Abstract
HIV-associated neurocognitive disorders (HAND) remain prevalent even with widespread use of combination antiretroviral therapy (ART), suggesting a potential role for co-morbidities in neurologic decline. Indeed, it is well established that ART drugs, particularly HIV protease inhibitors, can induce hyperlipidemia, lipodystrophy, and insulin resistance; all of which are associated with neurologic impairment. This study was designed to determine how metabolic dysfunction might contribute to cognitive impairment and to reveal specific metabolic co-morbidities that could be targeted to preserve brain function. Adult male C57BL/6 mice were thus treated with clinically relevant doses of lopinavir/ritonavir for 4 weeks, and subjected to thorough metabolic, neurobehavioral, and biochemical analyses. Data show that lopinavir/ritonavir resulted in manifestations of lipodystrophy, insulin resistance, and hyperlipidemia. Evaluation of neurologic function revealed cognitive impairment and increased learned helplessness, but not motor impairment following treatment with lopinavir/ritonavir. Further analyses revealed a significant linear relationship between cognitive performance and specific markers of lipodystrophy and insulin resistance. Finally, analysis of brain injury indicated that lopinavir/ritonavir treatment resulted in cerebrovascular injury associated with decreased synaptic markers and increased inflammation, and that the cerebral cortex was more vulnerable than the cerebellum or hippocampus. Collectively, these data reveal an intimate link between metabolic co-morbidities and cognitive impairment, and suggest that remediation of selective aspects of metabolic syndrome could potentially reduce the prevalence or severity HIV-associated neurocognitive disorders.
Keywords: HAND, HIV co-morbidities, HIV protease inhibitors, insulin resistance, lipodystrophy, metabolic syndrome
1. INTRODUCTION
In the US and other developed nations, survival rates associated with HIV infection have improved dramatically since the introduction of combination antiretroviral therapies (ART), which restrict viral replication, raise CD4 cell counts, prevent opportunistic infections, and improve and extend health-related quality of life (reviewed in (Quinn, 2008). However, HIV-associated neurocognitive disorders (HAND) still occur in conjunction with HIV infection and can range from subtle neuropsychological impairments to profoundly disabling HIV-associated dementia (Ances and Ellis, 2007), (Nath et al., 2008), (Power et al., 2009). While the incidence of the most severe neurologic forms of manifestations of HIV (e.g., AIDS dementia complex) have significantly declined with widespread application of ART (Sacktor et al., 2001), the incidence of milder syndromes remains quite high with estimates that 50% of HIV patients display neurocognitive dysfunction despite successful viral suppression (Heaton et al., 2011). This level of disability can undermine activities of daily living and threaten employment and self-care independence (Heaton et al., 2004). The physiologic basis for the continued high rates of HAND remain uncertain, but potential mechanisms include incomplete viral suppression in the central nervous system (CNS) due to poor CNS penetration of ART, possible direct neurotoxicity of drugs that do enter the CNS, and co-morbidities including metabolic syndrome and associated cerebrovascular pathology that undermine neurologic function.
While the advent of ART has revolutionized the care of HIV-positive patients, epidemiological data have revealed clinically and physiologically significant iatrogenic metabolic complications of these drugs. These metabolic disturbances produce clinical syndromes that can include dyslipidemia, insulin resistance, and lipodystrophy (reviewed in (Herman and Easterbrook, 2001), (Anuurad et al., 2009), which not only affect patient health, but also limit ART compliance (reviewed in (Schambelan et al., 2002). Furthermore, epidemiological and experimental studies reveal quite clearly that metabolic dysfunction can increase brain injury and decrease cognitive function (reviewed in (Fillit et al., 2008), (Bruce-Keller et al., 2009), (Haan and Wallace, 2004), (Middleton and Yaffe, 2009)). Indeed, recent reports indicate that HIV patients with metabolic compromise have a significantly greater risk of developing neurologic complications (Valcour et al., 2004), (Valcour et al., 2005), (Valcour et al., 2006), (Bandaru et al., 2007), (Valcour et al., 2011), but the extent to which and the mechanisms by which the metabolic effects of ART undermine neurologic function have not been directly examined.
Although multiple factors influence metabolism in HIV patients, protease inhibitors are thought to play a major and specific role in the development of metabolic co-morbidities, as protease inhibitors are well-known to profoundly affect insulin signaling, serum and hepatic triglycerides, body fat composition, and adipokine levels both in humans and mice (Pistell et al., 2010a), (Lenhard et al., 2000), (Tsiodras et al., 2000), (Hurwitz et al., 2004), (Prot et al., 2006), (Moyle, 2007), (Thomas and Smart, 2007),(Jiang et al., 2009). Indeed, previous work from our group has shown that the administration of clinically relevant doses of lopinavir/ritonavir to adult, male C57Bl/6 mice results in profound metabolic derangement, and also significant neurologic impairment (Pistell et al., 2010a). Thus, this study was designed to identify the exact relationship between specific metabolic co-morbidities and the development of neurologic impairment, and to reveal specific metabolic pathways that could be targeted therapeutically to protect the brain from further injury. To this end, C57BL/6 mice were given lopinavir/ritonavir daily for 4 weeks, and then extensively tested for both metabolic and neurologic function, as well as brain injury.
2. MATERIALS AND METHODS
2.1 Animal Treatments
The Institutional Animal Care and Use Committee at the Pennington Center approved all experimental protocols, which were compliant with NIH guidelines on the use of experimental animals. 6–8 month-old male C57Bl/6 mice were purchased from Charles River Laboratories (Wilmimgton, MA), and were housed in standard caging with 12:12 light: dark cycle and ad libitum access to food and water. Lopinavir/ritonavir (Kaletra®, Abbott Laboratories), was diluted in a vehicle of 10% ethanol/15% propylene glycol, and mice received daily administration of vehicle or lopinavir/ritonavir at 150/37.5 mg/kg via oral gavage. The dose was devised based on dosing guidelines for daily oral lopinavir/ritonavir in adult HIV patients (800/200 total mg or 10/2.5 mg/kg), and on body surface area (BSA) normalization factors (Pinkel, 1958), (Sawyer and Ratain, 2001), (Reagan-Shaw et al., 2007), which translate 10 mg/kg in humans to approximately 125 mg/kg in mice.
Body weight and composition (measured using a Bruker minispec LF90 time domain NMR analyzer, Bruker Optics, Billerica MA) were measured regularly. Blood glucose was measured in tail blood using a glucometer (Ascensia Elite, Bayer, Mishawaka, IN), and oral glucose tolerance was measured using a modified oral glucose tolerance test (OGTT). Briefly, mice were fasted for 4 hrs, baseline glucose was measured, and then mice were immediately administered glucose (2gm/kg) via oral gavage. Blood glucose was measured at 15, 30, 60 and 120 min, and area under the curve (AUC) was recorded as an index of glucose diposal. To measure circulating non-etserified fatty acids (NEFA) in the context of hyperglycemia, additional blood samples (~50 ul) were collected at 0 and 60 min by lancing the submandibular vein (Fernández et al., 2010), and NEFA were analyzed as described in Section 2.3. After behavioral testing, all mice were humanely euthanatized after a brief (6 hr) fast, and blood, brain, and adipose tissue were collected. Brain samples were further divided into anterior cortex (anterior 1/3 of cortex), hippocampus, and cerebellum. Data were compiled from 2 separate cohorts of mice, with a total of 9–20 animals in each group.
2.2 Behavioral analyses
Cognitive ability was tested behaviorally on all mice using the five-segmented, Stone T-maze as described in previously published reports (Pistell et al., 2009), (Pistell and Ingram, 2010). Briefly, mice must learn the correct sequence of 13 consecutive left and right turns to successfully escape the maze, and are motivated to escape because they are required to wade (not swim) through the maze. Mice were given 15 sequential trials in the T-maze in a single day such that the first trial was completed by all mice before proceeding to the second trial to prevent fatigue. Trials were recorded using video tracking software (Viewpoint Lifesciences, Inc), and the numbers of errors committed was recorded and used as the primary measure of learning. For the purpose of data analysis and presentation, acquisition data was averaged into 5 separate blocks of 3 trials each.
To assess learned helplessness, mice were tested using the Porsolt forced swim test (Porsolt et al., 1977), (Cryan et al., 2002), (Crowley et al., 2004). Mice were placed in transparent 17-cm diameter plastic tanks filled to a depth of 20 cm with 23–25°C water, and the amount of time spent mobile (active, escape-oriented behaviors such as swimming, rearing, or diving) versus immobile (floating or making only minimal movements to keep the head above water) was recorded and scored during the last four minutes of 6-minute trials by an observer blind to the condition of the mouse.
Motor function was analyzed using a Five Station Rota-Rod Treadmill for Mouse (Med-Associates, St. Albans, VT). Each mouse was given three trials during which the starting speed of the rotarod was 4 rpm, but the rod accelerated to 40 rpm over a period of five min. The amount of time the animal was able to remain on an accelerating rotating cylinder was recorded and used as the primary measure of motor function. The maximum trial length was 5 min and there was a 30 min rest period between each trial.
