Abstract
Idiopathic erythrocytosis is an uncommon disease, and is defined by an increase in red blood cell mass. The differential diagnosis of erythrocytosis is extensive, and can be divided into primary and secondary forms. Primary erythrocytoses are due to intrinsic defects in erythroid precursor cells and are characterized by low erythropoietin levels. Secondary erythrocytoses are extrinsic to erythroid progenitors and are characterized by either high or inappropriately normal erythropoietin levels. A distinct subset of secondary erythrocytoses are due to genetic mutations in key proteins of the oxygen sensing pathway. These proteins constitute the core molecular machinery of oxygen-sensing with respect to red blood cell control. Apart from assigning physiologic roles for these proteins, studies of these rare mutations have (i) revealed the exquisite sensitivity of this pathway to genetic perturbations, (ii) highlighted important functional regions of the proteins, and (iii) provided a basis for potentially targeting this pathway for therapeutic benefit.
Keywords: Erythrocytosis, Erythropoietin, Hypoxia Inducible Factor, Prolyl Hydroxylase Domain Protein, von Hippel Lindau Protein, Oxygen sensing, Polycythemia, Red cell control
Introduction
Deciphering the function of proteins and their roles in pathways is one of the main goals of biomedical research, not only from the point of view of basic biology but more importantly from the perspective of uncovering pathways that may ultimately be exploited for therapeutic benefit. In this regard, human genetics can offer important insights into proteins that play key roles in medically relevant pathways. In recent years, there have been advances in understanding the mechanism of oxygen sensing in mammals and its relevance to human disorders of red blood cell control. This has enhanced our understanding of the molecular mechanisms by which red blood cell mass is regulated in humans.
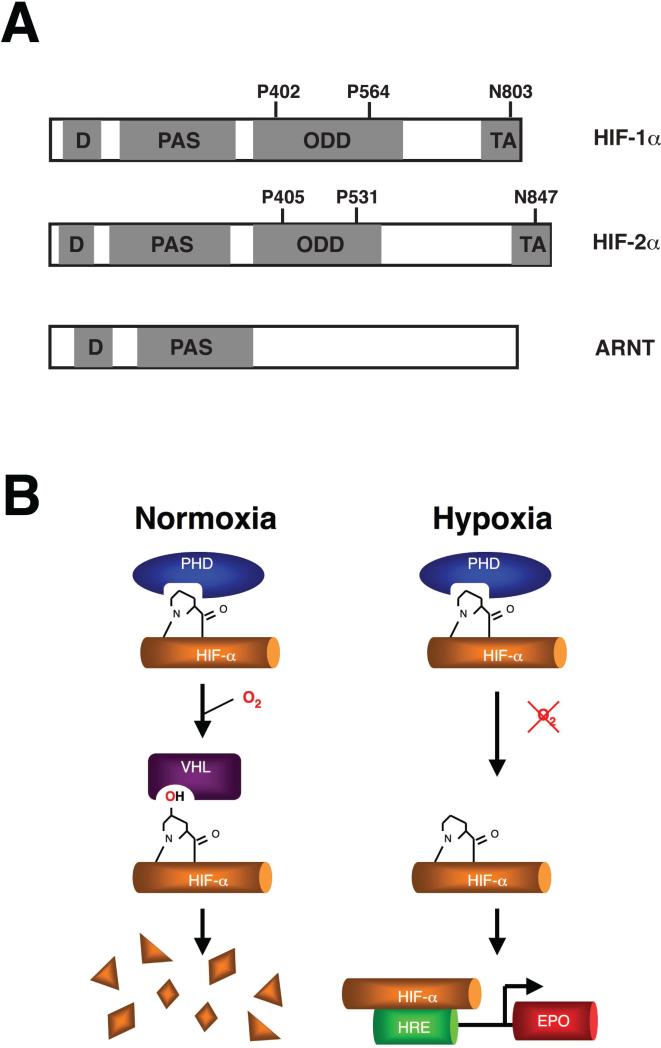
In metazoans, a universal response to hypoxia is the activation of a transcriptional response mediated by Hypoxia Inducible Factor (HIF) 1. HIF is a heterodimeric complex consisting of an α and a β subunit, both subunits of which are members of the Per-ARNT-Sim (PAS) family of proteins 2. At their N-terminii are two domains, DNA-binding and PAS domains, both of which are involved in dimerization (Figure 1A). The β subunit of HIF is identical to Aryl Hydrocarbon Nuclear Translocator (ARNT), and its protein level is insensitive to oxygen. The α subunit consists of three isoforms, HIF-1α, HIF-2α, and HIF-3α 3-6. In contrast to ARNT, the protein level of the α-subunit is exquisitely sensitive to oxygen. Under normoxic conditions (21% O2), HIF-α is rapidly degraded by the ubiquitin-proteasome pathway 7, 8. Under hypoxic conditions, this degradation is arrested and HIF-α consequently stabilized. The α subunits contain, in addition to the aforementioned domains, two other domains. One is a C-terminal transcriptional activation domain, which itself displays activity that increases under hypoxic conditions. The other is an oxygen-dependent degradation domain that can confer oxygen-dependent lability when fused to a heterologous protein 9-12.
Figure 1.
(A) Schematic diagrams of HIF-1α, HIF-2α, and ARNT. The DNA binding (D), Per-ARNT-Sim (PAS), oxygen-dependent degradation (ODD), and transcriptional activation (TA) domains are shaded and as indicated. The sites of prolyl (P402, P564, P405, P531) and asparaginyl hydroxylation (N803, N847) in HIF-1α and HIF-2α are indicated. (B) Regulation of HIF-α. Under normoxic conditions, HIF-α is prolyl hydroxylated, allowing binding of VHL which in turn promotes the degradation of HIF-α via ubiquitination and proteasomal degradation. Under hypoxic conditions, the prolyl hydroxylation is inhibited, thereby allowing stabilization of HIF-α and activation of HRE-driven genes, including the EPO gene.
When stabilized under hypoxia, the α subunit heterodimerizes with the β subunit, and this HIF complex then binds to the Hypoxia Response Elements (HREs) in the promoters and enhancers of genes involved in cellular, local, and systemic responses to hypoxia 13-15. Over one hundred such genes have been identified, and they promote a multiplicity of processes, including erythropoiesis, angiogenesis, increased glucose uptake, increased glycolysis, and decreased oxidative phosphorylation. These processes in aggregate promote ATP production from glucose under hypoxic conditions, augmented vasculature in hypoxic areas, and increased oxygen-carrying capacity of blood. The latter is mediated by HIF-mediated stimulation of the Erythropoietin (EPO) gene. This gene is considered a paradigm of an oxygen-regulated gene because of its high dynamic range of inducibility with respect to oxygen concentration, as well as the central importance of this growth factor to oxygen homeostasis 16. Indeed, HIF-1α was purified based on its capacity to bind to an oligonucleotide duplex comprising the Hypoxia Response Element (HRE) of the EPO gene 2. In this pathway, which is a classic negative feedback loop, decreased oxygen delivery to the kidney results in increased transcription of the EPO gene. The resulting increased EPO protein in the circulation then binds to the Erythropoietin Receptor (EPOR) present on erythroid progenitors in the bone marrow. This binding initiates an intracellular signaling cascade that then inhibits apoptosis, thereby expanding red blood cell mass 17. Increased red blood cell mass then improves oxygen delivery to the kidney, leading to downregulation of EPO production. The liver can also produce EPO, and up to 10% of circulating EPO can be of hepatic origin 18.
