Transcatheter intraarterial therapies have proved valuable in the battle against primary and secondary hepatic malignancies; the unique aspects of all such therapies are their minimal toxicity profiles and highly effective tumor responses while the normal hepatic parenchyma is spared.
Abstract
Transcatheter intraarterial therapies have proved valuable in the battle against primary and secondary hepatic malignancies. The unique aspects of all such therapies are their reduced toxicity profiles and highly effective tumor responses. These unique characteristics coupled with their minimally invasive nature provide an attractive therapeutic option in patients who may have previously had few alternatives. The concept of all catheter-based intraarterial therapies is to selectively deliver anticancer treatment to tumor(s). These therapies, which include transarterial embolization, intraarterial chemoinfusion, transarterial chemoembolization with or without drug-eluting beads, and radioembolization with use of yttrium 90, inflict lethal insult to tumors while preserving normal hepatic parenchyma. This is possible because hepatic neoplasms preferentially derive their blood supply from an arterial source while the majority of noncancerous liver is supplied by the portal vein. As part of the interventional oncology review series, in this article we describe the rationale behind each of these transcatheter therapies and provide a review of the existing medical literature.
© RSNA, 2011
Introduction
Transcatheter intraarterial therapies are image-guided local-regional therapies used for the treatment of patients with primary and metastatic tumors, especially those confined to the liver. The goal of these therapies, which include embolization, infusional therapy, chemoembolization with or without drug-eluting beads, and radioembolization using yttrium 90 (90Y), is to inflict lethal insult to tumor(s) by selectively delivering anticancer treatment to the arterial supply of the tumor. The anticancer effect of embolization is based on terminal arterial blockade and subsequent tumor ischemia. Chemoinfusion therapy involves local and targeted delivery of high concentrations of chemotherapeutic drugs directly to the tumor, whereas chemoembolization with or without drug-eluting beads combines local and targeted drug delivery with concurrent tumor-feeding artery embolization (1–3). Radioembolization combines delivery of internal radiation to the tumor with concomitant microembolization of its feeding artery (4–6). These therapies all appear to have some role in palliating and providing survival benefit in select patient populations (7,8). The role of these therapies has been established in the past decade.
Treatment of liver tumors by means of arterial embolization was initially proposed in the late 1970s, with the goal of controlling symptoms and local tumor growth by cutting off the tumor blood supply (9–11). Series from the late 1970s and early 1980s (12–14) highlighted the use of intraarterial infusion of chemotherapeutic drugs directly into the liver for primary and metastatic liver tumors, and from this, the addition of chemotherapy to arterial embolization was popularized (15–17). Chemoembolization emerged as standard of care for unresectable hepatocellular carcinoma (HCC) when two randomized controlled trials (7,8) demonstrated a significant survival benefit in patients undergoing this procedure versus best supportive care. Drug-eluting beads, polyvinyl alcohol–based microspheres that can be loaded with various types of chemotherapeutic agents, have more recently been developed in effort to improve the pharmacokinetic profile of the chemotherapeutic agents administered (2,18). Overcoming the shortcomings of the techniques of external beam radiation, radioembolization has separately emerged over the past decade (19).
The aim of this article is to provide an overview of transcatheter intraarterial therapies, particularly as they relate to hepatic interventions. The rationale behind each modality will be discussed, and the principles behind their therapeutic effect will be analyzed.
Technical Considerations
Since hepatic neoplasms preferentially derive their blood supply from an arterial source while the majority of noncancerous liver is supplied by the portal vein, transcatheter intraarterial therapies are possible (20,21). This allows for selective therapy delivery to tumors and protects against ischemic necrosis if the arterial supply is occluded, as is common following transarterial embolization and chemoembolization. Although portal vein thrombosis was once considered a contraindication to these hepatic arterial embolization procedures, recent publications have revealed a highly selective approach may be safe (22–24). Radioembolization, a much less embolic procedure, has proved safe and effective in patients with portal vein thrombosis (25,26).
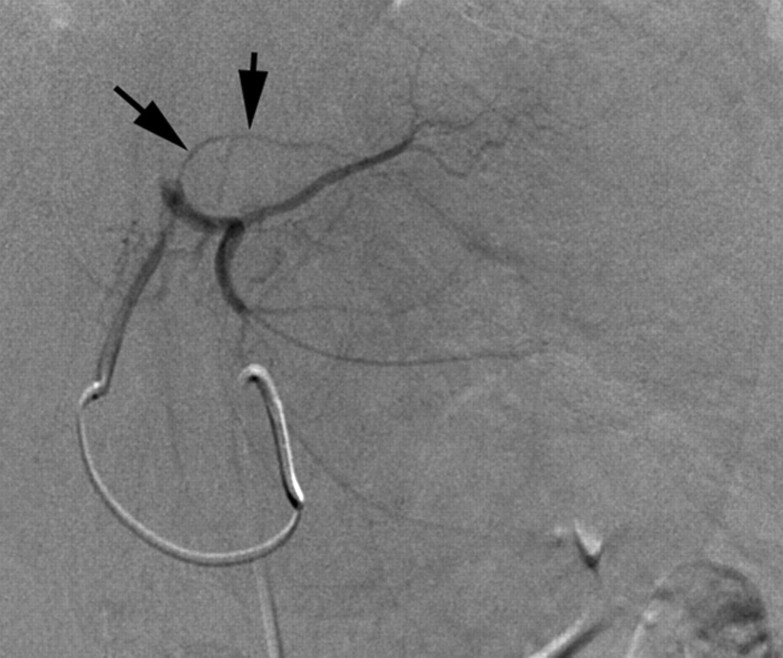
Optimal utilization of all transcatheter therapies requires knowledge of variant hepatic anatomy (27). This is critical for both appropriate targeted therapy delivery and the prevention of complications. Specifically, awareness of extrahepatic vessels arising from the hepatic arteries (28,29) is critical to prevent toxicities from nontarget embolization. Figure 1 delineates many of the extrahepatic arterial pathways that are of interest in these procedures. The prospective identification of these nontarget vessels can minimize the risk of adverse events related to the extrahepatic deposition of the therapeutic agent.
Figure 1a:
Common extrahepatic arteries that need to be recognized and potentially embolized prior to transcatheter intraarterial therapy. (a) Celiac angiogram reveals right gastric artery (arrows) originating from the proximal left hepatic artery. (b) Right hepatic angiogram reveals cystic artery (arrow) originating from the proximal right hepatic artery. (c) Celiac angiogram in a patient with a replaced right hepatic artery from the superior mesenteric artery. Arrows delineate a faint linear vessel coursing from the segment IV branch of the left hepatic artery. (d) Selective left hepatic angiogram better demonstrates the falciform artery (black arrows). The tumor blush is now visible (white arrows). (e) Left hepatic angiogram reveals an accessory left gastric artery (arrows). (f) Delayed phase selective angiogram of the accessory left gastric artery shows the draining coronary vein (arrow), confirming gastric perfusion. (g) Celiac angiogram reveals arterial-portal fistula in the right hepatic lobe. Off the left hepatic artery, there is a vessel coursing under the left hemidiaphragm (arrows). (h) Left hepatic angiogram better demonstrates this vessel (arrows) as the left inferior phrenic artery.
Figure 1b:
Common extrahepatic arteries that need to be recognized and potentially embolized prior to transcatheter intraarterial therapy. (a) Celiac angiogram reveals right gastric artery (arrows) originating from the proximal left hepatic artery. (b) Right hepatic angiogram reveals cystic artery (arrow) originating from the proximal right hepatic artery. (c) Celiac angiogram in a patient with a replaced right hepatic artery from the superior mesenteric artery. Arrows delineate a faint linear vessel coursing from the segment IV branch of the left hepatic artery. (d) Selective left hepatic angiogram better demonstrates the falciform artery (black arrows). The tumor blush is now visible (white arrows). (e) Left hepatic angiogram reveals an accessory left gastric artery (arrows). (f) Delayed phase selective angiogram of the accessory left gastric artery shows the draining coronary vein (arrow), confirming gastric perfusion. (g) Celiac angiogram reveals arterial-portal fistula in the right hepatic lobe. Off the left hepatic artery, there is a vessel coursing under the left hemidiaphragm (arrows). (h) Left hepatic angiogram better demonstrates this vessel (arrows) as the left inferior phrenic artery.
Figure 1c:
Common extrahepatic arteries that need to be recognized and potentially embolized prior to transcatheter intraarterial therapy. (a) Celiac angiogram reveals right gastric artery (arrows) originating from the proximal left hepatic artery. (b) Right hepatic angiogram reveals cystic artery (arrow) originating from the proximal right hepatic artery. (c) Celiac angiogram in a patient with a replaced right hepatic artery from the superior mesenteric artery. Arrows delineate a faint linear vessel coursing from the segment IV branch of the left hepatic artery. (d) Selective left hepatic angiogram better demonstrates the falciform artery (black arrows). The tumor blush is now visible (white arrows). (e) Left hepatic angiogram reveals an accessory left gastric artery (arrows). (f) Delayed phase selective angiogram of the accessory left gastric artery shows the draining coronary vein (arrow), confirming gastric perfusion. (g) Celiac angiogram reveals arterial-portal fistula in the right hepatic lobe. Off the left hepatic artery, there is a vessel coursing under the left hemidiaphragm (arrows). (h) Left hepatic angiogram better demonstrates this vessel (arrows) as the left inferior phrenic artery.
Figure 1d:

Common extrahepatic arteries that need to be recognized and potentially embolized prior to transcatheter intraarterial therapy. (a) Celiac angiogram reveals right gastric artery (arrows) originating from the proximal left hepatic artery. (b) Right hepatic angiogram reveals cystic artery (arrow) originating from the proximal right hepatic artery. (c) Celiac angiogram in a patient with a replaced right hepatic artery from the superior mesenteric artery. Arrows delineate a faint linear vessel coursing from the segment IV branch of the left hepatic artery. (d) Selective left hepatic angiogram better demonstrates the falciform artery (black arrows). The tumor blush is now visible (white arrows). (e) Left hepatic angiogram reveals an accessory left gastric artery (arrows). (f) Delayed phase selective angiogram of the accessory left gastric artery shows the draining coronary vein (arrow), confirming gastric perfusion. (g) Celiac angiogram reveals arterial-portal fistula in the right hepatic lobe. Off the left hepatic artery, there is a vessel coursing under the left hemidiaphragm (arrows). (h) Left hepatic angiogram better demonstrates this vessel (arrows) as the left inferior phrenic artery.
Figure 1e:
Common extrahepatic arteries that need to be recognized and potentially embolized prior to transcatheter intraarterial therapy. (a) Celiac angiogram reveals right gastric artery (arrows) originating from the proximal left hepatic artery. (b) Right hepatic angiogram reveals cystic artery (arrow) originating from the proximal right hepatic artery. (c) Celiac angiogram in a patient with a replaced right hepatic artery from the superior mesenteric artery. Arrows delineate a faint linear vessel coursing from the segment IV branch of the left hepatic artery. (d) Selective left hepatic angiogram better demonstrates the falciform artery (black arrows). The tumor blush is now visible (white arrows). (e) Left hepatic angiogram reveals an accessory left gastric artery (arrows). (f) Delayed phase selective angiogram of the accessory left gastric artery shows the draining coronary vein (arrow), confirming gastric perfusion. (g) Celiac angiogram reveals arterial-portal fistula in the right hepatic lobe. Off the left hepatic artery, there is a vessel coursing under the left hemidiaphragm (arrows). (h) Left hepatic angiogram better demonstrates this vessel (arrows) as the left inferior phrenic artery.
Figure 1f:
Common extrahepatic arteries that need to be recognized and potentially embolized prior to transcatheter intraarterial therapy. (a) Celiac angiogram reveals right gastric artery (arrows) originating from the proximal left hepatic artery. (b) Right hepatic angiogram reveals cystic artery (arrow) originating from the proximal right hepatic artery. (c) Celiac angiogram in a patient with a replaced right hepatic artery from the superior mesenteric artery. Arrows delineate a faint linear vessel coursing from the segment IV branch of the left hepatic artery. (d) Selective left hepatic angiogram better demonstrates the falciform artery (black arrows). The tumor blush is now visible (white arrows). (e) Left hepatic angiogram reveals an accessory left gastric artery (arrows). (f) Delayed phase selective angiogram of the accessory left gastric artery shows the draining coronary vein (arrow), confirming gastric perfusion. (g) Celiac angiogram reveals arterial-portal fistula in the right hepatic lobe. Off the left hepatic artery, there is a vessel coursing under the left hemidiaphragm (arrows). (h) Left hepatic angiogram better demonstrates this vessel (arrows) as the left inferior phrenic artery.