2.3 Clinical Chemistry
Whole blood was collected by cardiac puncture of terminally anesthetized mice, and was allowed to clot at 4°C overnight and then centrifuged at 3000×g for 30 minutes. Serum was collected and either analyzed immediately or aliquoted and stored at −80°C. Levels of total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, and nonesterified fatty acids (NEFA) in sera were measured colorimetrically using commercially available kits (Wako Chemicals, Richmond, VA). Adiponectin, leptin, and insulin levels in serum were all evaluated by ELISA in accordance with the manufacturer’s assay protocol (R&D Systems, Minneapolis, MN for adiponectin and leptin; and Crystal Chem Inc., Downers Grove IL for insulin). To measure total pools of adiponectin, serum samples were first denatured (boiled in SDS buffer for 5 minutes) to break down large complexes.
2.4 Measures of brain injury by Western blot and ELISA
Tissue samples were homogenized and processed for Western blot with chemiluminescence as described in previous reports (Bruce-Keller et al., 2011), (Pistell et al., 2010b). Blots were processed using the following primary antisera: anti-claudin-5 (1:400, Abcam Inc., Cambridge, MA), anti-ZO-1 (1:100, Abcam Inc.), anti-occludin (1:8000, Abcam Inc.), anti-MMP2 (1:1000, Abcam Inc.), anti-MMP-9 (1:1000, Abcam Inc.), anti-synapsin 1 (1:10000, Thermo Fisher Scientific, Pittsburg, PA), anti-phospho(S553)-synapsin 1 (1:10000, Abcam Inc.), anti-synapse associated protein 97 (1:2500, Abcam Inc.), anti-GFAP (1:5000, Abcam Inc.); anti-Iba-1 (1:500, Wako Chemicals USA Inc., Richmond, VA), and anti-tubulin (1:1000, Wako Chemicals USA Inc.). To ensure accurate quantification across multiple blots, samples from both liponavir/ritonaivr and vehicle mice were included in each individual blot. Data were first calculated as a ratio of expression over tubulin expression, which was included as an internal loading control, and then expression in liponavir/ritonavir mice was calculated and presented as percent expression in control mice.
For ELISA, brain tissue samples were homogenized (100 µg total protein/well) and analyzed as described previously (Bruce-Keller et al., 2001), (Pistell et al., 2010b) using commercially available kits (BD Biosciences, San Jose, CA for IL-1β and BDNF; R&D Systems, Minneapolis, MN for IL-6 and TNF-α).
2.5 Statistical analyses
All data were analyzed using Prism software (GraphPad Software, Inc., La Jolla, CA), and are displayed as mean ± standard error of measurement. Stone maze performance was analyzed with 2-way repeated measures ANOVA to determine main effects of trail block and treatment, followed by Bonferroni post-hoc comparisons to determine differences between lopinavir/ritonavir and vehicle groups. All other data were analyzed by 2-tailed, unpaired t-tests to determine specific differences between lopinavir/ritonavir and vehicle groups. Correlation analyses were used to determine statistical relationships of metabolic measurements with cognitive impairment (errors in trail block 4–6 of Stone maze), and were derived by calculating Pearson correlation coefficients with data evaluated as continuous variables without transformation. Statistical significance for all analyses was accepted at p < 0.05, and *, **, and *** represent p < 0.05, p < 0.01, and p < 0.001, respectively.
3. RESULTS
3.1 Metabolic effects of lopinavir/ritonavir
Combined lopinavir/ritonavir (Kaletra, Abbott Laboratories, Abbott Park, IL) is a cocktail prepared at a 4:1 ratio, and is very commonly used in clinical settings to manage HIV (reviewed in (Cvetkovic and Goa, 2003), and also has a strong association with metabolic derangement both in humans and in mice (Prot et al., 2006), (Chandwani and Shuter, 2008). The present study was designed to reveal the relationship between metabolic dysfunction and neurologic impairment to identify metabolic pathway(s) that might mediate brain injury. To this end, 6–8 month old, male C57BL/6 mice were administered a daily oral regimen of vehicle or lopinavir/ritonavir for 4 weeks as described in Methods. Metabolic data were collected, and for presentation purposes are thematically divided into syndromes separately describing lipodystrophy, insulin resistance, and dyslipidemia (Table 1). Data show that daily lopinavir/ritonavir resulted in significant changes in body composition in the mice, with a moderate but significant decrease in body weight (t(41) = 2.20, p = 0.0332), and a marked decrease in total fat mass in lopinavir/ritonavir-treated mice (t(41) = 2.85, p = 0.0069) as compared to vehicle-treated mice (Table 1). In keeping with the loss of adipose tissue, serum concentrations of the adipokines adiponectin (t(39) = 6.43, p < 0.0001) and leptin (t(39) = 4.62, p < 0.0001) were both significantly decreased in lopinavir/ritonavir-treated mice as compared to vehicle-treated mice (Table 1).
Table 1. Metabolic consequences of chronic L/R.
Male C57BL/6 mice were treated daily with vehicle or lopinavir/ritonavir (150/37.5 mg/kg body weight) for 28 days, after which mice were evaluated for metabolic changes reflecting lipodystrophy, insulin resistance, and hyperlipidemia as described in Methods. Values are mean and S.E.M. data collected from 9–20 animals over 2 separate cohorts.
| Vehicle | Lop/Rit | Change from Vehicle |
||
|---|---|---|---|---|
| Lipodystrophy | ||||
| Final body weight (gr) | 34.2 ± 0.7 | 32.2 ± 0.6* | ↓ 6% | |
| Final fat mass (%BW) | 17.8 ± 0.9 | 13.8 ± 1.0** | ↓ 22% | |
| Serum Adiponectin (µg/ml) | 41.6 ±.1.3 | 27.6 ± 1.8*** | ↓ 34% | |
| Serum Leptin (ng/m) | 6.3 ± 0.7 | 2.5 ± 0.5*** | ↓ 60% | |
| Insulin Resistance | ||||
| Fasting Glucose (mg/dl) | 152.6 ± 4.4 | 167.2 ± 4.5* | ↑10% | |
| Fasting Insulin (ng/ml) | 1.34 ± 0.2 | 2.3 ± 0.3** | ↑ 72% | |
| OGTT: Glu AUC | 17.7 ± 0.5 | 17.4 ± 0.4 | NS | |
| OGTT: 60 min NEFA (mEq/ml) | 0.59 ± 0.03 | 0.93 ± 0.04*** | ↑ 37% | |
| Hyperlipidemia | ||||
| Total Cholesterol (mg/dl) | 108.2 ± 2.8 | 132.1 ± 8.0** | ↑ 22% | |
| LDL Cholesterol(mg/dl) | 18.7 ± 2.7 | 29.8 ± 6.7 | NS | |
| HDL Cholesterol(mg/dl) | 55.7 ± 3.7 | 62.4 ± 5.6 | NS | |
| Triglycerides(mg/dl) | 31.6 ± 2.1 | 70.2 ± 7.9*** | ↑ 122% | |
| NEFA (mEq/L) | 0.96 ± 0.07 | 1.46 ± 0.18* | ↑ 52% | |
Data were analyzed by 2-tailed, unpaired t-tests, and *, **, and *** indicate significant (p<0.05, p<0.01, and p<0.001, respectively) differences noted in lopinavir/ritonavir-treated mice as compared to vehicle-treated mice.
AUC = area under the curve, NEFA = non-esterified fatty acids; NS = not significantly different from vehicle; OGTT = oral glucose tolerance test.
To determine the extent of insulin resistance and loss of glycemic control, studies focused on regulation of fasting glucose and glucose tolerance. At the end of the 28-day regimen of daily lopinavir/ritonavir, mice were fasted as described in Methods, and blood glucose and serum insulin were measured as described in Methods. Data show a modest but statistically significant increase in fasting blood glucose (t(38) = 2.34, p = 0.0245), and a robust increase in fasting insulin levels (t(39) = 3.09, p = 0.0037, Table 1). Evaluation of glucose tolerance, conversely, revealed no effect of the 28-day regimen of daily lopinavir/ritonavir on glucose disposal (Table 1). Finally, in light of the degree of lipodystrophy, the adipose response to glucose loading was evaluating by quantifying the serum levels of NEFA 60 minutes after glucose administration, as described in Methods. Data show that lopinavir/ritonavir-treated mice had significantly elevated serum NEFA levels following glucose administration as compared to vehicle-treated mice (t(34) = 7.01, p < 0.0001, Table 1).
Studies next assessed the panel of bioactive serum lipids in lopinavir/ritonavir-treated mice. Data show that lopinavir/ritonavir caused significant increases in total serum cholesterol (t(37) = 2.97, p = 0.0052), although levels of HDL- and LDL-cholesterol were not significantly affected (Table 1). Furthermore, statistical analyses confirm significant increases in circulating triglycerides (t(36) = 5.20, p < 0.0001) and NEFA (t(36) = 2.59, p = 0.0139) in lopinavir/ritonavir-treated mice as compared to vehicle-treated control mice (Table 1).
