Abstract
The nucleotide adenosine 5′-triphosphate (ATP) has classically been considered the cell's primary energy currency. Importantly, a novel role for ATP as an extracellular autocrine and/or paracrine signalling molecule has evolved over the past century and extensive work has been conducted to characterize the ATP-sensitive purinergic receptors expressed on almost all cell types in the body. Extracellular ATP elicits potent effects on vascular cells to regulate blood vessel tone but can also be involved in vascular pathologies such as atherosclerosis. While the effects of purinergic signalling in the vasculature have been well documented, the mechanism(s) mediating the regulated release of ATP from cells in the blood vessel wall and circulation are now a key target of investigation. The aim of this review is to examine the current proposed mechanisms of ATP release from vascular cells, with a special emphasis on the transporters and channels involved in ATP release from vascular smooth muscle cells, endothelial cells, circulating red blood cells, and perivascular sympathetic nerves, including vesicular exocytosis, plasma membrane F1/F0-ATP synthase, ATP-binding cassette (ABC) transporters, connexin hemichannels, and pannexin channels.
Keywords: Adenosine 5′-triphosphate (ATP), Purine, Vessel wall biology, Connexin hemichannels, Pannexins
1. Introduction
Classically, the nucleotide adenosine 5′-triphosphate (ATP) has been considered the major energy source in the cell. Through the metabolic breakdown of ingested nutrients, subsequent production of the electron carriers NADH and FADH2 in the citric acid cycle and the transfer of electrons through the electron transport chain, the mitochondrial ATP synthase synthesize ATP in the mitochondrial matrix, which is then transported to and accumulates in the cytosol at a concentration in the low mM range.1,2 The cell utilizes the high-energy content of this molecule for a multitude of functions including its requirement as a cofactor for active transport of molecules across the plasma membrane, actin–myosin cross bridge cycling during muscle contraction, and hydrolysis and transfer of its terminal phosphate group to proteins driven by protein kinases as a post-translational modification to modulate protein function. Through extensive characterization of the role of this nucleotide as an intracellular energy molecule, a novel role for ATP as a potent extracellular signalling molecule emerged nearly 50 years ago. Over the past five decades, extensive work has shown that ATP can be released from a number of different cell types and can exert effects via autocrine and/or paracrine signalling mechanisms. In 1971, Burnstock3 coined the term ‘purinergic’, referring to nerves in the autonomic nervous system that released neither noradrenaline nor acetylcholine upon stimulation, but instead the purine nucleotide ATP. This classification has since been expanded to encompass all aspects of purine signalling. While the effects of ATP as an extracellular signalling molecule have been extensively characterized, the mechanisms involved in its regulated release from different cell types remain an important topic of investigation.4–6
1.1. ATP signalling in the vasculature
The effects of ATP signalling have been observed throughout the vasculature in both homeostatic and pathological conditions, including reactive hyperaemia,7,8 hypoxia-induced vasodilation,9,10 alpha-adrenergic receptor mediated vasoconstriction,11 hypertension,12 VSMC proliferation in atherosclerosis, and arterial restenosis after balloon angioplasty.13,14 Elucidation of the mechanisms regulating purine nucleotide release in the cells comprising the vascular wall is critical for both understanding the pathogenesis of vascular diseases and the identification of novel drug interventions. In 1977, Forrester and Williams15 observed the release of ATP from cells in the cardiovascular system and the release of ATP has since been shown in all major cell types of the vessel wall including endothelial cells,9,16–19 vascular smooth muscle cells,11,20 perivascular sympathetic nerves,21–24 and circulating erythrocytes.10,25–27 Therefore, a keen understanding of the signalling mechanisms involved in the release of ATP is critical. In this review, we will focus on the possible mechanisms involved with the ATP release from endothelial and smooth muscle cells in the blood vessel wall, as well as circulating erythrocytes and perivascular sympathetic nerves that innervate the vasculature.
The foundation for an active role of purinergic signalling in the vasculature was first observed by Drury and Szent-Gyorgyi28 in 1929, who demonstrated that the adenine nucleotides and nucleosides, when injected intravenously into the circulatory system of dogs, produced a potent sinus bradycardia and a fall in blood pressure. This observation pioneered the concept that ATP may be an important signalling molecule in the cardiovascular system and could produce profound effects on cardiac output and blood pressure. Later, the identification and characterization of ATP-sensitive purinergic receptors at the plasma membrane of vascular smooth muscle and endothelial cells across vascular beds further suggested a role for ATP as an extracellular vasoactive signalling molecule which may contribute to blood pressure regulation at the level of the blood vessels themselves.29–32 Purinergic receptors have since been classified into two main families, designated as P1 and P2 receptors (with P annotating Purinergic). P1 receptors are further subclassified as A1, A2A, A2B, or A3 receptors, and are G-protein-coupled receptors that signal through either Gs or Gi and selectively bind the nucleoside adenosine to modulate levels of cAMP within the cell.33 The P2 receptors have been subclassified as either ionotropic P2X receptors, of which seven isoforms have been characterized to date (P2X1–7),34 or metabotropic P2Y receptors, which contain eight isoforms (P2Y1, 2, 4, 6, 11–14)35 (reviewed by Ralevic and Burnstock36). The P2X receptors are ligand gated ion channels which exhibit a high binding affinity for ATP over its metabolic breakdown products. P2X receptor activation by ATP induces a conformational change in the transmembrane channel that allows the influx of extracellular cations, including Na+ and Ca2+ leading to cellular depolarization. This depolarization in turn can activate voltage-gated calcium channels to facilitate further Ca2+ influx strengthening the depolarization.37,38 The P2Y family of receptors are G-protein-coupled receptors that differentially bind to ATP, ADP, or the uracil nucleotides UTP and UDP, depending on receptor subtype, and signal through Gq, Gi, or Gs ultimately producing changes in the concentration of intracellular cAMP or Ca2+. While numerous purinergic receptor isoforms have been identified in vascular cells, it has been shown that binding of ATP to P2X1 receptors localized on VSMCs causes an influx of Ca2+ that results in constriction of the blood vessel.39,40 Conversely, ATP in the blood vessel lumen can bind to P2Y1 and P2Y2 purinergic receptors localized to endothelial cells, which signals the production of inositol triphosphate (IP3) and release of Ca2+ from the endoplasmic reticulum via activation of IP3 receptors. This rise in intracellular calcium concentration leads to activation of endothelial cell nitric oxide synthase, resulting in the production of nitric oxide which feeds back on VSMCs to induce vasodilation.41–43 Contrary to this traditional classification of differential P2 receptor effects in the vascular wall, luminal perfusion of ATP has also been shown to elicit vasodilation via activation of endothelial cell P2X receptors,44 and ATP activation of smooth muscle cell P2Y purinergic receptors has been shown to promote vasoconstriction in different vascular beds.11 Altogether, these observations may suggest that purinergic signalling in the vasculature occurs at distinct micro-signalling domains where localized ATP release stimulates purinergic receptors based on their relative location to the site of release or that purinergic receptor expression in the vessel wall may vary depending on the vascular bed examined. Future studies are required to elucidate this observed difference. This dual control of vascular tone by the purine nucleotide ATP has been reviewed extensively by Burnstock.45
Extracellular purine nucleotide concentration is tightly regulated in the vasculature. The presence and activity of metabolic enzymes that degrade ATP to ADP, AMP and adenosine, as well as expression of multiple P1 and P2 receptors across vascular beds has sparked the question as to which nucleotide or nucleoside is the primary vasoactive molecule. Berne46 first proposed an active role for adenosine in regulating vascular tone by showing that the nucleoside can induce coronary vasodilation in the isolated perfused heart. Moreover, measurement of ATP levels in the venous effluent from the isolated rat hindlimb in response to hyperaemia induced by muscle contraction were undetectable, while adenosine levels were significantly increased.47 In a separate study, however, Hopwood and Burnstock29 observed that a significant portion of the dilation elicited by luminal ATP in the isolated perfused Langendorff heart preparation was resistant to P1 receptor blockade with the non-selective adenosine receptor antagonist PACPX, supporting the role that ATP acting on P2 receptors on endothelial cells elicits the observed vasodilatory response. Furthermore, the vasodilation observed in response to luminal ATP has been shown to be approximately 100 times more potent than that to adenosine.48 Several years later, Bunger et al.49 were able to carefully repeat these observations, but found the magnitude of difference significantly decreased compared with what was previously reported. Altogether, these observations illustrate the dynamic regulation of purine nucleotide metabolism and support the idea that ATP can elicit potent effects on vascular cells before its metabolic breakdown.
In the blood vessel lumen, ATP concentration has been shown to increase during periods of hypoxia and ischaemia. The source of this luminal ATP has been suggested to arise from liberation from circulating erythrocytes10,50 as well as the endothelial cells that line the vessel lumen.9,16,17 Locovei et al.50 reported ATP release from erythrocytes in response to low oxygen tension and osmotic stress, providing a luminal source of ATP, presumably to act on endothelial cell purinergic receptors to induce vasodilation, an increase in blood flow and O2 delivery to tissues. Increased shear stress on endothelial cells has also been shown to induce ATP release into the vessel lumen.9,16,19 At the smooth muscle cell-perivascular sympathetic nerve axis, ATP has been shown to be released as a co-transmitter along with noradrenaline from sympathetic nerves.21–23,51 Release of ATP from perivascular nerve terminals and increases in concentration of the nucleotide at adjacent vascular smooth muscle cells induces vasoconstriction through activation of P2X1 purinergic receptors.23,29 Sneddon and Burnstock23 reported a portion of the excitatory junction potentials and constrictions elicited by perivascular nerve stimulation that is insensitive to α-adrenergic receptor blockade and is mimicked by application of exogenous ATP, suggesting an essential role for this nucleotide in sympathetic nerve-induced contraction of blood vessels.
