Abstract
Aims
Pulmonary hypertension (PH) is a devastating condition for which no disease-modifying therapies exist. PH is recognized as proliferative disease of the pulmonary artery (PA). In the experimental newborn calf model of hypoxia-induced PH, adventitial fibroblasts in the PA wall exhibit a heightened replication index. Because elevated platelet-derived growth factor β receptor (PDGFβ-R) signalling is associated with PH, we tested the hypothesis that the activation of PDGFβ-R contributes to fibroblast proliferation and adventitial remodelling in PH.
Methods and results
Newborn calves were exposed to either ambient air (PB = 640 mmHg) (Neo-C) or high altitude (PB = 445 mm Hg) (Neo-PH) for 2 weeks. PDGFβ-R phosphorylation was markedly elevated in PA adventitia of Neo-PH calves as well as in cultured PA fibroblasts isolated from Neo-PH animals. PDGFβ-R activation with PDGF-BB stimulated higher replication in Neo-PH cells compared with that of control fibroblasts. PDGF-BB-induced proliferation was dependent on reactive oxygen species generation and extracellular signal-regulated kinase1/2 activation in both cell populations; however, only Neo-PH cell division via PDGFβ-R activation displayed a unique dependence on c-Jun N-terminal kinase1 (JNK1) stimulation as the blockade of JNK1 with SP600125, a pharmacological antagonist of the JNK pathway, and JNK1-targeted siRNA selectively blunted Neo-PH cell proliferation.
Conclusions
Our data strongly suggest that hypoxia-induced modified cells engage the PDGFβ-R-JNK1 axis to confer distinctively heightened proliferation and adventitial remodelling in PH.
Keywords: PDGFβ receptor, JNK1, Hypoxic pulmonary hypertension, Proliferation, Adventitial fibroblasts
1. Introduction
Pulmonary hypertension (PH) is a life-threatening disease for which symptomatic treatments lack disease-modifying effects. PH is increasingly recognized as a fibroproliferative disease of the pulmonary artery (PA).1,2 In the experimental newborn calf model of hypoxia-induced PH, adventitial fibroblasts exhibit a heightened replication index in the remodelled PA wall.3,4 We and others have demonstrated that cells of remodelled PA adventitia preserve the enhanced proliferative responses in culture.5,6 Recently, we have also reported that the increased replication rate of remodelled PA adventitial fibroblasts is due to the lack of a replication repressor signal of the protein kinase Cζ isozyme.7 These phenotypically modified cells might be the key participants in the PA adventitial remodelling process during the progression of PH. Although platelet-derived growth factor β receptor (PDGFβ-R) is a well-known contributor to the progression of PH,8–11 responses of the phenotypically altered adventitial cells to PDGFβ-R activation are unknown.
In spite of PDGFβ-R overexpression in PA adventitial layer in PH patients,12,13 the role of such an increase in receptor levels in the regulation of adventitial cell responses in PH remains unexplored. Since adventitial cells are important participants in the PA remodelling process in PH,1,2,14 gaining knowledge of the precise molecular mechanisms regulating adventitial cell proliferation in response to PDGFβ-R stimulation is the first critical step in the design of novel effective therapeutics for PH.
Chronic hypoxia exposure instigates vascular remodelling and subsequent PH through the generation of NADPH oxidase-derived reactive oxygen species (ROS), which have been demonstrated to be critical mediators of PDGFβ-R activation and downstream proliferative signalling cascades in PA smooth muscle cells (SMCs).15 In systemic sclerosis, PDGF has also been shown to generate ROS in fibroblasts as a part of the process of fibroblast to myofibroblast phenotype conversion,16,17 suggesting that PDGFβ-R-ROS signalling is an important regulator of fibroblast activation. However, the role of this pathway in the regulation of PA adventitial fibroblast phenotype during the development of hypoxia-induced PH awaits exploration.
MAP kinases, including extracellular signal-regulated kinase1/2 (ERK1/2), c-Jun NH2-terminal kinase1/2 (JNK1/2), and p38 MAP kinase, are the key intracellular signals for stimuli-induced cell proliferation, survival, and apoptosis.18,19 These signalling pathways also exert powerful influence on the vascular remodelling processes.20–22 Although PDGF-induced responses of vascular smooth muscle cells and responses to hypoxia of PA adventitial fibroblasts are mediated through the activation of ERK1/2, JNK1/2, and p38 MAP kinase,23–26 the role of these MAP kinases in PDGFβ-R-mediated PA adventitial fibroblast activation has not been studied.
Therefore, we tested the hypothesis that heightened levels of PDGFβ-R-mediated ROS and MAP kinase activation are key regulators of adventitial fibroblast replication in the PA during the development of hypoxia-induced PH. We evaluated the levels of both activated and total PDGFβ-R in the PA in situ as well as in PA adventitial fibroblasts isolated and cultured from neonatal control (Neo-C) and hypoxia-exposed (Neo-PH) calves. Proliferation, intracellular ROS levels, and MAP kinase activation patterns upon PDGFβ-R activation with PDGF-BB were then examined. We also used pharmacological inhibitors and siRNA antagonist strategies targeting JNK1 and JNK2 to differentiate the role of these two JNK isoforms in adventitial fibroblast replication in hypoxia-induced PH.
We found that hypoxia stimulates an increase in the levels and activation of PDGFβ-R in PA adventitial fibroblasts. While PDGF-BB-induced proliferation was dependent on generation of ROS and ERK1/2 activation in both Neo-C and Neo-PH cells, only proliferation of the Neo-PH cells, which was higher than that of control cells, displayed a unique dependence on transient JNK1 phosphorylation. Our results provide first-time evidence that chronic hypoxia exposure activates the PDGFβ-R-JNK1 axis as an integral process in fibroblasts during adventitial remodelling in PH.
2. Methods
For detailed methods please see Supplementary material online.
2.1. Animals
This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the University of Colorado Institutional Animal Care and Use Committee, # 41702005(07)2A. Veterinary care of newborn calves was conducted according to the institutional guidelines at the Department of Physiology, School of Veterinary Medicine, Colorado State University (Fort Collins, Colorado). Briefly, 1-day-old Holstein calves were maintained at the Fort Collins altitude (PB = 640 mmHg) (Neo-C) (n = 7) or in a hypobaric chamber at simulated altitude (PB = 445 mm Hg) (Neo-PH) (n = 7) for 2 weeks to develop a model of severe PH. Calves were euthanized by overdose of sodium pentobarbital (160 mg/kg body weight). Animal handling and lung extraction occurred according to the previously described method.3
2.2. Isolation of PA adventitial fibroblasts in culture
Cells from Neo-C and Neo-PH calves were isolated and maintained in culture according to our previously described protocol.14 Briefly, adventitial tissue was isolated postmortem, carefully dissected free of blood vessels and fat under a dissecting microscope, and cut into small pieces. The tissue pieces were then dispersed with Hank's Buffered Salt Solution containing elastase, collagenase, albumin, and soybean trypsin inhibitor. The isolated cells were passed through a 100 μm nylon cell strainer (Falcon) to remove any undigested tissue pieces, diluted in the MEM medium containing 10% foetal bovine serum (FBS) to inactivate the enzymes, and centrifuged at 900 rpm for 10 min. Using a light microscope and haemocytometer, we counted the cells and serially diluted the cell suspension in the media containing 30% foetal conditioned media and 10% FBS. Cells were plated at a density of 0.5 cells/well/0.2 ml in the 96-well plates. The cells were maintained in 96-well plates for 2 weeks. Once reached confluence, cells were trypsinized and transferred to 24-well culture dishes in the MEM medium containing 10% FBS. After reaching confluence for the second time, cells were transferred sequentially according to the growth rate into 12 wells followed by 6-well culture dishes, and then into 25 mm followed by 75-mm culture flasks. Each individual cell population was characterized first for their morphological appearance and then for the expression of SMC-specific markers such as SMC-specific actin and SMC-specific myosin. Any population positive for SMC-specific myosin might represent SMC and no further studies were performed on these cells. In addition, cells exhibiting a more epithelioid or cobblestone morphology were considered endothelial cells and were also not utilized in this study. In all experiments, fibroblasts were studied at passages 3–7 after cloning and amplification procedures.