Of the three HIF-α isoforms, HIF-1α and HIF-2α have been most extensively studied. Studies have shown that these proteins behave as classic transcriptional activators, with a DNA binding domain and a transcriptional activation domain. HIF-3α has been far less studied and studies show it to have inhibitory activity in certain contexts. For example, a splice variant, iPAS, inhibits HIF activity and maintains the cornea in an avascular state 19. HIF-1α and HIF-2α have overlapping but nevertheless distinct tissue expression profiles. HIF-1α is expressed in all organs and in a broad spectrum of cell types, whereas HIF-2α is substantially more restricted in an organ and cell-type specific manner 13. HIF-2α, for example, is expressed in endothelial cells, in the interstitial cells and podocytes of the renal cortex, hepatocytes of the liver, and cardiomyocytes of the heart 20.
Prolyl Hydroxylation and HIF Regulation
A notable advance in our understanding of the regulation of HIF came from the observation that prolyl hydroxylation targets the HIF α-subunit for degradation 21-24. Previously, prolyl hydroxylation was well-recognized as a postranslational modification of the extracellular protein collagen, being essential for the structural stability of this protein 25. This modification is catalyzed by the collagen 4-prolyl hydroxylases. The studies on HIF demonstrated that this modification serves an entirely distinct and novel purpose. In particular, site-specific prolyl hydroxylation of the α-subunit, occurring on Pro-402 and Pro-564 of HIF-1α, and Pro-405 and Pro-531 of HIF-2α, is necessary for its degradation under normoxia (Figure 1B). These hydroxylation events occur within a conserved LXXLAP motif (where L = leucine, X = any amino acid, A = alanine, P = proline, and underlining indicates the hydroxylacceptor proline). Mechanistically, this modification provides a recognition motif for the von Hippel Lindau (VHL) tumor suppressor protein, a component of an E3 ubiquitin ligase complex 26. The VHL complex also contains Elongin C and Elongin B, and has therefore also been referred to as the VCB complex. The remarkable ability of VHL to discriminate between hydroxylated and nonhydroxylated HIF-α is derived in large part from two specific hydrogen bonds from Ser-111 and His-115 of VHL to the hydroxyl group of the hydroxyprolyl residue 27, 28. Important contacts are also made between specific HIF-1α residues N-terminal and C-terminal to the hydroxylacceptor proline. The HIF-1α peptide binds in an extended conformation to the HIF-binding groove of VHL.
At the time these observations were made, they provided an immediate means to rationalize the hypoxic stabilization of HIF-α. Namely, prolyl hydroxylases comprise a family of enzymes that require molecular oxygen as an obligatory substrate in the hydroxylation reaction, along with 2-oxoglutarate and ascorbic acid 29. Hence, low oxygen concentrations would be predicted to lead to hypohydroxylation of HIF-α, with the degree of hypohydroxylation governed in a manner dependent on oxygen concentration and the Kd for oxygen of these enzymes. The situation, however, is undoubtedly more complex than this. Hypoxia also induces the formation of reactive oxygen species, and these reactive oxygen species are necessary for the activation of HIF-α 30-32. It therefore seems likely that lowered oxygen tension acting in concert with these reactive oxygen species decreases PHD activity, thereby stabilizing HIF-α.
Significantly, a separate hydroxylation event also regulates the transcriptional activity of HIF-α. This occurs on Asn-803 of HIF-1α and Asn-847 of HIF-2α (Figure 1A) 33. Under normoxia, this hydroxylation modification sterically blocks the interaction between HIF-α and CBP/p300 34. Under hypoxic conditions, just as hypohydroxylation of prolines results in the stabilization of HIF-α, hypohydroxylation of this specific asparaginyl residue allows the interaction of the transcriptional activation domain with CBP/p300, thereby unmasking the full activity of HIF.
Three enzymes have been identified that prolyl hydroxylate HIF-α: Prolyl Hydroxylase Domain Protein 1 [PHD1, also known as HIF Prolyl Hydroxylase 3 (HPH3) or Egg Laying Defective Nine 2 (EGLN2)], PHD2 (HPH2/EGLN1), and PHD3 (HPH1/EGLN3) 35-37. All three are 2-oxoglutarate dependent dioxygenases that share a conserved His-Asp-His triad of residues that chelates an active site iron. The prolyl hydroxylases can be modulated by iron concentrations, ascorbic acid levels, and metabolic intermediates of the citric acid cycle, including fumarate and succinate 38-40. A single enzyme has been identified that hydroxylates the critical asparagine of HIF-α: Factor Inhibiting HIF (FIH) 41-43. Like the PHDs, FIH is a 2-oxoglutarate dependent dioxygenase.
Subsequent in vitro studies surveyed the importance of residues in the vicinity of the HIF-1α hydroxylation sites for modification by the PHDs and FIH 44-46. An unexpectedly wide range of amino acid substitutions could be tolerated. In fact for both the prolyl and aspariginyl modifications, the only obligatory residue was the hydroxylacceptor residue itself. In vitro, all three PHDs have the capacity to hydroxylate HIF-1α and HIF-2α. PHD1 and PHD2, in addition, have the capacity to efficiently hydroxylate both sites of prolyl hydroxylation, while PHD3 displays a marked preference for the C-terminal hydroxylation site 37, 45. FIH has the capacity to hydroxylate the apariginyl hydroxylation sites of HIF-1α and HIF-2α, with the efficiency of the latter less than that of the former 47, 48.
The relevance of these proteins to human physiology is a central issue. In the case of VHL, its identification as a tumor suppressor gene established its importance even before the function of the protein was understood 49. The VHL syndrome is a tumor predisposition syndrome that is characterized by a triad of tumors—renal cell carcinoma, pheochromocytoma, and hemangioblastoma. It is inherited in an autosomal dominant manner, and conforms to Knudsen's two hit hypothesis in which germline mutation/inactivation of one allele is followed by sporadic mutation/inactivation of the second allele. The significance of this gene is underscored by the fact that approximately 50% of sporadic renal cell carcinomas also display mutation/inactivation of both alleles. VHL syndrome-associated mutations cluster in two regions, the α domain and β domain. The β domain corresponds to the HIF-binding domain while the α-domain corresponds to the Elongin C interaction domain. It might be noted that Ser-111 and His-115 are two residues in which mutations have been observed in VHL syndrome, reinforcing the critical importance of these residues in recognizing hydroxylproline 28.
Idiopathic Erythrocytosis
As studies on the tumor suppressor function of VHL were being conducted, a separate line of investigation into idiopathic erythrocytosis was proceeding that independently converged on VHL. Idiopathic erythrocytosis is characterized by an absolute increase in red cell mass, and current guidelines for its evaluation have been published 50. It is not a common condition. For example, a central registry for such patients comprising approximately two hundred patients exists in the United Kingdom and Ireland, which collectively have a population of 70 million people 51. This, however, is likely to underestimate the incidence, as other studies have provided an incidence estimate of 1.1 per 1000 52. The erythrocytoses can be divided into primary erythocytoses, in which the defect resides in the erythroid precursors and serum EPO levels are below normal, or the secondary erythrocytoses, which are extrinsic to erythroid precursors and in which serum EPO levels are either elevated or inappropriately normal.