It has been suggested that chronic increases in ATP in the vasculature could potentiate pathological conditions such as hypertension and atherosclerosis; therefore, it is essential that the extracellular concentration of this nucleotide is tightly regulated. ATP is actively metabolized by a class of catalytic enzymes known as ectonucleotidases. These membrane-bound proteins present their catalytic core to the extracellular side of the plasma membrane, allowing selective degradation of extracellular nucleotides. Cells can also release soluble nucleotidases or exonucleotidases into the extracellular milieu, which can contribute to ATP metabolism outside of the cell.52 The expression and activity of ectonucleotidases has been extensively characterized in the vasculature.53–56 Of the ectoenzymes present in the vasculature, ectonucleotide triphosphate diphosphohydrolase 1 (E-NTPDase, also termed CD39 or ecto-apyrase) plays a substantial role in the breakdown of ATP and ADP to produce AMP.54 The monophosphorylated nucleotide can then be metabolized further to adenosine by removal of its 5′-phosphate by the enzyme ecto-5′-nucleotidase (CD73).57 CD39 localized to arterial smooth muscle cells reduces the concentration of ATP, ADP, UTP, and UDP available for activation of P2 receptors and can reduce the extent and duration of constriction. It has been shown that injection of the purine nucleotide UDP into the circulation of mice lacking CD39 caused an initial vasodilation followed by a strong constriction and a significant increase in mean arterial pressure. Also, endothelium-denuded aortic rings from these mice showed a significant increase in constriction to adenine and uridine nucleotides when compared with controls that express a functional enzyme.12 In a separate study, Kauffenstein et al.58 observed a marked decrease in blood pressure upon intravenous injection of UTP at a sub-threshold concentration for vasoconstriction, consistent with decreased nucleotide breakdown and increased signalling through P2Y receptors on the endothelium. These studies indicate an essential role for ectonucleotidases in regulating the concentration of ATP both in the blood vessel lumen and in the extracellular space surrounding the vascular media and reinforce the dual effect of purines on peripheral resistance and blood pressure.
While it is now widely accepted that ATP in the vascular system plays important roles, the mechanisms of its release into the extracellular milieu are a topic of current investigation with much effort placed on identification of the channels or transporters responsible for its release from the different vascular cell types. The focus of this review is to examine the evidence for ATP release from vascular cells with an emphasis placed on vesicular exocytosis, ATP-binding cassette (ABC) transporters, the plasma membrane F1/F0-ATP synthase, connexin hemichannels, and the recently identified pannexin channels.
2. Mechanisms of ATP release
2.1. Vesicular exocytosis
Proteins synthesized in the endoplasmic reticulum and processed through the Golgi apparatus can be packaged into intracellular vesicles that traffic and fuse with the plasma membrane in a process termed vesicular exocytosis. This event can result in incorporation of membrane bound proteins into the plasma membrane or release of soluble proteins and intracellular molecules into the extracellular space. Vesicular exocytosis occurs on a second-to-second basis in secretory cell types to release soluble proteins, signalling molecules, and neurotransmitters important in maintaining homeostasis. In the vascular system, perivascular sympathetic nerve stimulation leads to the vesicular release of noradrenaline (NA) and produces an excitatory junction potential at the post-junctional membrane of innervated vascular smooth muscle cells and subsequent vasoconstriction. This response has been well characterized by the activation of α-adrenergic receptors localized at the post-junctional membrane of innervated smooth muscle cells and can be mimicked by application of exogenous NA. However, α-adrenergic receptor blockade at the post-junctional membrane has been shown to completely inhibit the response to exogenously applied NA but not that seen by sympathetic nerve stimulation, suggesting the action of a second signalling molecule, notably ATP.23 The observation by Westfall et al.59 that tritiated [H3] purine nucleotides are taken up by sympathetic nerves, along with NA, and released upon field stimulation of the guinea pig vas deferens suggests a key role for vesicular release of ATP from these nerves. The contraction following sympathetic nerve stimulation in the guinea pig vas deferens as well as the rat tail artery is biphasic, characterized by a fast initial contraction followed by a slower sustained contraction. Application of the P2 receptor antagonist arylazido aminopropionyl ATP (ANAPP3) inhibits the initial constriction, but has no effect on the secondary sustained contraction, indicating the presence of an ATP-mediated component. The opposite is seen during α-adrenergic receptor blockade with the α1-adrenergic receptor antagonist prazosin. The ATP-mediated contractile response is also eliminated by application of tetrodotoxin, a potent neurotoxin that inhibits voltage-gated sodium channels important for vesicular exocytosis at nerve terminals, further supporting release of ATP from sympathetic nerves by vesicular exocytosis.21
Endothelial cells lining the vessel lumen are constantly subjected to conditions of varying blood flow and shear stress. The release of vasoactive substances including ATP during conditions of increased shear stress has been extensively documented.9,16,60 The mechanism responsible for this event, however, is currently a topic of investigation. It has been proposed that similar to ATP release from sympathetic perivascular nerve terminals, endothelial cells may release ATP in a vesicular manner. Labelling of intracellular ATP with quinacrine, a quinolone-acridine derivative that has a high binding affinity for ATP,61,62 in cultured human umbilical vein endothelial cells (HUVECs) revealed a punctate staining pattern within the cell consistent with intracellular vesicles.63 Moreover, pretreatment of HUVECs with monensin, an inhibitor of vesicle formation at the Golgi, resulted in a significant decrease in quinacrine fluorescence when compared with untreated cells, consistent with localization of ATP to intracellular vesicles in these cells. Stimulation of quinacrine-labelled endothelial cells by increasing the shear stress at 10 or 25 dynes/cm2 lead to a rapid decrease in quinacrine fluorescence and increase in ATP concentration in the extracellular media, suggesting release of ATP stores via an exocytotic mechanism. In contrast, unstimulated cells showed little detectable ATP in the extracellular medium. Also, monensin was shown to abolish the release of ATP from cultured HUVECs exposed to a shear stress of 25 dynes/cm2. These observations suggest that vesicular exocytosis of ATP from endothelial cells could occur during changes in vessel blood flow and shear stress.
While evidence has surfaced suggesting the vesicular release of ATP from different tissues, the question has remained how ATP from the cytosol is packaged into secretory vesicles. Recently, a vesicular nucleotide transporter, termed VNUT, was identified in a number of secretory cell types.64 This transporter was found to be localized to intracellular vesicles and was active in pumping ATP into the vesicle lumen, utilizing a proton gradient established by the vacuolar-ATPase (v-ATPase). Hydrolysis of ATP by v-ATPase drives the translocation of protons across the vesicular membrane, concentrating them in the vesicle lumen.65 Although the establishment of a proton gradient creates both an electrochemical potential difference as well as pH gradient across the vesicular membrane, the activity of VNUT has been shown to be solely dependent on the electrochemical difference.64 While VNUT fulfils the role of a transporter for concentrating ATP into intracellular vesicles, it remains to be established whether this transporter is expressed and active in perivascular sympathetic nerves and endothelial cells.
2.2. ABC transporters
The ABC transporters are a class of integral membrane proteins that utilize the energy of ATP hydrolysis to facilitate the movement of a large array molecules across the plasma membrane of cells, including cholesterol, lipids and many hydrophobic drugs.66 Each member of an ABC transporter has two conserved intracellular ATP-binding domains that bind and hydrolyse ATP. Three of these proteins, the cystic fibrosis transmembrane conductance regulator (CFTR), the multidrug resistance gene product mdr (also known as P-glycoprotein), and the sulfonylurea receptor (SUR) have been suggested to not only utilize ATP as an energy source for active transport, but to physically transport the purine nucleotide out of the cell for autocrine/paracrine purinergic signalling.67–69 With the search for a channel or transporter that is responsible for ATP release in a multitude of cell types, the ABC transporters have become a potential candidate to fill this role. To date, however, much work is focused on determining whether these membrane transporters are capable of transporting ATP out of the cell themselves, or whether they regulate the activity of another channel or transporter responsible for the event.
In 1989, the gene responsible for cystic fibrosis (CF) was identified. Characterization of its gene product, CFTR, revealed a transmembrane transport protein belonging to the ABC transporter family that is involved in cellular chloride homeostasis.70–72 CFTR has been shown to produce a small Cl− current itself, and it is thought that activation of CFTR by cAMP-dependent protein kinase A (PKA) regulates the activity of a large-conductance outwardly rectifying chloride channel (ORCC), whose activity is also absent in CF patients lacking the functional CFTR gene. Elucidation of the mechanism mediating this event in epithelial cells has revealed that cAMP-dependent activation of CFTR results in the release of ATP, which can then bind and activate P2 purinergic receptors in an autocrine/paracrine signalling mechanism and stimulate Cl− efflux from the cell by activation of ORCCs.73 In agreement with these findings, whole-cell and inside-out patch clamp recordings of cells transfected with CFTR revealed ATP currents that were dependent on cAMP and PKA activation, which were absent in cells lacking the ABC transporter.68 Related studies show ATP currents in cells expressing the multiple drug resistance gene product P-glycoprotein, another member of the ABC transporter family, further supporting a functional role for this family of proteins in the release of ATP.67 While the major focus of the ATP release from CFTR has been in epithelial cell physiology, this transporter has been identified in vascular smooth muscle cells,74 endothelial cells,75 and circulating erythrocytes25 and platelets76 providing a potential conduit for ATP release into from these cells.
