Abstract
Aims
The objective of the present study is to elucidate the pathogenic role of eicosanoids in myocardial infarction (MI). The accumulation of eicosanoid metabolites in ischaemic myocardium has been demonstrated in animal models and patients with MI, and it occurs in parallel with the development of irreversible cardiac damage. However, the key question that remains unanswered is whether cardiac-generated eicosanoids are the cause or the consequence of cardiac cell damage in MI.
Methods and results
We used a clinically relevant animal model of MI and metabolic profiling to monitor the eicosanoid profile in ischaemic myocardium. We demonstrate that ischaemia induces the generation of prostanoids mainly through the cyclooxygenase (COX)-1 pathway in the myocardium. Cardiac-generated prostanoids, particularly prostaglandin D2 (PGD2), can directly induce apoptosis in cardiac myocytes. This effect involves the up-regulation of the pro-apoptotic gene, Fas ligand (FasL), in a D-type prostanoid receptor-independent manner. The treatment of the MI mice with low-dose aspirin effectively inhibits the ischaemia-induced prostanoid generation and FasL expression in the myocardium, leading to the reduction in cardiac apoptosis following cardiac ischaemia.
Conclusions
Cardiac ischaemia results in COX-1-mediated generation of prostanoids, which by inducing cardiac myocyte apoptosis, contribute to the cardiac cell loss following MI. The benefits of low-dose aspirin treatment in MI may be attributable, in part, to the inhibition of cardiac prostanoid generation and attenuation of apoptosis. Further understanding of the mechanisms underlying prostanoid-induced cardiac apoptosis may be of significant value in designing new therapeutic strategies to prevent aberrant cell loss following MI and subsequent progression to heart failure.
Keywords: Myocardial infarction, Eicosanoids, Prostanoids, Apoptosis, Metabolomics
1. Introduction
Acute myocardial infarction (MI) remains a leading cause of morbidity and mortality worldwide. Ischaemia of significant duration overwhelms myocardial cellular repair mechanisms and results in irreversible myocardial cell damage or death. It has long been recognized that the disturbance in membrane lipid metabolism may play an important role in the onset of irreversible myocardial damage.1 The eicosanoids consist mainly of the prostanoids, leukotrienes, and epoxyeicosatrienoic acids, which are a group of lipid mediators derived from the cell membrane component arachidonic acid (AA). The majority of AA in the heart is esterified to the sn-2 position of myocardial phospholipids. The accumulation of free AA and the eicosanoid metabolites in ischaemic myocardium has been demonstrated in animal models and patients with MI, suggesting the involvement of AA metabolites in the pathogenesis of cardiac injury.2 However, the key question that remains unanswered is whether cardiac-generated eicosanoids are the cause or the consequence of myocardial damage in MI.
Prostanoids, including prostaglandins (PGs) and thromboxanes (TX), are generated from AA by the enzyme cyclooxygenases (COXs), which exist as distinct isoforms referred to as COX-1 and -2.3 The importance of prostanoids in the pathogenesis of cardiovascular disease has been widely appreciated with the evidence that low-dose aspirin, which is a competitive inhibitor and covalently modifies the COX protein, reduces the incidence and mortality from MI.4 However, recent compelling evidence demonstrating an increase in cardiovascular events associated with the use of COX-2 selective inhibitors5 reinforces the fact that a remarkably diverse and contrasting range of biological effects can be attributable to these lipids,6 and there is much to be learned about the roles of prostanoids in the pathogenesis of MI. Recently, our laboratory has taken advantage of a new technique of metabolic profiling using liquid chromatography-tandem mass spectrometry (LC-MS/MS) to elucidate the contribution of AA metabolism in cardiovascular diseases.7,8 In the present study, using a similar approach, we directly test the overall hypothesis that cardiac-generated eicosanoids can induce cardiac myocyte apoptosis and contribute to the cardiac cell loss following MI. We quantified the profile of prostanoid release during cardiac ischaemia, and revealed a novel mechanism underlying prostanoid-mediated myocardial injury using multiple approaches.
2. Methods
All animal care and procedures were approved by the University of California, Davis Institutional Animal Care and Use Committee. The study conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Detailed in vivo experimental designs and methods are presented in the Supplementary material online.
2.1. Overall experimental designs
In the present study, we used a clinical relevant mouse model of MI and metabolic profiling to determine prostanoids generation in plasma and cardiac tissues following myocardial ischaemia. Western blot analyses and COX activity assay were used to determine the mechanism underlying the ischaemia-induced prostanoids generation. We further used in vitro cultured neonatal cardiomyocytes to directly test whether the cardiac-generated prostanoids were involved in mediating cardiac myocyte injury. In order to elucidate the mechanistic pathways involved in prostaglandin D2 (PGD2)-induced cardiac myocyte apoptosis, immunofluorescence confocal microscopy was used to determine the expression of PGD2 receptors in cardiac myocytes. The apoptosis PCR array was used to identify pro-apoptotic genes that were directly up-regulated by PGD2 treatment in cardiac myocytes. Furthermore, using the combination of prostanoid profiling, apoptosis assay, western blot, and echocardiography analysis, we tested whether the treatment of the MI mice with low-dose aspirin could inhibit the cardiac generation of prostanoids and attenuate ischaemia-induced myocardial apoptosis, which may lead to the preservation of cardiac function following MI.
2.2. MI model in mice
The MI model in mice was created using the procedure as previously described.9,10 Briefly, 12–16-week-old male C57Bl/6J mice (Charles River, Wilmington, MA) were anaesthetized with intraperitoneal ketamine 80 mg/kg and xylazine 6 mg/kg. Isoflurane was used to maintain the anaesthesia during the surgery. The depth of anaesthesia was monitored by toe pinch, respiratory, and heart rate. The left anterior descending (LAD) coronary artery was ligated and maintained for 45 min after which the occlusion was released. The sham-operated mice underwent the same procedure without tying the suture but left it around the LAD for 45 min. For aspirin treatment, the mice were randomized 3 days before surgery to receive aspirin (acetylsalicylic acid, ASA, Sigma) in drinking water (50 μg/mL) or water alone.