2.3. Immunohistochemistry
PDGFβ-R and phosphoPDGFβ-R levels in Neo-C and Neo-PH lungs were assessed by staining of 5 μm thick paraffin-embedded lung sections. Antibodies against PDGFβ-R and phosphoPDGFβ-R were used for lung sections in the dilution of 1:100. Antigen-antibody binding in the lung was visualized by immunoperoxidase reaction.
2.4. Immunoblot analysis
For the evaluation of PDGF-BB-induced activation of ERK1/2, JNK1/2, and p38MAP kinase, quiescent Neo-C and Neo-PH cells were stimulated with PDGF-BB (25 ng/mL) for different lengths of time. At the end of the experimental period, cells were harvested with the lysis buffer. Growth-arrested fibroblasts were also pre-incubated with either U0126 (inhibitor of MEK1/2) or SP600125 (JNK1/2 antagonist) for 1 h at 37°C, stimulated with PDGF-BB for 30 min and then cell lysates were collected for the examination of attenuation of either ERK1/2 activation or c-jun phosphorylation. Western blots for the detection of PDGFβ-R, phosphoPDGFβ-R, ERK1/2, phosphoERK1/2, JNK1/2, phosphoJNK1/2, p38MAP kinase, phosphop38MAP kinase, c-jun, and phosphoc-jun in the lysates of Neo-C and Neo-PH cells were performed according to our previously described method.7
2.5. Proliferation assay
Cells were plated in either 96-well plates (4 × 103 cells/well) or 24-well plates (20 × 103 cells/well) in 10% FBS containing media. After 72 h of growth arrest in 0.1% FBS containing media, cells were stimulated with PDGF-BB (25 ng/ml) for 48 h. Increase in cell numbers in response to PDGF-BB stimulation was evaluated by counting cells with haemocytometer. To examine the role of ROS and MAP kinases in PDGF-BB-induced fibroblast proliferation, quiescent cells were pre-incubated with ROS scavengers N-acetyl cysteine (NAC) and 4-Hydroxy-2,2,6,6-tetramethylpiperidinyloxy, TEMPOL) and inhibitors of MAP kinases (U0126 and SP600125) for 1h and then stimulated with PDGF-BB for 48 h. Fibroblast replication was evaluated either by Cell-Titer-96 Proliferation Assay (Promega) or cell counting.
2.6. ROS measurement
Intracellular hydrogen peroxide (H2O2) and superoxide levels were determined by dichlorofluorescein diacetate (DCFDA) and dihydroethidium (DHE), respectively. Both Neo-C and Neo-PH cells were plated in 96-well plates in 10% FBS containing media. After overnight incubation, fibroblasts were growth arrested with 0.1% FBS/MEM for 72 h. Quiescent cells were stimulated with PDGF-BB for 2 h. Either DCFDA or DHE (both at the concentration of 5 × 10−6 mol/L) was added to the cells for last 30 min of treatment. At the end of the experimental period, cells were washed with PBS and fluorescence of DCFDA (522 nm) and DHE (520/610 nm) were recorded.
2.7. siRNA transfection
Both Neo-C and Neo-PH fibroblasts were plated in 10% FBS containing media. After allowing the cells to attach overnight, the media were changed to 0.1% FBS/MEM. Fibroblasts were transfected with one of the three constructs: (i) non-targeting (scrambled) siRNA control, (ii) anti-JNK1 siRNA, or (iii) anti-JNK2 siRNA according to the manufacturer's protocol. siRNAs against bovine JNK1 (5′-GGAGCUAGAUCAUGAAAGAUU-3′), JNK2 (5′-GGAAAGAGCUAAUUUACAAUU-3′), and control siRNA (5′-UGGUUUACAUGUCGACUAA-3′) were used for transfection. After 48 h of transfection, Neo-C and Neo-PH cells were processed for either proliferation assay according to our above-mentioned method or western blot analysis for evaluation of JNK1/2 and ERK1/2 levels.
2.8. Data analysis
All data are expressed as arithmetic means ± SEM. Differences between groups were analysed by ANOVA followed by the Student–Newman–Keuls post hoc test for multiple comparisons. Probability value ≤0.05 was regarded as significant.
3. Results
3.1. Activation of PDGFβ-R increases fibroblast proliferation in remodelled PA adventitia
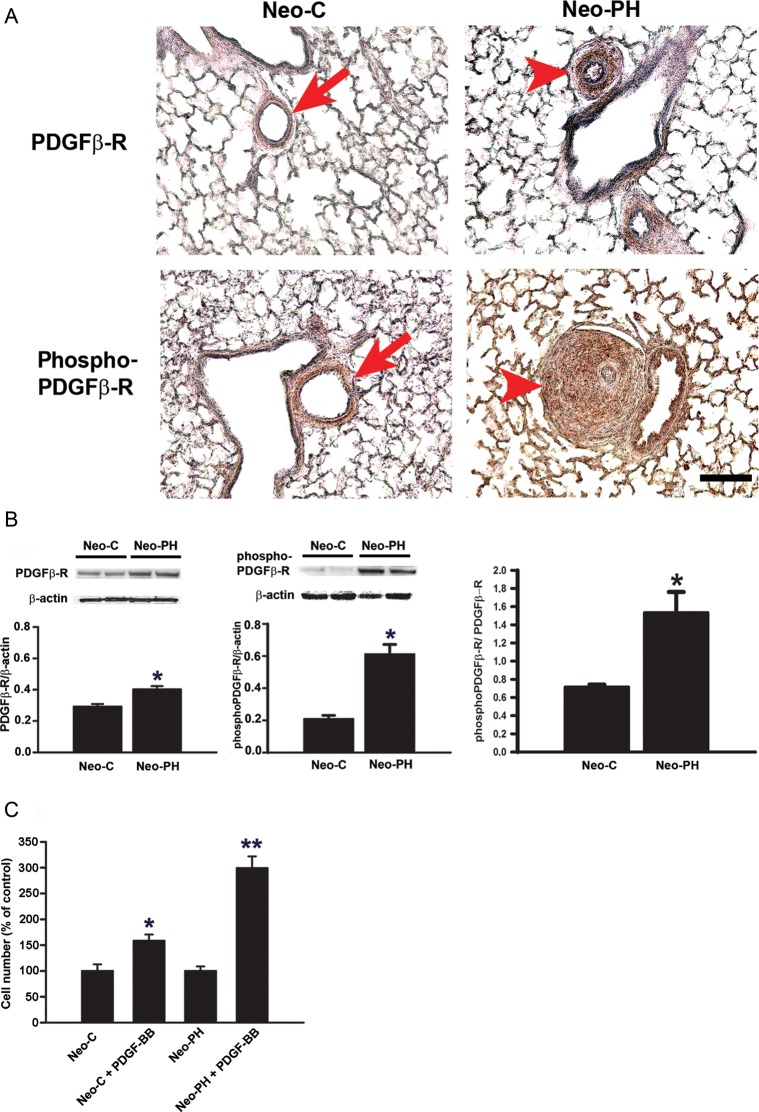
To evaluate the expression and activation of PDGFβ-R in the PA adventitia of Neo-C and Neo-PH calves, lung sections were examined immunohistochemically with anti-PDGFβ-R as well as anti-phosphoPDGFβ-R antibodies. Compared with Neo-C PA adventitia, which had weak staining intensity with these two anti-PDGFβ-R antibodies, both phosphorylated and total PDGFβ-R levels were substantially increased in the remodelled PA adventitial compartment of Neo-PH lungs (Figure 1A). These data suggest that chronic hypoxia exposure leads to increased expression and activation of PDGFβ-R in PA adventitia.
