Abstract
Background and Aims
The dwarf shrub Cassiope tetragona (Arctic bell-heather) is increasingly used for arctic climate reconstructions, the reliability of which depends on the existence of a linear climate–growth relationship. This relationship was examined over a high-arctic to sub-arctic temperature gradient and under multi-year artificial warming at a high-arctic site.
Methods
Growth chronologies of annual shoot length, as well as total leaf length, number of leaves and average leaf length per year, were constructed for three sites. Cassiope tetragona was sampled near its cold tolerance limit at Ny-Ålesund, Svalbard, at its assumed climatic optimum in Endalen, Svalbard, and near its European southern limit at Abisko, Sweden. Together these sites represent the entire temperature gradient of this species. Leaf life span was also determined. Each growing season from 2004 to 2010, 17 open top chambers (OTCs) were placed near Ny-Ålesund, thus increasing the daily mean temperatures by 1·23°C. At the end of the 2010 growing season, shoots were harvested from OTCs and control plots, and growth parameters were measured.
Key Results
All growth parameters, except average leaf length, exhibited a linear positive response (R2 between 0·63 and 0·91) to mean July temperature over the temperature gradient. Average leaf life span was 1·4 years shorter in sub-arctic Sweden compared with arctic Svalbard. All growth parameters increased in response to the experimental warming; the leaf life span was, however, not significantly affected by OTC warming.
Conclusions
The linear July temperature–growth relationships, as well as the 7 year effect of experimental warming, confirm that the growth parameters annual shoot length, total leaf length and number of leaves per year can reliably be used for monitoring and reconstructing temperature changes. Furthermore, reconstructing July temperature from these parameters is not hampered by divergence.
Keywords: Cassiope tetragona, climate proxy, ‘divergence problem’, experimental warming, growth variables, high- to sub-arctic, North Sweden, Svalbard, temperature reconstruction
INTRODUCTION
The arctic climate is characterized by a large variability in time and space, but the scarcity of long-term, high-resolution proxy and instrumental climate records restricts our knowledge of past arctic climate change (McBean et al., 2005). To increase our understanding of the drivers of arctic climatic variability, a more uniform spatial coverage of past climate is needed (ACIA, 2005). Such estimates can be obtained from proxy records, such as ice cores, tree rings and varved sediments. In treeless environments, such as the high arctic and at high altitudes, shrubs and dwarf shrubs provide an alternative for trees, and their dendrochronological potential for monitoring and reconstructing environmental and climate changes is increasingly studied (Woodcock and Bradley, 1994; Zalatan and Gajewski, 2006; Bär et al., 2008; Liang and Eckstein, 2009; Hallinger et al., 2010; Rayback et al., 2010; Schmidt et al., 2010; Blok et al., 2011; Liang et al., 2012).
Previous studies demonstrate that various annual growth parameters of the dwarf shrub Cassiope tetragona may provide high-resolution, arctic-wide climate proxy records (Callaghan et al., 1989; Havström et al., 1993, 1995; Johnstone and Henry, 1997; Aanes et al., 2002; Rayback and Henry, 2005, 2006; Welker et al., 2005; Rozema et al., 2009; Weijers et al., 2010).
If growth is, however, no longer temperature limited in the sub-arctic, as reported by Havström et al. (1993), linear growth temperature transfer functions might lead to less reliable temperature estimates, especially at the higher temperature margin of the geographical range of C. tetragona. Therefore, it is important to test the climate–growth association over wide temperature ranges and in long-term experimental settings, to assess whether this association is both temporally and spatially stable. Here, we test the climate–growth association in C. tetragona over three sites along a high- to sub-arctic European climatic gradient through the development of growth chronologies, and linear regression analyses between these chronologies and monthly climate parameters. We test whether the climate–growth relationship in C. tetragona is fully linear over the broad temperature range. Furthermore, the response of C. tetragona to 7 years (2004–2010) of artificial warming in open top chambers (OTCs) at a high arctic site was analysed to assess whether the growth response of C. tetragona to OTC warming in Svalbard, as reported by Rozema et al. (2009), is persistent over 7 years of experimental warming.
MATERIALS AND METHODS
Study plants
Cassiope tetragona has a circumarctic distribution and a prostrate growth habit. Annual growth parameters can be measured, as it forms smaller leaves at the beginning and end of each growing season, resulting in annual wave-like leaf patterns (Warming, 1908). Similarly, leaf scars are grouped closely together at each year boundary and, from the measurement of leaf scar distances, the annual number of leaves and shoot length can be derived (e.g. Rayback and Henry, 2006). Dead leaves may remain attached to the stems for up to 45 years and leaf scars can stay visible for periods exceeding 100 years. The relatively recent discovery of wintermarksepta (WMS) within C. tetragona stems has enabled the measurement of annual shoot length further back in time (>180 years; Weijers et al., 2010). Wintermarksepta consist of dark bands in the pith of the stems, which coincide with lows in leaf length and leaf scar distances, thereby demarking annual shoot length growth (Rozema et al., 2009; Weijers et al., 2010).
Study sites and sampling
Ny-Ålesund
For the construction of growth chronologies, 21 plant samples were collected on 21 August 2010, from apparently old C. tetragona stands [with the presence of long (>50 cm) shoots] about 5·5 km south-east of Ny-Ålesund (Table 1). The area is at the border between the middle arctic tundra zone (zone C), characterized by C. tetragona on leesides, and the northern arctic tundra zone (zone B), where C. tetragona is absent (Elvebakk, 1999; Walker et al., 2005). The stands were found on small, bowl-shaped, northeast-facing slopes, on a rocky plateau.
Table 1.
Environmental characteristics of the four research sites, climatic data from 1979–2008
| High Arctic |
Sub-Arctic |
|||
|---|---|---|---|---|
| Ny-Ålesund OTCs | Ny-Ålesund chronology | Endalen | Abisko | |
| Altitude (m asl) | 25 | 35 | 100 | 500 |
| Latitude N | 78°54′ | 78°54′ | 78°11′ | 68°20′ |
| Longitude E | 11°58′ | 12°10′ | 15°44′ | 18°51′ |
| Arctic sub-zone | Border B and C | Border B and C | C | E |
| Mean June temperature (°C) | 2·08 | 2·08 | 2·76 | 8·76 |
| Mean July temperature (°C) | 5·14 | 5·14 | 6·43 | 11·72 |
| Mean August temperature (°C) | 4·19 | 4·19 | 5·37 | 10·17 |
| Mean summer temperature (°C) | 3·81 | 3·81 | 4·85 | 10·22 |
| Mean annual precipitation sum (mm) | 408 | 408 | 190 | 337 |
| Species composition | Dominated by C. tetragona (about 10–15 % cover), Salix polaris (5–10 %) and the moss Sanionia uncinata (10–20 %). Saxifraga oppositifolia, Dryas octopetala and Oxyria digina are present, but less abundant (Rozema et al., 2006) | Mixed patches of C. tetragona, Dryas octopetala and Salix polaris, patches in which either C. tetragona or S. Polaris is dominant and individuals of Saxifraga oppositifolia among others growing outside these patches | C. tetragona (30 % cover), Salix polaris and Dryas octopetala. Patches of Empetrum nigrum s.l. and Betula nana are also present (Weijers et al., 2010) | Relatively rich dwarf shrub vegetation with patches of C. tetragona, Empetrum nigrum s.l., Vaccinium uliginosum, Rhododendron lapponicum and Salix hastata (Havström et al., 1993) |
| Number of plants analysed | 36 (2 × 18) | 21 | 32 | 12 |
The division of the Arctic into sub-zones is after Elvebakk (1999) and Walker et al. (2005).
