Abstract
Background and Aims
Apomictic plants are less dependent on pollinator services and able to occupy more diverse habitats than sexual species. However, such assumptions are based on temperate species, and comparable evaluation for species-rich Neotropical taxa is lacking. In this context, the Melastomataceae is a predominantly Neotropical angiosperm family with many apomictic species, which is common in the Campos Rupestres, endemism-rich vegetation on rocky outcrops in central Brazil. In this study, the breeding system of some Campo Rupestre Melastomataceae was evaluated, and breeding system studies for New World species were surveyed to test the hypothesis that apomixis is associated with wide distributions, whilst sexual species have more restricted areas.
Methods
The breeding systems of 20 Campo Rupestre Melastomataceae were studied using hand pollinations and pollen-tube growth analysis. In addition, breeding system information was compiled for 124 New World species of Melastomataceae with either wide (>1000 km) or restricted distributions.
Key Results
Most (80 %) of the Campo Rupestre species studied were self-compatible. Self-incompatibility in Microlicia viminalis was associated with pollen-tube arrest in the style, as described for other Melastomataceae, but most self-incompatible species analysed showed pollen-tube growth to the ovary irrespective of pollination treatment. Apomictic species showed lower pollen viability and were less frequent among the Campo Rupestre plants. Among the New World species compiled, 43 were apomictic and 77 sexual (24 self-incompatible and 53 self-compatible). Most apomictic (86 %) and self-incompatible species (71 %) presented wide distributions, whilst restricted distributions predominate only among the self-compatible ones (53 %).
Conclusions
Self-compatibility and dependence on biotic pollination were characteristic of Campo Rupestre and narrowly distributed New World Melastomataceae species, whilst apomictics are widely distributed. This is, to a certain extent, similar to the geographical parthenogenesis pattern of temperate apomictics.
Keywords: Apomixis, Melastomataceae, breeding system, Campo Rupestre, Cerrado, geographic distribution, rocky outcrops
INTRODUCTION
Different ecological and evolutionary factors such as the dispersal ability, tolerance to environment changes, population size, floral phenotypic plasticity, establishment and extinction dynamics may help to explain the geographic distribution of species (Brown et al., 1996). But breeding systems have been often viewed as a factor that may affect the persistency and distribution of plant species (Eckert et al., 2006; Barrett et al., 2008). Since the seminal papers by Baker (e.g. Baker, 1967), self-incompatibility and self-compatibility have been associated with distinct distribution patterns (Lowry and Lester, 2006), and it is believed that the apomictic species are capable of occupying more diverse environments, due to their greater independence of pollinator services, compared with the sexual and self-incompatible species (Bierzychudek, 1987; Hörandl and Paun, 2007; Hörandl et al., 2008). Although these assumptions are mostly based on temperate plant taxa, the Pleistocene climatic changes, which seem to have driven apomictics range expansion (geographical parthenogenesis sensu Hörandl, 2006), also affected the southern hemisphere and the idea may help in understanding the breeding system and biogeographical association of species-rich Neotropical groups.
The Melastomataceae is the seventh largest family of flowering plants, with around 166 genera and 4500 species of which some 3000 are Neotropical (Clausing and Renner, 2001). Centres of diversity for the family are distributed in two regions with different environmental conditions: the Andes in the north of South America, and the Brazilian central–south mountain ranges (Renner, 1993). In Brazil, there are >1500 species of Melastomataceae distributed in habitats ranging from moist forests to open plant formations of Cerrado, the Neotropical savanna region in central Brazil (Renner, 1989a; Romero and Martins, 2002).
In comparison with other tropical families the Melastomataceae are relatively conservative in their floral morphology and pollination system. Most species have hermaphrodite flowers with poricidal anthers and marked herkogamy, which favour allogamy (Renner, 1989a). Usually, pollen is the only resource offered and bees able to vibrate the anthers (buzz pollination) are the main pollinators (Proença, 1992).
Although allogamy is common in the family, there are also many apomictic and self-compatible species (Renner, 1989a). Indeed, apomixis appears to be more frequent in some groups of Melastomataceae than in the angiosperms as a whole (Goldenberg and Shepherd, 1998; Melo et al., 1999; Goldenberg, 2000a). In some of these species, apomixis has been related to the presence of polyembryony, polyploidy and high pollen sterility (Carman, 1997; Goldenberg and Shepherd, 1998; Mendes-Rodrigues and Oliveira, 2012).
Most of the apomictic species of Melastomataceae belong to the tribe Miconieae (88 %), and they have wider distributions than the sexual species of the same tribe (Goldenberg, 2000a). If this relationship between breeding system and geographic distribution applies to the family as a whole, one would anticipate that the species of Melastomataceae with restricted distributions are likely to be sexual species, dependent on pollination for their reproductive success. However, for the moment, there are only isolated studies on the reproductive biology of these endemic species (Andrade et al., 2007) to corroborate this hypothesis.
In the Cerrado region, some upland areas with rocky outcrops, the Campos Rupestres, present very specific conditions, such as shallow soils, low fertility and water availability (Vitta, 2002; Ribeiro and Walter, 2008). Such patchy and requiring environments, small distances and simple environmental barriers seem to be capable of generating considerable genetic differentiation (Lousada et al., 2011), and have produced a typical flora with considerable endemism (Harley and Simmons, 1986; Giulietti et al., 1987; Pirani et al., 2003; Stannard, 1995; Romero and Nakajima, 1999; Vitta, 2002). The Melastomataceae is one of the most common families of Campo Rupestre vegetation, especially such genera as Cambessedesia, Lavoisiera, Marcetia, Microlicia, Trembleya and Svitramia (Romero and Martins, 2002; Faria et al., 2006). However, information on the breeding biology of these taxa, and on the occurrence of apomixis, is still limited (Goldenberg, 2000a).
We investigated the breeding system of some Melastomataceae species of the central Brazilian Campos Rupestres and associated these features with their geographic distribution. We also complemented our field observations with data available in the literature for New World Melastomataceae species. We tested the hypothesis that apomixis is associated with species with wider distributions, whilst sexual breeding systems, and dependency on biotic pollinators, characterize endemic groups, such as those found in the Campo Rupestre highland flora.
