Abstract
D-serine, which is synthesized by the enzyme serine racemase (SR), is a co-agonist at the N-methyl-D-aspartate receptor (NMDAR). In an animal model of NMDAR hypofunction, the constitutive SR knockout (SR−/−) mouse, pyramidal neurons in primary somatosensory cortex (S1) had reductions in the complexity, total length, and spine density of apical and basal dendrites. We wondered whether the dendritic pathology required deprivation of D-serine throughout development or reflected the loss of D-serine only in adulthood. To address this question, we used mice homozygous for floxed SR in which we bred CaMKIICre2834, which is expressed in forebrain glutamatergic neurons starting at 3–4 weeks post-partum (nSR−/−). Our prior studies demonstrated that the majority of cortical SR is expressed in glutamatergic neurons. We found that similar to SR−/− mice pyramidal neurons in S1 of nSR−/− also had significantly reduced dendritic arborization and spine density, albeit to a lesser degree. S1 neurons of nSR−/− mice had reduced total basal dendritic length that was accompanied by less complex arborization. These characteristics were unaltered in the apical dendritic compartment. In contrast, spine density on S1 neurons was significantly reduced on apical, but not basal dendrites of nSR−/− mice. These results demonstrate that in adulthood neuronally derived D-serine, which is required for optimal activation of post-synaptic NMDAR activity, regulates pyramidal neuron dendritic arborization and spine density. Moreover, they highlight the glycine modulatory site (GMS) of the NMDAR as a potential target for therapeutic intervention in diseases characterized by synaptic deficits, like schizophrenia.
Keywords: D-serine, NMDA receptor, dendritic spines, somatosensory cortex, neuron
1. Introduction
D-amino acids are now well established as mediators and modulators of neuronal activity in mammals [6, 39]. The discovery of substantial levels of D-serine in the mammalian forebrain sparked its interest in neurobiology [14]. Activation of the N-methyl-D-aspartate receptor (NMDAR) requires the binding of either glycine or D-serine at the glycine modulatory site (GMS) on the NR1 subunit [17]. D-Serine is enriched in corticolimbic regions of the brain, where its localization closely parallels that of NMDARs [31]. Thus, D-serine is thought to be the primary forebrain co-agonist because it is concentrated in the forebrain and elimination of synaptic D-serine reduces NMDAR-mediated currents [23, 31].
The cloning and characterization of serine racemase (SR) demonstrated that D-serine is synthesized endogenously in the mammalian brain through the conversion of L- to D-serine [38]. Inactivation of the SR gene reduces cortical D-serine levels by ~85% [3]. Initial in vitro studies and immunohistochemical studies suggested that SR was present mainly in astrocytes, and therefore was the major source of D-serine in the brain [25, 31, 38]. However, recent immunohistochemical studies have suggested a more prominent neuronal SR expression, consistent with the localization of D-serine in neurons [10, 22]. Furthermore, mice with a conditional deletion of SR selectively in neurons or astrocytes demonstrate that the majority (~65%) of SR is expressed in forebrain glutamatergic neurons, particularly in the cortex and hippocampus [5].
NMDARs have been well established to regulate dendritic elaboration and spine formation in the developing nervous system [18]. Our previous work has shown that constitutive SR−/− mice, which lack SR throughout life and display reduced NMDAR function [3], have less complex dendritic arbors and reduced spine density on pyramidal neurons in the medial prefrontal cortex [9] and primary somatosensory cortex (S1) [1]. We wondered whether the dendritic pathology required deprivation of D-serine throughout development or reflected the loss of D-serine only in adulthood, since studies of addictive drugs [29] and of the estrous cycle [21] have shown marked and rapid alterations of dendrites in adulthood.
Therefore, we utilized mice that suppress SR expression beginning at 3–4 weeks post-partum in forebrain excitatory neurons (nSR−/−) to determine whether the neuronal pool of D-serine is an important regulator of adult pyramidal cell dendritic plasticity in S1.