Our research site for experimental warming is situated about 2 km southeast of Ny-Ålesund (Table 1), on an elevated plane at the south bank of Kongsfjorden. In total, 34 plots were marked. Half of them were randomly chosen as control plots. For the other half, temperature was increased by placing hexagonal OTCs with a diameter of 2·2 m and height of 50 cm (Marion et al., 1997). The OTCs were installed at the onset of each growing season (mid June) and removed at the end of the growing season (late August) in the years 2004–2010. In this way, snow accumulation in the OTCs during winter (Marion et al., 1997; Aerts et al., 2004) and undesired side effects, e.g. delayed snowmelt, are prevented. From each plot, three approx. 10 cm long C. tetragona shoots were randomly harvested on 19 August 2010. Afterwards, individual leaf lengths, leaf scar distances and WMS distances of 18 control shoots and 18 OTC shoots were measured.
Endalen
In June and August 2008 and in late August 2009, 40 plant samples were collected from the southeast-facing slope of the valley Endalen situated about 4 km southeast of Longyearbyen (Table 1). The site is located in the middle arctic tundra zone (zone C). It is among the warmest sites in Svalbard, where scattered occurrences of some low arctic taxa such as Betula nana and Empetrum nigrum sensu lato (s.l.) are also found.
Abisko
At the end of August 2009, 12 plant samples were collected approx. 2 km south-southeast of Abisko (Table 1) for the construction of growth chronologies. The site is characterized by a rich dwarf shrub community.
Climate data
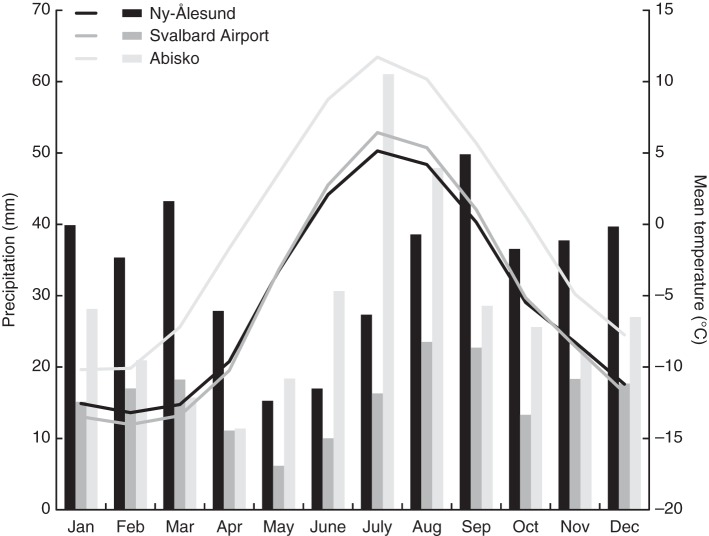
Information on the climate data sources used for each site and period, and weather stations are summarized in Table 2. Thirty year average (1979–2008) monthly precipitation sums and mean monthly temperatures for each site are plotted in Fig. 1.
Table 2.
Sources of the monthly climate parameters for each site and related weather station information
| Site | Data type (°C or mm) | Period | Station (code) | Location (N, E) | Altitude (m asl) | Source |
|---|---|---|---|---|---|---|
| Ny-Ålesund | Mean temperature and precipitation sums | January 1969–July 1974 | Ny-Ålesund (99900) | 78°55′, 11°52′ | 42 | eKlima database (DNMI, 2011) |
| Mean temperature and precipitation sums | August 1974–2011 | Ny-Ålesund (99910) | 78°55′, 11°55′ | 8 | eKlima database (DNMI, 2011) | |
| Endalen | Precipitation sums | 1958–1975 | Longyearbyen (99860) | 78°13′, 15°21′ | 37 | eKlima database (DNMI, 2011) |
| Mean temperature | 1912–July 1975 | Homogenized Svalbard Airport record (Nordli and Kohler, 2003) | 78°14′, 15°28′ | 8 | NORDKLIM | |
| Mean temperature and precipitation sums | August 1975–2011 | Svalbard Airport (99840) | 78°14′, 15°28′ | 8 | eKlima database (DNMI, 2011) | |
| Abisko | Mean temperature and precipitation sums | 1913–2009 | Abisko (18880) | 68°21′, 18°49′ | 388 | Abisko Scientific Research Station |
Fig. 1.
Thirty year average (1979–2008) monthly precipitation sums (bars) and monthly temperatures (lines) for each site.
Temperatures were monitored simultaneously in OTCs and control plots near Ny-Ålesund during three consecutive growing seasons with loggers (Tinytag Transit H temperature loggers; Gemini Data Loggers, Chichester, UK). Temperatures were measured in the centre of the plots every 15 min (2008) or hourly (2009 and 2010). The temperature loggers were placed at vegetation height, about 5 cm above soil level, underneath plastic shelters to avoid overestimation of temperatures due to direct solar radiation and damage by precipitation. At the start of the growing season in 2008, six loggers were placed in control plots and seven in OTC plots. In 2009, loggers were placed in two control plots and in one OTC, whereas in 2010, loggers were placed in four control plots and in four OTCs.
Measurement of annual growth parameters
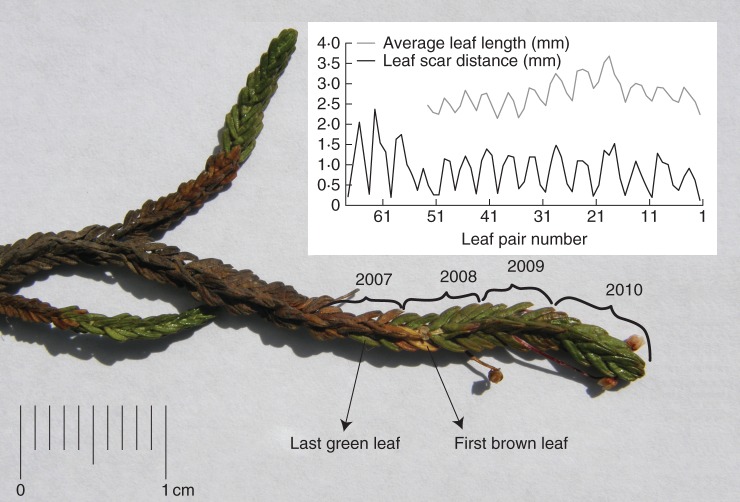
Measurement of total leaf length, number of leaves and average leaf length is time consuming and was performed on a single branch per harvested plant sample only. The leaves were removed from the top to the base of the stems (to keep track of their exact order) and their lengths were subsequently measured under ×30 magnification with a 0·1 mm precision (Fig. 2). Thereafter, the distances between leaf scars on one side of the stems were measured (×30 magnification, 0·1 mm precision, Fig. 2). Finally a thin layer of the stem was laterally removed with a scalpel to reveal the pith and the WMS therein. Subsequently, WMS distances (annual shoot length) were measured (×10 magnification, 0·1 mm precision). For further details on this method, see Rozema et al. (2009) and Weijers et al. (2010).
Fig. 2.
A branch of Cassiope tetragona. The separate annual leaf cohorts are indicated. Inset: average leaf length and leaf scar distance per leaf pair from old to young, with each wave representing 1 year of growth.
