Abstract
Background and Aims
The involvement of two steps in the physical dormancy (PY)-breaking process previously has been demonstrated in seeds of Fabaceae and Convolvulaceae. Even though there is a claim for a moisture-controlled stepwise PY-breaking in some species of Geraniaceae, no study has evaluated the role of temperature in the PY-breaking process in this family. The aim of this study was to determine whether a temperature-controlled stepwise PY-breaking process occurs in seeds of the winter annuals Geranium carolinianum and G. dissectum.
Methods
Seeds of G. carolinianum and G. dissectum were stored under different temperature regimes to test the effect of storage temperature on PY-break. The role of temperature and moisture regimes in regulating PY-break was investigated by treatments simulating natural conditions. Greenhouse (non-heated) experiments on seed germination and burial experiments (outdoors) were carried out to determine the PY-breaking behaviour in the natural habitat.
Key Results
Irrespective of moisture conditions, sensitivity to the PY-breaking step in seeds of G. carolinianum was induced at temperatures ≥20 °C, and exposure to temperatures ≤20 °C made the sensitive seeds permeable. Sensitivity of seeds increased with time. In G. dissectum, PY-break occurred at temperatures ≥20 °C in a single step under constant wet or dry conditions and in two steps under alternate wet–dry conditions if seeds were initially kept wet.
Conclusions
Timing of seed germination with the onset of autumn can be explained by PY-breaking processes involving (a) two temperature-dependent steps in G. carolinianum and (b) one or two moisture-dependent step(s) along with the inability to germinate under high temperatures in G. dissectum. Geraniaceae is the third of 18 families with PY in which a two-step PY-breaking process has been demonstrated.
Keywords: Geraniaceae, Geranium, physical dormancy, stepwise process, the autumn effect, timing of PY-break, two-step model, winter annual
INTRODUCTION
Breaking of physical dormancy (PY) in seeds at the onset of autumn is of survival advantage for winter annuals in that it provides them with favourable conditions for germination and establishment of seedlings (Taylor, 1996a, b). In the absence of physiological dormancy (PD), breaking of PY may lead to immediate germination of seeds upon imbibition (Baskin and Baskin, 1998). Germination of winter annual species in summer may result in loss of seedlings due to prevailing drought conditions (Baskin and Baskin, 1971). Therefore, in winter annuals with PY, timing of PY-break must be set to synchronize with the onset of autumn.
Taylor (1981) presented a temperature-dependent two-stage conceptual model for breaking of PY. In the first or pre-conditioning stage, the seeds are made sensitive to the second or the PY-breaking stage (Taylor, 2005). This two-stage model is known to occur in seeds of several annual species of Fabaceae [Medicago polymorpha (Taylor, 1996a, b), Ornithopus compressus (Taylor and Revell, 1999), Trifolium subterraneum (Taylor, 1981), Melilotus albus, Medicago lupulina, Lotus corniculatus and Trifolium repens (Van Assche et al., 2003)] and Convolvulaceae [Ipomoea lacunosa (Jayasuriya et al., 2008a), Ipomoea hederacea (Jayasuriya et al., 2009b) and Cuscuta australis (Jayasuriya et al., 2008b)]. The two-stage model consists of two distinct temperature and/or moisture-dependent processes (Taylor, 2005; Van Assche and Vandelook, 2006; Jayasuriya et al., 2009a). Involvement of two stages in breaking of PY can be used to explain the PY-breaking behaviour and timing of germination under natural conditions (Taylor, 2005).
Geranium carolinianum and Geranium dissectum are herbaceous winter annual species of Geraniaceae. Geranium carolinianum is native to eastern North America (Piper, 1906; Small, 1907; Aedo, 2000), while G. dissectum is native to Europe (Aedo et al., 1998; Rhoads and Block, 2007) and is an introduced species in North America (Piper and Beattie, 1915). Both species are widely distributed weeds in North America and usually grow in disturbed habitats such as roadsides, old fields, waste places, gardens and fallow and cultivated fields (McCready and Cooperrider, 1984; Abbas et al., 1995; Wilson and Clark, 2001). Moreover, both species are reported to be naturalized weeds in many parts of the world including Australia, China, Great Britain, Japan, Italy and South America (Mueller, 1885; Dunn, 1905; Macbride, 1949; Peng, 1978; Aedo et al., 1998, 2005; Benvenuti et al., 2001; Xu and Aedo, 2008; Nishida and Yamashita, 2009).
As in most species of Geraniaceae, PY is known to occur in seeds of G. carolinianum and G. dissectum (Meisert, 2002; Van Assche and Vandelook, 2006; Gama-Arachchige et al., 2010). Freshly matured seeds of both species also exhibit shallow non-deep PD, thus the seeds have combinational dormancy (PY + PD). However, the shallow PD is lost during a short after-ripening period (Baskin and Baskin, 1974; Gama-Arachchige et al., 2010). The water-gap (small opening) formed in the water-impermeable seed or fruit coat during breaking of PY allows the seed to take up water. Opening of the water-gap involves dislodgment or disruption of water-gap structures that act as environmental ‘signal detectors’ for germination (Baskin et al., 2000). The hinged-valve gap (water-gap) of the seeds of Geraniaceae is located near the micropyle. On breaking of PY, the changes in the palisade layer of the water-gap region are externally visible as a colour change from dark brown to brownish orange (Gama-Arachchige et al., 2010). Thus, seeds with a colour change in the water-gap region are permeable (Gama-Arachchige et al., 2010).
In a study of the ecological factors involved in breaking of PY in G. carolinianum, Baskin and Baskin (1974) concluded that PY-breaking takes place under dry or alternate wet–dry conditions at high summer temperatures. They concluded that the water-impermeable seed coat, conditional dormancy of the freshly matured embryo and the inability of seeds to germinate at high summer temperatures delay germination of seeds until autumn. However, examination of a sample of seeds from their study revealed that the seeds they used were G. dissectum, not G. carolinianum (N.S. Gama-Arachchige et al., unpubl. res.). Furthermore, a new preliminary study showed that unlike the seeds used by Baskin and Baskin (1974), PY of G. carolinianum can be broken under wet conditions, further supporting the fact that the seeds they used were G. dissectum (N.S. Gama-Arachchige et al., unpubl. res.). In a study of germination ecology of several species of Geraniaceae, including G. dissectum, Van Assche and Vandelook (2006) showed that subsequent drying of exhumed impermeable seeds in a desiccator for 1 week at approx. 20 °C markedly stimulated germination at 23 °C [see table 7 in Van Assche and Vandelook (2006)]. Meisert (2002) observed that seeds of certain species of Geraniaceae, including G. dissectum, became permeable under dry storage for 5 years at room temperature. Seeds of G. bicknellii, G. bohemicum and G. lanuginosum germinated (>90 %) after exposure to wet heat at 55–95 °C (Granstrom and Schimmel, 1993), suggesting that those three species do not require drying for the breaking of PY.
Occurrence of a temperature-dependent process with two steps in the breaking of PY is unknown in Geraniaceae. Furthermore, none of the previous studies has clearly explained the environmental factors involved in the timing of PY-break and germination of G. carolinianum under field conditions.
Therefore, the objectives of the current study on G. carolinianum and G. dissectum were to (1) determine the number of steps involved in the PY-breaking processes; (2) identify the temperature and moisture regimes that activate the dormancy-breaking process at each stage; and (3) develop a conceptual model for dormancy break and germination phenology under field conditions.
MATERIALS AND METHODS
Seed collection
Stems of Geranium carolinianum bearing mature fruits were collected at Spindletop Farm, Lexington, KY, USA, on 9 June 2010 (GC 2010) and 1 June 2011 (GC 2011). Similarly, stems of G. dissectum were collected from the same location on 30 May 2010 (GD 2010) and 20 May 2011 (GD 2011). They were covered with a mesh cloth and allowed to dry for 3 d inside a non-heated greenhouse. Seeds released naturally were collected and stored at room temperature (approx. 23 °C and 50–60 % relative humidity, dry storage) until used. According to imbibition tests, >98 % of fully matured seeds of both species were impermeable (data not shown). Therefore, fully matured seeds were used for all the experiments, which were started within 2 weeks of seed collection.