MATERIALS AND METHODS
Study areas
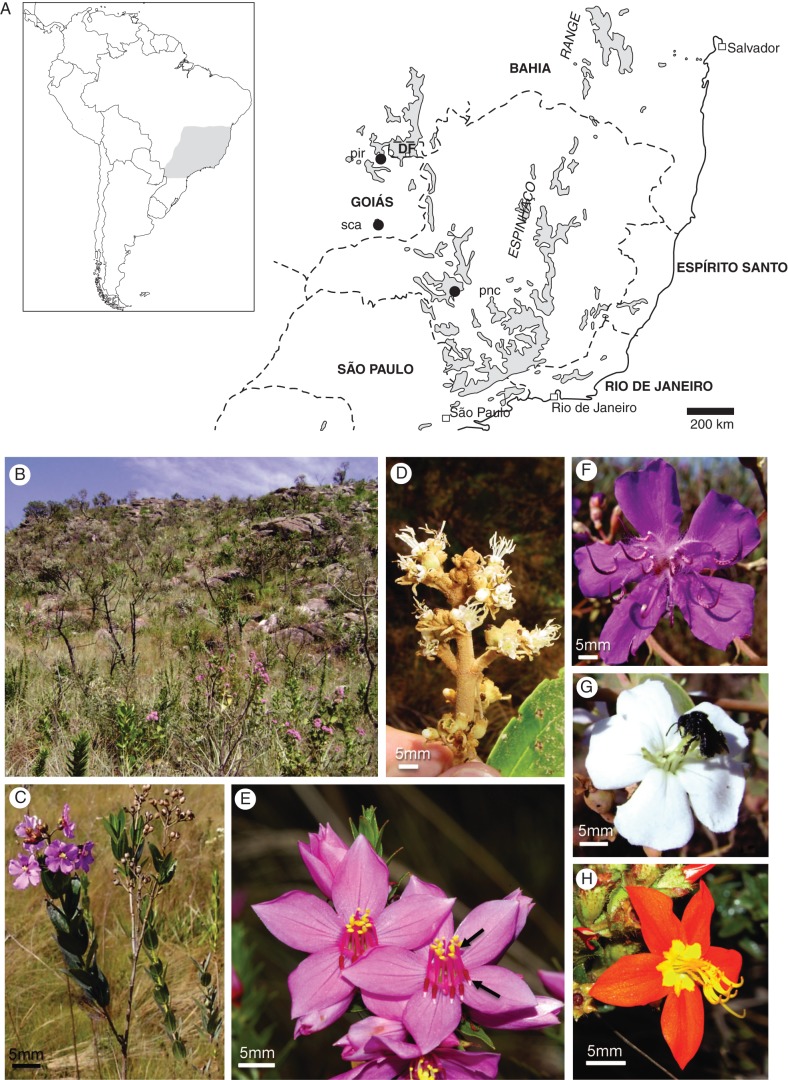
The field studies were carried out in three different areas (Fig. 1A): Serra de Caldas Novas State Park and Pirineus State Park, both in Goiás state, and the Serra da Canastra National Park, in Minas Gerais (Brazil). All Parks are in the Cerrado bioma and present diverse plant formations, from forest to open savanna grasslands, but are characterized by upland Campos Rupestres areas (Fig. 1B). The Serra de Caldas Novas State Park comprises approx. 12 500 ha and is located in the south east of Goiás (17°43′ to 17°50′S; 48°40′ to 48°42′W). The average altitude of the mountain range is approx. 1000 m, with differences of 150 m in relation to the surrounding areas. Average annual rainfall is approx. 1500 mm, concentrated from September to April, and average annual temperature ranges from 20 to 25 °C (Almeida and Sarmento, 1998). The Pirineus State Park comprises approx. 2800 hectares and is located in the centre of Goiás state (15°46′ to 15°50′S; 48°48′ to 48°53′W), at an altitude of 1300 m. The average annual rainfall is around 1200 mm, concentrated from October to April, and the average annual temperature varies from 21 to 24 °C (Oliveira et al., 2002). The Serra da Canastra National Park is the second largest conservation area in Minas Gerais state. It comprises approx. 200 000 ha and includes the municipalities of São Roque of Minas, Delfinópolis and Sacramento, in the south-west of the state (20°00′ to 20°30′S; 46°15′ to 47°00′W). Some peaks of the mountain range reach 1500 m. The annual rainfall is above 2000 mm and the average temperature of the coldest month is <16 °C and in the warmest month it does not exceed 22 °C (MMA/IBAMA, 2005).
Fig. 1.
Campo Rupestre areas specifically studied, environment and some of the species in these areas. (A) Shaded areas are 1000 m a.s.l. in Central Brazil where Campo Rupestre vegetation often appears (modified from Vasconcelos et al., 2003). Dots show the study areas; pir, Pirinópolis State Park; sca, Serra de Caldas Novas State Park; pnc, Parque Nacional da Serra da Canastra. (B) Campo Rupestre area on rocky outcrops at Serra da Canastra; (C) Svitramia hatschbachii habit; (D) flowers of Miconia rubiginosa; (E) flowers of Microlicia inquinans showing yellow connectives and anthers and less conspicuous anthers (arrows); (F) Tibouchina stenocarpa flower; (G) bee visiting Svitramia sp.; (H) flower of Cambessedesia regnelliana.
Breeding system
The breeding system of 20 Melastomataceae species from Brazilian Campos Rupestres were investigated by controlled hand-pollination experiments, analysis of pollen viability and observations of pollen-tube growth (e.g. Fig. 1C–H). The sampled taxa comprised ten species of the tribe Melastomeae [Macairea radula, Svitramia hatschbachii (Fig. 1C), S. minor, Svitramia sp. (Fig. 1G), Tibouchina anderssonii, T. frigidula, T. heteromalla, T. papyrus, T. stenocarpa (Fig. 1F) and T. villosissima], nine of the tribe Microlicieae [Cambessedesia espora, C. regnelliana (Fig. 1H), Lavoisiera imbricata, Microlicia fasciculata, M. inquinans (Fig. 1E), M. viminalis, Rhynchanthera grandiflora, Trembleya neopyrenaica and T. parviflora] and one of the tribe Miconieae [Miconia ferruginata (Fig. 1D)]. Voucher specimens were deposited at Herbarium of the University of Brasilia (UB), Herbarium Uberlandense (HUFU) and the Herbarium of the State University of Campinas (UEC).
The controlled pollination experiments were carried out using freshly opened flowers or pre-anthesis floral buds. Flowers previously isolated using nylon mesh bags were submitted to hand self-pollination, cross-pollination, and emasculation of pre-anthesis floral buds to test for autonomous apomixis (Goldenberg and Shepherd, 1998). Other flowers were bagged and left untreated to test for spontaneous self-pollination. The fruit set in all manipulated flowers was compared with that from natural pollinations of tagged flowers (Kearns and Inouye, 1993). It is important to notice, as discussed later, that this set of controlled pollinations do not allow testing for pseudogamous apomixis. But the Melastomataceae studied so far are autonomous apomictics.