2. Materials and Methods
2.1. Animals
Neuron-specific SRCKO (nSR−/−) mice were generated as previously described [5] using mice containing the floxed (fl) SR construct [3] and mice containing the Ca2+/calmodulin-dependent kinase II (CaMKIICre2834), which produces Cre expression in forebrain neurons beginning at postnatal day 17 and reaching near adult levels by day 34 [32]. nSR−/− mice were created by generating mice that expressed the CaMKIICre2834 transgene in SR fl/fl mice. All genetic constructs used in these experiments were maintained on a C57BL/6J mouse background (backcrossed ≥10 generations). The control mice used in these experiments were the SR fl/fl genotype that did not carry the Cre transgene. Animals were group housed in polycarbonate cages and maintained on a 12:12 h light/dark cycle in a temperature (22°C) and humidity controlled vivarium. Animals were given access to food and water ad libitum. All animal procedures were approved by the McLean Hospital Institutional Animal Care and Use Committee.
2.2. Golgi staining
Golgi-staining was performed on adult (n = 6 mice/genotype) using the FD Rapid GolgiStain Kit (FD NeuroTechnologies, Ellicot City, MD) as previously described [1].
2.3. Quantification of dendritic morphology and spine density analysis
Pyramidal neurons in the primary somatosensory cortex (S1) were chosen for reconstruction. Neurons were located in cortical layers 2/3 (L/2/3) between approximately 0.46 mm to 1.82 mm posterior to bregma (Paxinos & Franklin, 2001). Neurons selected for reconstruction were located in the middle third of the section. Neurons that were obscured by neighboring neurons and/or glia were excluded from analysis. Within each region examined, 4–5 neurons were reconstructed for each mouse. Neurons were traced at 100x on a Zeiss Axioskop40 microscope. The morphology of apical and basilar arbors, as well as spine density were quantified using Neurolucida (MBF Bioscience, Williston, VT). The experimenter was blind to genotype during tracing. Sholl analysis was performed using the Neurolucida Explorer software to assess dendritic complexity. Spines were counted on dendritic branches from 4–5 neurons per region in each animal. For apical dendrites, spines were counted on the apical trunk (last 40 μm) and oblique segments separately, because spine density has been shown to vary across these dendritic compartments [28]. One to two terminal basilar branches at each order were selected for spine counting.
2.4. Statistical analyses
The effect of genotype on total dendritic length and segment analyses were analyzed using two-tailed, unpaired Student’s t test. Dendritic complexity was analyzed using two-factor ANOVA with repeated measures, while spine density was compared using two-factor ANOVA. Significant F tests were followed up with Student’s t test comparisons. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Dendritic morphology is altered in S1 cortex of nSR−/− mice
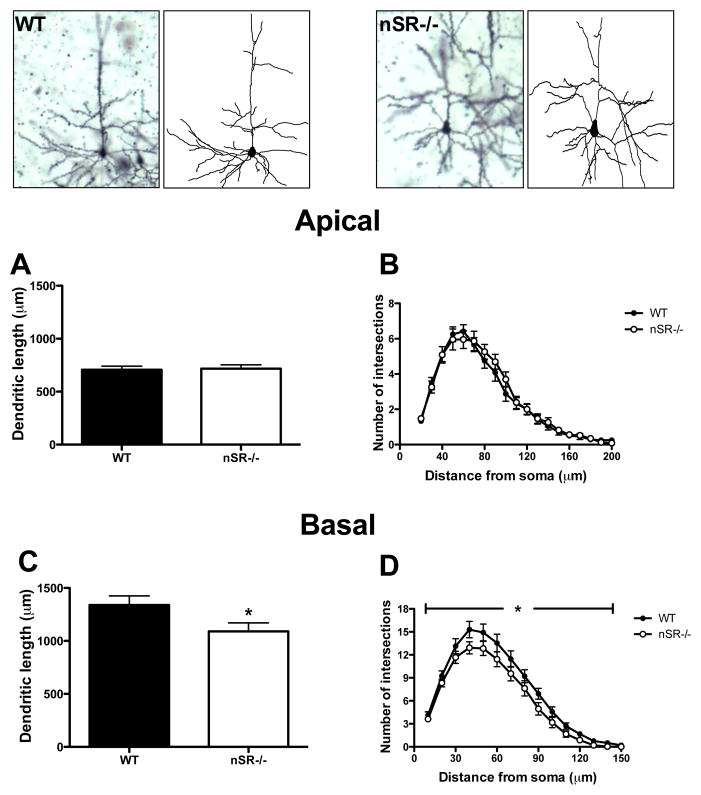
In S1, there were no differences in the total length of pyramidal neuron apical dendrites in nSR−/− mice as compared to WT mice (Fig. 1A; t(45) = 0.21, p = 0.84). There was also no difference in dendritic complexity between genotypes (Fig. 1B; distance from soma: F(18,45) = 102, p<0.0001 ; genotype: F(1,45) = 0.22, p = 0.64; distance x genotype: F(18,45) = 0.5, p = 0.96 ), which was assessed using Sholl analysis (the number of dendrite intersections for concentric circles centered at the cell body were counted at a progressively increasing 10 μm radius). In contrast to the apical tree, the characteristics of basal dendrites of neurons in nSR−/− mice were significantly affected. The total length of basal dendrites was lower in the neurons of nSR−/− mice (Fig. 1C; t(46) = 2.12, p = 0.04). They were also significantly less complex than those in WT mice (Fig. 1D; distance from soma: F(15,46) = 192.2, p < 0.0001; genotype: F(1,46) = 3.87, p = 0.05; distance x genotype: F(15,46) = 0.94, p = 0.52).
