Abstract
The phagocyte NADPH oxidase is a multi-component enzyme complex mediating microbial killing. We find that NADPH oxidase p47phox deficient (p47phox-/-) chronic granulomatous disease (CGD) mice develop lymph node hyperplasia even without obvious infection, where increased number of T and B lymphocytes is associated with increased percent of naïve cells and lower T:B cell ratio than wild type. Paradoxically, despite lymphoid hyperplasia in vivo, when lymphocytes are placed in culture, p47phox-/- CD8+ lymphocytes progress more rapidly to apoptosis than wild type. This is associated in cultured p47phox-/- CD8+ lymphocytes with induction of pro-apoptotic Bim and Puma expression, increased mitochondrial outer membrane permeabilization and depressed Bcl-2 expression. Addition of IL-7 to the culture partially corrects Bcl-2 levels in cultured p47phox-/- CD8+ lymphocytes and improves survival. Adding glucose oxidase to the culture to generate hydrogen peroxide along with IL-7 further improves p47phox-/- CD8+ lymphocyte survival, but only to 30% of wild type. We conclude that p47phox-/-CD8+ lymphocytes have an intrinsic survival defect likely in part related to the oxidase deficiency, but in vivo in lymph nodes of CGD mice there are microenvironmental factors yet to be delineated that suppress progression to apoptosis and allow accumulation of lymphocytes leading to lymphoid hyperplasia.
Keywords: p47phox, T cells, apoptosis, cytokines, apoptosis
Introduction
Within the complex microarchitecture of secondary lymphoid organs (SLOs) such as lymph nodes (LNs) and spleen, critical signals balance lymphocyte survival and homeostasis. Self-major histocompatibility molecule stimulation of the T cell receptor (TcR) and extrinsic signals provided by cytokines such as IL-7 and IL-15 control naïve T lymphocyte survival (1, 2). In addition, these signals facilitate continuous low-level division of resting naïve T cells during homeostatic proliferation to maintain T cell number and repertoire diversity (2). Homeostasis is disrupted during the mounting of an immune response allowing for clonal expansion of effector lymphocytes. Thereafter, the majority of effector cells die to prevent undesirable autoimmunity, and a subset survives as memory T cells (3, 4). Immune responses are mounted within SLOs to eradicate invading pathogens. However, impaired pathogen clearance in an immunocompromised host often results in sustained immune responses that produce local acute inflammatory responses and ultimately systemic chronic inflammation.
Resting naïve T cells recirculate between blood and lymphoid tissues while memory T cells migrate to nonlymphoid tissue sites. Furthermore, without antigen encounter, resting naïve T cells eventually die within a defined half-life (5, 6). Survival of resting naïve T cells during recirculation between inflamed and non-inflamed SLOs undoubtedly requires different signals than those required for survival during recirculation between non-inflamed SLOs. In this manuscript we examined the relationship between nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) oxidase deficiency and naïve T cell survival within SLOs. The phagocyte multicomponent NADPH oxidase mediates the single electron reduction of oxygen to superoxide anion resulting in the well-characterized respiratory burst which promotes innate cell killing of catalase positive microorganisms (7, 8). Several non-phagocytic cells have been reported to express ligand-dependent NADPH oxidase-like reactive oxygen species (ROS) (9). We reported T cells express each of the NADPH oxidase structural proteins and that TcR stimulation induces low level intracellular NADPH oxidase dependent hydrogen peroxide (10). Chronic granulomatous disease of childhood (CGD) is the genetically inherited primary immunodeficiency that is caused by defects of structural NADPH oxidase proteins (11). CGD patients are at increased risk of developing and succumbing to life threatening infection, and acute and chronic inflammatory diseases (12). We report here NADPH oxidase p47phox deficient (p47phox-/-, (13)) mice without evidence of gross infection develop reactive SLOs characterized by LN hyperplasia with increased B and T lymphocyte accumulations. Nearly half of spleen and LN resting p47phox-/- CD8+ lymphocytes rapidly die ex vivo, and during the initial 24 hours of in vitro culture significantly more resting LN p47phox-/- CD8+ lymphocytes undergo caspase independent cell death than splenic p47phox-/- CD8+ lymphocytes. BH3-only pro-apoptotic Bim and Puma protein expression is significantly reduced in LN p47phox-/- CD8+ lymphocytes. However, Bim and Puma expression are significantly enhanced in vitro, and dying p47phox-/- CD8+ lymphocytes from p47phox-/- LNs show increased mitochondrial outer membrane permeabilization and depressed Bcl-2 expression. These results indicate microenvironmental factors generated during ongoing immune responses in p47phox-/- mice provide signals that regulate naïve T lymphocyte homeostasis and survival, and may contribute to cellular immune dysfunction in CGD patients.
Results
LN hyperplasia in p47phox-/- mice
p47phox-/- mice have LN hyperplasia, increased total numbers of B and T lymphocytes and lower T:B cell ratios than age and sex matched wild type (WT) mice (Figure 1). However, p47phox-/- and WT CD4-CD8 ratios were comparable. Flow cytometry analysis showed p47phox-/- and WT LNs contained phenotypically naïve B220+CD27- B cells, and comparable phenotypically naïve CD44lowCD62Lhigh and CD25lowCD69low CD4+ and CD8+ T lymphocytes (Supplemental Figure 1).
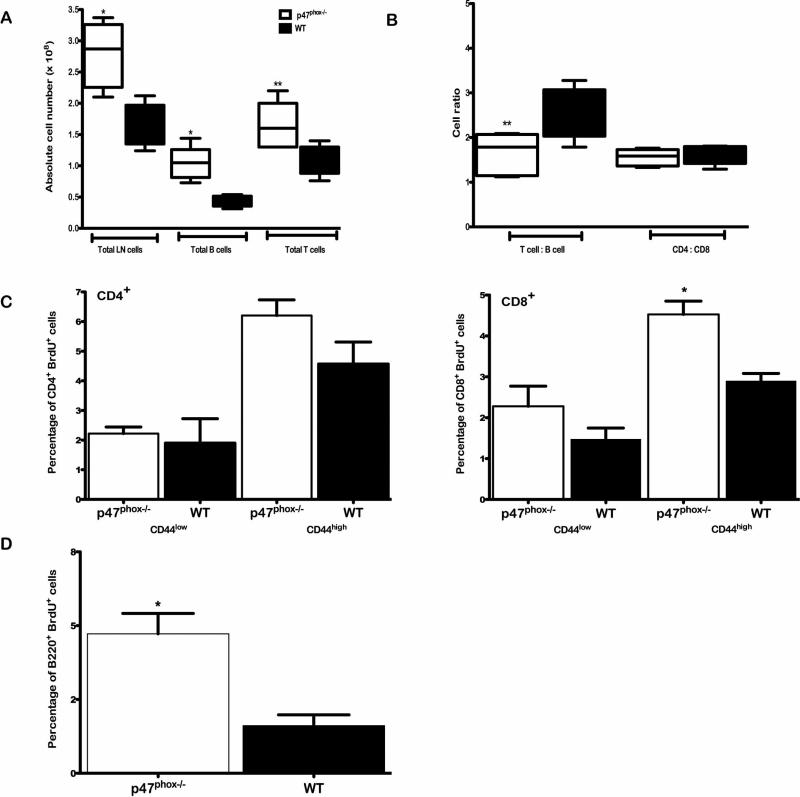
Figure 1.
LN hyperplasia and in vivo lymphocyte proliferation in p47phox-/- mice. Single cell suspensions of resting LN cells from WT and p47phox-/- mice were generated. Surface B220, CD3 CD8 and CD4 expression on peripheral LN cells of WT and p47phox-/- mice was assessed by flow cytometry. (A) The total number of cells as well as absolute number of B and T lymphocytes isolated from the peripheral LNs of WT and p47phox-/- mice were quantitated. (B) The ratio of T:B cells and CD4:CD8 T cells in the peripheral LNs of WT and p47phox-/- mice was quantitated. The data shown are the mean +/- SEM for 5 independent experiments with 3 mice mice/experiment. p47phox-/- responses are indicated by the open histograms and WT responses are indicated by the filled histograms. *p = 0.014, **p = 0.007. p47phox-/- and WT mice (n = 3) were fed BrdU in their drinking water at a concentration of 1 mg/ml for 5 days. Lymph nodes were harvested on day 5. Whole lymph node cell populations were stained with anti-CD4, CD8, CD44, B220 and BrdU antibodies and assessed by flow cytometry. (C) The percentage of CD44+low and CD44high -Brdu+ CD4 (left) and CD8 (right) lymphocytes in WT or p47phox-/- lymph nodes is shown. (D) The percentage of B220+-Brdu+ lymphocytes in WT or p47phox-/- lymph nodes is shown. *p < 0.02.
In vivo 5-bromo-2'deoxyuridine (BrdU) incorporation studies demonstrated peripheral LN CD4+ lymphocyte turnover was similar in p47phox-/- and WT mice, while p47phox-/- CD8+ CD44high T cells proliferated 1.5 times more than WT cells (Figure 1C). Peripheral LN p47phox-/- B cells also hyper-proliferated compared to WT B cells (Figure 1D). Collectively, this data shows that within the reactive p47phox-/- LN microenvironment there is increased B cell and memory CD8+ cell division along with an increased accumulation of naïve B and T lymphocytes.
