Abstract
Background and Aims
The phenolic composition of Coffea leaves has barely been studied, and therefore this study conducts the first detailed survey, focusing on mangiferin and hydroxycinnamic acid esters (HCEs).
Methods
Using HPLC, including a new technique allowing quantification of feruloylquinic acid together with mangiferin, and histochemical methods, mangiferin content and tissue localization were compared in leaves and fruits of C. pseudozanguebariae, C. arabica and C. canephora. The HCE and mangiferin content of leaves was evaluated for 23 species native to Africa or Madagascar. Using various statistical methods, data were assessed in relation to distribution, ecology, phylogeny and use.
Key Results
Seven of the 23 species accumulated mangiferin in their leaves. Mangiferin leaf-accumulating species also contain mangiferin in the fruits, but only in the outer (sporophytic) parts. In both leaves and fruit, mangiferin accumulation decreases with ageing. A relationship between mangiferin accumulation and UV levels is posited, owing to localization with photosynthetic tissues, and systematic distribution in high altitude clades and species with high altitude representatives. Analyses of mangiferin and HCE content showed that there are significant differences between species, and that samples can be grouped into species, with few exceptions. These data also provide independent support for various Coffea lineages, as proposed by molecular phylogenetic analyses. Sampling of the hybrids C. arabica and C. heterocalyx cf. indicates that mangiferin and HCE accumulation may be under independent parental influence.
Conclusions
This survey of the phenolic composition in Coffea leaves shows that mangiferin and HCE accumulation corresponds to lineage recognition and species delimitation, respectively. Knowledge of the spectrum of phenolic accumulation within species and populations could be of considerable significance for adaptation to specific environments. The potential health benefits of coffee-leaf tea, and beverages and masticatory products made from the fleshy parts of Coffea fruits, are supported by our phenolic quantification.
Key words: Arabica coffee, C. arabica, C. canephora, chlorogenic acids, Crop Wild Relatives (CWRs), coffee-leaf tea, hybridization, hydroxycinnamic acids, mangiferin, phenolic compounds, phylogeny, robusta coffee
INTRODUCTION
Coffee (Coffea) is the second most traded commodity after oil, accounting for exports worth an estimated US$15·4 billion in 2009/10, when some 93·4 million bags were shipped [International Coffee Organization (ICO), 2011], and has an estimated annual retail value exceeding US$70 billion (Vega et al., 2003; Lewin et al., 2004). In 2010, total coffee sector employment was estimated at about 26 million people in 52 producing countries [International Coffee Organization (ICO), 2011]. Coffea arabica L. (Arabica coffee) and C. canephora Pierre ex A. Froehner (robusta coffee) are the two species used in the production of coffee, providing approx. 70 and 30 % of commercial production, respectively [International Coffee Organization (ICO), 2011]. Due to their economic importance, the biochemical composition of Arabica and robusta coffee beans (i.e. seeds; see Materials and Methods) has been extensively studied, because of their implication in taste and cup quality (Belay, 2011). Less attention has been paid to other Coffea species, of which there are 124 occurring naturally in the Old Word (Davis et al., 2006, 2011; Davis, 2011). Several biochemical studies have focused on wild species, particularly those from Africa and Madagascar, although these works have generally focused on qualitative and quantitative assessments of sugar, lipid, caffeine and esters of hydroxycinnamic acid (HCEs) in green (unroasted) coffee beans (Clifford et al., 1989; Anthony et al., 1993; Campa et al., 2004, 2005; Dussert et al., 2008). In contrast to the considerable amount of research on green beans, there are relatively few studies concerned with the metabolite content of the other parts of the coffee plant, such as the leaves (Mondolot et al., 2006; Clifford et al., 2008), the outer fleshy layers of the fruit (Lopes and Monaco, 1979; Lopes et al., 1984; Lopes and Shepherd, 1991), and vascularized organs (Mahesh et al., 2007). Moreover, many of these studies are still focused on the crop species, and most exclusively on HCE content, when analysing phenolic compounds (Lepelley et al., 2007). Other than Arabica and robusta coffee, only one wild species, C. pseudozanguebariae Bridson, has been studied for its leaf phenolic content. High quantities of esters of feruloylquinic acid (FQA) were first described in fruit, green beans and leaves of this species (Bertrand et al., 2003), and then a C-glucosylxanthone, mangiferin, was isolated from the leaves (Talamond et al., 2008). This compound, which was initially isolated from the leaves, bark and peel of mango [Mangifera indica L. (Anacardiaceae)] (Baretto et al., 2008), is well known for its numerous pharmacological properties such as anti-inflammatory, antidiabetic, antihyperlipidaemic and neuroprotective activities (Garrido et al., 2004; Muruganandan et al., 2005; Campos-Esparza et al., 2009). In plants, mangiferin provides antioxidant and antimicrobial protection upon biotic stress (Franklin et al., 2009). More generally, phenolics are involved in the response to biotic and abiotic stresses (Santiago et al., 2000; Read et al., 2009), mainly due to their antioxidant properties (Moglia et al., 2008; Shahidi et al., 2010; Gallego-Giraldo et al., 2011). In this way, they may play a role in adaptation to environmental change (Boudet, 2007) and in coevolution with pests and diseases (Orians, 2000; Eyles et al., 2010). Phenolics could be critical to understanding plant–animal interactions, including those involved in the control of significantly destructive pests. Phenolics also have the potential to elucidate evolutionary history when scored as characters in phylogenetic analyses, as undertaken using other secondary metabolites (Wink, 2003; Dussert et al., 2008).
Although hydroxycinnamic acids (p-coumaric, caffeic, ferulic and sinapic acids) exist as a free form in cells, they usually occur in conjugated forms. The most common forms are glycosylated derivatives (Herrmann, 1989; Macheix et al., 1990) and esters of triterpenes (Bolzani et al., 1991) or quinic acid, shikimic acid or tartric acid (Ribereau-Gayon, 1968; Schuster and Herrmann, 1985; Manach et al., 2004), named HCEs. The esters formed between hydroxycinnamic acids and quinic acid are grouped under the generic name of chlorogenic acids, one of the most widespread in the plant kingdom being 5-O-caffeoylquinic acid (5-CQA), also called chlorogenic acid.
The aim of this study was to undertake a survey of the accumulation of phenolic compounds in the leaves of Coffea species, focusing on mangiferin and HCEs, in order to: (1) better understand the physical and temporal location of mangiferin within Coffea leaves, and make a comparison with accumulation in other plant tissues; (2) test for correlations between the accumulation of mangiferin and HCEs in leaf tissue; (3) assess the geographical and systematic (phylogenetic) distribution of leaf-accumulated mangiferin and HCEs within Coffea leaf tissue; and (4) briefly investigate the relationships between hybridization and phenolic accumulation (mangiferin and HCEs) in Coffea leaves. The first step in achieving these objectives was to develop a new high-performance liquid chromatography (HPLC) procedure to quantify mangiferin together with FQA. Identification of chemical structures was performed by liquid chromatography–mass spectrometry (LC-MS) analyses on a leaf extract of C. pseudozanguebariae, the first coffee species elucidated as mangiferin accumulating (Talamond et al., 2008). Mangiferin and HCE content was then evaluated in the leaves of 23 Coffea species (24 taxa), including two hybrids (C. arabica ‘Laurina’ and C. heterocalyx Stoff. cf.), originating from different localities in Africa and Madagascar (Table 1), the main centres of Coffea species diversity (Davis et al., 2006, 2011; Davis, 2011). The fruits of three species were tested for the temporal and physical accumulation of mangiferin.
Table 1.
Origin of the species studied and provenance of the genotypes analysed
| Species | Species range | Clade designation (and alliance, if applicable) | Genotype: native origin/germplasm collection (accession number/code) |
|---|---|---|---|
| From Africa | |||
| C. anthonyi Stoff. & F. Anthony | Cameroon, Congo | EC-Afr | Cameroon/IRD Montpellier (OD 71, OD 72) |
| C. arabica L. ‘Laurina’ [Bourbon Pointu] | Ethiopian, SE South Sudan | Hybrid origin: EC-Afr (C. eugenioides) × LG (C. canephora) | Ethiopia/IRD La Réunion (Ar 27, Ar 34, Ar 39) |
| C. canephora Pierre ex A. Froehner | Upper Guinea Region and Lower Guinea/Congolian Region | LG/C [canephora alliance] | Cameroon (BD)/IRD Montpellier (BD 62, BD 66) |
| C. canephora Pierre ex A. Froehner | Upper Guinea Region and Lower Guinea/Congolian Region | LG/C [canephora alliance] | Congo (BB)/IRD Montpellier (BB 62, BB 66, BB 67) |
| C. congensis A. Froehner | Lower Guinea/Congolian Region | LG/C [canephora alliance] | Cameroon/IRD Montpellier (CC 61, CC 66, CC70) |
| C. eugenioides S. Moore | Burundi, Rwanda, Zaire, southern South Sudan, Kenya, Tanzania, Uganda | EC-Afr | Tanzania/IRD Montpellier (DA 75, DA 77) |
| C. heterocalyx Stoff. cf. | SW Cameroon | Hybrid origin: × EC-Afr (C. eugenioides) × C. liberica | Central Africa/IRD Montpellier (JC 8, JC 62, JC 69) |
| C. humilis A. Chev. | Sierra Leone, Liberia, SW Ivory Coast | UG | Ivory Coast/IRD Montpellier (G 52, G 68, G 72) |
| C. liberica var. dewevrei (De Wild. & T.Durand) Lebrun | Lower Guinea/Congolian Region | LG/C [liberica alliance] | Central African Republic/IRD Montpellier (EB 52, EB 63) |
| C. liberica var. liberica Bull. ex Hiern | Upper Guinea Region and Lower Guinea/Congolian Region | LG/C [liberica alliance] | Ivory Coast/IRD Montpellier (EA 51, EA 61, EA 63) |
| C. mannii (Hook.f) A.P. Davis | Upper Guinea Region and Lower Guinea/Congolian Region | ‘African Psilanthus’ | Ivory Coast/IRD Montpellier (PSI-man) |
| C. pseudozanguebariae Bridson | Kenya, Tanzania, Mozambique | EA [2] | Tanzania/IRD Montpellier [via Divo, Côte d'Ivoire] (H 58, H 63) |
| C. racemosa Lour. | Mozambique, Mozambique Channel Is., KwaZulu-Natal, Zimbabwe | EA [3] | Mozambique/IRD Montpellier (IB 58, IB 58, IB 62) |
| C. salvatrix Swynn. & Phillipson | Malawi, Mozambique, Tanzania, Zimbabwe | EA [2] | Mozambique/IRD Montpellier (LB 57, LB 63) |
| C. sessiliflora Bridson | Kenya, Tanzania, | EA [3] | Mozambique/IRD Montpellier (PA 58, PA 67, PB 58) |
| C. stenophylla G. Don | Guinea, Ivory Coast, Sierra Leone | UG | Ivory Coast/IRD Montpellier (FB 53, FB 54, FB 61) |
| From Madagascar | |||
| C. leroyi A.P. Davis | Eastern Madagascar | IO [Madagascan Coffea] | Kianjavato (A.315) |
| C. andrambovatensis J.-F. Leroy | Eastern Madagascar | IO [Madagascan Coffea] | Kianjavato (A.227) |
| C. ankaranensis A.P. Davis & Rakotonas. | NE Madagascar | IO [Madagascan Coffea] | Kianjavato (A 808) |
| C. augagneuri Dubard | Northern Madagascar | IO [Madagascan Coffea] | Kianjavato (A.519) |
| C. millotii J.-F. Leroy | Eastern Madagascar | IO [Madagascan Coffea] | Kianjavato (A.219) |
| C. perrieri Drake ex Jum. & H. Perrier | Western and southern Madagascar | IO [Madagascan Coffea] | Kianjavato (A.305) |
| C. resinosa (Hook.f.) Radlk. | Eastern Madagascar | IO [Madagascan Coffea] | Kianjavato (A.915) |
| C. tsirananae J.-F. Leroy | Northern Madagascar | IO [Madagascan Coffea] | Kianjavato (A.515) |
| C. vohemarensis A.P. Davis & Rakotonas | Northern Madagascar | IO [Madagascan Coffea] | Kianjavato (A.977) |
Species range after Davis et al. (2006); clade designation, terminology and alliance after Maurin et al. (2007) and Davis et al. (2011). Clade terminology abbreviations: EA, East African clade (numbers in square brackets denote the three major clades within the EA clade); EC-Afr, East Central African clade; IO, Indian Ocean clade; LG/C, Lower Guinea/Congolian clade; UG, Upper Guinea clade. Region designation (i.e. Upper Guinea Region and Lower Guinea/Congolian Region) after White (1979, 1983).