The number of individuals and flowers used in controlled pollination varied among species, depending on their availability in the study areas (see Results), and the fruit development was verified about 2 months after the experiments. The ratio between the percentage of fruits resulting from self-pollination and cross-pollination was used to determine the index of self-incompatibility (ISI sensu Bullock, 1985) and species were considered self-incompatible whenever the ISI was lower than 0·20.
Pollen viability was estimated for each species using pollen of pre-anthesis buds fixed in FAA or ethanol 70 %. Five buds were collected from different individuals and 100 pollen grains per bud were counted. Some species presented anthers in two distinct whorls, one of them with yellow appendices on the connective and less conspicuous anthers (Fig. 1E). In these cases, pollen from each whorl was analysed separately. The percentage of viable pollen grains was calculated from the ratio of stained and non-stained grains by aceto-carmine (Kearns and Inouye, 1993; Goldenberg and Shepherd, 1998) and also from pollen morphology (collapsed vs. intact grains).
Post-pollination pollen-tube growth in the styles was analysed in 17 out of the 20 species studied, using previously fixed pistils, softened in NaOH or cleared with sodium hypochloride, rinsed thoroughly in water, stained with buffered aniline blue, and observed under fluorescence microscopy (Martin, 1959). For such observations of pollen-tube growth into the ovary in Cambessedesia regnelliana, Macairea radula, Miconia angelana, Microlicia inquinans, Rhynchanthera grandiflora, Svitramia hatschbachii, S. minor, Svitramia sp., Tibouchina papyrus and Tibouchina villosissima, we used pistils of self- and cross-pollinated flowers fixed at 24, 48 and 72 h after pollination. For Cambessedesia espora, Lavoisiera imbricata, Microlicia inquinans, M. viminalis, M. frigidula, Tibouchina heteromalla, T. stenocarpa, T. vilosissima and Trembleya parviflora, we observed pollen germination at 2, 12, 24, 36, 48, 60, 72 and 84 h after both self- and cross-pollination.
Geographical distribution
We searched the literature for all New World species of Melastomataceae with information detailing their breeding system, and all such taxa were referred to their tribes according to the classification system proposed by Renner (1993), Goldenberg et al. (2008) and Penneys et al. (2010). The species were also grouped according to their breeding system as self-compatible, self-incompatible or apomictic. For all species, information on their geographical distributions was compiled from taxonomic revisions, floristic studies, studies on reproductive biology and sites with databases of Brazilian and international herbaria (e.g. www.discoverlife.org; www.splink.cria.org.br; www.zipcodezoo.com).
The species were separated into two patterns of geographical distribution, based on the distance between the most distant populations, calculated using the ArcView software version 3·2. We recognized a wide distribution pattern, which included species with distance between populations >1000 km; and a restricted distribution pattern, which included species with the most distant populations <1000 km from each other. We tested if the number of species with either restricted or wide distribution was independent of the breeding system by using the chi-square independence test (Sokal and Rohlf, 1981).
RESULTS
Breeding system
Most of the species studied directly in the Campo Rupestre areas (80 %) were self-compatible (Table 1). The exceptions were Tibouchina aegopogon and Microlicia viminalis, which were self-incompatible, and Miconia ferruginata and Microlicia fasciculata, which were apomictic. Apomixis was described here for the tribe Microlicieae for the first time, as were the breeding system data for the highly endemic genus Svitramia. The number of treatments was somewhat limited for M. viminalis to assure self-incompatibility, but pollen tube analysis (below) seems to corroborate this conclusion. The few fruits formed after the treatment to test apomixis (emasculation of floral buds) in Cambessedesia regnelliana, Microlicia inquinans, Svitramia hatschbachii and Trembleya parviflora did not complete their development. Fruit formation from spontaneous self-pollination was observed only for some flowers in Tibouchina papyrus (6·8 %).
Table 1.
Fruit set of Melastomataceae species after controlled pollination (flowers treated inside parenthesis)
| Tribe | Species* | Controlled pollinations† |
ISI‡ | Reproductive system§ | PV¶ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ss | Ms | Cr | Em | Ct | |||||
| Melastomeae | Macairea radula2,3 | 0 (68) | 65·3 (121) | 82·9 (123) | 0 (122) | 89·7 (146) | 0·79 | SC | 88·3 ± 10·3 |
| Svitramia hatschbachii1 | 0 (60) | 56·3 (87) | 71·0 (76) | 4·0 (101) | 41·3 (104) | 0·79 | SC | 92·8 ± 3·8 | |
| S. minor1 | – | 61·8 (76) | 61·5 (78) | 0 (72) | 64·3 (84) | 1·00 | SC | 96·6 ± 4·9 | |
| Svitramia sp.1 | 0 (81) | 57·6 (66) | 33·8 (65) | 0 (63) | 68·3 (60) | 1·70 | SC | 96·7 ± 2·4 | |
| Tibouchina aegopogon2,3 | 0 (110) | 0 (20) | 38·5 (13) | 0 (74) | 62·3 (61) | 0·00 | SI | 97·5 ± 2·1 | |
| T. frigidula1 | 0 (80) | 65·0 (90) | 75·0 (80) | 0 (100) | 89·0 (100) | 0·87 | SC | 69·9 ± 12·5 (S) | |
| 78·5 ± 12·3 (P) | |||||||||
| T. heteromalla1 | 0 (100) | 72·0 (108) | 80·9 (110) | 0 (100) | 68·0 (100) | 0·89 | SC | 98·0 ± 1·5 (S) | |
| 96·0 ± 2·0 (P) | |||||||||
| T. papyrus3 | 6·8 (176) | 11·7 (94) | 45·0 (151) | 0 (197) | 30·1 (259) | 0·26 | SC | 65·8 ± 11·8 | |
| T. stenocarpa1 | 0 (88) | 21·2 (85) | 32·5 (89) | 0 (80) | 65·0 (80) | 0·65 | SC | 78·0 ± 5·3 | |