2.3. Assessment of prostanoid generation
Blood samples were collected from MI or sham-operated mice, immediately centrifuged at 1500 rcf for 10 min. The plasma was collected and frozen at −70°C until time of analysis. Heart tissues were collected from MI or sham-operated mice. Eicosanoids were extracted from the murine plasma and tissue extraction and analyzed with LC-MS/MS methods described previously.7 The technique allows us to determine multiple eicosanoid mediators generated through COX, lipoxygenase, and cytochrome P450 pathways simultaneously (see Supplementary material online, Table S1).
2.4. Langendorff perfusion apparatus
Adult mice were sacrificed using intraperitoneal ketamine 80 mg/kg and xylazine 6 mg/kg followed by rapid heart excision. The hearts were then perfused using a retrograde Langendorff method. After an equilibration period of 30 min, hearts in the MI group were subjected to 45 min of global ischaemia by turning off flow of perfusion solution to the heart, while hearts in the sham group were perfused continuously for another 45 min. Then all hearts were briefly rinsed with ice cold PBS and tissue prostanoids were extracted and determined as described above.
2.5. Western blot analysis
Heart tissues or cultured neonatal cardiac myocytes were collected in ice-cold lysis buffer, homogenized, and centrifuged at 1000 g for 5 min at 4°C to remove cell debris. The following primary antibodies were used: COX-1 1:200 from Cayman Chemical, COX-2 1:200 from Cayman Chemical, and FasL 1:300 from Cell Signaling.
2.6. Measurement of COX activity
The COX activity (COX-1 and -2) in tissue homogenates was determined using the COX activity kit (Cayman chemical). The COX-1 activity was calculated as the difference between the total COX activity in the sample without an inhibitor and the sample with Sc560, and the sample with DuP-697. Each sample and the COX standard were assayed in triplicate.
2.7. Mouse neonatal cardiac myocytes culture and prostanoid treatment
Primary cardiac myocytes culture was obtained from 1- to 2-day old mouse pups as previously described.11 The animals were anaesthesized with intraperitoneal pentobarbital (40 mg/kg) after which the hearts were rapidly excised. All cultures were serum starved for 24 h before starting the experiments. PGD2, PGI2, and PGE2 (Cayman Chemical) were dissolved in ethanol as 1000× stock solution and were added into culture medium (at concentrations from 10 nM to 100 μM) for 6 h.
2.8. Detection of cardiac myocyte apoptosis
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay was used to detect cardiac myocyte apoptosis in both cultured neonatal cardiac myocytes and cardiac tissues from the MI mice as previously described.9 At least 1500 DAPI-stained cells in multiple random fields were counted in each group to assess the fraction of apoptotic cells determined by dividing the number of TUNEL-positive cells by the total number of DAPI-positive cardiac myocyte nuclei.
2.9. Analysis of cardiac function by echocardiography
Echocardiograms were performed in conscious animals to assess the systolic function using M-mode and two-dimensional measurements as we have previously described.9,11 Fractional shortening (FS), a surrogate of systolic function, was calculated from left ventricle dimensions as follows: FS = ((EDD − ESD)/EDD) × 100%, where EDD and ESD represent end-diastolic and end-systolic dimensions, respectively.
2.10. Immunofluorescence confocal microscopic imaging
To detect the expression of PGD2 receptors in cardiac myocytes, cultured neonatal cardiac myocytes were fixed with 4% paraformaldehyde, incubated with primary antibodies for α-actinin (1:500, Sigma) together with D-type prostanoid receptor (DP-1) (1:300, Cayman Chemical) or the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2 or DP-2; 1:500, Thermo Scientific). Immunofluorescence stainings were visualized using a confocal microscope (Pascal Zeiss Confocal Microscope).
2.11. Apoptosis PCR array
RNA was isolated from cultured neonatal cardiac myocytes using the RNeasy mini kit (QIAGEN) according to manufacturer's instruction. cDNAs were prepared using the RT First Strand kit (SABiosciences) and loaded on Mouse Apoptosis PCR Array plates (SABiosciences). Real-Time PCR was performed on ABI: 7900HT system and data were analyzed using PCR Array Data Analysis Web Portal (SABiosciences).
2.12. Statistical analysis
Results are expressed as the mean ± SD. Statistical analysis was performed with Student's t-test and one-way ANOVA followed by Tukey or Games-Howell tests for post hoc comparison. Statistical significance was considered to be achieved when P < 0.05. All experiments were repeated at least three times.