Fig. 1.
Pyramidal neurons in the somatosensory cortex of SR−/− mice display perturbations in basilar dendritic morphology. The apical and basal dendrites of pyramidal neurons (4–5 neurons/subject) in the cortex from wild-type (WT; n= 6 mice; black) and nSR−/− (n = 6 mice; white) animals were compared. Golgi stained pyramidal neurons and computer-assisted reconstructions of representative neurons in WT (top left panels) and nSR−/− (top right panels) mice. (A) There was no significant difference in the total apical dendritic length of neurons between WT and nSR−/− mice (t(45) = 0.21, p = 0.84). (B) The apical dendrites of neurons from nSR−/− mice were not significantly less complex than WT mice (distance from soma: F(18,45) = 102, p<0.0001 ; genotype: F(1,45) = 0.22, p = 0.64; distance x genotype: F(18,45) = 0.5, p = 0.96 ). The basal dendrites of neurons from nSR−/− mice showed significant reductions in (C) total length (t(46) = 2.12, p = 0.04) and (D) complexity (distance from soma: F(15,46) = 192.2, p < 0.0001; genotype: F(1,46) = 3.87, p = 0.05; distance x genotype: F(15,46) = 0.94, p = 0.52). Asterisk (*) indicates significant difference from the WT group (p < 0.05). All values represent the mean ± SEM.
3.2. Spine density is reduced in S1 cortex of SR −/− mice, with no change in spine maturity
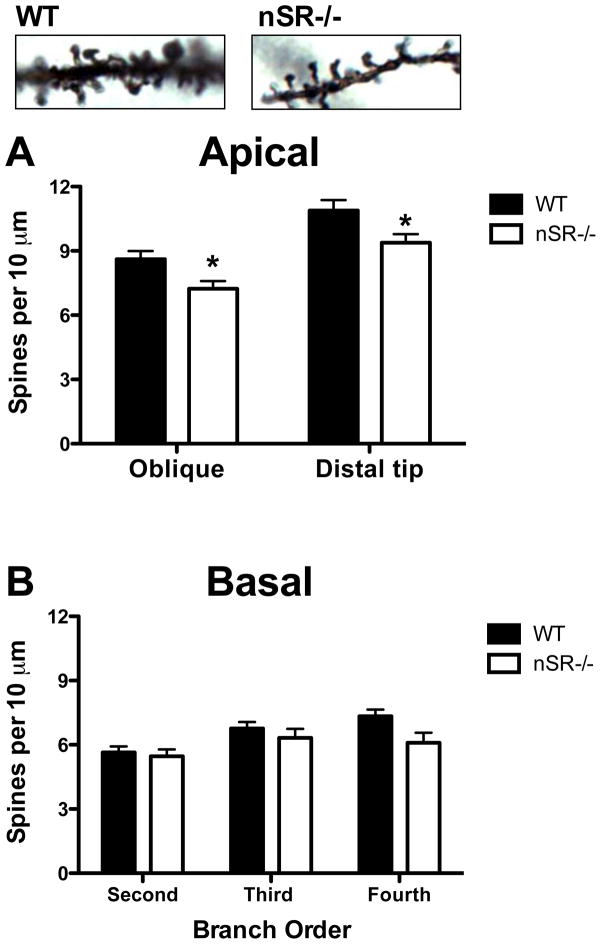
Pyramidal neurons from nSR−/− mice exhibited reductions in apical spine density on the distal portions of their apical dendrites (last 40 μm of the primary apical dendrite) and on oblique apical branches (Fig. 2A; genotype: F(1,47) = 9.05, p < 0.01; branch x genotype: F(1,47) = 0.07, p = 0.79). The distal sections of the primary dendrites exhibited higher spine density than the oblique branches for both WT and nSR−/− mice (branch type: F(1,47) = 81.52, p < 0.0001). However, there were no differences in spine density on basal dendrites across any branch order in SR−/− mice as compared to WT mice (Fig. 2B; branch order: F(2,46) = 11.91, p < 0.0001; genotype: F(1,46)= 2.23, p = 0.14; branch x genotype: F(2,46) = 2.31, p = 0.10).