Average leaf lengths were then calculated per pair, using either one or both of the leaves of each pair, as some pairs were incomplete (especially those on the older part of the shoots). The first leaf pair formed during a growing season is usually longer than the last of the previous season. Similarly, the shortest distance between leaf scars generally demarks the growing season end (Fig. 2). In this study, the annually summed leaf scar distances of each stem were plotted against the accompanying WMS distances, as a reference for the appointment of the exact year boundaries. Thereafter, the year boundaries for the average leaf lengths per pair were derived from those of the leaf scar distances. The average leaf lengths per pair between each boundary were then summed and multiplied by two, to calculate the total leaf length per year. Only years with at least one leaf of each pair present were taken into account. The number of leaves per year was derived from the number of leaf scars per year. Annual total leaf lengths divided by the number of leaves results in the annual average leaf length.
Chronology construction
Shoot length
Cassiope tetragona is a strongly branched species, and parts of the shrubs, especially older ones, may be missing, resulting in incomplete shrub chronologies (see Weijers et al., 2010). Therefore, annual shoot length measurement was carried out on multiple stems per shrub, to create as complete shoot length chronologies as possible for each shrub. Individual branch chronologies were initially plotted and visually cross-dated with branch chronologies of the same plant. Subsequently, a number of complete shrub chronologies were created for each site by averaging the individual branch chronologies of one shrub into a single chronology. These shrub chronologies were then averaged per site to create a master chronology for each site. The remaining individual branch chronologies of incomplete individuals could then be visually cross-dated against these master chronologies. Visual cross-dating was possible, because branches display similar growth patterns to branches of the same individual and others from the same site (see Weijers et al., 2010). After cross-dating, individual branch chronologies were averaged per shrub, before the construction of site chronologies, to prevent over-representation of single shrubs.
To separate reliable parts of the shoot length chronologies from parts with too low a sample size, the sub-sample signal strength (SSS; see Wigley et al., 1984) was calculated. The SSS is a parameter based on sample size and the average correlation between individual chronologies (rbar) and is a measure for the amount of signal captured by a sub-set of samples from the master chronology. We used the individual shrub chronologies to calculate rbar in ARSTAN (Cook, 1985). Parts of the site chronologies with SSS values below the generally accepted threshold of 0·85 (Wigley et al., 1984) were disregarded. Autocorrelation within the effective (SSS >0·85) standardized and raw site chronologies was calculated in PASW Statistics 17·0. Furthermore, mean sensitivity values, a measure for the year to year variability within growth chronologies (Fritts, 1976), were calculated.
Leaf parameters
As leaves only remain attached to the stems of C. tetragona for a limited time (up to 45 years), leaf parameter chronologies are much shorter than the shoot length chronologies. Site chronologies for each parameter were created by averaging each parameter per year per site.
Standardization
The shoot length chronologies are characterized by high autocorrelation, probably caused by juvenile growth trends (Rayback and Henry, 2006) and the leaf life span of several years of C. tetragona leaves (Weijers et al., 2010). Juvenile growth trends result from relatively slow growth during the first years of C. tetragona branches. Standardization of growth chronologies is necessary to remove such age-related autocorrelation. Shoot length chronologies were standardized by dividing the individual branch series with a horizontal line through their mean. This conservative method successfully removes autocorrelation attributable to previous growth, while leaving the temperature signal intact (Weijers et al., 2010). For this study and for Weijers et al. (2010), many other standardization methods were tested. However, all of those led to a partial loss of the temperature signal. To show the effect of such less conservative methods, the individual branch series from Endalen were detrended with a 32 year cubic spline, a commonly used method in dendrochronology.
After standardization, the individual branch chronologies were again first averaged per shrub, and then per site to form standardized site chronologies. Standardization was carried out in program ARSTAN, version 41d_XP, created by E.R. Cook and P.J. Krusic, Tree-Ring Laboratory, Palisades, New York, USA (Cook, 1985).
Leaf life span
Leaf senescence is slow and gradual in C. tetragona. Leaves can remain (partly) green for several years before they turn red, brown and then finally grey. In this study, the leaf life span was defined as the average between the number of years before the present in which the first brown leaf appears and the number of years before the present in which the last (partly) green leaf appears (Fig. 2).
Climate–growth analysis
Pearson correlation coefficients were calculated between the raw/standardized shoot length chronologies and local monthly climate data from January of the previous year through to October of the current year for the three sites, to pinpoint the most dominant factor(s) determining C. tetragona growth at each site. Subsequently, Pearson correlation coefficients were calculated between the most dominant monthly climate parameter across all sites and raw growth. In addition, monthly climate data and previous growth parameters that correlated significantly with the chronologies were used in a forward stepwise multiple regression as predictors to create growth–climate models for the three raw shoot length chronologies. Predictors were entered in the models in order of the strength of their correlation with the current year's growth (from strong to weak). Only predictors contributing significantly (P < 0·05) to the models in the presence of other predictors were retained.
Statistical comparison of OTCs vs. controls
Temperature differences between controls and OTCs were tested for significance with a paired samples t-test over three different time intervals: 24 h (0000–2400 h), 12 h (0600–1800 h) and 4 h (1000–1400 h). Differences between growth in controls and OTCs were assessed with one-way analyses of variance (ANOVAs) for each individual year, with treatment as between-subject factor. Furthermore, growth differences over five consecutive years without and 7 years with OTC warming were assessed with one-way ANOVAs with repeated measurements, with treatment as between-subject factor and time as within-subject factor. Average annual absolute differences in growth over the period of experimental warming were calculated and tested with paired samples t-tests. All tests were executed in PASW Statistics 17·0.
RESULTS
Climate difference
Although geographically relatively close to Endalen, represented by the Svalbard Airport weather station, the Ny-Ålesund climate record shows lower 30 year average June, July and August temperatures than in Endalen (Fig. 1, Table 1). Sub-arctic Abisko is substantially warmer than both sites in Svalbard (1979–2008; Table 1). All sites are relatively dry. Ny-Ålesund receives the most precipitation annually, although Abisko is more humid during summer (Fig. 1, Table 1). Endalen is the driest site.
Chronology development
We constructed four annually resolved growth chronologies (shoot length, total leaf length, number of leaves and average leaf length) for each site. All chronologies are new, except the Endalen shoot length chronology, which was previously used for July temperature reconstruction (Weijers et al., 2010).
The shoot length chronologies were 154, 169 and 49 years long for Ny-Ålesund, Endalen and Abisko, respectively (Table 3). The chronologies are characterized by intermediate (Endalen; 0·284) to high (Ny-Ålesund and Abisko; 0·360 and 0·353, respectively) mean sensitivity values (Grissino-Mayer, 2001) and intermediate (0·248–0·397) mean interseries correlation coefficients (rbar).
Table 3.
Statistical characteristics of the standardized (horizontal line through the mean) and raw shoot length chronologies for each site
| Ny-Ålesund | Endalen | Abisko | |
|---|---|---|---|
| Chronology length | 1857–2010 | 1840–2008 | 1961–2009 |
| Number of years | 154 | 169 | 49 |
| Sample size (plants/branches) | 21/111 | 32/213 | 12/74 |
| Average segment length (branches) (years) | 31 | 23·5 | 10·53 |
| Average time span plant chronologies (years) | 104·38 | 114·25 | 32·75 |
| Mean (mm mm−1 or mm) | 0·993 (3·529) | 1·00 (5·05) | 0·988 (9·267) |
| Standard deviation (mm mm−1 or mm) | 0·443 (1·562) | 0·342 (1·767) | 0·386 (3·593) |
| Mean sensitivity | 0·360 | 0·284 | 0·353 |
| First-order autocorrelation | 0·353** (0·669**) | 0·321** (0·662**) | 0·591* (0·730**) |
| rbar | 0·321 (0·397) | 0·276 (0·261) | 0·248 (0·315) |
| Effective chronology length, where SSS >0·85 | 1895–2010 (1881–2010) | 1876–2008 | 1980–2009 (1978–2009) |
| Minimal sample size, where SSS >0·85 | 7 (6) | 10 (11) | 7 (6) |
Values for the raw chronologies are placed in parentheses when different from those of the standardized chronologies. The values for Endalen are after Weijers et al. (2010). SSS is the sub-sample signal strength (Wigley et al., 1984) and rbar is the mean interseries correlation coefficient. Significance levels: **P < 0·0001; *P = 0·001.
