Abstract
Background and Aims
Cyanolichens are usually stated to be bipartite (mycobiont plus cyanobacterial photobiont). Analyses revealed green algal carbohydrates in supposedly cyanobacterial lichens (in the genera Pseudocyphellaria, Sticta and Peltigera). Investigations were carried out to determine if both cyanobacteria and green algae were present in these lichens and, if so, what were their roles.
Methods
The types of photobiont present were determined by light and fluorescence microscopy. Small carbohydrates were analysed to detect the presence of green algal metabolites. Thalli were treated with selected strengths of Zn2+ solutions that stop cyanobacterial but not green algal photosynthesis. CO2 exchange was measured before and after treatment to determine the contribution of each photobiont to total thallus photosynthesis. Heterocyst frequencies were determined to clarify whether the cyanobacteria were modified for increased nitrogen fixation (high heterocyst frequencies) or were normal, vegetative cells.
Key Results
Several cyanobacterial lichens had green algae present in the photosynthetic layer of the thallus. The presence of the green algal transfer carbohydrate (ribitol) and the incomplete inhibition of thallus photosynthesis upon treatment with Zn2+ solutions showed that both photobionts contributed to the photosynthesis of the lichen thallus. Low heterocyst frequencies showed that, despite the presence of adjacent green algae, the cyanobacteria were not altered to increase nitrogen fixation.
Conclusions
These cyanobacterial lichens are a tripartite lichen symbiont combination in which the mycobiont has two primarily photosynthetic photobionts, ‘co-primary photobionts’, a cyanobacterium (dominant) and a green alga. This demonstrates high flexibility in photobiont choice by the mycobiont in the Peltigerales. Overall thallus appearance does not change whether one or two photobionts are present in the cyanobacterial thallus. This suggests that, if there is a photobiont effect on thallus structure, it is not specific to one or the other photobiont.
Keywords: Zinc, heterocyst, carbohydrate, ribitol, photobiont, lichen, cyanolichen, cyanobacteria, green algae, phenotypic plasticity, Pseudocyphellaria, Peltigera, Sticta
INTRODUCTION
Lichens are a symbiotic association between a fungus (mycobiont) and a photosynthetic partner (photobiont). The exact form of the symbiosis remains controversial (Nash, 2008), but is commonly suggested to be mutualistic (Büdel and Scheidegger, 2008). Lichenization is a successful strategy for fungi, almost 20 % of all species being lichenized with a wide variety of photobionts and photobiont arrangements (Honegger, 2009). About 95 % of lichens are bipartite, and the mycobiont has a single photosynthetic partner which is either a green alga (around 85 % of lichens) or a cyanobacterium (around 10 % of lichens), and a small number of lichens have photobionts from other algal groups (Honegger, 2008). Another 3–4 % of lichens are tripartite, the so-called cephalodiate species, and have a green algal photobiont arranged as in bipartite species and a cyanobacterium that is confined to a special structure, the cephalodium, that can occur within the tissues of the lichen or on its upper or lower surface (see Brodo et al., 2001, for definition; Nash, 2008). Within the cephalodia the cyanobionts apparently become predominantly heterotrophic and are specialized for nitrogen fixation. In cephalodia, a high proportion of the cells are heterocysts, up to 35·6 % compared with 4–7 % in free-living cyanobacteria, but values of around 25–30 % are more common and the remaining vegetative cells appear not to be highly photosynthetic (Feige, 1976; Brodo and Richardson, 1978). Goebel (1926) showed that the large cephalodia of Peltigera aphthosa were not capable of independent life, indicating that the cyanobacteria within were apparently not photosynthetic. However, there are only a few cases where the actual functioning of the different photobionts has been investigated, so it is rare to know how much of the secondary cyanobiont is photosynthetic or centred on nitrogen fixation.
In recent years, the growth of molecular studies has not only restructured the taxonomy of many lichen groups but has also led to a greater understanding of the relationships between the photobionts and the mycobionts. The degree of selectivity shown by species for their photobionts has been investigated, and some studies show that the mycobiont exhibits little choice of its cyanobacterial partner (Wirtz et al., 2004), while others report strong selectivity (Paulsrud et al., 2000; Lohtander et al., 2003; Stenroos et al., 2006). There is also increasing evidence that a single lichen species may show genetic variability in its photobiont and that occurrence of particular strains may be linked to the ecology of the species (Yahr et al., 2006; Fernández-Mendoza et al., 2011). There is also growing interest in the evolution of the mycobiont/photobiont symbiosis and in which mycobiont–photobiont combination first evolved. In some groups, such as the Lobariaceae, it is suggested that the ancestral lichens were cyanobacterial only, and that green algae were a later addition (Miadlikowska and Lutzoni, 2004; Högnabba et al., 2009).
Members of the Peltigerales, which are all tripartite chlorolichens or cyanolichens, show an astonishing range of photobionts within single lichen species. In Lobaria amplissima, the cephalodia that can form external coralloid structures on the upper surface of the lichen have long been known to be similar to the thalli of the genus Dendriscocaulon (James and Henssen, 1976). Molecular studies have now confirmed that the mycobionts of Dendriscocaulon umhausense and the chlorolichen L. amplissima are identical (Stenroos et al., 2003; Högnabba et al., 2009) so that D. umhausense is actually the cyanobacterial form (cyanomorph) of L. amplissima. A small number of species in both the Lobariaceae and Peltigeraceae form photosymbiodemes (Fig. 1B), where single thalli occur with sectors that contain either cyanobacterial or green algal photobionts. The two forms can also occur as independent, separate thalli with either cyanobacterial (cyanomorph) or green algal (chloromorph) photobionts. In the past, the two forms were given full species status, e.g. the species pair Pseudocyphellaria rufovirescens (chlorolichen) and Ps. murrayi (cyanolichen) (Renner and Galloway, 1982). The classification of these photosymbiodemes and their independent forms has intrigued lichen taxonomists for some time, causing considerable discussion (Kaule, 1931; Renner and Galloway, 1982; Jørgensen, 1996, 1998; Laundon, 1996; Heidmarsson et al., 1997). However, several studies have shown that all these forms share a single mycobiont (Armaleo and Clerc, 1991; Goffinet and Bayer, 1997; Thomas et al., 2002) and under existing rules of nomenclature must be regarded as a single species. The forms have been reduced to synonomy in the latest Flora of New Zealand (Galloway, 2007).