Exaggerated death of p47phox-/- CD8+ lymphocytes
Numerous investigations have reported ROS mediates apoptosis in a variety of cells including T cells. Although investigations of activated T cell death concluded excess ROS disrupts mitochondrial membrane integrity by perturbing the balance of Bcl-2 anti and proapoptotic proteins, and antioxidants protect activated T cells from ROS mediated apoptosis (1, 6, 14-16), the role of ROS in naïve T cell survival is less clear. To determine whether NADPH oxidase ROS mediates resting T cell survival, we examined cytokine responses of LN derived T lymphocytes. Viable and non-viable lymphocytes from 24 hour cultures were differentially stained with DNA binding dyes and the absolute number of viable versus non-viable nucleated cells were quantitated based on cell size and staining properties using the Guava ViaCount assay (see methods). Cell count and viability were determined and the data normalized to the baseline values. After 24 hours of culture in IL-2, fewer resting p47phox-/- CD4+ and CD8+ lymphocytes remained viable than WT lymphocytes. Furthermore, there was significantly more cell death of resting p47phox-/- CD8+ lymphocytes (Figure 2).
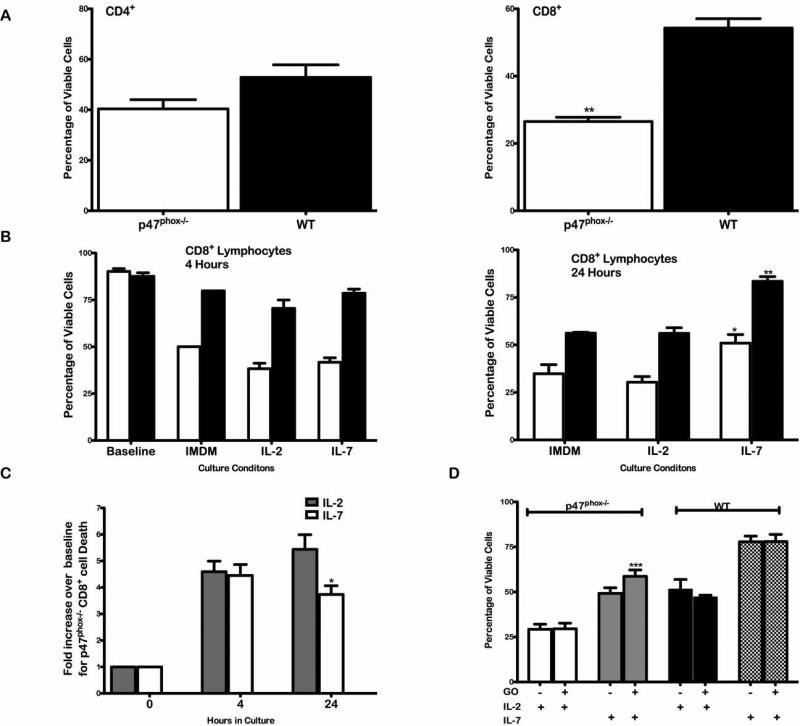
Figure 2.
In vitro cytokine activated lymphocyte survival. Fractionated CD4+ and CD8+ p47phox-/-and WT lymphocytes were cultured in IMDM alone or supplemented with IL-2 or IL-7 as indicated for 24 hours. The total number of viable and non-viable nucleated cells was measured using the Guava ViaCount assay. p47phox-/- responses are indicated by the open histograms and WT responses are indicated by the filled histograms. (A) The percentage of viable CD4+ (right) and CD8+ (left) p47phox-/- and WT lymphocytes after at 24 hours in culture. (B) The total number of viable and non-viable nucleated CD8+ cells was measure using the Guava ViaCount assay, and responses were normalized to the baseline values. The percentage of viable p47phox-/- and WT cells was quantitated at 4 hours (left) and 24 hours (right). (C) The fold increase in dead CD8+ lymphocyte cell number in the p47phox-/- cultures supplemented with IL-2 (open histogram) and IL-7 (hatched histogram) at 24 hours was quantitated. (D) Glucose-oxidase produced H2O2 enhances p47phox-/- CD8+ lymphocyte survival. Fractionated CD8+ p47phox-/-and WT lymphocytes were cultured with IL-2 or IL-7 supplemented culture medium with 1 pg/ml of glucose oxidase (GO) for 24 hours. The histograms show the percentage of viable cells at 24 hours. p47phox-/- IL-2 +/- GO (open histogram), p47phox-/- IL-7 +/- GO (grey filled histogram), WT IL-2 +/- GO (black filled histogram) and WT IL-7 +/- GO (hatched histogram). The data shown are the mean +/- SEM for 4 independent experiments with 3 mice mice/experiment. *p = 0.01, **p < 0.0001, ***p = 0.003.
Both IL-4 and IL-7 have been shown to provide survival signals for resting T cells in vitro (17-21), and studies have shown IL-7 protects resting T cells from apoptotic death by regulating anti and pro-apoptotic Bcl-2 factors (6, 22). Next we cultured resting p47phox-/- and WT lymphocytes in IL-7 to determine whether IL-7 could rescue dying CD8+ p47phox-/- lymphocytes. At baseline the percentage of viable p47phox-/- and WT CD8+ cells were comparable. Both WT and p47phox-/- CD8+ lymphocytes cultured in IL-7 for 24 hours showed significantly enhanced survival over cells cultured in IMDM alone or with IL-2 (Figure 2B), and p47phox-/- CD8+ lymphocytes cultured in IL-7 contained significantly fewer dead cells than lymphocytes cultured in IL-2 (Figure 2C). However, p47phox-/- CD8+ lymphocytes die at an accelerated rate in vitro even when cultured in IL-7, which prevents WT CD8+ cell death.
We previously reported TcR stimulated T cell blasts release a prolonged pulse of NADPH oxidase dependent hydrogen peroxide (H2O2) (10). Consequently, to assess whether the survival defect in p47phox-/- CD8+ lymphocytes could be rescued by exogenous H2O2, CD8+ cells from p47phox-/- and WT mice were incubated with the H2O2 generating enzyme glucose oxidase (GO) for 24 hours. GO is a flavoenzyme that catalyses the conversion of β-D-glucose to H2O2 and produces a continuous pulse of H2O2 (23, 24). The combination of GO (1 pg/ml) and IL-7 enhanced p47phox-/- lymphocyte survival 10% over that seen with IL-7 alone (Figure 2D, Supplemental Figure 2). While 10 ng/ml GO was toxic to p47phox-/- and WT CD8+ cells (data not shown), 1 pg/ml GO was not toxic and did not alter WT lymphocyte survival. Using lower concentrations of GO (see methods) did not improve p47phox-/- lymphocyte survival. These results indicate that exogenous replacement of intracellular H2O2 by enzymatic manipulation further enhances IL-7 mediated rescue of p47phox-/- CD8+ lymphocytes.
Pro-survival cytokines IL-2, IL-4, IL-7 and IL-15 are members of the IL-2 family of cytokines that share the common γc receptor subunit (25), and Park et al showed these cytokines suppress IL-7 receptor (IL-7R) transcription and expression in resting T cells (26). In order to determine p47phox-/- CD8 lymphocyte responsiveness to extrinsic cytokine stimulation, we examined cytokine regulated IL-7R expression. Freshly isolated resting WT and p47phox-/- cells express comparable levels of surface IL-7R. Similarly, viable WT and p47phox-/- cells down regulated IL-7R after overnight culture in IL-7, but retain surface IL-7R after overnight culture in IL-2 (Supplemental Figure 3). Collectively, this data indicates that while cytokine activated p47phox-/- CD8+ lymphocytes undergo an exaggerated postactivation cell death in vitro, IL-2 and IL-7 triggered common γc receptor pathways in p47phox-/- cells are responsive. In addition, the enhanced rescue of p47phox-/- CD8+ lymphocytes by the combination of IL-7 and GO suggests NADPH oxidase p47phox and/or NADPH oxidase mediated ROS may have a preferential role in regulating CD8+ lymphocyte survival.