Fig. 2.
Reduced apical spine density on pyramidal neurons in the somatosensory cortex of nSR−/− mice. Spine density was compared between wild-type (WT; n= 6 mice; black) and nSR−/− (n = 6 mice; white) animals. Apical dendritic spines on a Golgi-stained pyramidal neuron in a WT (top left panel) and nSR−/− mouse (top right panel). (A) Spine density on pyramidal neurons (4–5 neurons/subject) was reduced on both oblique branches and the distal tips of apical dendrites in nSR−/− mice. (B) Spine density on neurons (4–5 neurons/subject) was not reduced on any of the branch orders of basal dendrites in nSR−/− mice. Spine density was expressed as the number of spines per 10 μm of dendrite. Asterisk (*) indicates significant difference from the WT group (p < 0.05). All values represent the mean ± SEM.
4. Discussion
It has been shown by using SR−/− mice that the NMDAR co-agonist D-serine is an important regulator of dendritic arborization and spine density of S1 pyramidal neurons. However, in these mutants, SR is constitutively deleted in all cell types throughout their lifetime. Although initial findings suggested that SR and D-serine were present mainly in astrocytes, recent studies demonstrated that the majority of SR in the cortex and hippocampus is expressed in glutamatergic neurons. Thus, we utilized nSR−/− mice to determine whether neuronal D-serine is important for regulating dendritic characteristics. We indeed found that pyramidal neurons in S1 of nSR−/− also had significantly reduced dendritic arborization and spine density.
The cellular localization of SR and its product D-serine were initially thought to be found exclusively in astrocytes based on immunocytochemical staining and primary cell culture results [37]. More recent immunocytochemical studies indicate that SR protein can also be detected in neurons [22]. Furthermore, cell specific conditional deletion of SR in astrocytes or neurons indicates that at least 60 percent of cortical SR protein is localized to glutamatergic neurons whereas less than 20 percent is expressed in astrocytes [5]. Interestingly, the tissue content of D-serine is reduced by only 33% in the cortex in the nSR−/− mice, suggesting that neuronal synthesis is not the only determinant of D-serine concentration [5]. Therefore, it is not surprising that the nSR−/− dendritic phenotype is less severe than observed in SR−/− mice, which have an 85% reduction in brain D-serine levels [3]. The more modest phenotype in nSR−/− could also be attributed to the fact that these mutants do not have reduced SR and D-serine levels during the early critical developmental periods [5]; the CAMKIIα produces Cre expression in forebrain neurons beginning at postnatal day 17 and reaching near full expression by 34 days post-partum.
The complexity of dendritic branching is thought to represent the extent of neuronal connectivity. Dendritic arborization is enhanced by NMDAR-dependent neurotransmission and downstream Ca2+ sensitive signaling pathways [34, 35]. This known pathway for activity-dependent elaboration of dendritic structure suggests that the less complex dendritic trees of cortical pyramidal neurons in nSR−/− mice could be due to their constitutive NMDAR hypofunction.