| T. villosissima2 | 0 (6) | 12·6 (87) | 19·3 (109) | 0 (50) | 16·4 (116) | 0·65 | SC | 90·1 ± 5·6 | |
| Miconieae | Miconia ferruginata2,3 | 54·9 (408) | 100·0 (24) | 87·5 (32) | 50·5 (406) | 77·6 (308) | 1·14 | AP | 8·0 ± 0·9 |
| Microlicieae | Cambessedesia espora1 | 0 (30) | 72·5 (40) | 82·5 (40) | 0 (40) | 35·0 (40) | 0·88 | SC | 68·0 ± 12·5 |
| C. regnelliana1 | 0 (20) | 14·9 (74) | 28·9 (83) | 1·3 (74) | 33·0 (106) | 0·52 | SC | 96·7 ± 6·3 | |
| Lavoisiera imbricata1 | 0 (66) | 58·8 (68) | 88·9 (72) | 0 (89) | 80·4 (97) | 0·66 | SC | 89·0 ± 5·3 (S) | |
| 92·0 ± 6·0 (P) | |||||||||
| Microlicia fasciculata2,3 | – | 100·0 (5) | 33·3 (6) | 23·0 (22) | 32·0 (50) | 3·00 | AP | 1·9 ± 2·3 | |
| M. inquinans1 | 0 (40) | 55·1 (98) | 75·2 (105) | 0 (131) | 72·1 (136) | 0·73 | SC | 98·1 ± 1·4 | |
| M. viminalis1 | 0 (15) | 0 (15) | 13·3 (15) | 0 (15) | 40·0 (15) | 0·00 | SI | 97·0 ± 3·8 (S) | |
| 95·0 ± 4·4 (P) | |||||||||
| Rhynchanthera grandiflora2,3 | – | 24·5 (53) | 81·0 (47) | 0 (75) | 43·3 (67) | 0·30 | SC | 78·3 ± 8·2 | |
| Trembleya neopyrenaica3 | – | 57·7 (78) | 91·8 (61) | 0 (20) | 50·0 (38) | 0·63 | SC | 59·9 ± 13·4 | |
| T. parviflora1 | 0 (30) | 47·9 (48) | 60·8 (51) | 0 (60) | 5·7 (70) | 0·79 | SC | 98·0 ± 5·6 (S) | |
| 65·0 ± 11·0 (P) | |||||||||
* Study areas: 1 Serra da Canastra National Park; 2 Serra de Caldas Novas State Park; 3 Pirineus State Park.
† Ss, Spontaneous self-pollination; Ms, manual self-pollination; Cr, cross-pollination; Em, emasculation; Ct, control; –, treatment not performed; in parenthesis, the sample number in each treatment.
‡ ISI, index of self-incompatibility.
§ SC, Self-compatible; SI, self-incompatible; AP, apomictic.
¶ PV, Pollen viability (mean ± standard deviation); S, antisepalous stamens; P, antipetalous stamens.
Pollen viability, estimated by staining, showed a predominance of fertile pollen in populations of all sexual species (PV > 60 %, Table 1). For the species with stamens in two whorls there was no difference in the estimated fertility of pollen from the anthers of the antisepalous stamens in relation to antipetalous ones. Pollen viability estimated for the two apomictic species, Miconia ferruginata and Microlicia fasciculata, was very low, approx. 8 % and 2 %, respectively.
The analysis of pollen-tube growth showed self-incompatibility reaction along the style only in Microlicia viminalis. In general, pollen tubes, irrespective of pollination treatment, were observed at the base of the styles or penetrating the ovules 24 h after pollination (Table 2). Only two species, S. hatschbachii and C. regnelliana, showed slower pollen-tube growth in self- vs. cross-pollinated pistils: self-pollen tube arrival at the ovary was delayed by 24 h in the former species, and by 48 h in the latter.
Table 2.
Period between pollination and penetration of pollen tubes into the ovary and ovules after hand self-pollination (Ms), cross-pollination (Cr) or natural pollination (Np) in some Melastomataceae species of Campo Rupestre in Central Brazil
| Species | Period between pollination and penetration of pollen tubes |
|||
|---|---|---|---|---|
| <24 h | 24–48 h | 48–72 h | >72 h | |
| Into the ovary | ||||
| Cambessedesia regnelliana (few pollen tubes in all styles) | X (Cr) | . | X (Ms) | . |
| Macairea radula | X (Cr) | . | . | . |
| Miconia angelana | X (Cr) | X (Ms) | . | . |
| Microlicia inquinans | X (Cr) | . | . | . |
| Rhynchanthera grandiflora | X (Cr) | . | . | . |
| Svitramia hatschbachii | X (Cr) | X (Ms) | . | . |
| S. minor | X (Cr) | . | . | . |
| Svitramia sp. | X (Cr) | . | . | . |
| Tibouchina papyrus | X (Cr) | . | . | . |
| T. villosissima | X (Cr) | . | . | . |
| Into the ovule | ||||
| C. espora | . | X (Np) | . | . |
| Lavoisiera imbricata | . | . | X (Np) | . |
| Microlicia inquinans | . | . | X (Np) | . |
| M. viminalis | . | . | X (Np) | . |
| T. frigidula | . | . | X (Np) | . |
| T. heteromala | . | . | X (Np) | . |
| T. stenocarpa | . | . | . | X (Np) |
| Trembleya parviflora | X (Np) | . | . | . |
Geographical distribution
Based on the literature survey, information on geographical distribution and the breeding system was compiled for 124 Melastomataceae species, 41 of restricted and 83 of wide distribution (Appendix). The best represented tribe was Miconieae, with 59 species, followed by Melastomeae (27 spp.), Microlicieae (12 spp.), Rhexieae (11 spp.), Blakeeae (6 spp.), Henrietteeae (3 spp.), Merianieae (3 spp.) and Bertolonieae represented by only one species. Tribe placement of the three species of Cambessedesia is unresolved (Fritsch et al., 2004).
Among the species analysed, 43 were apomictic and 77 were sexual. For this latter group, 24 were self-incompatible and 53 were self-compatible. Four species presented mixed or contradictory results. The analysis of the breeding systems per tribe, regardless of the geographic distribution pattern, indicated that both apomixis and self-incompatibility are common in the tribe Miconieae, respectively in 61 % and 19 % of the species studied, whereas self-compatibility is predominant in the tribes Melastomeae and Microlicieae, occurring in 78 % and 67 % of the species, respectively. Most of the apomictic and self-incompatible species, 86 % and 71 %, respectively, presented wide distributions (Fig. 2), but the chi-square test showed the differences were significant only in the case of the apomictics (P < 0·0001 and P = 0·6848, respectively). A restricted distribution was predominant only among the self-compatible species (53 %) and, although some self-compatible species presented a wide distribution, distribution was significantly different when compared with the original sample ratio (P = 0·0022).