MATERIALS AND METHODS
Taxon sampling and plant material
The level of accumulation of HCEs and mangiferin was concomitantly studied. Experiments were performed on the leaves of 23 Coffea species (Table 1), representing 40 African genotypes and nine Madagascan genotypes (one genotype per species). Genotypes represent individuals taken as whole plants, seedlings or cuttings from wild populations or cultivation, and gathered under a common accession prefix with different numbers. The biosynthesis of secondary metabolites is largely dependent on environmental conditions, and so we used trees grown under near-identical culture conditions for the African and Madagascan genotypes. In this manner, a valid estimation of the genetic effect on the species diversity of phenolic compounds was possible. For the African species, leaves were collected from two or three genotypes for each species grown in tropical greenhouses (natural daylight, 25 °C night and 28 °C day temperature, 78–82 % relative humidity) at the IRD research centre in Montpellier (France), to replicate the same environment as found in Madagascar and Reunion (see below). For the Madagascan species, leaves were harvested from one tree per species grown at the FOFIFA coffee research station of Kianjavato (Madagascar), where trees are grown in the open ground. Only fully expanded leaves, from the second node below the apex of the growing shoot, were taken for the study of phenolic diversity. To study mangiferin content during leaf development, four species were used: C. arabica, C. canephora, C. eugenioides S. Moore and C. pseudozanguebariae. Leaves were sampled at three developmental stages: stage 1, young leaves from the apex; stage 2, leaves from the first node below the apex; and stage 3, leaves from the second node below the apex. Immediately after harvesting, leaves were weighed and then frozen in liquid nitrogen before being lyophilized for extraction. Fruits from C. arabica were harvested at Reunion Island (IRD collection). All the C. arabica samples used in this study were represented by the cultivar ‘Laurina’ (Bourbon Pointu). Although this entity is a cultivated mutant, the sequence profile of the species, based on the same markers as Maurin et al. (2007), is the same as wild origin material (A. Davis, unpubl. data). Coffea pseudozanguebariae and C. canephora fruits were obtained from the CNRA station (Divo, Ivory Coast). Fruits were sampled at three developmental stages: stage 1 (length <0·7 cm), fruit green with a partially formed seed (immature); stage 2 (length <1·2 cm), fruit yellowish green, pericarp (exocarp, mesocarp, endocarp) easily detached from the seed (semi-mature); and stage 3 (length >1·2 cm), fruit reddish yellow, pericarp easily detached from the seed (mature). Except for stage 1, the pericarp was isolated from the seed and each part was pooled (up to five fruits per species, per stage), weighed, and frozen in liquid nitrogen until extraction. Coffee beans are the seeds of the coffee fruit, the bulk of which is made up of endosperm. The fruit skin (exocarp), the fleshy part of the fruit (mesocarp), and the parchment, which is the crustaceous pyrene coat (endocarp), are all removed during processing.
Phenolic extraction
Extraction was performed using sonication (20 min, room temperature, 24 kHz, R.E.U.S-GEX 180, Contes, France) in 6 mL of MeOH/H2O (80:20, v/v) of 25 mg of plant material (lyophilized leaves or frozen fruits ground under liquid nitrogen with a mortar and pestle). After centrifugation (5 min, 5000 rpm), the methanol extract was collected and filtered (Millipore, 0·25 µm porosity) before analysis. Each extraction was realized in triplicate for leaves and fruit samples. Each sample was characterized by its mean content of mangiferin and HCEs, expressed as a percentage of fresh or dry weight.
Electrospray-mass spectrometry analysis of the samples
Electrospray-mass spectrometry (ESI-MS) was performed on a binary HPLC system (Waters 1525 µ, Waters, Manchester, UK) coupled with a Waters Micromass ZQ ESCi multimode ionization mass spectrometer (Micromass Ltd, Manchester, UK). Separation was performed on an XTerra MS C18 column (3·5 µm particle size, 2·1 mm × 100 mm) kept at 40 °C in a thermostatic oven (Gecko 2000, Cluzeau Info Labo, France) with a binary mobile phase gradient delivered at a total flow rate of 210 µL min−1. The mobile phase was composed of permuted water (solvent A) and acetonitrile (solvent B), both phases acidified by 0·1 % (v/v) formic acid in order to prevent the ionization of phenolic acids. The gradient profile evolved linearly from 5 % B to 40 % B in 40 min, increased exponentially to 100 % B from 40 to 50 min. After 5 min of isocratic elution at 100 % B, the mobile phase composition returned to 5 % B with 5 min re-equilibration.
The source and capillary were heated at 80 and 450 °C, respectively, and the capillary voltage was set to 3·0 kV. Nitrogen was used as the desolvatation gas (400 L h−1) and cone gas (50 L h−1). Spectra were acquired and accumulated over 60 s in the full scan mode over the m/z 50–1200 range, in both the negative and positive modes at cone voltages of –25, –60, +25 and 60 V, successively. Absorbance spectra over the range of 210–800 nm were acquired using a Waters 996 Photodiode Array Detector. Absorbance and mass spectra were handled using MassLynx 3·5 software (Micromass Ltd). The sensitivity of the mass spectrometer was optimized using the chlorogenic acid standard. 5-O-caffeoylquinic acid, mangiferin and 5-metoxyflavone were provided by Sigma-Aldrich (Buchs, Switzerland).
Clifford et al. (2008) showed that Coffea leaves had a proportionately greater content of cis-isomers relative to trans-isomers, compared with coffee beans, suggesting that UV-irradiation in vivo may also cause geometric isomerization. In our study, we noticed the presence of cis-isomers, especially in leaves of cultivated species grown outdoors during daylight hours, and returned to greenhouses after dark. In these cases cis-isomers never exceeded 10 % of the CGA content, and we decided to ignore these compounds for our study because: (1) most of the samples used in this study (all except those from Madagascar) were cultivated in greenhouses with very little or no UVb, and correspondingly very low quantities of cis-isomers; and (2) the Madagascan samples, which are cultivated outdoors, have CGAs in such low quantities that the cis-isomers are not quantifiable.
Phenolic quantification of samples
Quantification was carried out on 10 µL of extract using a HPLC system (Shimadzu LC 20, Japan) equipped with a photodiode array detector and consisting of a Eclipse XDB C18 (3·5 µm) column (100 mm × 4·6 mm, Agilent). The elution system (0·6 mL min−1) involved two filtered (0·2 µm pore size filter), sonicated and degassed solvents, namely solvents A (water/acetic acid, 98:2, v/v) and B (H2O/MeOH/acetic acid, 5:90:5 v/v/v). The linear gradient was: 0 min, 18 % solvent B; 0–5 min, 25 %; 5–8 min, 36 %; 8–10 min, isocratic; 10–13 min, 58 %; 13–16 min, 62 %; 16–21, 18 %; isocratic, 18 % till 25 min.
The calibration curve was plotted using three replicate points of standard solutions of mangiferin and 5-CQA from Sigma-Aldrich (St Louis, USA), and 3,5-dicaffeoylquinic acid (3,5-diCQA), a gift from Professor Andary {extracted from sunflower [Helianthus annuus L. (Asteraceae)], Laboratory of Natural Substances, Faculty of Pharmacy, Montpellier} at 10, 25, 50, 75 and 100 mg mL–1. Identification was performed by comparing spectra and retention times at 280, 320 and 360 nm. Quantification of mangiferin, 3-, 4- and 5-CQA, FQAs and 3,4-, 3,5- and 4,5-diCQA was undertaken at 320 nm by comparison with mangiferin, 5-CQA and 3,5-diCQA standards. The technique for FQA and mangiferin quantification is new, and is reported here for the first time. Except for seeds in which content was expressed as a percentage of fresh weight (g 100 g−1 f. wt), compound content was expressed as percentage of dry weight (g 100 g−1 d. wt).
Histochemical analysis
Small pieces of freshly collected Coffea leaves or fruits were embedded in 3 % agarose (type II EEO, Panreac) before cutting for histochemical examination. Cross-sections (40 µm) were obtained using a Leica VT 1000S vibrating blade microtome (frequency 7, speed 2). For mangiferin histolocalization, cross-sections were mounted in distilled water without any reagent. Transverse sections of specimens were viewed under a light microscope (Nikon Optiphot) with UV light (filter UV-1A: 365 nm excitation filter). In these conditions, mangiferin presents a strong yellow autofluorescence. Photographs were taken with a digital Nikon Coolpix 4500 camera.
Statistical analyses
All results were analysed using the Statistica software package (7·1 version, USA). The primary variables are: 3-CQA, 3-caffeoylquinic acid; 4-CQA, 4-caffeoylquinic acid; 5-CQA, 5-caffeoylquinic acid; 3,4-diCQA, 3,4-dicaffeoylquinic acid; 3,5-diCQA, 3,5-dicaffeoylquinic acid; 4,5-diCQA, 4,5-dicaffeoylquinic acid; FQAs, sum of 3-, 4- and 5-feruloylquinic acid; and mangiferin. Secondary variables were: CQAs, sum of the isomers of CQA; and HCEs, sum of the isomers of CQA and FQA. Statistical analysis was used to examine between-species variation, which was tested using one-way analysis of variance (ANOVA) with fixed effect. When the F-test was significant, a Newman and Keuls test was carried out to compare means. Standard deviations were computed from the residual mean square, the latter being the best estimate (unbiased and accurate) of the residual variance. Linear regression was performed to highlight relationships between some HCE isomers. Hierarchical clustering analysis (with weighted average grouping method and Euclidian distance as parameters) and principal component analysis (PCA) were applied to data in order to identify clusters and similarities between samples and species.
Clade and geographical terminology
The terminology for area-based clades largely follows Maurin et al. (2007): Upper Guinea (UG) clade, Lower Guinea/Congolian (LG/C) clade, East-Central African (EC-Afr) clade, East African (EA) clade; and Anthony et al. (2010) and Davis et al. (2011): Africa/Indian Ocean (A/IO) clade. The humid West and Central African forests are contained within the Guineo-Congolian Regional Centre of Endemism (White, 1983). Within this major region there are three sub-centres of endemism for humid forest species: (1) Upper Guinea; (2) Lower Guinea; and (3) Congolian (White, 1979). For practical purposes, the sub-centres (2) and (3) are often merged as the Lower Guinea/Congolian region, and this convention has been followed here. There are three exceptions to the area–clade relationship outlined by Maurin et al. (2007). Coffea canephora and C. liberica, (members of the LG/C clade) also occur in the Upper Guinea region; and C. anthonyi Stoff. & F.Anthony (a member of the EC-Afr clade) occurs in the Lower Guinea/Congolian region. The East-Central Africa area is comparable with the Lake Victoria Regional Mosaic [including most of Uganda, the whole of eastern Rwanda and Burundi, and small parts of Zaire, Kenya and Tanzania (White, 1983)], a predominantly high altitude region formed by the uplift of the Great Rift Valley. Coincidently, the vegetation of this area is ‘a meeting place of five distinct floras’ (White, 1983), including the Guineo-Congolian flora, and this may help to explain the relationship (EC-Afr clade; Maurin et al., 2007; Davis et al., 2011) of C. anthonyi with the high altitude East-Central Africa species, C. eugenioides and C. kivuensis Lebrun.
RESULTS
Identification of mangiferin and HCEs
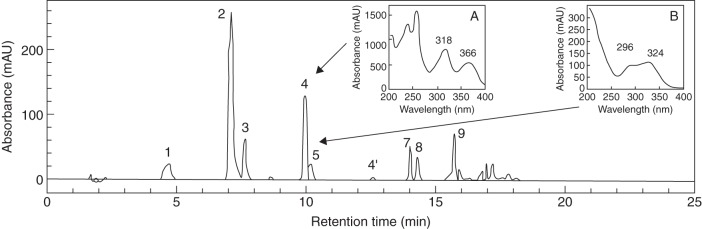
The HPLC quantitative method described herein allowed the separation of chlorogenic acids (CQAs, diCQAs and FQAs) and mangiferin in a methanol extract of C. pseudozanguebariae leaves (Fig. 1). Based on their retention time and UV absorbance spectra, the four different peak families were determined. Except for FQAs, all the compounds detected can be quantified, although mangiferin and an FQA isomer (peak 4 and 5) presented quite similar retention times (9·95 and 10·17 min, respectively). It was difficult to state the nature of FQA isomers in the absence of standards. We have therefore chosen to express FQA content as FQAs, cumulating 4- and 5-FQA content when both were presented (3-FQA was generally undetectable), without specifying the nature and the proportion of each isomer. Isomangiferin (peak 4′) was detected at 12·58 min. Because it was often present in trace amounts, this compound was not taken into account.
Fig. 1.
HPLC profile of a C. pseudozanguebariae leaf extract. Absorption profile obtained at 320 nm using the technical conditions for quantitative analysis. Peak 1 = 3-caffeoylquinic acid (3-CQA); peak 2 = 5-caffeoylquinic acid (5-CQA); peak 3 = 4-caffeoylquinic acid (4-CQA); peak 4 = mangiferin; peak 4' = isomangiferin; peak 5 = 5-feruloylquinic acid (5-FQA); peak 6 = 4-feruloylquinic acid (4-FQA); peak 7 = 3,4-dicaffeoylquinic acid (3,4 diCQA); peak 8 = 3,5-dicaffeoylquinic acid (3,5-diCQA); and peak 9 = 4,5-dicaffeoylquinic acid (4,5-diCQA). The absorption spectrum between 200 and 400 nm is indicated for mangiferin (A) and FQA (B).