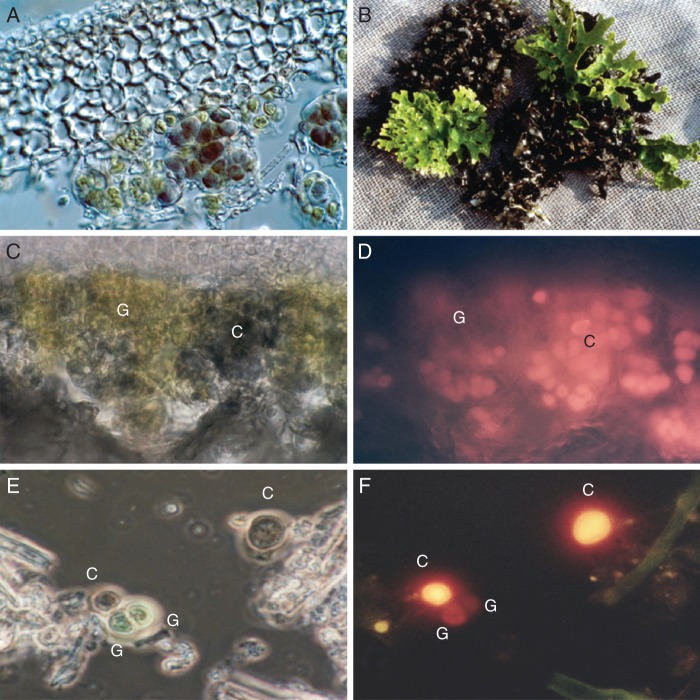
Fig. 1.
(A) Photomicrograph (DIC; Nomarski mode) of a vertical section through a thallus of the cyanobacterial lichen Ps. rufovirescens/cyan showing the green algae (green colour, left, lower centre) and cyanobacteria (dark colour, lower centre and right centre) beneath the upper cortex. (B) Thallus of a Ps. rufovirescens photosymbiodeme composed of sectors that are green algal (bright green colour) and cyanobacterial (dark colour). (C) Photomicrograph under normal light of a thick vertical section through a thallus of the cyanobacterial lichen Ps. rufovirescens/cyan; the green areas, G, are green algae and the darker areas, C, are cyanobacteria. (D) As in (C) but in fluorescence mode with green excitation light; only the cyanobacterial cells, C, fluoresce bright yellow/orange. (E) Photomicrograph under normal light of isolated photobionts from the cyanobacterial lichen Ps. rufovirescens/cyan; the labels C indicate cyanobacterial cells and G indicate green algal cells. (F) as in (E) but in fluorescence mode under green excitation light; the cyanobacterial cells fluoresce bright yellow/orange and the green algal cell shows almost no fluorescence.
In all of these studies, the assumption is made that only one form of tripartite lichen exists, the cephalodiate model: the lichen is a chlorolichen with the secondary cyanobiont confined to cephalodia. The alternative situation where the lichen is a cyanolichen (i.e. the primary photobiont is cyanobacterial and the visually dominant photobiont) but also has a chlorobiont present is not usually mentioned. A very small number of lichens have been recognized to have this structure, such as Euopsis granatina (Buedel and Henssen, 1988) and Muhria urceolata (Jørgensen and Jahns, 1987). Some Solorina species also show variable amounts of cyanobacteria in their thalli, and a complete cyanobiont layer can be formed below the chlorobiont layer. However, these are still referred to as having a cephaloidate structure (Buedel and Scheidegger, 2008). In the literature, these examples tend to be considered curiosities.
Here we provide evidence that the tripartite form, one in which the mycobiont has two ‘primary’ photosynthetic photobionts, a dominant cyanobiont and also a chlorobiont, both contributing substantially to the photosynthesis of the thallus, could be widespread in the Lobariaceae and Peltigeraceae. The existence of this tripartite form adds another level of complexity to mycobiont/photobiont relationships. It also contributes to our understanding of the development of morphological control in the lichen thallus and of the ecology of the species.
MATERIALS AND METHODS
Terminology
Photobionts
In this paper we will follow the nomenclature of Lange and Wagenitz (2003) and refer to green algal photobionts as chlorobionts, and cyanobacterial photobionts as cyanobionts. Bipartite lichens with chlorobionts are chlorolichens, and those with cyanobionts are cyanolichens. In addition, when a photobiont forms all or part of the photobiont layer below the upper cortex in the foliose lichen thallus and carries out the photosynthetic function of the lichen, we call it a ‘primary’ photobiont (Büdel and Scheidegger, 2008). If two different photobionts are present in the photosynthetic layer and both contribute to the photosynthetic function of the lichen, then we call them ‘co-primary photobionts’. The cyanobionts that are in the nitrogen-fixing cephalodia of some chlorolichens, which contribute little to photosynthesis, are referred to as ‘secondary’ photobionts.
Species pairs and photosymbiodemes
Although members of species pairs have been given separate names on occasion (Renner and Galloway, 1982), the demonstration that they share a common mycobiont (Armaleo and Clerc, 1991) means that they have now all been reduced to synonomy (Galloway, 2007). In this paper we will use the accepted name for each pair and add the appropriate suffix ‘chlor’ or ‘cyan’ after the name when referring to the chorolichen or cyanolichen. For example, the pair Ps. rufovirescens, chlorolichen with cephalodia, and P. murrayi, cyanolichen, has the single name Ps. rufovirescens which is annotated to give Ps. rufovirescens/chlor or Ps. rufovirescens/cyan when referring to the independent chlorolichen and cyanolichen thalli, respectively (Heidmarsson et al., 1997).
Material
Lichen species used and their collection sites are listed in Table 1. Lichens are categorized as cyanobacterial, or green algal with cephalodia, as in Galloway (2007). The reported chlorobionts are Dictyochloropsis (Trebouxiophyceae) for chlorolichens in the genera Pseudocyphellaria and Sticta. The cyanobionts in the cyanolichens and cephalodia of chlorolichens are Nostoc sp. Thalli were collected, and attached twigs, soil and epiphytes were removed on the same day in the laboratory, followed by washing with tap water, rinsing with distilled water and then air drying at room temperature. The air-dried samples were then stored in paper bags over silica gel and were used within 6 weeks of collection.
Table 1.