3.2 Behavioral effects of lopinavir/ritonavir
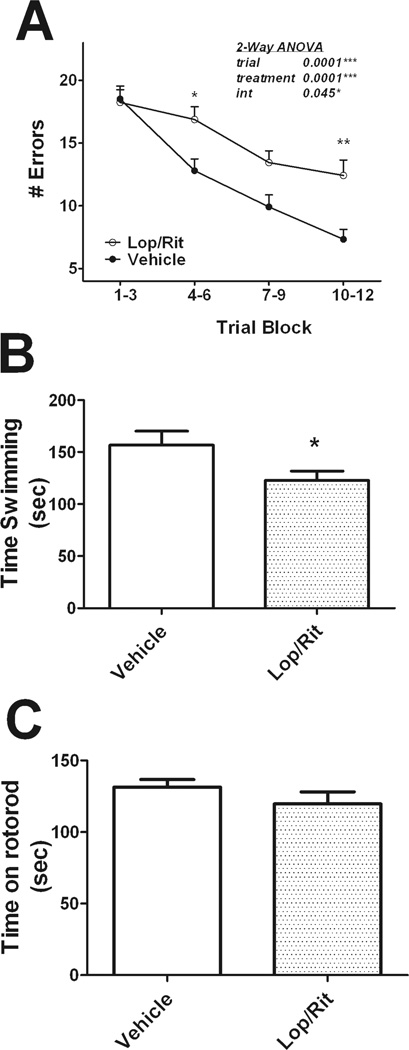
The effects of lopinavir/ritonavir on behavioral tasks of cognition, motor function, and learned helplessness were evaluated. Cognitive function was measured using the Stone T-maze, which is a task of procedural learning and memory that is not confounded by changes in motor impairment, feeding behavior, or nociception (Pistell and Ingram, 2010), (Pistell et al., 2010b), (Pistell et al., 2010a). Data show combined lopinavir/ritonavir treatment resulted in significant cognitive impairment (Fig 1A). Specifically, ANOVA on maze performance revealed significant main effects of trial number (F(3, 152) = 28.04, p < 0.0001) and treatment group (F(1,152) = 19.38, p < 0.0001) with a significant interaction (F(3,152) = 2.75, p = 0.0451). Post-hoc analyses revealed that lopinavir/ritonavir-treated mice committed significantly more errors in the trial blocks 4–6 and 10–12 as compared to vehicle treated mice (Fig. 1A). The degree of impairment caused by lopinavir/ritonavir is greater than the degree of impairment caused by diet-induced obesity in mice (Pistell et al., 2010b), but is roughly comparable to the degree of cognitive impairment noted in aged (25 month) mice compared to young (5 month) mice as measured in this task (Pistell et al., 2012).
Figure 1. Lopinavir/ritonavir affects cognition and learned helplessness, but not motor ability in mice.
Male C57BL/6 mice were treated daily with vehicle or lopinavir/ritonavir (150/37.5 mg/kg body weight) for 28 days, after which mice were evaluated behaviorally as described in Methods. Experiments were conducted in 9–20 animals per group over 2 separate cohorts. (A) Effects of lopinavir/ritonavir on cognitive performance in the Stone T-maze. Data show the number of errors committed over 15 trials of maze training and are means ± S.E.M. of average errors accrued over 3-trial blocks. Data were analyzed by 2-way ANOVA, and the insert depicts the significant main effects of trial number, treatment group, and the significant interaction between trial and treatment. *and ** indicate significant (p<0.05, p<0.01, respectively) increases in errors made by lopinavir/ritonavir-treated mice in trial blocks 4–6 and 10–12. (B) Effects of lopinavir/ritonavir on behavioral despair in Porsolt forced swim test. Data depict time spent in swimming/escape behavior, and were analyzed by 2-tailed, unpaired t-tests. * indicates the significant (p<0.05) decrease in swim time in lopinavir/ritonavir-treated mice as compared to vehicle-treated mice. (C) Effects of lopinavir/ritonavir on motor performance in the Rotarod test. Data depict the time mice were able to remain on the accelerating rotarod, and are mean ± S.E.M. of average time over 3 trails.
As HIV and HAND are associated with neuropsychiatric disorders and depression (reviewed in (Sherr et al., 2011)), we analyzed the effects of lopinavir/ritonavir in the Porsolt forced swim test of behavioral despair, as described in Methods. While this test does not measure depression per se, it is a common and established experimental measures of depression-like behavior in rodents (Cryan et al., 2002), (Crowley et al., 2004) with numerous reports showing its sensitivity to antidepressant drugs (Castagné et al., 2011). Mice were evaluated in this task at the end of the 4-week treatment regimen, and data show that treatment with lopinavir/ritonavir significantly decreased swim time (t(23) = 2.18, p = 0.0402, Fig 1B), suggesting an increase in depressive-like behavior.
Since both the Stone T-maze and Porsolt swim test involve physical activity, motor performance of vehicle- and lopinavir/ritonavir-treated mice was specifically addressed by testing mice on the rotarod, which measures the time the animal is able to remain on an accelerating rotating cylinder. Analysis of rotarod performance data did not reveal any impairment in motor function in lopinavir/ritonavir-treated mice as compared to vehicle-treated mice (Fig 1C).
3.3 Relationship of individual metabolic parameters to cognitive impairment
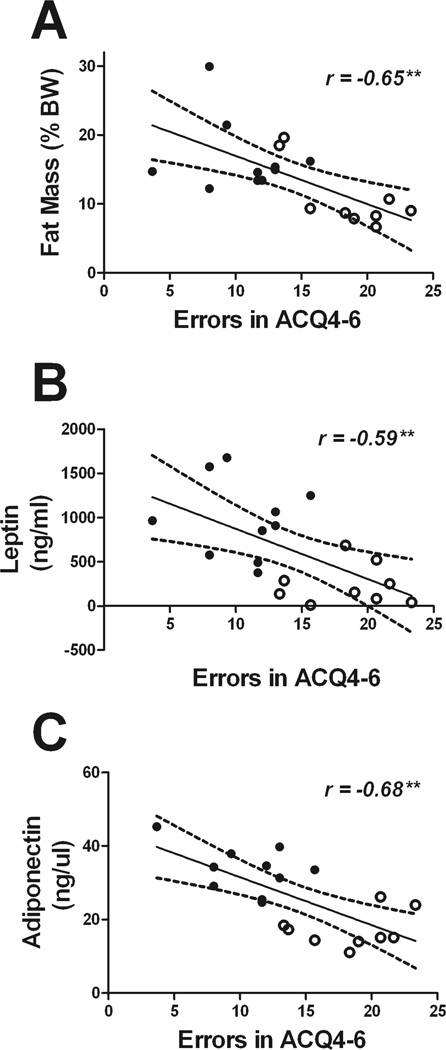
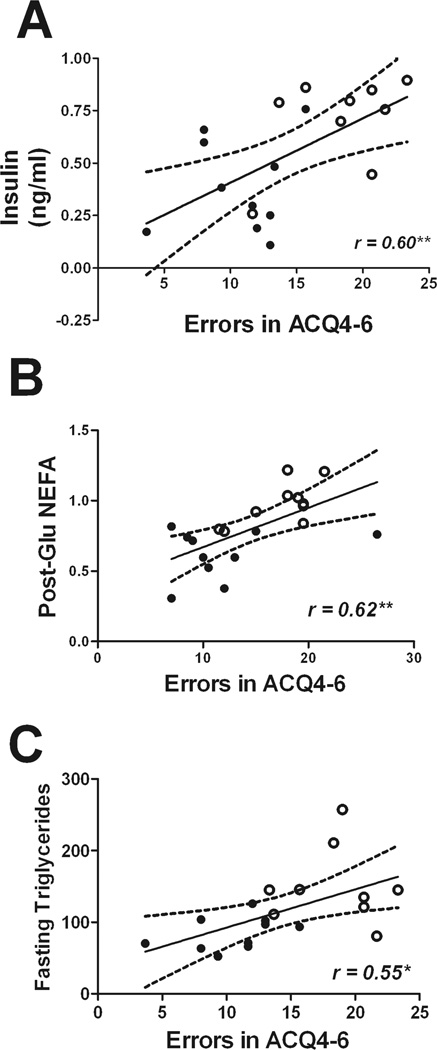
To determine how impaired metabolic pathways might lead to the cognitive abnormalities noted in these mice, correlation analyses were undertaken to determine the exact statistical relationship between individual metabolic indices and cognitive function. To this end, numerical values for metabolic measures were correlated against acquisition errors in the same individual vehicle- and lopinavir/ritonavir-treated mice. Data show that while no correlation could be found for body weight and acquisition errors (data not shown), other parameters of lipodystrophy showed a significant, inverse correlation with maze performance (Fig. 2). Specifically, acquisition errors in trial block 4–6 of the Stone maze correlated significantly with fat mass (r = −0.65, p = 0.0028, Fig. 2A), serum leptin (r = −0.59, p = 0.0081; Fig. 2B), and serum adiponectin (r = −0.68, p = 0.0012; Fig. 2C). Likewise, maze errors correlated significantly with fasting insulin (r = −0.60, p = 0.0062; Fig 3A) and also with post-glucose levels of NEFA (r = 0.62, p = 0.0033; Fig. 3B), but not with fasting glucose levels or OGTT AUC (data not shown). Finally, a significant linear relationship was detected between fasting triglycerides and acquisition errors (r = 0.55, p = 0.0158; Fig. 3C), but there was no significant linear relationship between acquisition errors in the Stone maze and total cholesterol, HDL-cholesterol, LDL-cholesterol, or fasting NEFA (data not shown).
Figure 2. Parameters of lipodystrophy correlate with maze performance in mice.
Scatter plots show the statistically significant linear relationship between errors in acquisition trail block 4–6 (errors in ACQ4–6) and (A) fat mass, (B) serum leptin, and (C) serum adiponectin in vehicle- and lopinavir/ritonavir-treated mice. Each point represents an individual subject, and closed circles depict vehicle-treated mice while open circles depict lopinavir/ritonavir-treated mice.
Figure 3. Parameters of insulin resistance and serum triglycerides correlate with maze performance in mice.