An analysis by ESI-MS was conducted to identify with certainty the compounds accumulated in the leaves but also in fruits (Table 2). An elution profile identical to that obtained by the HPLC quantitative method allowed the observation of nine major peaks by separation on an XTerra MS C18 column. Peaks 1, 2 and 3 shared typical chlorogenic acid (5-CQA) UV absorbance spectra with a shoulder at 240 nm and maxima at 324 nm. These peaks have the same m/z 353 [M-H]− and 355 [M + H]+ parent ions, and shared m/z 191, 179, 173 [M-H]− fragment ions corresponding to [quinic acid-H]−, [caffeic acid-H]− and [quinic acid-H-H2O]−, respectively. Peak 2 was assigned to 5-CQA as it co-eluted with chlorogenic acid standard and exhibited the same fragmentation pattern (Clifford et al., 2003). The very low m/z 173 relative intensity of peak 1 compared with peak 3 indicated that peak 1 can be assigned to 3-CQA and peak 3 to 4-CQA. Peak 4 showed the absorbance spectra of the mangiferin standard, with peaks at 241, 258, 318 and 366 nm and a shoulder at 275 nm within the acidified water/acetonitrile eluent. It exhibited the pseudomolecular parent ion m/z 423 [M + H]+ and 421 [M-H]– at a retention time of 16·26 min. In our system, the mangiferin standard gave the same fragmentation pattern as that obtained using the API 4000 LC-MS/MS system (Suryawanshi et al., 2007). In negative mode, the m/z 421 [M-H]− parent ion yielded to fragment ions at m/z 403, 331, 301, 273 and 259. The fragment ion at m/z 301 corresponded to glycoside lost [mangiferin-H-120]− and at m/z 273 to a supplementary [-CH2O] lost.
Table 2.
Electrospray ionization mass–spectrometry characterization of isomers
| Peak | RT (min) | [M-H]− (m/z) | ESI-MS fragments (m/z) | UV λmax (nm) | Abbreviation | Identification |
|---|---|---|---|---|---|---|
| 1 | 6·05 | 353 | 191 (100), 179 (82), 173 (1), 135 (10) | 240, 300sh, 324 | 3-CQA | 3-Caffeoylquinic acid |
| 2* | 10·03 | 353 | 191 (100), 179 (51) | 240, 300sh, 324 | 5-CQA | 5-Caffeoylquinic acid |
| 3 | 11·20 | 353 | 191 (20), 179 (98), 173 (100), 135 (12) | 240, 300sh, 324 | 4-CQA | 4-Caffeoylquinic acid |
| 4* | 16·26 | 421 | 403, 331 (93), 301, 271 | 258, 318, 366 | Mangif | Mangiferin |
| 5 | 17·90 | 367 | 193, 191, 149 | 296sh, 324 | 5-FQA | 5-Feruloylquinic acid |
| 6 | 29·29 | 367 | 193, 173 | 296sh, 324 | 4-FQA | 4-Feruloylquinic acid |
| 7 | 24·33 | 515 | 353, 191, 173, 161, 135 | 240, 300sh, 326 | 3,4-DiCQA | 3,4-Dicaffeoylquinic acid |
| 8 | 28·91 | 515 | 353, 191, 179, 161, 135 | 240, 300sh, 326 | 3,5-diCQA | 3,5-Dicaffeoylquinic acid |
| 9 | 31·63 | 515 | 353, 179 | 240, 300sh, 326 | 4,5-DiCQA | 4,5-Dicaffeoylquinic acid |
Peak assignments of the fresh pericarp extracts and green bean extracts using LC-ESI-MS. Characterization of phenolic compounds and mangiferin by UV absorbance spectrum and electrospray ionization-mass spectrometry detection (LC-DAD/ESI-MS). RT, retention time.
* Identified with standard compound.
Peaks 5 and 6 (as the latter was not detected in leaf samples, it is not shown in Fig. 1) exhibited typical UV spectra of hydroxycinnamic acids, with a shoulder at 296 nm and a maximum at 324 nm, characteristic of quinic acid derivatives. Both peaks showed the m/z 367 [M-H]− and 369 [M + H]+ parent ions, and shared the m/z 191 [M-H]− fragment ion, corresponding to [quinic acid-H]−. The more abundant fragments of 3-FQA, 5-FQA and 4-FQA are 193, 191 and 173, respectively. From comparison with a C. canephora grain extract, we deduced that peaks 5 and 6 can be assigned to 5-FQA and 4-FQA, respectively (Clifford et al., 2006; Matsui et al., 2007; Alonso-Salces et al., 2009; Jaiswal et al., 2010b). Peaks 7, 8 and 9 shared typical 5-CQA UV absorbance spectra. Having the same m/z 515 [M-H]− and 517 [M + H]+ parent ions, and a m/z 353 [M-H]− fragment ion assigned to CQAs, they corresponded to diCQAs. Peak 7 and 9 formed four major fragments at m/z 191 [quinic acid-H]-, 173 [quinic acid-H-H2O]-, 161 [caffeic acid-H-H2O]- and 135 [caffeic acid-H-CO2]-. Peak 7 and peak 9, exhibiting the highest 173 [M-H]− and 179 [M-H]− fragment, respectively, can be assigned to 3,4-diCQA and 4,5-diCQA. Peak 8 appeared to be 3,5-diCQA.
Jaiswal et al. (2010a) and Kuhnert et al. (2011) reported the presence of numerous minor chlorogenic acids in C. canephora beans, based on sinapic or methoxycinnamic acids. We also observed some of these secondary metabolites in C. canephora and C. pseudozanguebariae (their retention times are close to those of FQAs), although their concentrations were very low and content comparison between species was difficult.
Mangiferin localization in leaf and fruit tissues
Histochemical observations for the localization of mangiferin in leaves and fruits were performed on three species: C. pseudozanguebariae, C. arabica and C. canephora.
Leaves
Based on mangiferin autofluorescence properties, histochemical observations revealed a preferential localization of mangiferin in palisade and spongy (mesophyll) parenchyma of C. pseudozanguebariae leaves (Fig. 2A1). In comparison, the same transverse sections of leaves indicated that mangiferin is absent in C. canephora (Fig. 2A2), but present at a low concentration in C. arabica (Fig. 2A3).
Fig. 2.
Histochemical localization of mangiferin in leaves (A) and green fruit (B) of different Coffea species. Cross-sections were observed under UV light, ×400, except B4 (×40). (A) Leaves. Coffea pseudozanguebariae leaf blade (A1), showing a very high concentration of mangiferin preferentially localized in the cells forming the palisade and spongy parenchyma (mesophyll) (yellow arrows). Coffea canephora leaf blade (A2), no mangiferin accumulated (i.e. no specific yellow autofluorescence), allowing observation of chlorophyll (red autofluorescence), principally in palisade and spongy parenchyma. Blue fluorescences are specific for cuticle constituents and of caffeoylquinic acids (in epidermal cells). Coffea arabica ‘Laurina’ leaf blade (A3), showing that the presence of mangiferin (yellow autofluorescence) has attenuated the red autofluorescence of the chlorophyll. (B) Fruits. Coffea pseudozanguebariae (B1), yellow autofluorescence of transverse sections (T.S.) of immature fruits (stage 1), showing that mangiferin is extremely concentrated in the exocarp and mesocarp cells. Coffea canephora (B2), no mangiferin accumulated. Coffea arabica ‘Laurina’ (B3 and B4) mangiferin accumulation appears to be vacuolar and restricted to exocarp and external mesocarp cells (arrows) in young fruits. There is no mangiferin detected in the endocarp, integument (seed coat) and seed. Abbreviations: c, cuticle; chl, chlorophyll; e, epidermis; en, endocarp; ex, exocarp; in, integument; m, mesocarp; pp, palisade; s, seed; sp, spongy parenchyma (mesophyll).
Fruits
The intense yellow autofluorescence observed in the cells of the exocarp (outer fruit wall or skin) and the external layers of the mesocarp (soft, pulpy layer of the fruits) of young green fruits of C. pseudozanguebariae indicate a high content of mangiferin (Fig. 2B1). Mangiferin was not detected in the green fruit of C. canephora (Fig. 2B2). In C. arabica, mangiferin was weakly accumulated and preferentially localized in cells from the exocarp and external mesocarp (Fig. 2B3), as in C. pseudozanguebariae. Mangiferin was entirely absent from the seeds and endocarp (pyrene layer, or ‘parchment’) of the three species examined (Fig. 2B4).
Quantitative evaluation of mangiferin during leaf and fruit ageing
Mangiferin content was evaluated in leaves and fruits at different development stages. The study was undertaken on the same three species used in the histochemical observations (see above) plus C. eugenioides, one of the parent species of C. arabica, the unique amphidiploid species originating from hybridization between C. eugenioides and C. canephora (Lashermes et al., 1999; Maurin et al., 2007).
Leaves
In C. pseudozanguebariae, C. eugenioides and C. arabica, mangiferin was present at each development stage, but was not detected in C. canephora (Table 3). When present, mangiferin content decreased with leaf ageing [about 30 % less mangiferin content between young leaf (from the apex) and mature leaf (from node 2)]. Irrespective of the developmental stage, the highest mangiferin content was observed in leaves of C. eugenioides.
Table 3.
Mangiferin content (% d. wt) in leaves at three development stages of C. pseudozanguebariae, C. canephora, C. arabica L. cv. ‘Laurina’ and C. eugenioides
| Species | Stage 1 | Stage 2 | Stage 3 |
|---|---|---|---|
| C. pseudozanguebariae | 8·60 ± 1·15 | 8·03 ± 1·34 | 5·62 ± 0·87 |
| C. eugenioides | 11·39 ± 1·03 | 7·61 ± 0·73 | 6·43 ± 0·53 |
| C. arabica ‘Laurina’ | 1·11 ± 0·26 | 0·92 ± 0·32 | 0·89 ± 0·22 |
| C. canephora | 0 | 0 | 0 |
Stage 1 corresponds to leaves from the apex; and stages 2 and 3 to leaves from the first and second node from the apex, respectively. Values are expressed as a percentage of dry weight (% d. wt) and corresponded to the mean between three trees evaluated in triplicate.
Fruits
Mangiferin content was evaluated for the same species as above, except C. eugenioides, in fruits at different growth stages. As previously indicated by histochemical observations, quantitative analysis confirmed that mangiferin was actually present at all stages of development in the fruit of C. pseudozanguebariae and C. arabica (Table 4). In entire fruits from stage 1, mangiferin content was 5-fold lower in C. arabica than in C. pseudozanguebariae, where the average content of mangiferin is approx. 1·15 % of the fresh fruit weight. Mangiferin was not detected in the fruit of C. canephora. Even at the later stages of fruit maturity, a separate analysis revealed that mangiferin continued only to accumulate in the endocarp and outer layers of the mesocarp, and not the pyrene coat (endocarp) or the seed (mostly endosperm). As with leaves, mangiferin content decreased with ageing (maturity); stage 2 and 3 had >3-fold less mangiferin compared with stage 1.
Table 4.
Mangiferin content (% f. wt) during fruit development of C. pseudozanguebariae, C. arabica L. cv. ‘Laurina’ and C. canephora
| Stage 1 (ST1) | Stage 2 (ST5) | Stage 3 (ST6) | |||
|---|---|---|---|---|---|
| Species | Entire fruit | Pericarp | Seed | Pericarp | Seed |
| C. pseudozanguebariae | 1·13 ± 0·37 | 0·43 ± 0·15 | 0 | 0·61 ± 0·23 | 0 |
| C. arabica ‘Laurina’ | 0·23 ± 0·07 | 0·07 ± 0·02 | 0 | 0·05 ± 0·02 | 0 |
| C. canephora | 0 | 0 | 0 | 0 | 0 |
Stage 1 corresponds to the first stage of development of the perisperm; stage 2 to the completion of the development of the endosperm; and stage 3 to pericarp maturation; or ST1, ST5 and ST6 stages according to De Castro and Marraccini (2006), respectively. Content is expressed as a percentage of fresh weight (% f. wt). Evaluations have been made in triplicate on five entire fruits for the first stage, and on fruit fragmented between seeds and pericarp for stages 2 and 3.
Mangiferin and HCE content in leaves of wild coffee species
Mangiferin and HCE contents were analysed by liquid chromatography in mature leaves (taken from the second node below the apex of the shoot) of 49 genotypes belonging to 23 species originating from different regions of Africa and Madagascar (Table 1). The nine selected compounds were readily identified in the chromatograms (Table 5).
Table 5.