Figure 1g:
Common extrahepatic arteries that need to be recognized and potentially embolized prior to transcatheter intraarterial therapy. (a) Celiac angiogram reveals right gastric artery (arrows) originating from the proximal left hepatic artery. (b) Right hepatic angiogram reveals cystic artery (arrow) originating from the proximal right hepatic artery. (c) Celiac angiogram in a patient with a replaced right hepatic artery from the superior mesenteric artery. Arrows delineate a faint linear vessel coursing from the segment IV branch of the left hepatic artery. (d) Selective left hepatic angiogram better demonstrates the falciform artery (black arrows). The tumor blush is now visible (white arrows). (e) Left hepatic angiogram reveals an accessory left gastric artery (arrows). (f) Delayed phase selective angiogram of the accessory left gastric artery shows the draining coronary vein (arrow), confirming gastric perfusion. (g) Celiac angiogram reveals arterial-portal fistula in the right hepatic lobe. Off the left hepatic artery, there is a vessel coursing under the left hemidiaphragm (arrows). (h) Left hepatic angiogram better demonstrates this vessel (arrows) as the left inferior phrenic artery.
Figure 1h:
Common extrahepatic arteries that need to be recognized and potentially embolized prior to transcatheter intraarterial therapy. (a) Celiac angiogram reveals right gastric artery (arrows) originating from the proximal left hepatic artery. (b) Right hepatic angiogram reveals cystic artery (arrow) originating from the proximal right hepatic artery. (c) Celiac angiogram in a patient with a replaced right hepatic artery from the superior mesenteric artery. Arrows delineate a faint linear vessel coursing from the segment IV branch of the left hepatic artery. (d) Selective left hepatic angiogram better demonstrates the falciform artery (black arrows). The tumor blush is now visible (white arrows). (e) Left hepatic angiogram reveals an accessory left gastric artery (arrows). (f) Delayed phase selective angiogram of the accessory left gastric artery shows the draining coronary vein (arrow), confirming gastric perfusion. (g) Celiac angiogram reveals arterial-portal fistula in the right hepatic lobe. Off the left hepatic artery, there is a vessel coursing under the left hemidiaphragm (arrows). (h) Left hepatic angiogram better demonstrates this vessel (arrows) as the left inferior phrenic artery.
Technical considerations have been published for transarterial chemoembolization (with and without drug-eluting beads) and radioembolization (30–34). At present, radioembolization is potentially one of the more technically challenging of the transcatheter embolization procedures because of the risk of nontarget embolization. Given this, detailed angiographic considerations have been published (35,36). However, it is now being recognized that all loaded embolic therapies (90Y and drug-eluting beads) can result in severe adverse events when delivered to nontarget vascular territories. As experience with drug-eluting beads continues to increase, unexpected adverse events are now being identified (33). These findings reinforce the notion and concept that meticulous angiography and identification of collaterals should be undertaken prior to all transcatheter intraarterial therapies, no matter the embolic intent.
Transarterial Embolization
Background
The concept of treating liver malignancy by occluding its blood supply was introduced in the 1950s and continues to be the basis of transarterial embolization in the treatment of unresectable disease (37,38). In this procedure, an embolizing agent (eg, gelfoam, polyvinyl alcohol, acrylic copolymer gelatin particles) is administered via arterial catheter with the goal of completely occluding the tumor-feeding arterioles. The goal of this therapy is terminal arterial blockade. No other therapy (such as chemotherapy or radiation) is performed with this treatment. Findings of several experimental and clinical trials using various microsphere sizes have shown that although particles smaller than 40 μm preferentially (6- to 12-fold) accumulate in tumor vasculatures, they may pass through sinusoids and tumor-related arteriovenous shunts into the systemic circulation, and thus, may produce serious embolic complications (39,40).
Although embolization of the feeding arteries has been shown to induce tumor necrosis and cell death, recent research has shown that tumor ischemia and hypoxia may actually result in a cascade of detrimental events, including (a) stimulating neoangiogenesis by upregulating proangiogenic factors (eg, hypoxia-induced factor 1 alpha, vascular endothelial growth factor) and (b) providing a mechanism for resisting apoptosis (41,42). Further, hypoxia has been associated with metastasis and poor outcomes, with an unclear mechanism (43).
Clinical Evidence
In 1998, Brown et al (44) retrospectively evaluated survival outcomes in 46 patients with HCC treated with transarterial embolization over a 4-year period. This heterogeneous cohort underwent 86 sessions of transarterial embolization, in which 81% experienced postembolization syndrome. The authors concluded that particle embolization for HCC was well tolerated and demonstrated actuarial survivals of 50% and 33% at 1 and 2 years, respectively.
Bruix et al (45) prospectively reported findings in 80 patients enrolled in a randomized controlled trial treated with transarterial embolization. The investigators concluded that despite the ability of transarterial embolization to retard growth of the tumor, no survival benefit was seen between the two groups. In fact, the authors suggested abandoning this mode of therapy for HCC.
Covey et al (46) reported on the use of transarterial embolization in 45 patients with postoperative HCC tumor recurrence. In their cohort, 97% of patients had Okuda stage I disease. The median overall survival was 46 months, with 1-, 2-, and 5-year actuarial survival rates of 86%, 74%, and 47%, respectively. The median length of hospital stay was 3.2 days following therapy. The authors concluded that transarterial embolization following disease recurrence was an effective method of salvage therapy for patients with preserved hepatic function.
In one of the largest series reported, Maluccio et al (47) reported findings in 322 patients with inoperable HCC treated with transarterial embolization. The median survival for the entire cohort was 21 months from the first treatment. The 1-, 2-, and 3-year survival rates were 66%, 46%, and 33%, respectively. There was a 2.5% 30-day periprocedure mortality rate. Grade 3–4 complications included vascular dissections (n = 3), myocardial infarctions (n = 3), and contrast material–induced nephropathies (n = 9). Toxicities related to nontarget embolization included cholecystitis (n = 3), pancreatitis (n = 3), and hepatic decompensation (n = 9). The authors concluded that transarterial embolization with particles was effective in treating patients with unresectable HCC, and that particles alone may be the critical component of intraarterial embolotherapy.
Besides HCC, transarterial embolization has been used for pain and symptomatic control for neuroendocrine and sarcoma metastases to the liver (48). The principles and techniques of transarterial embolization have also been applied to nonhepatic sites. Introduced by Lalli et al in 1969 (49), emboliziation of hypervascular renal tumors has been used preoperatively to facilitate nephrectomy (50), as well as to palliate patients with unresectable renal cell carcinoma (51–53). Transcatheter arterial embolization has also proved successful and clinically effective in helping to minimize intraoperative and postoperative bleeding in patients with musculoskeletal tumors (54–57).
Intraarterial Chemoinfusion
Background
The rationale for regional chemotherapy is to maximize drug concentrations and tumor drug uptake in the target organ and minimize systemic toxicity (58). For regional drug delivery to be successful, several important principles regarding tumor biology, drug pharmacology, and delivery systems must be fulfilled (59).
The first concept is that regional delivery of a drug leads to increased local concentration of the drug. This only holds true for drugs demonstrating first-order kinetics (constant clearance) despite the higher dose. The second concept is that the increased local drug concentration of a drug leads to increased therapeutic response. While this may be true for cytotoxic drugs exhibiting a steep dose-response curve, it might not be so for targeted agents whose therapeutic effect occurs at a specific drug concentration (60). The third concept is that regional drug delivery leads to decreased systemic exposure of that drug. This depends on the extent of the metabolism or elimination of the drug during first-pass effect. Finally, other drug properties need to be considered. These include drug stability at room temperature, high solubility to be infused in small volumes, and compatibility with titanium, stainless steel, silicone rubber, and polyurethane (61).
Floxuridine (5-fluoro-2′-deoxyuridine), FUDR, is a good example of an ideal drug for arterial infusion therapy. FUDR is active against metastatic colorectal cancer. In vivo studies have shown a sigmoidal dose-response curve to FUDR (62), and the pharmacokinetics of FUDR is linear and not saturated even at dose rates greater than used clinically (63). Further, hepatic extraction of FUDR is higher following intraarterial administration versus intravenous administration (63).
Arterial port catheter systems for intraarterial chemotherapy were historically placed surgically. Advances in minimally invasive techniques have allowed for the percutaneous placement of these devices by interventional radiologists (64–66). Many recent studies have refined this technique, utilizing the common femoral artery and novel means to secure the catheter tip in the hepatic arterial tree (67–72).
Clinical Evidence
The principle of chemoinfusion has been studied most extensively in patients with colorectal cancer and unresectable liver metastases. Since 1987, findings of at least 10 prospective randomized phase III clinical trials (73–82) comparing systemic treatment versus chemoinfusion therapy have been published. These trials have consistently shown higher response rates for chemoinfusion (42%–62%) versus systemic chemotherapy (9%–21%). These trials were limited by small numbers, crossover design methodology, inclusion of patients with extrahepatic systemic disease, and difficulty in delivering treatment to patients in the chemoinfusion arm. Only two of these studies revealed a benefit in the overall survival.
The role of chemoinfusion therapy outside of metastatic colorectal cancer has been studied as well. Most recently, Okusaka et al (83) published findings of a randomized phase III trial comparing transarterial chemoembolization and infusion chemotherapy for the treatment of patients with unresectable HCC. In this prospective 161-patient study, there was no significant difference when the median overall survival time was compared between these two therapies. The authors suggested that treatment intensification by adding embolization did not increase survival over chemoinfusion therapy alone.
Intraarterial chemoinfusion therapy has also been applied to extrahepatic sites. In 1987, combined chemotherapy and radiation therapy (with systemic delivery of cisplatin) proved a viable treatment alternative to radical cystectomy in patients with invasive bladder carcinoma (84). To reduce systemic toxicities and improve response rate, intraarterial chemoinfusion was proposed (85). In a 1997 phase II trial, Mokarim et al (86) reported a 74% complete response rate and a 76.6% actuarial 5-year survival.
Isolated limb infusion therapy is a minimally invasive technique for delivering regional chemotherapy (most often melphalan and actinomycin-D) in patients with advanced and metastatic melanoma to a limb. In 2008, Kroon et al (87) published findings of a 14-year experience of 185 patients with advanced metastatic melanoma treated with isolated limb infusion therapy. The overall response rate was 84%, with median response duration of 13 months, comparable to that obtained with conventional isolated limb perfusion therapy. Subsequently, the same group reported on the safety and efficacy of repeat isolated limb infusion therapy in 48 patients who had recurrent or progressive disease after prior isolated limb infusion (88). Of note, isolated limb infusion therapy can also be used to treat other limb tumors, such as soft-tissue sarcomas and advanced or recurrent squamous and basal cell carcinomas (89,90).
Taking advantage of the principles of chemoinfusion, Armstrong et al (91) published a seminal study in 2006 demonstrating a therapeutic advantage to intraperitoneal chemotherapy in the treatment of optimally debulked stage III ovarian, fallopian tube, and primary peritoneal cancers. In this randomized study, patients receiving intraperitoneal chemotherapy had significantly longer overall survival (66 vs 50 months) and median progression-free survival (24 vs 18 months) than those receiving only intravenous chemotherapy. The following year, the National Institutes of Health issued a consensus statement advocating the use of intraperitoneal chemotherapy in patients with advanced ovarian cancer (92).
Results from a phase III randomized trial comparing percutaneous hepatic perfusion with melphalan to standard of care for patients with hepatic metastases from metastatic ocular or cutaneous melanoma were presented at the 2010 American Society of Clinical Oncology annual meeting (93). In the study patients, melphalan was infused into the hepatic artery via a percutaneously placed catheter with hepatic venous hemofiltration by using a retrohepatic, double-balloon catheter (Delcath Systems; Delcath, Stamford, Conn). Median hepatic progression-free survival was 245 days (95% confidence interval [CI]: 136, 267) for hepatic melphalan perfusion versus 49 days (95% CI: 43, 68) for standard of care (P < .001). Overall response rate was 34.1% (15 of 44) (95% CI: 20.5, 49.9) for hepatic melphalan perfusion versus 2.0% (one of 49) (95% CI: 0.1, 10.9) for standard of care (P < .001).