3. Results
3.1. A significant increase in plasma and cardiac-generated prostanoids following myocardial ischaemia
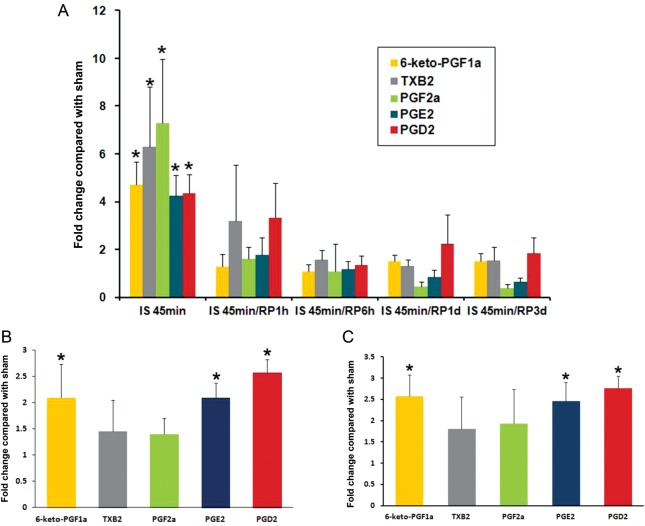
As a first step to assess the generation of prostanoids after MI, the measurement of the plasma levels of COX metabolites demonstrated significant increases of all prostanoids including PGE2, PGD2, PGF2α, 6-keto-PGF1α (a stable metabolite of PGI2), and TXB2 (a stable metabolite of TXA2) after 45 min of myocardial ischaemia, to a level four to seven times higher than sham-operated animals. These levels decreased gradually upon reperfusion (Figure 1A). To further determine whether the elevated plasma levels of prostanoids were generated from cardiac tissue, tissue prostanoids were measured from mouse hearts that were subjected to 45 min of ischaemia or sham operation. The plasma and cardiac tissue samples were obtained from the same animals. Tissue levels of PGD2, PGE2, and 6-keto-PGF1α were also significantly higher in the MI mice than that in the sham-operated mice, while the tissue level of TXB2 and PGF2α were not significantly different from sham-operated animals (Figure 1B). In order to exclude the possible interference from circulating blood cells, cardiac generation of prostanoids was further tested using a Langendorff perfusion system where isolated mouse hearts were perfused in the Langendorff mode and subjected to 45 min of no-flow ischaemia. The measurement of tissue prostanoids from mouse hearts after 45 min of no-flow ischaemia showed significant elevated tissue levels of PGD2, PGE2, and 6-keto-PGF1α compared with control (sham) hearts, a prostanoid pattern similar to the in vivo ischaemic hearts (Figure 1C).
Figure 1.
The assessment of plasma and cardiac tissue levels of prostanoids. (A) Plasma levels of prostanoids from mice subjected to 45 min of LAD ligation with or without subsequent reperfusion. Values are fold changes compared with sham-operated mice at the same time points. (B) Prostanoids levels from mouse cardiac tissues subjected to 45 min of ischaemia with LAD ligation. (C) Prostanoid levels from mouse cardiac tissue subjected to 45 min of global ischaemia with the Langendorff perfusion system. Values are fold changes compared with sham hearts, which were subjected to 45 min of continuous perfusion instead of ischaemia. Data are presented as the mean ± SD, n = 4 in each group, *P < 0.05 compared with sham.
3.2. No significant changes in cardiac COX expression or activity during myocardial ischaemia
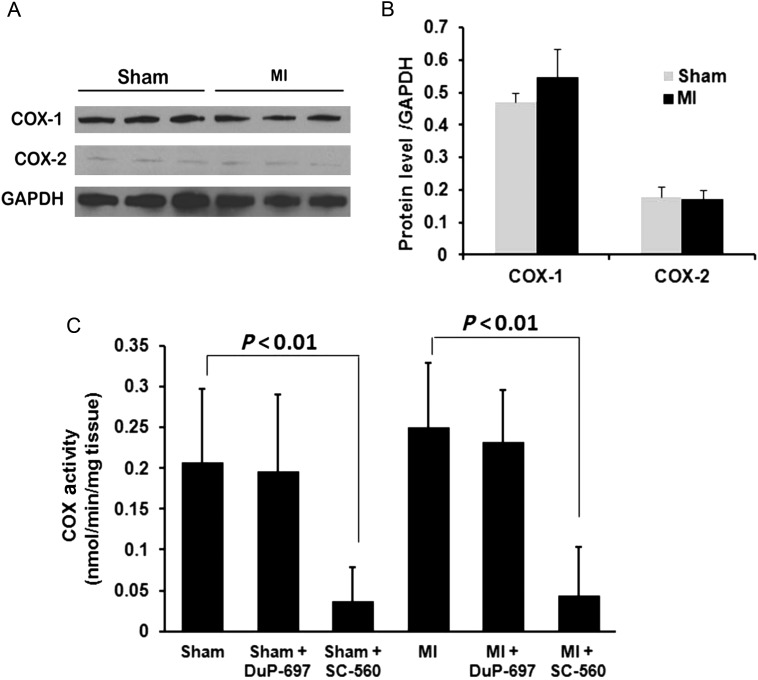
In order to determine whether the elevated levels of prostanoids in cardiac tissue after MI were due to the up-regulation or enhanced activity of the key enzyme COX, we examined mouse hearts for the expression of COX-1 and -2. Both COX-1 and -2 proteins were detected in cardiac tissues by western blot, with COX-1 expressing at a higher level than COX-2 expression. The expression levels of both COX-1 and -2 were not different between ischaemic hearts and sham-operated hearts (Figure 2A). The COX activity (COX-1 and -2) was measured in ischaemic and sham-operated heart tissues and revealed no significant difference between ischaemic and sham-operated hearts. Consistent with the protein expression levels, the COX activity was significantly inhibited by a COX-1 selective inhibitor, SC-560, but not a COX-2 selective inhibitor, DuP-697, in cardiac tissues from both sham and MI mice. Taken together, our data demonstrate that the significant increases in the levels of prostanoids in the cardiac tissues as well as in the plasma after 45 min of myocardial ischaemia are likely a result of increases in the availability of AA with no changes in the expression or activities of COX-1 and -2 (see Supplementary material online, Table S1).
Figure 2.
The expression of the COX protein and the COX activity in cardiac tissues. (A) Western blot analysis of COX-1 and -2 protein expression in cardiac tissues from mice subjected to sham operation (Sham) or LAD ligation for 45 min (MI). (B) The quantification of COX-1 and -2 expression in cardiac tissues from the sham or MI mice. (C) The COX activity was assessed in cardiac tissues from mice subjected to sham operation (Sham) or LAD ligation for 45 min (MI). The COX-1 and -2 activities were identified by adding a COX-1 inhibitor (SC560) or a COX-2 inhibitor (DuP-697), respectively. Data are presented as the mean ± SD (n = 3).