The most common cause of primary erythrocytosis is polycythemia vera, in which there is an acquired mutation in the JAK2 gene, typically V617F 53. Rarer familial forms of primary erythrocytosis can be due to mutations in the EPOR gene that render the encoded protein constitutively active 54, 55. The secondary erythrocytoses can be due to many different causes, including high affinity hemoglobin, chronic obstructive pulmonary disease, high altitude, right-to-left cardiac shunts, and EPO-producing tumors. Excellent recent reviews on these various forms of erythrocytosis have been published 18, 56.
VHL and Chuvash Polycythemia
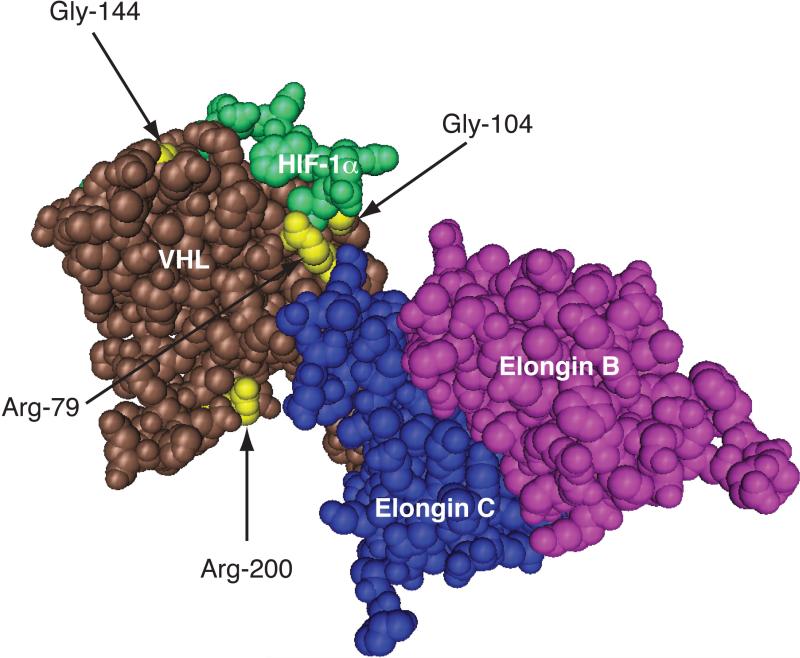
There is a distinct genetic subset of secondary erythrocytoses which will be the focus of the remainder of this review. The particular form of erythrocytosis eventually linked to VHL is termed Chuvash polycythemia. This is an autosomal recessive form of erythrocytosis that is endemic to Chuvashia, a republic in the former Soviet Union 57. A positional cloning approach demonstrated it to be due to an Arg200Trp mutation in the VHL gene 58. EPO levels in these patients are typically elevated. Notably, erythrocytosis is only rarely seen in VHL syndrome 59. Furthermore, the Chuvash mutation, in contrast to VHL syndrome-associated mutations, involves neither the crystallographically-determined HIF-binding groove nor its contact region with Elongin C, but instead appears on a face of the protein that is opposite from that of the HIF binding groove (Figure 2). The mutation leads to partial loss of activity towards HIF-α 58. Mechanistically, there are a number of considerations. (i) This mutation might induce a conformational change in VHL that alters its activity towards HIF-α. (ii) This residue may, in fact, be a contact residue with HIF-α, since recent studies indicate that longer peptides serve as better substrates than shorter ones 60, raising the possibility that the contact region of VHL for HIF-α might be more extensive than that provided by published VCB:HIF-1α peptide structures. (iii) The mutation destabilizes the VHL protein itself 61. (iv) The mutation alters substrate specificity, having a more pronounced detrimental effect on HIF-2α than on HIF-1α 61. Thus, there may be multiple factors that contribute to the partial loss of function seen with this mutation.
Figure 2.
Positions of select VHL-associated erythrocytosis mutations. Three-dimensional structure of the VCB complex bound to hydroxyproline-564 HIF-1α peptide (residues 556-575). The structure was generated using Cn3D from PDB coordinates (1LM8) deposited by Min et al28. The positions of Arg-79, Gly-104, Gly-144, and Arg-200 in VHL are shown in yellow.
The Chuvash mutation occurs in other populations as well. For example, it has been identified in patients of Pakistani descent 62. Subsequent work also identified a cohort in the Italian island of Ischia 63. Haplotype analysis has indicated that patients with the Chuvash mutation arose from a single founder 64, with one exception being an individual in Turkey 65. Additional studies have identified other VHL mutations associated with erythrocytosis (Table 1) 66-69. Some of these, intriguingly, cluster on the same face of the protein as the Chuvash mutation 69, suggesting that these mutations may have effects similar to those detailed above. Others, however, affect residues lying within or in the vicinity of the HIF-binding groove, such as Arg-79, Gly-104, and Gly-144 (Figure 2). Reports have also identified compound heterozygotes, and even individuals that are simple heterozygotes 62, 63, 65-69. The existence of simple VHL heterozygotes raises the possibility of other as yet to be identified mutations that affect the oxygen-sensing pathway. Collectively, these findings identify a subset of VHL mutations with a distinct phenotype, namely erythrocytosis as opposed to the tumor predisposition phenotype of VHL syndrome. The distinction, however, is not absolute. For example, the Arg200Trp as well as the Val130Leu mutation (Table 1) have been seen in some VHL syndrome kindreds 70. It should also be noted that Chuvash polycythemia, while principally a secondary erythrocytosis, also has features of primary erythrocytosis, evidence for which is seen in both patients and in a mouse model for the disease 58, 61. Finally, Chuvash polycythemia should not be considered a benign disease, as patients with this disease have premature morbidity and mortality due to cerebrovascular accidents and also are predisposed to pulmonary hypertension 71-73. While these patients do not develop the tumors characteristically associated with VHL disease, they do develop vertebral hemangiomas at increased frequency 71.
Table 1.