Mangiferin and hydroxycinnamic acid ester (HCE) composition of leaves (% d. wt)
| Species | n | Mangiferin | 3-CQA | 5-CQA | 4-CQA | CQA | FQAs | 3,4-diCQA | 3,5-diCQA | 4,5-diCQA | diCQA | HCE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| From Africa | ||||||||||||
| C. anthonyi | 2 | 12·056d | 0·041a | 1·700d | 0·094a | 1·834cd | 0·000a | 0·000a | 0·001a | 0·000a | 0·001a | 1·835bcd |
| C. canephora (Con) | 3 | 0·000a | 0·169bd | 1·305bcd | 0·220abcd | 1·694bcd | 0·064b | 0·095c | 0·209c | 0·065e | 0·370d | 2·127cd |
| C. canephora (Cam) | 2 | 0·000a | 0·339f | 1·306bcd | 0·443ef | 2·088d | 0·047ab | 0·086c | 0·147b | 0·087f | 0·320cd | 2·456d |
| C. congensis | 3 | 0·000a | 0·266e | 0·813abc | 0·361def | 1·439abcd | 0·000a | 0·021a | 0·064a | 0·010ab | 0·095a | 1·534abc |
| C. eugenioides | 2 | 5·676c | 0·095abc | 2·742e | 0·163abc | 3·001e | 0·000ab | 0·023a | 0·012a | 0·037bcd | 0·071a | 3·072e |
| C. heterocalyx cf. | 3 | 4·521b | 0·130bc | 0·992abc | 0·123ab | 1·246abc | 0·000a | 0·007a | 0·020a | 0·056de | 0·083a | 1·328ab |
| C. humilis | 3 | 0·000a | 0·235cde | 0·647ab | 0·305cde | 1·187abc | 0·181d | 0·019a | 0·009a | 0·021abc | 0·049a | 1·417abc |
| C. liberica var. liberica | 3 | 0·000a | 0·077ab | 1·524cd | 0·169abc | 1·770cd | 0·042ab | 0·005a | 0·018a | 0·022abc | 0·045a | 1·857bcd |
| C. liberica var. dewevrei | 2 | 0·000a | 0·070ab | 1·249bcd | 0·124ab | 1·443abcd | 0·017ab | 0·010a | 0·008a | 0·017abc | 0·034a | 1·494abc |
| C. mannii | 1 | 0·000 | 0·059 | 0·492 | 0·079 | 0·630 | 0·029 | 0·000 | 0·000 | 0·000 | 0·000 | 0·659 |
| C. pseudozanguebariae | 2 | 4·907bc | 0·062ab | 0·847abc | 0·094a | 1·003ab | 0·000a | 0·002a | 0·019a | 0·027abcd | 0·048a | 1·051a |
| C. racemosa | 3 | 0·000a | 0·226ce | 0·950abc | 0·439ef | 1·615bcd | 0·000a | 0·035ab | 0·164bc | 0·033bcd | 0·232b | 1·847bcd |
| C. salvatrix | 2 | 16·359e | 0·276e | 1·073abcd | 0·489f | 1·838cd | 0·000a | 0·013a | 0·049a | 0·044cde | 0·106a | 1·944bcd |
| C. sessiliflora | 3 | 0·026a | 0·165bcd | 0·348a | 0·303cde | 0·815a | 0·000a | 0·009a | 0·011a | 0·017abc | 0·038a | 0·853a |
| C. stenophylla | 3 | 0·000a | 0·375f | 0·479a | 0·331de | 1·185abc | 0·743d | 0·000a | 0·000a | 0·000a | 0·000a | 1·928bcd |
| C. arabica ‘Laurina’ | 3 | 0·566a | 0·098abc | 3·231f | 0·256bcd | 3·584f | 0·116c | 0·053b | 0·171bc | 0·036bcd | 0·260 bc | 3·960f |
| F | 253·450 | 30·665 | 28·002 | 13·833 | 24·297 | 271·760 | 17·742 | 24·109 | 12·032 | 27·759 | 27·145 | |
| P | <0·001 | <0·001 | <0·001 | <0·001 | <0·001 | <0·001 | <0·001 | <0·001 | <0·001 | <0·001 | <0·001 | |
| From Madagascar | ||||||||||||
| C. leroyi | 1 | 0 | 0·191 | 1·149 | 0·302 | 1·642 | 0·312 | 0·000 | 0·011 | 0·051 | 0·062 | 2·016 |
| C. andrambovatensis | 1 | 0 | 0·105 | 0·330 | 0·123 | 0·558 | 0·358 | 0·000 | 0·006 | 0·026 | 0·032 | 0·948 |
| C. ankaranensis | 1 | 0 | 0·179 | 0·751 | 0·369 | 1·298 | 0·000 | 0·000 | 0·003 | 0·029 | 0·032 | 1·330 |
| C. augagneuri | 1 | 0 | 0·148 | 0·257 | 0·339 | 0·744 | 0·000 | 0·053 | 0·023 | 0·030 | 0·106 | 0·850 |
| C. millotii | 1 | 0 | 0·040 | 0·577 | 0·107 | 0·724 | 0·000 | 0·000 | 0·000 | 0·000 | 0·000 | 0·724 |
| C. perrieri | 1 | 0 | 0·110 | 0·818 | 0·329 | 1·257 | 0·035 | 0·005 | 0·012 | 0·018 | 0·035 | 1·327 |
| C. resinosa | 1 | 0 | 0·136 | 0·335 | 0·160 | 0·631 | 0·000 | 0·002 | 0·004 | 0·003 | 0·009 | 0·640 |
| C. tsirananae | 1 | 0 | 0·341 | 0·509 | 0·283 | 1·133 | 0·000 | 0·002 | 0·005 | 0·000 | 0·007 | 1·140 |
| C. vohemarensis | 1 | 0 | 0·170 | 0·788 | 0·204 | 1·162 | 0·015 | 0·000 | 0·045 | 0·028 | 0·073 | 1·250 |
Means followed by the same letter were not significantly different at P = 0·05 according to the Newman and Keuls test. n = number of genotypes studied per species (or origin for C. canephora). CQA, total content in caffeoylquinic acid isomers; FQAs, total content in feruloylquinic acids; diCQA, total content in dicaffeoylquinic acid isomers; HCE, hydroxycinnamic acid esters, total content in CQA, FQA and diCQA. F and P. results of one-way analyses of variance.
Cam, Cameroon; Con, Congo.
Mangiferin content
Seven of the 15 African species accumulated mangiferin in their leaves (Table 5), i.e. C. anthonyi, C. arabica, C. eugenioides, C. heterocalyx cf., C. pseudozanguebariae, C. sessiliflora and C. salvatrix Swynn. & Philipson. Mangiferin content among these seven species was present in a range of 0·03–16·36 % d. wt. Coffea salvatrix and C. anthonyi accumulated the greatest quantities (16·36 and 12·06 % d. wt, respectively). None of the nine Madagascan species examined contained mangiferin.
HCE content
Among HCEs, the three isomers of CQA were the only compounds accumulated in the leaves of all species examined, FQAs and diCQAs being exclusive to individual species (Table 5). The HCE composition of C. canephora leaves was in agreement with previous results concerning the biosynthesis pathway of CQAs in C. canephora seedlings (Mahesh et al., 2007). That is, in mature leaves of C. canephora from Cameroon, the total CQAs represents about 2 % d. wt and >80 % of the content in HCEs. The average dry weight of the total HCE content varied from 0·85 % (C. sessiliflora) to 3·96 % (C. arabica) in the leaves of African species, and from 0·64 % [C. resinosa (Hook.f.) Radlk.] to 2·02 % (C. leroyi A.P.Davis) for the Madagascan species tested. CQAs were the major HCEs accumulated in the leaves of all the species examined. These two compounds (3- and 5-CQA) accounted for >80 % of the HCEs, except for two species: C. stenophylla G.Don, an African species from the Upper Guinean forests, and C. andrambovatensis J.-F. Leroy, a species from Eastern Madagascar. Both species were characterized by <62 % of HCEs in the form of CQAs, and a FQAs content higher (0·74 and 0·36 % d. wt, respectively) than that of 5-CQA (0·48 and 0·33 % d. wt). As in numerous other plants, 5-CQA appeared as the most abundant CQA isomer in Coffea species, except in C. augagneuri Dubard, a Madagascan species in which 4-CQA is preferentially accumulated. FQAs were not detected in >60 % of the genotypes, including C. pseudozanguebariae. Only five species presented FQAs content >0·1 % d. wt: C. arabica (0·12 %), C. humilis (0·18 %) and C. stenophylla (0·74 %) for African species, and C. leroyi (0·31 %) and C. andrambovatensis (0·36 %) for Madagascan species. DiCQAs accumulated weakly, but were detected in 90 % of the species. When evaluated, total content varied from 0·001 to 0·370 % d. wt in C. anthonyi and C. canephora leaves, respectively, and diCQAs were not detected in the leaves of C. stenophylla (from Africa) and C. millotii (from Madagascar). For the 20 species containing diCQAs, total content ranged from 0·61 % (C. tsirananae, a Madagascan species) to 13·12 % (C. canephora, an African species) of HCEs. For all the species, except C. augagneuri, 3,4-diCQA appeared as the minor isomer.
Statistical analyses
For each of the phenolic compounds analysed, interspecific variability was considerable, as estimated by the interspecific maximum/minimum ratio, which varied from 4 for 5-CQA (from 3·2 % for C. arabica, to 0·64 % for C. humilis), and 620 for mangiferin (from 16·36 % for C. salvatrix to 0·03 % for C. sessiliflora). There was a significant effect of species, as tested by ANOVA. A Newmann and Keuls test applied to the means of the African species indicated that the 5-CQA content of C. arabica was significantly greater than that of all other species (Table 5). In the same way, the FQA content of C. stenophylla and the 3,4-diCQA content of C. canephora were significantly higher compared with the other species. Differences were highly significant with mangiferin, so that values can be considered as species specific, i.e. C. anthonyi and C. salvatrix (Table 5). No particular relationship could be established between the content in the different compounds studied, except for 3-CQA and 4-CQA which showed a weak linear relationship (Fig. 3), although five African species did not present this relationship: C. arabica, C. heterocalyx cf., C. racemosa, C. salvatrix and C. stenophylla.
Fig. 3.
Linear regression between 4-CQA and 3-CQA contents in leaves. Analysis included 40 genotypes from the 15 African taxa.
When species were grouped according to their position within recent phylogenetic analyses of Coffea (Maurin et al., 2007; Anthony et al., 2010; Davis et al., 2011; see Table 1) and analysed statistically, the following groups were found to be significantly different for mangiferin accumulation (Table 6): (1) C. anthonyi, C. eugenioides (EC-Afr clade); and (2) C. pseudozanguebariae, C. salvatrix (EA[2] clade). For HCE distribution, the following groups were supported: (1) C. anthonyi, C. eugenioides (EC-Afr clade), on the basis of 5-CQA and total CQAs; and (2) C. humilis and C. stenophylla (UG clade), for FQAs (Table 6). A clustering analysis of all African species was performed using all HCE data (CQA isomers, FQAs and diCQA isomers) as variables.
Table 6.
Mangiferin and hydroxycinnamic acid ester (HCE) composition of leaves (% d. wt)
| Clade | n | Mangiferin | 3-CQA | 5-CQA | 4-CQA | CQA | FQA | 3,4-diCQA | 3,5-diCQA | 4,5-diCQA | diCQA | HCE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UG | 6 | 0a | 0·305b | 0·563a | 0·318ab | 1·186ab | 0·462b | 0·009a | 0·005a | 0·010a | 0·024a | 1·672abc |
| LG/C | 8 | 0a | 0·146ab | 1·189a | 0·230ab | 1·564a | 0·020a | 0·012a | 0·032a | 0·016a | 0·061a | 1·645abc |
| UG_LG/C | 5 | 0a | 0·237ab | 1·305a | 0·309ab | 1·852ac | 0·057a | 0·092c | 0·184b | 0·074c | 0·350c | 2·259bc |
| EC-Afr | 4 | 8·866b | 0·068a | 2·221b | 0·129ab | 2·418c | 0a | 0·011a | 0·006a | 0·018a | 0·036a | 2·453c |
| EA[2] | 4 | 10·633b | 0·169ab | 0·960a | 0·291ab | 1·421ab | 0a | 0·007a | 0·034a | 0·021a | 0·077a | 1·497ab |
| EA [3] | 6 | 0·013a | 0·195ab | 0·649a | 0·371b | 1·215ab | 0a | 0·022a | 0·087a | 0·036ab | 0·135a | 1·350ab |
| IO | 9 | 0a | 0·158ab | 0·613a | 0·246ab | 1·016ab | 0·080a | 0·007a | 0·012a | 0·021a | 0·039a | 1·136a |
| Non-designated species | ||||||||||||
| C. mannii | 1 | 0a | 0·059a | 0·492a | 0·079a | 0·630b | 0·029a | 0a | 0a | 0a | 0a | 0·659a |
| Hybrids | ||||||||||||
| C. arabica cv. ‘Laurina’ | 3 | 0·566a | 0·098a | 3·231c | 0·256ab | 3·584d | 0·116a | 0·053b | 0·171b | 0·036ab | 0·260b | 3·960d |
| C. heterocalyx cf. | 3 | 4·521a | 0·130ab | 0·0992a | 0·123ab | 1·246ab | 0·000a | 0·007a | 0·020a | 0·056bc | 0·083a | 1·328ab |
| F | 15·932 | 3·552 | 22·989 | 2·481 | 15·449 | 6·746 | 17·451 | 12·898 | 8·976 | 18·299 | 14·230 | |
| P | <0·001 | 0·003 | <0·001 | 0·024 | <0·001 | <0·001 | <0·001 | <0·001 | <0·001 | <0·001 | <0·001 |
Means followed by the same letter were not significantly different at P = 0·05 according to the Newman and Keuls test. n = number of genotypes studied for each biogeographic origin according to Maurin et al. (2007) and Davis et al. (2011).
F and P, results of one-way analyses of variance.
Clade terminology abbreviations: EA, East African clade (number in square brackets denote the three major clades within the EA clade); EC-Afr, East Central African clade; IO, Indian Ocean clade; LG/C, Lower Guineae/Congolian clade; UG, Upper Guinea clade.
Analyses using hierarchical clustering analysis, e.g. on all samples (49 genotypes), all African (40 genotypes) (neither analyses shown here), and those undertaken below, revealed weak correspondence with phylogenetic topologies based on parsimony and Bayesian analysis of molecular data (Maurin et al., 2007; Anthony et al., 2010; Davis et al., 2011). At the species level, however, clustering was remarkably good. In the separate analyses presented here, which are based on the major lineages of Coffea (Maurin et al., 2007; Anthony et al., 2010; Davis et al., 2011), the clustering ability of HCEs at the species and population level is clearly demonstrated. In Fig. 4A, which incorporates taxa from the Upper and Lower Guinea/Congolian regions (White, 1979, 1983) and two hybrids, only one sample of C. liberica var. dewevrei fails to cluster; the samples of C. canephora cluster according to their geographical origin (Congo and Cameroon). In Fig. 4B, using taxa from East Central Africa and East Africa, only two species do not cluster (C. anthonyi and C. salvatrix). In other analyses (not shown), e.g. all African samples, all samples of C. liberica (var. liberica and var. dewevrei) cluster according to their varietal designations.