Transarterial Chemoembolization
Background
Transarterial chemoembolization with use of anticancer drugs followed by Gelfoam was introduced by Yamada et al in the late 1970s (94,95). According to the Society of Interventional Radiology guidelines, chemoembolization is currently defined as the infusion of a mixture of chemotherapeutic agents with or without iodized oil, followed by embolization with particles (96). The concept of chemoembolization is to administer potent chemotherapeutic agent(s) into the hepatic arteries supplying the tumor. This is followed by embolization of the target vessels with agents such as gelfoam, polyvinyl alcohol, or acrylic copolymer gelatin particles (97). The intended purpose of embolization is twofold: to prevent washout of the drug at the site of the tumor and to induce ischemic necrosis. This necrosis might result in the failure of transmembrane pumps in tumor cells, allowing a greater absorption of chemotherapeutic agents by tumor cells. In a cell culture experiment, there was a greater uptake of a doxorubicin analog by hepatoma cells under hypoxic conditions (98). Chemoembolization offers the ability to expose tumors to high local chemotherapeutic agent concentrations with minimal systemic drug bioavailability.
Several variations of this technique have been demonstrated throughout the world; no standard protocol has been uniformly adopted. Centers have differed in the characteristics of the patients treated, the choice of the embolizing agent used, the choice and/or dose of the anticancer agents used, embolization end-points, and the schedule and/or interval of retreatment (99). In treating HCC, single-agent doxorubicin is commonly used worldwide, whereas the combination of mitomycin C, doxorubicin, and cisplatin is preferred in the United States. Irrespective of the agent used, it is typically emulsified in lipiodol, an oily contrast agent believed to increase intratumoral retention of the cytotoxic agent (100–102).
Iodized oil has been shown to act as a carrier of chemotherapeutic agents, which are released slowly from the lipiodol mixture (103,104). Further, iodized oil has been found to remain selectively in the neovasculature and extravascular spaces of liver tumors when injected into the hepatic artery (105,106). The iodized oil persists selectively in the tumor for a few weeks or months because of hemodynamic differences between hypervascular hepatic tumors and liver parenchyma and presumably because of the absence of Kupffer cells in tumors (107).
Clinical Evidence
Until 2002, there was a lack of compelling data that transarterial chemoembolization offered a survival benefit versus best supportive care (108–110). The use of this technique was based on promising phase II data showing safety and effectiveness of the technique according to the tumor response. However, in 2002, two landmark studies showed a statistically significant survival advantage with the use of transarterial chemoembolization versus best supportive care in selected patients with well-preserved liver functions (7,8). Llovet et al (8) prospectively studied the survival outcomes of patients treated with fixed interval (intention to treat) chemoembolization, embolization, and conservative measures. Survival outcomes for the three arms showed a survival benefit in stringently selected patients treated with chemoembolization and embolization versus those treated conservatively. In a second randomized controlled trial, Lo et al (7) reported findings of a select group of patients with unresectable HCC treated with transarterial chemoembolization or supportive care. The authors of that study concluded that transarterial chemoembolization significantly improves survival in select patients with unresectable HCC.
Transarterial chemoembolization for metastatic disease has been explored. In 1998, Tellez et al (111) reported findings on 30 patients with metastatic colorectal cancer treated with transarterial chemoembolization after failing standard of care chemotherapy. After transarterial chemoembolization, a radiographic response defined as a decrease in tumor density of 75% or a decrease in tumor size of 25% occurred in 63% of patients. In 95% of patients, there was at least 25% decrease from baseline carcinoembryonic antigen levels. All patients experienced postembolization syndrome. The authors concluded that transarterial chemoembolization is a feasible treatment modality for patients with colorectal metastasis to the liver who have experienced failure of systemic therapies. In 2006, Geschwind et al (112) demonstrated that transarterial chemoembolization can prolong survival of patients with colorectal metastases. Most of the patients in that cohort had previously been treated with systemic chemotherapy.
In 2003, Roche et al (109) reported on the outcome of 14 patients with liver neuroendocrine metastases treated with transarterial chemoembolization as a first-line nonsurgical treatment. Transarterial chemoembolization was performed with doxorubicin emulsified in lipiodol and gelatin sponge particles. Complete symptomatic response was achieved in seven cases and objective morphologic response was noted in 12 of 14 cases. The 5- and 10-year survival rates from diagnosis were 83% and 56%, respectively. More recently, Liapi et al analyzed imaging responses and determined survival outcome of 26 patients with neuroendocrine metastases treated with transarterial chemoembolization (113). Mean patient survival was 78 months. Despite the fact that tumor imaging characteristics significantly changed after treatment, indicating a successful response to therapy, partial response according to the World Health organization (WHO) and Response Evaluation Criteria in Solid Tumors (RECIST) was achieved in only 1∕3 of the cohort, suggesting that imaging response using traditional size measurements was inadequate. This concept of imaging response criteria is further elucidated in the Future Directions section of this article.
Giroux et al (114) performed transarterial chemoembolization in eight patients with liver metastasis from breast cancer. These patients presented with pain and/or liver dominant disease unresponsive to standard of care chemotherapy. After transarterial chemoembolization, five patients demonstrated some tumor regression and one had stable disease; 50% of the patients had symptomatic relief. All patients died within 13 months of treatment. The authors concluded that transarterial chemoembolization stabilizes or improves the liver tumor burden, which may palliate symptoms, but most patients develop other metastases, which eventually lead to death. Buijs et al (115) reported the radiologic response and survival outcome of 14 patients with hepatic metastatic breast cancer. In their study, the mean tumor size decreased by 18% after treatment and the median patient survival was 25 months.
In 2005, Burger et al (116) reported findings in 17 patients with unresectable cholangiocarcinoma treated with transarterial chemoembolization. The median survival was 23 months, with two of the patients being downstaged to resection. Minor complications were present in 12% of the patients, and a major complication resulting in death was seen in 6%. The authors concluded that transarterial chemoembolization was effective in prolonging survival in this patient population.
In 2006, Takayasu et al (117) published data from a large cohort study of 8510 HCC patients treated with transarterial chemoembolization (lipiodol, chemotherapy, gelatin sponge). Exclusion criteria were extrahepatic metastases and/or any previous treatment prior to the transarterial chemoembolization. The mean follow-up period was 1.77 years. The overall median survival was 34 months and 1-, 3-, 5-, and 7-year survival rates were 82%, 47%, 26%, and 16%, respectively. Multivariate analysis showed that prognosticators of survival included degree of liver damage, α-fetoprotein level, maximum tumor size, number of tumors, and portal vein invasion. Treatment-related mortality was 0.5%.
Drug-eluting Microspheres
Background
Chemoembolization with drug-eluting beads represents a relatively new mechanism of enhancing the delivery of potent anticancer agents to the site of the tumor. The concept of drug-eluting beads is to load polyvinyl alcohol–based microspheres with various types of chemotherapeutic agents and deliver them intraarterially in a manner similar to that of conventional chemoembolization. The unique properties of these beads allow for fixed dosing and the ability to release the anticancer agents in a sustained and controlled manner. Significant reductions of peak plasma concentrations have been observed with drug-eluting beads when compared with conventional chemoembolization, suggesting that a greater amount of the anticancer agent is being sequestered by the tumor versus distributing in the systemic circulation (118). This may result in a more pronounced tumor response, while diminishing the systemic bioavailability of the agent.
An example of drug-eluting beads is the doxorubicin-capable bead, or DC bead. DC beads can be loaded with doxorubicin to 25 mg/mL on hydrated beads by immersing them in a drug solution for 1 to 120 minutes (119). These beads have also been loaded with irinotecan and have been reported to be active and safe in patients with hepatic metastasis from colorectal cancer (120). A second example is a copolymer microsphere (QuadraSphere; Biosphere Medical, Rockland, Mass). Initial experiments with doxorubicin-loaded QuadraSphere microspheres have shown a safe pharmacokinetic profile and effective tumor killing in an animal model of liver cancer (121).
Clinical Evidence
In 2007, Varela et al (2) reported on the applicability, safety, and efficacy of drug-eluting beads in 27 patients with HCC. Objective responses of 66.6% were reported by using the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases guidelines. Postembolization syndrome was observed in 41% and 18% of treated patients after the first and second treatment, respectively. There were two cases of liver abscesses.
Similarly in 2007, Poon et al (122) reported about a phase I and II study in which drug-eluting beads were used for patients with incurable HCC and Child-Pugh class A cirrhosis. The phase I study was a dose-escalation study, from 25 to 150 mg of doxorubicin. The 150-mg dose was used for the phase II study. According to the modified RECIST criteria, by taking into account the extent of tumor necrosis, 63% of patients had a partial response and 7% had a complete response. The treatment-related complication rate was 11%. The authors concluded that drug-eluting beads represent a safe and effective treatment for HCC, justifying the initiation of a randomized phase III trial versus transarterial chemoembolization.
Malagari et al (123) have reported on 71 patients prospectively enrolled and treated segmentally with drug-eluting beads. All patients had underlying cirrhosis (Child-Pugh class A or B). According to the EASL guidelines, the reported complete and partial response of the cohort at 24 months was 16.1% and 66.2%, respectively. The overall survival at 30 months was 88.2%. All patients were observed to have varying degrees of self-limiting postembolization symptoms. Major procedure-related complications occurred in 4.2% of patients and included liver abscess and cholecystitis in 3.2%, as well as pleural effusion. The authors concluded that drug-eluting bead therapy was a safe and effective treatment option in patients with inoperable disease.
The first prospective phase II study in the United States in which drug-eluting beads were used for the treatment of patients with unresectable HCC was published in 2009. Twenty patients underwent 34 treatment sessions. RECIST and EASL response rates at 1 month were 10% and 60%, respectively. The remainder had stable disease. Overall survival rates at 1 and 2 years were 65% and 55%, respectively; the median overall survival was 26 months. The authors concluded that chemoembolization with drug-eluting beads is safe and effective in achieving local tumor control in patients with unresectable HCC (124).
Drug-eluting beads loaded with irinotecan are being investigated for the treatment of patients with colorectal hepatic metastases (125). Most recently, Martin et al (126) published results from a multicenter single-arm study of metastatic colorectal cancer patients who received drug-eluting beads with irinotecan after failing systemic chemotherapy. They reported adverse events in 28% (median grade of 2) and a 19-month overall survival. They concluded that this treatment was safe and effective in this subset of patients.
90Y Radioembolization
Background
External beam irradiation has historically played a limited role in the treatment of HCC because of the radiosensitive nature of normal hepatic tissue (127). With radiation doses exceeding 35 Gy, a clinical syndrome consisting of anicteric ascites, hepatomegaly, and elevated liver enzymes is observed weeks to months after therapy (127–129). Given this limitation and the need for higher doses to achieve tumoricidal effects, radioembolization with 90Y has emerged. Radioembolization is defined as the injection of micron-sized embolic particles loaded with a radioisotope by using percutaneous transarterial techniques. Fluoroscopic guidance, angiographic endpoints of embolization and stasis, and the need to modify the therapeutic delivery of radioactive microspheres based on angiographic findings make this treatment a true embolization procedure. Dosimetry planning, the administration and delivery of radiation, modification of dose based on tumor and hepatic volume, as well as the required knowledge of radiation effects on tissue, make this a brachytherapy procedure as well. Radioembolization therefore combines radiation with embolization.
Radioembolization can deliver radiation doses as high as 150 Gy without developing the clinical complications seen with external beam therapy (130–133). Radioembolization is a procedure in which glass or resin microspheres incorporating the radioactive isotope 90Y are directly infused into the hepatic arteries perfusing the tumor. Although first used in human subjects in the early 1960s (134), only within the past decade has it gained increasing awareness and usage (19).
Two radioembolic devices are commercially available. TheraSphere (glass microsphere) was approved in 1999 by the Food and Drug Administration under humanitarian device exemption for the treatment of unresectable HCC in patients who can have appropriately positioned hepatic arterial catheters (135). SIR-Spheres (resin microsphere) were granted full premarketing approval in 2002 from the Food and Drug Administration for the treatment of colorectal metastases in conjunction with intrahepatic FUDR (136).
90Y is a pure beta emitter that decays to stable zirconium 90. Its physical half-life is 64.2 hours (129,137–140). The emissions generated have a mean tissue penetration of 2.5 mm, with a maximum reach of 11 mm. This limited tissue penetration allows for local high-dose radiation with less risk of radiation-induced hepatic necrosis than may be seen with external beam therapy (141). In general, resin microspheres (20–60 μm in diameter) differ from glass microspheres (20–30 μm in diameter) in that they have a lower specific activity, lower specific gravity, and higher number of particles per treatment (141).
Clinical Evidence
Geschwind et al (129) reported findings in 80 patients with inoperable HCC treated with 90Y microspheres by using segmental, regional, and whole-liver approach. Median survival for Okuda stage I (68%) and stage II (38%) was 628 and 324 days, respectively. In 2005, Salem et al (142) reported on the tumor response, safety, and survival of 43 consecutive patients with inoperable HCC treated with 90Y during a 4-year period. Forty-seven percent had an objective tumor response, and the median survival for low-risk and high-risk patients was 20.8 and 11.1 months, respectively. The authors reported no procedure-related life-threatening events. Sangro et al (143) reported findings in 24 Child-Pugh class A patients with HCC who underwent 90Y radioembolization with resin microspheres. The authors observed reduction in the size of the tumor in 19 patients. There were no cases of postembolization syndrome, and all patients were discharged within 24 hours of treatment.