In the case of alternating incubation temperatures, high and low temperatures in the incubators were supplied on a 12 h/12 h daily basis under light/dark conditions (14 h/10 h; approx. 40 µmol m−2 s−1, 400–700 nm, cool white fluorescent light). The same photon irradiance and 24 h continuous light were used for constant temperatures.
Breaking of PY under dry storage
Experiments were carried out to investigate the effects of dry storage under constant and alternating temperatures and subsequent exposure to autumn temperatures (20/10 °C) on breaking of PY.
Seeds collected in 2011 were stored dry at constant temperatures of 5, 10, 15, 20, 25, 30, 35 and 40 °C and at alternating temperatures of 15/6, 20/10, 25/15, 30/15, 30/20 and 40/25 °C in Petri dishes. From seeds stored under each temperature, a sample of 200 was retrieved every month. One hundred seeds from each sample (five replicates of 20 seeds) were incubated at the same storage temperature on sand moistened by adding distilled water heated or cooled to the respective storage temperature. The remaining 100 seeds (five replicates of 20 seeds) were incubated at the average autumn temperature (20/10 °C) on sand moistened by adding distilled water at 20 °C. The number of imbibed seeds was counted after 14 d. The same procedure was repeated for five consecutive months.
Sensitivity of seeds of G. carolinianum to changes in temperature
To determine the increase of sensitivity in seeds of G. carolinianum to the change in temperature, GC 2011 were stored dry in Petri dishes under alternating temperature of 40/25 °C (to simulate summer soil temperatures) and under constant 30 °C (approximately the average of 40/25 °C). Seven hundred seeds each were retrieved from both storage temperatures at 0 (fresh), 2 and 4 months and were incubated on moist sand at 10, 15, 20, 25, 30, 35 and 40 °C (five replicates with 20 seeds in each). The number of imbibed seeds was counted after 14 d.
Breaking of PY by simulated natural temperatures
To determine the effect of temperatures that the seeds of G. carolinianum and G. dissectum would experience in nature during the PY-breaking period, seeds (GC 2011 and GD 2011) were subjected to a sequence of temperature conditions simulating the average daily maximum and minimum temperatures in Lexington in June (30/15 °C), July (30/20 °C), August (30/20 °C), September (25/15 °C) and October (20/10 °C) under constant wet and dry conditions.
Wet storage
Seeds were placed in Petri dishes filled with sand wetted with distilled water and their lids were sealed with Parafilm®. Then five replicates (with 20 seeds each) were subjected to the three temperature schemes shown in Table 1. Seeds were kept under each temperature for 1 month and then transferred to the next temperature. At the end of each month, the number of imbibed seeds was recorded. Distilled water was added as required to maintain the wet condition. The procedure was continued for five consecutive months.
Table 1.
Temperature conditions for each month of the three storage temperature schemes
| Month |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature scheme | June (1) | July (2) | August (3) | September (4) | October (5) | ||||
| 1. Alternating | 30/15 °C | → | 30/20 °C | → | 30/20 °C | → | 25/15 °C | → | 20/10 °C |
| 2. Constant high | 30 °C | → | 30 °C | → | 30 °C | → | 25 °C | → | 20 °C |
| 3. Constant low | 15 °C | → | 20 °C | → | 20 °C | → | 15 °C | → | 10 °C |
Dry storage
Five samples each containing five replicates of 20 seeds each from both species were placed on dry sand in Petri dishes. They were subjected to the three temperature schemes in Table 1. At the end of each month, one sample was watered and left under the same temperature, and all the other samples were moved to the next temperature in the sequence. This procedure was followed for five consecutive months. Each watered sample was observed for imbibition after 14 d.
Effect of moisture regime on breaking of PY in G. dissectum
To determine the effect of different moisture regimes on breaking of PY in G. dissectum, fresh mature seeds (GD 2011) were subjected to four moisture regimes in an incubator at 40 °C. Twenty samples each containing five replicates of 20 seeds were placed on sand in Petri dishes. Five samples each were subjected to the four moisture regimes. (1) Constant wet; seeds were kept under constant wet conditions by adding distilled water weekly. (2) Constant dry; seeds were kept dry. (3) Alternate wet–dry; seeds were alternated between wet and dry (2 weeks under each condition) for 10 weeks starting with the wet condition. (4) Alternate dry–wet; seeds were alternated between dry and wet (2 weeks under each condition) for 10 weeks starting with the dry condition. In the case of alternating moisture regimes, distilled water was added once a week during wet periods and no water was given during dry periods. For all moisture regimes, at the end of each 2 week interval, one sample was tested for imbibition at 40 °C, and in the case of alternate moisture regimes all the other samples were moved to the next moisture condition. Imbibition in each sample was recorded after 14 d.
Breaking of PY under semi-natural conditions
To determine the effect of temperature on timing of PY-break under semi-natural conditions, 100 seeds (GC 2010, GD 2010, GC 2011 and GD 2011) were placed on dry sand in plastic Petri dishes (five replicates of 20 seeds). The Petri dishes then were placed in trays filled with potting soil inside a non-heated greenhouse [second week of June 2010/11 (GC 2010 and GC 2011), first week of June 2010 (GD 2010) and last week of May 2011 (GD 2011)]. The water-gap region of seeds was observed weekly under a dissecting microscope. Seeds with colour change from dark brown to brownish orange in the water-gap region were considered permeable (Gama-Arachchige et al., 2010). Air temperature inside the greenhouse was recorded in 30 min intervals using a Thermochron ibutton® (DS 1921G#F50), and daily maximum and minimum temperatures were obtained from the recordings.
Effect of soil moisture regime on breaking of PY
To determine the effect of the soil moisture regime on breaking of PY in both species, seeds (GC 2010, GC 2011 and GD 2011) were maintained under three soil moisture regimes (wet, wet–dry and dry). Five replicates each containing 300 seeds from each species were prepared separately for each moisture regime by sowing the seeds on plastic trays (30 × 30× 5 cm) filled with a 3 cm layer of dry potting soil and covering them with 0·5 cm of the same dry soil layer. The trays containing G. carolinianum and G. dissectum were placed inside a non-heated greenhouse in the second week of June (2010/11) and the last week of May (2011), respectively. Seeds maintained under the wet soil moisture regime were watered to field capacity and were covered with aluminium foil to minimize evaporation of water. Soil in those trays was kept at or near field capacity until the end of the experiment by watering once a week. Seeds in the wet–dry regime were watered once a week to field capacity and allowed to dry under ambient conditions. Seeds in the dry regime were left without watering until the second week of October 2010/11, after which they were watered to field capacity once a week. Seed germination was checked at 7 d intervals until the end of the experiment. The air temperature inside the greenhouse was recorded at 30 min intervals using a Thermochron ibutton® (DS 1921G#F50), and daily maximum and minimum temperatures were obtained from the recordings.