Erythrocytes from CF patients with mutations in CFTR show marked reductions in ATP release upon membrane deformation, a stimulus known to induce ATP release from these cells. Also, incubation of erythrocytes from healthy donors with glibenclamide, a sulfonylurea drug shown to inhibit ABC transporters and the ATP-sensitive K+ channel (KATP), or niflumic acid, an inhibitor of cyclooxygenase-2 that has been proposed to inhibit CFTR, results in a significant decrease in deformation-induced ATP release.25 However, it should be noted that the pharmacology associated with these studies is complicated by non-specific drug interactions with targets other than CFTR. Nonetheless, the role for CFTR in ATP release from erythrocytes is further supported by studies showing that constitutive activation of PKA, a mechanism known to regulate CFTR activity, by incubation with the active S-stereoisomer of cAMP causes increased ATP release, whereas incubation with the inactive R-stereoisomer does not.77
The SUR, recently identified as a member of the ABC transporter family, has been shown to form a functional complex with the KATP channel.78 Buildup of intracellular ATP causes membrane depolarization by directly inhibiting potassium efflux through the constitutively active KATP channel. The sulfonylurea drug glibenclamide is known to inhibit potassium currents from the KATP channel. This event has been suggested to occur through direct action on SUR to inhibit ATP efflux from the cell and increase the intracellular concentration of ATP. Indeed, activation of KATP currents by diazoxide, a sulfonylurea known to activate KATP channels in pancreatic beta cells,79 is dependent on the presence of releasable intracellular ATP.80 In the rat pulmonary vasculature, flow-induced release of ATP by the endothelium and the resultant vasodilation can be attenuated by luminal perfusion with glibenclamide.81 This observation might be explained by inhibition of ATP release from SUR on endothelial cells, or by inhibition of ATP release from CFTR localized to these cells. Ultimately, inhibition of ATP release from either of these transporters could result in decreased autocrine/paracrine signalling through P2Y purinergic receptors on the endothelium which may explain the attenuated vasodilation. The release of ATP through CFTR in the vascular endothelium has not been directly examined, but the expression and Cl− channel activity of CFTR have been observed in these cells.75
While the studies presented above have suggested a role for ABC transporters in directly releasing ATP from cells, studies have emerged opposing this idea. Importantly, it has been shown that CFTR reconstituted in planar lipid bilayers failed to conduct ATP, and CFTR in stably transfected mammalian cell lines, as well as endogenous CFTR in intact organs and human lung cell lines was not involved in the release of ATP from these cells.82 These studies had a large impact in silencing the notion that ABC transporters may be involved in releasing ATP from cells; however, more recently, a novel study implicating CFTR in acidosis-induced ATP release from skeletal muscle cells has re-ignited the potential for ABC transporters in releasing ATP. In this study, the authors noted a significant decrease in the amount of ATP release from acidotic skeletal muscle cells upon blockade of CFTR with the selective CFTR inhibitor CFTRinh172 and by selective knockdown of CFTR with siRNA.83 These studies suggest a functional role for CFTR and other ABC transporters as ATP release channels. Expression of these proteins in the vascular endothelium as well as in vascular smooth muscle cells74,84 may provide a potential mechanism for ATP release from these cells, warranting further investigation into their functional role in the vascular system.
2.3. Plasma membrane F1F0-ATPase
The process of oxidative phosphorylation in the mitochondria sets up an electrochemical proton gradient across the inner mitochondrial membrane that is utilized by the mitochondrial F1/F0-ATP synthase (referred to as ATP synthase) to drive the synthesis of ATP in the mitochondrial matrix. This enzyme is composed of the F0 membrane bound subunit, which anchors the protein in the inner mitochondrial membrane and houses the pore where proton transport takes place, and the catalytic F1 subunit, which extends into the mitochondrial matrix and catalyses the formation of ATP from ADP and inorganic phosphate (for review see85). While it was originally thought that ATP synthase was localized and active only in the mitochondria, recent evidence has detected the enzyme at the plasma membrane of a number of cell types, including vascular endothelial cells.86–89 Angiostatin, a product of plasminogen cleavage in the fibrinolytic system, exhibits high affinity binding to the alpha and beta subunits of the F1 subunit of ATP synthase in membrane fractions from HUVECs and has been identified as a potent inhibitor of angiogenesis and tumour development.86,90,91 The anti-angiogenic effects of angiostatin are thought to be mediated by inhibition of ATP release as extracellular ATP is suggested to be a pro-angiogenic factor for endothelial cells.92 Binding of angiostatin to the F1 subunit of plasma membrane ATP synthase in endothelial cells inhibits its activity and the formation of extracellular ATP.93 In these cells, immunocytochemistry performed under non-permeablizing conditions revealed surface expression of the F1 subunit further indicating an extracellular localization of the ATP synthase catalytic head. In the vasculature, endothelial cells exposed to increases in blood flow and shear stress release ATP into the lumen to promote vasodilation. Measurement of ATP in the media from cultured human pulmonary artery endothelial cells exposed to increased flow conditions revealed a flow-dependent increase in ATP release that could be inhibited by angiostatin as well as by an antibody directed against the beta subunit of the F1 catalytic head.18 These observations present a potential mechanism by which endothelial cells in the blood vessel lumen can release ATP in response to haemodynamic forces. As ATP synthase utilizes a proton motive force for ATP generation, the mechanism of its activation by increased flow remains to be elucidated. In order to synthesize ATP at the extracellular surface of the endothelial cell plasma membrane, a proton gradient must be established across the cell membrane local to the ATP synthase, which could potentially affect the intracellular pH and have detrimental effects on the activity of a number of intracellular proteins by acidifying the regions of the cytosol. Importantly, as this enzyme catalyses the formation of ATP from ADP and inorganic phosphate, these metabolites must be present in the blood vessel lumen in concentrations sufficient to promote ATP synthase activity. The activity of ectonucleotidases and soluble nucleotidases in the plasma may also potentiate degradation of these precursor molecules. Nonetheless, the ectopic expression of the ATP synthase at the plasma membrane of vascular endothelial cells suggests that this protein may be involved in regulating vascular purinergic signalling events. Future studies may provide insights into this novel mechanism of ATP release from cells.
2.4. Connexin hemichannels
Connexins (Cx) are vertebrate gap junction proteins that form hexameric oligomers (called connexons) in the endoplasmic reticulum and Golgi, which are then trafficked to and inserted into the plasma membrane of cells.94 When a connexon from one cell is in close apposition to a connexon expressed on an adjacent cell, the two proteins can form non-covalent linkages between their extracellular loops, creating an intercellular gap junction channel that connects the cytosol of the two cells. Gap junctions mediate cell-to-cell communication by the transport of intracellular signalling molecules and second messengers less than 1 kDa, as well as propagation of membrane potential currents between joined cells.95–97 To date, about 20 mammalian connexin isoforms have be identified, and the role of these proteins in gap junctional communication has been extensively characterized. However, there have been reports of undocked connexons or hemichannels at the surface of a number of cell types, including astrocytes and glia in the central nervous system,98–100 circulating polymorphonuclear granulocytes101 and monocytes,102 vascular endothelial cells,19,103–105 and vascular smooth muscle cells.106 It has been proposed that these hemichannels may function as conduits for the transport of molecules, including ATP, between the cytosol of a cell and the extracellular space.107,108 Under resting physiological conditions, connexin hemichannels remain in a closed impermeable state, but it has been suggested that stimuli including decreases in extracellular Ca2+ ([Ca2+]e),109,110 strong membrane depolarizations,111,112 mechanical stimulation,104,105 and metabolic inhibition113 are capable of opening these hemichannels. Dye uptake experiments are a commonly used experimental technique to evaluate the permeability of membrane channels to dyes of varying molecular weights. Li et al.108 demonstrated that a decrease in [Ca2+]e resulted in cellular uptake of multiple low-molecular weight dyes in HeLa cells transfected to overexpress Cx43 and normal rat kidney cells. Moreover, dye uptake in a Novakoff hepatoma cell line was markedly reduced upon expression of an antisense Cx43 vector and significantly increased upon over expression of the protein, indicating activity of Cx43 hemichannels in mediating this event. Analysis of the biophysical properties of hemichannels composed of Cx43 revealed an ionic conductance of ∼220pS during electrophysiological patch clamp studies in HeLa cells overexpressing Cx43. This measured conductance was approximately twice that observed for gap junctions, indicating that the measured currents were produced by opening of connexin hemichannels and not gap junctions.111 Moreover, ATP liberation by C6 glioma cells overexpressing Cx43 was detected in both whole-cell and inside-out patch clamp studies coupled to bioluminescence assays, whereas no detectable ATP release was measured in cells expressing a truncation mutant or GFP-tagged Cx43. Both of these modifications have been shown to eliminate channel activity. Also, ATP release from Cx43 expressing cells was attenuated by the addition of two different non-specific gap junction blockers, gadolinium3+ and carbenoxolone.114 In addition, cultured astrocytes have been reported to release ATP through connexin hemichannels, inducing calcium wave propagation.107 Application of the gap junction inhibitor flufenamic acid attenuated both dye uptake and ATP release from these cells, suggesting a mechanism involving transport through connexin hemichannels. In a separate study, inflammatory insults such as tumour necrosis factor-α and interleukin-1β in the central nervous system induced ATP and glutamate liberation from astrocytes via Cx43 hemichannels.98 Altogether, these observations provide evidence for a role of connexin hemichannels in mediating ATP release from a number of different cell types.
To date, four connexin isoforms are abundantly expressed in the vascular smooth muscle and endothelial cells that comprise the blood vessel wall: Cx37, Cx40, Cx43, and Cx45.115,116 Gap junctions formed by these isoforms have been shown to have important roles in regulating cross talk between adjacent VSMCs and ECs, as well as between the two cell types at the myoendothelial junction (for review see 117). In particular, gap junctions comprised of Cx40 in the vascular endothelium are important for calcium wave propagation in conducted vasodilation.118 At the level of the myoendothelial junction, the point at which endothelial cells and smooth muscle cells come into contact through the internal elastic lamina, Cx40 and Cx43, form gap junctions that allow for the transfer of second messengers and ions between the two cell types to coordinate vasomotor responses.119 As these vascular cells highly express connexin proteins, it is possible that there exists a population of undocked connexin hemichannels that could provide a conduit for ATP release into the vessel lumen or extracellular milieu surrounding the vascular media.