Fig. 4.
Hierarchical clustering analyses of African Coffea taxa using seven HCE variables (see Table 5). (A) Taxa from the Upper and Lower Guinea/Congolian regions (White, 1979, 1983), and the hybrids C. arabica ‘Laurina’ and C. heterocalyx cf. (B) Taxa from East Central Africa and East Africa. (see Tables 1, 5 and 6, and text, for details).
A PCA undertaken on all African species using the seven HCE primary variables (Fig. 5) showed that two principal components have an eigenvalue of >1, and accounted for 39 and 32 % of the total variance, respectively. The score plot obtained for principal components 1 and 2 individualized eight species: C. anthonyi, C. arabica, C. canephora, C. eugenioides, C. racemosa, C. salvatrix, C. sessiliflora and C. stenophylla. Coffea canephora and C. racemosa genotypes were characterized by a high 3-CQA content, C. stenophylla by a high FQAs content, and C. eugenioides and C. anthonyi by high 5-CQA contents. The remaining species in the PCA analysis overlap with respect to their CQA, FQA and HCE profiles to a greater (C. heterocalyx cf. and C. liberica) or lesser (C. congensis and C. humilis) extent. In a PCA analysis including the Madagascan species (not shown) it was clear that they exhibited the lowest diversity for HCEs, and this was manifest by their distribution in the centre of the PCA plot.
Fig. 5.

Principal component analysis (PCA) using seven HCE variables (see Table 5). (A) Projection of the seven variables on the axes: 3-CQA, 4-CQA, 5-CQA, 3,4 diCQA, 3,5 diCQA, 4,5 diCQA and FQAs. (B) Score plot of the 40 African genotypes. (C) Enlargement of the central zone of the score plot. Grouped genotypes from individualized species are noted in the text. Abbreviations: ant, C. anthonyi; can 1, C. canephora (Congo); can 2, C. canephora (Cameroon); con, C. congensis; eug, C. eugenioides; het, C. heterocalyx cf.; hum, C. humilis; lib, C. liberica var. liberica; dew, C. liberica var. dewevrei; man, C. mannii; pseu, C. pseudozanguebariae; rac, C. racemosa; sal, C. salvatrix; ses, C. sessiliflora; sten, C. stenophylla; arab, C. arabica ‘Laurina’.
DISCUSSION
The accumulation of mangiferin in Coffea species
Mangiferin, a C-glucosylxanthone, has been recently described in leaves of the wild Coffea species C. pseudozanguebariae (Talamond et al., 2008). The present work confirms this result by a double analysis involving a new HPLC technique and LC-MS. In contrast to the study of Bertrand et al. (2003) dealing with FQA content of leaves and fruits in C. pseudozanguebariae, only traces of FQAs were recorded in leaves in which mangiferin was highly concentrated. Our analysis of fruit content in this species showed that mangiferin was present in the exocarp and mesocarp, and yet was undetectable in the endocarp (parchment) or the coffee bean (i.e. seed). The same result was obtained with one of the other leaf mangiferin-accumulating species, C. arabica (Table 4). In C. canephora, the absence of mangiferin in leaves corresponds to the absence of this compound in the fruit. Our data clearly demonstrate that mangiferin is differentially accumulated in separate organs of the coffee plant. In species that accumulate mangiferin, this compound is found in the leaves and outer layers of the fruit (exocarp and outer mesocarp) but not in the seed (or endocarp; see above), which is largely composed of endosperm. The differential accumulation within the fruit could be linked to origin, the endosperm being formed by the fertilization of maternal tissue, whereas the outer layers of the fruit are composed of vegetative (sporophytic) tissue of the inferior fruit (i.e. the receptacle). Another possibility is that mangiferin production is only associated with photosynthetic tissue, the receptacle and young fruit being green and photosynthetic; the internal parts of fruit (e.g. the ovary) not. Presumably, in mangiferin-accumulating species, the embryonic cotyledons contain no mangiferin and accumulation starts at a later leaf stage.
As most of the earlier biochemical analyses of Coffea have been undertaken on seeds, the lack of accumulation of mangiferin in this organ may explain why the compound has only been reported in a single species of Coffea, i.e. C. pseudozanguebariae, when the leaves were being studied (Talamond et al., 2008). In the analysis of leaf mangiferin content carried out here on 23 Coffea species (24 Coffea taxa; Table 5), seven African taxa were found to accumulate mangiferin: C. anthonyi, C. arabica, C. eugenioides, C. heterocalyx cf., C. pseudozanguebariae, C. salvatrix and C. sessiliflora. Coffea salvatrix and C. anthonyi accumulated the greatest quantities (16·36 and 12·06 % d. wt, respectively), which represents higher levels of mangiferin than have been detected in the leaves of mangoes, i.e. approx. 10 % d. wt (Baretto et al., 2008).
Systematic distribution of mangiferin in Coffea species
Comparison of leaf mangiferin content among 23 Coffea species (24 taxa), represented by 49 genotypes, shows that the level of mangiferin accumulation in leaves at the same growth stage is species specific in three species, C. anthonyi, C. eugenioides and C. salvatrix. These data agree with the morphological delimitation (Bridson, 1988; Stoffelen et al., 2009) and molecular characterization of these species (Maurin et al., 2007; Anthony et al., 2010; Davis et al., 2011), in that they represent distinct species. The presence/absence of mangiferin in leaves is also in good agreement with molecular phylogenetic studies of Coffea, as detailed below. The clade designation of each of the 24 taxa tested is shown in Table 1 (see also ‘Clade and geographical terminology’, above); a statistical analysis of the accumulation of mangiferin for each clade is shown in Table 6. Among the five non-hybrid African species, C. anthonyi and C. eugenioides belong to the EC-Afr clade, and C. pseudozanguebariae, C. salvatrix and C. sessiliflora belong to the EA clade. Coffea pseudozanguebariae, C. salvatrix and C. sessiliflora are further divided into the two distinct EA clades reported by Maurin et al. (2007): EA[2] (C. pseudozanguebariae and C. salvatrix) and EA[3] (C. sessiliflora), EA[2] being a predominantly mid- to high altitude East African clade, and EA[3] a predominantly low altitude East African clade (Davis et al., 2006; Maurin et al., 2007). As all the above clades have a strong geographical element, based on species/sample distribution, the mangiferin data are also aligned with the provenance of these African coffee species, at least in the species that we have sampled. The phylogenetic position of C. anthonyi, a species from the Lower Guinea/Congolian Region (Stoffelen et al., 2009) but located in the EC-Afr clade (Davis et al., 2011), violates an area–clade relationship, but, significantly, its phylogenetic position is supported by the mangiferin accumulation. Additionally, it is reported that East-Central Africa (comparable with the Lake Victoria Regional Mosaic) is a meeting place of five distinct floras (White, 1983), including the Guineo-Congolian flora (White, 1983). It should also be said that the exact provenance details for C. anthonyi are not yet fully known, and further work on the precise geographical distribution of this species is required.
Our sampling of major clades across the Coffea genus is not complete: material from Asian and Australasian clade, and the Mascarene clade (Davis et al., 2011) is lacking and, in addition, sampling within some clades is limited, except for the EC-Afr clade and the UG clade. Despite these sampling limitations, the accumulation of mangiferin shows a consistent pattern of phylogenetic correlation, being restricted to the EC-Afr clade (Lake Victoria Regional Mosaic) and the EA clade (East Africa).
In the analyses of Maurin et al. (2007) and Davis et al. (2011), the phylogenetic position of the UG clade (Upper Guinea species: C. humilis, C. stenophylla, C. togoensis A.Chev.) is incongruous, being placed with Lower Guinea/Congolian (LG/C clade) species in their internal transcribed spacer (ITS) region analyses and with the EC-Afr clade and EA clade with plastid data. We demonstrate here that the absence of mangiferin in the UG clade (i.e. C. stenophylla and C. humilis) is in agreement with the ITS analyses, which is logically consistent with geographical distance and phytogeographical distribution (White, 1979, 1983).
The lack of mangiferin in the early diverging lineages of Coffea (Maurin et al., 2007; Davis et al., 2011; M. Nowak et al., unpubl. data), represented here by C. mannii (from the ‘African Psilanthus clade’; Davis et al., 2011) and the LG/C clade, infers that the accumulation of mangiferin is a derived characteristic within Coffea, as it is only found in clades of more recent origin. Reduction or loss of mangiferin accumulation would have to be invoked in order to explain the absence (i.e. C. racemosa) and low level of accumulation (i.e. C. sessiliflora) in the EA[3] clade, and the absence in the Indian Ocean (I/O) clade (i.e. the Madagascan species), perhaps in response to the different environmental conditions in these low altitude species and clades.
The accumulation of HCEs in Coffea species
All of the HCEs identified here as present in Coffea leaves have also been identified in green (unroasted) coffee beans (Anthony et al., 1993; Campa et al., 2008). In leaves, the total amount of accumulated HCEs is highest in C. arabica (3·96 % d. wt) and its progenitor species, C. canephora (2·46 % d. wt) and C. eugenioides (3·07 % d. wt). This is not the case for green beans of these species, even though the values are among the highest in Coffea (Anthony et al., 1993; Campa et al., 2008). Specific comparison between HCE accumulation in leaves and green beans is not possible due to differences in taxonomic sampling and experimental procedures, although it is clear that accumulation values in both leaves and green beans for the HCEs are much higher in African species when compared with Madagascan species.
Systematic distribution of HCEs in Coffea species
Total CQA and 5-CQA (Table 5) convincingly groups the species C. anthonyi and C. eugenioides even though the amounts within each of these species are significantly different (Fig. 5). This corresponds to the placement of these species into the EC-Afr clade based on sequence data (Maurin et al., 2007; Davis et al., 2011), and the same grouping based on mangiferin accumulation (Table 5; see above). FQA accumulation supports the paring of C. humilis and C. stenophylla, which agrees with the molecular placement of these species into the UG clade (Maurin et al., 2007; Anthony et al., 2010; Davis et al., 2011). As with the mangiferin data, HCE accumulation supports a distinction between the UG clade and the EC-Afr clades (see above; Fig. 5). Hierarchical cluster analysis of all samples for the HCE data (not shown) shows the Upper Guinea species clustering with the Lower Guinea species.
Low values for most HCEs in the representatives of Madagascan species, observed in PCA analyses (not shown) as points clustered around the crossing point of the axes, support separation of these taxa from African Coffea. The separation of Madagascan from African Coffea species on the basis of low levels of HCE accumulation in green beans of Coffea has been reported by Anthony et al. (1993) and Campa et al. (2005). Phenolic data for both leaves and green beans thus support the assumption based on molecular results (Anthony et al., 2010; Hamon et al., 2011) that the Madagascan species represent a monophyletic lineage within Coffea. In addition, the presence of other families of phenolics has been identified in Madagascan species (Rakotomalala, 1992). Further analysis is underway to determine the nature of these compounds and include them in future analysis of biochemical diversity (A. Rakotondravao, unpubl. data). The generally low interspecific phenolic diversity of the Madagascan species, compared with the phenolic diversity across the genus, is comparable with low DNA sequence diversity obtained in molecular phylogenetic studies (Maurin et al., 2007; Anthony et al., 2010). It has been suggested that the Madagascan species, excluding the baracoffea alliance (Davis and Rakotnasolo, 2008; M. Nowak, unpubl. data), represent a recent radiation within Coffea (Maurin et al., 2007; Anthony et al., 2010).
Like mangiferin, HCEs have the ability to cluster species based on multiple samples (Table 5; Figs 4 and 5). For example, in the PCA analysis (Fig. 5), eight species are individualized based on seven HCE variables: C. anthonyi, C. arabica, C. canephora, C. eugenioides, C. racemosa, C. salvatrix, C. sessiliflora and C. stenophylla. These results are comparable with the discriminatory ability of fatty acid and sterol composition of seeds to group Coffea accessions into species groups (Dussert et al., 2008). The leaf HCE data also separate geographical samples of C. canephora: samples from the Congo share a more similar HCE profile with C. arabica, compared with representatives of the same species from Cameroon. This is consistent with geographical proximity, and possibly genetic similarity, given that C. canephora is implemented in the hybrid origin of C. arabica (Lashermes et al., 1999; Maurin et al., 2007).