Kulik et al (144) described a 35-patient cohort not amendable to transplantation, resection, or ablation that was treated with 90Y. Sixty-six percent of these patients were successfully downstaged to transplantation, resection, or radiofrequency ablation. Five of seven explants in the transplanted patients demonstrated complete necrosis at pathologic examination. Tumor partial response rate was 50% according to WHO criteria. Intention-to-treat survival for the entire cohort was 800 days. In 2008, Kulik et al (25) subsequently reported the findings from 21 patients with HCC bridged to transplantation with 90Y radioembolization. Complete necrosis was noted in 14 of 21 (66%) explants at pathologic examination.
The role of radioembolization in metastatic disease has been studied. Gray et al (145) published findings of a phase III randomized clinical trial of 74 patients that was conducted to assess whether a single injection of 90Y in combination with intrahepatic FUDR could increase the tumor response rate, time to disease progression in the liver, and survival when compared with FUDR alone. Patients with unresectable colon cancer treated with 90Y and hepatic artery chemotherapy exhibited longer time to progression. The 1-, 2-, 3-, and 5-year survival for patients receiving 90Y was 72%, 39%, 17%, and 3.5%, compared with 68%, 29%, 6.5%, and 0% for hepatic artery chemotherapy alone (P = .18). Van Hazel et al (146) reported findings from a randomized study in 21 patients (11 patients received 90Y plus 5-fluorouracil)/leucovorin), 10 patients received 5-fluorouracil/ leucovorin alone). The authors concluded that the administration of 90Y along with a standard chemotherapeutic regimen significantly increased treatment-related response (10 vs 0 patients demonstrated a partial response at follow-up CT), time to disease progression (18.6 vs 3.6 months), and survival (29.4 vs 12.8 months), when compared with chemotherapy alone.
In 2006, Kennedy et al (147) reported findings in 208 patients with inoperable, chemorefractory hepatic metastases from colorectal cancer. They found a computed tomography (CT) response rate of 35% and a positron emission tomography (PET) response rate of 91%. Survival rates were 10.5 months for responders and 4.5 months for nonresponders. Treatment-related toxicities included fatigue (45%), nausea and/or abdominal pain (30%), gastrointestinal ulceration (5%), and elevated bilirubin levels (4.5%).
Sato et al (180) recently reported findings in 137 patients with hepatic metastatic disease that was treated with 90Y microspheres. Primary sites (origins) included colon, neuroendocrine, and noncolorectal nonneuroendocrine cohort. According to WHO criteria, there was a 42.8% response rate (2.1% complete response, 40.7% partial response). The overall median survival was 300 days. One-year survival was 47.8%, and 2-year survival was 30.9%. Median survival was 15.2 months for patients with colorectal tumors, 25.9 months for those with neuroendocrine tumors, and 6.9 months for those with noncolorectal nonneuroendocrine tumors.
Salem et al (6) published their 291-patient single-center experience on the role of radioembolization in patients with HCC. They concluded that patients with Child-Pugh class A disease with or without vascular invasion benefited most from radioembolization. Time-to-progression and survival varied by tumor stage and liver function.
Comparison of Transcatheter Therapies
Few studies have effectively compared transcatheter therapies. The publications by Lo et al (7) and Llovet et al (8) in 2002 set the standard for these therapies by demonstrating a survival benefit in patients with unresectable HCC randomized to transcatheter chemoemoblization versus best supportive care. In the Llovet study, patients were randomized to transarterial chemoembolization, transarterial embolization, or conservative treatment groups. Proponents of transarterial embolization argue that if this study had not been stopped early (once a survival benefit for transarterial chemoembolization versus supportive care was identified), a similar survival advantage for transarterial embolization might have been realized. Marelli et al (99) compared the outcomes of transarterial embolization versus transarterial chemoembolization from three randomized controlled trials (8,148,149). The authors concluded that transarterial chemoembolization failed to demonstrate a survival benefit compared with transarterial embolization alone. The authors suggested that the observed tumor response and survival benefits seen in these patients may have been a result of induced ischemia rather than the cytotoxic effects of chemotherapy. However, authors of a recent prospective randomized comparison (150) of chemoembolization with doxorubicin-eluting beads and arterial embolization with BeadBlock (Biocompatibles, UK) for HCC concluded that although ischemia plays a role in the development of tumor necrosis, there is a clear additional benefit from the addition of doxorubicin. In that study, there was a complete response in 26.8% of patients in the drug-eluting bead group and 14% in the arterial embolization group at 6 months. Time to progression (± standard deviation) was longer for the drug-eluting bead group (42.4 weeks ± 9.5 and 36.2 weeks ± 9.0, respectively) (P = .008).
Studies have compared transarterial chemoembolization with radioembolization. Kooby et al (151) published a retrospective analysis on 71 patients with unresectable HCC treated with either chemoembolization or radioembolization between 1996 and 2006. They concluded that these therapies provided similar effectiveness and toxicity. Similarly, Carr et al (152) found therapeutic equivalence in survival when comparing radioembolization and chemoembolization in a two-cohort study of patients with unresectable HCC. In 2009, Lewandowski et al (153) compared the downstaging effectiveness of chemoembolization versus radioembolization in 86 patients with unresectable HCC. Disease in 58% of patients who underwent radioembolization was downstaged to stage T2, while that in 31% of those who underwent chemoembolized was downstaged (P = .02). Radioembolization was shown to be a better tool than chemoembolization for downstaging the disease from a size outside transplant criteria to a size within the Milan criteria for transplantation. Recently, Salem et al (4) demonstrated similar survival times for patients with unresectable HCC treated with transarterial chemoembolization or radioembolization. In that comparative effectiveness analysis, radioembolization resulted in longer time to progression and less toxicity than did chemoembolization (P < .05).
Findings of a randomized controlled trial (1) of 200 patients comparing conventional chemoembolization and chemoembolization with drug-eluting beads loaded with doxorubicin failed to show a significant survival difference. Chemoembolization with drug-eluting beads did show a higher rate of complete response, objective response, and disease control compared with conventional chemoembolization (27% vs 22%, 52% vs 44%, and 63% vs 52%, respectively, P > .05). There were fewer adverse events (including liver and systemic toxicities) after chemoembolization with drug-eluting beads than with conventional chemoembolization, and patients with advanced disease (Eastern Cooperative Oncology Group performance status > 0, Child-Pugh class B, bilobar disease) seemed to benefit from drug-eluting beads over conventional chemoembolization.
Future Directions
Background
The future of transcatheter therapies is promising. Ongoing research in this field incorporates advances in the knowledge of liver cancer biology, new concepts in targeting liver cancer, development of new drugs, improvement of intraarterial drug delivery techniques, and technological advances in imaging systems.
The preclinical rabbit VX2 model of liver cancer plays an important role in the improvement of catheter-based therapies. The VX2 tumor blood supply is almost entirely from the hepatic artery, similar to that of humans (154), and rabbit hepatic arteries are large enough to permit hepatic artery catheterization (155). This animal model may serve as a black box in which to develop and evaluate existing and new transcatheter therapies (156–158).
Combined Approaches
Transcatheter therapies, such as the ones discussed in this review, have great potential in combination with other treatment modalities such as ablation or systemic therapies. Both transarterial embolization and chemoembolization procedures have been combined with thermal ablation (Fig 2). In 2005, Maluccio et al (159) published findings of a comparative study of surgical resection versus transarterial embolization with ablation in patients with HCC. There were 40 patients who underwent surgical resection and 33 patients who underwent transarterial embolization and ablation. With a median follow-up of 23 months, the 1-, 3-, and 5-year actuarial overall survival rates were 97%, 77%, and 56% for the embolization and ablation group and 81%, 70%, and 58% for the surgical group, respectively. There was no significant difference in the overall survival rates. The authors concluded that transarterial embolization in combination with ablation is effective in treating solitary HCC tumors up to 7 cm and achieves overall survival rates similar to those of surgical resection in selected patients. Recently, Morimoto et al (160) published findings of a randomized study of 37 patients with solitary HCCs 3–5-cm in size treated with either chemoembolization and radiofrequency ablation or chemoembolization alone. They concluded that radiofrequency ablation combined with chemoembolization is more effective than radiofrequency ablation alone for extending the ablated area in fewer treatment sessions and for decreasing the local tumor progression rate.
Figure 2a:
Example of combination therapy with transcatheter chemoembolization and radiofrequency ablation. The patient has a history of HCC and underwent prior right hepatic lobe resection. Surveillance imaging revealed subcentimeter focus of tumor recurrence in the left hepatic lobe. A multidiscipline conference recommended percutaneous ablation, but the tumor could not be seen at US. (a) Pretreatment gadolinium-enhanced magnetic resonance (MR) image reveals a small arterial enhancing focus (arrow) in the left hepatic lobe. This tumor washed out at the delayed phases, consistent with recurrent HCC. (b) Left hepatic angiogram depicts a small hypervascular tumor (arrow) correlating with the preprocedure MR imaging. Transcatheter chemoembolization was performed to permit appropriate tumor targeting for percutaneous radiofrequency ablation. (c) Nonenhanced CT scan immediately after transcatheter chemoembolization reveals dense accumulation of ethiodol within the tumor (arrow). (d) Nonenhanced CT scan obtained during radiofrequency ablation depicts the electrode placed through the ethiodol-stained tumor (arrow).
Figure 2b:
Example of combination therapy with transcatheter chemoembolization and radiofrequency ablation. The patient has a history of HCC and underwent prior right hepatic lobe resection. Surveillance imaging revealed subcentimeter focus of tumor recurrence in the left hepatic lobe. A multidiscipline conference recommended percutaneous ablation, but the tumor could not be seen at US. (a) Pretreatment gadolinium-enhanced magnetic resonance (MR) image reveals a small arterial enhancing focus (arrow) in the left hepatic lobe. This tumor washed out at the delayed phases, consistent with recurrent HCC. (b) Left hepatic angiogram depicts a small hypervascular tumor (arrow) correlating with the preprocedure MR imaging. Transcatheter chemoembolization was performed to permit appropriate tumor targeting for percutaneous radiofrequency ablation. (c) Nonenhanced CT scan immediately after transcatheter chemoembolization reveals dense accumulation of ethiodol within the tumor (arrow). (d) Nonenhanced CT scan obtained during radiofrequency ablation depicts the electrode placed through the ethiodol-stained tumor (arrow).
Figure 2c:
Example of combination therapy with transcatheter chemoembolization and radiofrequency ablation. The patient has a history of HCC and underwent prior right hepatic lobe resection. Surveillance imaging revealed subcentimeter focus of tumor recurrence in the left hepatic lobe. A multidiscipline conference recommended percutaneous ablation, but the tumor could not be seen at US. (a) Pretreatment gadolinium-enhanced magnetic resonance (MR) image reveals a small arterial enhancing focus (arrow) in the left hepatic lobe. This tumor washed out at the delayed phases, consistent with recurrent HCC. (b) Left hepatic angiogram depicts a small hypervascular tumor (arrow) correlating with the preprocedure MR imaging. Transcatheter chemoembolization was performed to permit appropriate tumor targeting for percutaneous radiofrequency ablation. (c) Nonenhanced CT scan immediately after transcatheter chemoembolization reveals dense accumulation of ethiodol within the tumor (arrow). (d) Nonenhanced CT scan obtained during radiofrequency ablation depicts the electrode placed through the ethiodol-stained tumor (arrow).
Figure 2d:
Example of combination therapy with transcatheter chemoembolization and radiofrequency ablation. The patient has a history of HCC and underwent prior right hepatic lobe resection. Surveillance imaging revealed subcentimeter focus of tumor recurrence in the left hepatic lobe. A multidiscipline conference recommended percutaneous ablation, but the tumor could not be seen at US. (a) Pretreatment gadolinium-enhanced magnetic resonance (MR) image reveals a small arterial enhancing focus (arrow) in the left hepatic lobe. This tumor washed out at the delayed phases, consistent with recurrent HCC. (b) Left hepatic angiogram depicts a small hypervascular tumor (arrow) correlating with the preprocedure MR imaging. Transcatheter chemoembolization was performed to permit appropriate tumor targeting for percutaneous radiofrequency ablation. (c) Nonenhanced CT scan immediately after transcatheter chemoembolization reveals dense accumulation of ethiodol within the tumor (arrow). (d) Nonenhanced CT scan obtained during radiofrequency ablation depicts the electrode placed through the ethiodol-stained tumor (arrow).