Mutations in the VHL Gene Associated with Erythrocytosis
| Mutation | Amino Acid | Zygosity | References |
|---|---|---|---|
| c.598C>T | R200W | Homozygous | 58, 62, 63, 65 |
| c.598C>T | R200W | Heterozygous | 58, 62, 63, 65 |
| c.235C>T/562C>G | R79C/L188V | Compound Heterozygous | 66 |
| c.311G>T | G104V | Heterozygous | 65 |
| c.376G>T | D126Y | Heterozygous | 68 |
| c.388G>C/598C>T | V130L/R200W | Compound Heterozygous | 68 |
| c.430G>A | G144R | Heterozygous | 67 |
| c.523A>G | Y175C | Heterozygous | 66 |
| c.562C>G/598C>T | L188V/R200W | Compound Heterozygous | 69 |
| c.571C>G | H191D | Homozygous | 69 |
| c.574C>T/598C>T | P192A/R200W | Compound Heterozygous | 69 |
HIF-2α and Erythrocytosis
Studies on Chuvash polycythemia showed that a defect in the oxygen-sensing pathway can cause erythrocytosis in humans, and it raised the possibility the mutations in yet other genes, such as HIF-α itself, might underly erythrocytosis. In this regard, the specific isoform involved in EPO regulation has been a matter of substantial debate, and evidence has been presented for both HIF-1α and HIF-2α being important for EPO regulation. HIF-1α, as mentioned previously, was purified based on its capacity to bind an oligonucleotide duplex comprising the HRE of the EPO gene 2. In addition, subsequent studies showed that Hif-1α +/- mice had impaired Epo responses to chronic or intermittent hypoxia, pointing to a role of Hif-1α in Epo gene regulation 74, 75. Other studies have highlighted a central role for HIF-2α. Cell culture studies employing siRNA knockdown of HIF-1α and HIF-2α in Hep3B hepatoma and Kelly neuroblastoma cells demonstrated that at least in these cell types, HIF-2α was the isoform responsible for EPO regulation 76. A subsequent series of studies then collectively demonstrated an essential role for Hif-2α in Epo regulation in the mouse. One showed that complete knockout of Hif-2α results in anemia, which could be rescued by recombinant Epo 77. Another showed that liver-specific knockout of VHL induces erythrocytosis, which could be rescued by liver-specific knockout of Hif-2α but not Hif-1α 78. Conversely, liver-specific overexpression of Hif-2α but not Hif-1α could produce erythrocytosis 79. Finally, a recent report demonstrated that acute global deletion of Hif-2α but not Hif-1α produced anemia in adult mice 80.
All of these studies provided a strong impetus to examine HIF-1α and HIF-2α in patients with idiopathic erythrocytosis. A particular challenge in trying to identify a HIF-α isoform relevant to Epo production in humans was posed by the high degree of in vitro tolerance for mutations in the region surrounding the sites of hydroxylation 45, 46. Studies examining the HIF1A gene showed that a P582S polymorphism was not associated with phenotype 81. A subsequent study identified a point mutation in exon 12 of the HIF2A gene in a family with erythrocytosis 82. It was present in three generations of this kindred, with the mutation segregating with the phenotype. The index case in this family also had a history of deep venous thrombosis. The mutation, which is heterozygous, is predicted to change Gly-537 to Trp, a position just six residues away from the primary hydroxylation site (Pro-531). Functionally, this mutation significantly impairs the ability of HIF-2α to be recognized by PHD2, its capacity to be hydroxylated by PHD2, and its ability to be recognized by VHL following hydroxylation 82. Interestingly, the phenotype in this family bears similarity to that of Chuvash polycythemia, in which there are elevated serum EPO levels without the sequelae of VHL syndrome. It is also noteworthy that functionally, this mutation produced only a partial gain of function. The degree of HIF-2α stabilization in vitro was more than that of wild type HIF-2α, but less than that of a Pro531Ala mutant which abolishes hydroxylation at this residue. Gly-537 occupies a position that is unique with respect to the HIF-α isoforms but is evolutionarily conserved in HIF-2α. It is situated between two acidic residues, Asp-536 and Glu-538. These residues correspond to Asp-569 and Asp-570 of HIF-1α, and in the three-dimensional structure of VHL bound to HIF-1α, both of these residues are in a bulge region that separates the two sets of contact residues that are important in establishing specificity in this interaction 27, 28, 83. This study provides evidence for the importance of HIF proline hydroxylation in humans. The finding moreover identifies the primary site of hydroxylation, at Pro-531, as being critical for oxygen-dependent degradation of HIF-2α, and shows that a secondary site of hydroxylation at Pro-405 is not redundant, consistent with cell culture studies 24, 84. Subsequently, four additional exon 12 mutations have been identified in patients with erythocytosis 83. Interestingly, three of these four mutations change Gly-537 to Arg, and affect the same residue that is mutated in the family just described (Table 2).
Table 2.
Mutations in the HIF2A Gene Associated with Erythrocytosis
These findings identify HIF-2α as a critical transcription factor regulating EPO in humans. This may also be relevant as to why partial loss of VHL activity in Chuvash polycythemia is sufficient to cause erythrocytosis, while haploinsufficiency of VHL syndrome typically does not; namely, the Chuvash mutation has a preferentially more detrimental effect on HIF-2α than on HIF-1α 61. The study also raises a number of interesting issues. First, it is not clear whether the stabilized HIF-2α is upregulating EPO gene transcription in the kidney or liver, or both. Mouse studies indicate that in the liver, Hif-2α is sufficient to activate Epo gene transcription 78, 79. Corresponding studies have yet to be reported for the Epo-producing cells of the kidney. Nonetheless, the fact that acute global deletion in the mouse of Hif-2α but not Hif-1α results in anemia80 supports a critical role for HIF-2α in both the kidney and liver. Second, since the mutation leads to a gain of function, it is formally possible that a gain of function mutation in HIF-1α might also yield the same phenotype. If such a scenario were to occur, it would imply that HIF-1α can activate Epo gene transcription in the kidney; overexpression of Hif-1α in murine liver is not sufficient to induce Epo 79, 85. It should also be noted that a role for HIF-1α in Epo regulation may also exist in certain circumstances, such as embryogenesis, and chronic or intermittent hypoxia 74, 75, 86.
PHD2 and Erythrocytosis
These studies on HIF-2α and VHL raise the possibility of yet other erythrocytosis-associated mutations in the oxygen-sensing pathway, such as in the hydroxylases that regulate HIF-α turnover. That being said, a potential issue here is that of redundancy, given the multiple isoforms of PHD and the fact that one would anticipate, based on cell culture data, that they would all be constitutively active 87. Study of a family with hereditary erythrocytosis showed, in fact, that the PHDs are not redundant 88. This family harbored a heterozygous mutation in PHD2. The affected individuals in this family presented with isolated erythrocytosis, and EPO levels were inappropriately normal. This might be contrasted to those with erythrocytosis-associated HIF-2α or VHL mutations, in which EPO levels are typically high. The PHD2 mutation, predicted to change Pro-317 to Arg, is several amino acids away from two of the three active site iron-chelating residues, His-313 and Asp-315. Mutations were not seen in PHD1 or PHD3. Notably, the father in the PHD2 pedigree died of esophageal carcinoma, which might raise the possibility of loss of heterozygosity of PHD2. However, sequencing of DNA obtained from the tumor revealed this not to be the case.