Figure 1.
Chronic hypoxia exposure stimulates an increase in the levels of PDGFβ-R, phosphoPDGFβ-R, and PDGF-BB-stimulated proliferative responses in PA adventitial fibroblasts. (A) Immunohistochemical staining of paraffin-embedded lung sections of Neo-C and Neo-PH calves was performed using antibodies against PDGFβ-R and phosphoPDGFβ-R. Arrows indicate PA adventitia compartments of Neo-C animals. Arrowheads indicate remodelled adventitial layers of PA of Neo-PH calves. Scale bar = 50 µm. Representative photomicrographs are presented. Staining was performed in lung sections of three different Neo-C and three different Neo-PH calves. (B) PDGFβ-R and phosphoPDGFβ-R levels were measured by western immunoblot analysis in growth-arrested PA adventitial fibroblasts isolated from Neo-C and Neo-PH calves. Densitometric quantification of the bands using NIH Image J program and normalized to β-actin is shown below each blot. pPDGFβ-R levels were also normalized to the level of PDGFβ-R in both Neo-C and Neo-PH cells. Values are mean ± SEM from three independent experiments where cells isolated from three different Neo-C and Neo-PH calves were used. *P< 0.01 vs. Neo-C value. (C) Quiescent fibroblasts were stimulated with PDGF-BB (25 ng/mL) for 48 h and then counted. The proliferation rate is represented as % of untreated cells. Data are mean ± SEM from three independent experiments using fibroblasts isolated from three different Neo-C and Neo-PH calves. *P< 0.01 vs. untreated Neo-C cells; **P< 0.01 vs. untreated Neo-PH cells and PDGF-BB-treated Neo-C cells.
To provide further evidence for the role of PDGFβ-R in fibroproliferative responses in PH, fibroblast populations from PA adventitia of Neo-C and Neo-PH calves were isolated and cultured. PDGFβ-R expression and activation were assessed by western immunoblot analysis in lysates from quiescent Neo-C and Neo-PH cells. Although PDGFβ-R levels were increased by only 1.3-fold in Neo-PH cells compared with Neo-C populations, upregulation in basal PDGFβ-R phosphorylation in Neo-PH cells was much more pronounced (3.0-fold) (Figure 1B). The PhosphoPDGFβ-R/PDGFβ-R ratio also confirmed a significant (2-fold) increase in the activation of PDGFβ-R in Neo-PH cells compared with that in Neo-C fibroblasts (Figure 1B). These results strongly suggest that PDGFβ-R expression and activation are stimulated in PA adventitial fibroblasts by chronic hypoxia exposure.
To evaluate the role of PDGFβ-R activation in PA adventitial remodelling, quiescent Neo-C and Neo-PH fibroblasts were stimulated with the PDGFβ-R ligand, PDGF-BB, and replication rate was determined. Neo-PH fibroblasts showed 3.0-fold increase in cell numbers upon PDGF-BB stimulation compared with that of untreated cells (Figure 1C). PDGF-BB also induced Neo-C proliferation, but at a much lower rate (1.5-fold compared with 3.0-fold in Neo-PH populations) (Figure 1C). Taken together, these data suggest that hypoxia stimulates PDGFβ-R expression and activation to regulate augmented fibroblast replication in remodelled adventitial compartment of PA.
3.2. ROS mediate PDGF-BB-stimulated PA adventitial fibroblast proliferation
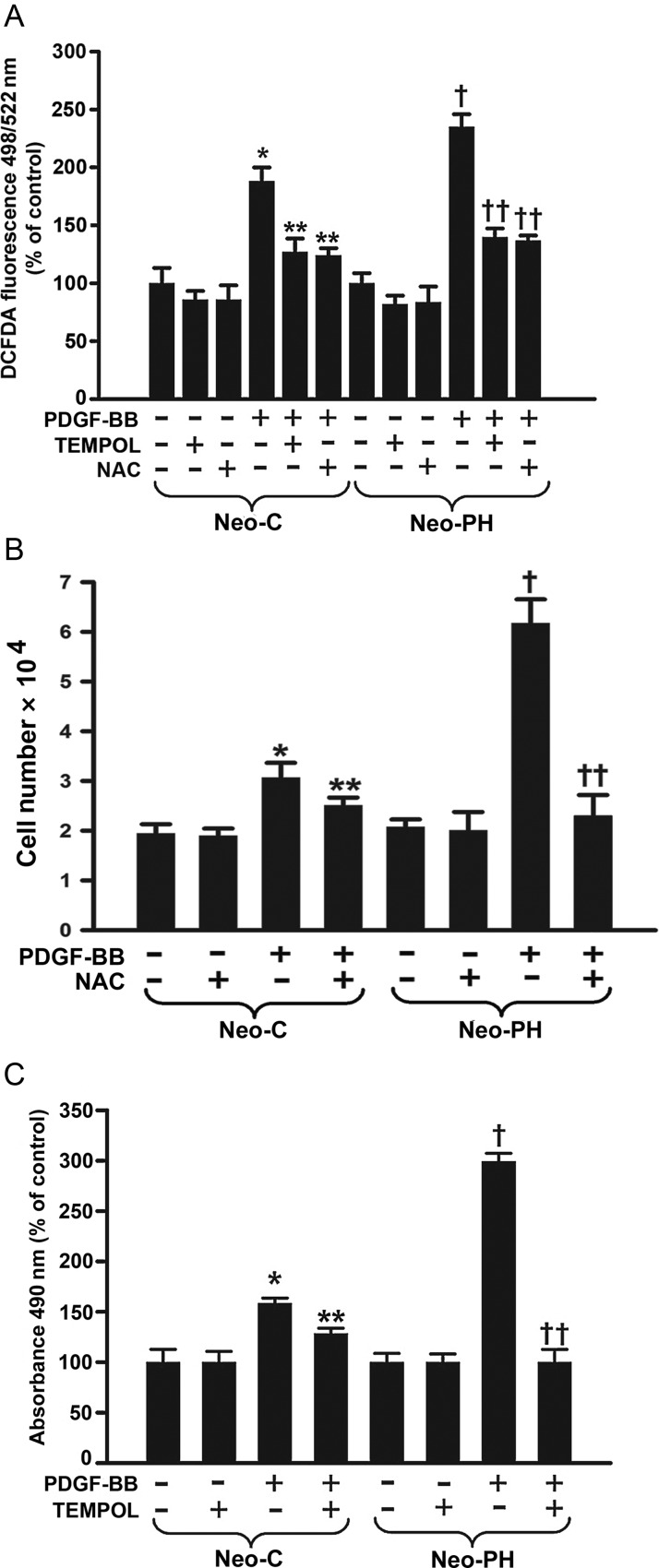
Intracellular H2O2 production in response to PDGFβ-R activation with PDGF-BB was measured after loading cells with DCFDA, which is oxidized by H2O2 to a highly fluorescent dichlorofluorescein. In both Neo-C and Neo-PH cells, PDGF-BB induced an increase in the intracellular H2O2 levels; however, the magnitude of H2O2 generation was significantly greater in Neo-PH cells compared with that of control fibroblasts (Figure 2A). Hydrogen peroxide generation in both Neo-C and Neo-PH cells induced by PDGFβ-R activation with PDGF-BB was attenuated by an antioxidant, NAC as well as a superoxide dismutase mimetic, TEMPOL (Figure 2A).