Resting naïve p47phox-/- CD8 lymphocytes undergo a rapid and profound apoptotic death
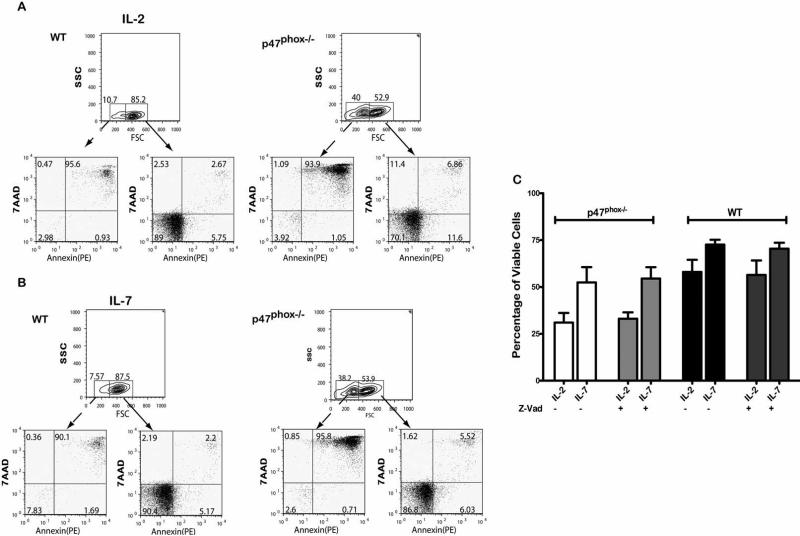
In order to clarify the basis for the exaggerated p47phox-/- CD8+ cell death we used flow cytometry to assess directly whether there was a difference in p47phox-/- CD8+ lymphocyte apoptosis. The forward and side scatter (FSC/SSC) properties of p47phox-/- cells cultured with IL-2 or IL-7 for 4 hours (Figure 3) and 24 hours (data not shown) showed significantly fewer p47phox-/- than WT CD8+ cells were viable and that the majority of the cells outside of the viable gate were in late apoptosis (Annexin+, 7AAD+). Among the cells in the viable gate (solid line arrows) nearly twice as many p47phox-/- as WT cells cultured in IL-2 were in early (Annexin+, 7AAD-) and late (Annexin+, 7AAD+) apoptosis. For cells cultured in IL-7, twice as many p47phox-/- as WT cells were in late apoptosis. Consistent with the viability data in Figure 2, p47phox-/- viable : late apoptotic cell ratios at 4 versus 24 hours revealed more p47phox-/- cells continue to die in IL-2 than in IL-7 (1.4± 0.07 vs 0.44 ± 0.16 p = 0.02 for IL-2, and 1.5 ± 0.13 vs 1± 0.21 for IL-7).
Figure 3.
Apoptosis of cytokine activated CD8+ lymphocytes. Fractionated CD8+ cells were cultured in IMDM supplemented with IL-2 or IL-7. At 3 hours cells were removed from the cultures, stained for surface CD8 and Annexin (x-axis), and 7AAD (y-axis) and assessed by flow cytometry. The dot plots are gated on the CD8+ cell population. The dot plots show the WT (top) and p47phox-/- (bottom) Annexin (x-axis) vs 7AAD (y-axis) staining for cells cultured in IL-2 (A) or Il-7 (B). One representative experiment of 4 is shown. The data shows the responses of 3-4 mice/experiment. (C) Fractionated CD8+ p47phox-/-and WT lymphocytes were cultured in IMDM supplemented with IL-2 or IL-7, with z-vad as indicated for 24 hours. The total number of viable and non-viable nucleated CD8+ cells was measure using the Guava ViaCount assay, and responses were normalized to the baseline values. The percentage of viable p47phox-/- and WT cells at 24 hours (right). The data shown are the mean +/- SEM for 4 independent experiments with 3 mice mice/experiment. *p = 0.01, **p < 0.0001.
We also activated p47phox-/- and WT CD8+ lymphocytes in the presence of the soluble pan caspase inhibitor Z-VAD-FMK, and found there was no difference in the survival of p47phox-/ CD8+ lymphocytes after 24 hours (Figure 3C). Collectively, this data indicates p47phox-/- CD8+ lymphocytes undergo a rapid and profound apoptosis, and the exaggerated in vitro p47phox-/ CD8+ lymphocyte cell death is triggered upstream of caspase mediated phenomenon.
Impaired survival of p47phox-/- CD8+ cells is due to an intrinsic apoptotic pathway defect
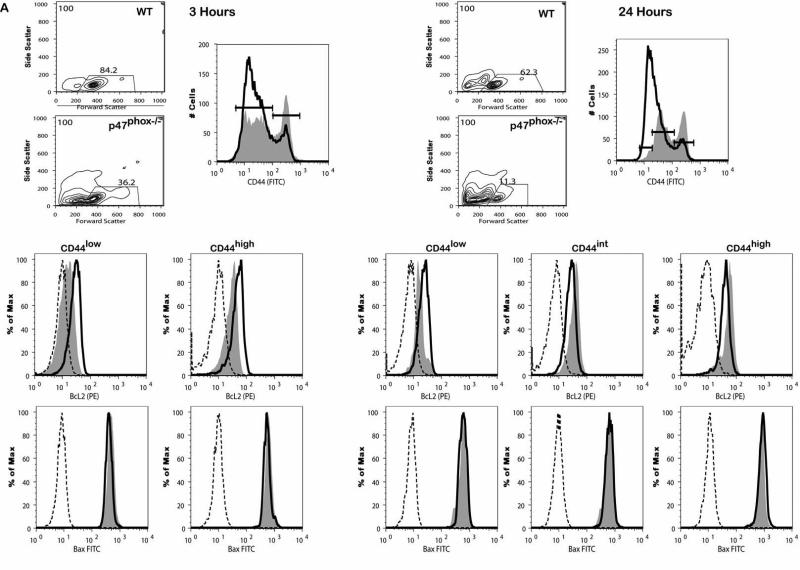
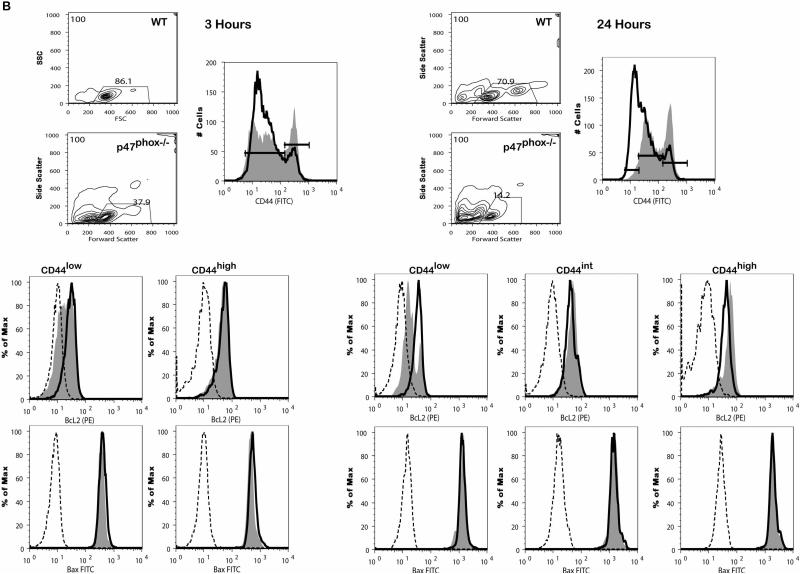
When purified resting naïve mouse T cells are placed into in vitro culture in the absence of required survival signals, intrinsic apoptotic pathways are initiated and T cells die rapidly due to the loss of control of mitochondrial homeostasis (27, 28). We cultured resting CD8+ lymphocytes from WT and p47phox-/- mice as described and examined anti-apoptotic Bcl-2 and pro-apoptotic Bax protein expression along with mitochondrial transmembrane potential (ΔΨm) and apoptosis in order to determine whether the exaggerated p47phox-/- CD8+ lymphocyte apoptosis was due to aberrant intrinsic apoptotic pathway function. For these investigations, we performed a multiparametric vitality and apoptosis study using the cyanine dye 1,1',3,3,3',3'-hexamethylindodicarbo-cyanine iodide (DiIc1(5)) to assess ΔΨm in combination with Annexin-V and 7-AAD to asses apoptosis. In addition, we used surface staining for CD44 to distinguish survival parameters of naïve (CD44low) verse activated (CD44high) CD8+ cells. Consistent with the apoptosis data in Figure 2, FSC/SSC properties of p47phox-/- cells after 3-4 hours of culture showed appreciably fewer p47phox-/- CD8 cells were viable (Figure 4). Additionally, as shown in histogram overlays of WT (bold lined histograms) and p47phox-/- (shaded filled histograms) CD8+ lymphocytes we also observed a dramatic and rapid shift of the p47phox-/- CD8+ lymphocyte CD44 profile. Although the baseline CD44 WT and p47phox-/- CD8+ lymphocyte profiles were essentially identical after just 3 hours in culture, approximately 1/3 of p47phox-/- CD8+ cells are CD44high suggesting that during in vitro culture there is a preferential loss of naive CD44low p47phox-/- CD8+ lymphocytes. Further, after 24 hours we found that among the remaining viable p47phox-/- CD8+ lymphocytes the majority of the cells were CD44moderate-high in each of the culture conditions.
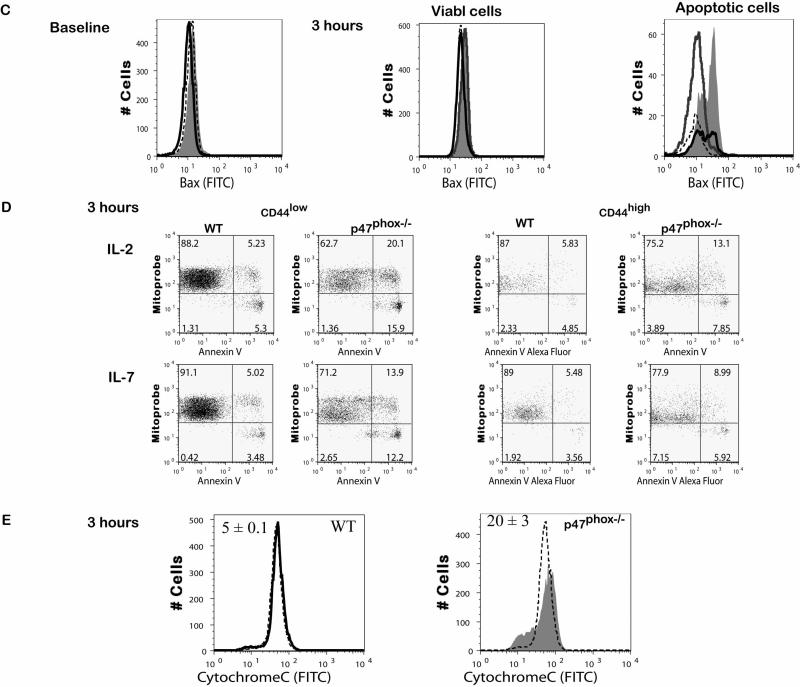
Figure 4.