The effects of hybridization on mangiferin and HCE accumulation
The sampling of two well-documented Coffea hybrids, C. arabica and C. heterocalyx cf., provides an opportunity for a preliminary study of the effects of hybridization on mangiferin and HCE accumulation. Coffea arabica is an allotetraploid, formed via the hybridization of C. eugenioides, a high altitude species from the EC-Afr clade, and C. canephora, a low to mid-altitude species from the LG/C clade. Coffea arabica is placed in the EC-Afr clade (i.e. with C. eugenioides) with ITS data (Lashermes et al., 1997; Maurin et al., 2007) and the LG/C clade (with C. canephora) with plastid data (Cros et al., 1998; Maurin et al., 2007). Coffea heterocalyx cf. (= C. sp. X of Lashermes et al., 1997; Cros et al., 1998; Table 1) has been identified (Cros et al., 1998; Maurin et al., 2007) as a hybrid between C. eugenioides (EC-Afr clade; ITS data) and C. liberica (LG/C clade; plastid data). Close examination of plastid sequence data for C. liberica var. dewevrei and C. heterocalyx cf. shows that the sequences of four plastid regions are almost identical, and thus this variety has been implemented as the closest parental taxon within C. liberica (Maurin et al., 2007). Direct examination of ITS and plastid sequence data produced by Maurin et al. (2007) for C. arabica and C. heterocalyx cf. shows that sequence divergence from their progenitors is minimal, e.g. 2 or 3 bp differences for 3222 bp of plastid data, and 2 or 3 bp difference for 831 bp for ITS. Based on these data and restriction fragment length polymorphism (RFLP) results (Lashermes et al., 1999), it has been assumed that C. arabica and C. heterocalyx cf. are of recent origin, although no dates of origin have been proposed. There are considerable differences between these taxa, however, as C. arabica is an allotetraploid species and occurs over a relatively large area in south-west Ethiopia and south-west South Sudan, and possibly northern Kenya (Davis et al., 2006), whereas C. heterocalyx cf. is of unknown origin and is so far only known from cultivation. The use of ITS and plastid sequence data represents a coarse means of investigating hybridization, and in most cases provides only a preliminary level of understanding of hybrid origin, age and history. Nevertheless, our results for these two taxa provide a useful starting point for investigating the effects of hybridization on the phenolic accumulation in Coffea.
For C. heterocalyx cf., our results show that this taxon accumulates a similar [although significantly different (at P = 0·05)] quantity of mangiferin (average 4·2 % d. wt) to C. eugenioides (average 5·7 % d. wt); C. liberica does not accumulate mangiferin. For the HCE data (Table 5), there are no statistical differences compared with C. liberica var. dewevrei (Table 5; Fig. 5); conversely, C. eugenioides shows profound differences in 5-CQA, total CQAs and total HCEs (Fig. 5). This pattern does not precisely follow any of the reported scenarios for relative chemical concentrations of parental chemicals in first-generation (F1) hybrids, reported either as absolute concentration or relative amounts (Orians, 2000), that is: (1) similar to that in one of the two parental taxa; (2) intermediate between those of the two parental taxa; (3) overexpressed or present in higher concentrations than in either parent; (4) underexpressed or present in lower concentrations than either parent; (5) deficient, in that hybrids lack chemicals that both parents produce; or (6) novel, in that hybrids may contain chemicals lacking in both parental taxa. A better fit would be inheritance of different chemical profiles from each parent: mangiferin from C. eugenioides and HCE content from C. liberica var. dewevrei, or it could be interpreted as a mixture of scenarios: (1) for HCE accumulation and (4) for mangiferin content. Because plastids (and plastid sequences) are uniparentally inherited in flowering plants, our results, in combination with the available sequence data (Lashermes et al., 1997; Cros et al., 1998; Maurin et al., 2007), could be used to argue that in C. heterocalyx cf., mangiferin and HCE accumulation is linked to independent parental influence, in agreement with studies on the accumulation of various secondary compounds in hybrids of other plant groups (Orians, 2000). In Coffea, the indication that HCE and mangiferin accumulation are derived from independent branches of the same metabolite pathway, together with the absence of competition for substrate availability, would certainly not violate this assumption (see below: ‘Correlation between HCE and mangiferin accumulation’). It has been demonstrated that maternal influence can be important in determining hybrid chemistry (Harney and Grant, 1963; Belzer and Ownbey, 1971; Spring and Schilling, 1990; Buschmann and Spring, 1995). For example, Buschmann and Spring (1995) found that some sesquiterpene lactones were absent in Helianthus debilis Nutt. × H. annuus L. hybrids but present in H. annuus × H. debilis crosses, with each hybrid more similar to the maternal taxon.
The situation observed here for C. arabica is more complicated. Mangiferin accumulation in C. arabica (average 0·57 % d. wt) is approx. 10-fold lower than in C. eugenioides (average 5·67 % d. wt). HCE accumulation of C. arabica shares similar values with both C. canephora and C. eugenioides (Table 5), and in these three species HCE amounts are much higher than in all the other Coffea species tested (Table 5). In the PCA analysis of the HCE data (Fig. 5), C. arabica falls between C. canephora and C. eugenioides, but clusters closer to the former, and is strongly influenced by 5-CQA content. The HCE profile of C. arabica, together with the presence of mangiferin, is consistent with the assumption that C. canephora and C. eugenioides are the progenitor species for this hybrid taxon. In a scenario following the model of hybrid influence in C. heterocalyx cf., we would assume that in C. arabica the ability for mangiferin accumulation was inherited from C. eugenioides, and its HCE chemistry from C. canephora, and this is weakly supported by mangiferin and HCE accumulation, respectively (Table 5; Fig. 5) This mode of inheritance is, however, far less convincing than for C. heterocalyx cf., given the intermediary of the HCE quantities and the 10-fold difference in mangiferin accumulation. An alternative scenario would be that the data represent chemical concentrations intermediate between those of the two parental taxa (Orians et al., 2000). In addition, it is reported that F2 and later generation hybrids are both qualitatively and quantitatively more variable than F1 hybrids (Orians, 2000), and this provides a third possibility, i.e. that early generations of C. arabica expressed HCE and mangiferin concentrations similar to the parental taxa, as in C. heterocalyx cf., and then divergence occurred through differential selection during subsequent generations. This model would not be concomitant with the sequence data (Maurin et al., 2007; A. Davis, unpubl. data), which shows negligible ITS and plastid sequence divergence between each hybrid and their parental taxa (see above), but this is not an issue given that most plastid and ITS markers lack the fidelity to discriminate the relatively short divergence times between tens, hundreds or even thousands of generations. Even though both C. arabica and C. heterocalyx cf. each possess only one parental ITS type (Lashermes et al., 1997; Cros et al., 1998; Maurin et al., 2007), the time periods for homogenization to either of the parental ITS types can vary considerably within closely related groups, especially where allopolyploids are present (Pillon et al., 2007). Moreover, ITS homogenization can be a rapid process (Ganley and Kobayashi, 2007), and may occur in <100 generations in natural hybrids (Franzke and Mummenho, 1999), and Song et al. (1995) have demonstrated that extensive genome changes can occur during each of just five generations. Based on the information, and if we accept the third scenario for C. arabica as proposed above, the phenolic data presented here would support an older hybrid origin, with a greater number of post-hybridization generations, for C. arabica, compared with C. heterocalyx cf. This would agree with the relatively wide natural distribution of C. arabica (Davis et al., 2006), and the fact that this hybrid taxon functions like a species. In comparison, C. heterocalyx cf. is only known from cultivation. The fact that C. arabica is an allotetraploid might have some bearing on its chemical inheritance, but so far the only conclusion made in this respect is that novel chemicals are more common in polyploid than diploid hybrids (Orians, 2000). This effect was not revealed by our experiments. It should also be said that we examined a cultivated accession of C. arabica ‘Laurina’ (Bourbon Pointu), a genetic mutant of cultivated stock, and that the effects of mutation could have some influence on HCE and mangiferin accumulation. The possible influence of gene silencing (Van Moerkercke et al., 2009) on the modification of metabolite production is unknown, and warrants investigation, particularly in controlled crosses of Coffea taxa.
Correlation between HCE and mangiferin accumulation
We found no statistical correlation between mangiferin and HCE accumulation in Coffea, other than independent support for phylogenetic groupings of species (see above). Mangiferin biosynthesis occurs via the benzoic acid (BA) biosynthetic route that leads to the formation of benzophenone derivatives (Abd El-Mawla and Beerhues, 2002; Liu et al., 2003; Long et al., 2009). This route is a branching occurring at the cinnamic acid step of the general phenylpropanoid pathway common to most of the phenolic compounds, including HCEs. The lack of correlation between metabolite contents has been previously observed in Petunia hybrida (Hook.) P.L.Vilm. (Solanaceae). In this example, the independence of the metabolite pathways and the absence of competition for substrate availability have been shown to be caused by the silencing of a gene encoding 3-ketoacyl-CoA thiolase (PhKAT1), which is involved in the benzenoid biosynthetic pathway and the production of BA (Van Moerkercke et al., 2009).
The function of mangiferin in Coffea species
Mangiferin is a phenolic compound and, as such, one might assume that it is involved in a response to stress. The peripheral localization of mangiferin in Coffea plant organs, its association with photosynthetic tissue and its accumulation at early stages of fruit formation strengthen a hypothesis of a protective action against UV-radiation in Coffea, as suggested for Aphloia theiformis (Vahl) Benn. (Aphloiaceae), a Madagascan shrub that abundantly accumulates mangiferin in leaves when plants develop under high light conditions (Danthu et al., 2010). Of the seven Coffea species (including the hybrid-derived species C. arabica, but excluding C. heterocalyx cf., which is of unknown natural origin) accumulating mangiferin, five are high altitude species or species that have high altitude populations, where high UV conditions might be encountered [altitude data from Davis et al. (2006), with new altitude records incorporated]: C. anthonyi (350–900 m; mangiferin 12·06 % d. wt), C. arabica (950–2000 m; 0·56 % d. wt), C. eugenioides (1000–2200 m; 5·68 % d. wt), C. pseudozanguebariae (0–800 m; 4·91 % d. wt) and C. salvatrix (400–1650; 16·36 % d. wt). Coffea sessiliflora (10–600 m; 0·03 % d. wt) would be the only exception, although this is a predominantly lowland species and only accumulates very small amounts of mangiferin. The influence of altitude on mangiferin accumulation has been studied in the genus Swertia L. (Gentianaceae): S. mussotii Franch. showed a weaker mangiferin content when plants were artificially cultivated at lower altitude (Yang et al., 2005); whereas S. franchetiana Harry Sm. showed no altitudinal trend but instead a significant latitudinal and longitudinal correlation (Yang et al., 2004). Measurement of ecological and geographical variables, including direct measurement of light levels on leaves, in conjunction with further biochemical analysis, would be necessary to test for mangiferin accumulation correlations in Coffea. The distribution of mangiferin-accumulating species within specific clades of Coffea may indicate that the onset of the xanthone pathway occurred independently in East-Central Africa and East Africa (EC-Afr clade and EA clade), and perhaps in predominantly high altitude clades, after the formation of the early diverging lineages in West and Central (Guinea/Congolian) Africa (LG/C clade and ‘African Psilanthus clade’) as identified by sequencing studies (Maurin et al., 2007; Davis et al., 2011; M. Nowak, unpubl data). Further sampling of African Coffea species would be required to test these ideas; investigation of high altitude Madagascan species, such as C. buxifolia A.Chev. (1250–2000 m), should also be undertaken. The precise distribution and influence of mangiferin in leaves of the commercial species, i.e. the predominantly high altitude (950–2000 m) C. arabica (0·56 % d. wt) and lower altitude [50–600(–1500) m] C. canephora (0 % d. wt) would be a priority, particularly given recent interest in climate change mitigation using improved varieties of these two species (Camargo, 2010).
The presence of mangiferin has also been cited for its antioxidant and antimicrobial properties and for its contribution to plant defence against biotic stress (Franklin et al., 2009). A better understanding of the biosynthetic pathway of xanthones and their glycosylated derivatives is essential to determine the level of involvement of mangiferin in stress response. In particular, it would be necessary to isolate the genes controlling this poorly understood pathway to study their expression level in individuals, populations and species occurring in contrasting environmental niches.
Phenolic accumulation in Coffea leaves and fruits and implications for human use
The leaves and soft outer fruit layers of C. arabica and C. canephora are used to produce ‘tea’ (leaves) and coffee-like (outer fruit layers) beverages, particularly in Africa and Asia (Wellman, 1961; Wrigley, 1988). This use is largely unpractised in Europe and America, although some specialist coffee and tea outlets sell limited amounts of coffee-leaf ‘tea’. In Africa, a hot beverage made from the outer parts of the fruits [‘skins’ or ‘husks’, i.e. the fruit skin (exocarp) and soft, fleshy part of the fruit (mesocarp)] is referred to as Qixr or Kishr, and in Yemen a hot drink called Qishr is made from coffee husks and spices. In an early report, Sloane (1694) started that: ‘ … the Arabians themselves in summer heats use these husks [C. arabica] roasted after the manner of Coffee-berries, esteeming that drink more cooling, it being sourish to the taste.’ Coffee husks are also widely used in Africa as a masticatory (Wellman, 1961; Wrigley, 1988; A. Davis, pers. observ.). The food and health values of non-seed Coffea products is not well known. Early literature (Anonymous, 1876) suggests that coffee-leaf ‘tea’ has health benefits. Our findings, which show high levels of HCEs in the leaves of both C. arabica and C. canephora, would support the assumption of a beneficial influence on human health, given that research demonstrates high antioxidant potential for chlorogenic acids (Johnston et al., 2003; Stalmach et al., 2006; Parras et al., 2007), which can prevent oxidative damage, cerebrovascular diseases, brain performance and mental health, ageing and cancer (Zang et al., 2003; Niggeweg et al., 2004; Jin et al., 2005; Mishima et al., 2005; Rajavelu et al., 2011), and have potential for chemotherapy (Belkaid et al., 2006; Cos et al., 2008). Like green tea and green coffee beans (Rajavelu et al., 2011), the main phenolic constituent of coffee leaves is 5-CQA, although in certain Coffea species this is considerably exceeded by mangiferin. As said in the Introduction, mangiferin is recognized for its numerous pharmacological properties such as anti-inflammatory, antidiabetic, antihyperlipidaemic and neuroprotective activities (Garrido et al., 2004; Muruganandan et al., 2005; Campos-Esparza et al., 2009). Information on the influence of consuming products made from the ‘husk’ of Coffea species is more elusive. It does seem compelling, however, that in C. arabica, where we demonstrate the accumulation of mangiferin in the husk and not the seed (endosperm), the former is sometimes used in favour of the latter in the production of a beverage, given that mangiferin has potential beneficial properties. We report higher levels of mangiferin in the ‘husk’ of C. eugenioides and C. pseudozanguebariae, compared with C. arabica, but have not been able to trace use of these species in beverage production or as a masticatory. Further research on the use and properties of coffee-leaf ‘tea’ and coffee-husk coffee would be worthwhile.