Figure 2.
PDGF-BB stimulates PA adventitial fibroblast proliferation through generation of intracellular ROS. (A) H2O2 levels were measured 2 h after PDGF-BB stimulation in both Neo-C and Neo-PH cells using DCFDA fluorescent dye and are represented as % of untreated cells. (B) Growth arrested cells were pretreated with N-acetyl cysteine (NAC) for 1 h, stimulated with PDGF-BB for 48h, and counted. (C) Quiescent fibroblasts were stimulated with PDGF-BB after pre-incubation with 4-Hydroxy-2,2,6,6-tetramethylpiperidinyloxy (TEMPOL), a SOD mimetic, for 1 h and proliferation assay was performed after 48 h. Data are mean ± SEM from three independent experiments using cells isolated from three different Neo-C and Neo-PH calves. *P< 0.001 vs. untreated Neo-C cells; **P< 0.01 vs. PDGF-BB treated Neo-C cells; †P< 0.01 vs. untreated Neo-PH cells; ††P< 0.01 vs. PDGF-BB treated Neo-PH cells.
Superoxide production in fibroblasts resulting from PDGFβ-R activation was also detected by DHE, which is converted by superoxide to fluorescent ethidium. The patterns of superoxide generation in both Neo-C and Neo-PH fibroblasts (Supplementary material online, Figure S1) mimic the findings for intracellular H2O2 production. In contrast, however, only TEMPOL, but not NAC, attenuated PDGF-BB-stimulated superoxide generation (Supplementary material online, Figure S1). Although, taken together, these data suggest that PDGFβ-R activation up-regulates ROS (H2O2 and superoxide) generation in PA fibroblasts, the magnitude of the increase in the intracellular ROS is substantially greater in Neo-PH cells compared with control fibroblasts.
To evaluate whether observed increases in ROS affect proliferation, PDGFβ-R-mediated replication was assessed in the presence of either NAC or TEMPOL by one of the two methods: cell counts (Figure 2B) or measuring cell metabolic activity (Figure 2C). Treatment with either NAC (Figure 2B) or TEMPOL (Figure 2C) inhibited PDGF-BB-induced increase in cell numbers in both Neo-C and Neo-PH cells. Collectively, these results suggest that PDGFβ-R-stimulated PA adventitial fibroblast proliferation is mediated through intracellular ROS. However, most importantly, the entirety of the PDGF-BB-induced proliferative response in adventitial fibroblasts from hypoxia-exposed remodelled PA can be explained by the greater magnitude in ROS levels.
3.3. Fibroblasts of remodelled adventitia exhibit distinctly different JNK1 activation patterns
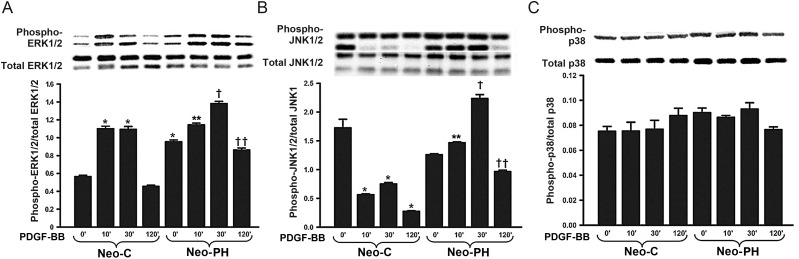
To dissect the mechanisms responsible for heightened Neo-PH cell replication, activation patterns of intracellular MAP kinases, ERK1/2, JNK1/2, and p38MAP kinase, were evaluated by individually examining the phosphorylation of each kinase. In control cells stimulated with PDGF-BB, maximal ERK1/2 phosphorylation was observed at 10 min of treatment, maintained up to 30 min, and then returned to baseline by 120 min (Figure 3A). In contrast, basal ERK1/2 phosphorylation levels of Neo-PH cells were nearly 2-fold greater than those of Neo-C fibroblasts (Figure 3A). Despite these high basal activation levels, Neo-PH cells remained responsive to PDGF-BB, albeit to a lesser degree than the control cells. In these Neo-PH fibroblasts, maximal ERK1/2 phosphorylation was observed after 30 min of PDGF-BB stimulation and returned to the basal levels by 120 min (Figure 3A).
Figure 3.
PDGF-BB selectively stimulates JNK1 phosphorylation in Neo-PH cells. Growth-arrested fibroblasts were treated with PDGF-BB for different lengths of time. Cell lysates were prepared and immunoblotted for phosphoERK1/2 (A), phosphoJNK1/2 (B), and phosphop38 MAP kinase (C). Protein bands were quantified according to our above-mentioned method and normalized to the levels of appropriate total MAP kinases. Data represent mean ± SEM of three independent experiments using Neo-C and Neo-PH fibroblasts isolated from three different control and hypoxia-exposed calves. *P< 0.001 vs. untreated Neo-C cells; **P< 0.01 vs. untreated Neo-PH cells; †P< 0.01 vs. Neo-PH cells stimulated with PDGF-BB for 10 min and Neo-C cells treated with PDGF-BB for 30 min; ††P< 0.01 vs. Neo-PH cells treated with PDGF-BB for 30 min.
JNK1/2 was strongly phosphorylated under basal conditions in both Neo-C and Neo-PH cells (Figure 3B). Stimulation of control cells with PDGF-BB caused a distinct decline in JNK1 activation (Figure 3B). In contrast, JNK1 phosphorylation was sharply enhanced by PDGF-BB treatment in Neo-PH cells (Figure 3B). Maximal increase (1.8-fold compared with basal levels) in JNK1 phosphorylation levels was observed after 30 min of stimulation. Dephosphorylation of JNK1 occurred by 120 min of PDGF-BB treatment (Figure 3B). JNK2 phosphorylation was unaffected by PDGF-BB stimulation of either Neo-C or Neo-PH cells (Supplementary material online, Figure S2).
Experiments examining the activation patterns of p38 MAP kinase showed that this kinase is not likely to play a role in PDGF-BB-induced proliferation of the adventitial fibroblasts. Specifically, p38 MAP kinase phosphorylation was not altered by PDGFβ-R activation in any of the cells studied (Figure 3C). Taken together, our data reveal three novel features regarding PDGFβ-R-mediated activation of adventitial fibroblasts: (i) ERK1/2 phosphorylation is required for both Neo-C and Neo-PH fibroblast stimulation, (ii) JNK1 activation occurs, but only in the Neo-PH cells, and (iii) p38 MAP kinase is not activated beyond its basal level during this activation phase.