Breaking of PY under natural conditions
To determine the timing of breaking of PY under natural conditions, three replicates of 100 seeds (GC 2010, GC 2011 and GD 2011) were placed in nylon mesh bags and buried at a depth of 2 cm in soil for 5 months [May to October (GD 2011) and June to November (GC 2010/11)] in an open area on the campus of the University of Kentucky in 2010 and 2011. The soil temperature at a depth of 2 cm was recorded at 30 min intervals using a Thermochron ibutton® (DS 1921G#F50) sealed in a plastic bag, and daily maximum and minimum temperatures were obtained from the recordings. Manual weeding of the buried area was done when necessary during the burial period. Three bags each were exhumed every month and the numbers of intact, dead, swollen and germinated seeds were counted. The micropylar region of intact seeds was observed under a dissecting microscope. Seeds with a colour change from dark brown to brownish orange in the water-gap region were considered permeable (Gama-Arachchige et al., 2010). Then, all the intact seeds without the colour change were placed on moist soil and incubated at 20 °C. The number of imbibed and germinated seeds was counted after 14 d. The permeable seed fractions (initial and after exposure to 20 °C) were calculated as follows:
Initial permeable seed percentage = [(Ci + Di + Gi + Si)/total] × 100
Final permeable seed percentage = [(Ci + Di + Gi + Si + Cf + Gf + Sf)/total] × 100
where Ci is the number of seeds with a colour change in the water-gap region at each sampling time; Di is the number of dead seeds at each sampling time; Gi is the number of germinated seeds at each sampling time; Si is the number of swollen seeds at each sampling time; Cf is the number of seeds with a colour change in the water-gap region after exposure to 20 °C; Gf is the number of germinated seeds after exposure to 20 °C; and Sf is the number of swollen seeds after exposure to 20 °C.
Statistical analysis
A completely randomized design was used in all experiments. Percentage imbibition and permeable fraction data were normalized by arcsine transformation prior to analysis. Data for percentage permeable seed fractions in burial experiments were compared using paired t-tests (P = 0·05). All other imbibition percentage and germination rate data were analysed by one-way analysis of variance (ANOVA). Duncan's mean separation procedure was used to compare treatments (P = 0·05). All analyses were carried out using SPSS ver. 19 software.
RESULTS
Breaking of PY under dry storage
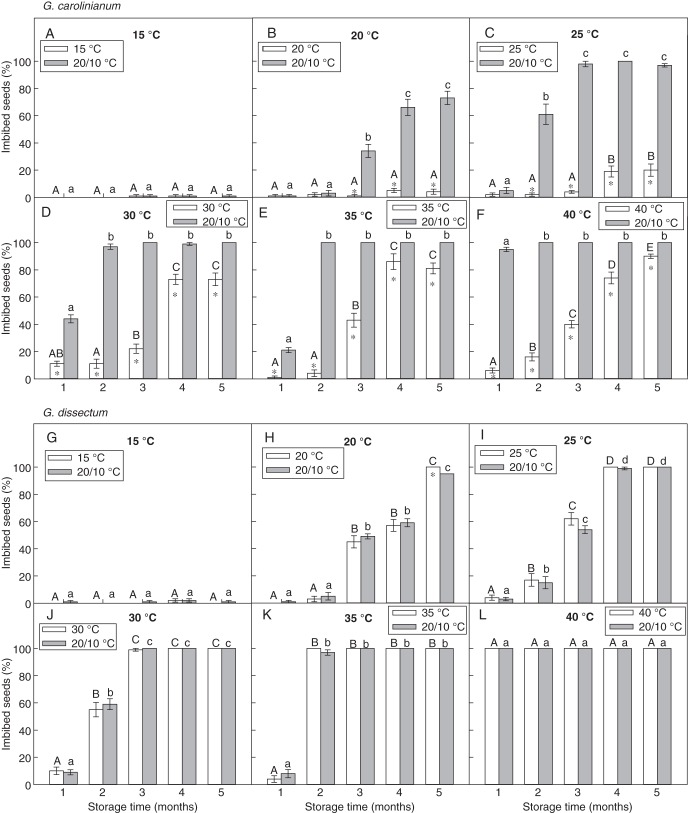
In both species, imbibition did not take place in seeds stored under dry conditions at constant temperatures below 15 °C even for 5 months (Fig. 1A, G; results for storage under 5 and 10 °C not shown). The minimum storage temperature at which imbibition occurred was 20 °C (Fig. 1B, H). With increasing storage temperature and time, the fraction of imbibed seeds increased (Fig. 1B–F, H–L). However, in G. carolinianum, incubation at 20/10 °C caused a significant increase in the fraction of imbibed seeds compared with imbibition at each storage temperature, whereas in G. dissectum, no such change in imbibition was observed.
Fig. 1.
Percentage of imbibed seeds (mean ± s.e.) of (A–F) G. carolinianum and (G–L) G. dissectum at constant temperature and at 20/10 °C after dry storage at different constant temperatures (15–40 °C) for 1 – 5 months. An asterisk indicates a significant difference in imbibition between the two incubation temperatures in each month. Different upper- and lower-case letters indicate a significant difference between different months in imbibition under a constant temperature and under 20/10 °C, respectively (P < 0·05).
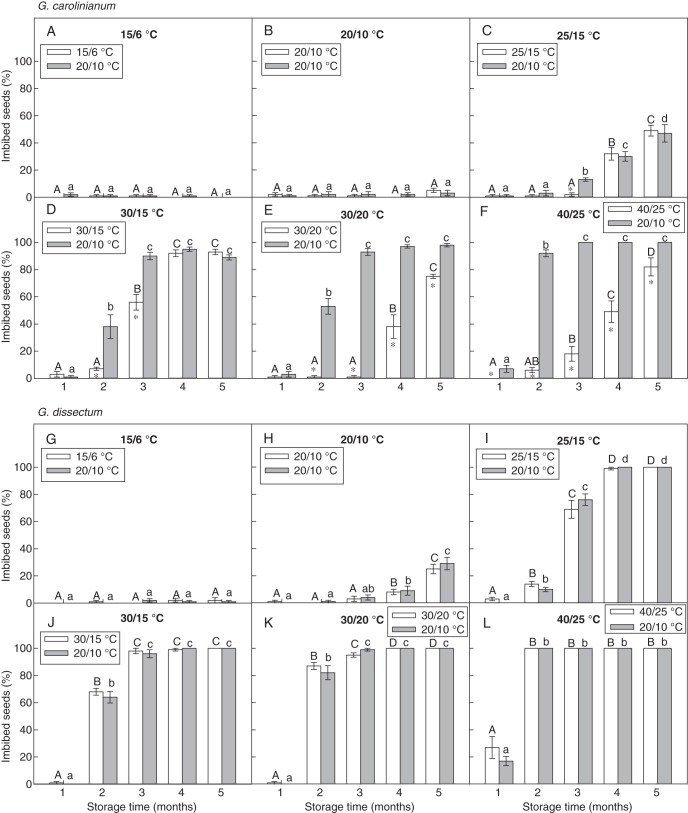
In G. carolinianum, <2 % of seeds stored under 15/6 and 20/10 °C imbibed (Fig. 2A, B). Generally, the percentage imbibition increased with increasing storage time (Fig. 2C–F). Higher imbibition percentages were observed at 30/15, 30/20 and 40/25 °C than at lower alternating temperatures (Fig. 2D–F). At 40/25 and 30/20 °C storage temperatures, the percentage of seeds that imbibed during subsequent incubation under 20/10 °C was significantly higher than those at the corresponding storage temperature after storage for 1 and 2 months, respectively. However, at 25/15 and 30/15 °C, no such significance was observed between percentage imbibition values after 3 months of storage (Fig. 2C, D).
Fig. 2.
Percentage of imbibed seeds (mean ± s.e.) of (A–F) G. carolinianum and (G–L) G. dissectum at alternating temperature and at 20/10 °C after dry storage at different alternating temperatures for 1–5 months. An asterisk indicates a significant difference in imbibition between the two incubation temperatures in each month. Different upper- and lower-case letters indicate a significant difference between different months in imbibition under a particular alternating temperature and under 20/10 °C, respectively (P < 0·05).
In contrast to G. carolinianum, no significant difference was observed in imbibition between seeds incubated under alternating storage temperatures and those incubated at 20/10 °C in G. dissectum (Fig. 2G–L). Also, imbibition was low (<9 %) in seeds stored at 15/6 and 20/10 °C (Fig. 2G, H). At the other storage temperatures, imbibition increased rapidly with storage time, reaching approx. 100 % after 2–4 months of storage (Fig. 2I–L).