Scatter plots show the statistically significant linear relationship between errors in acquisition trail block 4–6 (errors in ACQ4–6) and (A) fasting insulin, (B) circulating NEFA 60 minutes following oral glucose administration (Post-Glu NEFA), and (C) fasting triglycerides in vehicle- and lopinavir/ritonavir-treated mice. Each point represents an individual subject, and closed circles depict vehicle-treated mice while open circles depict lopinavir/ritonavir-treated mice.
3.4 Markers of brain injury in lopinavir/ritonavir-treated mice
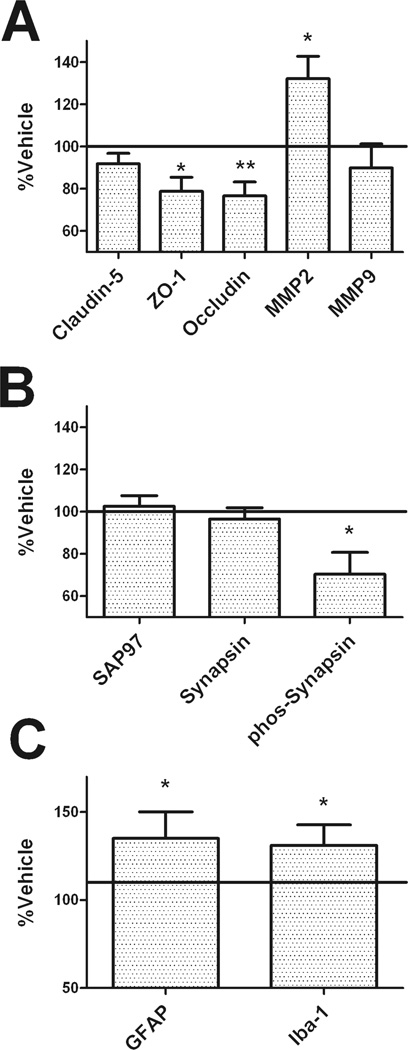
Experiments were next designed to determine the extent and type of brain injury caused by lopinavir/ritonavir. Analyses were thematically split into evaluations of cerebrovascular integrity, synaptic density, and reactive gliosis; and initial investigations were conducted in the anterior cortex as cortical injury has been shown to disrupt Stone maze performance in rats (Spangler et al., 1994). Cerebrovascular and blood-brain barrier integrity were evaluated by measuring the expression of tight junction proteins claudin-5, ZO-1, and occludin; as well as the matrix metalloproteinases MMP2 and MMP9, via Western blot as described in Methods. While there were no differences in caludin-5 expression between groups (Fig. 4A), 2-tailed, unpaired t-tests revealed significant decreases in ZO-1 (t(20) = 2.34, p = 0.0299) and occludin expression (t(20) = 2.91, p = 0.0087) in cortex homogenates from liponavir/ritonavir-treated mice as compared to vehicle-treated mice (Fig. 4A). Conversely, levels of MMP2 expression in frontal cortex of liponavir/ritonavir-treated mice were significantly increased (t(20) = 2.51, p = 0.0206) as compared to vehicle mice, while MMP9 was not affected (Fig. 4A).
Figure 4. Lopinavir/ritonavir induces brain injury in mice.
Male C57BL/6 mice were treated daily with vehicle or lopinavir/ritonavir (150/37.5 mg/kg body weight) for 28 days, after which markers of cerebrovascular integrity, synaptic density, and reactive gliosis were evaluated in tissue homogenates prepared from the frontal cortex as described in Methods. Data depict mean ± SEM expression in lopinavir/ritonavir-treated mice presented as % vehicle (100% line) on graph. Data were obtained from 9–20 mice/group, and were analyzed by 2-tailed, unpaired t-tests. (A) Expression of the tight junction proteins claudin-5, ZO-1, and occludin; and the matrix metalloproteinases MMP2 and MMP9. * and ** indicate significant (p < 0.05 and 0.01, respectively) changes in expression in lopinavir/ritonavir-treated mice as compared to vehicle. (B) Expression of the post-synaptic marker synapse associated protein 97 (SAP97), the pre-synaptic protein synapsin 1, and phosphorylated synapsin 1. * indicates significant (p < 0.05) the significant decrease in phosphorylated synapsin 1 expression in lopinavir/ritonavir-treated mice. (C) Expression of the glial markers glial fibrillary acidic protein (GFAP) and Iba-1. * indicates significant (p < 0.05) increases in GFAP and Iba-1 expression in lopinavir/ritonavir-treated mice.
Evaluations of synaptic density were based on altered expression of the post-synaptic protein synapse associated protein 97 (SAP97) and total and phosphorylated forms of the pre-synaptic protein synapsin 1 (SYN1). These specific markers were chosen as studies have shown that these proteins reflect most faithfully the number of synapses as determined by EM-based synapse counts (S.W. Scheff, personal communication). Quantification of SAP97 and total SYN1 expression revealed no differences in expression between groups (Fig. 4B). Conversely, phosphorylated SYN1 expression in frontal cortex of liponavir/ritonavir-treated mice was significantly decreased (t(20) = 2.14, p = 0.0447) as compared to vehicle mice
To determine if liponavir/ritonavir treatment affected glial reactivity in mice, the expression of astrocyte and microglial markers were evaluated using Western blot. The intermediate filament protein glial fibrillary acidic protein (GFAP) was used to evaluate astrocyte hypertrophy (O'Callaghan and Sriram, 2005), and evaluation of blots revealed significant increases in GFAP expression in liponavir/ritonavir-treated mice as compared to vehicle mice (t(20) = 2.31, p = 0.0319; Fig. 4C). Microglial reactivity was evaluated by measuring expression of Iba-1, which is a 17-kDa calcium binding protein specifically expressed in macrophages/microglia (Hilton et al., 2008), (Lee et al., 2008), (Zecca et al., 2008) that can be detected in denatured samples (Ahmed et al., 2007), (Vega-Avelaira et al., 2007). Evaluation of blots likewise revealed significant increases in Iba-1 expression in liponavir/ritonavir-treated mice as compared to vehicle mice (t(20) = 2.50, p = 0.0212; Fig. 4C).
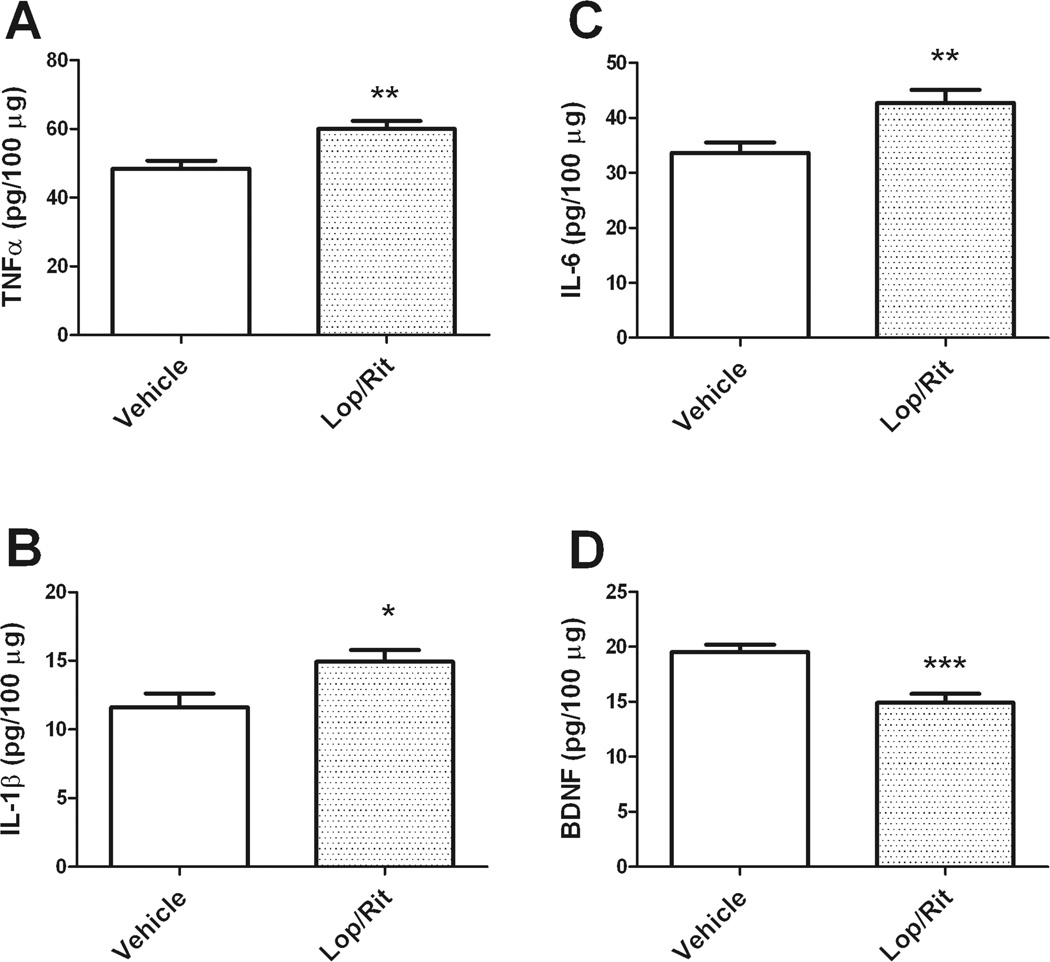
To then determine if liponavir/ritonavir enhanced brain inflammation and/or decreased brain growth factor levels, levels of the cytokines TNFα, IL-1β, and IL-6, and the growth factor BDNF were examined by ELISA. Statistical analyses of cytokine data reveal that homogenates derived from the anterior cortex of liponavir/ritonavir-treated mice contained significantly higher levels of TNFα (t(23) = 3.52, p = 0.0018), IL-1β (t(28) = 2.52, p = 0.0178), and IL-6 (t(24) = 2.87, p = 0.0084) as compared to levels in vehicle mice (Fig. 5). Furthermore, ELISA data show that liponavir/ritonavir administration was associated with significant decreases in cortical BDNF levels (t(19) = 4.22, p = 0.0005) compared to vehicle mice (Fig. 5D).