Intrinsic apoptotic pathway defect in cytokine activated p47phox-/- CD8+ lymphocytes. Fractionated CD8+ p47phox-/- and WT lymphocytes were cultured in IMDM supplemented with IL-2 (A) or IL-7 (B) for 24 hours. (A) At 3 hours (right) and 24 hours (left) cells were removed from the cultures, stained for surface CD8 and CD44, fixed, permeabilized and stained with anti-Bcl-2 or anti-Bax as indicated. The contour plots show the forward scatter verses side scatter cell phenotype and the analysis gates. The adjacent histograms show the flow cytometry phenotype of the CD8+CD44+ p47phox-/- (filled tinted histograms) and WT (bold lined histograms) cells and the isotype control staining for p47phox-/-(dotted line histogram) and WT (dashed line histogram) cells. The gates on the CD44 histograms correspond to the CD44low, CD44intermediate and CD44high populations used to generate the histograms showing the anti-Bcl-2 or anti-Bax staining for p47phox-/- (filled tinted histograms) and WT (bold lined histograms) cells and the isotype control staining for p47phox-/-(dotted line histogram) and WT (dashed line histogram) cells. One representative experiment of 3 is shown. The data shows the responses of 3 mice/experiment. (C) Proapoptotic conformational change in Bax. Fractionated CD8+ p47phox-/- and WT lymphocytes were cultured in IMDM supplemented with IL-2 for 3 hours then fixed, permeabilized and stained with conformation-specific anti-Bax (6A7) antibody as indicated. One of 2 representative experiments is shown. (D) Fractionated CD8+ p47phox-/- and WT lymphocytes were cultured in IMDM supplemented with IL-2 or IL-7 as indicated. At 3 hours cells were removed from the cultures, stained for surface CD8 and CD44, and Annexin, PI and Mitoprobe. The dot plots are gated on the CD8+ cell population. The dot plots show the CD44low (left) versus CD44high (right) populations of WT (top) and p47phox-/- (bottom) Mitoprobe (y-axis) vs PI (x-axis) and Mitoprobe (y-axis) vs annexin (x-axis) staining for cells cultured in IL-2 or Il-7 as indicated. One representative experiment of 3 is shown. (E) Cytochrome c release in p47phox-/- CD8+ lymphocytes at baseline (dashed line histograms) and 3 hours (WT: bold line histogram, p47phox-/-: filled tinted histogram) of culture with IL-2 as indicated. Fractionated CD8+ p47phox-/- and WT lymphocytes were cultured in IMDM supplemented with IL-2 for 3 hours then permeabilized with digitonin, fixed, immunostained with an anti-cytochrome c antibody. One of 3 representative experiments is shown. Numbers indicate percentage of cells (mean ± SEM) with low fluorescence (cytochrome c release) for the 3 replicates. The data shows the responses of 3-4 mice/experiment.
Baseline Bcl-2 expression for WT and p47phox-/- CD8+ cells were essentially identical (Supplemental Figure 4), however Bcl-2 expression was rapidly and dramatically reduced in CD44low and CD44high p47phox-/- CD8+ lymphocytes compared to WT lymphocytes cultured in IL-2 for 3 hours (Figure 4A). After 24 hours Bcl-2 expression in CD44moderate and CD44high p47phox-/- cells was comparable to WT CD8+ lymphocytes. For cells cultured in IL-7 (Figure 4B) we found Bcl-2 was rapidly and dramatically reduced in CD44low p47phox-/- CD8+ lymphocytes compared to WT CD8+ lymphocytes after 3 hours. However, Bcl-2 was comparable in WT and p47phox-/- CD44high CD8+ lymphocytes at 3 hours. After 24 hours, we found a small fraction of CD44low p47phox-/- cells cultured with IL-7 retained Bcl-2, and as with IL-2 cultured cells, Bcl-2 expression in CD44moderate and CD44high p47phox-/- cells was comparable to WT. Bax expression in p47phox-/- CD8+ lymphocytes was comparable to WT lymphocytes cultured in IL-2 and in IL-7 for each characterized CD44 population. Additionally, Bax undergoes proapoptotic conformational changes in both WT and p47phox-/- apoptotic CD8+ lymphocytes (Figure 4C). These results indicate the rapid loss of Bcl-2, preferentially among naïve CD44low cells, contributes to the profound death of p47phox-/- CD8+ lymphocytes in vitro. Furthermore, the ability of IL-7 to partially rescue p47phox-/- CD8+ lymphocytes from an exaggerated cell death in vitro, is due in part to its regulation of Bcl-2 protein levels especially among viable CD44high cells during the initial 3 hours of culture.
Finally when we examined cells for ΔΨm we observed that as rapidly as 3 hours fewer CD44low and CD44high p47phox-/- CD8+ lymphocytes than WT retained the cyanine iodide dye (MtP+Ann-,(27)), indicating significantly more mitochondrial outer membrane permeabilization (MOMP, Figure 4D, Table I). We also found that compared to IL-7 cultured cells significantly more CD44low p47phox-/- lymphocytes cultured with IL-2 showed reduced ΔΨm (Table I). In additional experiments we confirmed that in addition to the loss of ΔΨm dying p47phox-/- CD8+ lymphocytes also release mitochondrial cytochrome c (Figure 4E). For these analyses we used a quantitative flow cytometric assay to differentiate cells with intact mitochondria (high fluorescence) from cells that had released mitochondrial cytochrome c (low fluorescence) (29, 30). As indicated in the histograms in Figure 4E the majority of WT lymphocytes had intact mitochondria at baseline and after culture in IL-2. However, while the majority of p47phox-/- lymphocytes also had intact mitochondria at baseline after 3 hours in culture cytochrome c was released in the apoptotic p47phox-/- cells (21% ± 3 p47phox-/- vs 5 ± 0.1 WT, p = 0.005). Collectively these observations implicate a role for NADPH oxidase p47phox as an upstream effector of the mitochondrial apoptosis pathway via regulation of the anti-apoptotic protein Bcl-2.
Table 1.
Mitochondrial outer membrane permeabilization (MOMP) of cytokine stimulated CD8+ lymphocytes.
| CD44low | CD44high | |||||||
|---|---|---|---|---|---|---|---|---|
| Live (MtP+Ann-) | Apoptotic (MtP+Ann+) | Live (MtP+Ann-) | Apoptotic (MtP+Ann+) | |||||
| IL-2 | IL-7 | IL-2 | IL-7 | IL-2 | IL-7 | IL-2 | IL-7 | |
| p47phox-/- | 38 +/- 14 | 45 +/- 13 | 46 +/- 11 | 40 +/- 11 | 55 +/- 7.7 | 60 +/- 6.7 | 30 +/- 5.8 | 24 +/- 5 |
| Wild type | 76 +/- 8.6 | 78 +/- 9.7 | 16 +/- 6.8 | 17 +/- 7.5 | 79 +/- 4.5 | 83 +/- 3.5 | 10 +/- 2.6 | 12 +/- 2.7 |
Survival of p47phox-/- CD8+ cells is dependent upon the lymphoid microenvironment
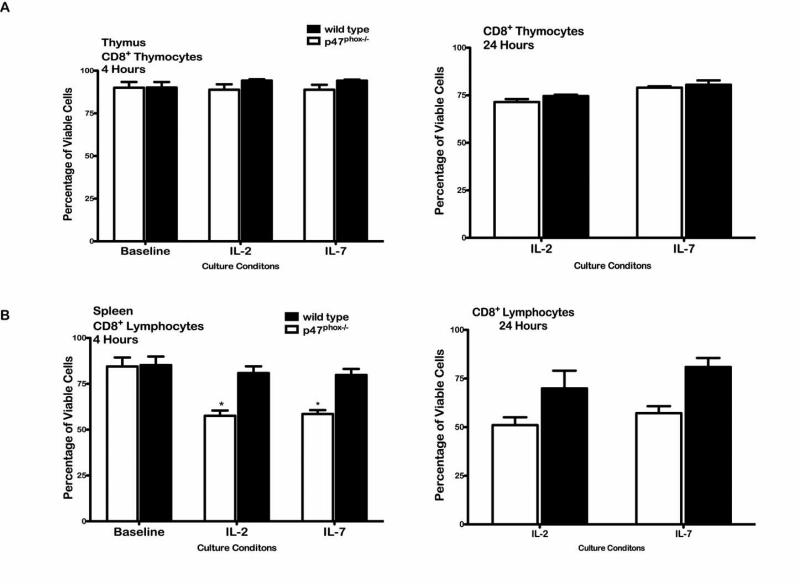
T lymphocyte homeostasis is tightly controlled by multiple regulatory checkpoints that restrain T lymphocyte differentiation within the thymus and expansion in the periphery. Post-thymic export resting naïve T cells are found concentrated in secondary lymphoid organs (SLOs). However, resting naïve T cells are not stationary but constantly traffic between SLOs in search of antigen thus providing an effective surveillance system within lymphoid organs. We compared survival of thymic, spleen and LN derived resting naïve p47phox-/- CD8+ lymphocytes to discern whether the observed survival defect could be linked to the NADPH oxidase p47phox-/- physiologic microenvironment (Figure 5). We found single positive p47phox-/- CD8+ thymocytes survived as well as WT thymocytes while significantly more splenic resting p47phox-/- CD8+ lymphocytes rapidly succumb during the initial 3-4 hours of in vitro culture in IL-2 or IL-7 similar to LN (Figure 2) p47phox-/- CD8+ lymphocytes. However, unlike LN p47phox-/- CD8+ lymphocytes the majority of the remaining splenic p47phox-/- CD8+ lymphocytes remained viable after 24 hours of culture in IL-2 or IL-7. Additionally, there was no difference in the in vitro survival of splenic p47phox-/- CD8+ lymphocytes cultured with IL-2 or IL-7 after 24 hours. Consequently, post-thymic survival of resting naïve p47phox-/- CD8+ lymphocytes is highly variable and significantly impacted by NADPH oxidase p47phox-/- SLO microenvironmental factors.