Lichen species used, their photobiont type and their collection sites
| Lichen species* | Photobiont | Collection site† |
|---|---|---|
| Collema leucocarpum | Cyanobacterial | 1 |
| Peltigera dolichorhiza | Cyanobacterial | 1 |
| Pseudocyphellaria carpoloma | Green algal + cephalodia | 5 |
| Ps. cinnamomea | Cyanobacterial | 3 |
| Ps. chloroleuca | Green algal + cephalodia | 5 |
| Ps. crocata | Cyanobacterial | 5 |
| Ps. dissimilis | Cyanobacterial | 1 and 2 |
| Ps. hookeri | Cyanobacterial | 4 |
| Ps. lividofusca/cyan | Cyanobacterial | 1 |
| Ps. lividofusca | Green algal + cephalodia | 1 |
| Ps. rufovirescens/cyan | Cyanobacterial | 1 |
| Ps. rufovirescens | Green algal + cephalodia | 1 and 2 |
| Sticta fuliginosa | Cyanobacterial | 1 |
| S. latifrons | Green algal + cephalodia | 4 |
* Nomenclature is as in Galloway (2007) except Ps. lividofusca/cyan and Ps. rufovirescens/cyan which were previously named Ps. knightii and P. murrayi, which are now synonymous with, and the cyanomorphs of, Ps. lividofusca and Ps. rufovirescens, respectively.
† Collections sites were: 1, Stubb's farm, Waitomo (NZMS1 N82 894 885); 2, Hakirimata Range, Ngaruawahia (NZMS 260 S14 988 915); 3, Wairere Falls (NZMS 260 T14 641 813); 4, Kauranga Valley, Coromandel (NZMS 260 T12 478 561); 5, Brophies Beach, Coromandel (NZMS 260 T11 528 847). Samples are lodged in the personal collections of T.G.A.G. and W.A.I.K.
Microscopy
Samples from the lobe margins of Ps. rufovirescens/cyan, Ps. lividofusca/cyan, Ps. dissimilis, Ps. hookeri, Ps. crocata and Sticta fuliginosa were soaked overnight in distilled water; they were then frozen into blocks of ice and sectioned on a freezing microtome to produce sectons of suitable thickness for photobiont determination. Photobionts from the same lichens were isolated using the technique of Green and Smith (1974) and suspended in distilled water for examination. Sections were examined with a Reichart Polyvar microscope operated in normal light, differential interference contrast (DIC; Nomarski) and fluorescence modes. In fluorescence mode, green light excitation was used (excitation BP 546/10, dichroic mirror DS 580, barrier filter LP 590) and this selectively causes cyanobacteria to produce a reddish fluorescence due to light absorbtion by phycobilins (Watras and Baker, 1988). Fluorescence by cyanobacteria not only showed that the cells were photosynthetically active but also revealed their presence in the photobiont layer containing both green algae and cyanobacteria.
Heterocyst frequency
Portions of thallus (10 mg) were taken and cut into strips (5 × 1 mm) which were then placed overnight in 10 % (w/v) chromium trioxide solution (Hitch and Millbank, 1975a). Small pieces of the macerated lichens were then further dispersed by a glass rod on a microscope slide. To assess the ratio of vegetative to heterocyst cells, the number of normal cells and heterocysts in intact chains of cells were counted in several fields until a total of 1000 cells had been reached. Heterocyst frequency is reported as a percentage of total cells (vegetative plus heterocysts).
Zn2+-resistant photosynthesis
Thallus samples were kept moist at 20 °C, under a photosynthetic photon fluence rate (PPFR) over the waveband 400–700 nm of 70 µmol m−2 s−1 for 12 h. CO2 exchange of the thalli was then measured using a closed loop infrared gas analyser system (Snelgar et al., 1981). Samples were placed in a cuvette at 16 °C, and NP (net photosynthesis) at 340 ppm CO2 determined at a PPFR of 70 µmol m−2 s−1 from the rate of decline in CO2 concentration. Dark respiration (DR) was determined at a PPFR of 0 µmol m−2 s−1 and 340 ppm CO2. The lichens were then removed and agitated in a 5 mm ZnSO4 solution for 30 min at 20 °C, with a PPFR of 200 µmol m−2 s−1. The thalli were rinsed with distilled water, and NP and DR were measured again. The Zn2+-resistant GP (gross photosynthesis = NP – DR) was determined after treatment as the percentage of original GP (Brown and Beckett, 1983). At these low concentrations, zinc has been shown to arrest electron transfer at the reducing side of the photosystem (PS) II reaction centre in cyanobacteria but not in green algae (Chaloub et al., 2005).
Carbohydrate analysis
Small molecular weight carbohydrates (sugars and sugar alcohols) were analysed by capillary gas–liquid chromatography (GLC). Lichen samples were placed in ampoules and frozen in liquid nitrogen either immediately after collection in the field or after 12 h pre-treatment (moist, 13 °C, PPFR of 70 µmol m−2 s−1) in the laboratory. Samples were then stored over silica gel following freeze drying. Extraction followed Cowan et al. (1979): 100 mg dry weight (d. wt) samples were homogenized in an all-glass Potter Elverhjem tissue grinder containing 2 mL of MCW (methanol, chloroform, water, 12:5:3 v/v/v). The homogenized sample plus a 2 mL rinse were centrifuged at 3500 rpm for 1 min and the supernatant saved. The pellet was resuspended in 2 mL of fresh solvent, centrifuged again and the supernatant saved. This was repeated twice more. The pooled supernatant was separated into two phases by the addition of 1 mL of chloroform and 1·5 mL of water containing 0·2 mg mL−1 perseitol as an internal standard. Following centrifugation at 5000 rpm for 5 min, the extract had separated into three distinct layers. The lower two layers were removed, leaving the upper methanol phase containing amino acids, organic acids, soluble carbohydrates and phosphate esters. The extract was further purified by addition of 2 mL of saturated solution of lead acetate, chilling for 30 min and filtering to remove the precipitated lead salts. The filtrate was evaporated to dryness under vacuum at 70 °C and then stored over silica gel for a minimum of 24 h to complete drying.
Trimethylsilyl ethers (TMS ethers) were prepared by letting the dried extract or a known quantity of carbohydrate standard dissolve in a small volume of pyridine (<1 mL) and TSIM (N-trimethylsilylimidazole, 50–100 µL) for 30 min. Toluene (10 mL) was added to assist the azeotropic removal of pyridine. After standing for a further 10 min, the samples were evaporated to dryness under vacuum at 50 °C. The residue was taken up in hexane (100 µL) for GLC analysis. Samples were injected into a Pye Unicam 4500 gas chromatograph fitted with an SGE unijector in split mode and flame ionization detection. A BP-1 bonded vitreous silica capillary column, 12·5 m long × 0·22 mm internal diameter, was used with hydrogen as carrier gas. Carbohydrates were identified by comparison of relative retention times with those of standard carbohydrates followed by co-chromatography with standards. Further confirmation was by running acetate derivatives and also using a different column, BP-10 with TMS ethers. Detector response to carbohydrates was determined using a sample containing equal weights of ribitol, mannitol and perseitol. These were run three times per day, and the resulting ratios, expressed relative to perseitol, were used to calculate carbohydrate quantities. Quantities as low as 0·0002 mg were detectable, but those <0·0005 mg are reported as trace levels. Individual carbohydrates are reported as percentage dry weight.