3.4. ROS scavengers selectively attenuate JNK1 phosphorylation in remodelled PA adventitial fibroblasts
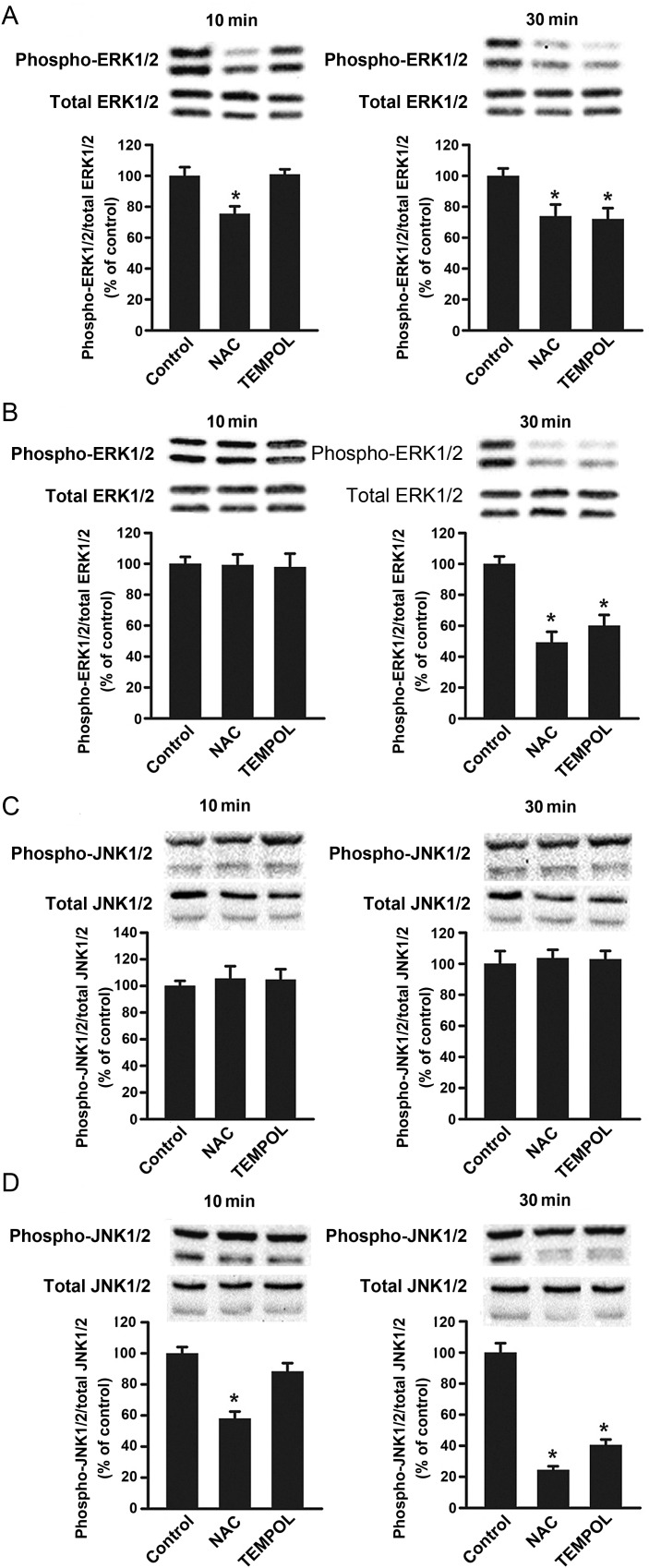
To examine whether ROS mediate their pro-proliferative effects in adventitial fibroblasts by activating ERK1/2 and JNK1/2, phosphorylation patterns of these kinases were evaluated in the presence of either NAC or TEMPOL in both Neo-C and Neo-PH cells. In Neo-C cells, NAC pretreatment blocked ERK1/2 phosphorylation at both 10 and 30 min of PDGF-BB treatment (Figure 4A). TEMPOL also inhibited ERK1/2 activation in these cells, but only at 30 min (Figure 4A). In Neo-PH populations, PDGF-BB-induced ERK1/2 phosphorylation (30 min) was reduced by both NAC and TEMPOL treatments (Figure 4B).
Figure 4.
Antioxidants abolish PDGF-BB-induced JNK1 phosphorylation in Neo-PH cells. Quiescent cells were pretreated with either NAC or TEMPOL for 1 h and then stimulated with PDGF-BB (25 ng/mL) for 10 or 30 min. ERK1/2 and JNK1/2 phosphorylation levels were assessed by western immunoblot analysis in Neo-C (A and C) and Neo-PH (B and D). Phosphorylated bands were quantified and normalized to those of total ERK1/2 and JNK1/2. Values are mean ± SEM of three independent experiments using cells cultured from three different Neo-C and Neo-PH calves. *P< 0.01 vs. control data.
In contrast to the nearly ubiquitous nature of NAC and TEMPOL inhibitory effects on ERK1/2 in PDGF-BB-stimulated Neo-C and Neo-PH adventitial fibroblasts, JNK1/2 phosphorylation was unaffected by either NAC or TEMPOL treatment in PDGF-BB-stimulated Neo-C cells (Figure 4C). This necessarily relates to the fact that PDGF-BB treatment reduces JNK1 phosphorylation in Neo-C cells thereby making such response an unlikely candidate for regulation by ROS, which appear to function by increasing (rather than decreasing) kinase phosphorylation. This unresponsiveness of the JNK1 phosphorylation to NAC and TEMPOL in Neo-C cells differs from a substantial inhibition of PDGF-BB-stimulated JNK1 activation by NAC treatment in Neo-PH fibroblasts (both at 10 and 30 min) (Figure 4D). JNK1 activation in these cells was also significantly attenuated by TEMPOL, but only at 30 min of stimulation (Figure 4D). Collectively, these data suggest that PDGF-BB-induced ERK1/2 activation is mediated through ROS in both Neo-C and Neo-PH cells. However, activation of the PDGFβ-R-ROS-JNK1 pathway occurs uniquely in fibroblasts of hypoxia-induced remodelled PA adventitia.
3.5. Attenuation of JNK1/2 activity with SP600125 selectively inhibits Neo-PH cell replication
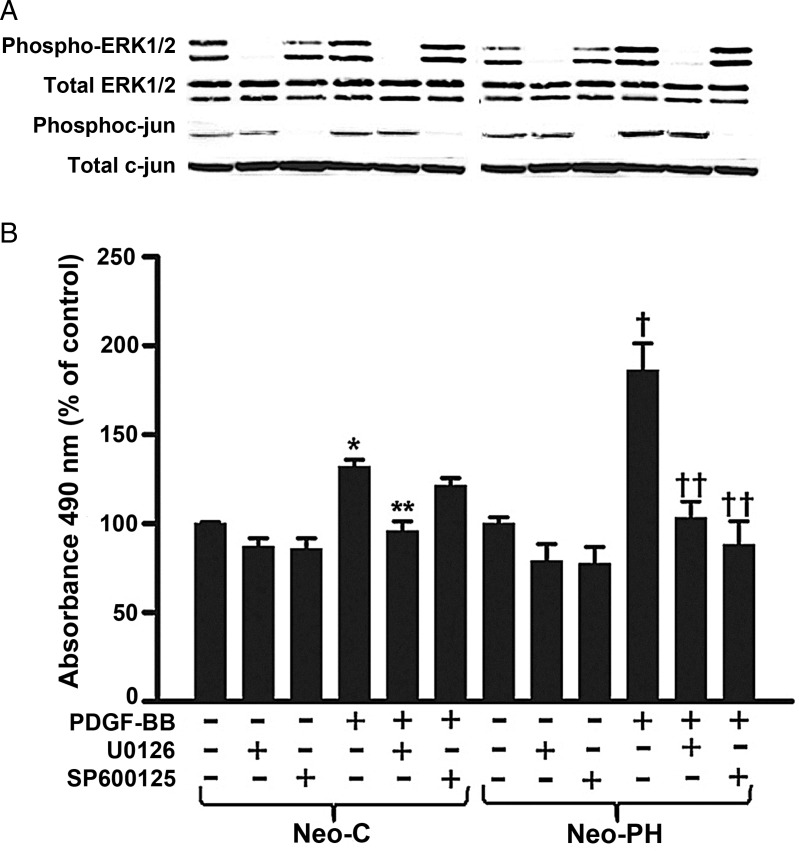
To substantiate further the role of ERK1/2 and JNK1 activation in heightened proliferation of Neo-PH fibroblasts, pharmacological antagonists, U0126 and SP600125, were used to block ERK1/2 and JNK1/2 pathways, respectively. Consistent with the role of ERK1/2 in such proliferative responses, U0126, inhibitor of MEK1/2, selectively attenuated PDGF-BB-induced ERK1/2 phosphorylation and replication in both Neo-C and Neo-PH cells (Figure 5A and B). In contrast, in the presence of SP600125, proliferative response to PDGF-BB stimulation was only blocked in Neo-PH fibroblasts (Figure 5B). Inhibitory action of SP600125 on JNK1/2 activity was confirmed by assessing the phosphorylation of c-Jun, a downstream target of JNK1/2. In Neo-C as well as Neo-PH cells, both basal and PDGF-BB-induced c-Jun phosphorylation levels were completely attenuated by SP600125, but not by the ERK1/2 inhibitor, U0126 (Figure 5A). Pharmacological inhibitor data provide further credence to the observations that PDGF-BB-induced Neo-C cell replication is mediated solely through ERK1/2 activation and that a distinguishing characteristic of stimulated Neo-PH cell proliferation lies in its regulation by combined activities of two members of the MAP kinase family—ERK1/2 and JNK1.