3.3. Cardiac-generated prostanoids-induced cardiac myocyte apoptosis
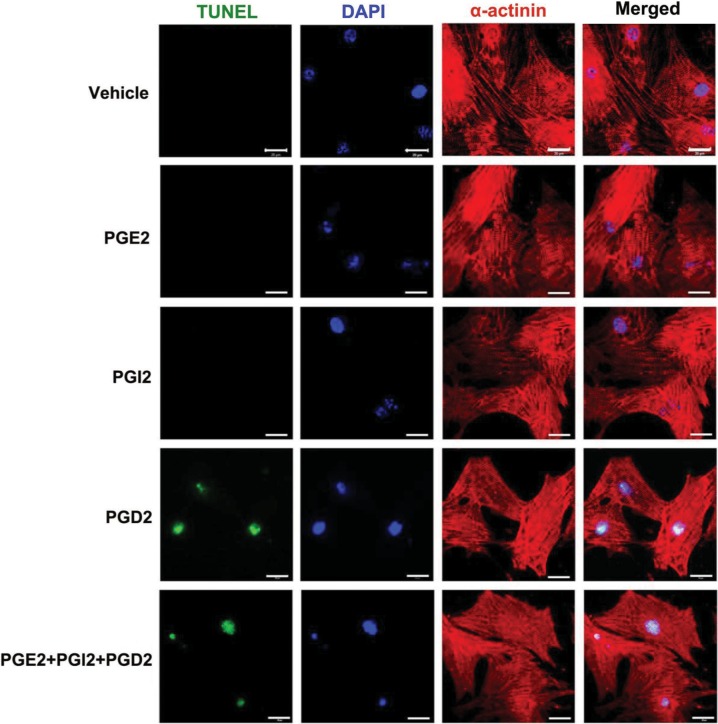
To directly test whether the cardiac-generated prostanoids are involved in mediating cardiac myocyte injury, cultured neonatal cardiac myocytes were treated with PGI2, PGE2, PGD2, and a combination of these three prostanoids that were found to be significantly elevated in ischaemic heart tissues compared with sham-operated hearts. Treatment with PGD2, but not PGI2 or PGE2, induced apoptosis in cardiac myocytes as assessed by TUNEL assays. The combination of PGE2/PGI2/PGD2 demonstrated the same effect as PGD2 alone (Figure 3).
Figure 3.
Effects of prostanoids on cardiac myocyte apoptosis. Isolated neonatal cardiac myocytes were cultured for 48 h and serum starved for 24 h before starting the experiments. PGD2, PGI2, and PGE2 (Cayman Chemical) were dissolved in ethanol as 1000× stock solution and were added into culture medium at a concentration of 10 μM individually or in combination (10 μM each). In control experiments, cells were treated with the same volume of vehicle (ethanol) that was also diluted 1000× in the culture medium. After 6h of incubation, cells were fixed with 4% paraformaldehyde, treated with proteinase K, permeabilized in 0.01% Triton-X-100. Permeabilized cells and sections were then incubated with TUNEL reaction mixture (Roche In Situ Cell Death Detection Kit, Fluorescein) at 37°C for 1h. This was immediately followed by incubation with primary antibody for α-actinin (1:500, Sigma) overnight at 4°C, then Alex-Fluor-555-labeled secondary antibody (1:300, Invitrogen). The nuclei were counter stained by DAPI in the mounting media. Scale bar = 20 μm.
3.4. Aspirin treatment reduced prostanoids generation and cardiac apoptosis following myocardial ischaemia
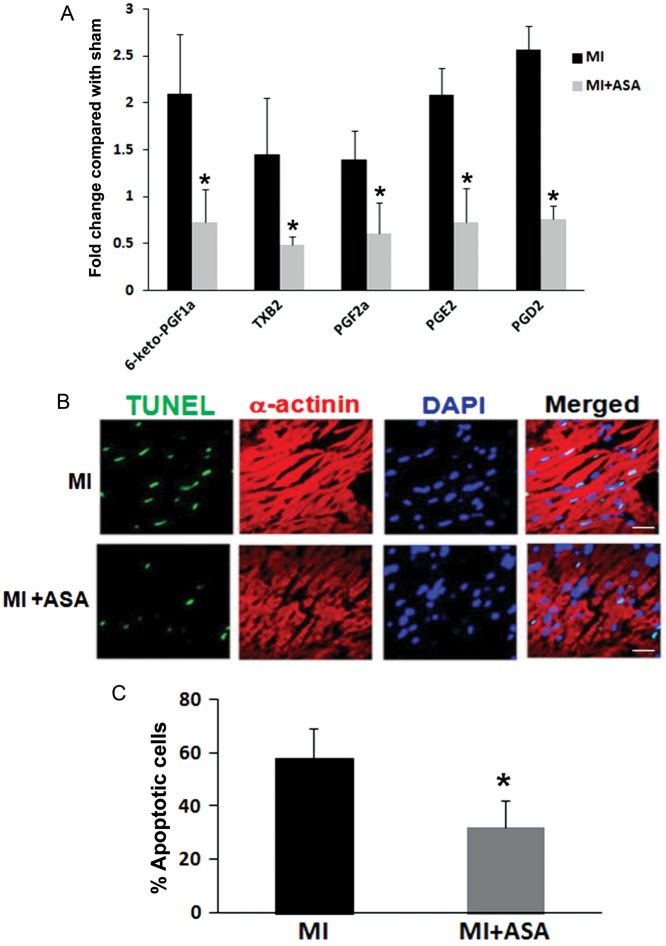
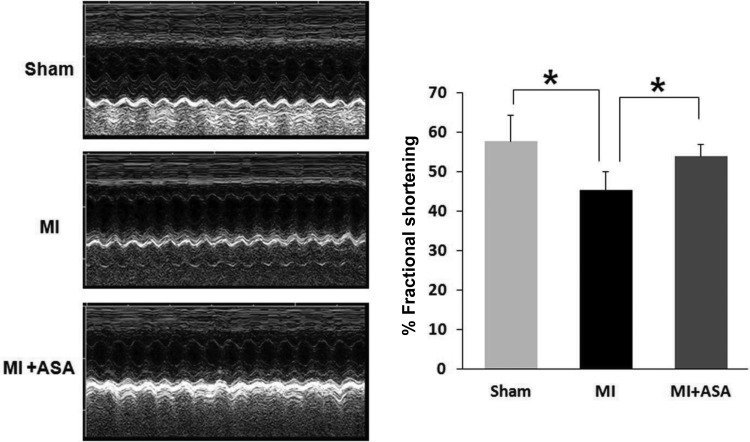
In order to further determine the possible impact of prostanoid-induced cardiac apoptosis in ischaemic injury in vivo, the MI mice were treated with low-dose aspirin, a COX inhibitor that is widely used clinically in preventing and treating cardiovascular diseases. Aspirin treatment of the MI mice significantly inhibited the generation of prostanoids in ischaemic heart tissues (Figure 4A). In line with the reduced production of prostanoids in cardiac tussues, the TUNEL assay detected significantly less apoptosis in ischaemic heart tissue in aspirin-treated MI mice compared with untreated MI mice (Figure 4B and C). In vivo cardiac function was analysed using echocardiography. Consistently, there was a significant reduction in the FS in the MI mice compared with sham-operated animals. Pre-treatment with aspirin significantly attenuated the reduction in FS in the MI mice (Figure 5).