The Abisko shrubs were shorter and younger than those from Svalbard, resulting in a relatively short shoot length chronology. First-order autocorrelation in the shoot length chronologies remained significant after standardization.
Statistics of the leaf parameter chronologies and their correlation with the local shoot length chronologies are summarized in Table 4. The largest average total leaf length and number of leaves were found in Abisko, and the smallest in Ny-Ålesund. Average leaf lengths were, in contrast, the longest in Ny-Ålesund. The total leaf length and number of leaves were positively correlated with shoot length at all sites. Average leaf length was only positively correlated with shoot length at Ny-Ålesund.
Table 4.
Summarized statistics of the leaf parameter chronologies for each site
| Ny-Ålesund | Endalen | Abisko | |
|---|---|---|---|
| Total leaf length | |||
| Chronology length | 1967–2010 | 1971–2008 | 1996–2009 |
| Effective chronology length | 1986–2010 | 1983–2008 | 2002–2009 |
| Sample size (no. of branches) | 19 | 20 | 12 |
| Minimal samples size | 8 | 8 | 9 |
| Average length (years) | 24·6 | 20·5 | 9·8 |
| Mean (mm) | 37·0 | 42·8 | 81·4 |
| Standard deviation (mm) | 14·4 | 15·2 | 29·0 |
| r/R2 (vs. shoot length) | 0·93/0·86** | 0·68/0·46** | 0·91/0·83* |
| Number of leaves | |||
| Chronology length | 1957–2010 | 1968–2008 | 1992–2009 |
| Effective chronology length | 1981–2010 | 1980–2008 | 1999–2009 |
| Sample size (no. of branches) | 19 | 20 | 12 |
| Minimal samples size | 10 | 8 | 9 |
| Average length (years) | 31·4 | 25·2 | 12·5 |
| Mean (n) | 9·31 | 11·6 | 22·6 |
| Standard deviation (n) | 2·52 | 2·6 | 5·5 |
| r/R2 (vs. shoot length) | 0·93/0·87** | 0·83/0·68** | 0·91/0·83** |
| Average leaf length | |||
| Chronology length | 1967–2010 | 1971–2008 | 1996–2009 |
| Effective chronology length | 1986–2010 | 1983–2008 | 2002–2009 |
| Sample size (no. of branches) | 19 | 20 | 12 |
| Minimal samples size | 8 | 8 | 9 |
| Average length (years) | 24·6 | 20·5 | 9·8 |
| Mean (mm) | 3·73 | 3·57 | 3·48 |
| Standard deviation (mm) | 0·70 | 0·64 | 0·55 |
| r/R2 (vs. shoot length) | 0·80/0·64** | 0·37/0·14NS | –0·37/0·14NS |
NS, not significant; *P = 0·002; **P < 0·001.
Climate–growth analysis
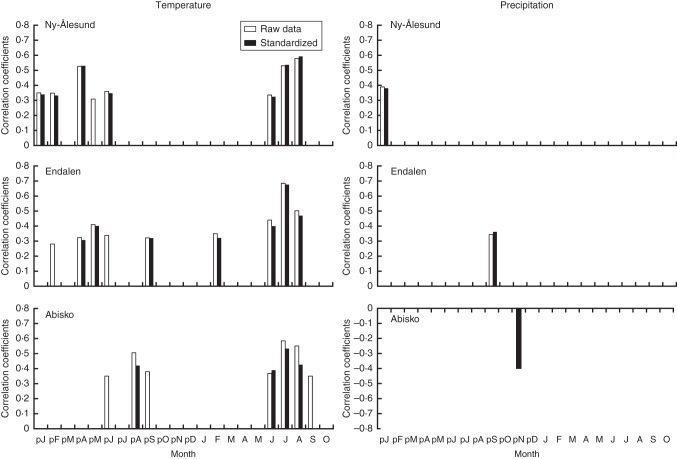
Shoot length was most strongly correlated with current growing season temperatures. June, July and August temperature correlated positively with raw and standardized growth at all sites (Fig. 3). Overall, July temperature appears to be the most important factor determining growth, as it showed the strongest correlation with growth at Endalen (raw, r = 0·685, P < 0·0001; standardized, r = 0·676, P < 0·0001) and Abisko (raw, r = 0·585, P < 0·001; standardized, r = 0·533, P = 0·002), and the second strongest after August temperature at Ny-Ålesund (raw, r = 0·530, P = 0·0004; standardized, r = 0·537, P = 0·0003).
Fig. 3.
Correlations between the raw (grey bars)/standardized (black bars) annual shoot length chronologies and monthly mean temperature (left graphs) and monthly precipitation sums (right graphs) from previous January (pJ) through current October (O). The climate data for Ny-Ålesund covers the period 1969–2010 (n = 41), for Endalen the period 1958–2008 (n = 50), for Abisko (raw measurements) the period 1978–2009 (n = 32) and for Abisko (standardized chronology) the period 1980–2009 (n = 30); significant coefficients are shown (P < 0·05, two-sided).
The significant correlations between shoot length growth and monthly climatic parameters outside the growing season were generally weaker than those during the season. Only a few monthly precipitation sums correlated significantly with growth.
The models resulting from the forward stepwise multiple regression analyses explaining raw shoot length growth with monthly climate data and previous growth as predictors are listed in Table 5. Results from the Pearson correlation analyses were confirmed with mean August temperature as the strongest climatic predictor at Ny-Ålesund and mean July temperature as the strongest climatic predictor at Endalen and Abisko. Previous growth of 5 and 6 years, of 1, 2 and 5 years, and of 1 year prior to current growth, contributed to current shoot length growth at Ny-Ålesund, Endalen and Abisko, respectively.
Table 5.
Statistics of the growth–climate models resulting from forward stepwise multiple regression analyses with significantly correlated monthly climate data and previous growth parameters as predictors of the current year's raw shoot length at the three sites
| Unstandardized coefficients | Standardized coefficients | |||||||
|---|---|---|---|---|---|---|---|---|
| Location | Model | B | s.e. | Beta | t | Significance | R2 | R2-adjusted |
| Ny-Ålesund | (Constant) | –1·690 | 0·878 | –1·926 | 0·063 | 0·65 | 0·62 | |
| AugustT | 0·849 | 0·191 | 0·485 | 4·455 | 0·000 | |||
| Growtht=–5 | 0·329 | 0·124 | 0·312 | 2·658 | 0·012 | |||
| Growtht=–6 | 0·328 | 0·124 | 0·308 | 2·649 | 0·012 | |||
| Endalen | (Constant) | –1·028 | 0·510 | –2·018 | 0·047 | 0·70 | 0·68 | |
| JulyT | 0·533 | 0·078 | 0·426 | 6·861 | 0·000 | |||
| SepTt=–1 | 0·160 | 0·052 | 0·183 | 3·050 | 0·003 | |||
| Growtht=–1 | 0·187 | 0·078 | 0·190 | 2·406 | 0·018 | |||
| Growtht=–2 | 0·189 | 0·076 | 0·192 | 2·471 | 0·015 | |||
| Growtht=–5 | 0·236 | 0·069 | 0·241 | 3·421 | 0·001 | |||
| Abisko | (Constant) | –2·789 | 2·459 | –1·134 | 0·266 | 0·62 | 0·60 | |
| JulyT | 0·553 | 0·237 | 0·309 | 2·336 | 0·027 | |||
| Growtht=–1 | 0·591 | 0·132 | 0·592 | 4·474 | 0·000 | |||
The values for Endalen are after Weijers et al. (2010). T, average monthly temperature (°C); t = –1, t = –2. t = – 5 and t = –6 indicate the number of years before current growth.