More than 90% of all excitatory synapses in the central nervous system are on dendritic spines [13]. Thus, the reduced spine density of S1 pyramidal neurons in nSR−/− mice would be expected to lead to glutamatergic transmission abnormalities [13]. For example, postsynaptic NMDARs are necessary for the induction of t-LTP [4], a timing-dependent form of potentiation at S1 L4-L2/3 synapses that occurs in adulthood [2]. Furthermore, as excitatory activity influences the density of dendritic spines [41], reduced NMDAR-dependent signaling in the nSR−/− mice could be responsible for the reductions in spine density. The NMDAR hypofunction observed in nSR−/− mice is most likely due to lower neuronal-dependent D-serine release. D-serine can be released from both astrocytes [24, 31] and neurons [30], although at least in the hippocampus, it appears that neuronal D-serine release is the major contributor to the induction of LTP [5] (but see [16]).
Interestingly, the apical and basal dendritic compartments of L2/3 S1 pyramidal neurons were differentially affected in nSR−/− mice, with apical dendrites having reduced spine density and basal dendrites having reduced complexity and length. This dichotomy could be due to the type of inputs each compartment receives. L2/3 neurons are major integrators of sensory information and receive their input mostly from other L2/3 pyramidal neurons and L4 spiny stellate neurons [20]. These inputs project almost exclusively to the basal and apical oblique dendrites. The basal arbors of L2/3 neurons receive the majority of synaptic input from L4, suggesting that L4 spiny neuron to L2/3 pyramidal cell synapses act as a gate for the lateral spread of excitation in L2/3 [11]. However, inputs to the apical tuft are thought to be feedback connections from higher cortical areas and nonspecific thalamic nuclei [12]. In addition to excitatory neurotransmission, differential inhibitory inputs could be contributing to this dissociation in apical and basal dendritic perturbations in nSR−/− mice. For example, evidence suggests that the majority of L1 interneurons inhibit L2/3 pyramidal neurons at the level of the apical dendritic arbor [40]. In particular, neurogliaform cells, which have a large and dense axonal arborization in L1 and thus strongly inhibit L2/3 pyramidal neurons [40], are ideally positioned to control synaptic integration in distal apical dendrites of L2/3 pyramidal neurons [19]. Moreover, L2/3 interneurons also project to and inhibit firing of L2/3 pyramidal neurons [15]. Finally, this type of dissociation between apical and basal dendritic compartments is not without precedence. Our group [9] and others [7, 26, 27, 33, 36] have previously reported dissociations in the effects of genetic or environmental manipulations on the length and spine density of apical and basal dendrites in the prefrontal cortex and hippocampus.
5. Conclusions
These results demonstrate that neuronally synthesized D-serine, which contributes to the activation of NMDARs and is required for the induction of NMDAR-dependent LTP in the hippocampus, is also important for the dendritic arborization and spine density of L2/3 pyramidal neurons in S1 cortex. The time dependent nature of the nSR−/− mutation, in that D-serine is not reduced until early adulthood, suggests that the spine deficits can develop at this late stage and thus might be reversible with postnatal D-serine treatment. Thus, it highlights the glycine modulatory site of the NMDAR as a potential target for therapeutic intervention in diseases characterized by synaptic deficits, like schizophrenia [8].
Highlights.
Neuronal D-serine deficiency impairs NMDA receptor function
Mice lacking neuronal D-serine have cortical neurons with less complex dendrites
Mice lacking neuronal D-serine have neurons with reduced dendritic spine density
Acknowledgments
We would like to thank Drs. Sabina Berretta, Francine M. Benes, and Ole Isacson for the generous use of their equipment and software. We also thank Harry Pantazopoulos for technical assistance, as well as Jiamin Feng for animal colony maintenance and genotyping. This work was supported by a postdoctoral National Research Service Award F32 MH090697 and an Andrew P. Merrill Research Fellowship awarded to DTB, and grants R01MH05190 and P50MH0G0450, as well as an unrestricted grant from Bristol-Myers Squibb to JTC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balu DT, Basu AC, Corradi JP, Cacace AM, Coyle JT. The NMDA receptor co-agonists, d-serine and glycine, regulate neuronal dendritic architecture in the somatosensory cortex. Neurobiol Dis. 2012;45:671–682. doi: 10.1016/j.nbd.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee A, Meredith RM, Rodriguez-Moreno A, Mierau SB, Auberson YP, Paulsen O. Double dissociation of spike timing-dependent potentiation and depression by subunit-preferring NMDA receptor antagonists in mouse barrel cortex. Cereb Cortex. 