Figure 4.
Treatment with aspirin significantly decreased the generation of cardiac prostanoids and cardiac apoptosis in the MI mice. (A) Cardiac-generated prostanoids from the MI mice with or without aspirin treatment (ASA 50 μg/mL in drinking water). Data are presented as the mean ± SD, n = 3 in each group, *P < 0.05 comparing MI with MI + ASA. (B) The detection of cardiac myocyte apoptosis in the ischaemic area in the MI mice with or without aspirin treatment. Cardiac tissues were collected after 45 min of ischaemia (LAD ligation) followed by 6h of reperfusion. Cardiac apoptosis were quantified in Figure 3. Scale bar = 20 μm. (C) The quantification of percentage of apoptotic cells in the ischaemic area from the MI mice and the MI mice treated with aspirin. Data were presented as the mean ± SD, n = 3 in each group, *P < 0.05 comparing treatment and no treatment groups.
Figure 5.
The assessment of cardiac function by echocardiography. Echocardiographic analysis was performed in sham operated, MI, and MI treated with aspirin (ASA 50 μg/mL in drinking water) for 3 days after MI (45 min of ischaemia). FS, a surrogate of systolic function, was calculated from left ventricle dimensions as follows: FS = ((EDD − ESD)/EDD) × 100%. Data shown are the mean ± SD, n= 3 for each group, *P < 0.05 comparing sham vs. MI and MI vs. MI + ASA.
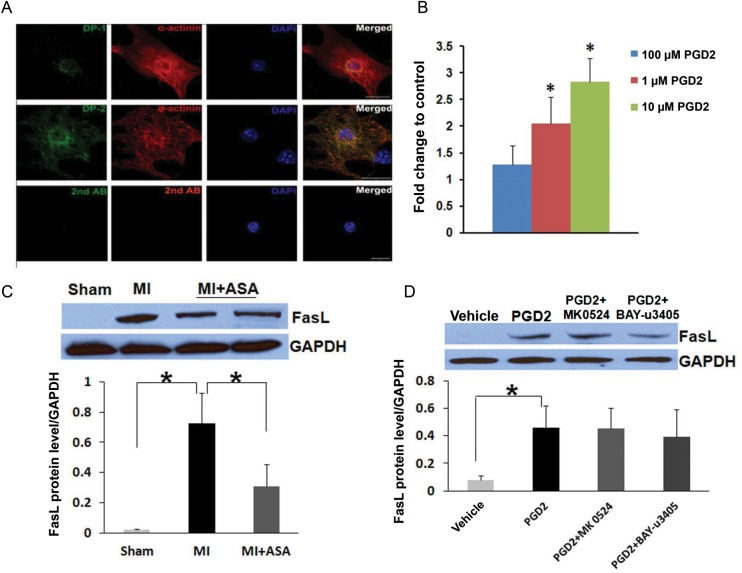
3.5. Expression of PGD2 receptors and PGD2-regulated gene in cardiac myocytes
Next, we investigated the mechanism of PGD2-induced apoptosis of cardiac myocytes. We first assessed the expression of PGD2 receptors, DP-1, and DP-2 in cardiac myocytes using immunofluorescence staining. Both DP-1 and DP-2 receptors were expressed in cardiac myocytes. DP-1 staining showed a perinuclear pattern, while DP-2 expression was present on the cell membrane (Figure 6A). Next PCR array analysis was performed to identify genes in the apoptotic pathways that were regulated in cardiac myocytes by PGD2 treatment. Treatment of cardiac myocytes with PGD2 significantly up-regulated the mRNA level of pro-apoptotic gene Fas ligand (FasL) in a dose-dependent manner (Figure 6B). Western Blot analysis demonstrated the up-regulation of FasL protein in MI mice compared with sham mice, and pre-treatment with aspirin resulted in a significant decrease in the FasL protein level (Figure 6C). In cultured neonatal cardiac myocytes, PGD2 treatment significantly up-regulated the FasL protein level in these cells. However, the pretreatment of cultured cardiac myocytes with DP-1-specific antagonist MK-0524 (100 nM) or DP-2-specific antagonist BAY-u3405 (100 nM) did not result in a significant attenuation in the FasL protein level (Figure 6D).
Figure 6.