Figure 5.
In vitro cytokine activated thymocyte and splenic lymphocyte survival. Fractionated CD8+ p47phox-/-and WT single positive thymocytes or splenic lymphocytes were cultured in IMDM supplemented with IL-2 or IL-7 as indicated for 24 hours. The total number of viable and non-viable nucleated CD8+ cells was measure using the Guava ViaCount assay, and responses were normalized to the baseline values. p47phox-/- responses are indicated by the open histograms and WT responses are indicated by the filled histograms. (A) The percentage of viable CD8+ p47phox-/- and WT thymocytes after at 4 (left) and 24 (right) hours in culture. (B) The percentage of viable CD8+ splenic p47phox-/- and WT lymphocytes after at 4 (left) and 24 (right) hours in culture. The data shown are the mean +/- SEM for 3 independent experiments with 3-4 mice mice/experiment. *p < 0.0001, **p < 0.02.
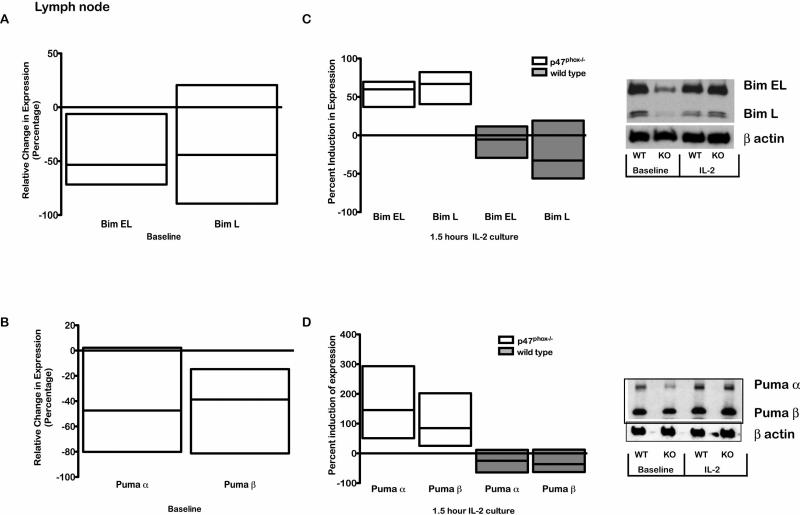
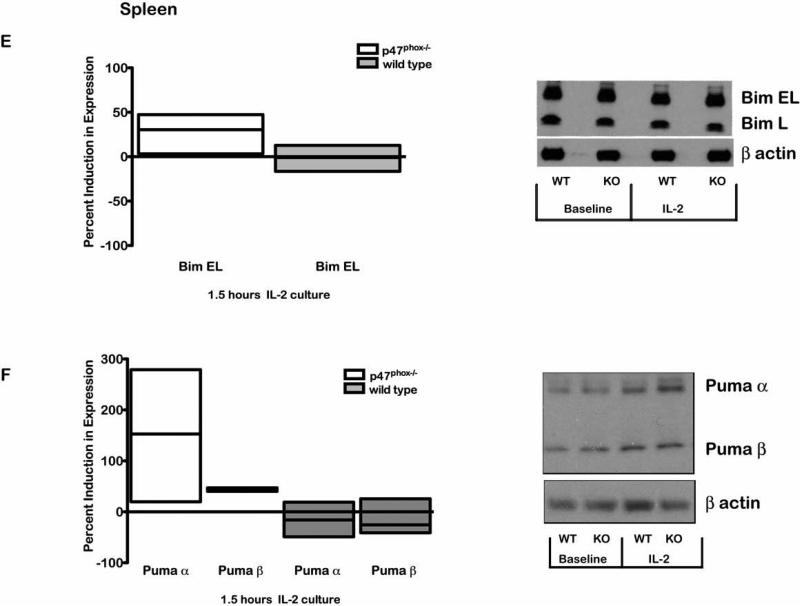
While WT and p47phox-/- LN CD8+ lymphocytes have comparable baseline Bcl-2 and Bax expression, Bcl-2 rapidly declines in p47phox-/- CD8+ lymphocytes in vitro while Bax remains stable. We also found that anti-apoptotic Mcl-1 expression was comparable in resting naïve WT and p47phox-/- LN CD8+ lymphocytes at baseline and after in vitro culture (Data not shown). The observed dysregulation of the intrinsic pathway in vitro lead us to question whether BH3-only proteins that regulate Bcl-2 (31); maybe aberrant in p47phox-/- CD8+ lymphocytes. We found baseline Puma and Bim EL expression were significantly reduced in LN p47phox-/- CD8+ lymphocytes relative to WT lymphocytes. However, after 2 hours of in vitro culture, LN p47phox-/- CD8+ lymphocytes expressed significantly more Bim EL and Bim L as well as Puma (Figure 6). For spleen, WT and p47phox-/- CD8+ lymphocytes showed comparable Bim and Puma expression at baseline (Data not shown). However, similar to LN, Bim EL and Puma were significantly induced in vitro in splenic p47phox-/- CD8+ lymphocytes compared to WT (Figure 6). These results suggest resting naïve p47phox-/- CD8+ within p47phox-/- SLOs are signaled to suppress pro-apoptotic protein expression.
Figure 6.
Secondary lymphoid organ factors alter BH3-only Bim and Puma expression in p47phox-/- CD8+ lymphocytes. Fractionated CD8+ p47phox-/-and WT lymphocytes were cultured in IMDM supplemented with IL-2 for 1.5 hours. Baseline and 1.5 hour western analysis for Bim or Puma expression was performed as described in the methods. Bars show the quantitative analysis (mean ± sem) of lymph node and spleen lymphocyte protein expression. The percentage change in LN p47phox-/-CD8+ lymphocyte Bim (A) or Puma (B) expression compared to WT CD8+ lymphocyte protein expression. The percentage induction in Bim (C) or Puma (D) expression in lymph node p47phox-/- CD8+ lymphocytes at 1.5 hours of culture compared to baseline protein expression. The percentage induction in Bim (E) or Puma (F) expression in splenic p47phox-/ CD8+ lymphocytes at 1.5 hours of culture compared to baseline protein expression. The data shown are the mean +/- SEM for 3 independent experiments with 3-4 mice mice/experiment. Representative western blot for Bim or Puma expression in lymph node and spleen are shown adjacent to the bar graphs.
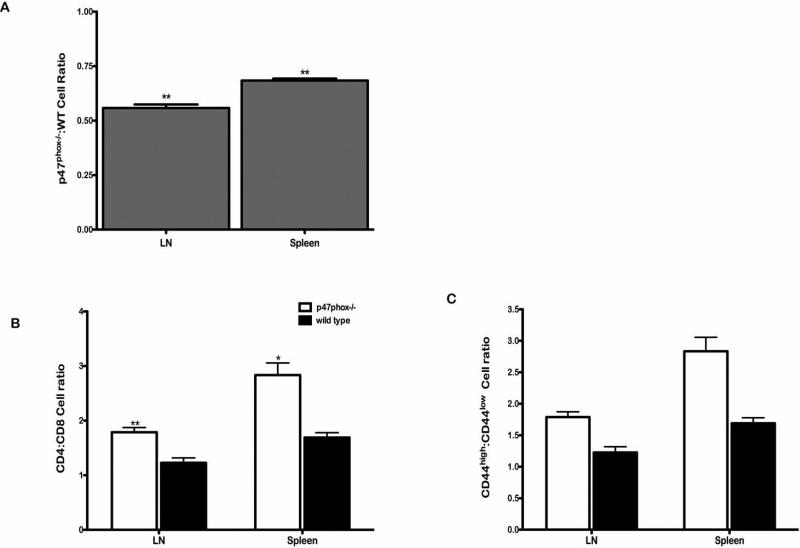
In order to assess the fate of p47phox-/- T cells within normal SLO microenvironments we adoptively transferred equal numbers of resting WT and p47phox-/- splenocytes into a WT recipient. Similar to what we observed in vitro the ratio of p47phox-/-: WT lymphocytes recovered from the LNs and spleens of recipient mice 24 hours post adoptive transfer indicates a profound loss of p47phox-/- cells (Figure 7). We also observed a greater loss of p47phox-/- CD8+ cells than CD4+ cells and found that there was a greater loss of naïve p47phox-/- CD44lowCD8+ lymphocytes than CD44high lymphocytes in vivo.