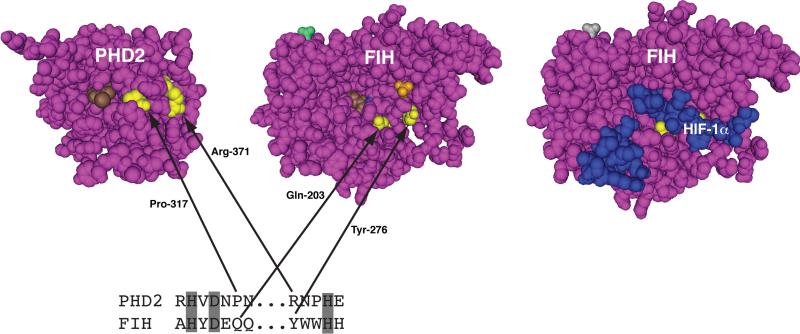
Functionally, the mutation impaired PHD2 binding to HIF-2α as well as HIF-1α, and substantially diminished prolyl hydroxylase activity, thereby suggesting that near-haploinsufficiency of PHD2 is sufficient to cause the phenotype 88. Consistent with this, there was no evidence of a dominant negative effect. In the three-dimensional structure of PHD2, Pro-317 is in the vicinity of the active site (Figure 3). PHD2 and FIH are both 2-oxoglutarate dependent dioxygenases and share a common overall fold, and comparision of the three dimensional structure of PHD2 with FIH in the absence or presence of bound HIF-α peptide strongly suggests that P317 is likely to be a contact residue (Figure 3). Several potential mechanisms by which this mutation might impair activity include (i) steric interference with HIF-α binding, (ii) perturbation of HIF-α binding at sites distant from this residue, or (iii) impairment of binding of the active site iron.
Figure 3.
Positions of PHD2-associated erythrocytosis missense mutations. Three-dimensional structures of PHD2, FIH, and the FIH:HIF-1α (786-826) complex, generated using Cn3D from PDB coordinates (2G1M, 1H2N, and 1H2L) deposited by McDonough et al 98 and Elkins et al 99. Arg-371 and Pro-317 in PHD2, and Tyr-276 and Gln-203 in FIH are highlighted in yellow. In the PHD2 structure, Compound A (a 2-oxoglutarate competitive inhibitor) is shown in brown. In the FIH structure, 2-oxoglutarate and sulfate are shown in brown and orange, respectively. In the FIH:HIF-1α (786-826) structure, the HIF-1α peptide is blue. Note that only HIF-1α residues 795-806 and 813-822 are resolved. At bottom is a comparison of residues 312-318 and 371-375 of PHD2 (separated by ...), and residues 198-204 and 276-280 of FIH. Shading indicates iron-chelating residues.
A subsequent study showed a different heterozygous mutation in PHD2, R371H (Table 3) 89. This patient, like the others, had isolated erythrocytosis and an inappropriately normal EPO level. Functional studies again indicated substantial dimunition of PHD2 binding to HIF-2α and HIF-1α as well as diminished hydroxylase activity, though perhaps not as substantial as with the P317R mutation. In the three dimensional structure of PHD2, Arg-371 is more distant from the active site iron than Pro-317, though still in the vicinity of the active site. Consideration of the three-dimensional structure suggests that Arg-371, as with Pro-317, is likely to be a contact residue and therefore a component of an active site binding groove (Figure 3).
Table 3.
Mutations in the PHD2 Gene Associated with Erythrocytosis
Additional studies have identified three additional heterozygous mutations in PHD2 (Table 3) 90. Two of these are frameshift mutations, while the third is a nonsense mutation. The two frameshift mutations produce truncated proteins lacking the catalytic domain, and would therefore be predicted to produce catalytically inactive proteins. The nonsense mutation removes the last 50 residues from the protein, suggesting that this C-terminal tail is essential for catalysis, protein folding, or both. While the possibility of a dominant negative effect of any of these mutations cannot be ruled out, it would seem that these PHD2 mutations taken together all point to haploinsufficiency as the cause of erythrocytosis in these patients. This, in turn, suggests that PHD2 protein levels must be precisely maintained at appropriate levels in order to properly regulate EPO.
The finding that PHD2 is critical for EPO control in humans has been validated in the mouse 91, 92. Acute global knockout of Phd2 using an floxed Phd2 allele and a tamoxifen-inducible cre recombinase transgene results in dramatic rises in Epo, hemoglobin, and hematocrit in the adult mouse. These mice display extramedullary hematopoeisis involving both the liver and spleen 92. Epo mRNA levels are markedly elevated in the kidney but not the liver, thereby identifying the former as the source of Epo in this mouse model 91, 92. Interestingly, Phd2 +/- mice do not display erythrocytosis, indicating that haploinsufficiency is not sufficient to cause the phenotype in mice, and therefore the dose response curve for PHD2 levels and Epo differs between mouse and humans 92. Phd1 -/- and Phd3 -/- mice do not display erythrocytosis. However, Phd1 -/-; Phd3 -/- mice do display modest erythrocytosis, and in contrast to the Phd2 -/- mice, the Phd1 -/-; Phd3 -/- mice do not display elevated Epo levels 92. Hif-2α protein levels are elevated in the livers but not kidneys of Phd1 -/-; Phd3 -/- mice; in these mice, Hif-1α is not elevated in either organ. Hif-1α protein levels, in contrast, are elevated in the kidneys and livers of Phd2 -/- mice. In these mice, one report indicates that Hif-2α is elevated in both organs as well 91, while another indicates it to be elevated in neither 92, which might raise the possibility that Hif-1α might regulate Epo in the mouse kidney. This observation, however, must be interpreted with caution, since HIF-2α is expressed in only a subset of cells in the kidney and may escape detection under the experimental conditions employed. Further studies will be required to resolve this issue.
Implications
Taken together, these studies on idiopathic erythrocytosis identify the PHD2:HIF-2α:VHL pathway as the core molecular machinery regulating EPO in humans. VHL mutations at this point appear to be more common than either PHD2 or HIF-2α mutations. With regard to VHL, its association with Elongin C or Elongin B might raise the question of whether mutations in these proteins might be associated with erythrocytosis. It should be noted however, that at least in the context of renal cell carcinoma, mutations in these proteins have not been identified 93.
The finding that mutations in VHL can produce two different phenotypes, a cancer predisposition phenotype and an erythrocytosis phenotype, now raises the question of whether PHD2 and HIF-2α will follow this same paradigm. With regard to PHD2, missense mutations in PHD2 have in fact been reported in endometrial carcinomas and sarcomas 94. A caveat here, though, is that the catalytic consequences of these mutations are not known. Mutations have not yet been reported in HIF-2α in human tumors.
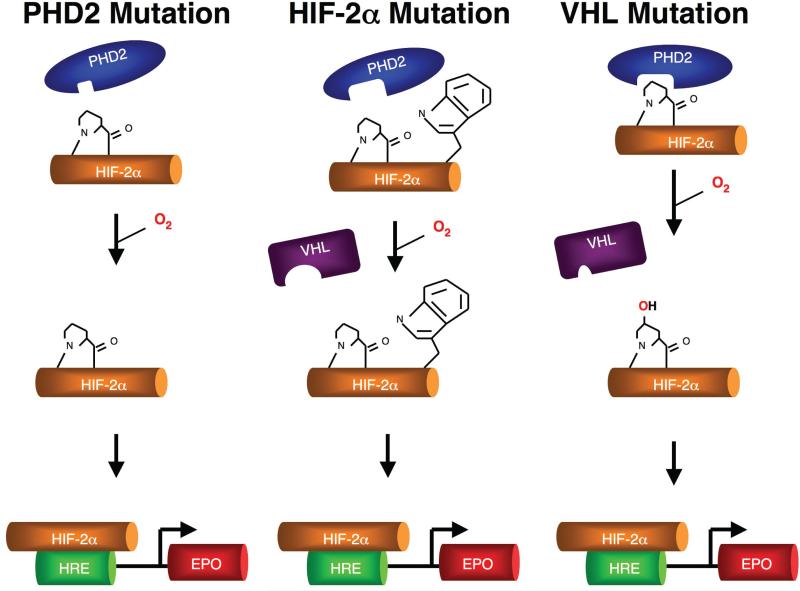
The findings highlight how exquisitely fine-tuned the oxygen sensing pathway is in the EPO pathway (Figure 4). Seemingly mild perturbations in PHD2-induced hydroxylation of HIF-2α, or the subsequent interaction of hydroxylated HIF-2α with VHL are sufficient to perturb the pathway. Moreover, the findings identify functionally critical regions in these proteins. In PHD2, these residues appear to contribute to a potential active site binding groove. In HIF-2α, these residues are in close vicinity to the primary hydroxylacceptor site. The numerous mutations seen in VHL define these as important residues; Arg-200, the residue mutated in Chuvash polycythemia, is a particularly noteworthy.