Figure 5.
U0126 and SP600125 attenuate Neo-PH cell proliferation. (A) Growth-arrested fibroblasts were pretreated with either U0126 or SP600125 for 1 h, stimulated with PDGF-BB (25 ng/mL) for 30 min, and then phosphorylation of ERK1/2 and c-jun was assessed by western immunoblot analysis. (B) Quiescent Neo-C and Neo-PH cells were pre-incubated for 1 h with either U0126 or SP600125, and then treated with PDGF-BB. Cell proliferation assay was performed after 48 h stimulation. Values are mean ± SEM of three independent experiments done with three different fibroblast populations cultured from three different control and hypoxia-exposed calves. *P< 0.01 vs. untreated Neo-C cells; **P< 0.01 vs. PDGF-BB-treated Neo-C cells; †P< 0.01 vs. untreated Neo-PH cells; ††P< 0.01 vs. PDGF-BB-treated Neo-PH cells.
3.6. JNK1-targeted siRNA selectively attenuates Neo-PH proliferation
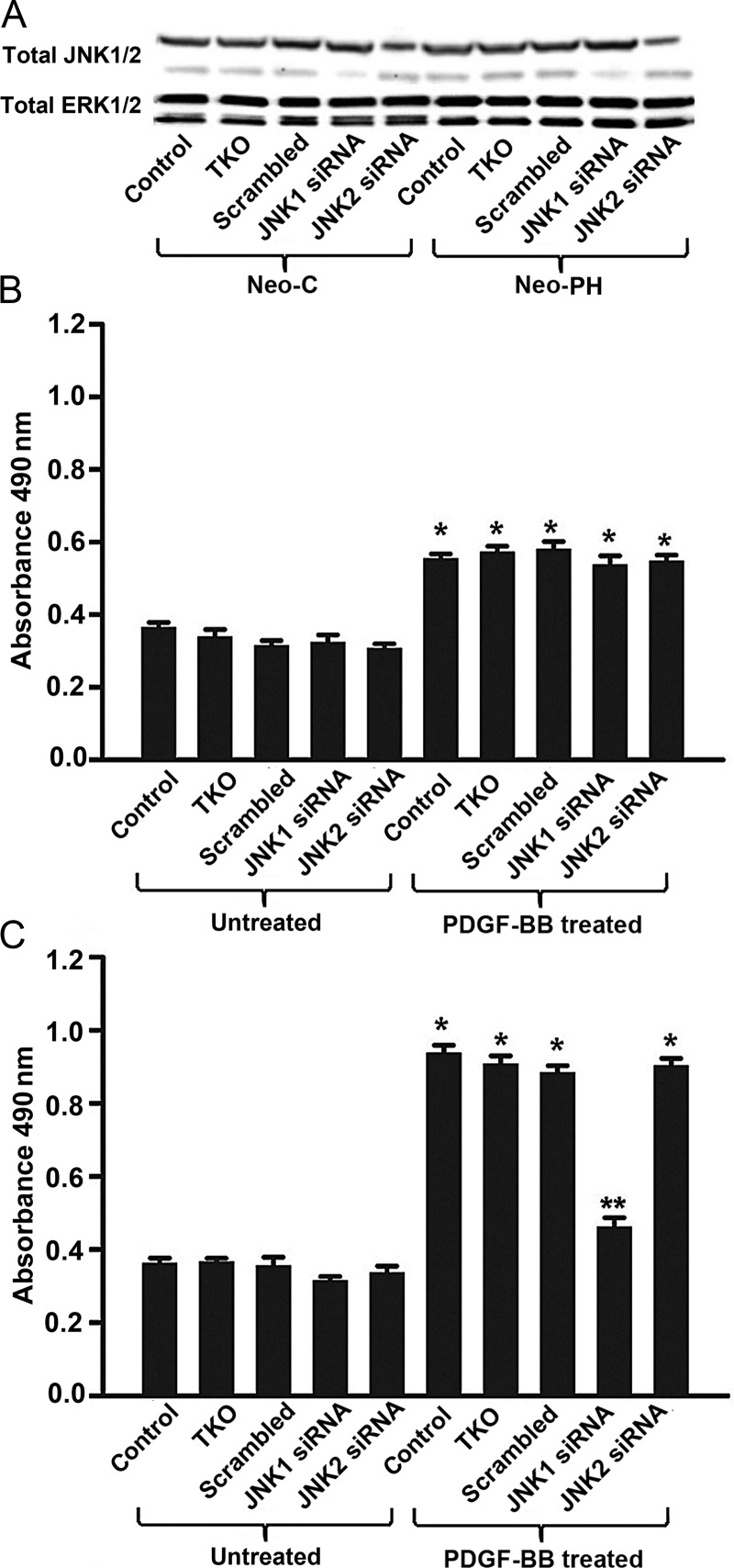
To interfere with JNK1 signalling occurring during PDGFβ-R activation by PDGF-BB in a sequence-specific fashion, fibroblast replication was evaluated in the presence of JNK1-targeting siRNA. In both Neo-C and Neo-PH cells, JNK1 and JNK2 protein levels examined by immunoblots were decreased in the presence of JNK1- and JNK2-targeting siRNAs, respectively (Figure 6A). Selectivity of the siRNAs toward JNKs was confirmed in Neo-C and Neo-PH cells by evaluating total ERK1/2 protein levels, which were unaffected by these siRNA treatments (Figure 6A). Efficiency of siRNA transfection in fibroblasts was assessed using siGLO Red, which indicated transfection of nearly 100% of the cells under conditions used here (Supplementary material online, Figure S3). In concordance with above-mentioned experiments in Neo-C fibroblasts employing pharmacological inhibition, PDGF-BB-stimulated proliferation was not altered by either JNK1- or JNK2-targeted siRNAs (Figure 6B). In addition, although, in Neo-PH cells, either scrambled (control) or JNK2-targeted siRNA did not affect replication (Figure 6C), JNK1-targeting siRNA selectively blocked the PDGF-BB-induced increase in proliferation of these cells (Figure 6C)—a result parallel to analogous blockade of proliferation achieved with JNK1/2 inhibitor, SP600125 (Figure 5B). Collectively, our results strongly support the idea that JNK1 activation plays an important role in the regulation of fibroblast proliferation during PA adventitial remodelling process in hypoxia-induced PH.
Figure 6.