Several recent studies have examined the potential for endothelial cells and smooth muscle cells to release ATP from connexin hemichannels. It has been shown that propagation of calcium waves in confluent monolayers of bovine corneal endothelial cells occurs in response to mechanical stimulation in which a glass micropipette is used to mechanically perturb the plasma membrane of a cell.105 In these studies, application of the gap junction blocker flufenamic acid and the connexin mimetic peptide Gap26 significantly reduced calcium wave propagation. Dye transfer was not affected, indicating that the release of a paracrine signalling molecule from connexin hemichannels elicits a calcium response in an adjacent cell and not direct transfer of calcium from one cell to another in this system. Further investigation revealed the release of ATP from these cells upon mechanical stimulation, as well as increased calcium wave propagation in the presence of the CD39 inhibitor ARL67156. The results of this study suggest a role for a distinct pool of connexin hemichannels at the non-junctional membrane of endothelial cells in the cornea that are capable of releasing ATP to promote calcium wave propagation through a paracrine signalling mechanism during mechanical stress. In the juxtaglomerular vasculature of the renal system, glomerular endothelial cells (GENCs) propagate calcium waves to regulate renal blood flow and the glomerular filtration rate through the release of ATP and purinergic signalling mechanisms.120 Mechanical stimulation of cultured GENC resulted in the formation of calcium waves that were inhibited by the gap junction uncoupler 18α-glycyrrhetinic acid (18α-GA) and by siRNA knockdown of Cx40 in these cells. As Cx40 is highly expressed and is capable of coupling GENCs through gap junctions, the contributions of ATP release and purinergic signalling mechanisms to the observed calcium wave were evaluated by application of an ATP scavenging cocktail consisting of hexokinase, a glycolytic enzyme that utilizes high amounts of ATP, and apyrase to degrade extracellular ATP. During mechanical stimulation in the presence of the cocktail, calcium wave propagation between adjacent GENCs was greatly attenuated with no significant effect on dye transfer between cells, indicating a role for extracellular ATP in mediating calcium wave propagation. The authors utilized an ATP biosensor assay to measure ATP release from cultured GENCs. The biosensor system consisted of loading PC12 cells, which express a variety of purinergic receptors, with the calcium indicator dye Fluo-4 and applying them to a confluent monolayer of GENCs. In response to the mechanical stimuli, GENCs released ATP, which bound to purinergic receptors on the PC12 cells, thereby eliciting an increase in intracellular calcium as detected by Fluo-4 fluorescence, which enabled direct quantification of ATP release from the endothelial cells. This response was attenuated by inhibiting the purinergic receptors on PC12 cells with the non-selective P2 purinergic receptor antagonist suramin, which validated the specificity of their biosensor system to extracellular ATP. The mechanically induced release of ATP from GENCs was abolished by siRNA knockdown of Cx40 and could be restored by addition of exogenous ATP to the system.104 Altogether, these different studies suggest that these endothelial cells express undocked connexin hemichannels at the plasma membrane, which are capable of releasing ATP in response to mechanical stimulation.
While hypoxia has been suggested to increase flow-induced ATP release from endothelial cells,9 it has also been suggested that hypoxia inhibits ATP liberation by Cx43 hemichannels in the endothelium.19 The authors of the latter study concluded that hypoxia decreased ATP release from cultured endothelial cells by down regulation of Cx43 mRNA and hemichannel expression at the plasma membrane, as well as an upregulation of Cx43 phosphorylation at Ser368,121 which has been shown to inhibit channel activity. This observation suggests that connexin hemichannels may be implicated in ATP release under certain conditions; however, they do not play a role in hypoxia-induced ATP release from endothelial cells.
Connexin hemichannels may play an important role in the liberation of ATP during pathologies, such as acute infection, inflammation, and atherosclerosis. Endothelial cells exposed to the gram-positive bacterial wall component peptidoglycan (PDG) derived from Staphylococcus epidermidis have been shown to release ATP through Cx43 hemichannels.103 This study revealed an increase in Cx43 mRNA as well as induction of the pro-inflammatory cytokine interleukin-6 and Toll like receptor 2 in endothelial cells in response to PDG. The induction of this inflammatory cascade was coupled to increased Cx43 hemichannel activity at the plasma membrane of these cells and release of ATP into the media.
During atherosclerosis, circulating monocytes adhere to and migrate across the vascular endothelium where they differentiate into macrophages and foam cells in the subintimal space, eventually leading to atherosclerotic plaque formation and narrowing of the vessel lumen (for review see 122). A novel study by Wong et al.102 indicated a protective role for ATP released from circulating monocytes via Cx37 hemichannels during atherosclerosis. Complete genetic knockout of Cx37 in the proatherogenic ApoE−/− mouse line resulted in a marked increase in atherosclerotic lesion formation and area. Further investigation revealed that monocytes from the Cx37−/−/ApoE−/− mice had a significant decrease in basal ATP release when compared with those from mice expressing functional Cx37. This reduction in ATP liberation from monocytes lacking Cx37 resulted in a significant increase in monocyte adhesion to cultured endothelial cells, supporting their in vivo evidence for increased plaque formation and size. Monocyte adhesion was also increased in response to connexin hemichannel blockade by 18α-GA, and the connexin mimetic peptides Gap26 and Gap27. In a separate study, activation of neutrophils with fMLP (N-formyl Met-Leu-Pro) induced ATP release that resulted in decreased endothelial cell permeability.101 Release of ATP from these cells was significantly reduced upon addition of the Cx43-specific mimetic peptide 43Gap26 as well as from Cx43−/− neutrophils, indicating a release mechanism involving Cx43 hemichannels. The released ATP was actively degraded to adenosine by the activity of CD39 expressed on the neutrophils themselves and CD73 expressed on endothelial cells. Binding of adenosine to A2a adenosine receptors on the endothelial cells resulted in decreased paracellular permeability. The mechanisms surrounding A2a adenosine receptor-mediated changes in endothelial cell permeability have also been investigated.123 While the data presented above suggest a role for ATP release from Cx hemichannels in circulating inflammatory cells as a protective mechanism against infiltration into the vascular wall, a host of literature suggests that ATP promotes vascular smooth muscle cell migration and proliferation through purinergic signalling mechanisms.13,14,124 It has also recently been shown that fibroblasts release ATP from connexin hemichannels promoting profibrotic responses in the heart125 and these observations may suggest a role for resident vascular fibroblasts to release ATP via this mechanism to promote purinergic signalling in the blood vessel wall. Taken together, connexin hemichannels may be important for regulating ATP release in the blood vessel lumen to modulate endothelial cell permeability, while differential ATP release mechanisms in the vascular media may be involved in modulating vascular smooth muscle cell phenotype, such as ATP released from parasympathetic nerves.126
Altogether, the data presented above support a potential role for connexin hemichannels in ATP release under pathological (e.g. inflammatory insults, atherosclerosis, decreased [Ca2+]e) and possibly physiological conditions; however, it remains to be elucidated as to whether connexin hemichannels play a role in ATP release from vascular cells for the regulation of vascular tone and blood pressure.
2.5. Pannexin channels
In 2000, a novel protein family was identified termed pannexins (Panx) that are orthologs of the invertebrate gap junction proteins, the innexins, and share a similar membrane topology to mammalian gap junction proteins, connexins.127 To date, three pannexin isoforms have been identified (Panx1, Panx2, and Panx3), and the distribution of each across different tissues and cell types is a current topic of investigation. Panx1 and Panx3 are believed to assemble into hexamers, while Panx2 may form heptamers or octamers.128,129 The pannexins are assembled in the ER and Golgi and are transported to the plasma membrane in a fashion similar to the connexins; however, the pannexins do not form gap junctions and therefore their nomenclature is designated as pannexin channels and not hemichannels.130 This key difference is likely due to the fact that the pannexins are highly glycosylated on their extracellular loops, which may impede docking with pannexin channels on neighbouring cells.131,132 Therefore, it has been suggested that pannexins form membrane channels that allow transport of molecules between the intra- and extracellular space. Upon characterization of the properties of the channels formed by pannexins in a heterologous expression system, it was observed that unlike connexin hemichannels, pannexin channels are not regulated by [Ca2+]e, allowing these channels to open under physiological [Ca2+]e.133 Expression of Panx1 in xenopus oocytes revealed maxi anion currents upon membrane depolarization with KCl, and patch clamp studies demonstrated the permeability of these channels to ATP.134 This observation has since prompted multiple studies investigating the role of Panx1 channels in ATP release from a number of cell types, including: astrocytes, glial cells and neurons in the central nervous system,135–140 T-cells,141–143 airway epithelia,144,145 taste cells,146 keratinocytes,147 circulating erythrocytes,27,50 and vascular smooth muscle cells and endothelial cells.11,148 While Panx1 channels constitute ATP release channels that function under physiological conditions, there have also been reports implicating these proteins in apoptosis and cell death,149 as well as in activation of T-cells during inflammation.141–143 Therefore, much effort is currently devoted to identifying the mechanisms that regulate pannexin channel permeability to purines and other intracellular molecules, as well as potential binding partners that may regulate the release of ATP through these channels.