The expression of PGD2 receptors in cardiac myocytes and up-regulation of FasL expression in cardiac myocytes treated with PGD2. (A) The expression of DP-1 and DP-2 in cardiac myocytes were detected using immunofluorescence confocal microscopy (green). Cardiac myocytes were identified by co-staining for α-actinin (red). Negative controls were incubated with secondary antibody only. Scale bar = 20 μm. (B) mRNA levels of FasL was up-regulated by PGD2 treatment in cultured cardiac myocytes in a dose-dependent manner. Data are presented as the mean ± SD, n = 3 in each group, *P < 0.05 compared with vehicle (ethanol) treated cells. (C) The expression of the FasL protein in cardiac tissue from sham, MI, and MI treated with aspirin. The quantification of the FasL protein level is shown in the lower panel (n = 3 in each group and *P < 0.05 comparing MI with sham and MI + ASA with MI). (D) The expression of the FasL protein in cultured cardiac myocytes treated with vehicle (ethanol), PGD2, PGD2 with DP-1 receptor antagonist (MK0524), or PGD2 with DP-2 receptor antagonist (BAY-u3405). The quantification of FasL protein levels is shown in the lower panel (n = 3 in each group and *P < 0.05 comparing vehicle treated with PGD2 treated cells).
4. Discussion
4.1. Critical roles of eicosanoids in cardiovascular diseases
Eicosanoids are pivotal molecules involved in diverse signalling cascades regulating homeostatic biological functions as well as mediating pathological processes such as inflammation, pain, and cardiovascular diseases.12 Drugs developed to target these signalling pathways represent >25% of annual pharmaceutical sales worldwide. COX, the principal target of non-steroidal anti-inflammatory drugs, is the key and rate-limiting enzyme in the conversion of AA to prostanoids (including PGs, prostacyclins, and TX). Two distinct COX isoforms, COX-1 and -2, have been characterized. COX-1, which is constitutively expressed in most tissues, is considered classically as the isoform primarily responsible for prostanoid synthesis involved in homeostatic functions.13 In contrast, COX-2 is mainly induced in response to inflammatory stimuli; therefore, is considered as the most appropriate target for anti-inflammatory drugs.14,15 Whereas aspirin, the prototypic COX-1/-2 inhibitor, has been clearly demonstrated to be cardioprotective and reduces the incidence and mortality from MI,4,5,16 unexpectedly, an abundance of recent clinical evidence has shown that selective COX-2 inhibition can increase the risk of cardiovascular events.5,17 The seemingly disparate effects among COX inhibitors have highlighted the need for a better understanding of the role that COX and prostanoids play in the initiation and propagation of MI.6,18,19
4.2. Use of metabolic profiling to explore the roles of eicosanoids in cardiac ischaemia
Although eicosanoids are well-known key mediators involved in thrombogenesis and initiation of MI, the precise role they play in the pathogenesis of cardiac damage after MI has not been fully elucidated. Interestingly, it has long been recognized that prolonged cardiac ischaemia leads to the release of free AA and its eicosanoid metabolites, and it occurs in parallel with the development of irreversible cardiac damage.2,20–24 Up to date, however, there is no clear answer to the critical question of whether the eicosanoids released after cardiac ischaemia are the consequence or the cause of cardiac cell damage. The new technique of metabolic profiling using LC-MS/MS, which allows the determination of multiple eicosanoid molecules simultaneously, has proved to be very valuable in elucidating the effects of AA metabolites in inflammation and cardiovascular diseases.7–9 Here we took advantage of this new technique to explore the role of eicosanoids in MI, with a special focus on their effects on cardiac myocytes following MI.
The assessment of plasma levels of prostanoids demonstrated significant increases in all COX pathway metabolites including PGE2, PGD2, PGF2α, 6-keto-PGF1α, and TXB2 after 45 min of myocardial ischaemia, while the assessment of tissue levels of prostanoids showed significant increases in PGE2, PGD2, and 6-keto-PGF1α (Figure 1). This result indicates that high levels of PGE2, PGD2, and PGI2 were generated from ischaemic cardiac tissue, while the increased plasma levels of PGF2α and TXB2 were most likely generated from circulating blood cells, particularly leukocytes and platelets. The expression of both COX-1 and -2 proteins was detected in cardiac tissues, with the COX-1 protein being expressed at a higher level than the COX-2 protein (Figure 2A). Expressions of neither COX-1 nor COX-2 proteins were significantly altered after 45 min of ischaemia. Moreover, the COX activity in cardiac tissues was not different between sham and MI mice, and the COX activity in both sham and MI mouse hearts was significantly inhibited by the COX-1 inhibitor SC-560, but not the COX-2 inhibitor DuP-697 (Figure 2B). This result suggests that the increased generation of prostanoids from cardiac tissue in MI mice was mainly attributable to the increased release of AA (see Supplementary material online, Table S1) and the generation of prostanoids from cardiac tissue was mainly smediated by COX-1, and not COX-2 enzyme.