Fig. 2.
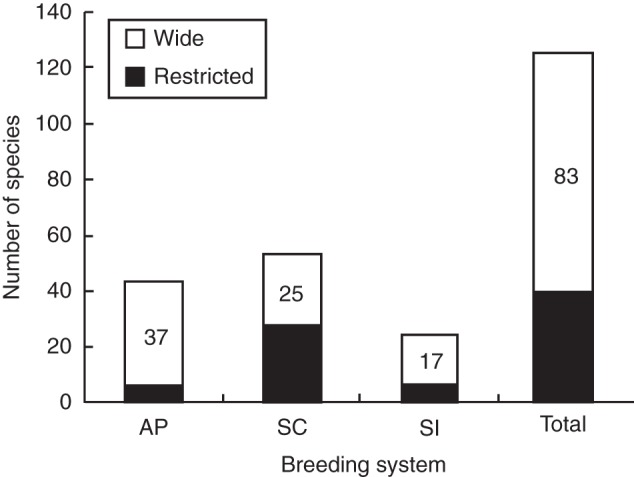
Reproductive system and geographical distribution of a sample of 124 species (83 of wide and 41 of restricted distribution) of New World Melastomataceae. Abbreviations: AP, apomictic; SC, self-compatible; SI, self-incompatible species; Total, all species.
DISCUSSION
The breeding system of the Melastomataceae studied here confirms some trends already described for these Neotropical plants (Renner, 1989a): a great reproductive diversity in an apomixis-rich group. These trends may be important for understanding the general characteristics of Cerrado species as a whole, and of the endemic Campo Rupestre species in particular. The general picture, to a certain extent, parallels the geographical parthenogenesis pattern described to the northern hemisphere apomictics. We discuss below specific aspects of both parts of the study and, finally, briefly consider the consequences for the evolution of Neotropical biodiversity.
Campo Rupestre reproductive biology
Most of the Campo Rupestre Melastomataceae studied here were self-compatible, which contrasts with the studies for other Neotropical woody species that are mostly allogamous (Oliveira and Gibbs, 2000; Machado et al., 2006; Vamosi et al., 2006). However, self-compatibility does not necessarily imply high levels of autogamy (sensu Richards, 1986), since autogamy is possibly restricted by poricidal anthers and herkogamy (Renner, 1989a).
Nevertheless, some spontaneous self-pollination (autonomous autogamy sensu Harder and Barrett, 2006) may occur when pollen released through mechanical movement of the anthers by wind or rain falls on the stigma of the flower (Renner, 1989a). Tibouchina papyrus, despite the poricidal anthers, produced some fruits after spontaneous self-pollination (6·8 %). Fruit set after spontaneous self-pollination was also observed in M. angelana (Santos et al., 2010), M. minutiflora (Goldenberg and Shepherd, 1998), M. sintenisii (Renner, 1989a) and Rynchanthera dichotoma (Guimarães and Ranga, 1997). In these cases pores may be larger which would facilitate pollination (Goldenberg and Varassin, 2001).
In self-compatible species, selfing also results from pollinators′ activity. The number of flowers opened at the same time is important for the reproductive success of the species dependent on biotic vectors, since it increases the attraction and visitation by pollinators (Mitchell, 1994; Williams et al., 2001). But visits will result frequently in geitonogamous self-pollination (Williams, 2007). Hence, the high fruit set under natural conditions in some of the species of this study is probably the result of both, cross-pollination and geitonogamy. Selfing may occur less frequently in C. regnelliana and S. hatschbachii due to the difference in the speed of pollen-tube growth. As in Miconia angelana (Santos et al., 2010), self-pollinated flowers are unlikely to form fruits, because the pollen tubes do not have sufficient time to reach and penetrate the ovules before the flower starts to senesce. This mechanism has also been reported for several species of Lythraceae and Spigeliaceae as a cryptic self-incompatibility and may reduce inbreeding (Eckert and Allen, 1997; Erbar and Leins, 1999; Erbar, 2003).
The frequency of apomictics recorded in our sample was much smaller than that recorded for the family as a whole (Renner, 1989a) and for the tribe Miconieae in particular (Goldenberg and Shepherd, 1998; Melo and Machado, 1998). But in both cases pollen viability was very low and apomixis was autonomous. Low pollen viability has been related to the occurrence of autonomous apomixis (Richards, 1986; Renner, 1989a; Carman, 1997; Goldenberg and Shepherd, 1998; Goldenberg and Varassin, 2001), but not to sporophytic pseudogamous apomixis, in which pollen viability is usually high (Mendes-Rodrigues et al., 2005). Low pollen viability has been found in several apomictic Melastomataceae species, but showed unexpectedly wide variation (Baumgratz and Silva, 1988; Goldenberg and Shepherd, 1998; Goldenberg and Varassin, 2001) when compared with better studied autonomous apomictic groups (e.g. Taraxacum; van Baarlen et al., 2000).
It is important to emphasize that the technique used to estimate the pollen viability is based on staining. Thus, stained grains are interpreted as being viable, although they are not necessarily able to germinate and sire seeds (e.g. Carvalho and Oliveira, 2003). Despite these limitations, this methodology has been used widely and allows pollen grains with cytoplasmatic contents to be identified and is therefore functional either for pollination or bee foraging (Kearns and Inouye, 1993). In this sense, many autonomous apomictics are, as the Melastomataceae species studied, useless as a floral resource to pollen-collecting bees.
Pollen is the most important floral resource offered by the Campo Rupestre Melastomataceae, although some nectar may be offered in small amounts by some species (e.g. Santos et al., 2010). Morphological and functional pollen dimorphism has been postulated for the Melastomataceae (Luo et al., 2008), a division-of-labour hypothesis which may optimize pollen transfer. In Lavoisiera imbricata, Microlicia fasciculata, M. inquinans, M. viminalis, Trembleya neopyrenaica and T. parviflora the two conspicuously different whorls of stamens could be associated with such a functional pollen dimorphism (Faegri and van der Pijl, 1979). The results for pollen viability did not show any distinction between pollen from the different whorls, although functional dimorphism cannot be ruled out since we did not test germinability and fruit set using each kind of pollen. Similar results were reported for Tibouchina pulchra (Brito, 2010), other Melastomataceae (Renner, 1989a) and many other plant groups (Endress, 1994). It is possible that the two types of stamens result simply in deposition of pollen in a larger area of the visitor′s body, increasing the chances of pollination.