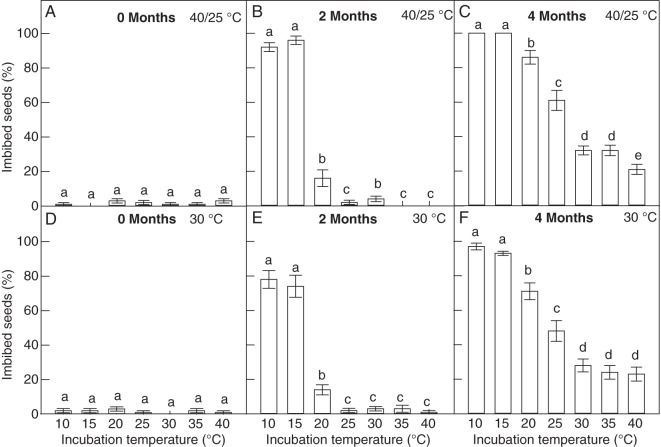
Sensitivity of seeds of G. carolinianum to changes in temperature
Only 0–3 % of fresh seeds (0 months) imbibed at all incubation temperatures (Fig. 3A, D). Seeds stored at 40/25 °C for 2 months showed significant imbibition at 10 and 15 °C (>92 %; Fig. 3B). The highest temperature at which a significant imbibition was observed was 20 °C (16 %). At higher incubation temperatures, imbibition was very low (<4 %). After 4 months of storage, imbibition had increased at all the incubation temperatures (Fig. 3C). At ≤20 °C, >86 % of the seeds had imbibed, while at 25 °C, 61 % of them had done so. At incubation temperatures >25 °C, the imbibed seed fraction remained between 20 and 30 %. An identical pattern, but lower percentages, of imbibition was observed for seeds stored at 30 °C (Fig. 3E, F).
Fig. 3.
Percentage of imbibed seeds (mean ± s.e.) of G. carolinianum incubated at different constant temperatures after dry storage (A–C) at 40–25 °C and (D–F) at 30 °C. Different letters indicate significant differences in imbibition percentages between the incubation temperatures (P < 0·05).
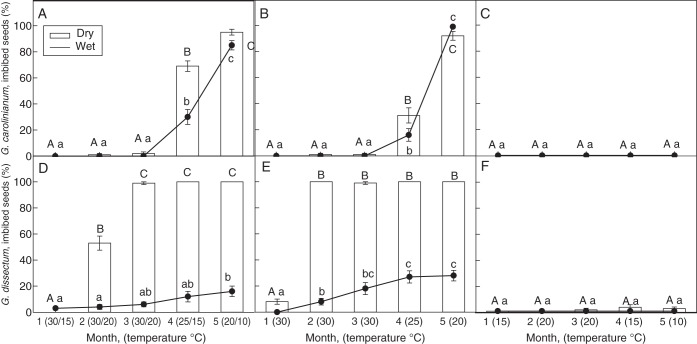
Breaking of PY by simulated natural temperatures
In G. carolinianum, imbibition increased drastically in seeds transferred to 25/15 °C (September), from approx. 0 to 70 % in dry-stored seeds and from approx, 0 to 30 % in wet-stored seeds (Fig. 4A). On transfer of seeds to 20/10 °C (October), imbibition increased to >95 % in seeds stored under both dry and wet conditions. Unlike in G. carolinianum, imbibition (53 %) was first observed in G. dissectum when dry-stored seeds were transferred to 30/20 °C (July) (Fig. 4D). Under the same conditions the following month (August), the fraction of imbibed seeds increased up to 100 %. In wet-stored seeds, imbibition increased only up to 16 % even after 5 months of storage under the same alternating temperature scheme.
Fig. 4.
Percentage of imbibed seeds (mean ± s.e.) of (A–C) G. carolinianum and (D–F) G. dissectum stored under dry and wet conditions (as indicated) under temperature sequences: (A, D) alternating; (B, E) daily maximum; and (C, F) daily minimum temperature simulating natural temperature conditions of each month. Different upper- and lower-case letters indicate significant differences in imbibition percentages between the incubation temperatures under dry storage and wet storage, respectively (P < 0·05).
At simulated monthly maximum temperatures under wet and dry conditions, imbibition up to 16 and 31 %, respectively, was observed in G. carolinianum after transfer of seeds to 25 °C (Fig. 4B). Subsequently, at 20 °C imbibition increased to >90 %. In the case of G. dissectum, all the dry-stored seeds imbibed after 2 months of storage at 30 °C, while only 28 % of the wet-stored seeds imbibed even after 5 months of storage (Fig. 4E). Imbibition of both species was negligible after wet and dry storage with the average monthly minimum temperature scheme (Fig. 4C, F).
Effect of moisture regime on breaking of PY in G. dissectum
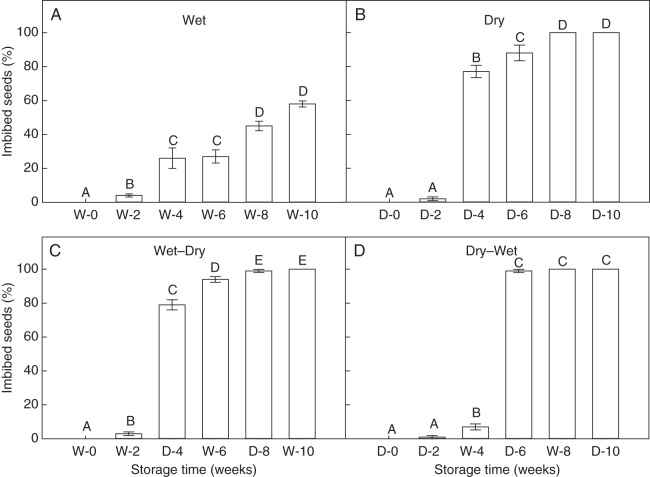
After the first 2 weeks of storage under all four moisture regimes at 40 °C, a low percentage of seeds imbibed (<4 %; Fig. 5A–D). During the remaining 8 weeks at the constant wet regime, the percentage of imbibed seeds increased gradually to approx. 60 % whereas under the constant dry regime, it increased rapidly to 100 % (Fig. 5A, B). After 4 weeks of storage (following the first dry period) under the wet–dry regime, a significantly higher percentage of seeds imbibed (approx. 80 %) (Fig. 5C). During the following 2 weeks of wet storage, imbibition increased to 94 %. After 4 weeks of storage under the dry–wet regime (Fig. 5D) (i.e. after the first wet period), a significant but low percentage of seeds imbibed (7 %). A marked increase in imbibition was then observed in the following dry period (100 %).
Fig. 5.
Percentage of imbibed seeds (mean ± s.e.) of G. dissectum at 40 °C, after storage under different moisture regimes at 40 °C. The moisture conditions of each week during the storage period are indicated on the x-axis: W, wet; and D, dry. Different letters indicate significant differences between imbibition percentages within each moisture regime (P < 0·05).