Figure 5. Effects of lopinavir/ritonavie on cytokine and growth factor levels in mouse brain.
Male C57BL/6 mice were treated daily with vehicle or lopinavir/ritonavir (150/37.5 mg/kg body weight) for 28 days, and the effects of lopinavir/ritonavir the cytokines (A) TNFα, (B) IL-1β, (C) IL-6 and (D) the growth factor BDNF in cortex were evaluated by ELISA as described in Methods. Data are means and SEM, with 9–20 individual mice per group, and were analyzed by 2-tailed, unpaired t-tests. *, **, and *** indicate significant (p<0.05, p<0.01, and p<0.001, respectively) differences in expression in lopinavir/ritonavir-treated mice as compared to vehicle.
To determine if the CNS effects of liponavir/ritonavir administration were uniform throughout the brain, homogenates prepared from the cerebellum and hippocampus of all experimental animals and probed for protein expression as was described for cortex. Data show that the hippocampus was relatively spared from the adverse effects of liponavir/ritonavir administration, as of all markers of cerebrovascular and synaptic integrity measured, only claudin-5 was significantly decreased (t(20) = 2.95, p = 0.0079) in liponavir/ritonavir-treated mice (Table 2). Furthermore, while there was no change in expression of the microglial marker Iba-1, there was an unexpected but statistically significant decrease in GFAP expression (t(20) = 2.46, p = 0.0232) in the hippocampus of liponavir/ritonavir-treated mice as compared to vehicle-treated mice (Table 2). Similarly, data indicate that the cerebellum was likewise relatively unaffected, as there was no change in expression of any marker of cerebrovascular or synaptic integrity when measured in cerebella of liponavir/ritonavir-treated mice, although there was again a statistically significant decrease in cerebellar GFAP expression (t(20) = 2.15, p = 0.0437; Table 2). While samples prepared from the hippocampus and cerebellum of mice were also probed for TNFα, IL-1β, IL-6, and BDNF, expression of these proteins was beneath the threshold of detection in these brain regions using our facilities (data not shown).
Table 2. Western blot data from Hippocampus and Cerebellum.
Male C57BL/6 mice were treated daily with vehicle or lopinavir/ritonavir (150/37.5 mg/kg body weight) for 28 days, after which the expression of markers of brain injury in hippocampus and cerebellum were measured by Western blot as described in Methods.
| Hippocampus | Cerebellum | ||
|---|---|---|---|
| Markers of Cerebrovascular Injury | |||
| Claudin-5 | 83.26 ± 5.7** | 92.3 ± 9.3 | |
| ZO-1 | 97.1 ± 11.9 | 114.1 ± 6.6 | |
| Occludin | 96.4 ± 4.1 | 103.5 ± 6.0 | |
| MMP2 | 93.5 ± 8.5 | 106.5 ± 4.2 | |
| MMP9 | 106.1 ± 6.6 | 119.1 ± 16.7 | |
| Markers of Synaptic Injury | |||
| SAP97 | 104.9 ± 2.9 | 106.7 ± 12.7 | |
| Total Synapsin | 95.1 ± 5.4 | 91.7 ± 8.4 | |
| Phosphorylated Synapsin | 91.1 ± 9.1 | 110.3 ± 5.3 | |
| Markers of Reactive Gliosis | |||
| GFAP | 90.4 ± 1.9* | 67.2 ± 8.1** | |
| Iba-1 | 93.9 ± 4.7 | 116.0 ± 9.4 | |
Data show mean ± SEM expression in lopinavir/ritonavir-treated mice presented as % vehicle, and were analyzed by 2-tailed, unpaired t-tests.
* and ** indicate significant (p<0.05 and p<0.01, respectively) differences noted in lopinavir/ritonavir-treated mice as compared to vehicle-treated mice.
4. DISCUSSION
Epidemiological data indicate that the overall prevalence of HAND has not been significantly reduced by ART (McArthur et al., 2003), (McArthur, 2004), and even in the context of widespread ART availability, HIV remains the most common preventable and treatable cause of neurologic impairment in patients under 50 (Ances and Ellis, 2007). However, as ART regimens are generally successful in maintaining low viral load, these epidemiological trends indicate that new mediators of neurologic dysfunction may have emerged in the ART era. Data from this study suggests that ART regimens could indirectly contribute to neurologic decline in HIV-positive patients via the development of specific metabolic co-morbidities. While it is recognized that the metabolic complications of ART contribute to premature atherosclerosis and cardiovascular risk in HIV patients, the net effect of metabolic co-morbidities on neurologic function in HIV patients is yet poorly understood. However, data in this manuscript are in general agreement with reports published over the past decade suggesting that HIV patients with metabolic compromise have increased prevalence of cognitive disturbances (Valcour et al., 2004), (Valcour et al., 2005), (Valcour et al., 2006), (Valcour et al., 2011), and further extend these studies by demonstrating an intimate link between specific metabolic co-morbidities and disruptions in cognitive function.
One of the most robust and rapid effects of lopinavir/ritonavir in mice was the dramatic decline in total fat mass. This observation is in keeping with clinical observations of HIV patients, as the HIV FRAM study revealed that HIV infected men with lipoatrophy had significantly less peripheral subcutaneous adipose and also less visceral adipose tissue (Bacchetti et al., 2005). While the link between fat loss and brain function has not been well studied, adipocytes participate in many physiologic processes via the secretion of adipokines (Frühbeck, 2008), (Rocha and Libby, 2008), (Ahima and Osei, 2008), and in particular, adiponectin and leptin may have potentially important roles in the brain (Harvey et al., 2005), (Oomura et al., 2006), (Harvey, 2007), (Ouchi and Walsh, 2007), (Chen et al., 2009). Our data show that total fat mass and circulating adiponectin and leptin levels are profoundly decreased by lopinavir/ritonavir treatment, and also demonstrate the statistically significant inverse correlation of these indices with cognitive impairment. Thus, these data strongly suggest that healthy, functioning adipose tissue is prerequisite for optimal cognitive performance, potentially via adipokine release. This potential scenario is supported by the presence of leptin receptors in extra-hypothalamic regions of the brain, including the cortex, hippocampus, and brain stem (Elmquist et al., 1998), (Tartaglia et al., 1995), (Fei et al., 1997). Indeed, studies have identified a role for leptin in cognitive processes (reviewed in (Harvey et al., 2005), (White et al., 2009b). In further relation to the data in this manuscript, leptin has been shown to modulate inflammatory signaling in microglia (Pinteaux et al., 2007), (Tang et al., 2007) and to regulate depression-related behaviors in mice (Guo et al., 2012). Similarly, adiponectin is a highly pleiotropic adipokine that likely supports optimal neurologic function through multiple mechanisms. Adiponecin has both anti-inflammatory (Ouchi and Walsh, 2007) and neuroprotective (Jung et al., 2006) properties, and is significantly decreased in HIV-positive patients with lipodystrophy (Kinlaw and Marsh, 2004), (Barbaro, 2007), (Chen et al., 2009). Furthermore, adiponectin deficiency is associated with exaggerated inflammatory response in human disease conditions (Hillenbrand et al., BMC Surg.), (Venkatesh et al., 2009) and has also been linked to microcirculatory disturbances (Ouedraogo et al., 2007) and blood brain-barrier deterioration (Vachharajan et al., 2012). Thus, data in this manuscript corroborate with available data to suggest that replacement therapy regimens with recombinant adipokines, or with recently developed peptide-based agonists for leptin (Kovalszky et al., 2010) and adiponectin (Otvos et al., 2011) receptors, could be potentially highly beneficial in the treatment and/or prevention of HAND in HIV patients.
Disruption of adipocyte function and altered adipokine release is also associated with microvascular dysfunction (reviewed in (Houben et al., 2012)), and data presented in this manuscript indicate that lopinavir/ritonavir treatment causes significant cerebrovascular dysfunction, particularly in the cortex. The physical seal of the blood brain barrier is maintained by inter-endothelial tight junction complexes (reviewed in (Nico and Ribatti, 2012)) comprised of plasma membrane spanning proteins (occludin) and scaffold cytoplasmic proteins (ZO-1), both of which are decreased by lopinavir/ritonavir treatment. Vascular pathology has received considerable attention as a participant in HAND (Foley et al., 2010), and it is quite reasonable to propose that that metabolic co-morbidities exacerbate HIV-related brain injury via disruption of cerebrovascular and blood brain barrier integrity. For example, published studies have shown that neurocognitive impairment in adult HIV patients is correlated with cardiovascular disease, hypertension, and hypercholesterolemia; but not with more conventional risk factors for dementia, including hepatitis C infection, alcohol abuse, CD4 cell counts, viral load, or CNS penetration of ART regimens (Wright et al., 2010). Other studies have shown that HIV-positive subjects with untreated cardiovascular disease have significantly reduced processing speed, recall, and executive functioning relative to those on medication (Foley et al., 2010). Data also suggest that cerebrovascular disease plays a greater role in the cognitive compromise of aging HIV-infected individuals as compared to the normal aging population (McMurtray et al., 2007), and indeed, a recent imaging study of coronary artery calcium accumulation in HIV-infected patients suggested that vascular “age” was increased in over 40% of patients, with a mean increase of 15 years over chronological age (Guaraldi et al., 2009). Evaluated collectively, these data strongly suggest that the lower neurocognitive performance noted in lopinavir/ritonavir-treated mice may be mediated at least in part via disruption of cerebrovascular homeostasis.