RESULTS
Carbohydrates
The contents of low molecular weight sugars and polyols (mono- and disaccharides) in 11 lichens, eight cyanolichens and three chlorolichens are given in Table 2. The percentage dry weight of these carbohydrates varied widely among the species, and ranged from a low of 1·31 % d. wt in Sticta latifrons to a high of 18·21 % d. wt in Peltigera dolichoriza. All the Pseudocyphellaria species had moderate to high contents, ranging from 3·68 to 15·32 % d. wt. Two of the species were analysed after immediate freezing in the field, Ps. crocata, the lowest content for the genus, and S. latifrons, the absolute lowest content. The other lichens were pre-treated in the laboratory (moist, 13 °C, PPFR of 70 µmol m−2 s−1) and this increased the carbohydrate content (data not shown). That mannitol generally was the most abundant carbohydrate was not unexpected because it is known to be a major small molecule storage carbohydrate in mycobionts (Lewis and Smith, 1967; da Silva et al., 1993). The mobile carbohydrate that moves between photobiont and mycobiont depends on the photobiont (Richardson et al., 1968). In cyanolichens (Nostoc) it is glucose, which was found at low levels in all the cyanolichens except Ps. crocata (Table 2). It was also detected in two of the chlorolichens, but this was not unexpected as they also contain cyanobacteria in cephalodia. The mobile carbohydrate in chlorolichens is ribitol, and this was found at higher concentrations, up to 5·70 % d. wt in Ps. lividofusca/chlor. This difference between the amounts of mobile carbohydrates for cyanobacteria and green algae is also not unexpected as the transfer rates are much slower in chlorolichens (Smith, 1975). Arabitol was present at moderate values, 1·83 % d. wt in Ps. lividofusca, as was sorbitol in almost all the cyanolichens, up to 0·59 % d. wt in Ps. rufovirescens/cyan. It is probable that the latter two carbohydrates are involved in the metabolism of mannitol in the mycobiont. A surprise in several cyanolichens was the presence of ribitol, which is not known at all as a cyanobacterial product, yet it was present in all the cyanobacterial species except for Collema leucocarpum (Table 2). As expected, ribitol was very high in chlorolichens as a proportion of the total carbohydrates, ranging from 29·0 % in S. latifrons to 42·0 % in Ps. rufovirescens/chlor. These levels were approached by some cyanolichens (40·6 % in Ps. crocata, 35·0 % in Ps. lividofusca/cyan) but remained around 0·2–3·1 % in others (Table 3).
Table 2.
Content (% d. wt) of small molecular weight carbohydrates in cyanobacterial (C) and green algal (G) lichens
| Low molecular weight carbohydrates (% d. wt) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lichen species | Photobiont | Erythritol* | Ribitol† | Glucose‡ | Arabitol | Mannitol | Sorbitol | Fructose | Myo-inositol | Volemitol? | Sucrose | Trehalose | Total |
| Ps. cinnamomea | C | – | 0·08 | t | 0·01 | 3·87 | 0·55 | 0·04 | 0·01 | 0·05 | 0·05 | 0·48 | 5·18 |
| Ps. crocataF | C | – | 1·50 | t | 0·06 | 1·91 | 0·02 | 0·02 | 0·01 | 0·02 | 0·03 | 0·12 | 3·68 |
| Ps. dissimilis | C | – | 0·01 | 0·08 | 0·01 | 6·25 | 0·51 | 0·05 | 0·06 | 0·1 | 0·53 | 0·26 | 7·90 |
| Ps. lividofusca/cyan | C | – | 1·75 | 0·02 | 0·54 | 2·14 | 0·09 | 0·21 | 0·05 | 0·07 | 0·04 | 0·04 | 4·94 |
| Ps. rufovirescens/cyan | C | – | 2·68 | 0·02 | 0·16 | 5·06 | 0·59 | 0·01 | 0·04 | 0·12 | 0·05 | – | 8·72 |
| Sticta fuliginosa | C | – | 0·14 | 0·01 | 0·05 | 2·09 | 1·53 | t | 0·42 | 0·13 | 0·01 | 0·02 | 4·39 |
| Collema leucocarpum | C | – | – | 0·23 | t | 1·35 | – | 0·07 | t | – | 0·08 | 0·15 | 1·88 |
| Peltigera dolichoriza | C | 0·47 | 0·03 | 0·01 | 0·29 | 8·23 | – | 0·19 | 0·06 | 1·45 | 0·04 | 7·45 | 18·21 |
| S. latifronsF | G | – | 0·38 | – | 0·22 | 0·64 | – | 0·01 | 0·01 | 0·03 | 0·02 | 0·01 | 1·31 |
| Ps. lividofusca | G | – | 5·70 | t | 1·83 | 6·99 | – | 0·02 | 0·01 | 0·19 | 0·44 | 0·14 | 15·32 |
| Ps. rufovirescens | G | – | 2·26 | 0·02 | 0·88 | 2·00 | 0·01 | 0·05 | 0·02 | 0·04 | 0·04 | 0·01 | 5·32 |
Known mobile carbohydrates released by photobiont: * Hyalococcus; † Trebouxiophyceae; ‡ Nostoc sp;
F lichen frozen in field; –, none detected; t, trace only; ?, tentative identification by co-chromatography only.
Table 3.