In any case, self-compatibility and dependence on pollen-collecting bees for pollination seems to be a general trend amongst the Campo Rupestre Melastomataceae. Although somewhat isolated by this patchy environment, these species seldom rely on autogamy or apomixis for reproductive assurance.
Distributional correlates
The numerous studies on the breeding systems in the Melastomataceae (Renner, 1989a; Borges, 1991; Goldenberg and Shepherd, 1998; Goldenberg and Varassin, 2001; Fracasso and Sazima, 2004; Fracasso, 2008; Pereira et al., 2011; Brito and Sazima, 2012) still encompass <5 % of the Neotropical species. But the present survey almost doubled the number of analysed species in Renner (1989a) and despite this still-limited sample, some trends can be clearly discerned. Apomixis is very common and apomictic species are widely distributed. The relationship between geographical distribution and breeding system for the Melastomataceae compiled here mostly comply with the pattern suggested by Goldenberg and Shepherd (1998) for the species of the tribe Miconieae.
Most apomictic species of the Melastomataceae surveyed did present wide distribution. Apomixis provides reproductive assurance and independence of pollinators, allowing uniparental reproduction favouring colonization of new areas (Baker, 1967). It also retains some advantages of seed production, such as dispersal ability and dormancy (Renner, 1989a). Moreover, many apomictics are polyembryonic and such taxa may benefit from the reproductive compensation or bet-hedging effect that polyembryony can provide (Mendes-Rodrigues and Oliveira, 2012). It is believed that, by presenting these characteristics, the apomictic species in general are able to occupy diverse environments (Bierzychudek, 1987; Hörandl and Paun, 2007). This could explain why many apomictic species of Melastomataceae are pioneers in disturbed areas and habitats, or even aggressive invasive weeds in exotic environments such as Clidemia hirta (Peters, 2001) and Miconia calvescens (Meyer, 1998; Baruch et al., 2000).
Another explanation for the wider distribution of apomictic species can be found in its relation with polyploidy. Apomixis in Melastomataceae, especially in species of the tribe Miconieae, is related to polyploidy (Goldenberg and Shepherd, 1998). By joining distinct genomes in a hybrid or creating genomes with duplicated chromosomes, polyploidy can both trigger apomixis (Carman, 1997) and conserve genetic heterogeneity, which may provide the ability for these species to colonize new environments (Lowry and Lester, 2006; Hörandl and Paun, 2007). Pleistocene climatic changes (Prado and Gibbs, 1993) but also other geohistoric processes such as fire pressure in C4-dominated ecosystems (Simon et al., 2009) may have provided the opportunity for the expansion of apomictic taxa.
Wide distributions were also common among self-incompatible species in our survey. These species may be better colonizers of new environments due to genetic variability provided by mandatory cross-pollination (Lowry and Lester, 2006). This breeding system ensures cross-fertilization (Richards, 1986), allowing adaptation to the environment and evolutionary changes by increasing the genetic variability (Barrett et al., 2008). But some plants with restricted distribution were also self-incompatible including some of the endemic Campo Rupestre Melastomataceae studied here.
The restricted distribution pattern predominated only in those Melastomataceae species in our survey with sexual reproduction and self-compatibility. It is believed that gene flow is limited in self-compatible species, allowing the emergence of highly specialized endemism (Lowry and Lester, 2006). In fact, this breeding system has been reported in many rare species with restricted distributions (Bernardello et al., 1999; Kaye, 1999; Vieira and Grabelos, 2003; Andrade et al., 2007). It conforms also with the endemism-rich Campo Rupestre areas where limited gene flow associated with selfing may lead to population differentiation and speciation (Lousada et al., 2011).
However, self-compatible species present a bimodal distribution, which may obscure the effect of mating system on range size (Lowry and Lester, 2006; Williams, 2007). Some species may have their allelic variability reduced by self-compatibility and, consequently, would have lower ability to occupy different habitats and show restricted distribution. Other species may have considerable genetic variability also fixed by self-compatibility, which would also provide reproductive assurance, dispersal ability and wider distribution. Both kinds of outcomes have been reported for different taxa (Lowry and Lester, 2006). These scenarios may explain the occurrence of some self-compatible species of Melastomataceae with wide geographical distribution such as Rynchanthera grandiflora (Aubl.) DC. and Leandra regnelii (Triana) Cogn. (Goldenberg and Varassin, 2001) and Tibouchina pulchra Cogn. and T. sellowiana Cogn. (Pereira et al., 2011; Brito and Sazima, 2012).
It is important to notice that the methods used here may fail to detect differences between self-compatible sexuals and pseudogamic apomictics. As many tropical apomictic species are pseudogamous sporophytic apomictics (Carman, 1997; Mendes-Rodrigues et al., 2005), results for hand-pollination treatments in pseudogamous apomictics would be similar to those of self-compatible species (Oliveira et al., 1992) and apomixis could be detected only by histological (Mendes-Rodrigues et al., 2005) or molecular methods (Martins and Oliveira, 2003). In this sense, widely distributed self-compatible Melastomataceae could be cryptic pseudogamic apomictics, but, as mentioned before, the Melastomataceae studied so far are autonomous apomictics.
We conclude that, on the one hand, apomixis seems to be associated with ample distribution in the New World Melastomataceae, supporting the idea that this breeding system may be more important for the persistency and diversity of Neotropical plants than previously thought (Allem, 2003). As in temperate environments, these apomictics may have been favoured for occupying Cerrado and other tropical biomes, which have expanded since the last glacial maximum (Ramos et al., 2009; Simon et al., 2009). On the other hand, species with restricted distribution, as the Campo Rupestre endemic species studied here, are mostly sexual and dependent on biotic pollination. Speciation and diversity in these areas seems to be driven by differentiation and restricted pollen flow, but not by autogamy or apomixis.
ACKNOWLEDGEMENTS
We thank the ICMBIO (formerly IBAMA) for granting the license for research and Joaquim Maia Neto, director of the Serra da Canasta National Park, for his kind hospitality and facilities provided in the Park. We thank Felipe Amorim, Pietro K. Maruyama, Ana Paula Caetano, Daniela Simão, Diana Sampaio and Clesnan Mendes-Rodrigues for field assistance and help during different parts of the work, Renato Goldenberg for his help with systematics and distribution of the plants and early discussions on the basic idea of the work, and Peter E. Gibbs for final discussions and English revision. This work was supported by the National Council for Scientific Research (CNPq 479421/2004-8, 371970/2006–7, 302452/2008-7 and 486091/2007-4) and also CAPES and FAPESP PhD grants. The work was part of the MSc dissertation of the first author at the Ecology Post-Graduation program of the Universidade Federal de Uberlândia; and included data of the PhD theses of the second author, at the Botany Post-grad program of Universidade Estadual de Campinas, and of the third author at the Botany Post-grad program at Universidade de Brasilia.