Transcatheter therapies, combined with systemic therapies, particularly in those with metastatic disease, are also likely to improve survival. Systemic therapies may also play a role in potentiating the effects of transcatheter therapies. Analogous to the traditional chemotherapy and radiation paradigm, radiosensitizing chemotherapy agents might be combined with radioembolization. In a recently published prospective randomized, multicenter phase III trial, Hendlisz et al (161) reported that radioembolization with 90Y resin microspheres plus 5-fluorouracil is well tolerated and significantly improves time-to-liver progression and time to progression than does 5-fluorouracil alone. There are many ongoing studies combining systemic and transcatheter therapies, including one by Geschwind (162) that combined bevacizumab with transarterial chemoembolization for the treatment of HCC, as well as a dose-escalating study by Hickey et al (163) that used Xeloda with 90Y glass microspheres.
Advances in Therapeutics
It is anticipated that delivered agents will become more potent, translating into higher efficacy and survival benefit. Furthermore, new therapies will be developed that may be more tumor specific or potent. These therapies may involve the delivery of genetic information. Recent research has investigated the targeted delivery of gene therapy to the liver by means of isolated hepatic perfusion or via the portal vein (164,165). The delivery of gene therapies and other future therapies will likely use nanotechnology. Nanocomposites could also be tagged to be tumor specific, tumor avid, visible at time of delivery, and carry a specific therapy (166). Finally, new classes of drugs delivered intraarterially could lead to markedly more potent tumor kill than conventional chemotherapeutic agents. For example, a new class of anticancer drugs, such as 3-bromopyruvate, specifically targeting tumor metabolism could be infused locally by means of transcatheter delivery for increased potency (157). By disrupting the ability of the cancer cell to generate energy, but leaving normal cells intact, this new approach is extremely promising. Early preclinical testing in the rabbit VX2 tumor model has proved quite effective, leading to significant survival benefit and even cure of the animals. Clinical trials should begin in the near future (167).
Advances in Imaging
Transcatheter therapies will be aided by advances in imaging. Because of limitations of conventional digital subtraction angiographic (DSA) imaging in completely targeting tumors, several techniques have been used to more successfully and completely target tumors during transcatheter delivery of a therapeutic agent. In 2005, Rhee et al (168) performed CT with an arterial catheter placed selectively into a feeding vessel. Iodinated contrast medium was manually injected via this hepatic arterial catheter while the patient was imaged in the CT scanner. This “catheter-directed CT angiography” successfully assisted superselective radioembolization, facilitating tumor targeting and sparing normal hepatic parenchyma. This technique is easily applicable to other therapies. In fact, several studies have demonstrated similar success with C-arm angiographic CT for chemoembolization and other transcatheter therapies (Fig 3) (169–171). The utility of combining DSA with MR imaging has also been demonstrated, resulting in catheter repositioning in nearly 50% of cases for better tumor therapy (172,173). More recently, Larson et al (174) have reported on the use of a new technique to measure changes in tumor perfusion during transcatheter therapies.
Figure 3a:
Images in a patient with unresectable HCC. (a) Gadolinium-enhanced MR image reveals a hypervascular tumor (arrow) in the right hepatic lobe. (b) C-arm CT image with the catheter selectively positioned in a tumor-feeding branch reveals marked tumor perfusion (arrow) and minimal perfusion to the adjacent parenchyma, enabling the uninvolved parenchyma to be spared exposure to toxic therapeutics.
Figure 3b:
Images in a patient with unresectable HCC. (a) Gadolinium-enhanced MR image reveals a hypervascular tumor (arrow) in the right hepatic lobe. (b) C-arm CT image with the catheter selectively positioned in a tumor-feeding branch reveals marked tumor perfusion (arrow) and minimal perfusion to the adjacent parenchyma, enabling the uninvolved parenchyma to be spared exposure to toxic therapeutics.
Advances in Assessing Response to Therapy
Follow-up imaging is typically performed 4–6 weeks after therapy, while maximum imaging response is often seen 3–4 months after therapy. Typical imaging findings following each of the transcatheter therapies discussed in this review are demonstrated in Figures 4–8. Evaluation of treatment efficacy for all transcatheter-based therapies has been traditionally performed with radiologic measurement of tumor size as proposed by the WHO or RECIST guidelines (175,176). Both sets of guidelines rely on anatomic criteria for the evaluation of tumor response. However, the accuracy and completeness of these guidelines have been challenged several times. The expert panel of the Barcelona 2000 EASL conference emphasized that tumor necrosis following treatment with local-regional liver-directed therapies may not be paralleled by a reduction in tumor diameter and recommended that estimations of tumor response should account for necrosis, which can readily be estimated by the area of nonenhancement at contrast-enhanced CT or MR imaging (177). Furthermore, the panel stressed that tumor diameter measurements should reflect the area of viable tumor, rather than the overall diameter. More recently, the WHO and RECIST guidelines have been also challenged by new forms of targeted therapies that do not result in tumor size changes that qualify as complete or partial response, but lead to intratumoral functional changes and cellular necrosis that can be measured at functional imaging (diffusion-weighted MR imaging, dynamic contras- enhanced MR imaging, PET, and single photon emission computed tomography) (113). Since transcatheter-based therapies are targeted therapies that may lead to both anatomic and functional intratumoral changes, it is essential that future imaging evaluation of tumor response routinely incorporates both anatomic and functional information of the tumor burden. A recent analysis has also sought to simplify tumor response assessment by describing the index tumor as a biomarker of response (178). Finally, a recent study has been published combining both size and necrosis criteria for assessing response to local-regional therapies, with pathology explants serving as the standard, and the authors concluded that the optimal method of response assessment must include the early findings of necrosis (EASL) and the time-dependent changes in size (RECIST/WHO guidelines) (179).
Figure 4a:
(a) Contrast-enhanced CT scan reveals infiltrating right hepatic lobe tumor invading the portal vein (arrows). (Image courtesy of Karen Brown, MD, Memorial Sloan-Kettering Cancer Center.) (b) After transarterial embolization, contrast-enhanced CT scan reveals marked necrosis and clear demarcation of this previously infiltrating tumor (arrows). Small gas bubbles within the tumor are an indication of necrosis. (Image courtesy of Karen Brown, MD, Memorial Sloan-Kettering Cancer Center.)
Figure 8a:
(a) Gadolinium-enhanced MR image reveals a 5.8-cm hypervascular HCC (arrow) in the right hepatic lobe. (b) Following radioembolization, MR image reveals necrosis and decreased tumor size (4.6 cm) (arrow), which is consistent with a favorable response to therapy. The pattern of necrosis is in a segmental distribution. This has been termed radiation segmentectomy.
Figure 4b:
(a) Contrast-enhanced CT scan reveals infiltrating right hepatic lobe tumor invading the portal vein (arrows). (Image courtesy of Karen Brown, MD, Memorial Sloan-Kettering Cancer Center.) (b) After transarterial embolization, contrast-enhanced CT scan reveals marked necrosis and clear demarcation of this previously infiltrating tumor (arrows). Small gas bubbles within the tumor are an indication of necrosis. (Image courtesy of Karen Brown, MD, Memorial Sloan-Kettering Cancer Center.)
Figure 5a:
(a) Contrast-enhanced CT scan reveals multifocal 2–3-cm bilobar hepatic metastases from colorectal cancer. (Image courtesy of Karen Brown, MD, Memorial Sloan-Kettering Cancer Center.) (b) Following chemoinfusion therapy with FUDR, contrast-enhanced CT scan reveals disappearance of these tumors, which is consistent with a complete response. (Image courtesy of Karen Brown, MD, Memorial Sloan-Kettering Cancer Center.)
Figure 5b:
(a) Contrast-enhanced CT scan reveals multifocal 2–3-cm bilobar hepatic metastases from colorectal cancer. (Image courtesy of Karen Brown, MD, Memorial Sloan-Kettering Cancer Center.) (b) Following chemoinfusion therapy with FUDR, contrast-enhanced CT scan reveals disappearance of these tumors, which is consistent with a complete response. (Image courtesy of Karen Brown, MD, Memorial Sloan-Kettering Cancer Center.)
Figure 6a:
(a) Gadolinium-enhanced MR image reveals a 3.2-cm hypervascular HCC (arrow) in the right hepatic lobe. (b) Nonenhanced CT scan immediately after transarterial chemoembolization demonstrates dense ethiodol staining with the tumor (arrow). (c) Gadolinium-enhanced MR image after transcatheter chemoembolization, reveals necrosis and tumor size reduction to 2.3 cm (arrow), which is consistent with a favorable response to therapy.
Figure 6b:
(a) Gadolinium-enhanced MR image reveals a 3.2-cm hypervascular HCC (arrow) in the right hepatic lobe. (b) Nonenhanced CT scan immediately after transarterial chemoembolization demonstrates dense ethiodol staining with the tumor (arrow). (c) Gadolinium-enhanced MR image after transcatheter chemoembolization, reveals necrosis and tumor size reduction to 2.3 cm (arrow), which is consistent with a favorable response to therapy.
Figure 6c:
(a) Gadolinium-enhanced MR image reveals a 3.2-cm hypervascular HCC (arrow) in the right hepatic lobe. (b) Nonenhanced CT scan immediately after transarterial chemoembolization demonstrates dense ethiodol staining with the tumor (arrow). (c) Gadolinium-enhanced MR image after transcatheter chemoembolization, reveals necrosis and tumor size reduction to 2.3 cm (arrow), which is consistent with a favorable response to therapy.
Figure 7a:
(a) Gadolinium-enhanced MR image reveals a 6.5-cm hypervascular HCC (arrow) in the right hepatic lobe. (b) Following chemoembolization with drug-eluting beads, gadolinium-enhanced MR image reveals complete necrosis (arrow), which is consistent with a favorable response to therapy. The tumor has only minimally decreased in size (6.1 cm).
Figure 7b:
(a) Gadolinium-enhanced MR image reveals a 6.5-cm hypervascular HCC (arrow) in the right hepatic lobe. (b) Following chemoembolization with drug-eluting beads, gadolinium-enhanced MR image reveals complete necrosis (arrow), which is consistent with a favorable response to therapy. The tumor has only minimally decreased in size (6.1 cm).
Figure 8b:
(a) Gadolinium-enhanced MR image reveals a 5.8-cm hypervascular HCC (arrow) in the right hepatic lobe. (b) Following radioembolization, MR image reveals necrosis and decreased tumor size (4.6 cm) (arrow), which is consistent with a favorable response to therapy. The pattern of necrosis is in a segmental distribution. This has been termed radiation segmentectomy.
Conclusion
Transcatheter intraarterial therapies have proved valuable in the battle against primary and secondary hepatic malignancies. The unique aspects of all such therapies are their minimal toxicity profiles and highly effective tumor responses while normal hepatic parenchyma is spared. These unique characteristics coupled with their minimally invasive nature provide an attractive therapeutic option for patients who may have previously had few alternatives. Despite these major advances in catheter techniques, imaging, and administration, further investigation remains warranted.
Essentials.
• Transcathter intraarterial therapies have proved valuable in the battle against primary and secondary hepatic malignancies.
• The concept of all catheter-based intraarterial therapies is to selectively deliver anticancer treatment to tumor(s).
• Transcathter intraarterial therapies demonstrate highly effective tumor responses with reduced toxicity profiles.
• Transcatheter intraarterial therapies have great potential in combination with other treatment modalities such as ablation or systemic therapies.
Disclosures of Potential Conflicts of Interest: R.J.L. No potential conflicts of interest to disclose. J.F.G. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: paid consultant to Biosphere Medical, Biocompatibles, Bayer HealthCare, and Guerbet; institution has grants/grants pending from Biosphere Medical, Biocompatibles, Bayer HealthCare, Philips Medical, MDS Nordion, Context Vision, and the RSNA. Other relationships: none to disclose. E.L. No potential conflicts of interest to disclose. R.S. Financial activities related to the present article: paid consultant to Sirtex Medical, MDS Nordion, and Biocompatibles. Financial activities not related to the present article: institution has grants/grants pending from Biocompatibles and MDS Nordion; author received payment for lectures including service on speakers bureaus from Sirtex Medical and MDS Nordion. Other relationships: none to disclose.
Received September 3, 2008; revision requested November 11; revision received September 2, 2010; accepted November 10; final version accepted December 28.
E.L. was supported by the National Institutes of Health (grant 5T32EB006351).