During cellular apoptosis, dying cells release nucleotides into the extracellular space to promote phagocyte chemotaxis, engulfment, and clearance. Recent evidence has implicated Panx1 channels in mediating ATP release during this process. Chekeni and co-authors observed activation of Panx1 channels in apoptotic cells induced by caspase cleavage of the intracellular carboxy-tail of Panx1. More recent evidence has characterized the importance of the Panx1 C-tail in the regulation of channel permeability.150 This event resulted in ATP release into the extracellular milieu and recruitment of phagocytes. The release of ATP as well as monocyte and leucocyte recruitment to cells stimulated with anti-Fas or UV light, both of which are known pro-apoptotic stimuli, were significantly attenuated upon knockdown of Panx1 with Panx1 siRNA, indicating that these channels are the conduit for ATP release and promotion of apoptotic cell clearance.149 While this study indicates a role for ATP release from Panx1 in cell death, ATP release from Panx1 has been shown to be protective during pathological insults such as ischaemia. In astrocytes, ischaemic stress has been shown to suppress the release of ATP to protect against cellular death and promote survival. This protection from ischaemic insult was suggested to occur through inhibition of ATP release from Panx1 channels, by a negative feedback mechanism involving the P2X7 purinergic receptor.136 Prolonged activation of P2X7 receptors results in the opening of a large pore permeable to divalent cations such as Ca2+ that eventually leads to cell death.151–153 This study revealed that ischaemic insult to cultured cortical astrocytes resulted in initial ATP release from Panx1 channels that in turn activated P2X7 receptors, inducing closure of Panx1 channels, which prevented the loss of integral intracellular constituents and averted cell death. Together, these studies identify differential roles for Panx1 in pathological states.
While evidence is accumulating that supports pannexins as ATP release channels across cell types, their expression and activity in the vasculature are a current topic of investigation. Initial studies have identified key roles for Panx1 channels in regulating ATP release from circulating erythrocytes during hypoxia and during membrane deformation,27,50 from vascular smooth muscle cells upon α1D-adrenergic receptor activation,11 and from endothelial cells in response to thrombin stimulation.148 Liberation of ATP from these cells occurs under physiological calcium concentrations and may play pivotal roles in regulating vessel tone and peripheral resistance. Initial evidence has suggested that ATP release from circulating erythrocytes in response to low oxygen tension occurs through the ABC-transporter CFTR25 (see ATP binding cassette transporters section); however, recent studies have presented conflicting evidence for the ability of ABC transporters to release ATP.154,155 A recent study by Locovei et al. demonstrated that ATP release during depolarization of erythrocytes by high extracellular K+, as well as decreased oxygen tension, was inhibited by the gap junction blocker carbenoxolone. The authors noted that the erythrocytes in this study had no detectable Cx43 expression, further suggesting that carbenoxolone inhibited Panx1 channels and not Cx hemichannels. While carbenoxolone has been shown to inhibit connexins as well as pannexins, the EC50 for pannexin inhibition is ∼5 µM, whereas incubation of xenopus oocytes expressing Cx46 (the model connexin isoform for examining properties of connexin hemichannels) with >10 µM carbenoxolone had no effect on hemichannel current.133 In a separate study, inhibition of ATP release from erythrocytes exposed to low oxygen tension was evaluated in the presence of three separate inhibitors of pannexin channels, carbenoxolone, probenecid, and the blocking peptide 10Panx1.27 This study revealed that the treatment of human erythrocytes with any of these three inhibitors significantly attenuated low oxygen tension-induced ATP release, further supporting a functional role for Panx1 channels in ATP release from circulating erythrocytes under these conditions.
Vascular smooth muscle cells in small arteries that are involved in regulation of blood flow and blood pressure have been shown to contract in response to α-adrenergic receptor stimulation. Sympathetic nervous impulses elicit release of the catecholamine norepinephrine from nerve terminals innervating vascular beds resulting in activation of the α1D-adrenergic receptor, an increase in smooth muscle cell [Ca2+]i and vasoconstriction, ultimately producing an increase in peripheral resistance and blood pressure.156 The Isakson laboratory recently identified a novel role for Panx1 channels in regulating the α1-adrenergic receptor mediated response.11 In this study, stimulation of isolated pressurized thoracodorsal arteries with the α1-adrenergic receptor agonist phenylephrine (PE) resulted in strong vasoconstriction, consistent with previous observations. Recent characterization of the thoracodorsal artery has shown that it is highly innervated by adrenergic nerves, develops spontaneous tone, and responds to various vasoactive agents similar to other small resistance artery models.157 In the presence of three different pannexin inhibitors probenecid, mefloquine, and 10Panx1 blocking peptide, the constriction to PE was significantly reduced. Also, application of apyrase to the superfusion bath surrounding the blood vessel resulted in a similar inhibition of contraction. Finally, stimulation of cultured human coronary arterial vascular smooth muscle cells with PE induced ATP release from these cells that were significantly attenuated by pretreatment of the cells with the 10Panx1 blocking peptide. Together, these results identify a novel role for vascular smooth muscle cell Panx1 channels in releasing ATP during α1-adrenergic receptor activation, a process that is essential for the regulation of vascular tone and blood pressure. These results indicate a potential therapeutic approach for blood pressure regulation. It was also observed that expression of Panx1 in endothelial cells of the blood vessel wall was significantly enhanced. In addition, Godecke et al.148 recently demonstrated that HUVECs release ATP in response to thrombin, which was sensitive to carbenoxolone and knockdown of endogenous Panx1. Furthermore, analysis of the expression patterns for the different pannexin isoforms in the smooth muscle and endothelial cells across the arterial tree suggests increased expression in smooth muscle cells as vessel diameter decreases, supporting the idea that ATP release from Panx1 channels in vascular smooth muscle cells of small arteries and arterioles may play an important role in regulating blood flow.158
To date, little is known about the role of Panx channels in the regulation of physiological processes, in particular those involving the vasculature and blood flow dynamics. However, characterization of the pharmacological properties of a number of gap junction inhibitors has suggested that some of the previous studies identifying connexin hemichannels as the cellular pathway for ATP release may potentially be due to activity of Panx channels.159 Further investigation into the expression patterns and regulatory mechanisms governing Panx channel activity in vascular endothelial and smooth muscle cells will help reveal novel signalling pathways that may participate in the regulation of vascular tone, peripheral resistance, and blood pressure, which could provide new targets for therapeutic intervention for the treatment of hyper/hypotension.
3. Conclusion
The release of intracellular ATP into the extracellular space promotes signalling through purinergic receptors that have been extensively characterized in multiple cell types and experimental systems. Importantly, evidence for purinergic signalling in the regulation of vascular tone at both vascular smooth muscle and endothelial cells warrants investigation into how this purine nucleotide is released from the cells. Current studies have proposed mechanisms of ATP release by vesicular exocytosis, transport through ABC transporters, a plasma membrane F1/F0-ATP synthase, and liberation via connexin hemichannels or pannexin channels (Figure 1). The stimuli used to evoke ATP release from vascular cells in these studies have varied and importantly it must be noted that quantification of ATP in cell supernatants can easily be confounded by stimuli that induce cell lysis. Because cells contain millimolar amounts of cytosolic ATP, rupture of even a single cell in a given preparation can release a sufficient amount of ATP to elicit physiological effects (i.e. Ca2+ wave propagation160) and may dramatically skew the quantification and interpretation of ATP released from cells. In particular, it has recently been noted that increases in shear stress on endothelial cells can induce cell lysis, which may be the rational for the noted increase in extracellular ATP.161 It is also not known whether the different pharmacological agents employed to evaluate the mechanisms of ATP release have effects on lytic-ATP release. It is therefore essential that experiments evaluating ATP release by quantification in cell supernatants be controlled for lytic-ATP release. Nonetheless, the studies reviewed above have examined ATP release from cells in the vasculature by multiple mechanisms and promote the idea that differential release mechanisms may be employed during specific physiological and pathological situations. However, the recently identified Panx1 channels have been shown to be closed at resting membrane potentials and are capable of opening in the presence of physiological extracellular calcium, in response to α1-adrenergic receptor activation and hypoxia and are ubiquitously expressed throughout the vasculature. These results support the notion that Panx channels may be the physiologic ATP release channels in the vascular system. The identification of Panx1 in the vascular endothelium raises the possibility that ATP released from these cells during periods of increased shear stress and hypoxia, or in response to other signalling mechanisms such as thrombin-induced ATP release, may occur by activation of these channels. Further investigation into the regulatory mechanisms of Panx channels in the vascular endothelium and smooth muscle cells, as well as circulating erythrocytes, may reveal new insight on the control of peripheral vascular resistance and blood pressure and introduce new drug targets for the treatment of vascular disease.
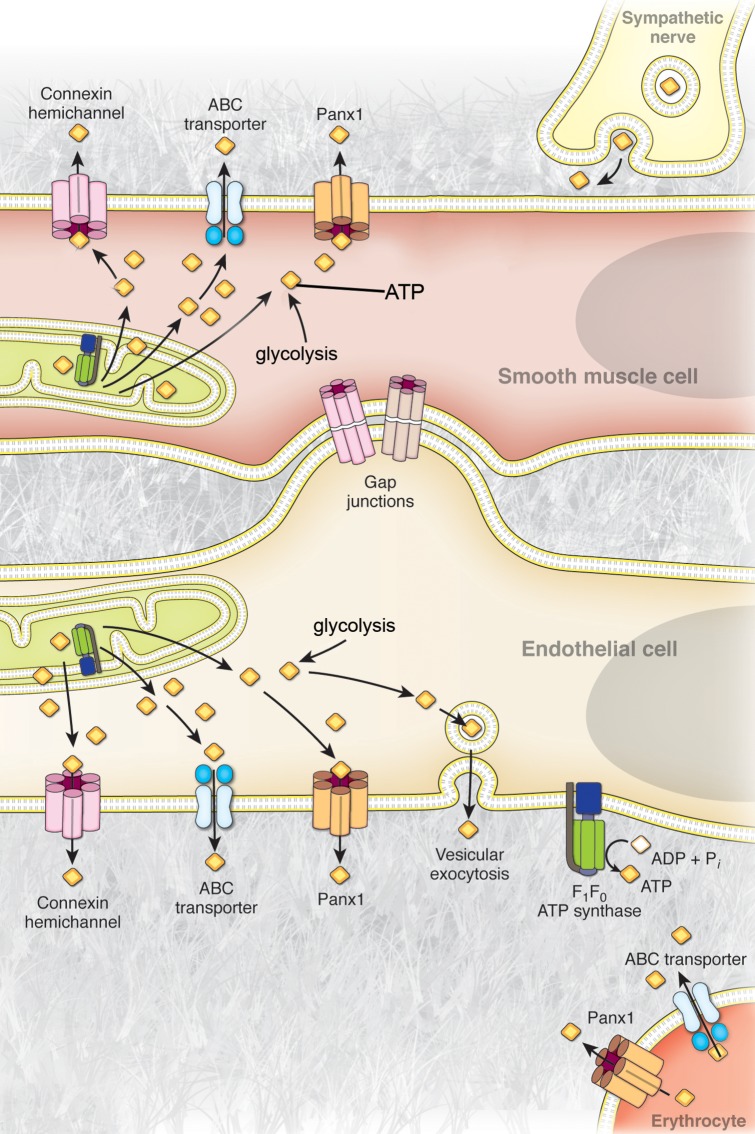
Figure 1.