Insulin resistance is typically defined as decreased sensitivity to the metabolic actions of insulin such that greater than normal amounts are required to obtain a quantitatively normal response. Thus, even though glucose tolerance was normal in lopinavir/ritonavir-treated mice, the elevations in fasting insulin reflect significant insulin resistance in these mice. Insulin resistance was one of the first reported metabolic complications of ART (Justman et al., 2003), and even with newer antiretrovirals with safer metabolic profiles, the prevalence of insulin resistance in HIV patients ranges from 25% to over 65% (Das, 2011) and is likely to increase as the HIV-infected population ages. Insulin resistance is a key biochemical determinant of disease, as it is underlies and thus predicts the development of cardiovascular disease, diabetes, and hypertension. More recent studies have linked insulin resistance with dementia and frailty (Barzilay et al., 2007), (Abbatecola et al., 2007). For example, insulin resistance is associated with poor performance on cognitive tasks including the Mini-Mental State Exam (MMSE) and Trail Making Tests (TMT) (Abbatecola et al., 2007). Indeed, the brain is an important physiological target of insulin (Niswender, 2011), but the net effects of wide fluctuations in blood glucose and/or insulin on brain physiology are very poorly understood. It is important to note that hyperinsulinemia might remain undiagnosed for a long period, but yet is a clinically significant target as changes in lifestyle and treatment can improve insulin sensitivity and thus potentially prevent clinical progression.
While data in the manuscript support previous data documenting the adverse effects of protease inhibitors on metabolic function, it is not clear whether lopinavir, or ritonavir, or the combination is ultimately responsible for triggering the chain of events leading to metabolic and cognitive dysfunction. However, numerous reports have shown that administration of only ritonavir, even at doses used in boosted regimens, can result in significant metabolic dysfunction both in humans (Périard et al., 1999), (Cohen, 2005) and in mice (Guo et al., 2009), (Riddle et al., 2003), (Goetzman et al., 2003), (den Boer et al., 2006), (Vyas et al., 2010). For example, studies in humans have shown that ritonavir dramatically increases triglyceride and cholesterol levels, and that the combination of ritonavir with either nelfinavir or saquinavir does not further elevate plasma lipids (Périard et al., 1999). Furthermore, mouse studies have repeatedly demonstrated the induction of lipodystrophy, hyperlipidemia, and/or insulin resistance in mice treated with doses of ritonavir (10–60 mg/kg) comparable to levels used in this study (Riddle et al., 2003), (Guo et al., 2009), (Goetzman et al., 2003), (den Boer et al., 2006), (Vyas et al., 2010). Based on available data, therefore, it is theorized that ritonavir is primarily responsible for the development of adverse co-morbidities described in this study. It is also possible that ritonavir and/or lopinavir mediate direct neurotoxicity in the CNS. However, this is quite unlikely given the well-established inability of these drugs to cross the blood-brain barrier (Varatharajan and Thomas, 2009). Indeed, our previously published investigations of lopinavir/ritonavir in mice verified that levels of lopinavir in brain were nearly 200-fold lower than levels in serum (Pistell et al., 2010a), and experiments in which cultured primary neurons or glia were exposed to lopinavir/ritonavir at concentrations found in brain (and up to 50-fold higher) revealed no effects of these drugs on in vitro neurotoxic or inflammatory signaling (AJB-K, unpublished data). Thus, it seems reasonable to conclude that the adverse neurologic effects of lopinavir/ritonavir administration to mice are mediated via the development of metabolic dysfunction, and data in the manuscript further point to potentially key roles for lipodystrophy and insulin resistance.
5. CONCLUSIONS
It is well-established that ART can cause metabolic syndrome, and data in this manuscript suggest that such metabolic co-morbidities might participate in the development of neurologic and cognitive dysfunction in HIV patients. These data are in keeping with published studies documenting significant metabolic dysfunction in mice treated with combined lopinavir/ritonavir in similar dose ranges (Prot et al., 2006), (Pistell et al., 2010a), and are also consistent with the growing body of literature describing the sensitivity of the brain to metabolic dysfunction both in human (Elias et al., 2003), (Elias et al., 2005), (Waldstein and Katzel, 2006) and animal studies (Baran et al., 2005), (Winocur and Greenwood, 2005), (Granholm et al., 2008), (Bruce-Keller et al., 2009). (White et al., 2009a), (Pistell et al., 2010b). While data in this study suggest an important role for lipodystrophy and/or insulin resistance in neurocognitive decline, there are additional metabolic risk factors that could adversely affect the brain. For example, it is well established that chronic kidney disease patients undermines neurologic function. For example, up to 70 percent of hemodialysis patients ages 55 years and older have moderate to severe chronic cognitive impairment, and recent studies reveal a strong correlation between estimated glomerular filtration rate (GFR) and cognitive function in kidney disease patients (reviewed in (Murray, 2008)). It should also be pointed out that HIV infection directly affects the brain, independently of perturbations to metabolic function. For example, the duration of HIV infection, viral load (particularly in the CNS) and immunologic status may all predispose patients to HAND (reviewed in (Ellis et al., 2009), (Letendre et al., 2010)). Thus, while there are likely multiple physiologic mechanisms at play, results from this study reinforce the link between metabolic and neurologic function, and suggest that successful remediation of metabolic dysfunction in HIV patients could decrease the incidence and/or severity of HAND.
Highlights.
Lopinavir/ritonavir causes lipodystrophy and insulin resistance in mice
Lopinavir/ritonavir impairs cognition and increases depressive behavior
Lopinavir/ritonavir induces cerebrovascular injury and synapse loss
Cognitive impairment correlates with lipodystrophy and insulin resistance
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Barry Robert for expert veterinary assistance related to lopinavir/ritonavir administration. This work was supported by grants from the NIH (NS46267 and AG05119), and also used PBRC Core facilities (Animal Phenotyping) that are funded by the NIH (P20-RR021945 and P30-DK072476).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbatecola AM, Ferrucci L, Marfella R, Paolisso G. Insulin resistance and cognitive decline may be common soil for frailty syndrome. Arch. Intern. Med. 2007;167:2145–2146. doi: 10.1001/archinte.167.19.2145-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Osei SY. Adipokines in obesity. Front. Horm. Res. 2008;36:182–197. doi: 10.1159/000115365. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J. Histochem. Cytochem. 2007;55:687–700. doi: 10.1369/jhc.6A7156.2007. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin. Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Anuurad E, Semrad A, Berglund L. Human immunodeficiency virus and highly active antiretroviral therapy-associated metabolic disorders and risk factors for cardiovascular disease. Metab. Syndr. Relat. Disord. 2009;7:401–410. doi: 10.1089/met.2008.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D, Saag M, Scherzer R, Shlipak M, Tien P (FRAM)., S.o.F.R.a.M.C.i.H.I. Fat distribution in men with HIV infection. J. Acquir. Immune Defic. Syndr. 2005;40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, McArthur JC, Sacktor N, Cutler RG, Knapp EL, Mattson MP, Haughey NJ. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68:1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran SE, Campbell AM, Kleen JK, Foltz CH, Wright RL, Diamond DM, Conrad CD. Combination of high fat diet and chronic stress retracts hippocampal dendrites. Neuroreport. 2005;16:39–43. doi: 10.1097/00001756-200501190-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaro G. Visceral fat as target of highly active antiretroviral therapy-associated metabolic syndrome. Curr. Pharm. Des. 2007;13:2208–2213. doi: 10.2174/138161207781039661. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch. Intern. Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Barger SW, Moss NI, Pham JT, Keller JN, Nath A. Proinflammatory and pro-oxidant properties of the HIV protein Tat in a microglial cell line: attenuation by 17beta-estradiol. J. Neurochem. 2001;78:1315–1324. doi: 10.1046/j.1471-4159.2001.00511.x. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Gupta S, Knight AG, Beckett TL, McMullen JM, Davis PR, Murphy MP, Van Eldik LJ, St Clair D, Keller JN. Cognitive impairment in humanized APP×PS1 mice is linked to Aβ(1–42) and NOX activation. Neurobiol. 2011 doi: 10.1016/j.nbd.2011.07.012. Dis Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keller JN, Morrison CD. Obesity and vulnerability of the CNS. Biochim. Biophys. Acta. 2009;1792:395–400. doi: 10.1016/j.bbadis.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2011;8:10A. doi: 10.1002/0471142301.ns0810as55. [DOI] [PubMed] [Google Scholar]
- Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther. Clin. Risk Manag. 2008;4:1023–1033. doi: 10.2147/tcrm.s3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Liao WQ, Xu N, Xu H, Wen JY, Yu CA, Liu XY, Li CL, Zhao SM, Campbell W. Adiponectin protects against cerebral ischemia-reperfusion injury through anti-inflammatory action. Brain Res. 2009;1273:129–137. doi: 10.1016/j.brainres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Cohen CJ. Ritonavir-boosted protease inhibitors, Part 2: cardiac implications of lipid alterations. AIDS Read. 2005;15:528–532. 537–528. [PubMed] [Google Scholar]
- Crowley JJ, Jones MD, O'Leary OF, Lucki I. Automated tests for measuring the effects of antidepressants in mice. Pharmacol. Biochem. Behav. 2004;78:269–274. doi: 10.1016/j.pbb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Cryan J, Markou A, Lucki Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63:769–802. doi: 10.2165/00003495-200363080-00004. [DOI] [PubMed] [Google Scholar]
- Das S. Insulin Resistance and Diabetes in HIV Infection. Recent Pat. Antiinfect. Drug Discov. 2011;6:260–268. doi: 10.2174/157489111796887846. [DOI] [PubMed] [Google Scholar]
- den Boer MA, Berbée JF, Reiss P, van der Valk M, Voshol PJ, Kuipers F, Havekes LM, Rensen PC, Romijn JA. Ritonavir impairs lipoprotein lipase-mediated lipolysis and decreases uptake of fatty acids in adipose tissue. Arterioscler. Thromb. Vasc. Biol. 2006;26:124–129. doi: 10.1161/01.ATV.0000194073.87647.10. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham Heart Study. Int. J. Obes. Relat. Metab. Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Obesity, diabetes and cognitive deficit: the Framingham Heart Study. Neurobiol. Aging. 2005;26:11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Calero P, Stockin MD. HIV infection and the central nervous system: a primer. Neuropsychol. Rev. 2009;19:144–151. doi: 10.1007/s11065-009-9094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J. Comp. Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc. Natl. Acad. Sci. U.S.A. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández I, Peña A, Del Teso N, Pérez V, Rodríguez-Cuesta J. Clinical biochemistry parameters in C57BL/6J mice after blood collection from the submandibular vein and retroorbital plexus. J. Am. Assoc. Lab. Anim. Sci. 2010;49:202–206. [PMC free article] [PubMed] [Google Scholar]
- Fillit H, Nash DT, Rundek T, Zuckerman A. Cardiovascular risk factors and dementia. Am. J. Geriatr. Pharmacother. 2008;6:100–118. doi: 10.1016/j.amjopharm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Foley J, Ettenhofer M, Wright MJ, Siddiqi I, Choi M, Thames AD, Mason K, Castellon S, Hinkin CH. Neurocognitive functioning in HIV-1 infection: effects of cerebrovascular risk factors and age. Clin. Neuropsychol. 2010;24:265–285. doi: 10.1080/13854040903482830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods Mol. Biol. 2008;456:1–22. doi: 10.1007/978-1-59745-245-8_1. [DOI] [PubMed] [Google Scholar]
- Goetzman ES, Tian L, Nagy TR, Gower BA, Schoeb TR, Elgavish A, Acosta EP, Saag MS, Wood PA. HIV protease inhibitor ritonavir induces lipoatrophy in male mice. AIDS Res. Hum. Retroviruses. 2003;19:1141–1150. doi: 10.1089/088922203771881248. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J. Alzheimers Dis. 2008;14:133–145. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G, Zona S, Alexopoulos N, Orlando G, Carli F, Ligabue G, Fiocchi F, Lattanzi A, Rossi R, Modena M, Esposito R, Palella F, Raggi P. Coronary aging in HIV-infected patients. 2009;49:1756–1762. doi: 10.1086/648080. [DOI] [PubMed] [Google Scholar]
- Guo M, Lu Y, Garza JC, Li Y, Chua SC, Zhang W, Lu B, Lu XY. Forebrain glutamatergic neurons mediate leptin action on depression-like behaviors and synaptic depression. Transl. Psychiatry. 2012;2:e83. doi: 10.1038/tp.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wong S, Pudney J, Jasuja R, Hua N, Jiang L, Miller A, Hruz PW, Hamilton JA, Bhasin S. Acipimox, an inhibitor of lipolysis, attenuates atherogenesis in LDLR-null mice treated with HIV protease inhibitor ritonavir. Arterioscler. Thromb. Vasc. Biol. 2009;29:2028–2032. doi: 10.1161/ATVBAHA.109.191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MN, Wallace R. Can dementia be prevented? Brain aging in a population-based context. Ann. Rev. Public Health. 2004;25:1–24. doi: 10.1146/annurev.publhealth.25.101802.122951. [DOI] [PubMed] [Google Scholar]
- Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr. Opin. Pharmacol. 2007;7:643–647. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Shanley LJ, O'Malley D, Irving AJ. Leptin: a potential cognitive enhancer? Biochem. Soc. Trans. 2005;33:1029–1032. doi: 10.1042/BST20051029. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur J, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I, Group H. The impact of HIV-associated neuropsychological impairment on everyday functioning. J. Int. Neuropsychol. Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Herman JS, Easterbrook PJ. The metabolic toxicities of antiretroviral therapy. Int. J. STD AIDS. 2001;12:555–562. doi: 10.1258/0956462011923714. [DOI] [PubMed] [Google Scholar]
- Hillenbrand A, Knippschild U, Weiss M, Schrezenmeier H, Henne-Bruns D, Huber-Lang M, Wolf AM. BMC Surg. Sepsis induced changes of adipokines and cytokines - septic patients compared to morbidly obese patients. 2010;10:26. doi: 10.1186/1471-2482-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton GD, Stoica BA, Byrnes KR, Faden AI. Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma. J. Cereb. Blood Flow Metab. 2008;28:1845–1859. doi: 10.1038/jcbfm.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben AJ, Eringa EC, Jonk AM, Serne EH, Smulders YM, Stehouwer CD. Perivascular Fat and the Microcirculation: Relevance to Insulin Resistance, Diabetes, and Cardiovascular Disease. Curr. Cardiovasc. Risk Rep. 2012;6:80–90. doi: 10.1007/s12170-011-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz BE, Klimas NG, Llabre MM, Maher KJ, Skyler JS, Bilsker MS, McPherson-Baker S, Lawrence PJ, Laperriere AR, Greeson JM, Klaus JR, Lawrence R, Schneiderman N. HIV, metabolic syndrome X, inflammation, oxidative stress, and coronary heart disease risk : role of protease inhibitor exposure. Cardiovasc. Toxicol. 2004;4:303–316. doi: 10.1385/ct:4:3:303. [DOI] [PubMed] [Google Scholar]
- Jiang B, Hebert VY, Khandelwal AR, Stokes KY, Dugas TR. HIV-1 antiretrovirals induce oxidant injury and increase intima-media thickness in an atherogenic mouse model. Toxicol. Lett. 2009;187:164–171. doi: 10.1016/j.toxlet.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TW, Lee JY, Shim WS, Kang ES, Kim JS, Ahn CW, Lee HC, Cha BS. Adiponectin protects human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity. Biochem. Biophys. Res. Commun. 2006;343:564–570. doi: 10.1016/j.bbrc.2006.02.186. [DOI] [PubMed] [Google Scholar]
- Justman JE, Benning L, Danoff A, Minkoff H, Levine A, Greenblatt RM, Weber K, Piessens E, Robison E, Anastos K. Protease inhibitor use and the incidence of diabetes mellitus in a large cohort of HIV-infected women. J. Acquir. Immune Defic. Syndr. 2003;32:298–302. doi: 10.1097/00126334-200303010-00009. [DOI] [PubMed] [Google Scholar]
- Kinlaw WB, Marsh B. Adiponectin and HIV-lipodystrophy: taking HAART. Endocrinology. 2004;145:484–486. doi: 10.1210/en.2003-1513. [DOI] [PubMed] [Google Scholar]
- Kovalszky I, Surmacz E, Scolaro L, Cassone M, Ferla R, Sztodola A, Olah J, Hatfield MP, Lovas S, Otvos LJ. Leptin-based glycopeptide induces weight loss and simultaneously restores fertility in animal models. Diabetes Obes. Metab. 2010;12:393–402. doi: 10.1111/j.1463-1326.2009.01170.x. [DOI] [PubMed] [Google Scholar]
- Lee CH, Hwang IK, Lee IS, Yoo KY, Choi JH, Lee BH, Won MH. Differential immunoreactivity of microglial and astrocytic marker protein in the hippocampus of the seizure resistant and sensitive gerbils. J. Vet. Med. Sci. 2008;70:1405–1409. doi: 10.1292/jvms.70.1405. [DOI] [PubMed] [Google Scholar]
- Lenhard JM, Croom DK, Weiel JE, Spaltenstein A, Reynolds DJ, Furfine ES. Dietary fat alters HIV protease inhibitor-induced metabolic changes in mice. J. Nutr. 2000;130:2361–2366. doi: 10.1093/jn/130.9.2361. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top. HIV Med. 2010;18:45–55. [PMC free article] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: an evolving disease. J. Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- McMurtray A, Nakamoto B, Shikuma C, Valcour V. Small-vessel vascular disease in human immunodeficiency virus infection: The Hawaii aging with HIV cohort study. Cerebrovascular Disease. 2007;24:236–241. doi: 10.1159/000104484. [DOI] [PubMed] [Google Scholar]
- Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch. Neurol. 2009;66:1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle G. Metabolic issues associated with protease inhibitors. J. Acquir. Immune Defic. Syndr. 2007;45(Suppl 1):S19–S26. doi: 10.1097/QAI.0b013e31806007ed. [DOI] [PubMed] [Google Scholar]
- Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: an occult burden. Adv. Chronic Kidney Dis. 2008;15:123–132. doi: 10.1053/j.ackd.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, McArthur J. Evolution of HIV dementia with HIV infection. Int Rev Psychiatry. 2008:25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- Nico B, Ribatti D. Morphofunctional aspects of the blood-brain barrier. Curr. Drug Metab. 2012;13:50–60. doi: 10.2174/138920012798356970. [DOI] [PubMed] [Google Scholar]