CONCLUSIONS
We have demonstrated mangiferin accumulation in seven African Coffea taxa: C. anthonyi, C. arabica, C. eugenioides, C. heterocalyx cf., C. pseudozanguebariae, C. salvatrix and C. sessiliflora. Previously the presence of mangiferin had only been detected in a single species, i.e. C. pseudozanguebariae (Talamond et al., 2008). Histochemical methods show that in Coffea mangiferin is localized in leaves and outer layers of the fruit, in the same regions as the photosynthetic tissue. These data, in conjunction with published work on other mangiferin-accumulating species, and the systematic distribution of mangiferin in high altitude clades and species of Coffea, suggest an association with altitude.
Statistical analysis of mangiferin and HCE accumulation identified significant difference between species, and also provided the means to place samples into: (1) clades established on the basis of molecular phylogenetic analyses (Maurin et al., 2007; Anthony et al., 2010; Davis et al., 2011; Hamon et al., 2011); (2) species-specific groups (with exceptions) agreeing with molecular and morphological delimitation; and (3) population groups (for C. canephora). It is clear that leaf phenolic data have considerable phylogenetic utility in Coffea, at a broad range of taxonomic levels. Combining phenolic data with larger, independent data sets, such as DNA sequence data sets, is likely to be especially rewarding. Knowledge of the spectrum of phenolic accumulation in leaves and fruits of coffee species and populations might be of significance for understanding insect herbivory, and environmental adaptation, including climate change mitigation.
Sampling of two known hybrid taxa, C. arabica and C. heterocalyx cf., provided an opportunity to make a preliminary assessment of the effects of hybridization on the phenolic composition of Coffea leaves. We suggest that mangiferin and HCE accumulation are controlled by independent parental influence, an assumption that is not a variance with the indication that these two groups of compounds are derived from independently regulated metabolite pathways. Further research with controlled crossings and their influence on phenolic accumulation in leaves, and seeds (beans), of Coffea could be of significant value for coffee breeding and crop development, including breeding strategies for climate change mitigation (Camargo, 2010; Jaramillo et al., 2011).
The potential health benefits of coffee-leaf ‘tea’, and beverages and masticatory products made from the fleshy parts of Coffea fruit, are supported by our findings on the basis of high accumulation of phenolic compounds in coffee leaves, although the accumulated influence of these compounds in the human body would require further research.
ACKNOWLEDGEMENTS
We would like to thank the National Center of Applied Research and Rural Development (Foibe Fikarohana ampiharina amin'ny Fampandrosoana ny eny Ambanivohitra: FOFIFA), and the Ueshima Coffee Corporation (UCC) Japan, for allowing us to access the collections at Kianjavato Coffee Research Station (FOFIFA), and Dokou Akaffou from the CNRA station of DIVO (Ivory Coast), for use of material. The authors also wish gratefully to acknowledge: Y. Baissac (UM2) for LC-MS support; T. Roscoe (CNRS) for critical reading and commenting on an earlier version of this contribution; C. Andary (Faculty of Pharmacy) for his generous gift of a 3,5-diCQA sample; P. Hamon and M. Noirot (IRD) for support and advice; and two anonymous reviewers for their constructive comments. This work was partially funded by a grant from the Agence Universitaire Francophone (AUF).
LITERATURE CITED
- Abd El-Mawla AMA, Beerhues L. Benzoic acid biosynthesis in cell cultures of Hypericum androsaemum. Planta. 2002;214:727–733. doi: 10.1007/s004250100657. [DOI] [PubMed] [Google Scholar]
- Alonso-Salces RM, Guillou C, Berrueta LA. Liquid chromatography coupled with ultraviolet absorbance detection, electrospray ionization, collision-induced dissociation and tandem mass spectrometry on a triple quadrupole for the on-line characterization of polyphenols and methylxanthines in green coffee beans. Rapid Communications in Mass Spectrometry. 2009;23:363–383. doi: 10.1002/rcm.3884. [DOI] [PubMed] [Google Scholar]
- Anonymous. Coffee-leaf tea. British Medical Journal. 1876;2(830):691. [Google Scholar]
- Anthony F, Diniz LEC, Combes M-C, Lashermes P. Adaptive radiation in Coffea subgenus Coffea L. (Rubiaceae) in Africa and Madagascar. Plant Systematics and Evolution. 2010;285:51–64. [Google Scholar]
- Anthony F, Noirot M, Clifford MN. Biochemical diversity in the genus Coffea L.: chlorogenic acids, caffeine, and mozambioside contents. Genetic Resources and Crop Evolution. 1993;40:61–70. [Google Scholar]
- Barreto JC, Trevisan MTS, Hull WE, et al. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.) Journal of Agricultural and Food Chemistry. 2008;56:5599–5610. doi: 10.1021/jf800738r. [DOI] [PubMed] [Google Scholar]
- Belay A. Some biochemical compounds in coffee beans and methods developed for their analysis. International Journal of the Physical Sciences. 2011;6:6373–6378. [Google Scholar]
- Belkaid A, Currie J-C, Desgagnés J, Annabi B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell International. 2006;6:7. doi: 10.1186/1475-2867-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzer NF, Ownbey M. Chromatographic comparisons of Tragopogon species and hybrids. American Journal of Botany. 1971;58:791–802. [Google Scholar]
- Bertrand C, Noirot M, Doulbeau S, de Kochko A, Hamon S, Campa C. Chlorogenic acid content swap during fruit maturation in Coffea pseudozanguebariae. Qualitative comparison with leaves. Plant Science. 2003;165:1355–1361. [Google Scholar]
- Bolzani V da S, Trevisan LMV, Young CM. Caffeic acid esters and triterpenes of Alibertia macrophylla. Phytochemistry. 1991;30:2089–2091. [Google Scholar]
- Boudet AM. Evolution and current status of research in phenolic compounds. Phytochemistry. 2007;68:2722–2735. doi: 10.1016/j.phytochem.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Bridson DM. In: Coffea. Polhill RM, editor. Rotterdam/Brookfield: Balkema; 1988. pp. 415–474. Flora of Tropical East Africa, Rubiaceae,2. [Google Scholar]
- Buschmann H, Spring O. Sesquiterpene lactones as a result of interspecific hybridization in Helianthus species. Phytochemistry. 1995;39:367–371. [Google Scholar]
- Camargo MBP. The impact of climatic variability and climate change on Arabic coffee crop in Brazil. Bragantia. 2010;69:239–247. [Google Scholar]
- Campa C, Ballester JF, Doulbeau S, Dussert S, Hamon S, Noirot M. Trigonelline and sucrose diversity in wild Coffea species. Food Chemistry. 2004;88:39–43. [Google Scholar]
- Campa C, Doulbeau S, Dussert S, Hamon S, Noirot M. Qualitative relationship between caffeine and chlorogenic acid contents among wild Coffea species. Food Chemistry. 2005;93:135–139. [Google Scholar]
- Campa C, Rakotomalala J-JR, de Kochko A, Hamon S. Chlorogenic acids: diversity in green beans of wild coffee species. In: Hemantaranjan A, editor. Advances in plant physiology. Vol. 10. Jodhpur: Scientific Publishers; 2008. pp. 421–437. [Google Scholar]
- Campos-Esparza MR, Sánchez-Gómez MV, Matute C. Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium. 2009;45:358–368. doi: 10.1016/j.ceca.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Clifford MN, Williams T, Bridson DM. Chlorogenic acids and caffeine as possible taxonomic markers in Coffea and Psilanthus. Phytochemistry. 1989;28:829–838. [Google Scholar]
- Clifford MN, Johnston KL, Knight S, Kuhnert N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. Journal of Agricultural and Food Chemistry. 2003;51:2900–2911. doi: 10.1021/jf026187q. [DOI] [PubMed] [Google Scholar]
- Clifford MN, Knight S, Surucu B, Kuhnert N. Characterization by LC-MSn of four new classes of chlorogenic acids in green coffee beans: dimethoxycinnamoylquinic acids, diferuloylquinic acids, caffeoyl-dimethoxycinnamoylquinic acids, and feruloyl-dimethoxycinnamoylquinic acids. Journal of Agricultural and Food Chemistry. 2006;54:1957–1969. doi: 10.1021/jf0601665. [DOI] [PubMed] [Google Scholar]
- Clifford MN, Kirkpatrick J, Kuhnert N, Roorendaal H, Salgado PR. LC-MSn analysis of the cis isomers of chlorogenic acids. Food Chemistry. 2008;106:379–385. [Google Scholar]
- Cos P, Maes L, Vlietinck A, Pieters L. Plant-derived leading compounds for chemotherapy of human immunodefiency virus (HIV) infection – an update (1998–2007) Planta Medica. 2008;74:1323–1337. doi: 10.1055/s-2008-1081314. [DOI] [PubMed] [Google Scholar]
- Cros J, Combes MC, Trouslot P, Anthony F, Hamon S, Charrier A, Lashermes P. Phylogenetic analysis of chloroplast DNA variation in Coffea L. Molecular Phylogenetics and Evolution. 1998;9:109–117. doi: 10.1006/mpev.1997.0453. [DOI] [PubMed] [Google Scholar]
- Danthu P, Lubrano C, Flavet L, et al. Biological factors influencing production of xanthones in Aphloia theiformis. Chemistry and Biodiversity. 2010;7:140–150. doi: 10.1002/cbdv.200900199. [DOI] [PubMed] [Google Scholar]
- Davis AP. Psilanthus mannii, the type species of Psilanthus, transferred to Coffea. Nordic Journal of Botany. 2011;29:471–472. [Google Scholar]
- Davis AP, Rakotonasolo F. A taxonomic revision of the baracoffea alliance: nine remarkable Coffea species from western Madagascar. Botanical Journal of the Linnean Society. 2008;158:355–390. [Google Scholar]
- Davis AP, Govaerts R, Bridson DM, Stoffelen P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae) Botanical Journal of the Linnean Society. 2006;152:465–512. [Google Scholar]
- Davis AP, Tosh J, Ruch N, Fay MF. Growing coffee: Psilanthus (Rubiaceae) subsumed on the basis of plastid and nuclear DNA sequences; implications for the size, morphology, distribution and evolutionary history of Coffea. Botanical Journal of the Linnean Society. 2011;167:357–377. [Google Scholar]
- De Castro RD, Marraccini P. Cytology, biochemistry and molecular changes during coffee fruit development. Brazilian Journal of Plant Physiolgy. 2006;18:175–199. [Google Scholar]
- Dussert S, Laffargue A, de Kochko A, Joët T. Effectiveness of the fatty acid and sterol composition of seeds for the chemotaxonomy of Coffea subgenus Coffea. Phytochemistry. 2008;69:2950–2960. doi: 10.1016/j.phytochem.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Eyles A, Bonello P, Ganley R, Mohammed C. Induced resistance to pests and pathogens in trees. New Phytologist. 2010;185:893–908. doi: 10.1111/j.1469-8137.2009.03127.x. [DOI] [PubMed] [Google Scholar]
- Franklin G, Conceição LFR, Kombrink E, Dias ACP. Xanthone biosynthesis in Hypericum perforatum cells provides antioxidant and antimicrobial protection upon biotic stress. Phytochemistry. 2009;70:60–68. doi: 10.1016/j.phytochem.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Franzke A, Mummenho K. Recent hybrid speciation in Cardamine (Brassicaceae) – conversion of nuclear ribosomal ITS sequences in statu nascendi. Theoretical Applied Genetics. 1999;98:831–834. [Google Scholar]
- Gallego-Giraldo L, Jikumaru Y, Kamiya Y, Tang Y, Dixon RA. Selective lignin down regulation leads to constitutive defence response expression in alfalfa (Medicago sativa L.) New Phytologist. 2011;190:627–639. doi: 10.1111/j.1469-8137.2010.03621.x. [DOI] [PubMed] [Google Scholar]
- Ganley ARD, Kobayashi T. Highly efficient concerted evolution in the ribosomal DNA repeats: total rDNA repeat variation revealed by whole-genome shotgun sequence data. Genome Research. 2007;17:184–191. doi: 10.1101/gr.5457707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido G, González D, Lemusa Y, et al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG®) Pharmacological Research. 2004;50:143–149. doi: 10.1016/j.phrs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Hamon P, Duroy P-O, Dubreuil-Tranchant C, et al. Two novel Ty1-copia retrotransposons isolated from coffee trees can effectively reveal evolutionary relationships in the Coffea genus (Rubiaceae) Molecular Genetics and Genomics. 2011;285:447–460. doi: 10.1007/s00438-011-0617-0. [DOI] [PubMed] [Google Scholar]
- Harney PM, Grant WF. Biochemical anomaly in flower extracts of interspecific hybrids between Lotus species. Science. 1963;142:1061. doi: 10.1126/science.142.3595.1061. [DOI] [PubMed] [Google Scholar]
- Herrmann K. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Critical Reviews in Food Science and Nutrition. 1989;28:315–347. doi: 10.1080/10408398909527504. [DOI] [PubMed] [Google Scholar]