Mechanisms of ATP release from cells of the blood vessel wall. Representative illustration of the currently proposed mechanisms of ATP release from endothelial cells, vascular smooth muscle cells, perivascular sympathetic nerves, and circulating erythrocytes in the vascular system. Cytosolic ATP available for release from these cells is generated by glycolysis in the cytosol and oxidative phospohorylation in the mitochondria. In sympathetic nerves, ATP release has been shown to occur via vesicular exocytosis. Vascular smooth muscle cells have been suggested to release ATP through membrane transporters and channels, including connexin hemichannels and pannexin channels, as well as potentially by ABC transporters. Vascular endothelial cells have been proposed to release ATP via vesicular exocytosis, ABC-transporters, connexin hemichannels, pannexin channels, and by direct synthesis at the extracellular plasma membrane by a cell surface F1/F0-ATP synthase. Circulating erythrocytes have been suggested to release ATP through ABC transporters and via pannexin channels.
Conflict of interest: none declared.
Funding
This work was supported by National Institutes of Health grants HL088554 and HL107963 (B.E.I.), American Heart Association Scientist Development Grant (B.E.I.), an American Heart Association postdoctoral fellowship (M.B.), and a National Institutes of Health Cardiovascular Training Grant (A.W.L.).
References
- 1.Gribble FM, Loussouarn G, Tucker SJ, Zhao C, Nichols CG, Ashcroft FM. A novel method for measurement of submembrane ATP concentration. J Biol Chem. 2000;275:30046–30049. doi: 10.1074/jbc.M001010200. [DOI] [PubMed] [Google Scholar]
- 2.Larcombe-McDouall J, Buttell N, Harrison N, Wray S. In vivo pH and metabolite changes during a single contraction in rat uterine smooth muscle. J Physiol. 1999;518(Pt 3):783–790. doi: 10.1111/j.1469-7793.1999.0783p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnstock G. Neural nomenclature. Nature. 1971;229:282–283. doi: 10.1038/229282d0. [DOI] [PubMed] [Google Scholar]
- 4.Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM. Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol. 2011;61:221–261. doi: 10.1016/B978-0-12-385526-8.00008-4. [DOI] [PubMed] [Google Scholar]
- 5.Li A, Banerjee J, Leung CT, Peterson-Yantorno K, Stamer WD, Civan MM. Mechanisms of ATP release, the enabling step in purinergic dynamics. Cell Physiol Biochem. 2011;28:1135–1144. doi: 10.1159/000335865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal. 2009;5:433–446. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrester T, Lind AR. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol. 1969;204:347–364. doi: 10.1113/jphysiol.1969.sp008917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1140–R1148. doi: 10.1152/ajpregu.90822.2008. [DOI] [PubMed] [Google Scholar]
- 9.Bodin P, Burnstock G. Synergistic effect of acute hypoxia on flow-induced release of ATP from cultured endothelial cells. Experientia. 1995;51:256–259. doi: 10.1007/BF01931108. [DOI] [PubMed] [Google Scholar]
- 10.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 11.Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, et al. Pannexin1 regulates alpha1-adrenergic receptor- mediated vasoconstriction. Circ Res. 2011;109:80–85. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D'Orleans-Juste P, et al. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. 2010;85:204–213. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erlinge D. Extracellular ATP: a growth factor for vascular smooth muscle cells. Gen Pharmacol. 1998;31:1–8. doi: 10.1016/s0306-3623(97)00420-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang DJ, Huang NN, Heppel LA. Extracellular ATP and ADP stimulate proliferation of porcine aortic smooth muscle cells. J Cell Physiol. 1992;153:221–233. doi: 10.1002/jcp.1041530202. [DOI] [PubMed] [Google Scholar]
- 15.Forrester T, Williams CA. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J Physiol. 1977;268:371–390. doi: 10.1113/jphysiol.1977.sp011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodin P, Bailey D, Burnstock G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol. 1991;103:1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodin P, Burnstock G. ATP-stimulated release of ATP by human endothelial cells. J Cardiovasc Pharmacol. 1996;27:872–875. doi: 10.1097/00005344-199606000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto K, Shimizu N, Obi S, Kumagaya S, Taketani Y, Kamiya A, et al. Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1646–H1653. doi: 10.1152/ajpheart.01385.2006. [DOI] [PubMed] [Google Scholar]
- 19.Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsuragi T, Tokunaga T, Ogawa S, Soejima O, Sato C, Furukawa T. Existence of ATP-evoked ATP release system in smooth muscles. J Pharmacol Exp Ther. 1991;259:513–518. [PubMed] [Google Scholar]
- 21.Lew MJ, White TD. Release of endogenous ATP during sympathetic nerve stimulation. Br J Pharmacol. 1987;92:349–355. doi: 10.1111/j.1476-5381.1987.tb11330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sneddon P, Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984;100:85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- 23.Sneddon P, Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984;106:149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- 24.Sneddon P, Westfall DP. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- 26.Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, et al. Rabbit erythrocytes release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Rep. 2009;61:183–190. doi: 10.1016/s1734-1140(09)70020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, et al. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2011;299:H1146–H1152. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopwood AM, Burnstock G. ATP mediates coronary vasoconstriction via P2x-purinoceptors and coronary vasodilatation via P2y-purinoceptors in the isolated perfused rat heart. Eur J Pharmacol. 1987;136:49–54. doi: 10.1016/0014-2999(87)90777-1. [DOI] [PubMed] [Google Scholar]
- 30.Houston DA, Burnstock G, Vanhoutte PM. Different P2-purinergic receptor subtypes of endothelium and smooth muscle in canine blood vessels. J Pharmacol Exp Ther. 1987;241:501–506. [PubMed] [Google Scholar]
- 31.Kennedy C, Delbro D, Burnstock G. P2-purinoceptors mediate both vasodilation (via the endothelium) and vasoconstriction of the isolated rat femoral artery. Eur J Pharmacol. 1985;107:161–168. doi: 10.1016/0014-2999(85)90055-x. [DOI] [PubMed] [Google Scholar]
- 32.Ralevic V, Mathie RT, Alexander B, Burnstock G. Characterization of P2X- and P2Y-purinoceptors in the rabbit hepatic arterial vasculature. Br J Pharmacol. 1991;103:1108–1113. doi: 10.1111/j.1476-5381.1991.tb12308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fredholm BB, AP IJ, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 35.von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 37.Li M, Kawate T, Silberberg SD, Swartz KJ. Pore-opening mechanism in trimeric P2X receptor channels. Nat Commun. 2010;1:44. doi: 10.1038/ncomms1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, et al. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 39.Benham CD. ATP-activated channels gate calcium entry in single smooth muscle cells dissociated from rabbit ear artery. J Physiol. 1989;419:689–701. doi: 10.1113/jphysiol.1989.sp017893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamont C, Vial C, Evans RJ, Wier WG. P2X1 receptors mediate sympathetic postjunctional Ca2+ transients in mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2006;291:H3106–H3113. doi: 10.1152/ajpheart.00466.2006. [DOI] [PubMed] [Google Scholar]
- 41.Buvinic S, Briones R, Huidobro-Toro JP. P2Y(1) and P2Y(2) receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol. 2002;136:847–856. doi: 10.1038/sj.bjp.0704789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raqeeb A, Sheng J, Ao N, Braun AP. Purinergic P2Y2 receptors mediate rapid Ca(2+) mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium. 2011;49:240–248. doi: 10.1016/j.ceca.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 43.da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation. 2009;119:871–879. doi: 10.1161/CIRCULATIONAHA.108.764571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrington LS, Mitchell JA. Novel role for P2X receptor activation in endothelium-dependent vasodilation. Br J Pharmacol. 2004;143:611–617. doi: 10.1038/sj.bjp.0706004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burnstock G. Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol Rep. 2008;60:12–20. [PubMed] [Google Scholar]
- 46.Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- 47.Bockman EL, Berne RM, Rubio R. Release of adenosine and lack of release of ATP from contracting skeletal muscle. Pflugers Arch. 1975;355:229–241. doi: 10.1007/BF00583686. [DOI] [PubMed] [Google Scholar]
- 48.Winbury MM, Papierski DH, Hemmer ML, Hambourger WE. Coronary dilator action of the adenine-ATP series. J Pharmacol Exp Ther. 1953;109:255–260. [PubMed] [Google Scholar]
- 49.Bunger R, Haddy FJ, Gerlach E. Coronary responses to dilating substances and competitive inhibition by theophylline in the isolated perfused guinea pig heart. Pflugers Arch. 1975;358:213–224. doi: 10.1007/BF00587218. [DOI] [PubMed] [Google Scholar]
- 50.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnstock G. Noradrenaline and ATP as cotransmitters in sympathetic nerves. Neurochem Int. 1990;17:357–368. doi: 10.1016/0197-0186(90)90158-p. [DOI] [PubMed] [Google Scholar]
- 52.Todorov LD, Mihaylova-Todorova S, Westfall TD, Sneddon P, Kennedy C, Bjur RA, et al. Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature. 1997;387:76–79. doi: 10.1038/387076a0. [DOI] [PubMed] [Google Scholar]
- 53.Jorgensen S. Breakdown of adenine and hypoxanthine nucleotides and nucleosides in human plasma. Acta Pharmacol Toxicol. 1956;12:294–302. [PubMed] [Google Scholar]