JNK1-targeting siRNA inhibits Neo-PH fibroblast proliferation. Quiescent Neo-C and Neo-PH cells were transiently transfected with either anti-JNK1 or anti-JNK2 or scrambled siRNA using TransIT TKO. (A) Cell lysates were collected 48h post-transfection and immunoblotted for JNK1/2 and ERK1/2. (B and C) Transfected Neo-C (B) and Neo-PH (C) cells were stimulated with PDGF-BB for 48 h and then proliferation assay was performed. Values are mean ± SEM of three independent experiments using fibroblasts isolated from three separate Neo-C and Neo-PH calves. *P< 0.01 vs. untreated control cells; **P< 0.01 vs. PDGF-BB treated cells.
4. Discussion
The present study demonstrates that adventitial fibroblasts of hypoxia-stimulated remodelled PA acquire the following three characteristics not observed in control fibroblasts: (i) elevated expression and activation of PDGFβ-R, (ii) marked increase in PDGF-BB-induced ROS generation, and (iii) greatly increased proliferation with PDGF-BB-stimulation. We are also reporting for the first time that fibroblasts from the remodelled PA obtain the unique capacity for selective activation of JNK1 in a response to PDGFβ-R activation with PDGF-BB. Furthermore, we show that interference with the signalling via this PDGFβ-R-JNK1 axis with either SP600125 or siRNA-targeting JNK1 selectively blocks replication of remodelled PA adventitial cells. Therefore, our data strongly suggest that PDGFβ-R-stimulated JNK1 might be the key intracellular mediator of fibroproliferative responses in chronic PH. Therapeutic blockade of JNK1 selectively in fibroblasts might consequently reduce or even prevent marked adventitial remodelling characteristic of hypoxia-induced PH and serve as a stepping stone toward restoration of vascular homeostasis in the PA.
Although increased levels of PDGFβ-R have been reported in PA adventitial cells of PH patients and experimental models of PH,12,13 the molecular mechanisms underlying adventitial fibroblast activation by PDGFβ-R remain unexplored. However, the knowledge regarding the intracellular signalling pathways engaged in PDGFβ-R-mediated adventitial cell responses is absolutely critical for the development of new ‘reverse remodelling’ strategies for PH as adventitial fibroblasts have been shown to be the cells in the PA wall which are first to proliferate in the earliest stages of hypoxia-induced PH. Findings of the current study are further supported by our earlier reports,7,27 demonstrating that chronic hypoxia exposure induces phenotypic alteration in PA adventitial fibroblasts. It is conceivable that up-regulation in PDGFβ-R phosphorylation in PA adventitial cells might be in the future used as a marker of hypoxia-stimulated modification of adventitial fibroblast phenotype. This in turn encourages questioning whether chronic hypoxia exposure stimulates PDGFβ-R activation in adventitial cells by either up-regulating tyrosine kinase activity or down-regulating protein tyrosine phosphatase activity, or affecting both. How actions of such receptor-regulatory moieties might influence PH pathophysiology remains to be explored. Thus, heightened PDGFβ-R phosphorylation in adventitial cells might underscore the presence of highly-activated specialized cell populations in hypoxia-induced remodelled PA adventitia.
Our findings that the PDGFβ-R-ROS signalling is important for excessive cell proliferation in the PA of calves exposed to chronic hypoxia are supported by a study in a murine model of PH, in which chronic intermittent hypoxia stimulates generation of NADPH oxidase-derived ROS, increases the activity of PDGFβ-R, and induces proliferation of smooth muscle cells.15 Our work, however, provides novel progress toward understanding of the mechanisms governing these responses with a focus on an adventitial fibroblast. Furthermore, we present here a novel downstream molecular target of the PDGFβ-R-ROS signalling axis, JNK1, a kinase that is selectively responsible for major increases in fibroblast proliferation observed in this model of severe PH.
In the present studies, we have also found that ROS production upon PDGF-BB stimulation is significantly greater in Neo-PH cells compared with that in the control fibroblasts. However, the magnitude of differences in ROS levels between Neo-PH and Neo-C fibroblasts is rather small. In contrast, the degree of differences in the proliferative responses between these two fibroblast populations is fairly large (Figure 2). In this case, the concentrations of ROS achieved might dictate the type of corresponding cellular response. In support of this notion is the fact that excessive production of ROS has been shown to induce oxidative stress, a detrimental process that can lead to cellular damage, whereas beneficial effects of ROS occur at low/moderate concentrations and control healthy physiological responses in cells.28 This report strongly supports our findings showing that a modest increase in ROS generation might elicit a sufficient signal, which in turn mediates replicative responses in Neo-PH cells with a greater magnitude than the elevation in ROS itself. Therefore, the small elevation in ROS production in Neo-PH cells might be the key driver of heightened proliferative phenotype of adventitial cells during the remodelling process in PH.
ERK1/2 phosphorylation is a major downstream signal activated by stimulating PDGFβ-R.29 In our study focusing on fibroblasts, we have found that PDGFβ-R activation induces conventional ERK1/2 phosphorylation via ROS generation in both Neo-C and Neo-PH cells, a process that leads to replication of these cells. However, the most striking finding of the current report points to JNK1 as the pro-proliferative kinase in Neo-PH cells. JNK pathways have established roles in apoptotic signalling, but might under certain circumstances, including responses to hypoxic stimulation described here, also contribute to cell proliferation and migration.25,30 In the present study, we have found that inhibition of JNK1 with JNK1-targeting siRNA blocks selectively PDGF-BB-stimulated increase in cell numbers in Neo-PH cells, suggesting that JNK1 functions as a replication regulator for these cells. Transient JNK1 phosphorylation induced by activating PDGFβ-R in Neo-PH cells might be due to the phenotypic modification caused by hypoxia, which in this model functions as a stimulator of proliferation rather than an inducer of apoptosis as seen in other cell types. In contrast, in Neo-C cells, JNK1 is not phosphorylated by PDGFβ-R. In the future studies, it will be important to investigate the pathways involved in differential PDGFβ-R-JNK1 activation patterns in adventitial cells to attempt to find a more selective target to be pursued for therapeutic development of novel PH treatments. Recent studies in JNK-deficient mice demonstrating that JNK1 acts as a profibrogenic kinase during hepatic fibrosis31 also support our data that identify JNK1 as a fibroblast activator. Since we have reported earlier that JNK regulates hypoxia-induced differentiation of fibroblasts to myofibroblasts in PA adventitia,26 we will next explore whether mice deficient in JNK1 will develop blunted hypoxia-induced PH due to the interference with the adventitial remodelling process.
Within the complex pattern of vessel remodelling in PH, adventitial fibroblasts expressing increased levels of activated PDGFβ-R contribute significantly to the observed pathology. Signalling events centred on selective activation of JNK1 make this kinase an important molecular target for interventional strategies in PH. Both experimental and clinical evidence of therapeutic efficacy of imatinib, a PDGFβ-R inhibitor, represent one novel and promising approach for the treatment of PH.10,32 However, imatinib treatment may be complicated by significant cardiotoxicity, which may result in overt heart failure.33 Therefore, it is crucial to thoroughly characterize PDGF signalling pathways specifically in adventitial cells of the vascular wall in order to identify more specific targets for pharmacological intervention in PH. Our present data strongly suggest that modulating the activity of JNK1 selectively in fibroblasts is a case in point.
Supplementary data
Supplementary data are available at Cardiovascular Research online.
Funding
This work was supported by the National Institutes of Health HL64917 (M.D.), HL014985-38 (K.R.S.), HL084923-04 (K.R.S.), and HL007171-39 (K.R.S.).