- Niswender KD. Basal insulin: beyond glycemia. Postgrad. Med. 2011;123:27–37. doi: 10.3810/pgm.2011.07.2301. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin. Drug Saf. 2005;4:433–442. doi: 10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li X, Kohno D, Uramura K, Sougawa H, Yada T, Wayner M, Sasaki K. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;11:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Otvos LJ, Haspinger E, La Russa F, Maspero F, Graziano P, Kovalszky I, Lovas S, Nama K, Hoffmann R, Knappe D, Cassone M, Wade J, Surmacz E. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC Biotechnol. 2011;11:90. doi: 10.1186/1472-6750-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, Chan L, Goldstein BJ, Scalia R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J. Clin. Invest. 2007;117:1718–1726. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périard D, Telenti A, Sudre P, Cheseaux JJ, Halfon P, Reymond MJ, Marcovina SM, Glauser MP, Nicod P, Darioli R, Mooser V. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- Pinkel D. The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res. 1958;18:853–856. [PubMed] [Google Scholar]
- Pinteaux E, Inoue W, Schmidt L, Molina-Holgado F, Rothwell NJ, Luheshi GN. Leptin induces interleukin-1beta release from rat microglial cells through a caspase 1 independent mechanism. J. Neurochem. 2007;102:826–833. doi: 10.1111/j.1471-4159.2007.04559.x. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Gupta S, Knight AG, Domingue M, Uranga RM, Ingram DK, Kheterpal I, Ruiz C, Keller JN, Bruce-Keller AJ. Metabolic and neurologic consequences of chronic lopinavir/ritonavir administration to C57BL/6 mice. Antiviral Res. 2010a;88:334–342. doi: 10.1016/j.antiviral.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Ingram DK. Development of a water-escape motivated version of the Stone T-maze for mice. Neuroscience. 2010;166:61–72. doi: 10.1016/j.neuroscience.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010b;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Nelson CM, Miller MG, Spangler EL, Ingram DK, Devan BD. Striatal lesions interfere with acquisition of a complex maze task in rats. Behav. Brain Res. 2009;197:138–143. doi: 10.1016/j.bbr.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Spangler EL, Kelly-Bell B, Miller MG, de Cabo R, Ingram DK. Age-associated learning and memory deficits in two mouse versions of the stone T-maze. Neurobiol. 2012 doi: 10.1016/j.neurobiolaging.2011.12.004. Aging Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. 1977;229:327–336. [PubMed] [Google Scholar]
- Power C, Boissé L, Rourke S, Gill MJ. NeuroAIDS: an evolving epidemic. Can. J. Neurol. Sci. 2009;36:285–295. doi: 10.1017/s0317167100007009. [DOI] [PubMed] [Google Scholar]
- Prot M, Heripret L, Cardot-Leccia N, Perrin C, Aouadi M, Lavrut T, Garraffo R, Dellamonica P, Durant J, Le Marchand-Brustel Y, Binétruy B. Long-term treatment with lopinavir-ritonavir induces a reduction in peripheral adipose depots in mice. Antimicrob. Agents Chemother. 2006;50:3998–4004. doi: 10.1128/AAC.00625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TC. HIV epidemiology and the effects of antiviral therapy on long-term consequences. AIDS Patient Care STDS. 2008;22(Suppl 3):S7–S12. doi: 10.1097/01.aids.0000327510.68503.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Riddle TM, Fichtenbaum CJ, Hui DY. Leptin replacement therapy but not dietary polyunsaturated fatty acid alleviates HIV protease inhibitor-induced dyslipidemia and lipodystrophy in mice. J. Acquir. Immune Defic. Syndr. 2003;33:564–570. doi: 10.1097/00126334-200308150-00003. [DOI] [PubMed] [Google Scholar]
- Rocha VZ, Libby P. The multiple facets of the fat tissue. Thyroid. 2008;18:175–183. doi: 10.1089/thy.2007.0296. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, Becker JT, Cohen B, McArthur JC, Study MAC. HIV-associated neurologic disease incidence changes:: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- Sawyer M, Ratain MJ. Body surface area as a determinant of pharmacokinetics and drug dosing. Invest. New Drugs. 2001;19:171–177. doi: 10.1023/a:1010639201787. [DOI] [PubMed] [Google Scholar]
- Schambelan M, Benson CA, Carr A, Currier JS, Dubé MP, Gerber JG, Grinspoon SK, Grunfeld C, Kotler DP, Mulligan K, Powderly WG, Saag MS Society-USA., I.A. Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: recommendations of an International AIDS Society-USA panel. J. Acquir. Immune Defic. Syndr. 2002;31:257–275. doi: 10.1097/00126334-200211010-00001. [DOI] [PubMed] [Google Scholar]
- Sherr L, Clucas C, Harding R, Sibley E, Catalan J. HIV and depression--a systematic review of interventions. Psychol. Health Med. 2011;16:493–527. doi: 10.1080/13548506.2011.579990. [DOI] [PubMed] [Google Scholar]
- Spangler EL, Heller B, Hengemihle J, Muth NJ, Jones BE, Garofalo P, Ingram DK. Thrombosis of parietal, but not striate, cortex impairs acquisition of a 14-unit T-maze in the rat. Physiol. Behav. 1994;56:95–101. doi: 10.1016/0031-9384(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Tang CH, Lu DY, Yang RS, Tsai HY, Kao MC, Fu WM, Chen YF. Leptin-induced IL-6 production is mediated by leptin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase Akt, NF-kappaB, and p300 pathway in microglia. J. Immunol. 2007;179:1292–1302. doi: 10.4049/jimmunol.179.2.1292. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Thomas CM, Smart EJ. How HIV protease inhibitors promote atherosclerotic lesion formation. Curr. Opin. Lipidol. 2007;18:561–565. doi: 10.1097/MOL.0b013e3282ef604f. [DOI] [PubMed] [Google Scholar]
- Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch. Intern. Med. 2000;160:2050–2056. doi: 10.1001/archinte.160.13.2050. [DOI] [PubMed] [Google Scholar]
- Vachharajan iV, Cunningham C, Yoza B, Carson JJ, Vachharajani TJ, McCall C. Adiponectin-deficiency exaggerates sepsis-induced microvascular dysfunction in the mouse brain. Obesity (Silver Spring) 2012;20:498–504. doi: 10.1038/oby.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Maki P, Bacchetti P, Anastos K, Crystal H, Young M, Mack W, Cohen M, Golub E, Tien P. Insulin Resistance and Cognition among HIV-infected and HIV-uninfected Adult Women - The Women's Interagency HIV Study. AIDS Res. Hum. Retroviruses. 2011 doi: 10.1089/aid.2011.0159. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Sacktor NC, Paul RH, Watters MR, Selnes OA, Shiramizu BT, Williams AE, Shikuma CM. Insulin resistance is associated with cognition among HIV-1-infected patients: the Hawaii Aging With HIV cohort. J. Acquir. Immune Defic. Syndr. 2006;43:405–410. doi: 10.1097/01.qai.0000243119.67529.f5. [DOI] [PubMed] [Google Scholar]
- Valcour VG, Shikuma CM, Shiramizu BT, Williams AE, Watters MR, Poff PW, Grove JS, Selnes OA, Sacktor NC. Diabetes, insulin resistance, and dementia among HIV-1-infected patients. J. Acquir. Immune. Defic. Syndr. 2005;38:31–36. doi: 10.1097/00126334-200501010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS Patient Care STDS. 2004;18(Suppl 1):S79–S86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82:A99–A109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Avelaira D, Moss A, Fitzgerald M. Age-related changes in the spinal cord microglial and astrocytic response profile to nerve injury. Brain Behav. Immun. 2007;21:617–623. doi: 10.1016/j.bbi.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Venkatesh B, Hickman I, Nisbet J, Cohen J, Prins J. Changes in serum adiponectin concentrations in critical illness: a preliminary investigation. Crit. Care. 2009;13:R105. doi: 10.1186/cc7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas AK, Koster JC, Tzekov A, Hruz PW. Effects of the HIV protease inhibitor ritonavir on GLUT4 knock-out mice. J. Biol. Chem. 2010;285:36395–36400. doi: 10.1074/jbc.M110.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein SR, Katzel LI. Interactive relations of central versus total obesity and blood pressure to cognitive function. Int. J. Obes. (Lond.) 2006;30:201–207. doi: 10.1038/sj.ijo.0803114. [DOI] [PubMed] [Google Scholar]
- White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, Bruce-Keller AJ. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol. Dis. 2009a;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for the programming effect of a high-fat diet on offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009b;35:3–13. doi: 10.1152/ajpregu.91015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol. Aging. 2005;26:46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Wright EJ, Grund B, Robertson K, Brew BJ, Roediger M, Bain MP, Drummond F, Vjecha MJ, Hoy J, Miller C, Penalva de Oliveira AC, Pumpradit W, Shlay JC, El-Sadr W, Price RW, Group ISS. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology. 2010;75:864–873. doi: 10.1212/WNL.0b013e3181f11bd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Wilms H, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J, Lucius R. Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson's disease. Acta Neuropathol. 2008;116:47–55. doi: 10.1007/s00401-008-0361-7. [DOI] [PubMed] [Google Scholar]