2009;19:2959–2969. doi: 10.1093/cercor/bhp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, Snyder SH, Bergeron R, Coyle JT. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 2009;14:719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benneyworth MA, Li Y, Basu AC, Bolshakov VY, Coyle JT. Cell Selective Conditional Null Mutations of Serine Racemase Demonstrate a Predominate Localization in Cortical Glutamatergic Neurons. Cell Mol Neurobiol. 2012 doi: 10.1007/s10571-012-9808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- 7.Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007;17:1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- 8.Coyle JT, Balu D, Benneyworth M, Basu A, Roseman A. Beyond the dopamine receptor: novel therapeutic targets for treating schizophrenia. Dialogues Clin Neurosci. 2010;12:359–382. doi: 10.31887/DCNS.2010.12.3/jcoyle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeVito LM, Balu DT, Kanter BR, Lykken C, Basu AC, Coyle JT, Eichenbaum H. Serine racemase deletion disrupts memory for order and alters cortical dendritic morphology. Genes Brain Behav. 2011;10:210–222. doi: 10.1111/j.1601-183X.2010.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding X, Ma N, Nagahama M, Yamada K, Semba R. Localization of D-serine and serine racemase in neurons and neuroglias in mouse brain. Neurol Sci. 2011;32:263–267. doi: 10.1007/s10072-010-0422-2. [DOI] [PubMed] [Google Scholar]
- 11.Feldmeyer D, Lubke J, Silver RA, Sakmann B. Synaptic connections between layer 4 spiny neurone-layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column. J Physiol. 2002;538:803–822. doi: 10.1113/jphysiol.2001.012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 13.Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto A, Nishikawa T, Oka T, Takahashi K. Endogenous D-serine in rat brain: N-methyl-D-aspartate receptor-related distribution and aging. J Neurochem. 1993;60:783–786. doi: 10.1111/j.1471-4159.1993.tb03219.x. [DOI] [PubMed] [Google Scholar]
- 15.Helmstaedter M, Staiger JF, Sakmann B, Feldmeyer D. Efficient recruitment of layer 2/3 interneurons by layer 4 input in single columns of rat somatosensory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8273–8284. doi: 10.1523/JNEUROSCI.5701-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Kwon HB, Sabatini BL. Glutamate induces de novo growth of functional spines in developing cortex. Nature. 2011;474:100–104. doi: 10.1038/nature09986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkum ME, Waters J, Sakmann B, Helmchen F. Dendritic spikes in apical dendrites of neocortical layer 2/3 pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:8999–9008. doi: 10.1523/JNEUROSCI.1717-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubke J, Roth A, Feldmeyer D, Sakmann B. Morphometric analysis of the columnar innervation domain of neurons connecting layer 4 and layer 2/3 of juvenile rat barrel cortex. Cereb Cortex. 2003;13:1051–1063. doi: 10.1093/cercor/13.10.1051. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H. Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol. 2008;510:641–654. doi: 10.1002/cne.21822. [DOI] [PubMed] [Google Scholar]
- 23.Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci U S A. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. The European journal of neuroscience. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- 27.Oyagi A, Oida Y, Kakefuda K, Shimazawa M, Shioda N, Moriguchi S, Kitaichi K, Nanba D, Yamaguchi K, Furuta Y, Fukunaga K, Higashiyama S, Hara H. Generation and characterization of conditional heparin-binding EGF-like growth factor knockout mice. PLoS One. 2009;4:e7461. doi: 10.1371/journal.pone.0007461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat. 1970;127:321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- 29.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg D, Kartvelishvily E, Shleper M, Klinker CM, Bowser MT, Wolosker H. Neuronal release of D-serine: a physiological pathway controlling extracellular D-serine concentration. Faseb J. 2010;24:2951–2961. doi: 10.1096/fj.09-147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Luscher B. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 33.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K, Soderling TR, Goodman RH, Impey S. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci U S A. 2008;105:9093–9098. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci U S A. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolosker H, Dumin E, Balan L, Foltyn VN. D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J. 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- 40.Wozny C, Williams SR. Specificity of synaptic connectivity between layer 1 inhibitory interneurons and layer 2/3 pyramidal neurons in the rat neocortex. Cereb Cortex. 2011;21:1818–1826. doi: 10.1093/cercor/bhq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61:247–258. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]