APPENDIX
Reproductive system and geographical distribution of New World Melastomataceae species
| Reproductive system† |
|||||||
|---|---|---|---|---|---|---|---|
| Tribe* | Species | SC | SI | AP | Reference‡ | Geographical distribution | Reference‡ |
| Bertolonieae | Bertolonia marmorata (Naudin) Naudin | X | 33 | Restricted (Bahia; Pernambuco, BR) | 7 | ||
| Blakeeae | Blakea anomala Donn. Sm. | X | 23 | Restricted (Costa Rica) | 45 | ||
| B. chlorantha Almeda | X | 22 | Restricted (Costa Rica) | 45 | |||
| B. gracilis Hemls. | X | 23 | Restricted (Nicaragua; Costa Rica; Panamá) | 45 | |||
| B. tuberculata Donn. Sm. | X | 23 | Restricted (Costa Rica; Panamá) | 45 | |||
| Topobea brenesii Standl. | X | 23 | Restricted (Costa Rica) | 45 | |||
| T. maurofernandeziana Cogn. | X | 23 | Restricted (Nicaragua; Costa Rica; Panamá) | 45 | |||
| Henrietteae | Bellucia acutata Pilg. | X | 33 | Wide | 34 | ||
| B. grossularioides (L.) Triana | X | 33 | Wide | 34 | |||
| Henriettea succosa (Aubl.) DC. | X | 25 | Wide | 48 | |||
| Melastomeae | Aciotis acuminifolia (Mart. ex DC.) Triana | X | 33 | Wide | 12 | ||
| Desmocelis villosa (Aubl.) Naudin | X | 28 | Wide | 48 | |||
| Macairea radula (Bonpl.) DC. | X | 1 | Wide | 34 | |||
| M. theresiae Cogn. | X | 33 | Restricted (Pará; Amazonas, BR) | 34 | |||
| Marcetia taxifolia (A.St.-Hil) DC. | X | 30 | Wide | 24 | |||
| Melastoma affine D.Don | X | 17 | Wide | 17 | |||
| Monochaetum amabile Almeda | X | 3 | Restricted (Costa Rica) | 45 | |||
| M. floribundum Naudin | X | 3 | Wide | 45 | |||
| M. neglectum Almeda | X | 3 | Restricted (Costa Rica; Panamá) | 45 | |||
| M. talamancense Almeda | X | 3 | Restricted (Costa Rica) | 45 | |||
| M. vulcanicum Cogn. | X | 3 | Restricted (Costa Rica) | 45 | |||
| Nepsera aquatica (Aubl.) Naudin | X | 33 | Wide | 48 | |||
| Pterolepis glomerata (Rottb.) Miq. | X | 31 | Wide | 36 | |||
| Sandemania hoehnei (Cogn.) Wurdack | X | 33 | Wide | 32 | |||
| Svitramia hatschbachii Wurdack | X | 1 | Restricted (South and Southwest of Minas Gerais, BR) | 37 | |||
| S. minor R. Romero & A.B. Martins | X | 1 | Restricted (Serra da Canastra-Minas Gerais, BR) | 37 | |||
| Svitramia sp. | X | 1 | Restricted (Serra da Canastra-Minas Gerais, BR) | 37 | |||
| Tibouchina aegopogon (Naudin) Cogn. | X | 1 | Wide | 18 | |||
| T. cerastifolia Cogn. | X | 16 | Wide | 18 | |||
| T. frigidula (DC.) Cogn. | X | 1 | Wide | 18 | |||
| T. heteromalla (D.Don) Cogn. | X | 1 | Wide | 18 | |||
| T. papyrus (Pohl) Toledo | X | 1 | Restricted (Pirineus-Goiás, BR) | 18 | |||
| T. sellowiana Cogn. | X | 16 | Wide | 18 | |||
| T. semidecandra (Schrank & Mart. ex DC.) Cogn. | X | 16 | Restricted (São Paulo; Minas Gerais; Rio de Janeiro, BR) | 18 | |||
| T. stenocarpa (Schrank & Mart. ex DC.) Cogn. | X | 1; 15 | Wide | 18 | |||
| T. trichopoda (DC.) Baill. | X | 30 | Wide | 18 | |||
| T. villosissima (Triana) Cogn. | X | 1 | Restricted (Minas Gerais; Goiás, BR) | 18 | |||
| Merianieae | Adelobotrys rachidotricha Brade | X | 33 | Restricted (Amazonas, BR) | 46 | ||
| Graffenrieda latifolia (Naudin) Triana | X | 42 | Restricted (Venezuela; Eastern Caribbean) | 57 | |||
| Miconieae | Clidemia bullosa DC. | X | 27 | Wide | 48 | ||
| C. capitellata (Bonpl.) D.Don | X | 31 | Wide | 48 | |||
| C. epibaterium DC. | X | 33 | Wide | 48 | |||
| C. fendleri Cogn. | X | 42 | Restricted (Venezuela) | 48 | |||
| C. hirta (L.) D.Don | X | 27 | Wide | 48 | |||
| C. novemnervia (DC.) Triana | X | 33 | Wide | 48 | |||
| C. rubra (Aubl.) Mart. | X | 33 | Wide | 48 | |||
| C. ruddae Wurdack | X | 4 | Restricted (México) | 45 | |||
| Conostegia macrantha O.Berg ex Triana | X | 22 | Restricted (Costa Rica; Panamá) | 45 | |||
| Eriocnema fulva Naudin | X | 5 | Restricted (Nova Lima-Minas Gerais, BR) | 5 | |||
| Leandra australis (Cham.) Cogn. | X | 16 | Wide | 43 | |||
| L. dasytricha (A.Gray) Cogn. | X | 16 | Wide | 43 | |||
| L. involucrata DC. | X | 41 | Restricted (Minas Gerais, BR) | 43 | |||
| L. lacunosa Cogn. | X | 15 | Wide | 43 | |||
| L. melastomoides Raddi | X | 16 | Wide | 43 | |||
| L. purpurascens (DC.) Cogn. | X | 16 | Wide | 43 | |||
| L. regnellii (Triana) Cogn. | X | 16 | Wide | 43 | |||
| Maieta guianensis Aubl. | X | 33 | Wide | 29 | |||
| Miconia alata (Aubl.) DC. | X | 33 | Wide | 14 | |||
| M. albicans (Sw.) Triana | X | 15 | Wide | 14 | |||