4.3. The pro-apoptotic effect of cardiac-generated prostanoids is mainly attributable to PGD2
The effect of cardiac-generated prostanoids on cardiac injury following MI was assessed by apoptosis assay. The loss of cardiac myocytes by apoptosis is an important mechanism in the development of cardiac injury following ischaemia.25 However, the mechanisms and signalling pathways that lead to apoptosis following ischaemic stimuli in cardiac cells are not fully elucidated. Previous studies showed that during the acute phase of myocardial ischaemia reperfusion (<6 h), apoptosis occurs mainly in cardiac myocytes, and is limited to ischaemic regions.26,27 It has been previously reported that prostanoids are potent inducers of apoptosis in non-cardiomyocytes.28–31 We hypothesize that prostanoids that accumulate in the myocardium after prolonged ischaemia may be involved in triggering post-ischaemic myocardial cell death. Our data demonstrated that when cultured neonatal cardiac myocytes were incubated with a combination of PGD2, PGE2, and PGI2, which are the prostanoids that were increased in ischaemic myocardium, they underwent apoptosis. The same effect could be observed when cardiac myocytes were treated with PGD2, but not PGI2 or PGE2, alone (Figure 3). Although PGI2 and PGE2 have been implicated in cardioprotection,32,33 their effects have been suggested as originating from the action on non-cardiomyocytes and more likely involved in inflammatory response that becomes more apparent with longer reperfusion. Our data indicates that the net effect of cardiac-generated prostanoids is pro-apoptotic and prostanoids may represent one of the stimuli that drive cardiomyocytes into apoptosis. The treatment of MI mice with low-dose aspirin, which inhibited the cardiac generation of all prostanoids, attenuated myocardial apoptosis (Figure 4) and effectively prevented the decline of cardiac function (Figure 5), providing further credence to the hypothesis that cardiac-generated prostanoids may play a significant role in cardiac injury following MI. Daily low-dose (<100 mg day−1) aspirin is relatively selective for COX-1 and is a well-established and prevailing treatment for the prevention of arterial thrombosis by irreversibly inhibiting the formation of thromboxane A2, a potent aggregatory agent. Our data demonstrated that low-dose aspirin treatment could inhibit the formation of prostanoids from ischaemic heart and prevent cardiac apoptosis. This reveals a previously unrecognized mechanism by which aspirin, in addition to its anti-thrombosis effect, confers cardioprotection against MI, an effect not shared with COX-2 inhibitors.
Aspirin can reduce inflammation in the ischaemic hearts and thereby play a role in myocardial cell death. Our study demonstrated that aspirin treatment led to the reduction in cardiac apoptosis that occurred within 6h after the onset of ischaemia, while inflammatory response after the ischaemic event becomes apparent during 12–24 h after the onset of ischaemia. Therefore, our study suggests that aspirin treatment can prevent myocardial apoptosis in addition to its anti-inflammatory effects. On the other hand, apoptotic cell death is among the factors that can cause the initiation of inflammatory cell infiltration resulting in further myocardial cell damage in the form of both apoptosis and necrosis. The reduction of early myocardial apoptosis with aspirin treatment may contribute to the overall anti-inflammatory effects with aspirin treatment.
4.4. PGD2 up-regulated FasL expression in cardiac myocytes.
Apoptosis is a highly regulated programme of cell death and can be mediated by two central pathways.34,35 The death receptor/extrinsic pathway transmits death signals from relatively specialized ligands such as FasL or tumor necrosis factor-α and links external stimuli to intracellular apoptotic cell death machinery. In contrast, the mitochondrial/intrinsic pathway transduces death signals that originate both outside and inside the cell and utilizes mitochondria and endoplasmic reticulum to propel cell death via a distinct set of molecules. Both the extrinsic and intrinsic apoptosis pathways have been shown to be critical in the pathogenesis of MI and heart failure.36–38 Our data suggests that the pro-apoptotic effect of cardiac-generated prostanoids is mainly attributed to PGD2 (Figure 3). The biological activities of PGD2 are mediated through two distinct G protein-coupled receptors, DP-1 receptor and the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2 or DP-2).39 We have detected the expression of both DP-1 and DP-2 in cardiac myocytes (Figure 6A). The treatment of neonatal cardiac myocytes with PGD2 resulted in an increased mRNA level of FasL (Figure 6B). Western blot analysis demonstrated an increased expression of FasL in cardiac tissue from the MI mice compared with sham, and aspirin treatment significantly attenuated FasL expression in the MI mice (Figure 6C). The treatment of cardiac myocytes with neither DP-1-specific antagonist MK0524 (10 nM–10 μM) nor DP-2-specific antagonist BAY-u3405 (10 nM–10 μM) resulted in the attenuation of FasL expression (Figure 6D) or prevention of PGD2-induced apoptosis (data not shown). This suggests that PGD2 may induce cardiac myocyte apoptosis through a mechanism that is independent of DP receptors. Further investigation into the mechanisms involved in PGD2-induced cardiac myocyte apoptosis will provide novel insights into the events that trigger cardiac myocyte death during MI and may assist in the design of pharmacological interventions aimed at reducing cardiac injury and subsequent development of heart failure following MI.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
H.Q. was supported by American Heart Association Western States Affiliate postdoctoral fellowship award. This work was supported by the Department of Veteran Affairs Merit Review Grant and the National Institutes of Health Grants (HL85844, HL85727) to N.C., National Institutes of Health Grants (ES02710, ES04699) to B.D.H., and National Institutes of Health Grant (DC010917) to E.Y.