- 54.Yagi K, Shinbo M, Hashizume M, Shimba LS, Kurimura S, Miura Y. ATP diphosphohydrolase is responsible for ecto-ATPase and ecto-ADPase activities in bovine aorta endothelial and smooth muscle cells. Biochem Biophys Res Commun. 1991;180:1200–1206. doi: 10.1016/s0006-291x(05)81323-3. [DOI] [PubMed] [Google Scholar]
- 55.Cooper DR, Lewis GP, Lieberman GE, Webb H, Westwick J. ADP metabolism in vascular tissue, a possible thrombo-regulatory mechanism. Thromb Res. 1979;14:901–914. doi: 10.1016/0049-3848(79)90008-2. [DOI] [PubMed] [Google Scholar]
- 56.Pearson JD, Carleton JS, Gordon JL. Metabolism of adenine nucleotides by ectoenzymes of vascular endothelial and smooth-muscle cells in culture. Biochem J. 1980;190:421–429. doi: 10.1042/bj1900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 58.Kauffenstein G, Furstenau CR, D'Orleans-Juste P, Sevigny J. The ecto-nucleotidase NTPDase1 differentially regulates P2Y1 and P2Y2 receptor-dependent vasorelaxation. Br J Pharmacol. 2010;159:576–585. doi: 10.1111/j.1476-5381.2009.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Westfall DP, Stitzel RE, Rowe JN. The postjunctional effects and neural release of purine compounds in the guinea-pig vas deferens. Eur J Pharmacol. 1978;50:27–38. doi: 10.1016/0014-2999(78)90250-9. [DOI] [PubMed] [Google Scholar]
- 60.Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194(Pt 3):335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olson L, Alund M, Norberg KA. Fluorescence-microscopical demonstration of a population of gastro-intestinal nerve fibres with a selective affinity for quinacrine. Cell Tissue Res. 1976;171:407–423. doi: 10.1007/BF00220234. [DOI] [PubMed] [Google Scholar]
- 62.Irvin JL, Irvin EM. The interaction of quinacrine with adenine nucleotides. J Biol Chem. 1954;210:45–56. [PubMed] [Google Scholar]
- 63.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nelson N, Perzov N, Cohen A, Hagai K, Padler V, Nelson H. The cellular biology of proton-motive force generation by V-ATPases. J Exp Biol. 2000;203:89–95. doi: 10.1242/jeb.203.1.89. [DOI] [PubMed] [Google Scholar]
- 66.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 67.Abraham EH, Prat AG, Gerweck L, Seneveratne T, Arceci RJ, Kramer R, et al. The multidrug resistance (mdr1) gene product functions as an ATP channel. Proc Natl Acad Sci USA. 1993;90:312–316. doi: 10.1073/pnas.90.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reisin IL, Prat AG, Abraham EH, Amara JF, Gregory RJ, Ausiello DA, et al. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem. 1994;269:20584–20591. [PubMed] [Google Scholar]
- 69.Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, et al. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- 70.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 71.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 72.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 73.Stutts MJ, Chinet TC, Mason SJ, Fullton JM, Clarke LL, Boucher RC. Regulation of Cl- channels in normal and cystic fibrosis airway epithelial cells by extracellular ATP. Proc Natl Acad Sci USA. 1992;89:1621–1625. doi: 10.1073/pnas.89.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robert R, Norez C, Becq F. Disruption of CFTR chloride channel alters mechanical properties and cAMP-dependent Cl- transport of mouse aortic smooth muscle cells. J Physiol. 2005;568:483–495. doi: 10.1113/jphysiol.2005.085019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tousson A, Van Tine BA, Naren AP, Shaw GM, Schwiebert LM. Characterization of CFTR expression and chloride channel activity in human endothelia. Am J Physiol. 1998;275:C1555–C1564. doi: 10.1152/ajpcell.1998.275.6.C1555. [DOI] [PubMed] [Google Scholar]
- 76.Mattoscio D, Evangelista V, De Cristofaro R, Recchiuti A, Pandolfi A, Di Silvestre S, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) expression in human platelets: impact on mediators and mechanisms of the inflammatory response. FASEB J. 2010;24:3970–3980. doi: 10.1096/fj.10-159921. [DOI] [PubMed] [Google Scholar]
- 77.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol. 2001;281:C1158–C1164. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]
- 78.Aguilar-Bryan L, Nichols CG, Rajan AS, Parker C, Bryan J. Co-expression of sulfonylurea receptors and KATP channels in hamster insulinoma tumor (HIT) cells. Evidence for direct association of the receptor with the channel. J Biol Chem. 1992;267:14934–14940. [PubMed] [Google Scholar]
- 79.Henquin JC, Charles S, Nenquin M, Mathot F, Tamagawa T. Diazoxide and D600 inhibition of insulin release. Distinct mechanisms explain the specificity for different stimuli. Diabetes. 1982;31:776–783. doi: 10.2337/diab.31.9.776. [DOI] [PubMed] [Google Scholar]
- 80.Larsson O, Ammala C, Bokvist K, Fredholm B, Rorsman P. Stimulation of the KATP channel by ADP and diazoxide requires nucleotide hydrolysis in mouse pancreatic beta-cells. J Physiol. 1993;463:349–365. doi: 10.1113/jphysiol.1993.sp019598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hassessian H, Bodin P, Burnstock G. Blockade by glibenclamide of the flow-evoked endothelial release of ATP that contributes to vasodilatation in the pulmonary vascular bed of the rat. Br J Pharmacol. 1993;109:466–472. doi: 10.1111/j.1476-5381.1993.tb13592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, et al. Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science. 1996;271:1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- 83.Tu J, Le G, Ballard HJ. Involvement of the cystic fibrosis transmembrane conductance regulator in the acidosis-induced efflux of ATP from rat skeletal muscle. J Physiol. 2010;588:4563–4578. doi: 10.1113/jphysiol.2010.195255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun J, Usune S, Zhao Y, Migita K, Katsuragi T. Multidrug resistance protein transporter and Ins(1,4,5)P-sensitive Ca(2)+-signaling involved in adenosine triphosphate export via Gq protein-coupled NK-receptor stimulation with neurokinin A. J Pharmacol Sci. 2010;114:92–98. doi: 10.1254/jphs.10145fp. [DOI] [PubMed] [Google Scholar]
- 85.Devenish RJ, Prescott M, Rodgers AJ. The structure and function of mitochondrial F1F0-ATP synthases. Int Rev Cell Mol Biol. 2008;267:1–58. doi: 10.1016/S1937-6448(08)00601-1. [DOI] [PubMed] [Google Scholar]
- 86.Moser TL, Stack MS, Asplin I, Enghild JJ, Hojrup P, Everitt L, et al. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci USA. 1999;96:2811–2816. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chi SL, Pizzo SV. Cell surface F1Fo ATP synthase: a new paradigm? Ann Med. 2006;38:429–438. doi: 10.1080/07853890600928698. [DOI] [PubMed] [Google Scholar]
- 88.Das B, Mondragon MO, Sadeghian M, Hatcher VB, Norin AJ. A novel ligand in lymphocyte-mediated cytotoxicity: expression of the beta subunit of H+ transporting ATP synthase on the surface of tumor cell lines. J Exp Med. 1994;180:273–281. doi: 10.1084/jem.180.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cavelier C, Ohnsorg PM, Rohrer L, von Eckardstein A. The beta-chain of cell surface F(0)F(1) ATPase modulates apoA-I and HDL transcytosis through aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:131–139. doi: 10.1161/ATVBAHA.111.238063. [DOI] [PubMed] [Google Scholar]
- 90.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 91.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Cao Y, et al. Angiostatin: a circulating endothelial cell inhibitor that suppresses angiogenesis and tumor growth. Cold Spring Harb Symp Quant Biol. 1994;59:471–482. doi: 10.1101/sqb.1994.059.01.052. [DOI] [PubMed] [Google Scholar]
- 92.Gerasimovskaya EV, Woodward HN, Tucker DA, Stenmark KR. Extracellular ATP is a pro-angiogenic factor for pulmonary artery vasa vasorum endothelial cells. Angiogenesis. 2008;11:169–182. doi: 10.1007/s10456-007-9087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, et al. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci USA. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koval M. Pathways and control of connexin oligomerization. Trends Cell Biol. 2006;16:159–166. doi: 10.1016/j.tcb.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gilula NB, Reeves OR, Steinbach A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972;235:262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- 96.Bennett MV, Goodenough DA. Gap junctions, electrotonic coupling, and intercellular communication. Neurosci Res Program Bull. 1978;16:1–486. [PubMed] [Google Scholar]
- 97.Loewenstein WR. The cell-to-cell channel of gap junctions. Cell. 1987;48:725–726. doi: 10.1016/0092-8674(87)90067-5. [DOI] [PubMed] [Google Scholar]
- 98.Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, et al. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem. 2011;118:826–840. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Decrock E, De Vuyst E, Vinken M, Van Moorhem M, Vranckx K, Wang N, et al. Connexin 43 hemichannels contribute to the propagation of apoptotic cell death in a rat C6 glioma cell model. Cell Death Differ. 2009;16:151–163. doi: 10.1038/cdd.2008.138. [DOI] [PubMed] [Google Scholar]
- 100.De Vuyst E, Wang N, Decrock E, De Bock M, Vinken M, Van Moorhem M, et al. Ca(2+) regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium. 2009;46:176–187. doi: 10.1016/j.ceca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 101.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 102.Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, et al. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12:950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 103.Robertson J, Lang S, Lambert PA, Martin PE. Peptidoglycan derived from Staphylococcus epidermidis induces Connexin43 hemichannel activity with consequences on the innate immune response in endothelial cells. Biochem J. 2010;432:133–143. doi: 10.1042/BJ20091753. [DOI] [PubMed] [Google Scholar]