Figure 4.
Three distinct types of mutations in the oxygen-sensing pathway can cause erythrocytosis. Mutations in PHD2 impair hydroxylation of HIF-2α (left), a mutation (Gly537Trp) in HIF-2α impairs PHD2-induced hydroxylation as well as subsequent recognition by VHL (middle), and mutations in VHL impair its capacity to induce HIF-2α degradation (right).
There are important therapeutic implications to this work. Targeting of PHD2 is a potential approach for treating anemia. It is noteworthy that patients with PHD2 and HIF-2α mutations present with isolated erythrocytosis. At the same time, it will be important to take into account a number of considerations. First, Chuvash polycythemia is accompanied by exaggerated cardiopulmonary responses to hypoxia, and these patients are predisposed to pulmonary hypertension 72, 73. It is not clear to what extent these are HIF-dependent versus HIF-independent effects, particularly since VHL has functions aside from promoting HIF-α ubiquitination 95. Second, it is presently uncertain whether there are HIF-independent pathways that might augment erythropoiesis under PHD2 control, and whether such HIF-independent pathways, if they were to exist, overlap with HIF-independent functions of VHL. Third, the human mutations observed produce only partial loss-of-function (PHD2) or partial gain-of-function (HIF-2α), and it is not clear whether full inhibition of prolyl hydroxylation—if this is the goal—will have different consequences.
In this regard, even partial PHD2 inhibition may be sufficient to induce erythrocytosis. The fact that the PHDs share a highly conserved catalytic domain might make isoform-selective inhibition of PHD especially challenging. However, the domains are not identical, and there is evidence that there are determinants that convey differences in specificity between the three isoforms. For example, a loop in PHD2 that is distinct from that in PHD3 is responsible for the ability of PHD2 to hydroxylate both the primary and secondary sites of hydroxylation in HIF-1α 96, 97. PHD3, in contrast, displays a marked preference for the primary site of hydroxylation.
Moreover, the findings now raise the possibility of pursuing inhibitors not only selective for PHD2, but moreover, selective for inhibition of HIF-2α over HIF-1α. Intriguingly, the Gly537Trp mutation in HIF-2α occurs in a residue that is not conserved in HIF-1α. In the three dimensional structure of VHL bound to HIF-1α, this occurs in a linker region that separates two highly conserved regions that establish specific contacts with VHL. An analogous situation might apply to the interaction of HIF-α to PHD2, namely, there are critical, highly conserved contacts both N- and C-terminal to the hydroxylacceptor Pro, raising the possibility that Gly-537 might occupy a space unique to this isoform. If such a circumstance were to exist, targeting such a region might allow a more selective inhibition of HIF-2α over HIF-1α. It must be emphasized, however, that our understanding of the biology of the PHDs is far from complete, reinforcing the need for more studies in this area.
Studies of rare human diseases, such as idiopathic erythrocytosis, can elucidate fundamental homeostatic pathways. In light of this, the continued study patients with idiopathic erythrocytosis will likely continue to expand our knowledge of oxygen-sensing in humans.
Practice Points
A subset of patients with secondary erythrocytosis have mutations in genes of the oxygen sensing pathway.
Cases of idiopathic erythrocytosis with high or normal EPO levels and no identifiable secondary cause should be investigated for the possibility of genetic mutations in PHD2, HIF2A, and VHL.
Research Agenda
Explore whether cases of unexplained erythrocytosis may harbor mutations in yet other genes of the oxygen-sensing pathway.
Examine the biologic consequences of alterations in PHD2, HIF2A, and VHL in experimental systems.
Acknowledgments
I am grateful to Dr. Terence R. J. Lappin and Dr. Melanie J. Percy for comments on the manuscript. Work in the author's laboratory that is described in this review is supported by NIH grant R01-CA090261.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Dr. Lee receives research grant support from GlaxoSmithKline.
References
- 1.Semenza GL. Life with oxygen. Science. 2007;318:62–4. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 2.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 1998;7:205–13. [PMC free article] [PubMed] [Google Scholar]
- 4.Ema M, Taya S, Yokotani N, et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia- inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A. 1997;94:4273–8. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogenesch JB, Chan WK, Jackiw VH, et al. Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem. 1997;272:8581–93. doi: 10.1074/jbc.272.13.8581. [DOI] [PubMed] [Google Scholar]
- 6.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 7.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–7. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 8.Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–9. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 9.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2- dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–92. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pugh CW, O'Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ. Activation of hypoxia-inducible factor-1; definition of regulatory domains within the alpha subunit. J Biol Chem. 1997;272:11205–14. doi: 10.1074/jbc.272.17.11205. [DOI] [PubMed] [Google Scholar]
- 11.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253–60. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 12.Ema M, Hirota K, Mimura J, et al. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. Embo J. 1999;18:1905–14. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–7. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 15.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–78. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 16.Stockmann C, Fandrey J. Hypoxia-induced erythropoietin production: a paradigm for oxygen-regulated gene expression. Clin Exp Pharmacol Physiol. 2006;33:968–79. doi: 10.1111/j.1440-1681.2006.04474.x. [DOI] [PubMed] [Google Scholar]