Abbreviations:
- EASL
- European Association for the Study of the Liver
- HCC
- hepatocellular carcinoma
- RECIST
- Response Evaluation Criteria in Solid Tumors
- WHO
- World Health Organization
References
- 1.Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33(1):41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007;46(3):474–481 [DOI] [PubMed] [Google Scholar]
- 3.Lewandowski RJ, Mulcahy MF, Kulik LM, et al. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology 2010;255(3):955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011;140(2):497-507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol 2006;29(4):522–529 [DOI] [PubMed] [Google Scholar]
- 6.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138(1):52–64 [DOI] [PubMed] [Google Scholar]
- 7.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35(5):1164–1171 [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359(9319):1734–1739 [DOI] [PubMed] [Google Scholar]
- 9.Tadavarthy SM, Knight L, Ovitt TW, Snyder C, Amplatz K. Therapeutic transcatheter arterial embolization. Radiology 1974;112(1):13–16 [DOI] [PubMed] [Google Scholar]
- 10.Allison DJ, Modlin IM, Jenkins WJ. Treatment of carcinoid liver metastases by hepatic-artery embolisation. Lancet 1977;2(8052-8053):1323–1325 [DOI] [PubMed] [Google Scholar]
- 11.Wheeler PG, Melia W, Dubbins P, et al. Non-operative arterial embolisation in primary liver tumours. BMJ 1979;2(6184):242–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed ML, Vaitkevicius VK, Al-Sarraf M, et al. The practicality of chronic hepatic artery infusion therapy of primary and metastatic hepatic malignancies: ten-year results of 124 patients in a prospective protocol. Cancer 1981;47(2):402–409 [DOI] [PubMed] [Google Scholar]
- 13.Misra NC, Jaiswal MS, Singh RV, Das B. Intrahepatic arterial infusion of combination of mitomycin-C and 5-fluorouracil in treatment of primary and metastatic liver carcinoma. Cancer 1977;39(4):1425–1429 [DOI] [PubMed] [Google Scholar]
- 14.Patt YZ, Mavligit GM, Chuang VP, et al. Percutaneous hepatic arterial infusion (HAI) of mitomycin C and floxuridine (FUDR): an effective treatment for metastatic colorectal carcinoma in the liver. Cancer 1980;46(2):261–265 [DOI] [PubMed] [Google Scholar]
- 15.Patt YZ, Chuang VP, Wallace S, Benjamin RS, Fuqua R, Mavligit GM. Hepatic arterial chemotherapy and occlusion for palliation of primary hepatocellular and unknown primary neoplasms in the liver. Cancer 1983;51(8):1359–1363 [DOI] [PubMed] [Google Scholar]
- 16.Charnsangavej C, Chuang VP, Wallace S, Soo CS, Bowers T. Work in progress: transcatheter management of primary carcinoma of the liver. Radiology 1983;147(1):51–55 [DOI] [PubMed] [Google Scholar]
- 17.Hirai K, Kawazoe Y, Yamashita K, et al. Arterial chemotherapy and transcatheter arterial embolization therapy for non-resectable hepatocellular carcinoma. Cancer Chemother Pharmacol 1989;23(Suppl):S37–S41 [DOI] [PubMed] [Google Scholar]
- 18.Constantin M, Fundueanu G, Bortolotti F, Cortesi R, Ascenzi P, Menegatti E. Preparation and characterisation of poly(vinyl alcohol)/cyclodextrin microspheres as matrix for inclusion and separation of drugs. Int J Pharm 2004;285(1-2):87–96 [DOI] [PubMed] [Google Scholar]
- 19.Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. III. Comprehensive literature review and future direction. J Vasc Interv Radiol 2006;17(10):1571–1593 [DOI] [PubMed] [Google Scholar]
- 20.Gyves JW, Ziessman HA, Ensminger WD, et al. Definition of hepatic tumor microcirculation by single photon emission computerized tomography (SPECT). J Nucl Med 1984;25(9):972–977 [PubMed] [Google Scholar]
- 21.Bierman HR, Byron RL, Jr, Kelley KH, Grady A. Studies on the blood supply of tumors in man. III. Vascular patterns of the liver by hepatic arteriography in vivo. J Natl Cancer Inst 1951;12(1):107–131 [PubMed] [Google Scholar]
- 22.Georgiades CS, Hong K, D’Angelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol 2005;16(12):1653–1659 [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Guo RP, Lai E, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol 2011;18(2):413-420 [DOI] [PubMed] [Google Scholar]
- 24.Deohodar A, Covey AM, Thornton R, et al. Safety and efficacy of transcatheter arterial embolization with particles only in the treatment of hepatocellular carcinoma with portal vein thrombus. J Interv Oncol 2010;3(1):3-11 [Google Scholar]
- 25.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47(1):71–81 [DOI] [PubMed] [Google Scholar]
- 26.Iñarrairaegui M, Thurston KG, Bilbao JI, et al. Radioembolization with use of yttrium-90 resin microspheres in patients with hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol 2010;21(8):1205–1212 [DOI] [PubMed] [Google Scholar]
- 27.Michels N. Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. Philadelphia, Pa: Lippincott Williams & Wilkins, 1955 [Google Scholar]
- 28.Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology 2002;224(2):542–547 [DOI] [PubMed] [Google Scholar]
- 29.Song SY, Chung JW, Lim HG, Park JH. Nonhepatic arteries originating from the hepatic arteries: angiographic analysis in 250 patients. J Vasc Interv Radiol 2006;17(3):461–469 [DOI] [PubMed] [Google Scholar]
- 30.Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol 2002;13(9 Pt 2):S211–S221 [DOI] [PubMed] [Google Scholar]
- 31.Liapi E, Georgiades CC, Hong K, Geschwind JF. Transcatheter arterial chemoembolization: current technique and future promise. Tech Vasc Interv Radiol 2007;10(1):2–11 [DOI] [PubMed] [Google Scholar]
- 32.Salem R, Lewandowski RJ, Sato KT, et al. Technical aspects of radioembolization with 90Y microspheres. Tech Vasc Interv Radiol 2007;10(1):12–29 [DOI] [PubMed] [Google Scholar]
- 33.Liapi E, Geschwind JF. Transcatheter arterial chemoembolization for liver cancer: is it time to distinguish conventional from drug-eluting chemoembolization? Cardiovasc Intervent Radiol 2011;34(1):37-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon B, Soulen MC, Baum RA, Haskal ZJ, Shlansky-Goldberg RD, Cope C. Chemoembolization of hepatocellular carcinoma with cisplatin, doxorubicin, mitomycin-C, ethiodol, and polyvinyl alcohol: prospective evaluation of response and survival in a U.S. population. J Vasc Interv Radiol 1999;10(6):793–798 [DOI] [PubMed] [Google Scholar]
- 35.Liu DM, Salem R, Bui JT, et al. Angiographic considerations in patients undergoing liver-directed therapy. J Vasc Interv Radiol 2005;16(7):911–935 [DOI] [PubMed] [Google Scholar]
- 36.Lewandowski RJ, Sato KT, Atassi B, et al. Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol 2007;30(4):571–592 [DOI] [PubMed] [Google Scholar]
- 37.Markowitz J. The hepatic artery. Surg Gynecol Obstet 1952;95(5):644–646 [PubMed] [Google Scholar]
- 38.Doyon D, Mouzon A, Jourde AM, Regensberg C, Frileux C. Hepatic, arterial embolization in patients with malignant liver tumours (author’s transl) [in French] Ann Radiol (Paris) 1974;17(6):593–603 [PubMed] [Google Scholar]
- 39.Bastian P, Bartkowski R, Köhler H, Kissel T. Chemo-embolization of experimental liver metastases. Part I: distribution of biodegradable microspheres of different sizes in an animal model for the locoregional therapy. Eur J Pharm Biopharm 1998;46(3):243–254 [DOI] [PubMed] [Google Scholar]
- 40.Brown KT. Fatal pulmonary complications after arterial embolization with 40-120- micro m tris-acryl gelatin microspheres. J Vasc Interv Radiol 2004;15(2 Pt 1):197–200 [DOI] [PubMed] [Google Scholar]
- 41.Kim KW, Bae SK, Lee OH, Bae MH, Lee MJ, Park BC. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res 1998;58(2):348–351 [PubMed] [Google Scholar]
- 42.Wu XZ, Xie GR, Chen D. Hypoxia and hepatocellular carcinoma: the therapeutic target for hepatocellular carcinoma. J Gastroenterol Hepatol 2007;22(8):1178–1182 [DOI] [PubMed] [Google Scholar]
- 43.Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 2006;440(7088):1222–1226 [DOI] [PubMed] [Google Scholar]
- 44.Brown KT, Nevins AB, Getrajdman GI, et al. Particle embolization for hepatocellular carcinoma. J Vasc Interv Radiol 1998;9(5):822–828 [DOI] [PubMed] [Google Scholar]
- 45.Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology 1998;27(6):1578–1583 [DOI] [PubMed] [Google Scholar]
- 46.Covey AM, Maluccio MA, Schubert J, et al. Particle embolization of recurrent hepatocellular carcinoma after hepatectomy. Cancer 2006;106(10):2181–2189 [DOI] [PubMed] [Google Scholar]
- 47.Maluccio MA, Covey AM, Porat LB, et al. Transcatheter arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2008;19(6):862–869 [DOI] [PubMed] [Google Scholar]
- 48.Maluccio MA, Covey AM, Schubert J, et al. Treatment of metastatic sarcoma to the liver with bland embolization. Cancer 2006;107(7):1617–1623 [DOI] [PubMed] [Google Scholar]
- 49.Lalli AF, Peterson N, Bookstein JJ. Roentgen-guided infarctions of kidneys and lungs: a potential therapeutic technic. Radiology 1969;93(2):434–435 [DOI] [PubMed] [Google Scholar]
- 50.Kaisary AV, Williams G, Riddle PR. The role of preoperative embolization in renal cell carcinoma. J Urol 1984;131(4):641–646 [DOI] [PubMed] [Google Scholar]
- 51.Nurmi M, Satokari K, Puntala P. Renal artery embolization in the palliative treatment of renal adenocarcinoma. Scand J Urol Nephrol 1987;21(2):93–96 [DOI] [PubMed] [Google Scholar]
- 52.Park JH, Kim SH, Han JK, Chung JW, Han MC. Transcatheter arterial embolization of unresectable renal cell carcinoma with a mixture of ethanol and iodized oil. Cardiovasc Intervent Radiol 1994;17(6):323–327 [DOI] [PubMed] [Google Scholar]
- 53.Maxwell NJ, Saleem Amer N, Rogers E, Kiely D, Sweeney P, Brady AP. Renal artery embolisation in the palliative treatment of renal carcinoma. Br J Radiol 2007;80(950):96–102 [DOI] [PubMed] [Google Scholar]
- 54.Broaddus WC, Grady MS, Delashaw JB, Jr, Ferguson RD, Jane JA. Preoperative superselective arteriolar embolization: a new approach to enhance resectability of spinal tumors. Neurosurgery 1990;27(5):755–759 [PubMed] [Google Scholar]
- 55.Prabhu VC, Bilsky MH, Jambhekar K, et al. Results of preoperative embolization for metastatic spinal neoplasms. J Neurosurg 2003;98(2 Suppl):156–164 [DOI] [PubMed] [Google Scholar]
- 56.Kwon JH, Shin JH, Kim JH, et al. Preoperative transcatheter arterial embolization of hypervascular metastatic tumors of long bones. Acta Radiol 2010;51(4):396–401 [DOI] [PubMed] [Google Scholar]
- 57.Yang HL, Chen KW, Wang GL, et al. Pre-operative transarterial embolization for treatment of primary sacral tumors. J Clin Neurosci 2010;17(10):1280–1285 [DOI] [PubMed] [Google Scholar]
- 58.Collins JM. Pharmacologic rationale for regional drug delivery. J Clin Oncol 1984;2(5):498–504 [DOI] [PubMed] [Google Scholar]
- 59.Melendez FDH, Kemeny N. Pharmacologic principles of regional therapy in the traetment of liver metastases or primary liver tumors. In: Geschwind JFH, Soulen MC, eds. Interventional oncology: principles and practice. Cambridge, England: Cambridge University Press, 2008; 38–52 [Google Scholar]
- 60.Arteaga CL, Baselga J. Clinical trial design and end points for epidermal growth factor receptor-targeted therapies: implications for drug development and practice. Clin Cancer Res 2003;9(5):1579–1589 [PubMed] [Google Scholar]
- 61.Ensminger WD. Intrahepatic arterial infusion of chemotherapy: pharmacologic principles. Semin Oncol 2002;29(2):119–125 [DOI] [PubMed] [Google Scholar]
- 62.Park JG, Collins JM, Gazdar AF, et al. Enhancement of fluorinated pyrimidine-induced cytotoxicity by leucovorin in human colorectal carcinoma cell lines. J Natl Cancer Inst 1988;80(19):1560–1564 [DOI] [PubMed] [Google Scholar]