Ribitol content, heterocyst frequency and Zn2+-resistant photosynthesis of cyano- and chlorolichens
| Lichen species | Photobionts | Ribitol content (% total carbohydrates)* | Heterocyst frequency (% total cells) | Zn2+-resistant photosynthesis (% original GP) |
|---|---|---|---|---|
| Ps. carpoloma | Chlorobiont + cephalodia | – | 26·2 ± 0·4 | – |
| Ps. lividofusca | Chlorobiont + cephalodia | 37·0 | – | – |
| Ps. chloroleuca | Chlorobiont + cephalodia | – | 25·0 ± 0·3 | – |
| Ps. rufovirescens | Chlorobiont + cephalodia | 42·0 | 26·3 ± 0·1 | 105·0 |
| Sticta latifrons | Chlorobiont + cephalodia | 29·0 | – | – |
| Ps. crocata | Cyanobiont | 40·6 | – | 13·8 |
| Ps. hookeri | Cyanobiont | – | – | 18·5 |
| Ps. lividofusca/cyan | Cyanobiont | 35·0 | 6·2 ± 0·3 | – |
| Ps. rufovirescens/cyan | Cyanobiont | 30·8 | 6·5 ± 0·2 | 0·2 |
| Ps. cinnamomea | Cyanobiont | 1·0 | 3·8 ± 0·4 | 17·6 |
| Ps. dissimilis† | Cyanobiont | 0·2 | 4·8 ± 0·6 | 22·7 |
| Sticta fuliginosa | Cyanobiont | 3·1 | 5·7 ± 0·2 | 12·2 |
| Peltigera dolichorhiza | Cyanobiont | 2·5 | 5·6 ± 0·2 | 1·1 |
| Collema leucocarpum | Cyanobiont | 0·0 | 5·4 ± 0·4 | 6·6 |
* Small carbohydrates are those composed of one or two molecules.
† Data from Snelgar and Green (1981).
–, not measured.
Microscopy
Microscopic examination of cyanolichens which contained ribitol (Ps. rufovirescens/cyan, Ps. lividofusca/cyan, Ps. dissimilis, Ps. hookeri, Ps. crocata and S. fuliginosa) revealed that green algal cells, often in large quantities, were present in the photobiont zone of the lichen thalli just beneath the upper cortex (Ps. rufovirescens/cyan, Fig. 1A). The green algae did not form a defined layer but appeared to be in cell clusters within the cyanobacterial layer. The cyanobacterial cells appeared normal in form under the light microscope, and when excited with green light produced a red fluorescence, indicating that they were photosynthetically active with light uptake by the phycobilins with energy transfer to the photosystems (Fig. 1C, D). Isolated cyanobacterial cells also showed this fluorescence (Fig. 1E, D). The cyanobiont appears, therefore, to be a normal primary photobiont, i.e. fully photosynthetic. Although green algal cells were found in the cyanobacterial partners of photosymbiodemes when they were growing separately, no green algae were found in cyanobacterial sectors of joined thalli (Fig. 1B).
Zn2+-resistant photosynthesis
The photosynthesis of both cyano- and chlorolichens shows differential sensitivity to zinc solutions. Whilst photosynthesis of cyanobacteria is almost totally inhibited at zinc concentrations >1 mm, there is no effect, or sometimes an enhancement, on photosynthesis in green algal lichens (Brown and Beckett, 1983). Treatment with zinc solutions thus provides a means to differentiate between the photosynthesis of green algal cells and cyanobacteria in mixed populations. After 30 min treatment with 5 mm ZnSO4 solution, the photosynthetic rate of the chlorolichen Ps. rufovirescens/chlor had increased slightly, whilst in the cyanolichen Peltigera dolichorhiza it had dropped to 1·1 % of the original GP (Table 3). Several cyanolichens retained a proportion of their original GP, as high as 30·2 % in the case of Ps. rufovirescens/cyan. The residual Zn2+-resistant photosynthesis confirmed that the green algae in these lichens were photosynthetically active.
Heterocyst frequency
The heterocyst frequencies in the independently growing lichens were as expected from the existing literature (Kershaw, 1985). Heterocyst frequencies in the cephalodia of chlorolichen species were high, ranging from 25·0 to 26·3 %, whilst the frequencies in the normal cyanobionts in the photobiont layer of all the lichens were between 3·8 and 6·5 %. The presence of green algae in the cyanolichen seemed to have little or no effect on the cyanobacteria, with no shift to the higher frequencies and greater heterotrophy of cyanobacteria specialized to fix nitrogen in cephalodia (Rai et al., 2000). However, there was evidence of such a change occurring as the distance between green algae and cyanobacteria decreased at the junctions between green algal and cyanobacterial sectors of photosymbiodemes (Table 4). The heterocyst frequency was 7·4 % at 10–20 mm from the green algal sector and this increased to 14·8 % at 0–2 mm. A similar phenomenon has been reported by Hitch and Millbank (1975b), and their results are also shown in Table 4. As noted earlier, unlike the independent cyanolichen partner of the photosymbiodeme, the cyanobacterial sectors of a joined thallus had no green algae present.
Table 4.
Change in heterocyst frequency of a cyanobacterial photobiont with distance from a green algal photobiont
| Heterocyst frequency (%) | ||
|---|---|---|
| Distance to green algal photobiont |
||
| Associations | 5–10 mm | 0–2 mm |
| Pseudocyphellaria rufovirescens | 7·4 ± 0·2 | 14·8 ± 0·9 |
| Dendriscocaulon umhausense/Lobaria amplissima* | 1·9, 2·5, 4·0. 5·7 | 10·2, 15·2 |
| Solorina spongiosa* | 5·8, 4·9, 7·1 | 28·3, 43·1, 45·5, 54·7 |
| Sticta dufourii/S. canariensis* | 3·1, 4·1 | 12·4, 13·3, 18·0 |
* Data from Hitch and Millbank (1975b); frequencies are individual counts.
DISCUSSION
‘Co-primary’ photobionts
Lichen species with cyanobacteria as a primary photobiont have always been referred to as being bipartite, i.e. there are only two symbionts present, the mycobiont and the cyanobiont. A small group of lichens, about 3–4 % of all species, have been called tripartite, with a mycobiont, a primary chlorobiont and a secondary cyanobiont that occurs in structures called cephalodia and appears to be specialized for nitrogen fixation. It now appears that another group of tripartite lichens exists, those previously always referred to as cyanolichens but which contain two primary photobionts, a chlorobiont and a cyanobiont, within the same photobiont layer in the thallus. We propose that these cyanolichens should be called chloro-cyanolichens.