- 17.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–55. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Hodges VM, Rainey S, Lappin TR, Maxwell AP. Pathophysiology of anemia and erythrocytosis. Crit Rev Oncol Hematol. 2007;64:139–58. doi: 10.1016/j.critrevonc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Makino Y, Cao R, Svensson K, et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414:550–4. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 20.Wiesener MS, Jurgensen JS, Rosenberger C, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. Faseb J. 2003;17:271–3. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 21.Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A. 2001;98:9630–5. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 23.Ivan M, Kondo K, Yang H, et al. HIFalpha Targeted for VHL-Mediated Destruction by Proline Hydroxylation: Implications for O2 Sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 24.Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. Embo J. 2001;20:5197–206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kivirikko KI, Myllyharju J. Prolyl 4-hydroxylases and their protein disulfide isomerase subunit. Matrix Biol. 1998;16:357–68. doi: 10.1016/s0945-053x(98)90009-9. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis [see comments]. Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 27.Hon WC, Wilson MI, Harlos K, et al. Structural basis for the recognition of hydroxyproline in HIF-1alpha by pVHL. Nature. 2002;417:975–8. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 28.Min JH, Yang H, Ivan M, et al. Structure of an HIF-1alpha -pVHL complex: hydroxyproline recognition in signaling. Science. 2002;296:1886–9. doi: 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- 29.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 30.Brunelle JK, Bell EL, Quesada NM, et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–14. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Mansfield KD, Guzy RD, Pan Y, et al. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–9. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzy RD, Hoyos B, Robin E, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–8. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–61. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 34.Freedman SJ, Sun ZY, Poy F, et al. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc Natl Acad Sci U S A. 2002;99:5367–72. doi: 10.1073/pnas.082117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivan M, Haberberger T, Gervasi DC, et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S A. 2002;99:13459–64. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–40. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 37.Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 38.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 39.Isaacs JS, Jung YJ, Mole DR, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–53. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Knowles HJ, Raval RR, Harris AL, Ratcliffe PJ. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003;63:1764–8. [PubMed] [Google Scholar]
- 41.Hewitson KS, McNeill LA, Riordan MV, et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem. 2002;277:26351–5. doi: 10.1074/jbc.C200273200. [DOI] [PubMed] [Google Scholar]
- 42.Lando D, Peet DJ, Gorman JJ, et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–71. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–86. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linke S, Stojkoski C, Kewley RJ, et al. Substrate requirements of the oxygen-sensing asparaginyl hydroxylase factor-inhibiting hypoxia-inducible factor. J Biol Chem. 2004;279:14391–7. doi: 10.1074/jbc.M313614200. [DOI] [PubMed] [Google Scholar]
- 45.Li D, Hirsila M, Koivunen P, et al. Many amino acid substitutions in a hypoxia-inducible transcription factor (HIF)-1alpha-like peptide cause only minor changes in its hydroxylation by the HIF prolyl 4-hydroxylases: substitution of 3,4-dehydroproline or azetidine-2-carboxylic acid for the proline leads to a high rate of uncoupled 2-oxoglutarate decarboxylation. J Biol Chem. 2004;279:55051–9. doi: 10.1074/jbc.M410287200. [DOI] [PubMed] [Google Scholar]
- 46.Huang J, Zhao Q, Mooney SM, Lee FS. Sequence determinants in hypoxia-inducible factor-1alpha for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J Biol Chem. 2002;277:39792–800. doi: 10.1074/jbc.M206955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan Q, Bartz S, Mao M, Li L, Kaelin WG., Jr The hypoxia-inducible factor 2alpha N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol Cell Biol. 2007;27:2092–102. doi: 10.1128/MCB.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bracken CP, Fedele AO, Linke S, et al. Cell-specific regulation of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha stabilization and transactivation in a graded oxygen environment. J Biol Chem. 2006;281:22575–85. doi: 10.1074/jbc.M600288200. [DOI] [PubMed] [Google Scholar]
- 49.Kaelin WG. Von hippel-lindau disease. Annu Rev Pathol. 2007;2:145–73. doi: 10.1146/annurev.pathol.2.010506.092049. [DOI] [PubMed] [Google Scholar]
- 50.McMullin MF, Bareford D, Campbell P, et al. Guidelines for the diagnosis, investigation and management of polycythaemia/erythrocytosis. Br J Haematol. 2005;130:174–95. doi: 10.1111/j.1365-2141.2005.05535.x. [DOI] [PubMed] [Google Scholar]
- 51.Lee FS, Percy MJ, McMullin MF. Oxygen sensing: recent insights from idiopathic erythrocytosis. Cell Cycle. 2006;5:941–5. doi: 10.4161/cc.5.9.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finazzi G, Gregg XT, Barbui T, Prchal JT. Idiopathic erythrocytosis and other non-clonal polycythemias. Best Pract Res Clin Haematol. 2006;19:471–82. doi: 10.1016/j.beha.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–83. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 54.de la Chapelle A, Traskelin AL, Juvonen E. Truncated erythropoietin receptor causes dominantly inherited benign human erythrocytosis. Proc Natl Acad Sci U S A. 1993;90:4495–9. doi: 10.1073/pnas.90.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Percy MJ, McMullin MF, Roques AW, et al. Erythrocytosis due to a mutation in the erythropoietin receptor gene. Br J Haematol. 1998;100:407–10. doi: 10.1046/j.1365-2141.1998.00550.x. [DOI] [PubMed] [Google Scholar]
- 56.Gordeuk VR, Stockton DW, Prchal JT. Congenital polycythemias/erythrocytoses. Haematologica. 2005;90:109–16. [PubMed] [Google Scholar]
- 57.Ang SO, Chen H, Gordeuk VR, et al. Endemic polycythemia in Russia: mutation in the VHL gene. Blood Cells Mol Dis. 2002;28:57–62. doi: 10.1006/bcmd.2002.0488. [DOI] [PubMed] [Google Scholar]
- 58.Ang SO, Chen H, Hirota K, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–21. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 59.Wiesener MS, Seyfarth M, Warnecke C, et al. Paraneoplastic erythrocytosis associated with an inactivating point mutation of the von Hippel-Lindau gene in a renal cell carcinoma. Blood. 2002;99:3562–5. doi: 10.1182/blood.v99.10.3562. [DOI] [PubMed] [Google Scholar]
- 60.Koivunen P, Hirsila M, Kivirikko KI, Myllyharju J. The length of peptide substrates has a marked effect on hydroxylation by the hypoxia-inducible factor prolyl 4-hydroxylases. J Biol Chem. 2006;281:28712–20. doi: 10.1074/jbc.M604628200. [DOI] [PubMed] [Google Scholar]
- 61.Hickey MM, Lam JC, Bezman NA, Rathmell WK, Simon MC. von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2alpha signaling and splenic erythropoiesis. J Clin Invest. 2007;117:3879–89. doi: 10.1172/JCI32614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Percy MJ, McMullin MF, Jowitt SN, et al. Chuvash-type congenital polycythemia in 4 families of Asian and Western European ancestry. Blood. 2003;102:1097–9. doi: 10.1182/blood-2002-10-3246. [DOI] [PubMed] [Google Scholar]
- 63.Perrotta S, Nobili B, Ferraro M, et al. Von Hippel-Lindau-dependent polycythemia is endemic on the island of Ischia: identification of a novel cluster. Blood. 2006;107:514–9. doi: 10.1182/blood-2005-06-2422. [DOI] [PubMed] [Google Scholar]
- 64.Liu E, Percy MJ, Amos CI, et al. The worldwide distribution of the VHL 598C>T mutation indicates a single founding event. Blood. 2004;103:1937–40. doi: 10.1182/blood-2003-07-2550. [DOI] [PubMed] [Google Scholar]
- 65.Cario H, Schwarz K, Jorch N, et al. Mutations in the von Hippel-Lindau (VHL) tumor suppressor gene and VHL-haplotype analysis in patients with presumable congenital erythrocytosis. Haematologica. 2005;90:19–24. [PubMed] [Google Scholar]