- 104.Toma I, Bansal E, Meer EJ, Kang JJ, Vargas SL, Peti-Peterdi J. Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1769–R1776. doi: 10.1152/ajpregu.00489.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2005;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- 106.Song M, Yu X, Cui X, Zhu G, Zhao G, Chen J, et al. Blockade of connexin 43 hemichannels reduces neointima formation after vascular injury by inhibiting proliferation and phenotypic modulation of smooth muscle cells. Exp Biol Med (Maywood) 2009;234:1192–1200. doi: 10.3181/0902-RM-80. [DOI] [PubMed] [Google Scholar]
- 107.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 108.Li H, Liu TF, Lazrak A, Peracchia C, Goldberg GS, Lampe PD, et al. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thimm J, Mechler A, Lin H, Rhee S, Lal R. Calcium-dependent open/closed conformations and interfacial energy maps of reconstituted hemichannels. J Biol Chem. 2005;280:10646–10654. doi: 10.1074/jbc.M412749200. [DOI] [PubMed] [Google Scholar]
- 111.Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Functioning of cx43 hemichannels demonstrated by single channel properties. Cell Commun Adhes. 2003;10:245–249. doi: 10.1080/cac.10.4-6.245.249. [DOI] [PubMed] [Google Scholar]
- 112.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sanchez HA, Orellana JA, Verselis VK, Saez JC. Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells. Am J Physiol Cell Physiol. 2009;297:C665–C678. doi: 10.1152/ajpcell.00200.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, et al. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hakim CH, Jackson WF, Segal SS. Connexin isoform expression in smooth muscle cells and endothelial cells of hamster cheek pouch arterioles and retractor feed arteries. Microcirculation. 2008;15:503–514. doi: 10.1080/10739680801982808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haefliger JA, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall. Cardiovasc Res. 2004;62:345–356. doi: 10.1016/j.cardiores.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 117.Johnstone S, Isakson B, Locke D. Biological and biophysical properties of vascular connexin channels. Int Rev Cell Mol Biol. 2009;278:69–118. doi: 10.1016/S1937-6448(09)78002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, et al. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86:649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- 119.Isakson BE. Localized expression of an Ins(1,4,5)P3 receptor at the myoendothelial junction selectively regulates heterocellular Ca2+ communication. J Cell Sci. 2008;121:3664–3673. doi: 10.1242/jcs.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nishiyama A, Rahman M, Inscho EW. Role of interstitial ATP and adenosine in the regulation of renal hemodynamics and microvascular function. Hypertens Res. 2004;27:791–804. doi: 10.1291/hypres.27.791. [DOI] [PubMed] [Google Scholar]
- 121.Bao X, Altenberg GA, Reuss L. Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am J Physiol Cell Physiol. 2004;286:C647–C654. doi: 10.1152/ajpcell.00295.2003. [DOI] [PubMed] [Google Scholar]
- 122.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Umapathy NS, Fan Z, Zemskov EA, Alieva IB, Black SM, Verin AD. Molecular mechanisms involved in adenosine-induced endothelial cell barrier enhancement. Vasc Pharmacol. 2010;52:199–206. doi: 10.1016/j.vph.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Erlinge D, You J, Wahlestedt C, Edvinsson L. Characterisation of an ATP receptor mediating mitogenesis in vascular smooth muscle cells. Eur J Pharmacol. 1995;289:135–149. doi: 10.1016/0922-4106(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 125.Lu D, Soleymani S, Madakshire R, Insel PA. ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y2 receptors. FASEB J. 2012 doi: 10.1096/fj.12-204677. Advance Access published March 13, 2012, doi:10.1096/fj.12-204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Southwell BR, Chamley-Campbell JH, Campbell GR. Tropic interactions between sympathetic nerves and vascular smooth muscle. J Auton Nerv Syst. 1985;13:343–354. doi: 10.1016/0165-1838(85)90022-0. [DOI] [PubMed] [Google Scholar]
- 127.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–R474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 128.Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, et al. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem. 2010;285:24420–24431. doi: 10.1074/jbc.M110.115444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–31743. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- 130.Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, et al. Pannexin channels are not gap junction hemichannels. Channels (Austin) 2011;5:193–197. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Penuela S, Bhalla R, Nag K, Laird DW. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell. 2009;20:4313–4323. doi: 10.1091/mbc.E09-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, et al. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120:3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 133.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 134.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 135.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, et al. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- 136.Iwabuchi S, Kawahara K. Functional significance of the negative-feedback regulation of ATP release via pannexin-1 hemichannels under ischemic stress in astrocytes. Neurochem Int. 2011;58:376–384. doi: 10.1016/j.neuint.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 137.Kawamura M, Jr., Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J Neurosci. 2010;30:3886–3895. doi: 10.1523/JNEUROSCI.0055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte ‘hemichannels. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Madry C, Haglerod C, Attwell D. The role of pannexin hemichannels in the anoxic depolarization of hippocampal pyramidal cells. Brain. 2010;133:3755–3763. doi: 10.1093/brain/awq284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woehrle T, Yip L, Manohar M, Sumi Y, Yao Y, Chen Y, et al. Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol. 2010;88:1181–1189. doi: 10.1189/jlb.0410211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, et al. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116:3475–3484. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 144.Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, et al. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286:26277–26286. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–5906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Celetti SJ, Cowan KN, Penuela S, Shao Q, Churko J, Laird DW. Implications of pannexin 1 and pannexin 3 for keratinocyte differentiation. J Cell Sci. 2010;123:1363–1372. doi: 10.1242/jcs.056093. [DOI] [PubMed] [Google Scholar]
- 148.Godecke S, Roderigo C, Rose CR, Rauch BH, Godecke A, Schrader J. Thrombin-induced ATP release from human umbilical vein endothelial cells. Am J Physiol Cell Physiol. 2012;302:C915–C923. doi: 10.1152/ajpcell.00283.2010. [DOI] [PubMed] [Google Scholar]
- 149.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sandilos JK, Chiu YH, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, et al. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C terminal autoinhibitory region. J Biol Chem. 2012 doi: 10.1074/jbc.M111.323378. Advance Access published February 6, 2012; doi:10.1074/jbc.M111.323378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Farrell AW, Gadeock S, Pupovac A, Wang B, Jalilian I, Ranson M, et al. P2X7 receptor activation induces cell death and CD23 shedding in human RPMI 8226 multiple myeloma cells. Biochim Biophy Acta. 2010;1800:1173–1182. doi: 10.1016/j.bbagen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 152.Constantinescu P, Wang B, Kovacevic K, Jalilian I, Bosman GJ, Wiley JS, et al. P2X7 receptor activation induces cell death and microparticle release in murine erythroleukemia cells. Biochim Biophys Acta. 2010;1798:1797–1804. doi: 10.1016/j.bbamem.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 153.Brough D, Le Feuvre RA, Iwakura Y, Rothwell NJ. Purinergic (P2X7) receptor activation of microglia induces cell death via an interleukin-1-independent mechanism. Mol Cell Neurosci. 2002;19:272–280. doi: 10.1006/mcne.2001.1054. [DOI] [PubMed] [Google Scholar]
- 154.Abraham EH, Okunieff P, Scala S, Vos P, Oosterveld MJ, Chen AY, et al. Cystic fibrosis transmembrane conductance regulator and adenosine triphosphate. Science. 1997;275:1324–1326. doi: 10.1126/science.275.5304.1324. [DOI] [PubMed] [Google Scholar]
- 155.Grygorczyk R, Tabcharani JA, Hanrahan JW. CFTR channels expressed in CHO cells do not have detectable ATP conductance. J Membr Biol. 1996;151:139–148. doi: 10.1007/s002329900065. [DOI] [PubMed] [Google Scholar]
- 156.Jackson WF, Boerman EM, Lange EJ, Lundback SS, Cohen KD. Smooth muscle alpha1D-adrenoceptors mediate phenylephrine-induced vasoconstriction and increases in endothelial cell Ca2+ in hamster cremaster arterioles. Br J Pharmacol. 2008;155:514–524. doi: 10.1038/bjp.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Billaud M, Lohman AW, Straub AC, Parpaite T, Johnstone SR, Isakson BE. Characterization of the thoracodorsal artery: morphology and reactivity. Microcirculation. 2012;19:360–372. doi: 10.1111/j.1549-8719.2012.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lohman AW, Billaud M, Straub AC, Johnstone SR, Best AK, Lee MY, et al. Expression of pannexin isoforms in the systemic murine arterial network. J Vasc Res. 2012 doi: 10.1159/000338758. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.D'Hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B. Pannexins, distant relatives of the connexin family with specific cellular functions? BioEssays. 2009;31:953–974. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- 160.Isakson BE, Evans WH, Boitano S. Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am J Physiol Lung Cell Mol Physiol. 2001;280:L221–L228. doi: 10.1152/ajplung.2001.280.2.L221. [DOI] [PubMed] [Google Scholar]
- 161.Burghoff S, Schrader J. Secretome of human endothelial cells under shear stress. J Proteome Res. 2011;10:1160–1169. doi: 10.1021/pr100937a. [DOI] [PubMed] [Google Scholar]