The presence of the two types of photobiont can be seen in microscopic sections of the cyanobacterial species (Fig. 1A). The occurrence of ribitol at an often high proportion of the extractable carbohydrates (up to 42·0 % d. wt, Table 3) confirms that active green algae are present. Ribitol is a good marker for species of the green algal family Trebouxiophyceae as it is the mobile compound translocated between photobiont and mycobiont in this group (Matthes and Feige, 1983) and has never been reported as a cyanobacterial product (Palmqvist, 2002). The response of GP when thalli were challenged with Zn2+ solution showed that both photobionts are behaving as primary photobionts. The majority of the photosynthesis is derived from the cyanobacteria and is suppressed by the addition of Zn2+, while a sometimes substantial amount of GP, up to 30·2 %, remains and must originate from the green algae (Table 3). The low heterocyst frequency of the cyanobiont, around 3·8–6·5 % (Table 3), also confirms that the cyanobacterium is primarily autotrophic and has not become specialized for nitrogen fixation, as occurs in cephalodia. The four lines of evidence together confirm that some of the ‘cyanolichen’ species in the Peltigerales are tripartite with co-primary photobionts.
Co-primary photobionts may be widespread in the Peltigerales. Here we report their occurrence in five Pseudocyphellaria, one Sticta and one Peltigera species (Table 3). Brown and Beckett (1983) report substantial Zn2+-resistant photosynthesis from three Sticta, three Peltigera, one Nephroma and one Lobaria species, all from Europe.
Photobiont and morphology
James and Henssen (1976) reviewed the morphotypes (photosymbiodemes) in the Peltigerales and concluded that the photobiont plays an important role in determining the form of the lichen thallus. They based this conclusion on the considerable differences in form found for cephalodia, particularly in Sticta species, and which they described in detail including examples of apparently independently living cephalodia-like forms of Dendriscocaulon (James and Henssen, 1976). This conclusion has been generally accepted since then (Honegger, 1992). Recent molecular data confirm that Dendriscocaulon and Sticta, and also that all photosymbiodemes tested, have a single mycobiont (Högnabba et al., 2009). The new tripartite structure represents another type of photobiont combination in the Peltigerales. For a single species such as Ps. rufovirescens, there exists an independently living cyanobacterial form with two primary photobionts (tripartite), a cyanobacterial form in a sectored thallus with a single cyanobacterial primary photobiont (bipartite), and a green algal form with a green algal primary photobiont and cyanobacteria (tripartite) in cephalodia where they are specialized for nitrogen fixation. The cyanobacteria thus occur in three arrangements: alone, as a primary photobiont with green algae, and in cephalodia with green algae. The common feature with all of these combinations is the single mycobiont, and it appears, therefore, that it must be the mycobiont that determines the form of the thallus and arrangement of the photobionts (Richardson, 1999). It was also noted that the cyanobacterial sector of the joined photosymbiodemes do not appear to have green algae present unlike in the free-living cyanolichen. We have no explanation for this situation which needs to be further checked and may represent yet another level of control by the mycobiont.
Phenotypic plasticity
Phenotypic plasticity is defined as: ‘the property of a genotype to produce different phenotypes when exposed to different environments’. This seems to describe the situation for some members of the Peltigerales where a single mycobiont shows considerable flexibility and can alter its nutritional strategy by forming lichen thalli with different photobiont combinations apparently under the influence of the environment. Unfortunately, it is not at all clear which environmental factor, or factors, drives the morphological changes in these lichens, i.e. stimulates the mycobiont variously to support growth of cyanobionts or chlorobionts. James and Henssen (1976) looked at the ecology of several species pairs and generally concluded that the cyanobacterial forms occur in shaded, consistently humid environments. There are large differences in the response of the green algal and cyanobacterial forms to water availability. Green algal thalli can reactivate from high humidity alone, while the cyanobacterial thalli need rainfall (Lange and Kilian, 1985; Lange et al., 1986, 1988). Also, at high thallus water contents, the net photosynthesis of the green algal forms is strongly depressed, whereas the cyanobacterial forms are much less affected (Green et al., 1993). As a result, under very wet conditions, the thalli with cyanobionts outperform the forms with chlorobionts, and vice versa under humid but drier conditions (Green et al., 1993). The very different water regimes inside and outside these evergreen forests may thus be the driver for the plasticity in the lichens.
There are an increasing number of examples of plasticity in lichens due to the presence of different strains of individual photobiont species. In the fruticose species Ramalina farinacea, it has been found that the mycobiont constantly associates with two different genetic lineages of the chlorobiont Trebouxia sp. and showing again a high level of flexibility (del Hoyo et al., 2011). The two lineages have slightly different physiological performances, they differ in their tolerance of oxidative stress, and it is suggested that this improves the physiological flexibility for the lichen. If the proportions of the different chlorobiont lineages changed with habitat then this would also be an example of phenotypic plasticity; however, this is not yet known. Cetraria aculeata shows very high phenotypic plasticity. It is a sterile sorediate dispersed species so that the possibility of vertical transfer of genotypes exists in order that dispersed photobionts could be selected. However, it appears that this widely dispersed bipolar species utilizes locally adapted populations of photobionts (Fernández-Mendoza et al., 2011). A similar situation is also proposed for Lobaria pulmonaria where photobionts appear to be selected for the different ecologies of the various phorophytes (Werth and Sork, 2010).
Mycobiont flexibility in photobiont choice
Photobiont choice appears to be a mycobiont character and therefore inheritable, unlike photobiont characters (Hill, 2009). In recent years, more detailed molecular analyses of the Peltigerales have started to appear, and in Miadlikowska and Lutzoni (2004) two groupings, Peltigeraceae (Peltigera and Solorina) and the Lobariaceae (Pseudocyphellaria, Lobaria and Sticta), are shown as being closely related, and these are the groups that show the broad range of photobiont relationships. These two families may represent recent evolutionary divergence (Miadlikowska and Lutzoni, 2004), with the mycobiont gaining the ability to better exploit the environment by using a range of photobionts.
There is growing evidence that the ancestor of the Peltigerales (Miadlikowska and Lutzoni, 2004; Högnabba et al., 2009) had only cyanobionts and that the chlorobionts were added later. The presence of tripartite lichens with co-primary photobionts does not diminish this hypothesis but strengthens it by showing a level of versatility in the mycobionts that allows both chloro- and cyanobionts in the thalli.
ACKNOWLEDGEMENTS
The University of Waikato is thanked for supporting this research as part of the Masters programme of F.H.; The Board and Staff of Te Urewera National Park are thanked for assistance in the field and granting permission to collect in the Park.