The correlations between July temperature and shoot length, total leaf length and number of leaves were linear over the climate gradient (P < 0·0001 in all cases; Fig. 4). There was no significant relationship between July temperature and average leaf length (P = 0·920).
Fig. 4.
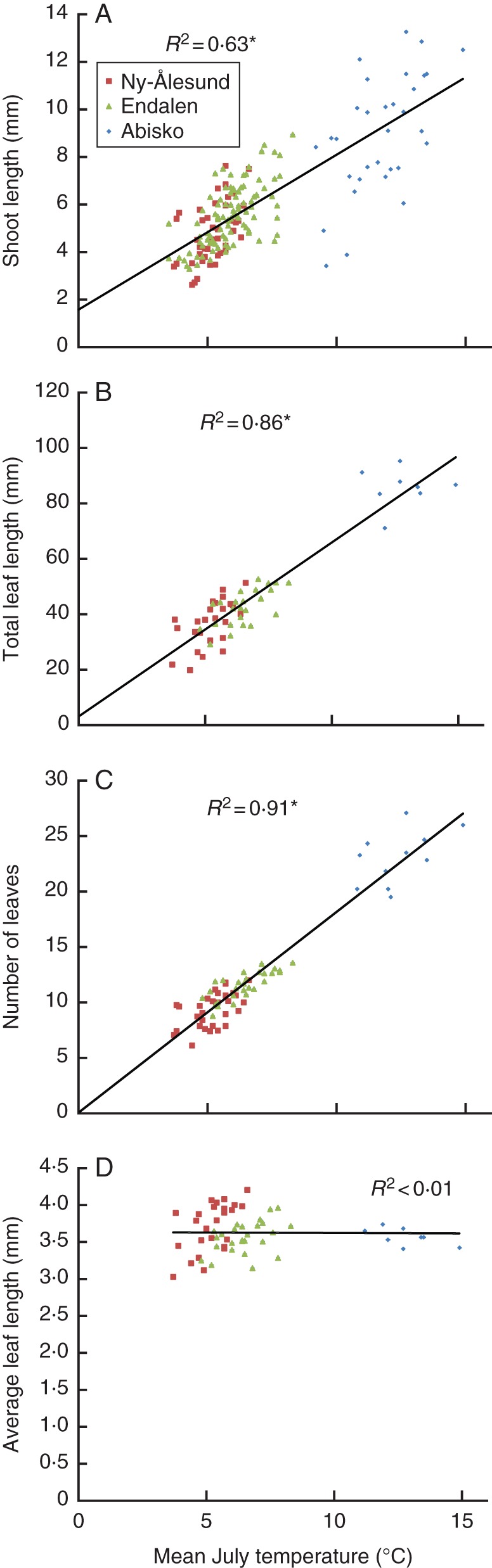
Relationships between average July temperature at all sites and four annual growth parameters of C. tetragona: (A) shoot length (n = 166), (B) total leaf length (n = 58), (C) number of leaves (n = 68) and (D) average leaf length (n = 58). The straight lines are the best-fit linear regressions through all data points. Data points from Ny-Ålesund, Endalen and Abisko are as indicated in the key. *P < 0·0001.
Temperature difference in OTCs vs. controls
Air temperatures were on average 1·5, 1·2 and 1·0°C higher (2008, 2009 and 2010, respectively) in OTCs compared with controls measured over a 24 h time interval (P < 0·0001; Table 6). Temperature differences were larger during the daytime, and the largest difference occurred during the 4 h around noon (1000–1400 h).
Table 6.
Start and end date of temperature measurements in control and OTC plots in Ny-Ålesund and the average differences in air temperature at vegetation height between OTCs and controls
| Start | End | ΔT (°C) 24 h | ΔT (°C) 12 h | ΔT (°C) 4 h | |
| 2008 | June 9 | August 21 | 1·5 | 2·0 | 2·3 |
| 2009 | June 28 | August 17 | 1·2 | 1·7 | 2·1 |
| 2010 | June 15 | August 18 | 1·0 | 1·3 | 1·4 |
Differences were tested with paired t-test for three different time intervals: 24 h (0000–2400 h), 12 h (0600–1800 h) and 4 h (1000–1400 h). All temperature differences were highly significant with P-values < 0·0001.
Growth differences in OTCs vs. controls
The results of the repeated measures ANOVA over the period 2004–2010 suggests that OTC placement led to an immediate increase in shoot length (P = 0·009), total leaf length (P = 0·010) and average leaf length (P = 0·004) (Fig. 5). There was also a tendency for the number of leaves to increase (P = 0·056). From 2005 onwards, significantly more leaves were produced in OTCs (P = 0·035). No difference in growth was found in the period before OTC instalment (1999–2003). When the growth differences are tested separately for each year, a similar picture emerges (Fig. 5). Again, there were no differences in growth prior to OTC placement.
Fig. 5.
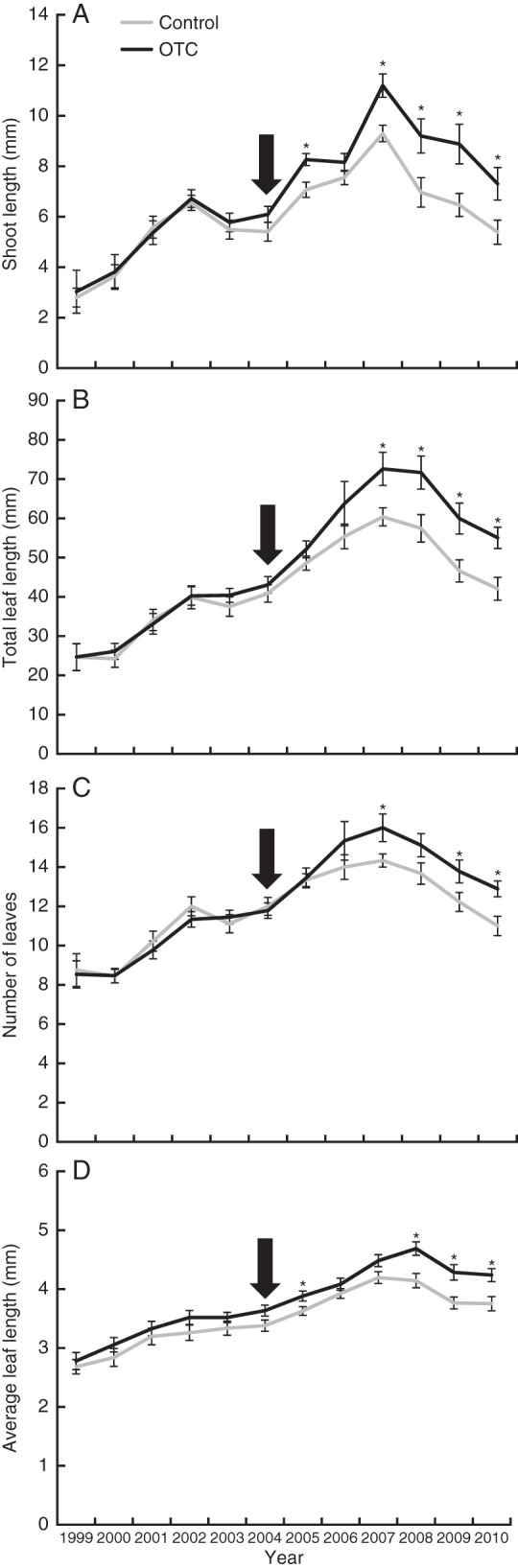
Average growth in 17 control and OTC plots, each between 1999 and 2010 near Ny-Ålesund. Arrows designate the start of the artificial warming in 2004. Error bars indicate the s.e.m. Annual shoot length (A), total leaf length (B), number of leaves (C) and average leaf length (D) were assessed for at least one shoot per plot (n = 18). Significant growth differences are marked by an asterisk (one-way ANOVA, P < 0·05).