- International Coffee Organization (ICO) World Coffee Trade. 2011 [http://www.ico.org/trade_e.asp?section=About_Coffee. ]. Accessed 3 January 2012. [Google Scholar]
- Jaiswal R, Patras MA, Eravuchira PJ, Kuhnert N. Profile and characterization of the chlorogenic acids in green robusta coffee beans by LC-MSn: identification of seven new classes of compounds. Journal of Agricultural and Food Chemistry. 2010a;58:8722–8737. doi: 10.1021/jf1014457. [DOI] [PubMed] [Google Scholar]
- Jaiswal R, Sovdat T, Vivan F, Kuhnert N. Profiling and characterization by LC-MSn of the chlorogenic acids and hydroxycinnamoylshikimate esters in Mate (Ilex paraguariensis) Journal of Agricultural and Food Chemistry. 2010b;58:5471–5484. doi: 10.1021/jf904537z. [DOI] [PubMed] [Google Scholar]
- Jaramillo J, Muchugu E, Vega FE, Davis AP, Borgemeister C, Chabi-Olaye A. Some like it hot: the influence and implications of climate change on coffee berry borer (Hypothenemus hampei) and coffee production in East Africa. PLoS One. 2011;6:e24528. doi: 10.1371/journal.pone.0024528. http://dx.doi.org/10.1371/journal.pone.0024528 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin UH, Lee JY, Kang SK, Kim JK, Park WH, Kim JG, Moon SK, Kim CH. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sciences. 2005;77:2760–2769. doi: 10.1016/j.lfs.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. American Journal of Clinical Nutrition. 2003;78:728–733. doi: 10.1093/ajcn/78.4.728. [DOI] [PubMed] [Google Scholar]
- Kuhnert N, Jaiswal R, Eravuchira PJ, El-Abassy RM, von der Kammer B, Materny A. Scope and limitations of principal component analysis of high resolution LC-TOF-MS data: the analysis of the chlorogenic acid fraction in green coffee beans as a case study. Analytical Methods. 2011;3:144–155. doi: 10.1039/c0ay00512f. [DOI] [PubMed] [Google Scholar]
- Lashermes P, Combes M-C, Trouslot P, Charrier A. Phylogenetic relationships of coffee-tree species (Coffea L.) as inferred from ITS sequences of nuclear ribosomal DNA. Theoretical and Applied Genetics. 1997;94:947–955. [Google Scholar]
- Lashermes P, Combes MC, Robert J, Trouslot P, D'Hont A, Anthony F, Charrier A. Molecular characterisation and origin of the Coffea arabica L. genome. Molecular and General Genetics. 1999;261:259–266. doi: 10.1007/s004380050965. [DOI] [PubMed] [Google Scholar]
- Lepelley M, Cheminade G, Tremillon N, Simkin A, Caillet V, McCarthy J. Chlorogenic acid synthesis in coffee: an analysis of CGA content and real-time RT-PCR expression of HCT, HQT, C3H1, and CCoAOMT1 genes during grain development in C. canephora. Plant Science. 2007;172:978–996. [Google Scholar]
- Lewin B, Giovannucci D, Varangis P. Coffee markets: new paradigms in global supply and demand. 2004 International Bank for Reconstruction and Development, Agriculture and Rural Development Discussion Paper 3. [Google Scholar]
- Liu B, Falkenstein-Paul H, Schmidt W, Beerhues L. Benzophenone synthase and chalcone synthase from Hypericum androsaemum cell cultures: cDNA cloning, functional expression, and site-directed mutagenesis of two polyketide synthases. The Plant Journal. 2003;34:847–855. doi: 10.1046/j.1365-313x.2003.01771.x. [DOI] [PubMed] [Google Scholar]
- Long MC, Nagegowda DA, Kaminaga Y, et al. Involvement of snapdragon benzaldehyde dehydrogenase in benzoic acid biosynthesis. The Plant Journal. 2009;59:256–265. doi: 10.1111/j.1365-313X.2009.03864.x. [DOI] [PubMed] [Google Scholar]
- Lopes CR, Monaco LC. Chemotaxonomic studies of some species of the genus Coffea. Journal of Plantation Crops. 1979;7:6–14. [Google Scholar]
- Lopes CR, Shepherd GP. Phylogenetic studies of some species of the genus Coffea – 1 – numerical analysis of flavonoid compounds. Brazilian Journal of Genetics. 1991;14:425–435. [Google Scholar]
- Lopes CR, Musche R, Hec M. Identificação de pigmentos flavonóides e ácidos fenólicos nos cultivares Bourbon e Caturra de Coffea arabica. Brazilian Journal of Genetics. 1984;7:657–669. [Google Scholar]
- Macheix J-J, Fleuriet A, Billot J. Changes and metabolism of phenolic compounds in fruits. In: Macheix J-J, Fleuriet A, Billot J, editors. Fruit phenolics. Boca Raton, FL: CRC Press; 1990. pp. 149–237. [Google Scholar]
- Mahesh V, Million-Rousseau R, Ullmann P, et al. Functional characterization of two p-coumaroyl ester 3'-hydroxylase genes from coffee tree: evidence of a candidate for chlorogenic acid biosynthesis. Plant Molecular Biology. 2007;64:145–159. doi: 10.1007/s11103-007-9141-3. [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Rémésy C, Jimenez L. Polyphenols: food source and bioavailability. American Journal of Clinical Nutrition. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Nakamura S, Kondou N, Takasu Y, Ochiai R, Masukawa Y. Liquid chromatography-electrospray ionization-tandem mass spectrometry for simultaneous analysis of chlorogenic acids and their metabolites in human plasma. Journal of Chromatography B. 2007;858:96–105. doi: 10.1016/j.jchromb.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Maurin O, Davis AP, Chester M, Mvungi EF, Jaufeerally-Fakim Y, Fay MF. Towards a phylogeny for Coffea (Rubiaceae): identifying well-supported lineages based on nuclear and plastid DNA sequences. Annals of Botany. 2007;100:1565–1583. doi: 10.1093/aob/mcm257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima S, Inoh Y, Narita Y, et al. Identification of caffeoylquinic acid derivatives from Brazilian propolis as constituents involved in induction of granulocytic differentiation of HL-60 cells. Bioorganic and Medicinal Chemistry. 2005;13:5814–5818. doi: 10.1016/j.bmc.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Moglia A, Lanteri S, Comino C, Acquadro A, de Vos R, Beekwilder J. Stress-induced biosynthesis of dicaffeoylquinic acids in globe artichoke. Journal of Agricultural and Food Chemistry. 2008;56:8641–8647. doi: 10.1021/jf801653w. [DOI] [PubMed] [Google Scholar]
- Mondolot L, La Fisca P, Buatois B, Talansier E, de Kochko A, Campa C. Caffeoylquinic acid content and histolocalization in Coffea canephora developing leaves. Annals of Botany. 2006;98:33–40. doi: 10.1093/aob/mcl080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruganandan S, Srinivasan K, Gupta S, Gupta PK, Laj J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. Journal of Ethnopharmacology. 2005;97:497–501. doi: 10.1016/j.jep.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Niggeweg R, Michael AJ, Martin C. Engineering plants with increased levels of the antioxidant chlorogenic acids. Nature Biotechnology. 2004;22:746–754. doi: 10.1038/nbt966. [DOI] [PubMed] [Google Scholar]
- Orians CM. The effects of hybridization in plants on secondary chemistry: implications for the ecology and evolution of plant–herbivore interactions. American Journal of Botany. 2000;87:1749–1756. [PubMed] [Google Scholar]
- Parras P, Martínez-Tomé M, Jiménez AM, Murci MA. Antioxidant capacity of coffees of several origins brewed following three different procedures. Food Chemistry. 2007;102:582–592. [Google Scholar]
- Pillon Y, Fay MF, Hedrén M, et al. Evolution and temporal diversification of Western European polyploid species complexes in Dactylorhiza (Orchidaceae) Taxon. 2007;56:1185–1208. [Google Scholar]
- Rajavelu A, Tulyasheva Z, Jaiswal R, Jeltsch A, Kuhnert N. The inhibition of the mammalian DNA methyltransferase 3a (Dnmt3a) by dietary black tea and coffee polyphenols. BMC Biochemistry. 2011;12(16) doi: 10.1186/1471-2091-12-16. http://dx.doi.org/10.1186/1471-2091-12-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakotomalala JJR. 1992 Diversité biochimique des caféiers: analyse des acides hydroxycinnamiques, bases puriques et diterpènes glycosidiques. Particularités des caféiers sauvages de la région malgache (Mascarocoffea Chev.). PhD thesis, University of Montpellier 2, France. [Google Scholar]
- Read J, Sanson GD, Caldwell E, et al. Correlations between leaf toughness and phenolics among species in contrasting environments of Australia and New Caledonia. Annals of Botany. 2009;103:757–767. doi: 10.1093/aob/mcn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribereau-Gayon P. Les composés phénoliques des végétaux. Paris: Dunod; 1968. [Google Scholar]
- Santiago LJM, Louro RP, De Oliveira DE. Compartmentation of phenolic compounds and phenylalanine ammonia-lyase in leaves of Phyllanthus tenellus Roxb. and their induction by copper sulphate. Annals of Botany. 2000;86:1023–1032. [Google Scholar]
- Schuster B, Herrmann K. Hydroxybenzoic and hydroxycinnamic acid derivatives in soft fruits. Phytochemistry. 1985;24:2761–2764. [Google Scholar]
- Shahidi F, Chandrasekara A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochemistry Reviews. 2010;9:147–170. [Google Scholar]
- Sloane H. An account of a prodigiously large feather of the bird cuntur, brought from Chili, and supposed to be a kind of vultur; and of the coffee-shrub. Philosophical Transactions. 1694;18:61–64. [Google Scholar]
- Song K, Lu P, Tang K, Osborn TC. Rapid genome change in synthetic polyploids of Brassicaceae and its implications for polyploid evolution. Proceedings of the National Academy of Sciences, USA. 1995;92:7719–7723. doi: 10.1073/pnas.92.17.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring O, Schilling EE. Chemosystematic investigation of the annual species of Helianthus (Asteraceae) Biochemical Systematics and Ecology. 1989;17:519–528. [Google Scholar]
- Stalmach A, Mullen W, NagaiI C, Crozier A. On-line HPLC analysis of the antioxidant activity of phenolic compounds in brewed, paper-filtered coffee. Brazilian Journal of Plant Physiology. 2006;18:253–262. [Google Scholar]
- Stoffelen P, Noirot M, Couturon E, Bontems S, De Block P, Anthony F. Coffea anthonyi, a new self-compatible Central African coffee species, closely related to an ancestor of Coffea arabica. Taxon. 2009;58:133–140. [Google Scholar]
- Suryawanshi S, Asthana RK, Gupta RC. Simultaneous estimation of mangiferin and four secoiridoid glycosides in rat plasma using liquid chromatography tandem mass spectrometry and its application to pharmacokinetic study of herbal preparation. Journal of Chromatography, B. 2007;858:211–219. doi: 10.1016/j.jchromb.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Talamond P, Mondolot L, Gargadennec A, et al. First report on mangiferine (C-glucosyl-xanthone) isolated from leaves of a wild coffee plant, Coffea pseudozanguebariae (Rubiaceae) Acta Botanica Gallica. 2008;155:513–519. [Google Scholar]
- Van Moerkercke A, Schauvinhold I, Pichersky E, Haring MA, Schuurink RC. A plant thiolase involved in benzoic acid biosynthesis and volatile benzenoid production. The Plant Journal. 2009;60:292–302. doi: 10.1111/j.1365-313X.2009.03953.x. [DOI] [PubMed] [Google Scholar]
- Vega FE, Rosenquist E, Collins W. Global project needed to tackle coffee crisis. Nature. 2003;435:343. doi: 10.1038/425343a. [DOI] [PubMed] [Google Scholar]
- Wellman FL. Coffee, botany, cultivation and utilization. London: Leonard Hill Books; 1961. [Google Scholar]
- White F. The Guineo-Congolian Region and its relationships to other phytochoria. Bulletin du Jardin Botanique National de Belgique. 1979;49:11–55. [Google Scholar]
- White F. The vegetation of Africa. A descriptive memoir to accompany the Unesco/AETFAT/UNSO vegetation map of Africa. Paris: Unesco; 1983. [Google Scholar]
- Wink M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry. 2003;64:3–19. doi: 10.1016/s0031-9422(03)00300-5. [DOI] [PubMed] [Google Scholar]
- Wrigley G. Coffee – tropical agriculture series. Harlow, UK: Longman Scientific & Technical; 1988. [Google Scholar]
- Yang H, Duan Y, Hu F, Liu J. Lack of altitudinal trends in phytochemical constituents of Swertia franchetiana (Gentianaceae) Biochemical Systematics and Ecology. 2004;32:861–866. [Google Scholar]
- Yang H, Ding C, Duan Y, Liu J. Variation of active constituents of an important Tibet folk medicine Swertia mussotii Franch. (Gentianaceae) between artificially cultivated and naturally distributed. Journal of Ethnopharmacology. 2005;98:31–35. doi: 10.1016/j.jep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Zang L-Y, Cosma G, Gardner H, Castranoca V, Vallyathan V. Effect of chlorogenic acid on hydroxyl radical. Molecular and Cellular Biochemistry. 2003;247:205–210. doi: 10.1023/a:1024103428348. [DOI] [PubMed] [Google Scholar]