- 63.Ensminger WD, Rosowsky A, Raso V, et al. A clinical-pharmacological evaluation of hepatic arterial infusions of 5-fluoro-2′-deoxyuridine and 5-fluorouracil. Cancer Res 1978;38(11 Pt 1):3784–3792 [PubMed] [Google Scholar]
- 64.Hildebrandt B, Pech M, Nicolaou A, et al. Interventionally implanted port catheter systems for hepatic arterial infusion of chemotherapy in patients with colorectal liver metastases: a phase II study and historical comparison with the surgical approach. BMC Cancer 2007;7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakamoto N, Arai Y, Takeuchi Y, Takahashi M, Tsurusaki M, Sugimura K. Ultrasound-guided radiological placement of central venous port via the subclavian vein: a retrospective analysis of 500 cases at a single institute. Cardiovasc Intervent Radiol 2010 Apr 14. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Arai Y, Endo T, Sone Y, et al. Management of patients with unresectable liver metastases from colorectal and gastric cancer employing an implantable port system. Cancer Chemother Pharmacol 1992;31(Suppl):S99–S102 [DOI] [PubMed] [Google Scholar]
- 67.Deschamps F, Rao P, Teriitehau C, et al. Percutaneous femoral implantation of an arterial port catheter for intraarterial chemotherapy: feasibility and predictive factors of long-term functionality. J Vasc Interv Radiol 2010;21(11):1681–1688 [DOI] [PubMed] [Google Scholar]
- 68.Hashimoto M, Watanabe O, Takahashi S, Watarai J, Sato T, Yamamoto Y. Efficacy and safety of hepatic artery infusion catheter placement without fixation in the right gastroepiploic artery. J Vasc Interv Radiol 2005;16(4):465–470 [DOI] [PubMed] [Google Scholar]
- 69.Tajima T, Yoshimitsu K, Kuroiwa T, et al. Percutaneous femoral catheter placement for long-term chemotherapy infusions: preliminary technical results. AJR Am J Roentgenol 2005;184(3):906–914 [DOI] [PubMed] [Google Scholar]
- 70.Grosso M, Zanon C, Mancini A, et al. Percutaneous implantation of a catheter with subcutaneous reservoir for intraarterial regional chemotherapy: technique and preliminary results. Cardiovasc Intervent Radiol 2000;23(3):202–210 [DOI] [PubMed] [Google Scholar]
- 71.Herrmann KA, Waggershauser T, Sittek H, Reiser MF. Liver intraarterial chemotherapy: use of the femoral artery for percutaneous implantation of catheter-port systems. Radiology 2000;215(1):294–299 [DOI] [PubMed] [Google Scholar]
- 72.Tanaka T, Arai Y, Inaba Y, et al. Radiologic placement of side-hole catheter with tip fixation for hepatic arterial infusion chemotherapy. J Vasc Interv Radiol 2003;14(1):63–68 [DOI] [PubMed] [Google Scholar]
- 73.Hohn DC, Stagg RJ, Friedman MA, et al. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol 1989;7(11):1646–1654 [DOI] [PubMed] [Google Scholar]
- 74.Kemeny N, Daly J, Reichman B, Geller N, Botet J, Oderman P. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma: a randomized trial. Ann Intern Med 1987;107(4):459–465 [DOI] [PubMed] [Google Scholar]
- 75.Chang AE, Schneider PD, Sugarbaker PH, Simpson C, Culnane M, Steinberg SM. A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg 1987;206(6):685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wagman LD, Kemeny MM, Leong L, et al. A prospective, randomized evaluation of the treatment of colorectal cancer metastatic to the liver. J Clin Oncol 1990;8(11):1885–1893 [DOI] [PubMed] [Google Scholar]
- 77.Martin JK, Jr, O’Connell MJ, Wieand HS, et al. Intra-arterial floxuridine vs systemic fluorouracil for hepatic metastases from colorectal cancer. A randomized trial. Arch Surg 1990;125(8):1022–1027 [DOI] [PubMed] [Google Scholar]
- 78.Rougier P, Laplanche A, Huguier M, et al. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long-term results of a prospective randomized trial. J Clin Oncol 1992;10(7):1112–1118 [DOI] [PubMed] [Google Scholar]
- 79.Allen-Mersh TG, Earlam S, Fordy C, Abrams K, Houghton J. Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet 1994;344(8932):1255–1260 [DOI] [PubMed] [Google Scholar]
- 80.Lorenz M, Müller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol 2000;18(2):243–254 [DOI] [PubMed] [Google Scholar]
- 81.Kerr DJ, McArdle CS, Ledermann J, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet 2003;361(9355):368–373 [DOI] [PubMed] [Google Scholar]
- 82.Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 2006;24(9):1395–1403 [DOI] [PubMed] [Google Scholar]
- 83.Okusaka T, Kasugai H, Shioyama Y, et al. Transarterial chemotherapy alone versus transarterial chemoembolization for hepatocellular carcinoma: a randomized phase III trial. J Hepatol 2009;51(6):1030–1036 [DOI] [PubMed] [Google Scholar]
- 84.Jakse G, Fritsch E, Frommhold H. Hyperfractionated, accelerated radiotherapy and concurrent chemotherapy in locally advanced bladder cancer. Eur Urol 1987;13(1-2):22–25 [DOI] [PubMed] [Google Scholar]
- 85.Mokarim A, Uetani M, Sakamoto I, Hayashi N, Nomata K, Ohtani H. Transarterial infusion of cisplatin and doxorubicin in bladder cancer. Acta Oncol 1997;36(2):175–181 [DOI] [PubMed] [Google Scholar]
- 86.Mokarim A, Uetani M, Hayashi N, et al. Combined intraarterial chemotherapy and radiotherapy in the treatment of bladder carcinoma. Cancer 1997;80(9):1776–1785 [DOI] [PubMed] [Google Scholar]
- 87.Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes following isolated limb infusion for melanoma: a 14-year experience. Ann Surg Oncol 2008;15(11):3003–3013 [DOI] [PubMed] [Google Scholar]
- 88.Kroon HM, Lin DY, Kam PC, Thompson JF. Efficacy of repeat isolated limb infusion with melphalan and actinomycin D for recurrent melanoma. Cancer 2009;115(9):1932–1940 [DOI] [PubMed] [Google Scholar]
- 89.Hegazy MA, Kotb SZ, Sakr H, et al. Preoperative isolated limb infusion of Doxorubicin and external irradiation for limb-threatening soft tissue sarcomas. Ann Surg Oncol 2007;14(2):568–576 [DOI] [PubMed] [Google Scholar]
- 90.Moncrieff MD, Kroon HM, Kam PC, Stalley PD, Scolyer RA, Thompson JF. Isolated limb infusion for advanced soft tissue sarcoma of the extremity. Ann Surg Oncol 2008;15(10):2749–2756 [DOI] [PubMed] [Google Scholar]
- 91.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354(1):34–43 [DOI] [PubMed] [Google Scholar]
- 92.Marth C, Walker JL, Barakat RR, et al. Results of the 2006 Innsbruck International Consensus Conference on intraperitoneal chemotherapy in patients with ovarian cancer. Cancer 2007;109(4):645–649 [DOI] [PubMed] [Google Scholar]
- 93.Pingpank JF, Hughes MS, Alexander HR, et al. A phase III random assignment trial comparing percutaneous hepatic perfusion with melphalan (PHP-mel) to standard of care for patients with hepatic metastases from metastatic ocular or cutaneous melanoma [abstr]. J Clin Oncol 2010;28(18 suppl):LBA8512 [Google Scholar]
- 94.Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 1983;148(2):397–401 [DOI] [PubMed] [Google Scholar]
- 95.Yamada R, Nakatsuka H, Nakamura K, et al. Hepatic artery embolization in 32 patients with unresectable hepatoma. Osaka City Med J 1980;26(2):81–96 [PubMed] [Google Scholar]
- 96.Brown DB, Gould JE, Gervais DA, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2007;18(12):1469–1478 [DOI] [PubMed] [Google Scholar]
- 97.Coldwell DM, Stokes KR, Yakes WF. Embolotherapy: agents, clinical applications, and techniques. RadioGraphics 1994;14(3):623–643; quiz 645–646 [DOI] [PubMed] [Google Scholar]
- 98.Kruskal JB, Hlatky L, Hahnfeldt P, Teramoto K, Stokes KR, Clouse ME. In vivo and in vitro analysis of the effectiveness of doxorubicin combined with temporary arterial occlusion in liver tumors. J Vasc Interv Radiol 1993;4(6):741–747 [DOI] [PubMed] [Google Scholar]
- 99.Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? a systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol 2007;30(1):6–25 [DOI] [PubMed] [Google Scholar]
- 100.Bhattacharya S, Dhillon AP, Winslet MC, et al. Human liver cancer cells and endothelial cells incorporate iodised oil. Br J Cancer 1996;73(7):877–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhattacharya S, Novell JR, Winslet MC, Hobbs KE. Iodized oil in the treatment of hepatocellular carcinoma. Br J Surg 1994;81(11):1563–1571 [DOI] [PubMed] [Google Scholar]
- 102.Terayama N, Matsui O, Gabata T, et al. Accumulation of iodized oil within the nonneoplastic liver adjacent to hepatocellular carcinoma via the drainage routes of the tumor after transcatheter arterial embolization. Cardiovasc Intervent Radiol 2001;24(6):383–387 [DOI] [PubMed] [Google Scholar]
- 103.Raoul JL, Heresbach D, Bretagne JF, et al. Chemoembolization of hepatocellular carcinomas: a study of the biodistribution and pharmacokinetics of doxorubicin. Cancer 1992;70(3):585–590 [DOI] [PubMed] [Google Scholar]
- 104.Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology 1989;170(3 Pt 1):783–786 [DOI] [PubMed] [Google Scholar]
- 105.Konno T, Maeda H, Iwai K, et al. Effect of arterial administration of high-molecular-weight anticancer agent SMANCS with lipid lymphographic agent on hepatoma: a preliminary report. Eur J Cancer Clin Oncol 1983;19(8):1053–1065 [DOI] [PubMed] [Google Scholar]
- 106.Nakamura H, Tanaka T, Hori S, et al. Transcatheter embolization of hepatocellular carcinoma: assessment of efficacy in cases of resection following embolization. Radiology 1983;147(2):401–405 [DOI] [PubMed] [Google Scholar]
- 107.Kan Z, McCuskey PA, Wright KC, Wallace S. Role of Kupffer cells in iodized oil embolization. Invest Radiol 1994;29(11):990–993 [DOI] [PubMed] [Google Scholar]
- 108.Madden MV, Krige JE, Bailey S, et al. Randomised trial of targeted chemotherapy with lipiodol and 5-epidoxorubicin compared with symptomatic treatment for hepatoma. Gut 1993;34(11):1598–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pelletier G, Roche A, Ink O, et al. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol 1990;11(2):181–184 [DOI] [PubMed] [Google Scholar]
- 110.A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med 1995;332(19):1256–1261 [DOI] [PubMed] [Google Scholar]
- 111.Tellez C, Benson AB, 3rd, Lyster MT, et al. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer 1998;82(7):1250–1259 [DOI] [PubMed] [Google Scholar]
- 112.Geschwind J, Hong K, Georgiades C. Utility of transcatheter arterial chemoembolization for liver dominant colorectal metastatic adenocarcinoma in the salvage setting. Presented at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium, San Francisco, Calif, January 26–28, 2006 [Google Scholar]
- 113.Liapi E, Geschwind JF, Vossen JA, et al. Functional MRI evaluation of tumor response in patients with neuroendocrine hepatic metastasis treated with transcatheter arterial chemoembolization. AM J Roentgenol 2008;190(1):67–73 [DOI] [PubMed] [Google Scholar]
- 114.Giroux MF, Baum RA, Soulen MC. Chemoembolization of liver metastasis from breast carcinoma. J Vasc Interv Radiol 2004;15(3):289–291 [DOI] [PubMed] [Google Scholar]
- 115.Buijs M, Kamel IR, Vossen JA, Georgiades CS, Hong K, Geschwind JF. Assessment of metastatic breast cancer response to chemoembolization with contrast agent enhanced and diffusion-weighted MR imaging. J Vasc Interv Radiol 2007;18(8):957–963 [DOI] [PubMed] [Google Scholar]
- 116.Burger I, Hong K, Schulick R, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol 2005;16(3):353–361 [DOI] [PubMed] [Google Scholar]
- 117.Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006;131(2):461–469 [DOI] [PubMed] [Google Scholar]
- 118.Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res 2006;12(8):2563–2567 [DOI] [PubMed] [Google Scholar]
- 119.Lewis AL, Gonzalez MV, Lloyd AW, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol 2006;17(2 Pt 1):335–342 [DOI] [PubMed] [Google Scholar]