On average, C. tetragona shoots in OTCs grew 1·63 mm longer each year compared wiht those in controls (6·81 mm year−1 in controls, 8·44 mm year−1 in OTCs; paired samples t-test, P = 0·001). Average total annual leaf length in OTCs was 59·73 mm and in controls 50·18 mm (9·55 mm year−1 difference, P = 0·002). Also, 1·11 more leaves were formed annually in OTCs (14·05 vs. 12·94, P = 0·012) and leaves were 0·36 mm longer (4·19 vs. 3·83 mm, P = 0·001).
Leaf life span
The average leaf life span at Ny-Ålesund (3·71 years) did not differ from that at Endalen (3·76 years) (Fig. 6). The leaf life span at Abisko was significantly shorter (2·29 years on average) than at both locations on Svalbard (one-way ANOVA, P < 0·001). The average leaf life span in OTCs (2·75 years) seems slightly shorter than that in controls (3·06 years), but this difference is not significant (one-way ANOVA, P = 0·10).
Fig. 6.
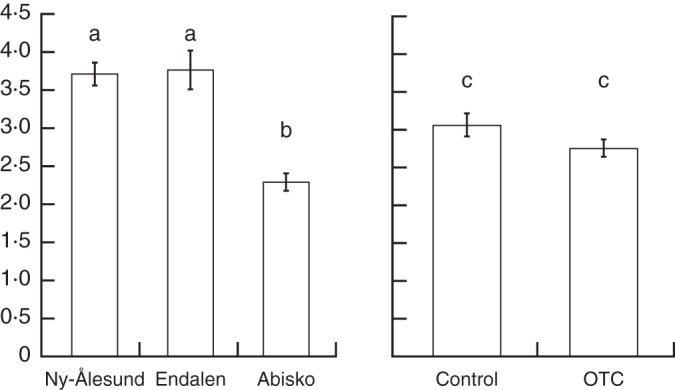
Average leaf life span (years) with standard error of the mean at the three research sites (left) and in the control and OTC plots (right); different letters indicate significant differences.
Standardization
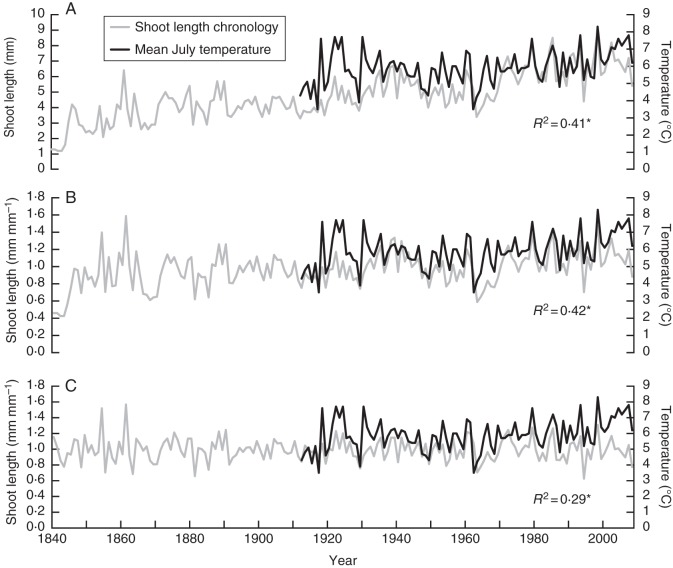
The raw Endalen shoot length chronology, the same chronology standardized with a horizontal line through the mean, and detrended with a 32 year cubic spline, are plotted together with local July temperature in Fig. 7. Detrending with a 32 year cubic spline resulted in a partial loss of the temperature signal, as indicated by the relatively low R2 of 0·29, and arguably in an artificial divergence problem, with temperatures typically diverting upward from the shoot length indices from the 1960s onward. Other standardization methods, besides the horizontal line through the mean, led to similar results.
Fig. 7.
Annual shoot length chronology for Endalen and Svalbard, together with local mean July temperature from the Svalbard Airport series. (A) No standardization applied; (B) standardized by dividing each individual branch chronology by a horizontal line through its mean; (C) standardized by dividing the individual branch chronologies by a fitted 32-year cubic spline; *P < 0·0001.
DISCUSSION
By comparing the growth response of C. tetragona to temperatures along a biogeographical climatic gradient, we show that its response to July temperature is linear between 4 and 15°C, which approximately represents the entire gradient of the species. Also, the growth response of C. tetragona to artificial warming is shown to be persistent over 7 years. Both findings show that annual growth parameters of this species provide a reliable source for past arctic temperature. Furthermore, it shows that reconstruction of mean July temperature based on these parameters, apart from average leaf length, is not hampered by divergence.
No divergence
A major problem in dendroclimatology is the ‘divergence problem’: a decreased sensitivity to rising temperatures of growth indices, typically after 1960 (Jacoby and D'Arrigo, 1995; Briffa et al., 1998; D'Arrigo et al., 2008). This dissociation between growth indices and temperature may have an ecophysiological background, e.g. increased drought stress due to increasing temperatures (Wilmking et al., 2004; D'Arrigo et al., 2008). Alternatively, detection of divergence could sometimes be erroneous (Esper and Frank, 2009) and represent a direct result of standardization techniques (see Cook et al., 1990) used to remove age-related growth trends from tree-ring time series. Divergence is problematic in climate reconstructions, as it hampers the assessment of the magnitude of past warm periods compared with the current climate (Loehle, 2009).
We believe that low-frequency temperature changes within the assessed temperature gradient are in principle captured by raw C. tetragona growth parameters (except average leaf length), due to their persistent growth response. Standardization should therefore be conservative in order to retain such low frequency climate signals and prevent invalid detection of divergence (Fig. 7).
We found no relationship between July temperature and average leaf length. Locally, there were, however, positive correlations between July temperature and average leaf length at Ny-Ålesund (r = 0·556, P = 0·004) and Endalen (r = 0·446, P = 0·022). Likewise, there was an immediate response of average leaf length to OTC warming. This is in line with the fact that average leaf length strongly correlated with temperature-driven shoot length growth in Ny-Ålesund, but not in Abisko, Sweden (Table 4). Thus, while temperature seems to be a limiting factor for leaf length growth at the species' northern range margin, it seems to be controlled by other factors at its southern range margin.
The relationship between July temperature and shoot length is weaker than the relationships of total leaf length and the number of leaves to July temperature (Fig. 4). This is mainly due to outliers at the early part of the Abisko shoot length chronology. Here, relatively warm mean July temperatures (between about 9 and 13°C) resulted in lower than expected growth. This might be a result of relatively poor replication (6–10 plants), although SSS values suggest otherwise, or of juvenile growth trends.