- 66.Bento MC, Chang KT, Guan Y, et al. Congenital polycythemia with homozygous and heterozygous mutations of von Hippel-Lindau gene: five new Caucasian patients. Haematologica. 2005;90:128–9. [PubMed] [Google Scholar]
- 67.Randi ML, Murgia A, Putti MC, et al. Low frequency of VHL gene mutations in young individuals with polycythemia and high serum erythropoietin. Haematologica. 2005;90:689–91. [PubMed] [Google Scholar]
- 68.Pastore YD, Jelinek J, Ang S, et al. Mutations in the VHL gene in sporadic apparently congenital polycythemia. Blood. 2003;101:1591–5. doi: 10.1182/blood-2002-06-1843. [DOI] [PubMed] [Google Scholar]
- 69.Pastore Y, Jedlickova K, Guan Y, et al. Mutations of von Hippel-Lindau tumor-suppressor gene and congenital polycythemia. Am J Hum Genet. 2003;73:412–9. doi: 10.1086/377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olschwang S, Richard S, Boisson C, et al. Germline mutation profile of the VHL gene in von Hippel-Lindau disease and in sporadic hemangioblastoma. Hum Mutat. 1998;12:424–30. doi: 10.1002/(SICI)1098-1004(1998)12:6<424::AID-HUMU9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 71.Gordeuk VR, Sergueeva AI, Miasnikova GY, et al. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004;103:3924–32. doi: 10.1182/blood-2003-07-2535. [DOI] [PubMed] [Google Scholar]
- 72.Bushuev VI, Miasnikova GY, Sergueeva AI, et al. Endothelin-1, vascular endothelial growth factor and systolic pulmonary artery pressure in patients with Chuvash polycythemia. Haematologica. 2006;91:744–9. [PubMed] [Google Scholar]
- 73.Smith TG, Brooks JT, Balanos GM, et al. Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology. PLoS Med. 2006;3:e290. doi: 10.1371/journal.pmed.0030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu AY, Shimoda LA, Iyer NV, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691–6. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai Z, Manalo DJ, Wei G, et al. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation. 2003;108:79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- 76.Warnecke C, Zaborowska Z, Kurreck J, et al. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. Faseb J. 2004;18:1462–4. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- 77.Scortegagna M, Ding K, Zhang Q, et al. HIF-2{alpha} regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood. 2005;105:3133–40. doi: 10.1182/blood-2004-05-1695. [DOI] [PubMed] [Google Scholar]
- 78.Rankin EB, Biju MP, Liu Q, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim WY, Safran M, Buckley MR, et al. Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. Embo J. 2006;25:4650–62. doi: 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gruber M, Hu CJ, Johnson RS, et al. Acute postnatal ablation of Hif-2{alpha} results in anemia. Proc Natl Acad Sci U S A. 2007;104:2301–6. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Percy MJ, Mooney SM, McMullin MF, et al. A common polymorphism in the oxygen-dependent degradation (ODD) domain of hypoxia inducible factor-1alpha (HIF-1alpha) does not impair Pro-564 hydroxylation. Mol Cancer. 2003;2:31. doi: 10.1186/1476-4598-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Percy MJ, Furlow PW, Lucas GS, et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358:162–8. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Percy MJ, Beer PA, Campbell G, et al. Novel Exon 12 mutations in the HIF2A gene associated with erythrocytosis. Blood. 2008 doi: 10.1182/blood-2008-02-137703. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chan DA, Sutphin PD, Yen SE, Giaccia AJ. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2005;25:6415–26. doi: 10.1128/MCB.25.15.6415-6426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takeda K, Cowan A, Fong GH. Essential role for prolyl hydroxylase domain protein 2 in oxygen homeostasis of the adult vascular system. Circulation. 2007;116:774–81. doi: 10.1161/CIRCULATIONAHA.107.701516. [DOI] [PubMed] [Google Scholar]
- 86.Yoon D, Pastore YD, Divoky V, et al. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006;281:25703–11. doi: 10.1074/jbc.M602329200. [DOI] [PubMed] [Google Scholar]
- 87.Appelhoff RJ, Tian YM, Raval RR, et al. Differential Function of the Prolyl Hydroxylases PHD1, PHD2, and PHD3 in the Regulation of Hypoxia-inducible Factor. J Biol Chem. 2004;279:38458–65. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 88.Percy MJ, Zhao Q, Flores A, et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 2006;103:654–9. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Percy MJ, Furlow PW, Beer PA, et al. A novel erythrocytosis-associated PHD2 mutation suggests the location of a HIF binding groove. Blood. 2007;110:2193–6. doi: 10.1182/blood-2007-04-084434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Al-Sheikh M, Moradkhani K, Lopez M, Wajcman H, Prehu C. Disturbance in the HIF-1alpha pathway associated with erythrocytosis: Further evidences brought by frameshift and nonsense mutations in the prolyl hydroxylase domain protein 2 (PHD2) gene. Blood Cells Mol Dis. 2007 doi: 10.1016/j.bcmd.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 91.Minamishima YA, Moslehi J, Bardeesy N, et al. Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood. 2008;111:3236–44. doi: 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takeda K, Aguila HL, Parikh NS, et al. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008;111:3229–35. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clifford SC, Astuti D, Hooper L, et al. The pVHL-associated SCF ubiquitin ligase complex: molecular genetic analysis of elongin B and C, Rbx1 and HIF-1alpha in renal cell carcinoma. Oncogene. 2001;20:5067–74. doi: 10.1038/sj.onc.1204602. [DOI] [PubMed] [Google Scholar]
- 94.Kato H, Inoue T, Asanoma K, et al. Induction of human endometrial cancer cell senescence through modulation of HIF-1alpha activity by EGLN1. Int J Cancer. 2006;118:1144–53. doi: 10.1002/ijc.21488. [DOI] [PubMed] [Google Scholar]
- 95.Roberts AM, Ohh M. Beyond the hypoxia-inducible factor-centric tumour suppressor model of von Hippel-Lindau. Curr Opin Oncol. 2008;20:83–9. doi: 10.1097/CCO.0b013e3282f310de. [DOI] [PubMed] [Google Scholar]
- 96.Flashman E, Bagg EA, Chowdhury R, et al. Kinetic rationale for selectivity towards N- and C-terminal oxygen dependent degradation domain substrates mediated by a loop region of HIF prolyl hydroxylases. J Biol Chem. 2007 doi: 10.1074/jbc.M707411200. [DOI] [PubMed] [Google Scholar]
- 97.Villar D, Vara-Vega A, Landazuri MO, Del Peso L. Identification of a region on hypoxia-inducible-factor prolyl 4-hydroxylases that determines their specificity for the oxygen degradation domains. Biochem J. 2007;408:231–40. doi: 10.1042/BJ20071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McDonough MA, Li V, Flashman E, et al. Cellular oxygen sensing: Crystal structure of hypoxia-inducible factor prolyl hydroxylase (PHD2). Proc Natl Acad Sci U S A. 2006;103:9814–9. doi: 10.1073/pnas.0601283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elkins JM, Hewitson KS, McNeill LA, et al. Structure of factor-inhibiting hypoxia-inducible factor (HIF) reveals mechanism of oxidative modification of HIF-1 alpha. J Biol Chem. 2003;278:1802–6. doi: 10.1074/jbc.C200644200. [DOI] [PubMed] [Google Scholar]