LITERATURE CITED
- Armaleo D, Clerc P. Lichen chimeras: DNA analysis suggests that one fungus forms two morphotypes. Experimental Mycology. 1991;15:1–10. [Google Scholar]
- Brodo IM, Richardson DHS. Chimeroid associations in the genus Peltigera. Lichenologist. 1978;10:157–170. [Google Scholar]
- Brodo IM, Sharnoff SD, Sharnoff S. Lichens of North America. New Haven, CT: Yale University Press; 2001. [Google Scholar]
- Brown DH, Beckett RP. Differential sensitivity of lichens to heavy metals. Annals of Botany. 1983;52:51–57. [Google Scholar]
- Büdel B, Henssen A. Trebouxia aggregata und Gloeocapsa sanguinea, Phycobionten in Euopsis granatina (Lichinaceae) Plant Systematics and Evolution. 1988;158:235–241. [Google Scholar]
- Büdel B, Scheidegger C. Thallus morphology and anatomy. In: Nash TH III, editor. Lichen biology. 2nd edn. Cambridge: Cambridge University Press; 2008. pp. 40–68. [Google Scholar]
- Chaloub RM, de Magalhães CCP, Dos Santos CP. Early toxic effects of zinc on PSII of Synechocystis aquatilis f. aquatilis (Cyanophyceae) Journal of Phycology. 2005;41:1162–1168. [Google Scholar]
- Cowan DA, Green TGA, Wilson AT. Lichen metabolism. 1. The use of tritium labelled water in studies of anhydrobiotic metabolism in Ramalina celastri and Peltigera polydactyla. New Phytologist. 1979;82:489–503. [Google Scholar]
- Da Silva MDC, Iacomini M, Jablownski E, Gorin PAJ. Carbohydrate, glycopeptide and protein components of the lichen Sticta sp. and effect of storage. Phytochemistry. 1993;33:547–552. doi: 10.1016/0031-9422(93)85446-x. [DOI] [PubMed] [Google Scholar]
- Feige GB. Untersuchungen zur Physiologie der Cephalodien der Flechte Peltigera aphthosa (L.) Willd. II. Das photosynthetische 14C-Markierungsmunster und der Kohlenhydrattransfer zwischen Phycobiont und Mycobiont. Zeitschrift für Pflanzenphysiologie. 1976;80:386–394. [Google Scholar]
- Fernández-Mendoza F, Domaschke S, Garcia MA, Jordan P, Martin MP, Printzen C. Population structure of mycobionts and photobionts of the widespread lichen Cetraria aculeata. Molecular Ecology. 2011;20:1208–1232. doi: 10.1111/j.1365-294X.2010.04993.x. [DOI] [PubMed] [Google Scholar]
- Galloway DJ. 2nd edn including lichen-forming and lichenicolous fungi. Vols 1 and 2. Lincoln. New Zealand: Manaaki Whenua Press; 2007. Flora of New Zealand lichens, revised. [Google Scholar]
- Goebel K. Morphologische und biologische Studien. VII. Ein Beitrag zur Biologie der Flechten. Leiden: EJ Brill; 1926. [Google Scholar]
- Goffinet B, Bayer AJ. Characterization of mycobionts of photomorph pairs in the Peltigerineae (lichenized ascomycetes) based on internal transcribed spacer sequences of the nuclear ribosomal DNA. Fungal Genetics and Biology. 1997;21:228–237. doi: 10.1006/fgbi.1997.0977. [DOI] [PubMed] [Google Scholar]
- Green TGA, Büdel B, Heber U, Meyer A, Zellner H, Lange OL. Differences in photosynthetic performance between cyanobacterial and green algal components of lichen photosymbiodemes measured in the field. New Phytologist. 1993;125:723–731. doi: 10.1111/j.1469-8137.1993.tb03921.x. [DOI] [PubMed] [Google Scholar]
- Green TGA, Smith DC. Lichen physiology XIV. Differences between lichen algae in symbiosis and in isolation. New Phytologist. 1974;73:753–766. [Google Scholar]
- Heidmarsson S, Mattsson J-E, Moberg R, Nordin A, Santesson R, Tibell L. Classification of lichen photomorphs. Taxon. 1997;46:519–520. [Google Scholar]
- Hill DJ. Asymmetric co-evolution in the lichen symbiosis caused by a limited capacity for adaptation in the photobiont. Botanical Review. 2009;75:326–338. [Google Scholar]
- Hitch CJB, Millbank JW. Nitrogen metabolism in lichens. VI. The blue-green phycobiont content, heterocyst frequency and nitrogenase activity in Peltigera species. New Phytologist. 1975a;74:473–476. [Google Scholar]
- Hitch CJB, Millbank JW. Nitrogen metabolism in lichens. VII. Nitrogenase activity and heterocyst frequency in lichens with blue-green phycobionts. New Phytologist. 1975b;75:239–244. [Google Scholar]
- Högnabba F, Stenroos S, Thell A. Phylogenetic relationship and evolution of photobiont associations in the Lobariaceae (Peltigerales, Lecanoromycetes, Ascomycota) In: Thell A, Seaward MRD, Feuerer T, editors. Diversity of lichenology. Bibliotheca Lichenologica. Vol. 100. 2009. pp. 157–187. [Google Scholar]
- Honegger R. Lichens: mycobiont–photobiont relationships. In: Reisser W, editor. Algae and symbioses: plants, animals, fungi, viruses, interactions explored. Bristol, UK: Biopress Limited; 1992. pp. 255–275. [Google Scholar]
- Honegger R. Morphogenesis. In: Nash TH III, editor. Lichen biology. 2nd edn. Cambridge: Cambridge University Press; 2008. pp. 69–93. [Google Scholar]
- Honegger R. Lichen-forming fungi and their photobionts. In: Deising H, editor. The mycota V. Plant relationships. Berlin: Springer-Verlag; 2009. pp. 307–333. [Google Scholar]
- del Hoyo A, Álvarez R, del Campo EM, Gasulla F, Barreno E, Casano LM. Oxidative stress induces distinct physiological responses in the two Trebouxia phycobionts of the lichen Ramalina farinacea. Annals of Botany. 2011;107:109–118. doi: 10.1093/aob/mcq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James PW, Henssen A. The morphological and taxonomical significance of cephalodia. In: Brown DH, Hawksworth DL, Bailey RH, editors. Lichenology, progress and problems. London: 1976. pp. 27–77. [Google Scholar]
- Jørgensen PM. On the nomenclature of lichen phototypes. Taxon. 1996;45:663–664. [Google Scholar]
- Jørgensen PM. What shall we do with the blue-green counterparts? Lichenologist. 1998;30:351–356. [Google Scholar]
