs with different resistance profiles should be used, and treatment should be started as early as possible. This minimizes the chances that resistant mutants pre-exist (14) and maximizes the chances that a relatively efficient CTL response is generated (27). If treatment is started later, drug administration could be combined with vaccination to sustain a CTL response during therapy and thus to prevent the outgrowth of the resistant strains. In the most optimal case, such a therapy regime would result in the generation of efficient and sustained immunity that can suppress virus load below the level of detection without the need for drugs.
Footnotes
See companion paper on page 7681 in issue 14 of volume 97.
References
- 1.Larder B A, Darby G, Richman D D. Science. 1989;243:1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- 2.Richman D D. Trends Microbiol. 1994;2:401–407. doi: 10.1016/0966-842x(94)90619-x. [DOI] [PubMed] [Google Scholar]
- 3.Coffin J M. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 4.Stilianakis N I, Boucher C A, De Jong M D, Van Leeuwen R, Schuurman R, De Boer R J. J Virol. 1997;71:161–168. doi: 10.1128/jvi.71.1.161-168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonhoeffer S, May R M, Shaw G M, Nowak M A. Proc Natl Acad Sci USA. 1997;94:6971–6976. doi: 10.1073/pnas.94.13.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Condra J H, Schleif W A, Blahy O M, Gabryelski L J, Graham D J, Quintero J C, Rhodes A, Robbins H L, Roth E, Shivaprakash M, et al. Nature (London) 1995;374:569–571. doi: 10.1038/374569a0. [DOI] [PubMed] [Google Scholar]
- 7.Kepler T B, Perelson A S. Proc Natl Acad Sci USA. 1998;95:11514–11519. doi: 10.1073/pnas.95.20.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bangsberg D R, Hecht F M, Charlebois E D, Zolopa A R, Holodniy M, Sheiner L, Bamberger J D, Chesney M A, Moss A. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 9.Miller L G, Hays R D. AIDS Read. 2000;10:177–185. [PubMed] [Google Scholar]
- 10.Gallant J E. J Am Med Assoc. 2000;283:1329–1334. [Google Scholar]
- 11.Markowitz M. J Am Med Assoc. 2000;283:250–251. [Google Scholar]
- 12.Paris D, Ledergerber B, Weber R, Jost J, Flepp M, Opravil M, Ruef C, Zimmerli S. AIDS Res Hum Retroviruses. 1999;15:1631–1638. doi: 10.1089/088922299309676. [DOI] [PubMed] [Google Scholar]
- 13.Wahl L M, Nowak M A. Proc R Soc London Ser B. 2000;267:835–843. doi: 10.1098/rspb.2000.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribeiro R M, Bonhoeffer S. Proc Natl Acad Sci USA. 2000;97:7681–7686. doi: 10.1073/pnas.97.14.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonhoeffer S, Nowak M A. Proc R Soc London Ser B. 1997;264:631–637. doi: 10.1098/rspb.1997.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Mendoza C, Soriano V, Perez-Olmeda M, Rodes B, Casas E, Gonzalez-Lahoz J. J Hum Virol. 1999;2:344–349. [PubMed] [Google Scholar]
- 17.Frost S D, McLean A R. AIDS. 1994;8:323–332. doi: 10.1097/00002030-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 18.McLean A R, Emery V C, Webster A, Griffiths P D. AIDS. 1991;5:485–489. doi: 10.1097/00002030-199105000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, et al. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 20.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, et al. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, et al. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalams S A, Goulder P J, Shea A K, Jones N G, Trocha A K, Ogg G S, Walker B D. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wodarz D, May R M, Nowak M A. Int Immunol. 2000;12:467–477. doi: 10.1093/intimm/12.4.467. [DOI] [PubMed] [Google Scholar]
- 24.Wodarz D, Nowak M A. Proc Natl Acad Sci USA. 1999;96:14464–14469. doi: 10.1073/pnas.96.25.14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz G M, Nixon D F, Trkola A, Binley J, Jin X, Bonhoeffer S, Kuebler P J, Donahoe S M, Demoitie M A, Kakimoto W M, et al. J Clin Invest. 1999;104:R13–R18. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lisziewicz J, Rosenberg E, Lieberman J, Jessen H, Lopalco L, Siliciano R, Walker B, Lori F. N Engl J Med. 1999;340:1683–1684. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- 27.Lifson J D, Rossio J L, Arnaout R, Li L, Parks T L, Schneider D M, Kiser R F, Coalter V J, Walsh G, Imming R, et al. J Virol. 2000;74:2584–2593. doi: 10.1128/jvi.74.6.2584-2593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]