Figure 7.
Unrestrained death of p47phox-/- lymphocyte in WT secondary lymphoid organs. Single cell suspensions of resting LN cells from WT and p47phox-/- mice were generated and pooled. 10 × 106 LN cells were transferred into Ly5.1 congenic recipient mice (n = 3). After 24 hours the recipients we sacrificed, lymph nodes and spleen were harvested and stained for surface markers. Cells were gated on CD45.2+ CD45.1-, and then analyzed for CD4, CD8 and CD44 staining amongst WT (Thy1.1+, Thy1.2-) or p47phox-/- (Thy1.2+, Thy1.1-) donors T cells in the (A) LNs and (B) spleens The results show the cell ratios for p47phox-/-:WT donor cells (gray histogram), and the CD4:CD8 and CD8+ CD44high:CD44low for p47phox-/- or WT cells as indicated.
In summary, these observations implicate a role for NADPH oxidase p47phox as an upstream effector of the mitochondrial apoptosis pathway via regulation of Bcl-2 protein family members. Furthermore, the increase death of adoptively transferred p47phox-/- CD8+ lymphocytes within WT mouse SLOs suggest signals generated within the complex NADPH oxidase p47phox-/- SLO microenvironment restrain CD8+ lymphocyte apoptosis.
Discussion
Previous observations revealed T cells express each of the NADPH oxidase structural proteins; pg91phox, p22phox, p47phox and p67phox and that TcR stimulation induces low level intracellular NADPH oxidase dependent H2O2 (10). These observations suggested a role for NADPH oxidase mediated ROS in T cell function. We report post-thymic resting naïve p47phox-/- CD8+ cells exhibit an intrinsic survival defect. We link the defect to higher levels of intrinsic apoptotic pathway activity in p47phox-/- CD8+ lymphocytes in vitro and showed naïve CD44low p47phox-/- lymphocytes have lower Bcl-2 levels that are only partially rescued by pro-survival cytokine IL-7. We also found in vitro p47phox-/- CD8+ lymphocyte survival could be distinguished by BH3-only Bim and Puma protein expression. While Bim and Puma were significantly reduced in isolated LN p47phox-/- CD8+ lymphocytes, protein expression in splenic p47phox-/- CD8+ lymphocytes was comparable to WT lymphocytes. Interestingly, both proteins were induced in vitro in LN and splenic p47phox-/- CD8+ lymphocytes indicating enhanced Bim and Puma expression regulates the rapid in vitro death of p47phox-/- cells.
Supplementing cultures with exogenous IL-7 in vitro normally regulates cellular Bcl-2 proteins to maintain equilibrium of anti and pro-apoptotic proteins. However, even with the addition of IL-7 leading to a demonstrative reduction in resting p47phox-/- lymphocyte death after 24 hours in culture the overall survival of p47phox-/- lymphocytes was only 61%, p < 0.0001, of WT cells. Thus, indicating that additional and as yet defined signaling events may be aberrant in p47phox-/- lymphocytes. Based on our observation that NADPH oxidase dependent H2O2 is differentially regulated in activated T cell blasts (10), we asked whether the aberrant death of p47phox-/- CD8+ cells was due to a H2O2 deficiency. To avoid the potential of causing oxidative stress in our in vitro culture mode (32), we used glucose oxidase (GO) rather than a H2O2 bolus to restore intracellular H2O2 in p47phox-/- cells. However, one of the inherent limitations of this in vitro experimental design is that we cannot control the timing of H2O2 generation. Subsequently, it is unclear whether the partial rescue of p47phox-/- CD8+ cells relative to WT cells is due to a disparity in the timing of H2O2 delivery. Finding the optimal survival of p47phox-/- CD8+ cells only occurred when both GO and IL-7 were used indicates neither is sufficient to support resting p47phox-/- CD8+ cell survival. However, finding that low-level H2O2 enhances IL-7 mediated p47phox-/- CD8+cell survival suggests an optimum physiologic H2O2 concentration released at the appropriate time during cytokine stimulation maybe a previously unrecognized pivotal, albeit partial, signal for lymphocyte survival. We plan to examine these questions in future investigations.
Immune responses are mounted within SLOs to eradicate invading pathogens. However, impaired pathogen clearance in immunocompromised host often results in sustained immune responses. Furthermore, the persistent immune challenge experienced by immunocompromised host undermines the need to maintain a consistent level of naïve T cells capable of regulating homeostasis. Our findings putatively implicate a role for dynamic physiologic cues generated within reactive SLO microenvironments that balance and modulate the transition to effector cells without exhausting naïve CD8+ lymphocytes. We propose NADPH oxidase p47phox and/or NADPH oxidase mediated ROS is a regulator of these adaptive immune phenomenon. The mechanisms appear to involve the regulated suppression of pro-survival members of the Bcl-2 protein family that protect post-thymic naïve CD8+ lymphocytes from Bim and Puma driven apoptosis as well as via mechanisms associated with removal of in vivo suppressive factor(s) that allow enhanced apoptotic BH3-only protein expression in vitro. This indicates that characterization of molecular pathways that are controlled by NADPH oxidase p47phox and/or NADPH oxidase mediated ROS may discern previously unrecognized targets for the development of immunomodulatory therapies.
Methods
Mice
p47phox deficient mice have been described (13). Congenic p47phox deficient mice are on a C57BL/6NTac background. Wild-type control mice (C57BL/6NTac) were obtained from Taconic Farms (Hudson, NY). Animal care was provided in accordance with Institutional Animal Care and Use Committee procedures approved by NIAID/NIH. NADPH oxidase-deficient mice were housed in aseptic conditions. Tissues showing gross evidence of infection were discarded and not used for the investigations reported.
Cell Isolation
Lymph node single-cell suspensions were prepared from peripheral mouse LNs or thymus. CD4+ and CD8+ T cells were negatively selected using the Dynal bead mouse CD4 or CD8 negative isolation kit (Invitrogen Corp, Carlsbad, CA) according to the manufacturer's protocol. The purity of each cell population was >98% as determined by flow cytometry.
Cell Culture and Stimulation
Lymph node purified CD4 or CD8 T lymphocytes were cultured at a concentration of 1 × 106 cell/ml, as indicated, were cultured in IMDM complete: IMDM (Gibco/ Invitrogen Corp) containing 10% FBS (Hyclone Laboratories, Logan, UT), 2.0 mM L-glutamine (Hyclone), 50 μM β-mercaptoethanol (βME, Sigma Aldrich, St. Louis, MO), and 100 units/ml of penicillin and streptomycin 10 units/mL (Gibco/ Invitrogen Corp) supplemented with 10-units/mL rhIL-2 (Rohmann-LaRoche Inc, Nutley, NJ), or 100 ng/mL IL-7 (PeproTech, Rocky Hill, NJ). For initial experiments, cells were cultured using a range of rhIL-2 from 10 - 100 units/ml. There was no difference in the relative cell viability differences between wild type and p47phox-/- cells using 100 units/ml of IL-2, subsequently for later experiments the cells were cultured using 10 units/ml IL-2.
Caspase Inhibition
Lymph node purified CD8+ T cells were cultured in IMDM complete and supplemented with 10 units/mL rhIL-2 or 100 ng/mL IL-7, and treated with 50 μM Z-VAD (OMe)-FMK (EMD Chemicals, San Diego, CA) or 10 μg/mL.
Cell Counts and Viability
Resting CD4+ or CD8+ LN cells were incubated with IL-2 or IL-7 as indicated, and cell count and viability were determined. Viable and non-viable lymphocytes from cultures were differentially stained with DNA binding dyes and the absolute number of viable versus non-viable nucleated cells were quantitated based on cell size and staining properties using the Guava ViaCount assay. Cells were diluted with the Guava Viacount (Guava Technologies, Hayward, CA) reagent in a total volume of 400 μL at the indicated time intervals. Cell counts, and viable and dead cell numbers were determined using Guava ViaCount Software on the Guava PCA (Guava Technologies).