- 120.Aliberti C, Tilli M, Benea G, Fiorentini G. Trans-arterial chemoembolization (TACE) of liver metastases from colorectal cancer using irinotecan-eluting beads: preliminary results. Anticancer Res 2006;26(5B):3793–3795 [PubMed] [Google Scholar]
- 121.Lee KH, Liapi EA, Cornell C, et al. Doxorubicin-loaded QuadraSphere microspheres: plasma pharmacokinetics and intratumoral drug concentration in an animal model of liver cancer. Cardiovasc Intervent Radiol 2010;33(3):576–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Poon RT, Tso WK, Pang RW, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 2007;5(9):1100–1108 [DOI] [PubMed] [Google Scholar]
- 123.Malagari K, Alexopoulou E, Chatzimichail K, et al. Transcatheter chemoembolization in the treatment of HCC in patients not eligible for curative treatments: midterm results of doxorubicin-loaded DC bead. Abdom Imaging 2008;33(5):512–519 [DOI] [PubMed] [Google Scholar]
- 124.Reyes DK, Vossen JA, Kamel IR, et al. Single-center phase II trial of transarterial chemoembolization with drug-eluting beads for patients with unresectable hepatocellular carcinoma: initial experience in the United States. Cancer J 2009;15(6):526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Taylor RR, Tang Y, Gonzalez MV, Stratford PW, Lewis AL. Irinotecan drug eluting beads for use in chemoembolization: in vitro and in vivo evaluation of drug release properties. Eur J Pharm Sci 2007;30(1):7–14 [DOI] [PubMed] [Google Scholar]
- 126.Martin RC, Joshi J, Robbins K, et al. Hepatic intra-arterial injection of Drug-Eluting Bead, Irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol 2010;18(1):192–198 [DOI] [PubMed] [Google Scholar]
- 127.Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med 1965;93:200–208 [PubMed] [Google Scholar]
- 128.Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 1995;31(5):1237–1248 [DOI] [PubMed] [Google Scholar]
- 129.Geschwind JF, Salem R, Carr BI, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology 2004;127(5 Suppl 1):S194–S205 [DOI] [PubMed] [Google Scholar]
- 130.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys 2004;60(5):1552–1563 [DOI] [PubMed] [Google Scholar]
- 131.Yorke ED, Jackson A, Fox RA, Wessels BW, Gray BN. Can current models explain the lack of liver complications in Y-90 microsphere therapy? Clin Cancer Res 1999;5(10 Suppl):3024s–3030s [PubMed] [Google Scholar]
- 132.Dawson LA, McGinn CJ, Normolle D, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol 2000;18(11):2210–2218 [DOI] [PubMed] [Google Scholar]
- 133.Dawson LA, McGinn CJ, Lawrence TS. Conformal chemoradiation for primary and metastatic liver malignancies. Semin Surg Oncol 2003;21(4):249–255 [DOI] [PubMed] [Google Scholar]
- 134.Ariel IM. Treatment of inoperable primary pancreatic and liver cancer by the intra-arterial administration of radioactive isotopes (Y90 radiating microspheres). Ann Surg 1965;162:267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.TheraSphere Yttrium-90 microspheres [package insert]. Kanata, Canada: MDS Nordion, 2004 [Google Scholar]
- 136.SIR-Spheres Yttrium-90 microspheres [package insert]. Lane Cove, Australia: SIRTeX Medical, 2004 [Google Scholar]
- 137.Andrews JC, Walker SC, Ackermann RJ, Cotton LA, Ensminger WD, Shapiro B. Hepatic radioembolization with yttrium-90 containing glass microspheres: preliminary results and clinical follow-up. J Nucl Med 1994;35(10):1637–1644 [PubMed] [Google Scholar]
- 138.Sarfaraz M, Kennedy AS, Cao ZJ, et al. Physical aspects of yttrium-90 microsphere therapy for nonresectable hepatic tumors. Med Phys 2003;30(2):199–203 [DOI] [PubMed] [Google Scholar]
- 139.Dancey JE, Shepherd FA, Paul K, et al. Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nucl Med 2000;41(10):1673–1681 [PubMed] [Google Scholar]
- 140.Salem R, Thurston KG, Carr BI, Goin JE, Geschwind JF. Yttrium-90 microspheres: radiation therapy for unresectable liver cancer. J Vasc Interv Radiol 2002;13(9 Pt 2):S223–S229 [DOI] [PubMed] [Google Scholar]
- 141.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. I. Technical and methodologic considerations. J Vasc Interv Radiol 2006;17(8):1251–1278 [DOI] [PubMed] [Google Scholar]
- 142.Salem R, Lewandowski RJ, Atassi B, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol 2005;16(12):1627–1639 [DOI] [PubMed] [Google Scholar]
- 143.Sangro B, Bilbao JI, Boan J, et al. Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2006;66(3):792–800 [DOI] [PubMed] [Google Scholar]
- 144.Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 2006;94(7):572–586 [DOI] [PubMed] [Google Scholar]
- 145.Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 2001;12(12):1711–1720 [DOI] [PubMed] [Google Scholar]
- 146.Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004;88(2):78–85 [DOI] [PubMed] [Google Scholar]
- 147.Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys 2006;65(2):412–425 [DOI] [PubMed] [Google Scholar]
- 148.Chang JM, Tzeng WS, Pan HB, Yang CF, Lai KH. Transcatheter arterial embolization with or without cisplatin treatment of hepatocellular carcinoma: a randomized controlled study. Cancer 1994;74(9):2449–2453 [DOI] [PubMed] [Google Scholar]
- 149.Kawai S, Okamura J, Ogawa M, et al. Prospective and randomized clinical trial for the treatment of hepatocellular carcinoma: a comparison of lipiodol-transcatheter arterial embolization with and without adriamycin (first cooperative study). The Cooperative Study Group for Liver Cancer Treatment of Japan. Cancer Chemother Pharmacol 1992;31(Suppl):S1–S6 [DOI] [PubMed] [Google Scholar]
- 150.Malagari K, Pomoni M, Kelekis A, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2010;33(3):541–551 [DOI] [PubMed] [Google Scholar]
- 151.Kooby DA, Egnatashvili V, Srinivasan S, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2010;21(2):224–230 [DOI] [PubMed] [Google Scholar]
- 152.Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer 2010;116(5):1305–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 2009;9(8):1920–1928 [DOI] [PubMed] [Google Scholar]
- 154.Ramirez LH, Juliéron M, Bonnay M, et al. Stimulation of tumor growth in vitro and in vivo by suramin on the VX2 model. Invest New Drugs 1995;13(1):51–53 [DOI] [PubMed] [Google Scholar]
- 155.Geschwind JF, Artemov D, Abraham S, et al. Chemoembolization of liver tumor in a rabbit model: assessment of tumor cell death with diffusion-weighted MR imaging and histologic analysis. J Vasc Interv Radiol 2000;11(10):1245–1255 [DOI] [PubMed] [Google Scholar]
- 156.Gu T, Li CX, Feng Y, Wang Q, Li CH, Li CF. Trans-arterial gene therapy for hepatocellular carcinoma in a rabbit model. World J Gastroenterol 2007;13(14):2113–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vali M, Liapi E, Kowalski J, et al. Intraarterial therapy with a new potent inhibitor of tumor metabolism (3-bromopyruvate): identification of therapeutic dose and method of injection in an animal model of liver cancer. J Vasc Interv Radiol 2007;18(1 Pt 1):95–101 [DOI] [PubMed] [Google Scholar]
- 158.Rhee TK, Young JY, Larson AC, et al. Effect of transcatheter arterial embolization on levels of hypoxia-inducible factor-1alpha in rabbit VX2 liver tumors. J Vasc Interv Radiol 2007;18(5):639–645 [DOI] [PubMed] [Google Scholar]
- 159.Maluccio M, Covey AM, Gandhi R, et al. Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J Vasc Interv Radiol 2005;16(7):955–961 [DOI] [PubMed] [Google Scholar]
- 160.Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 2010;116(23):5452–5460 [DOI] [PubMed] [Google Scholar]
- 161.Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010;28(23):3687–3694 [DOI] [PubMed] [Google Scholar]
- 162.Geschwind JF. Bevacizumab and chemoembolization in treating patients with liver cancer that cannot be removed by surgery. Bethesda, Md: National Cancer Institute, 2006 [Google Scholar]
- 163.Hickey RM, Mulcahy MF, Lewandowski R, Salem R. Dose-escalating study of yttrium 90 microspheres with capecitabine for intrahepatic cholangiocarcinoma or metastatic disease to the liver [abstr]. J Clin Oncol 2010;28(15 suppl):TPS216 [Google Scholar]
- 164.Kinoshita H, Watanabe A, Hisayasu S, Suzuki S, Shimada T. Targeted gene delivery to selected liver segments via isolated hepatic perfusion. J Surg Res 2010;160(1):47–51 [DOI] [PubMed] [Google Scholar]
- 165.de Roos WK, Fallaux FJ, Marinelli AW, et al. Isolated-organ perfusion for local gene delivery: efficient adenovirus-mediated gene transfer into the liver. Gene Ther 1997;4(1):55–62 [DOI] [PubMed] [Google Scholar]
- 166.Bhattacharyya S, Kudgus RA, Bhattacharya R, Mukherjee P. Inorganic nanoparticles in cancer therapy. Pharm Res 2010 Nov 23. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vali M, Vossen JA, Buijs M, et al. Targeting of VX2 rabbit liver tumor by selective delivery of 3-bromopyruvate: a biodistribution and survival study. J Pharmacol Exp Ther 2008;327(1):32–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rhee TK, Omary RA, Gates V, et al. The effect of catheter-directed CT angiography on yttrium-90 radioembolization treatment of hepatocellular carcinoma. J Vasc Interv Radiol 2005;16(8):1085–1091 [DOI] [PubMed] [Google Scholar]
- 169.Virmani S, Ryu RK, Sato KT, et al. Effect of C-arm angiographic CT on transcatheter arterial chemoembolization of liver tumors. J Vasc Interv Radiol 2007;18(10):1305–1309 [DOI] [PubMed] [Google Scholar]
- 170.Meyer BC, Frericks BB, Albrecht T, Wolf KJ, Wacker FK. Contrast-enhanced abdominal angiographic CT for intra-abdominal tumor embolization: a new tool for vessel and soft tissue visualization. Cardiovasc Intervent Radiol 2007;30(4):743–749 [DOI] [PubMed] [Google Scholar]
- 171.Wallace MJ, Murthy R, Kamat PP, et al. Impact of C-arm CT on hepatic arterial interventions for hepatic malignancies. J Vasc Interv Radiol 2007;18(12):1500–1507 [DOI] [PubMed] [Google Scholar]
- 172.Wilson MW, Kerlan RK, Jr., Fidelman NA, et al. Hepatocellular carcinoma: regional therapy with a magnetic targeted carrier bound to doxorubicin in a dual MR imaging/conventional angiography suite—initial experience with four patients. Radiology 2004;230(1):287–293 [DOI] [PubMed] [Google Scholar]
- 173.Vogl TJ, Balzer JO, Mack MG, Bett G, Oppelt A. Hybrid MR interventional imaging system: combined MR and angiography suites with single interactive table: feasibility study in vascular liver tumor procedures. Eur Radiol 2002;12(6):1394–1400 [DOI] [PubMed] [Google Scholar]
- 174.Larson AC, Wang D, Atassi B, et al. Transcatheter intraarterial perfusion: MR monitoring of chemoembolization for hepatocellular carcinoma—feasibility of initial clinical translation. Radiology 2008;246(3):964–971 [DOI] [PubMed] [Google Scholar]
- 175.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47(1):207–214 [DOI] [PubMed] [Google Scholar]
- 176.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92(3):205–216 [DOI] [PubMed] [Google Scholar]
- 177.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35(3):421–430 [DOI] [PubMed] [Google Scholar]
- 178.Riaz A, Miller FH, Kulik LM, et al. Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. JAMA 2010;303(11):1062–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Riaz A, Memon K, Miller FH, et al. Role of the EASL, RECIST, and WHO response guidelines alone or in combination for hepatocellular carcinoma: radiologic-pathologic correlation. J Hepatol doi:10.1016/j.jhep.2010.10.004. Published October 23, 2010. Accessed December 13, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres—safety, efficacy, and survival. Radiology 2008;247(2):507–515 [DOI] [PubMed] [Google Scholar]