Supplementary Material
References
- 1.Katz AM, Messineo FC. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res. 1981;48:1–16. doi: 10.1161/01.res.48.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Chien KR, Han A, Sen A, Buja LM, Willerson JT. Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation-reacylation cycle and the depletion of membrane phospholipids. Circ Res. 1984;54:313–322. doi: 10.1161/01.res.54.3.313. [DOI] [PubMed] [Google Scholar]
- 3.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuster V, Sweeny JM. Aspirin: a historical and contemporary therapeutic overview. Circulation. 2011;123:768–778. doi: 10.1161/CIRCULATIONAHA.110.963843. [DOI] [PubMed] [Google Scholar]
- 5.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50(Suppl.):S423–428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu JY, Li N, Yang J, Qiu H, Ai D, Chiamvimonvat N, et al. Metabolic profiling of murine plasma reveals an unexpected biomarker in rofecoxib-mediated cardiovascular events. Proc Natl Acad Sci USA. 2010;107:17017–17022. doi: 10.1073/pnas.1011278107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Liu JY, Qiu H, Harris TR, Sirish P, Hammock BD, et al. Use of metabolomic profiling in the study of arachidonic acid metabolism in cardiovascular disease. Congest Heart Fail. 2011;17:42–46. doi: 10.1111/j.1751-7133.2010.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Liu JY, Timofeyev V, Qiu H, Hwang SH, Tuteja D, et al. Beneficial effects of soluble epoxide hydrolase inhibitors in myocardial infarction model: insight gained using metabolomic approaches. J Mol Cell Cardiol. 2009;47:835–845. doi: 10.1016/j.yjmcc.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics. 2004;16:349–360. doi: 10.1152/physiolgenomics.00041.2003. [DOI] [PubMed] [Google Scholar]
- 11.Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, et al. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103:18733–18738. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 13.Zidar N, Dolenc-Strazar Z, Jeruc J, Jerse M, Balazic J, Gartner U, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the normal human heart and in myocardial infarction. Cardiovasc Pathol. 2007;16:300–304. doi: 10.1016/j.carpath.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- 15.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 16.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007;50:470–479. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- 17.FitzGerald GA. COX-2 in play at the AHA and the FDA. Trends Pharmacol Sci. 2007;28:303–307. doi: 10.1016/j.tips.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Patrono C, Baigent C. Low-dose aspirin, coxibs, and other NSAIDS: a clinical mosaic emerges. Mol Interv. 2009;9:31–39. doi: 10.1124/mi.9.1.8. [DOI] [PubMed] [Google Scholar]
- 19.Iniguez MA, Cacheiro-Llaguno C, Cuesta N, Diaz-Munoz MD, Fresno M. Prostanoid function and cardiovascular disease. Arch Physiol Biochem. 2008;114:201–209. doi: 10.1080/13813450802180882. [DOI] [PubMed] [Google Scholar]
- 20.Van der Vusse GJ, Reneman RS, van Bilsen M. Accumulation of arachidonic acid in ischemic/reperfused cardiac tissue: possible causes and consequences. Prostaglandins Leukot Essent Fatty Acids. 1997;57:85–93. doi: 10.1016/s0952-3278(97)90497-x. [DOI] [PubMed] [Google Scholar]
- 21.Engels W, van Bilsen M, de Groot MJ, Lemmens PJ, Willemsen PH, van der Vusse GJ, et al. Ischemia-induced arachidonic acid accumulation and prostanoid release during reperfusion in the isolated rat heart. Prog Clin Biol Res. 1989;301:175–179. [PubMed] [Google Scholar]
- 22.Hirsh PD, Hillis LD, Campbell WB, Firth BG, Willerson JT. Release of prostaglandins and thromboxane into the coronary circulation in patients with ischemic heart disease. N Engl J Med. 1981;304:685–691. doi: 10.1056/NEJM198103193041201. [DOI] [PubMed] [Google Scholar]
- 23.Coker SJ, Parratt JR, Ledingham IM, Zeitlin IJ. Thromboxane and prostacyclin release from ischaemic myocardium in relation to arrhythmias. Nature. 1981;291:323–324. doi: 10.1038/291323a0. [DOI] [PubMed] [Google Scholar]
- 24.Gurbel PA, Murugesan SR, Lowry DR, Serebruany VL. Plasma thromboxane and prostacyclin are linear related and increased in patients presenting with acute myocardial infarction. Prostaglandins Leukot Essent Fatty Acids. 1999;61:7–11. doi: 10.1054/plef.1999.0064. [DOI] [PubMed] [Google Scholar]
- 25.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–956. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- 27.Bialik S, Geenen DL, Sasson IE, Cheng R, Horner JW, Evans SM, et al. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J Clin Invest. 1997;100:1363–1372. doi: 10.1172/JCI119656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu F, Wang P, Kontrogianni-Konstantopoulos A, Konstantopoulos K. Prostaglandin (PG)D2 and 15-deoxy-Delta(12,14)-PGJ2, but not PGE2, mediate shear-induced chondrocyte apoptosis via protein kinase A-dependent regulation of polo-like kinases. Cell Death Differ. 2010;17:1325–1334. doi: 10.1038/cdd.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bishop-Bailey D, Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Delta12, 14-prostaglandin J2. J Biol Chem. 1999;274:17042–17048. doi: 10.1074/jbc.274.24.17042. [DOI] [PubMed] [Google Scholar]
- 30.Jeffrey JE, Aspden RM. Cyclooxygenase inhibition lowers prostaglandin E2 release from articular cartilage and reduces apoptosis but not proteoglycan degradation following an impact load in vitro. Arthritis Res Ther. 2007;9:R129. doi: 10.1186/ar2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang PC, Kung FL, Huang DM, Li TK, Fan JR, Pan SL, et al. Induction of Fas clustering and apoptosis by coral prostanoid in human hormone-resistant prostate cancer cells. Eur J Pharmacol. 2006;542:22–30. doi: 10.1016/j.ejphar.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 32.Shinmura K, Tamaki K, Sato T, Ishida H, Bolli R. Prostacyclin attenuates oxidative damage of myocytes by opening mitochondrial ATP-sensitive K+ channels via the EP3 receptor. Am J Physiol Heart Circ Physiol. 2005;288:H2093–2101. doi: 10.1152/ajpheart.01003.2004. [DOI] [PubMed] [Google Scholar]
- 33.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, et al. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci USA. 2000;97:10197–10202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitsis RN, Narula J. Introduction-cell death in heart failure. Heart Fail Rev. 2008;13:107–109. doi: 10.1007/s10741-008-9080-3. [DOI] [PubMed] [Google Scholar]
- 35.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 36.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 37.Camara AK, Bienengraeber M, Stowe DF. Mitochondrial approaches to protect against cardiac ischemia and reperfusion injury. Front Physiol. 2011;2:13. doi: 10.3389/fphys.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorn GW, II, Scorrano L. Two close, too close: sarcoplasmic reticulum-mitochondrial crosstalk and cardiomyocyte fate. Circ Res. 2010;107:689–699. doi: 10.1161/CIRCRESAHA.110.225714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuligoi R, Sturm E, Luschnig P, Konya V, Philipose S, Sedej M, et al. CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology. 2010;85:372–382. doi: 10.1159/000313836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.