Supplementary Material
Acknowledgements
The authors thank Steve Hofmeister and Sandi Walchak for harvesting the bovine PA tissue; Dr Kristin M. Shields (University of Colorado Denver, Denver, CO, USA) for the assistance of immunoperoxidase staining of PDGFβ-R and phospho-PDGFβ-R in bovine lung sections.
Conflict of interest: none declared.
References
- 1.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 2.Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–1539. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenmark KR, Fasules J, Voelkel NF, Henson J, Tucker A, Wilson H, et al. Severe pulmonary hypertension and arterial adventitial changes in newborn calves at 4300 m. J Appl Physiol. 1987;62:821–830. doi: 10.1152/jappl.1987.62.2.821. [DOI] [PubMed] [Google Scholar]
- 4.Belknap JK, Orton EC, Ensley B, Tucker A, Stenmark KR. Hypoxia increases bromodeoxyuridine labeling indices in bovine neonatal pulmonary arteries. Am J Respir Cell Mol Biol. 1997;16:366–371. doi: 10.1165/ajrcmb.16.4.9115746. [DOI] [PubMed] [Google Scholar]
- 5.Das M, Dempsey EC, Bouchey D, Reyland ME, Stenmark KR. Chronic hypoxia induces exaggrerated growth responses in pulmonary artery adventitial fibroblasts: potential contribution of specific protein kinase C isozymes. Am J Respir Cell Mol Biol. 2000;22:15–25. doi: 10.1165/ajrcmb.22.1.3536. [DOI] [PubMed] [Google Scholar]
- 6.Welsh DJ, Peacock AJ, MacLean M, Harnett M. Chronic hypoxia induces constitutive p38 mitogen-activated protein kinase activity that correlates with enhanced cellular proliferation in fibroblasts from rat pulmonary but not systemic arteries. Am J Respir Crit Care Med. 2001;164:282–289. doi: 10.1164/ajrccm.164.2.2008054. [DOI] [PubMed] [Google Scholar]
- 7.Das M, Burns N, Wilson SJ, Zawada WM, Stenmark KR. Hypoxia exposure induces the emergence of fibroblasts lacking replication repressor signals of PKCζ in the pulmonary artery adventitia. Cardiovasc Res. 2008;78:440–448. doi: 10.1093/cvr/cvn014. [DOI] [PubMed] [Google Scholar]
- 8.Humbert M, Monti G, Fartoukh M, Magnan A, Brenot F, Rain B, et al. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J. 1998;11:554–559. [PubMed] [Google Scholar]
- 9.Perros F, Montani D, Dorfmuller P, Durand-Gasselin I, Tcherakian C, Pavec JL, et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:81–88. doi: 10.1164/rccm.200707-1037OC. [DOI] [PubMed] [Google Scholar]
- 10.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, et al. Tie2-mediated loss of peroxisome proliferator-activated receptor-γ in mice cause PDGF receptor-β-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1082–L1090. doi: 10.1152/ajplung.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones R, Capen D, Jacobson M. PDGF and microvessel wall remodeling in adult lung: imaging PDGF-Rβ and PDGF-BB molecules in progenitor smooth muscle cells developing in pulmonary hypertension. Ultrastruct Pathol. 2006;30:267–281. doi: 10.1080/01913120600820336. [DOI] [PubMed] [Google Scholar]
- 13.Overbeek MJ, Boonstra A, Voskuyl AE, Vonk MC, Vonk-Noordegraaf A, van Berkel MPA, et al. Platelet-derived growth factor receptor-β and epidermal growth factor receptor in pulmonary vasculature of systemic sclerosis-associated pulmonary arterial hypertension versus idiopathic pulmonary arterial hypertension and pulmonary veno-occulusive disease: a case-control study. Arthritis Res Ther. 2011;13:R61. doi: 10.1186/ar3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das M, Dempsey EC, Reeves JT, Stenmark KR. Selective expansion of fibroblast subpopulations from pulmonary artery adventitia in response to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L976–L986. doi: 10.1152/ajplung.00382.2001. [DOI] [PubMed] [Google Scholar]
- 15.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TM, et al. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol. 2009;40:601–609. doi: 10.1165/rcmb.2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sveglian S, Cancello R, Sambo P, Luchetti M, Paroncini P, Orlandini G, et al. Platelet-derived growth factor and reactive oxygen species (ROS) regulate Ras protein levels in primary human fibroblasts via ERK1/2: amplification of ROS and Ras in systemic sclerosis fibroblasts. J Biol Chem. 2005;280:36474–36482. doi: 10.1074/jbc.M502851200. [DOI] [PubMed] [Google Scholar]
- 17.Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354:2667–2676. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 18.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 19.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 20.Xu Q, Liu Y, Gorospe M, Udelsman R, Holbrook NJ. Acute hypertension activates mitogen-activated protein kinases in arterial wall. J Clin Invest. 1996;97:508–514. doi: 10.1172/JCI118442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izumi Y, Kim S, Namba M, Yasumoto H, Miyazaki H, Hoshiga M, et al. Gene transfer of dominant negative mutants of extracellular signal-regulated kinase and c-Jun NH2-terminal kinase prevents neointimal formation in balloon-injured rat artery. Circ Res. 2001;88:1120–1126. doi: 10.1161/hh1101.091267. [DOI] [PubMed] [Google Scholar]
- 22.Force T, Pombo CM, Avruch JA, Bonventre JV, Kyriakis JM. Stress-activated protein kinases in cardiovascular disease. Circ Res. 1999;78:947–953. doi: 10.1161/01.res.78.6.947. [DOI] [PubMed] [Google Scholar]
- 23.Graf K, Xi XP, Yang D, Fleck E, Hsueh WA, Law RE. Mitogen-activated protein kinase activation is involved in platelet-derived growth factor directed migration by vascular smooth muscle cells. Hypertension. 1997;29:334–339. doi: 10.1161/01.hyp.29.1.334. [DOI] [PubMed] [Google Scholar]
- 24.Garat CV, Fankell D, Erickson PF, Reusch JE, Bauer NN, McMurtry IF, et al. Platelet-derived growth factor BB induces nuclear export and proteasomal degradation of CREB via phosphatidylinositol 3-kinase/Akt signaling in pulmonary artery smooth muscle cells. Mol Cell Biol. 2006;26:4934–4948. doi: 10.1128/MCB.02477-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das M, Bouchey DM, Moore M, Hopkins D, Nemenoff R, Stenmark KR. Hypoxia-induced proliferative responses of pulmonary artery adventitial fibroblasts is dependent on G-protein mediated activation of MAP kinases. J Biol Chem. 2001;276:15631–15640. doi: 10.1074/jbc.M010690200. [DOI] [PubMed] [Google Scholar]
- 26.Short M, Nemenoff RA, Zawada WM, Stenmark KR, Das M. Hypoxia induces differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts. Am J Physiol Cell Physiol. 2004;286:C416–C425. doi: 10.1152/ajpcell.00169.2003. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, et al. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol. 2011;187:2711–2722. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Jurek A, Heldin CH, Lennartsson J. Platelet-derived growth factor-induced signaling pathways interconnect to regulate the temporal pattern of ERK1/2 phosphorylation. Cell Signal. 2011;1:280–287. doi: 10.1016/j.cellsig.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell. 2004;15:713–725. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 31.Kluwe J, Pradere J-P, Gwak G-Y, Mencin A, Minicis SD, Osterreicher CH, et al. Modulation of hepatic fibrosis by c-Jun-N-Terminal kinase inhibition. Gastroenterology. 2010;138:347–359. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005;353:1412–1413. doi: 10.1056/NEJMc051946. [DOI] [PubMed] [Google Scholar]
- 33.Kerkela R, Grazette L, Yacobi R, IIiescu C, Patten R, Beahm C, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.