Breaking of PY under semi-natural conditions
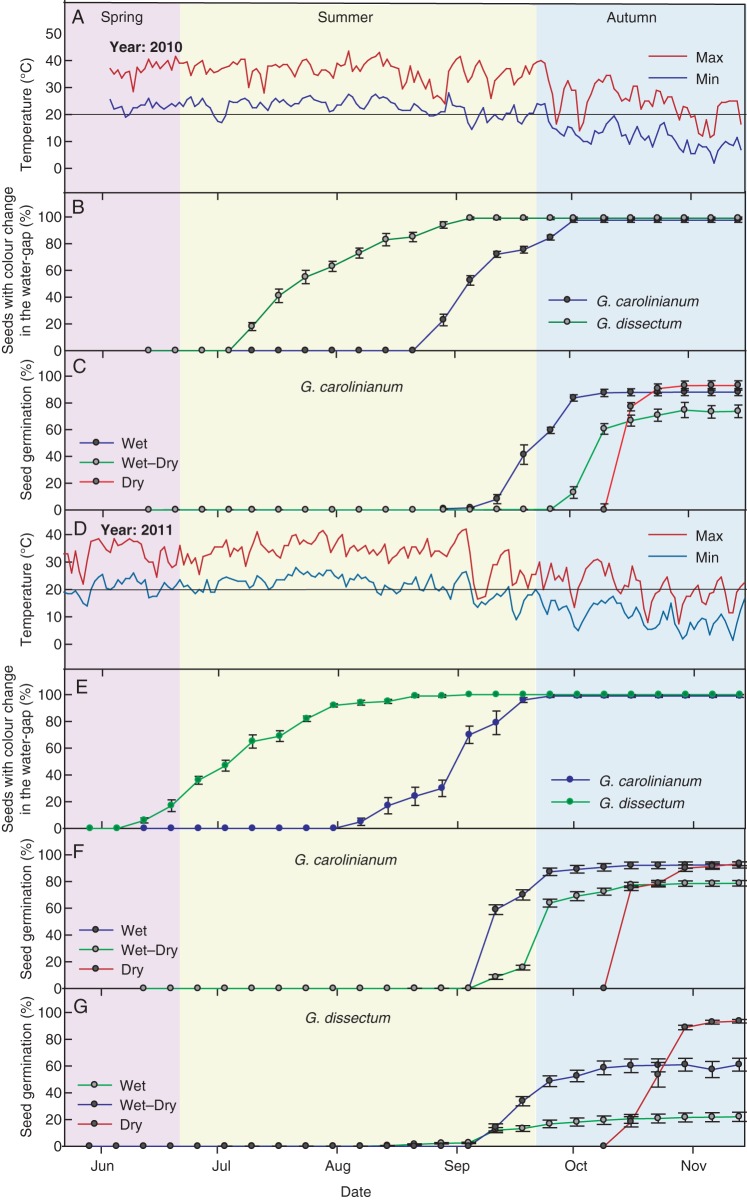
In 2010, the minimum temperature recorded inside the greenhouse during the first 12 weeks after sowing the seeds was 17·5 °C (Fig. 6A). During that period, no colour change was observed in the water-gap region of the seeds of G. carolinianum (GC 2010) (Fig. 6B). A colour change was first observed in 22 % of seeds in the last week of August, following a weekly minimum temperature of 19·5 °C (Fig. 6A, B). Thereafter, the percentage of seeds with colour change gradually increased during late summer to early autumn, coinciding with the decrease in temperature. By the first week of October (early autumn), all seeds showed a colour change.
Fig. 6.
PY-breaking of G. carolinianum and G. dissectum seeds under greenhouse conditions in 2010 and 2011. (A, D) Daily minimum and maximum air temperatures in the non-heated greenhouse in 2010 and 2011, respectively. Percentage (mean ± s.e.) of seeds with colour change from dark brown to brownish orange in the water-gap region of G. carolinianum and G. dissectum under dry conditions, (B) in 2010 and (E) 2011. Seed germination percentage (mean ± s.e) in G. carolinianum under the three moisture regimes in (C) 2010 and (F) 2011. (G) Seed germination percentage (mean ± s.e.) of G. dissectum in 2011 under the three moisture regimes.
In 2011, the minimum temperature recorded inside the greenhouse during the first 7 weeks after sowing the seeds was 17 °C (Fig. 6D). During that period, no colour change was observed in the water-gap region of the seeds of G. carolinianum (GC 2011) (Fig. 6E). A colour change was first observed in 5 % of seeds in the first week of August, following a weekly minimum temperature of 21 °C (Fig. 6D, E). Thereafter, the percentage of seeds with colour change gradually increased during late summer to early autumn, coinciding with the decrease in temperature. By the last week of September (end of summer), all the seeds showed a colour change.
In both years, the colour change in G. dissectum was first observed about 2 months earlier (i.e. early summer) than that in G. carolinianum. The percentage of seeds with colour change gradually increased during summer and reached 100 % by the end of summer (Fig. 6B, E).
Effect of soil moisture regime on breaking of PY
In both years, G. carolinianum kept under the constant wet and wet–dry moisture regimes began to germinate in early September (late summer) and reached maximum germination by early October (>80 %; early autumn) as the temperature began to decrease (Fig. 6C, F). However, germination under the wet–dry regime was lower than that under the constant wet regime. After watering in early October, seeds kept under the dry–moisture regime started to germinate within a week and reached >93 % by early November (Fig. 6C, F).
In G. dissectum (GD 2011), timing of germination under the three moisture regimes was similar to that of GC 2010 and GC 2011 (Fig. 6G). However, compared with G. carolinianum, germination of G. dissectum was lower under constant wet (22 %) and wet–dry (61 %) moisture regimes (Fig. 6G).
Breaking of PY under natural conditions
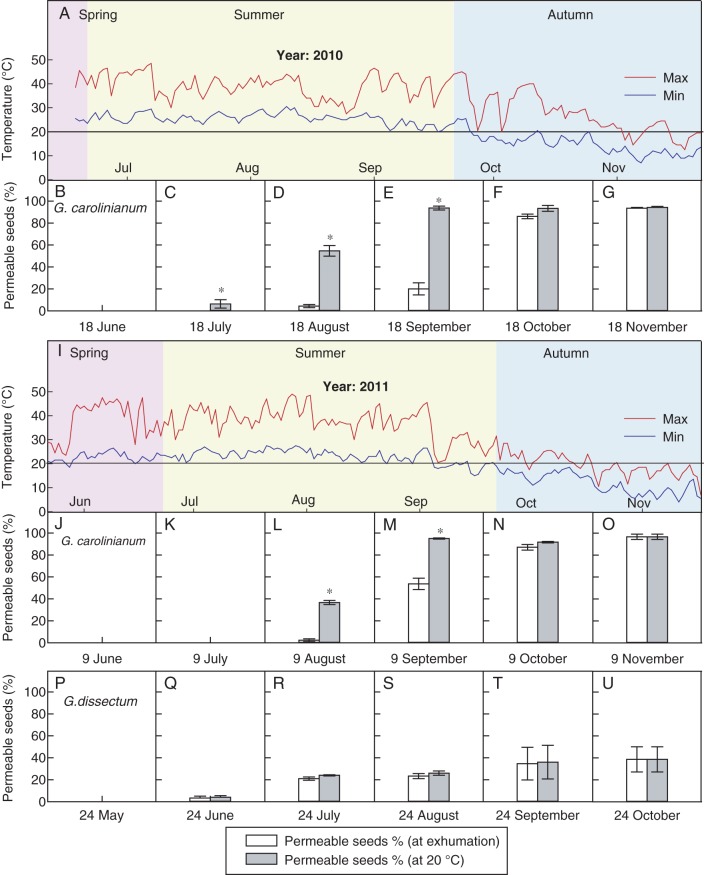
In G. carolinianum (GC 2010 and GC 2011), neither fresh seeds nor those exhumed after 1 month of burial (July) were permeable (Fig. 7B, C, J, K). When these seeds were transferred to 20 °C, only those that had been subjected to 1 month burial in 2010 became permeable (4 %). After transferring the seeds that had been buried for 2 (August) or 3 (September) months to 20 °C, a significant increase in the fraction of permeable seeds was observed (>36 and >94 %, respectively; Fig. 7D, E, L, M). Further, a considerable permeable fraction (>20 %) was observed in seeds exhumed after 3 months of burial (September) following the monthly minimum temperatures of 20 (2010) and 18 °C (2011) (Fig. 7A, E, I, M). More than 90 % of the seeds exhumed after 4 (October) or 5 (November) months of burial were permeable. However, transfer of seeds to 20 °C did not cause a significant increase in the permeable fraction (P < 0·05; Fig. 7F, G, N, O).