- Jørgensen PM, Jahns HM. Muhria, a remarkable new lichen genus from Scandinavia. Notes from the Royal Botanic Garden Edinburgh. 1987;44:581–599. [Google Scholar]
- Kaule A. Die Cephalodien der Flechten. Flora. 1931;126:1–44. [Google Scholar]
- Kershaw KA. Physiological ecology of lichens. Cambridge: Cambridge University Press; 1985. [Google Scholar]
- Lange OL, Green TGA, Ziegler H. Water status related photosynthesis and carbon isotope discrimination in species of the lichen genus Pseudocyphellaria with green or blue-green photobionts and in photosymbiodemes. Oecologia. 1988;75:494–501. doi: 10.1007/BF00776410. [DOI] [PubMed] [Google Scholar]
- Lange OL, Kilian E. Reaktivierung der Photosynthese trockener Flechten durch Wasserdampfaufnahme aus dem Luftraum: Artspezifisch unterschiedliches Verhalten. Flora. 1985;176:7–23. [Google Scholar]
- Lange OL, Wagenitz G. What is a ‘phycolichen’? Differences and changes in the meaning of an old lichenological term. Lichenologist. 2003;35:341–345. [Google Scholar]
- Lange OL, Kilian E, Ziegler H. Water vapor uptake and photosynthesis of lichens: performance differences in species with green and blue-green algae as phycobionts. Oecologia. 1986;71:104–110. doi: 10.1007/BF00377327. [DOI] [PubMed] [Google Scholar]
- Laundon JR. Lichen photomorphs: the good, the bad, and the ugly. Taxon. 1996;45:665. [Google Scholar]
- Lewis DH, Smith DC. Sugar alcohols (polyols) in fungi and green plants I. Distribution, physiology and metabolism. New Phytologist. 1967;66:143–184. [Google Scholar]
- Lohtander K, Oksanen I, Rikkinen J. Genetic diversity of green algal and cyanobacterial photobionts in Nephroma (Peltigerales) Lichenologist. 2003;35:325–339. [Google Scholar]
- Miadlikowska J, Lutzoni F. Phylogenetic classification of Peltigeralean fungi (Peltigerales, Ascomycota) American Journal of Botany. 2004;91:449–464. doi: 10.3732/ajb.91.3.449. [DOI] [PubMed] [Google Scholar]
- Matthes U, Feige GB. Ecophysiology of lichen symbiosis. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Physiological plant ecology III: responses to the chemical and biological environment. Berlin: Springer-Verlag; 1983. pp. 423–468. [Google Scholar]
- Nash TM. Lichen biology. 2nd edn. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Palmqvist K. Cyanolichens – carbon metabolism. In: Rai AN, Bergman B, Rasmussen U, editors. Cyanobacteria in symbiosis. Dordrecht: Kluwer Publications. 2002. pp. 73–96. [Google Scholar]
- Paulsrud P, Rikkinen J, Lindblad P. Spatial patterns of photobiont diversity in some Nostoc-containing lichens. New Phytologist. 2000;146:291–299. doi: 10.1046/j.1469-8137.2000.00647.x. [DOI] [PubMed] [Google Scholar]
- Rai AN, Söderbäck E, Bergman B. Cyanobacterium–plant symbioses. New Phytologist. 2000;147:449–481. doi: 10.1046/j.1469-8137.2000.00720.x. [DOI] [PubMed] [Google Scholar]
- Renner B, Galloway DJ. Phycosymbiodemes in Pseudocyphellaria in New Zealand. Mycotaxon. 1982;16:197–231. [Google Scholar]
- Richardson DHS. War in the world of lichens: parasitism and symbiosis as exemplified by lichens and lichenicolous fungi. Mycological Research. 1999;103:641–650. [Google Scholar]
- Richardson DHS, Hill DJ, Smith DC. Lichen physiology. XI. The role of the alga in determining the pattern of carbohydrate movement between lichen symbionts. New Phytologist. 1968;67:469–486. [Google Scholar]
- Smith DC. Symbiosis and the biology of lichenised fungi. Symposia of the Society for Experimental Biology. 1975;29:373–405. [PubMed] [Google Scholar]
- Snelgar WP, Green TGA. Ecologically-linked variation in morphology, acetylene reduction, and water relations in Pseudocyphellaria dissimilis. New Phytologist. 1981;87:403–411. [Google Scholar]
- Snelgar WP, Green TGA, Wilkins AL. Carbon dioxide exchange in lichens: resistances to CO2 uptake at different thallus water contents. New Phytologist. 1981;88:353–361. [Google Scholar]
- Stenroos S, Högnabba F, Myllys L, Hyvönen J, Thell A. High sensitivity in symbiotic associations of lichenized ascomycetes and cyanobacteria. Cladistics. 2006;22:230–238. [Google Scholar]
- Stenroos S, Stocker-Wörgötter E, Yoshimura I, Myllys L, Thell A, Hyvönen J. Culture experiments and DNA sequence data confirm the identity of Lobaria photomorphs. Canadian Journal of Botany. 2003;81:232–247. [Google Scholar]
- Thomas MD, Ryan D, Galloway DJ. Observations on phylo-genetic relationships within Lobariaceae Chevall. (Lecanorales, Ascomycota) in New Zealand, based on ITS-5·8S molecular sequence data. In: Llimona X, Lumbsch HT, Ott S, editors. Progress and problems in lichenology at the turn of the millennium-IAL4. Bibliotheca Lichenologica. Vol. 82. 2002. pp. 123–138. [Google Scholar]
- Watras CJ, Baker AL. Detection of planktonic cyanobacteria by tandem in vivo fluorometry. Hydrobiologia. 1988;169:77–84. [Google Scholar]
- Werth S, Sork VL. Identity and genetic structure of photobiont of the epiphytic lichen Ramalina menziesii on three oak species in Southern California. American Journal of Botany. 2010;97:821–830. doi: 10.3732/ajb.0900276. [DOI] [PubMed] [Google Scholar]
- Wirtz N, Lumbsch HT, Green TGA, Türk R, Pintado A, Sancho LG, Schroeter B. Lichen fungi have low cyanobiont selectivity in maritime Antarctica. New Phytologist. 2003;160:177–183. doi: 10.1046/j.1469-8137.2003.00859.x. [DOI] [PubMed] [Google Scholar]
- Yahr R, Vilgalys R, DePriest PT. Geographic variation in algal partners of Cladonia subtenuis (Cladoniaceae) highlights the dynamic nature of a lichen symbiosis. New Phytologist. 2006;171:847–860. doi: 10.1111/j.1469-8137.2006.01792.x. [DOI] [PubMed] [Google Scholar]