Antibodies, Immunofluorescent Staining and Flow Cytometry
Fluorescein isothiocyanate (FITC) conjugated monoclonal antibodies (mAB) against CD11b (Mac-1), Gr-1, B220, CD44, CD25, (BD Pharmingen,); R-phycoerythrin (PE) conjugated mAB against B220, CD27, CD62L, CD69, CD127 (IL-7R), (BD Pharmingen); Allophycocyanin (APC) conjugated CD11b, CD3, CD4, CD8 were used. Cells were stained with the designated FITC (1:250), APC (1:250), or PE (1:500) conjugated mAb or subclass control for 25 minutes at room temperature in 1-2% FBSHBSS containing purified rat anti-mouse CD16/CD32 Fc Block (BD Pharmingen) to minimize nonspecific binding. For analysis all cells were resuspended in 1-2% FBS-HBSS containing propidium iodide (50 ng/ml, Invitrogen) to distinguish viable cells. Cell acquisition was performed on the Becton Dickinson FACSort or FACSCalibur (BD Biosciences, San Jose, CA) using CellQuest Pro software (BD Biosciences). Analysis was performed using FlowJo software (FlowJo, LCC, Ashland, OR).
Adoptive transfer
Single cell suspensions were made from LNs from p47phox-/- mice (CD45.2 Thy 1.2) and Thy1.1+ wild type (WT) C57/BL6 mice. Ten million of each were injected IV into CD45.1 WT C57/BL6 recipients. LNs and spleen were harvested from recipients at 24 hours and stained for surface markers CD45.1 PE, Thy.1 PerCp, CD44 FITC (BD Pharmingen), CD4 Pacific Blue, CD8 Alexa Flour 700, CD45.2 Alexa Flour 750, Thy1.2 Pe-Cy5 (eBioscience, San Diego, CA). Cells were gated on CD45.2+ CD45.1-, and then analyzed for CD4, CD8 and CD44 staining amongst WT (Thy1.1+, Thy1.2-) or KO (Thy1.2+, Thy1.1-) donors. Cell acquisition was performed on the Becton Dickinson LSR II (BD Biosciences) using CellQuest Pro software (BD Biosciences). Analysis was performed using FlowJo software (FlowJo, LCC, Ashland, OR).
Brd U Stimulation
p47phox-/- and wild type mice were fed 5-bromo-2'deoxyuridine (BrdU, BD Pharmingen) in their drinking water at a concentration of 1 mg/ml for 5 days. LNs were harvested on day 5. Whole lymph node cell populations were stained with anti-CD4, CD8, CD44, B220 and BrdU antibodies (BD Pharmingen). Cell acquisition was performed on the Becton Dickinson LSRII (BD Biosciences, San Jose, CA) using CellQuest Pro software (BD Biosciences). Analysis was performed using FlowJo software (FlowJo, LCC, Ashland, OR).
Bcl-2 and Bax Staining
Cells were washed twice in 1% FBS/PBS and surface stained for CD8 (1:500) and CD44 (1:125-500) as indicated. Cells were fixed according to the Cytofix/Cytoperm Plus Kit (BD Pharmingen) manufacturer's protocol. Cells were stained for intracellular Bcl-2 (BD Pharmingen) or Bax (Santa Cruz, Santa Cruz, CA) according to the manufacturer's protocol. Conformational change in the Bax protein was assessed by intracellular immunostaining using a specific antibody that recognizes only the proapoptotic conformation: anti-Bax (6A7; BD Pharmingen) using the method described by Gómez-Benito et al (29). Briefly, cells were cultured with IL-2 supplemented medium for 3 hours. Then, 1 × 106 cells were fixed with 0.5% paraformaldehyde in PBS for15 minutes at 4°C and incubated for 25 minutes at room temperature with 0.5 μg of anti-Bax or an mouse IgG isotype control (BD Pharmingen) in 100 μl of PBS containing 0.1% saponin and 5% goat serum. Cells were washed with 0.03% saponin in PBS and incubated with a FITC-labeled anti-mouse IgG antibody (BD Pharmingen) at room temperature. Cell acquisition was performed on the Becton Dickinson FACSCalibur (BD Biosciences, San Jose, CA) using CellQuest Pro software (BD Biosciences). Analysis was performed using FlowJo software (FlowJo, LCC, Ashland, OR).
Cytochrome C release
Quantitative analysis of cytochrome c release from mitochondria was assessed by a modification of the method described by Waterhouse and Trapani (29, 30). Briefly, cells were cultured with IL-2 supplemented medium for 3 hours. Then, 1 × 106 cells were permeabilized with 100 μl of digitonin (50 μg/ml in PBS containing 100 mM KCl) for 5 minutes on ice, fixed in 4% paraformaldehyde in PBS for 20 minutes at room temperature, and resuspended in 3% bovine serum albumin, 0.05% saponin in PBS (blocking buffer). Cells were incubated overnight at 4°C with a 1:500 dilution of anti-cytochrome c antibody (InnoCyte™ Flow Cytometric Cytochrome c Release Kit, Calbiochem, EMD Chemicals, Inc., Gibbstown, NJ) in blocking buffer, washed, and then incubated with a 1:300 dilution of FITC-labeled anti-mouse IgG antibody (InnoCyte™ Flow Cytometric Cytochrome c Release Kit, Calbiochem). Control cells were stained with a 1:300 dilution of the FITC-labeled anti-mouse IgG antibody in the absence of a primary antibody. Cell acquisition was performed on the Becton Dickinson FACSCalibur (BD Biosciences) using CellQuest Pro software (BD Biosciences). Analysis was performed using FlowJo software (FlowJo).
Cell Death Analysis
Lymph node purified CD8+ T cells were cultured as indicated. Cells were harvested at the designated time, washed with 1%FBS/PBS and stained for surface CD8 and CD44 (BD Pharmingen). Mitochondrial membrane integrity and cell death were determined using the Mitoprobe kit (Invitrogen) or the Annexin V: PE Apoptosis Detection Kit I (BD Pharmalgen) according to the manufactures protocols.
Glucose Oxidase Stimulation
Lymph node purified CD8+ T cells were cultured in IMDM complete without βME and supplemented with 10-units/mL rhIL-2 or 100 ng/mL IL-7. Cells were treated with the following range of glucose oxidase (GO, Sigma-Aldrich) log 10 concentration from 10 ng/ml – 1 pg/ml, and log2 concentrations from 0.3 - 1.2 pg/mL. Cells counts were determined at 24 hours using Guava ViaCount Software on the Guava PCA (Guava Technologies).
Cell fractionation and Western blot
After CD8 isolation cells were counted and re-suspended at 1 × 106 cells/mL in IMDM complete with 10 units/mL rhIL-2 (Rohmann-LaRoche Inc.) for 1.5 hours, then washed with cold PBS. Cells were lysed in ice-cold Mammalian Protein Extraction Reagent (Pierce Biotechnology, Rockford IL), 1X mammalian protease inhibitor (Sigma-Aldrich) and 5mM EDTA followed by sonication for 10 minutes. Insoluble material was removed by centrifugation at 12,000 g at 4°C for 10 minutes. Equivalent amounts of protein were denatured in Laemmli buffer for direct resolution by 10-20% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE), and transferred to a nitrocellulose membrane (Millipore Corporation, Billerica, MA). Immunoblotting was performed using anti-Puma Ab, anti-Bim Ab, anti-BMF Ab, (Cell Signaling Technology, Danvers, MA), anti-Mcl-1 Ab, (Abcam Inc., Cambridge, MA) anti-actin Ab (Sigma-Aldrich), HRP conjugated anti-rabbit Ab, and HRP conjugated anti-mouse Ab (GE Healthcare, Piscataway, NJ). Immunoreactive proteins were visualized using an ECL detection kit (GE Healthcare) upon exposure to BioMax™ light film (Kodak). For the relative quantification of the proteins scanned images were analyzed using ImageJ (33).
Statistical analysis
Means and standard error of the mean (SEM) for cell count and viability were determined. Differences between the group means were analyzed by the Student's t. (Prism 4, GraphPad Software, Inc. San Diego, CA).
Supplementary Material
Supplemental Figure 1. Ex vivo Flow cytometry immunophenotype of resting peripheral LN B cells from WT and p47phox-/- mice stained for surface B220-CD27. The dot plot is gated on the whole LN cell population from the p47phox-/- mice. The histograms are gated on B220+ cells. The histograms indicate WT (tinted histogram) and p47phox-/- (bold line histogram) cells, or isotype control (dashed line histogram) staining for surface CD27. One representative experiment of five is shown. (D) Ex vivo flow cytometry immunophenotype of resting peripheral LN cells from WT and p47phox-/- mice stained for surface CD4-CD8-CD44-CD62L or CD4-CD8-CD69-CD25. The dot plots are gated on the whole LN cell population. Surface staining for WT (top row) or p47phox-/- (bottom row) CD4+ cells (left) and CD8+ cells (right) are shown. One representative experiment of five is shown. The data shows the responses of 3-4 mice/experiment.
Supplemental Figure 2. Glucose-oxidase produced H2O2 enhances p47phox-/- CD8+ lymphocyte survival. Individual experimental results of enhanced cell viability in Figure 3D for resting p47phox-/- CD8+ lymphocytes cultured in IL-7 supplemented IMDM +/- 1 pg/ml GO.
Supplemental Figure 3. IL-2 and IL-7 triggered common γc receptor pathways in p47phox-/- cells are responsive. Fractionated CD8+ p47phox-/-and WT lymphocytes were cultured with IL-2 or IL-7 supplemented culture medium. At 24 hours cells were removed from the cultures and stained for surface CD8, CD44 and CD127 (IL-7R). Flow cytometry phenotype of CD8+ propidium iodide negative cells at baseline (A) and following overnight culture with IL-7 (B) or IL-2 (C). The histograms are gated on CD8+ propidium iodide negative cells. Histograms show the surface CD127 staining for p47phox-/- (filled tinted histogram) and WT (bold lined histogram) CD8+ lymphocytes and isotype control (thin lined histograms). One representative experiment of three is shown. The data shows the responses of 3-4 mice/experiment.