Fig. 7.
PY-breaking in buried seeds of G. carolinianum in 2010/11 and G. dissectum in 2011. (A, I) Daily minimum and maximum soil temperatures at 2 cm soil depth in 2010 and 2011, respectively. Percentage (mean ± s.e.) of permeable seeds of G. carolinianum of (B) fresh seeds and after incubation at 20 °C; (C–G) at the time of exhuming and after incubation at 20 °C, in 2010. Percentage (mean ± s.e.) of permeable seeds of G. carolinianum of (J) fresh seeds and after incubation at 20 °C; (K–O) at the time of exhuming and after incubation at 20 °C, in 2011. Percentage (mean ± s.e.) of permeable seeds of G. dissectum of (P) fresh seeds and after incubation at 20 °C; (Q–U) at the time of exhuming and after incubation at 20 °C, in 2011. An asterisk indicates a significant difference between treatments.
All the fresh seeds of G. dissectum (GD 2011) were impermeable and they did not become permeable even after incubation at 20 °C (Fig. 7P). During the 5 month burial period, the permeable fraction of seeds increased up to 39 % (Fig. 7Q–U). None of the samples exhumed during the burial period showed a significant increase in permeability after incubation at 20 °C (P < 0·05).
DISCUSSION
Storage of G. carolinianum seeds at constant temperatures ≥20 °C followed by incubation at 20/10 °C resulted in a significant increase in the permeable seed fraction compared with seeds incubated under constant temperatures (P < 0·05). This observation suggests the involvement of two steps in breaking of PY in G. carolinianum seeds. Insensitive seeds are made sensitive at temperatures ≥20 °C during step-I, followed by step-II, where sensitive seeds become permeable at lower temperatures (i.e. 20/10 °C). However, under alternating storage temperatures with the daily maximum >20 °C and minimum <20 °C (e.g. 25/15, 30/15 °C), seeds became permeable without the requirement for incubation at 20/10 °C. Also, under alternating temperatures in which the daily minimum was ≥20 °C (e.g. 30/20, 40/25 °C) a significantly lower percentage of seeds became permeable than at 20/10 °C (P < 0·05). Thus, it can be assumed that seeds achieve permeability when the alternating temperature itself satisfies the temperature requirements for the completion of step-I (by the daily maximum) and step-II (by the daily minimum). Therefore, the requirement for step-II is not necessarily an alternating temperature, but it has to be ≤20 °C.
Fresh insensitive seeds of G. carolinianum did not become permeable at any of the incubation temperatures (10–40 °C; Fig. 3A, D). Dry storage under 40/25 °C (mean summer soil temperatures) or constant 30 °C for 2 months induced sensitivity. However, they became permeable only at temperatures ≤20 °C during subsequent incubation. Four months of dry storage at the same temperatures increased the sensitivity of seeds. A significantly high percentage of seeds (>61) became permeable at temperatures ≤25 °C but not at temperatures ≥30 °C (Fig. 3C, F). In relation to the mean temperature at step-I, sensitive seeds responded to a temperature decrease rather than an increase in step-II. Thus, sensitive seeds do not require alternating temperatures to become permeable at step-II. Furthermore, the amplitude of the decline in temperature required in step-II decreases and eventually may reach zero as seeds become more and more sensitive with time. Therefore, during the progression from the less sensitive stage to the highly sensitive stage, the temperature range at which seeds can become permeable (completion of step-II) gradually increases from low to high.
Simulation of temperatures of each month during summer and autumn provides further evidence for the occurrence of two steps in the PY-breaking process of G. carolinianum. During the first 3 months of storage, step-I may be completed due to high summer temperatures. However, since seeds had low sensitivity during June (30/15 °C) and were not exposed to temperatures <20 °C during July and August (30/20 °C), step-II might have been suspended during the first 3 months of summer. After exposing sensitive seeds to autumn temperatures (25/15 and 20/10 °C), seeds became permeable upon the completion of step-II. Results were similar in the simulation of constant high temperatures of each month. In this temperature simulation experiment, the storage moisture condition (wet or dry) did not affect step-I or step-II in PY-breaking of G. carolinianum.
Based on the results of laboratory experiments, 20 °C was selected as the temperature threshold for the completion of step-II for greenhouse and burial experiments. Similar to laboratory experiments, seeds sown in the non-heated greenhouse that were exposed to hot summer temperatures showed the colour change in the water-gap (= PY-break) of G. carolinianum when the daily minimum temperature declined to ≤20 °C in mid to late summer. In early summer, those seeds did not respond to low temperatures, possibly because they were insensitive or less sensitive to low temperatures at that time.
Increase of sensitivity to low temperature (≤20 °C) in G. carolinianum was also observed in seeds buried in the soil. Seeds exhumed in early summer did not respond to 20 °C, while seeds exhumed during mid to late summer responded significantly (P < 0·05). Even though seeds were sensitive to low temperature during mid to late summer, a majority of them did not become permeable in the soil, since the minimum soil temperature remained >20 °C. When the minimum soil temperature dropped below 20 °C in early autumn, seeds became permeable.
Seeds of G. dissectum become permeable under temperatures ≥20 °C (dry) without the requirement for a subsequent low temperature (20/10 °C) treatment. Similarly, the colour change in the water-gap region (= PY-break) of seeds occurred much earlier in the non-heated greenhouse under dry conditions than in G. carolinianum in summer under high temperatures. These results suggest that in contrast to G. carolinianum, breaking of PY in G. dissectum involves only a single step under one temperature regime (≥20 °C).
According to temperature simulation and moisture regime experiments conducted in the laboratory and greenhouse, it is evident that seeds of G. dissectum achieve permeability regardless of the moisture conditions (dry or wet storage). However, the dry condition is much more effective than the wet condition in breaking of PY (Figs 4D, E and 5A, B). Also, the initial moisture treatment affects the PY-breaking behaviour. If the seeds are initially exposed to a wet period, subsequent drying can make a significant fraction of them permeable (Fig. 5C). On the other hand, exposure of seeds initially to a dry period followed by a wet period delays their becoming permeable until the next dry period (Fig. 5D). However, the similar fractions of permeable seeds observed under constant dry (after 4 weeks) and wet–dry (2 weeks wet followed by 2 weeks dry) storage indicate that during the wet period, seeds progress towards PY-break, possibly by becoming sensitive to drying (Fig. 5B, C).
During the 5 month period of burial in the soil at 2 cm depth, <40 % of G. dissectum seeds became permeable. It is possible that the moisture or the high relative humidity in soil delays permeability in >60 % of the seeds. Furthermore, Van Assche and Vandelook (2006) demonstrated that when immediately buried, fresh seeds of several species of Geraniaceae (including G. dissectum) remain impermeable until they are exposed to drying. This may be considered a moisture-dependent, conditional stepwise PY-breaking process (Table 2). However, we found that breaking of PY in G. dissectum can take place in a single step under any moisture regime although at a different rate. Therefore, induction of sensitivity in seeds under wet conditions may be an essential step for seeds buried in soil but not for the seeds lying on the soil surface since they are constantly exposed to dry conditions during summer. The requirement for drying for breaking of PY may be an environmental cue that makes seeds permeable only from the upper layers of soil during summer, while seeds in the deeper moist layers maintain a soil seed bank (Van Assche and Vandelook, 2006).
Table 2.