The observed linear July temperature–shoot length relationship confirms an earlier reported linear relationship (R2 = 0·98) between mean July temperature and the average number of leaves formed per year by C. tetragona at nine different sites across the Arctic, located in Svalbard, Lapland, and the Canadian Arctic (Havström et al., 1995).
Experimental warming
In contrast, Havström et al. (1993) found no response of C. tetragona growth to short-term (three growing seasons; open and closed) greenhouse warming at a lowland site near Abisko [450 m above sea level (asl)]. At the same site, growth did respond to nutrient addition. At a high elevation site near Abisko (1150 m asl; 68°20′N, 18°41′E) and near Ny-Ålesund, Svalbard (10 m asl; 78°56′N, 11°50′E), the growth responses were opposite: increased growth as a result of warming and no response to fertilization (Havström et al., 1993). This suggests a ‘divergence problem’ with a potential ecophysiological cause in C. tetragona. However, the lack of response at the lowland Abisko site may have been influenced by the lower ambient PAR and air humidity in the small plastic greenhouses (100 cm diameter) used, the low number of treatment replicates (six), the use of indexed growth (growth relative to the average growth prior to the start of the experiment) and the short duration of the experiment. Sometimes growth responses become noticeable only after several seasons of warming (Walker et al., 2006). Molau (1997) also found no effect of warming on C. tetragona growth at a high-elevation site (1000 m asl) near Abisko, Sweden, and Johnstone (1995) reported no effect of 2 year OTC warming on shoot elongation on Ellesmere Island in arctic Canada. However, in these studies, growth was measured non-destructively in the field, which makes it harder to detect differences in growth (J. Rozema et al., unpubl. data). In contrast, we found a significant positive effect on all measured growth parameters of OTC warming in Ny-Ålesund (Fig. 5), which confirms the findings of Rozema et al. (2009).
When the differences in growth are tested for each year separately, it is shown that the OTCs had no significant effect on growth in 2006. This growing season was exceptionally long. Except for some light frost in May, maximum temperatures were above freezing from late April (7·8°C on April 27) until the middle of September. Thus, OTC placement from early June to late August only increased temperatures during a part of the growing season. This could partly explain the relatively small difference in growth between control and OTC plots that year. Also, January of 2006 was unusually warm, with maximum temperatures exceeding 0°C on 19 d, leaving plants vulnerable after snowmelt to subsequent severe frost (Bokhorst et al., 2009). Indeed, C. tetragona growth was lower than expected in 2006 at both Ny-Ålesund and Endalen (Weijers et al., 2010). Therefore, plants might have had to invest in recovery from winter damage, resulting in relatively poor growth and a smaller difference between OTC and control plants.
Leaf life span
Leaf life span is negatively correlated to growing season length and temperature (Kudo et al., 2001). The considerably higher average summer temperatures in Abisko compared with those in Svalbard (Table 1) may therefore explain the 40 % shorter leaf life span there. The temperature difference between Endalen and Ny-Ålesund did not result in a different leaf life span. In the same way, the similar difference in temperature between OTC and control plots did not lead to a significant decrease in leaf life span. Leaf life span seems to affect autoregressive patterns within C. tetragona shoot length chronologies (Weijers et al., 2010). The fifth year prior to current growth was the strongest of the previous growth parameters explaining current growth at Endalen (Table 5). The influence of this year on current growth may be best explained through the allocation of resources from senescing leaves, since the average leaf life span at this location was 3·76 years and the last green leaf was on average found in the fifth year (Weijers et al., 2010). Current growth at Ny-Ålesund was equally influenced by growth of the fifth and sixth year before the present (Table 5), suggesting a longer leaf life span in its recent history.
Concluding remarks
The lack of divergence between July temperature and raw annual C. tetragona growth, measured as shoot length, total leaf length and number of leaves, as well as the species' persistent growth response to 7 years of experimental warming, shows the capability of these parameters to capture large-scale changes in mean July temperature. Furthermore, it confirms their suitability to serve as an arctic-wide temperature proxy. Annual average leaf length should be disregarded as a temperature proxy due to insensitivity to temperature at the southern distribution limit of C. tetragona. As raw growth showed a linear response to mean July temperature, we believe that low-frequency changes in July temperature are in principle captured by C. tetragona growth parameters, except for average leaf length. Standardization of C. tetragona growth chronologies should therefore be conservative, in order to retain such trends and prevent possible erroneous detection of dissociation between annual growth parameters and temperature.
ACKNOWLEDGEMENTS
We acknowledge Kings Bay AS, Ny-Ålesund, for permission to install OTCs and control plots. We are indebted to Professor Dr T.V. Callaghan, Director Royal Swedish Academy of Sciences, Abisko Scientific Research Station, for providing the OTC panels. We thank James Weedon for collecting plants near Abisko. Furthermore, we are indebted to Mr Wojtek Moskal, Norsk Polarinstitutt, and Mr Nick Cox, BAS-NERC, Cambridge, UK, for their indispensable logistical support in Ny-Ålesund. We thank the anonymous reviewers, whose constructive comments led to great improvement of the manuscript. The research presented is part of project 851·40·051 ‘Long-lived evergreen shrubs from polar ecosystems as monitors of present and past climate change’, funded by The Netherlands Organization for Scientific Research (NWO) as part of the International Polar Year 2007–2008.
LITERATURE CITED
- Aanes R, Saether BE, Smith FM, Cooper EJ, Wookey PA, Oritsland NA. The Arctic Oscillation predicts effects of climate change in two trophic levels in a high-arctic ecosystem. Ecology Letters. 2002;5:445–453. [Google Scholar]
- ACIA. Arctic climate impact assessment. Cambridge: 2005. [Google Scholar]
- Aerts R, Cornelissen JHC, Dorrepaal E, van Logtestijn RSP, Callaghan TV. Effects of experimentally imposed climate scenarios on flowering phenology and flower production of subarctic bog species. Global Change Biology. 2004;10:1599–1609. [Google Scholar]
- Bär A, Pape R, Bräuning A, Löffler J. Growth-ring variations of dwarf shrubs reflect regional climate signals in alpine environments rather than topoclimatic differences. Journal of Biogeography. 2008;35:625–636. [Google Scholar]
- Blok D, Sass-Klaassen U, Schaepman-Strub G, Heijmans MMPD, Sauren P, Berendse F. What are the main climate drivers for shrub growth in Northeastern Siberian tundra? Biogeosciences. 2011;8:1169–1179. [Google Scholar]
- Bokhorst SF, Bjerke JW, Tømmervik H, Callaghan TV, Phoenix GK. Winter warming events damage sub-Arctic vegetation: consistent evidence from an experimental manipulation and a natural event. Journal of Ecology. 2009;97:1408–1415. [Google Scholar]
- Briffa KR, Schweingruber FH, Jones PD, Osborn TJ, Shiyatov SG, Vaganov EA. Reduced sensitivity of recent tree-growth to temperature at high northern latitudes. Nature. 1998;391:678–682. [Google Scholar]