Supplemental Figure 4. (A) Resting LN cell Bcl-2 expression. LN cells were stained for surface CD8, fixed, permeabilized and stained with anti-Bcl-2. The histograms are gated on the CD8+ cells. Bcl-2 expression of CD8+p47phox-/- (filled tinted histograms) and wild type (bold lined histograms) cells and the isotype control staining for p47phox-/-(dotted line histogram) and wild type (dashed line histogram) cells is shown. (B) 3 hour CD44low, and CD44high overlaid histograms showing anti-Bcl-2 staining for p47phox-/- (filled tinted histograms) and wild type (bold lined histograms) cells and the isotype control staining for p47phox-/-(dotted line histogram) and wild type (dashed line histogram) cells. Histograms are plotted to indicate‘# Cells’ (the number of cells (y-axis) within each bin of the histogram). Images correspond to the histograms in Figure 7A and 7B as indicated.
Acknowledgement
We would like to thank Kevin Gardner and Richard Youle for helpful discussions and critique of an earlier draft of this manuscript. We also thank Harry Malech and Michael Davis for careful review of this manuscript.
Funding
This research was supported by the Division of Intramural Research of the National Institutes of Health/National Institute of Allergy and Infectious Diseases.
Abbreviations
- ROS
Reactive oxygen species
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate
- p47phox-/-
p47phox deficient
- H2O2
hydrogen peroxide
- CGD
Chronic Granulomatous Disease
- LNs
lymph nodes
- BrdU
5-bromo-2'deoxyuridine
- FSC/SSC
forward and side scatter
- GO
glucose oxidase
- ΔΨμ
mitochondrial transmembrane potential
- MOMP
mitochondrial outer membrane permeabilization
- SLOs
secondary lymphoid organs
References
- 1.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002 Apr;109(Suppl):S97–107. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]
- 2.Guimond M, Fry TJ, Mackall CL. Cytokine signals in T-cell homeostasis. J Immunother. 2005 Jul-Aug;28(4):289–294. doi: 10.1097/01.cji.0000165356.03924.e7. [DOI] [PubMed] [Google Scholar]
- 3.Van Parijs L, Abbas AK. Science. 5361. Vol. 280. New York, NY: Apr 10, 1998. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. pp. 243–248. [DOI] [PubMed] [Google Scholar]
- 4.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nature reviews. 2002 Jan;2(1):11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 5.Marrack P, Bender J, Hildeman D, Jordan M, Mitchell T, Murakami M, Sakamoto A, Schaefer BC, Swanson B, Kappler J. Homeostasis of alpha beta TCR+ T cells. Nat Immunol. 2000 Aug;1(2):107–111. doi: 10.1038/77778. [DOI] [PubMed] [Google Scholar]
- 6.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 7.Babior BM. The respiratory burst oxidase. Adv Enzymol Relat Areas Mol Biol. 1992;65:49–95. doi: 10.1002/9780470123119.ch2. [DOI] [PubMed] [Google Scholar]
- 8.Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004 Oct;122(4):277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 9.Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11(4):173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 10.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004 Aug;5(8):818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 11.Assari T. Medical immunology. Vol. 5. London, England: 2006. Chronic Granulomatous Disease; fundamental stages in our understanding of CGD. p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenzweig SD. Inflammatory Manifestations in Chronic Granulomatous Disease (CGD). J Clin Immunol. 2008 Jan 12; doi: 10.1007/s10875-007-9160-5. [DOI] [PubMed] [Google Scholar]
- 13.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995 Sep 1;182(3):751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildeman DA. Regulation of T-cell apoptosis by reactive oxygen species. Free Radic Biol Med. 2004 Jun 15;36(12):1496–1504. doi: 10.1016/j.freeradbiomed.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, Marrack PC. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999 Jun;10(6):735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 16.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 17.Vella A, Teague TK, Ihle J, Kappler J, Marrack P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J Exp Med. 1997 Jul 21;186(2):325–330. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Science. 5466. Vol. 288. New York, NY: Apr 28, 2000. Control of homeostasis of CD8+ memory T cells by opposing cytokines. pp. 675–678. [DOI] [PubMed] [Google Scholar]
- 20.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005 Jun 1;174(11):6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 21.Kishimoto H, Sprent J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4+ T cells. J Immunol. 1999 Aug 15;163(4):1817–1826. [PubMed] [Google Scholar]
- 22.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine & growth factor reviews. 2005 Aug-Oct;16(4-5):513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Bentley R. The Enzymes. 2nd ed. Academic Press; New York: 1963. [Google Scholar]
- 24.Bentley R. Methods in Enzymology. Vol. 9. Academic Press; New York: 1966. [Google Scholar]
- 25.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev. 2004 Dec;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 26.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004 Aug;21(2):289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, Green DR. Science. 5745. Vol. 310. New York, NY: Oct 7, 2005. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. pp. 66–67. [DOI] [PubMed] [Google Scholar]
- 28.Green DR, Kroemer G. Science. 5684. Vol. 305. New York, NY: Jul 30, 2004. The pathophysiology of mitochondrial cell death. pp. 626–629. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Benito M, Marzo I, Anel A, Naval J. Farnesyltransferase inhibitor BMS-214662 induces apoptosis in myeloma cells through PUMA up-regulation, Bax and Bak activation, and Mcl-1 elimination. Molecular pharmacology. 2005 Jun;67(6):1991–1998. doi: 10.1124/mol.104.007021. [DOI] [PubMed] [Google Scholar]
- 30.Waterhouse NJ, Trapani JA. A new quantitative assay for cytochrome c release in apoptotic cells. Cell death and differentiation. 2003 Jul;10(7):853–855. doi: 10.1038/sj.cdd.4401263. [DOI] [PubMed] [Google Scholar]
- 31.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008 Jan;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 32.Antunes F, Cadenas E, Brunk UT. Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem J. 2001 Jun 1;356(Pt 2):549–555. doi: 10.1042/0264-6021:3560549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasband WS. ImageJ. 1997-2007 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Ex vivo Flow cytometry immunophenotype of resting peripheral LN B cells from WT and p47phox-/- mice stained for surface B220-CD27. The dot plot is gated on the whole LN cell population from the p47phox-/- mice. The histograms are gated on B220+ cells. The histograms indicate WT (tinted histogram) and p47phox-/- (bold line histogram) cells, or isotype control (dashed line histogram) staining for surface CD27. One representative experiment of five is shown. (D) Ex vivo flow cytometry immunophenotype of resting peripheral LN cells from WT and p47phox-/- mice stained for surface CD4-CD8-CD44-CD62L or CD4-CD8-CD69-CD25. The dot plots are gated on the whole LN cell population. Surface staining for WT (top row) or p47phox-/- (bottom row) CD4+ cells (left) and CD8+ cells (right) are shown. One representative experiment of five is shown. The data shows the responses of 3-4 mice/experiment.
Supplemental Figure 2. Glucose-oxidase produced H2O2 enhances p47phox-/- CD8+ lymphocyte survival. Individual experimental results of enhanced cell viability in Figure 3D for resting p47phox-/- CD8+ lymphocytes cultured in IL-7 supplemented IMDM +/- 1 pg/ml GO.
Supplemental Figure 3. IL-2 and IL-7 triggered common γc receptor pathways in p47phox-/- cells are responsive. Fractionated CD8+ p47phox-/-and WT lymphocytes were cultured with IL-2 or IL-7 supplemented culture medium. At 24 hours cells were removed from the cultures and stained for surface CD8, CD44 and CD127 (IL-7R). Flow cytometry phenotype of CD8+ propidium iodide negative cells at baseline (A) and following overnight culture with IL-7 (B) or IL-2 (C). The histograms are gated on CD8+ propidium iodide negative cells. Histograms show the surface CD127 staining for p47phox-/- (filled tinted histogram) and WT (bold lined histogram) CD8+ lymphocytes and isotype control (thin lined histograms). One representative experiment of three is shown. The data shows the responses of 3-4 mice/experiment.
Supplemental Figure 4. (A) Resting LN cell Bcl-2 expression. LN cells were stained for surface CD8, fixed, permeabilized and stained with anti-Bcl-2. The histograms are gated on the CD8+ cells. Bcl-2 expression of CD8+p47phox-/- (filled tinted histograms) and wild type (bold lined histograms) cells and the isotype control staining for p47phox-/-(dotted line histogram) and wild type (dashed line histogram) cells is shown. (B) 3 hour CD44low, and CD44high overlaid histograms showing anti-Bcl-2 staining for p47phox-/- (filled tinted histograms) and wild type (bold lined histograms) cells and the isotype control staining for p47phox-/-(dotted line histogram) and wild type (dashed line histogram) cells. Histograms are plotted to indicate‘# Cells’ (the number of cells (y-axis) within each bin of the histogram). Images correspond to the histograms in Figure 7A and 7B as indicated.