Life form, time of germination and conditions involved in the two-step model for breaking of PY
| Breaking of PY |
|||||
|---|---|---|---|---|---|
| Family/species | Life form | Germination | Step I | Step II | Reference |
| Convolvulaceae | |||||
| Cuscuta australis* | A | Summer | 15/6 °C (dry) | 35/20 °C (wet) | Jayasuriya et al. (2008a) |
| Ipomoea hederacea* | SA | Summer | ≥25 °C | 35/20 °C (dry) | Jayasuriya et al. (2009b) |
| Ipomoea lacunosa* | SA | Summer | ≥20 °C (wet) | 35 °C (RH >90 %) | Jayasuriya et al. (2008b) |
| Fabaceae | |||||
| Medicago polymorpha | WA | Autumn | 60/15 °C | 35/10 °C | Taylor (1996a, b) |
| Medicago lupulina | SA | Spring | 5 °C | 15/6 °C | Van Assche et al. (2003) |
| Melilotus albus* | B | Spring | 5 °C | 15/6 °C | Van Assche et al. (2003) |
| Ornithopus compressus* | WA | Autumn | 60/15 °C | 48/15 °C | Taylor and Revell (1999) |
| Trifolium subterraneum | WA | Autumn | ≥15 °C | 60/15 °C | Taylor (1981) |
| Geraniaceae | |||||
| Geranium carolinianum | WA | Autumn | ≥20 °C | ≤20 °C | This study |
| Erodium cicutarium | WA | Autumn | If seeds buried in soil (wet conditions) | Drying | Van Assche and Vandelook (2006) |
| Geranium columbinum | WA | Autumn | If seeds buried in soil (wet conditions) | Drying | Van Assche and Vandelook (2006) |
| Geranium dissectum† | WA | Autumn | If seeds buried in soil (wet conditions) | Drying | Van Assche and Vandelook (2006) |
| Geranium lucidum | WA | Autumn | If seeds buried in soil (wet conditions) | Drying | Van Assche and Vandelook (2006) |
| Geranium molle | WA | Autumn | If seeds buried in soil (wet conditions) | Drying | Van Assche and Vandelook (2006) |
| Geranium pusillum | WA | Autumn | If seeds buried in soil (wet conditions) | Drying | Van Assche and Vandelook (2006) |
A, Annual; B, Biennial; SA, summer annual; WA, winter annual; RH, relative humidity.
* Species known to have sensitivity cycling (sensitive ↔ insensitive).
† The present study shows that seeds of G. dissectum can become permeable under wet conditions without drying, hence they also have a one-step PY-breaking process.
Of the 18 families with PY (Baskin and Baskin, 1998; Nandi, 1998; Baskin et al., 2000, 2006; Baskin, 2003; Horn, 2004; Koutsovoulou et al., 2005), involvement of two steps in PY-breaking has been demonstrated only for Fabaceae and Convolvulaceae (Table 2). Also, Van Assche and Vandelook (2006) suggested the existence of a moisture-dependent, stepwise PY-breaking process in Geraniaceae. However, our study shows that seeds of G. dissectum do not behave in accordance with this pattern. Therefore, we suggest it to be considered a conditional two-step PY-breaking process.
Our study is the first report of a temperature-dependent, two-step PY-breaking process in Geraniaceae (G. carolinianum). Neither of the steps in the PY-breaking process of G. carolinianum were affected by the soil moisture regime, and both of them can be completed at constant temperatures (Table 2). Moreover, highly sensitive seeds may not require a second temperature condition to complete step-II. Therefore, it is possible that some seeds may become permeable without the second treatment.
Winter annuals in Fabaceae that have been reported as having a two-stage PY-breaking process require alternating temperatures in both step-I and step-II (except Trifolium subterraneum; only in step-II) to complete the process (Table 2). However, the effect of constant low temperatures in step-II has not been tested in the studies with Fabaceae species. Therefore, it is possible that those species may respond to the decline in temperature in early autumn below a certain threshold (as in G. carolinianum). PY-breaking of the summer annual Ipomoea lacunosa requires low temperatures in the first step and high temperatures in the second step, which is the reverse of the PY-breaking requirement in the winter annual G. carolinianum. This pattern may ensure that the PY-break takes place during spring and summer in I. lacunosa and during early autumn in G. carolinianum.
Cycling between sensitivity and insensitivity in PY-breaking previously has been reported in some species of Fabaceae and Convolvulaceae (Table 2). Neither seeds of G. carolinianum nor those of G. dissectum demonstrated such cycling behaviour. Thus, they depend on a stepwise PY-breaking behaviour in timing their germination.
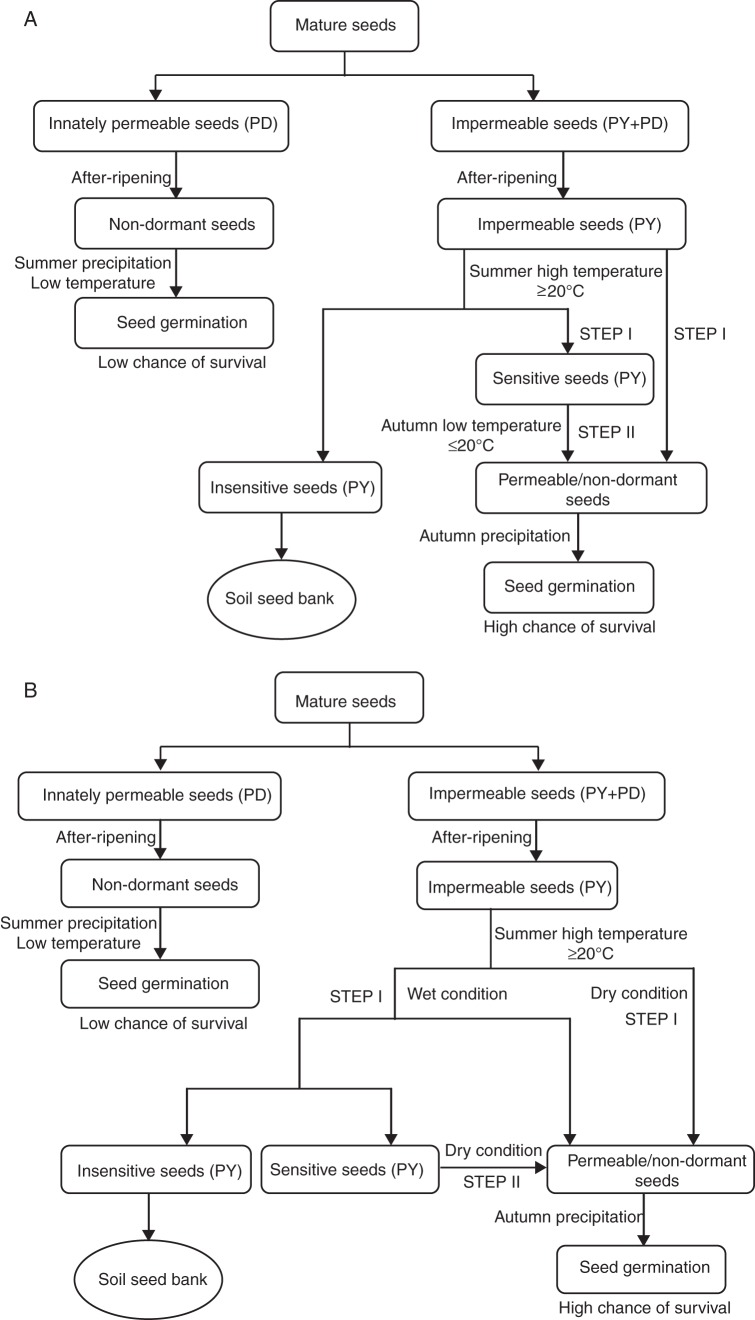
In G. carolinianum, breaking of PY involves two moisture-independent steps regulated by temperature (Fig. 8A). Low temperature is the environmental cue that triggers PY-breaking in late summer and subsequent germination in early autumn. In G. dissectum, PY-breaking takes place in early summer, either in two steps or in a single step, the number of steps being determined by the moisture condition of the environment (Fig. 8B). However, the inability of imbibed seeds to germinate under high temperatures delays germination until autumn (Supplementary Data Fig. S1). By means of these different dormancy-breaking strategies, seedling establishment in the two species is ensured to occur under favourable environmental conditions in autumn.