- Callaghan TV, Carlsson BA, Tyler NJC. Historical records of climate-related growth in Cassiope tetragona from the Arctic. Journal of Ecology. 1989;77:823–837. [Google Scholar]
- Cook ER. A time series analysis approach to tree-ring standardization. 1985 PhD thesis, University of Arizona, Tuscon, USA. [Google Scholar]
- Cook E, Briffa K, Shiyatov S, Mazepa V. Methods of dendrochronology: applications in the environmental sciences. Dordrecht: Kluwer Academic; 1990. Tree-ring standardization and growth-trend estimation; pp. 104–123. [Google Scholar]
- D'Arrigo R, Wilson R, Liepert B, Cherubini P. On the ‘divergence problem’ in northern forests: a review of the tree-ring evidence and possible causes. Global and Planetary Change. 2008;60:289–305. [Google Scholar]
- DNMI. eKlima: free access to weather- and climate data from Norwegian Meteorological Institute from historical data to real time observations. 2011 http://eklima.met.no , accessed 15 February 2012. [Google Scholar]
- Elvebakk A. The species concept in the high north – a panarctic flora initiative. Oslo: The Norwegian Academy of Science and Letters; 1999. Bioclimatic delimitation and subdivision of the Arctic; pp. 81–112. [Google Scholar]
- Esper J, Frank D. Divergence pitfalls in tree-ring research. Climatic Change. 2009;94:261–266. [Google Scholar]
- Fritts HC. Tree rings and climate. Caldwell, NJ: The Blackburn Press; 1976. [Google Scholar]
- Grissino-Mayer HD. Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree-Ring Research. 2001;57:205–221. [Google Scholar]
- Hallinger M, Manthey M, Wilmking M. Establishing a missing link: warm summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytologist. 2010;186:890–899. doi: 10.1111/j.1469-8137.2010.03223.x. [DOI] [PubMed] [Google Scholar]
- Havström M, Callaghan TV, Jonasson S. Differential growth responses of Cassiope tetragona, an arctic dwarf-shrub, to environmental perturbations among three contrasting high- and subarctic sites. Oikos. 1993;66:389–402. [Google Scholar]
- Havström M, Callaghan TV, Jonasson S, Svoboda J. Little Ice-Age temperature estimated by growth and flowering differences between subfossil and extant shoots of Cassiope tetragona, an arctic heather. Functional Ecology. 1995;9:650–654. [Google Scholar]
- Jacoby GC, D'Arrigo RD. Tree ring width and density evidence of climatic and potential forest change in Alaska. Global Biogeochem. Cycles. 1995;9:227–234. [Google Scholar]
- Johnstone J. Responses of Cassiope tetragona, a high arctic evergreen dwarf shrub, to variations in growing season temperature and growing season length at Alexandra Fiord, Ellesmere Island. 1995 MSc thesis, The University of British Columbia, Vancouver. [Google Scholar]
- Johnstone JF, Henry GHR. Retrospective analysis of growth and reproduction in Cassiope tetragona and relations to climate in the Canadian High Arctic. Arctic and Alpine Research. 1997;29:459–469. [Google Scholar]
- Kudo G, Molau U, Wada N. Leaf-trait variation of tundra plants along a climatic gradient: an integration of responses in evergreen and deciduous species. Arctic Antarctic and Alpine Research. 2001;33:181–190. [Google Scholar]
- Liang E, Eckstein D. Dendrochronological potential of the alpine shrub Rhododendron nivale on the south-eastern Tibetan Plateau. Annals of Botany. 2009;104:665–670. doi: 10.1093/aob/mcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang E, Lu X, Ren P, Li X, Zhu L, Eckstein D. Annual increments of juniper dwarf shrubs above the tree line on the central Tibetan Plateau: a useful climatic proxy. Annals of Botany. 2012;109:721–728. doi: 10.1093/aob/mcr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehle C. A mathematical analysis of the divergence problem in dendroclimatology. Climatic Change. 2009;94:233–245. [Google Scholar]
- Marion GM, Henry GHR, Freckman DW, et al. Open-top designs for manipulating field temperature in high-latitude ecosystems. Global Change Biology. 1997;3:20–32. [Google Scholar]
- McBean G, Alekseev G, Chen D, et al. Arctic Climate Impact Assessment. Cambridge: Cambridge University Press; 2005. Arctic climate: past and present; pp. 22–60. [Google Scholar]
- Molau U. Responses to natural climatic variation and experimental warming in two tundra plant species with contrasting life forms: Cassiope tetragona and Ranunculus nivalis. Global Change Biology. 1997;3:97–107. [Google Scholar]
- Nordli PO, Kohler J. Daily observations at Green Harbour, Grønfjorden, Spitsbergen. The early 20th century warming. 2003 Norwegian Meteorological Institute, Klima Report 12/03. [Google Scholar]
- Rayback SA, Henry GHR. Dendrochronological potential of the Arctic dwarf-shrub Cassiope tetragona. Tree-Ring Research. 2005;61:43–53. [Google Scholar]
- Rayback SA, Henry GHR. Reconstruction of summer temperature for a Canadian High Arctic site from retrospective analysis of the dwarf shrub, Cassiope tetragona. Arctic, Antarctic, and Alpine Research. 2006;38:228–238. [Google Scholar]
- Rayback SA, Lini A, Berg DL. Multiple climate signals characterize Cassiope mertensiana chronologies for a site on Mount Rainier, Washington, USA. Physical Geography. 2010;31:79–106. [Google Scholar]
- Rozema J, Boelen P, Doorenbosch M, et al. A vegetation, climate and environment reconstruction based on palynological analyses of high arctic tundra peat cores (5000–6000 years BP) from Svalbard. Plant Ecology. 2006;182:155–173. [Google Scholar]
- Rozema J, Weijers S, Broekman R, et al. Annual growth of Cassiope tetragona as a proxy for Arctic climate: developing correlative and experimental transfer functions to reconstruct past summer temperature on a millennial time scale. Global Change Biology. 2009;15:1703–1715. [Google Scholar]
- Schmidt N, Baittinger C, Kollmann J, Forchhammer M. Consistent dendrochronological response of the dioecious Salix arctica to variation in local snow precipitation across gender and vegetation types. Arctic, Antarctic, and Alpine Research. 2010;42:471–475. [Google Scholar]
- Walker DA, Raynolds MK, Daniëls FJA, et al. The circumpolar Arctic vegetation map. Journal of Vegetation Science. 2005;16:267–282. [Google Scholar]
- Walker MD, Wahren CH, Hollister RD. Plant community responses to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences, USA. 2006;103:1342–1346. doi: 10.1073/pnas.0503198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warming E. The structure and biology of Arctic flowering plants. I. Ericineae (Ericaceae, Pyrolaceae). I. Morphology and biology. Copenhagen: Commission for Scientific Research in Greenland; 1908. [Google Scholar]
- Weijers S, Broekman R, Rozema J. Dendrochronology in the High Arctic: July air temperatures reconstructed from annual shoot length growth of the circumarctic dwarf shrub Cassiope tetragona. Quaternary Science Reviews. 2010;29:3831–3842. [Google Scholar]
- Welker JM, Rayback S, Henry GHR. Arctic and North Atlantic Oscillation phase changes are recorded in the isotopes (delta O-18 and delta C-13) of Cassiope tetragona plants. Global Change Biology. 2005;11:997–1002. [Google Scholar]
- Wigley TML, Briffa KR, Jones PD. On the average value of correlated time-series, with applications in dendroclimatology and hydrometeorology. Journal of Climate and Applied Meteorology. 1984;23:201–213. [Google Scholar]
- Wilmking M, Juday GP, Barber VA, Zald HSJ. Recent climate warming forces contrasting growth responses of white spruce at treeline in Alaska through temperature thresholds. Global Change Biology. 2004;10:1724–1736. [Google Scholar]
- Woodcock H, Bradley RS. Salix arctica (Pall.): its potential for dendroclimatological studies in the High Arctic. Dendrochronologia. 1994;12:11–22. [Google Scholar]
- Zalatan R, Gajewski K. Dendrochronological potential of Salix alaxensis from the Kuujjua River area, western Canadian Arctic. Tree-Ring Research. 2006;62:75–82. [Google Scholar]