Fig. 8.
Conceptual models for breaking seed dormancy in (A) G. carolinianum and (B) G. dissectum.
SUPPLEMENTARY DATA
LITERATURE CITED
- Abbas HK, Tanaka T, Duke SO, Boyette CD. Susceptibility of various crop and weed species to AAL-toxin, a natural herbicide. Weed Technology. 1995;9:125–130. [Google Scholar]
- Aedo C. The genus Geranium L. (Geraniaceae) in North America. I. Annual species. Anales Jardín Botánico de Madrid. 2000;58:39–82. [Google Scholar]
- Aedo C, Garmendia FM, Pando F. World checklist of Geranium L. (Geraniaceae) Anales Jardín Botánico de Madrid56: 1998:211–252. [Google Scholar]
- Aedo C, Fiz O, Alarcon ML, Navarro C, Aldasoro JJ. Taxonomic revision of Geranium sect. Dissecta (Geraniaceae) Systematic Botany. 2005;30:533–558. [Google Scholar]
- Baskin CC. Breaking physical dormancy in seeds – focussing on the lens. New Phytologist. 2003;158:229–232. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Baskin JM, Baskin CC. Germination of winter annuals in July and survival of the seedlings. Bulletin of the Torrey Botanical Club. 1971;98:272–276. [Google Scholar]
- Baskin JM, Baskin CC. Some eco-physiological aspects of seed dormancy in Geranium carolinianum L. from central Tennessee. Oecologia. 1974;16:209–219. doi: 10.1007/BF00345883. [DOI] [PubMed] [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Baskin JM, Baskin CC, Li X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology. 2000;15:139–152. [Google Scholar]
- Baskin JM, Baskin CC, Dixon KW. Physical dormancy in the endemic Australian genus Stylobasium, a first report for the family Surianaceae (Fabales) Seed Science Research. 2006;16:229–232. [Google Scholar]
- Benvenuti S, Macchia M, Miele S. Quantitative analysis of emergence of seedlings from buried weed seeds with increasing soil depth. Weed Science. 2001;49:528–535. [Google Scholar]
- Dunn ST. Alien flora of Britain. Hatton Garden, London: West, Newman and Company; 1905. [Google Scholar]
- Gama-Arachchige NS, Baskin JM, Geneve RL, Baskin CC. Identification and characterization of the water gap in physically dormant seeds of Geraniaceae, with special reference to Geranium carolinianum. Annals of Botany. 2010;105:977–990. doi: 10.1093/aob/mcq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granstrom A, Schimmel J. Heat-effects on seeds and rhizomes of a selection of boreal forest plants and potential reaction to fire. Oecologia. 1993;94:307–313. doi: 10.1007/BF00317103. [DOI] [PubMed] [Google Scholar]
- Horn JW. The morphology and relationships of the Sphaerosepalaceae (Malvales) Botanical Journal of the Linnean Society. 2004;144:1–40. [Google Scholar]
- Jayasuriya KMGG, Baskin JM, Baskin CC. Cycling of sensitivity to physical dormancy-break in seeds of Ipomoea lacunosa (Convolvulaceae) and ecological significance. Annals of Botany. 2008;101:341–352. doi: 10.1093/aob/mcm285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasuriya KMGG, Baskin JM, Geneve RL, Baskin CC, Chien CT. Physical dormancy in seeds of the holoparasitic angiosperm Cuscuta australis (Convolvulaceae, Cuscuteae): dormancy-breaking requirements, anatomy of the water gap and sensitivity cycling. Annals of Botany. 2008;102:39–48. doi: 10.1093/aob/mcn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasuriya KMGG, Baskin JM, Baskin CC. Sensitivity cycling and its ecological role in seeds with physical dormancy. Seed Science Research. 2009;19:3–13. [Google Scholar]
- Jayasuriya KMGG, Baskin JM, Geneve RL, Baskin CC. Sensitivity cycling and mechanism of physical dormancy break in seeds of Ipomoea hederacea (Convolvulaceae) International Journal of Plant Sciences. 2009;170:429–443. [Google Scholar]
- Koutsovoulou K, Vassiliades D, Yannitsaros A, Thanos CA. Seed germination of Bieberstenia orphanidis Boiss. Proceedings of the Greek Botanical Society. 2005;10:331–337. (in Greek with English abstract) [Google Scholar]
- Macbride JF. Flora of Peru. Chicago, IL: Field Museum Press; 1949. [Google Scholar]
- McCready GA, Cooperrider TS. The Geraniaceae of Ohio. Castanea. 1984;49:138–141. [Google Scholar]
- Meisert A. Physical dormancy in Geraniaceae seeds. Seed Science Research. 2002;12:121–128. [Google Scholar]
- Mueller BFV. The plants of New South Wales. Sydney: Thomas Richard, Government Printer; 1885. [Google Scholar]
- Nandi OI. Ovule and seed anatomy of Cistaceae and related Malvanae. Plant Systematics and Evolution. 1998;209:239–264. [Google Scholar]
- Nishida T, Yamashita N. Developing a pre-entry weed risk assessment system for use in Japan. Biological Invasions. 2009;11:1319–1333. [Google Scholar]
- Peng CI. Some new records for the flora of Taiwan. Botanical Bulletin of Academia Sinica. 1978;19:83–86. [Google Scholar]
- Piper CV. XI. Smithsonian Institution: Washington, DC, 379; 1906. Flora of the state of Washington. Contributions from the United States National Herbarium. [Google Scholar]
- Piper CV, Beattie RK. Flora of the northwest coast. Lancaster, PA: New Era Printing Company; 1915. [Google Scholar]
- Rhoads AF, Block TA. The plants of Pennsylvania. Philadelphia, PA: University of Pennsylvania Press; 2007. [Google Scholar]
- Small JK. North American Flora. Bronx Park, NY: The New York Botanical Garden; 1907. [Google Scholar]
- Taylor GB. Effect of constant temperature treatments followed by fluctuating temperatures on the softening of hard seeds of Trifolium subterraneum L. Australian Journal of Plant Physiology. 1981;8:547–558. [Google Scholar]
- Taylor GB. Effect of the environment in which seeds are grown and softened on the incidence of autumn seed softening in two species of annual medics. Australian Journal of Agricultural Research. 1996;47:141–159. [Google Scholar]
- Taylor GB. Incidence and measurement of autumn seed softening within Medicago polymorpha L. Australian Journal of Agricultural Research. 1996;47:575–586. [Google Scholar]
- Taylor GB. Hardseededness in Mediterranean annual pasture legumes in Australia: a review. Australian Journal of Agricultural Research. 2005;56:645–661. [Google Scholar]
- Taylor GB, Revell CK. Effect of pod burial, light, and temperature on seed softening in yellow serradella. Australian Journal of Agricultural Research. 1999;50:1203–1209. [Google Scholar]
- Van Assche JA, Vandelook FEA. Germination ecology of eleven species of Geraniaceae and Malvaceae, with special reference to the effects of drying seeds. Seed Science Research. 2006;16:283–290. [Google Scholar]
- Van Assche JA, Debucquoy KLA, Rommens WAF. Seasonal cycles in the germination capacity of buried seeds of some Leguminosae (Fabaceae) New Phytologist. 2003;158:315–323. [Google Scholar]
- Wilson MV, Clark DL. Controlling invasive Arrhenatherum elatius and promoting native prairie grasses through mowing. Applied Vegetation Science. 2001;4:129–138. [Google Scholar]
- Xu L, Aedo C. Geraniaceae. In: Wu ZY, Raven PH, Hong DY, editors. Flora of China. Vol. 11. Beijing: Science Press/St Louis, MO: Missouri Botanical Garden Press; 2008. pp. 8–31. (Oxalidaceae through Aceraceae) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.