| M. angelana R.Romero & R.Goldenberg | X | 40 | Restricted (Serra da Canastra-Minas Gerais, BR) | 38 | |||
| M. araguensis Wurdack | X | 42 | Restricted (Venezuela) | 48 | |||
| M. argyrophylla DC. | X | 33 | Wide | 14 | |||
| M. campestris Benth. & Triana | X | 33 | Wide | 14 | |||
| M. chamissois Naudin | X | 44 | Wide | 14 | |||
| M. ciliata (Rich.) DC. | X | 26 | Wide | 14 | |||
| M. discolor DC. | X | X | 8 | Wide | 14 | ||
| M. dodecandra Cogn. | X | 42 | Wide | 14 | |||
| M. egensis Cogn. | X | 33 | Wide | 14 | |||
| M. elegans Cogn. | X | 8 | Wide | 14 | |||
| M. fallax DC. | X | 15 | Wide | 14 | |||
| M. ferruginata DC. | X | 1 | Wide | 14 | |||
| M. ibaguensis (Bonpl.) Triana | X | 13 | Wide | 14 | |||
| M. langsdorffii Cogn. | X | X | 15; 41 | Wide | 14 | ||
| M. latecrenata (DC.) Naudin | X | 16 | Wide | 14 | |||
| M. lepidota DC. | X | 33 | Wide | 14 | |||
| M. ligustroides (DC.) Naudin | X | 15 | Wide | 14 | |||
| M. minutiflora (Bonpl.) DC. | X | 15 | Wide | 14 | |||
| M. pepericarpa DC. | X | 15 | Wide | 14 | |||
| M. petropolitana Cogn. | X | 16 | Wide | 14 | |||
| M. pohliana Cogn. | X | 15 | Wide | 14 | |||
| M. prasina (Sw.) DC. | X | 10; 33 | Wide | 14 | |||
| M. pusilliflora (DC.) Naudin | X | 16 | Wide | 14 | |||
| M. rubiginosa (Bonpl.) DC. | X | 15 | Wide | 14 | |||
| M. sellowiana Naudin | X | 41 | Wide | 14 | |||
| M. sintenisii Cogn. | X | 10 | Restricted (Porto Rico) | 45 | |||
| M. spinulosa Naudin | X | 42 | Restricted (Venezuela) | 48 | |||
| M. stenostachya DC. | X | 15 | Wide | 14 | |||
| M. stephananthera Ule | X | 31 | Wide | 14 | |||
| M. sylvatica Schldl. | X | 42 | Wide | 48 | |||
| M. theaezans (Bonpl.) Cogn. | X | 8 | Wide | 14 | |||
| M. tomentosa (Rich.) D.Don | X | 33 | Wide | 14 | |||
| M. trianae Cogn. | X | 33 | Restricted (São Paulo; Minas Gerais; Espírito Santo, BR) | 14 | |||
| M. tuberculata (Naudin) Triana | X | 6;42 | Restricted (Venezuela) | 48 | |||
| Ossaea amygdaloides (DC.) Triana | X | 16 | Wide | 43 | |||
| O. confertiflora (DC.) Triana | X | 16 | Wide | 43 | |||
| Tococa bullifera Mart. & Schrank ex DC. | X | 33 | Wide | 28 | |||
| Tococa guianensis Aubl. (syn. T. formicaria Mart.) | X | 44 | Wide | 28 | |||
| Tococa coronata Benth. (syn. T. longisepala Cogn.) | X | 33 | Restricted (Amazônia, BR) | 28 | |||
| Microlicieae | Centradenia floribunda Planch. | X | 33 | Restricted (Guatemala; El Salvador; Honduras) | 45 | ||
| C. grandifolia Endl. ex Walp. | X | 2 | Restricted (South of México; Costa Rica) | 45 | |||
| C. paradoxa (Kraenzl.) Almeda | X | 2 | Restricted (Costa Rica; Panamá) | 45 | |||
| Lavoisiera imbricata (Thunb.) DC. | X | 1 | Wide | 48 | |||
| Microlicia fasciculata Mart. ex Naudin | X | 1 | Wide | 39 | |||
| M. inquinans Naudin | X | 1 | Restricted (Serra da Canastra-Minas Gerais, BR) | 37 | |||
| M. viminalis (DC.) Triana | X | 1 | Wide | 39 | |||
| Rhynchanthera dichotoma (Desr.) DC. | X | 19 | Wide | 35 | |||
| R. grandiflora (Aubl.) DC. | X | 1 | Wide | 35 | |||
| R novemnervia DC. | X | 9 | Wide | 35 | |||
| Trembleya neopyrenaica Naudin | X | 1 | Restricted (Goiás, BR) | 24 | |||
| T. parviflora (D.Don) Cogn. | X | X | 1; 44 | Wide | 24 | ||
| Rhexieae | Rhexia alifanus Walt. | X | 20 | Wide | 20 | ||
| R. aristosa Britton | X | 20 | Wide | 20 | |||
| R. cubensis Griseb. | X | 20 | Wide | 20 | |||
| R. lutea Walt. | X | 20 | Wide | 20 | |||
| R. mariana L. | X | 33 | Wide | 20 | |||
| R. nashii Small | X | 20 | Wide | 20 | |||
| R. nuttallii James | X | 20 | Restricted (Flórida, USA) | 20 | |||
| R. parviflora Chapm. | X | 20 | Restricted (Flórida, USA) | 20 | |||
| R. petiolata Walt. | X | 20 | Wide | 20 | |||
| R. salicifolia Kral & Bostick | X | 20 | Restricted (Flórida, USA) | 20 | |||
| R. virginica L. | X | X | 20; 21 | Wide | 20 | ||
| ** | Cambessedesia espora (A.St.-Hil. ex Bonpl.) DC. | X | 1 | Wide | 24 | ||
| ** | C. hilariana (Kunth) DC. | X | 11 | Wide | 24 | ||
| ** | C. regnelliana Cogn. | X | 1 | Restricted (Minas Gerais; Goiás, BR) | 24 | ||
* Classification in tribe according to Renner (1993), Penneys et al. (2010) and Fritsch et al. (2004); ** unresolved polytomy (Fritsch et al., 2004).
† SC, Self-compatible; SI, self-incompatible; AP, apomictic.
‡ Source references: 1, This study; 2, Almeda (1977); 3, Almeda (1978); 4, Almeda and Chuang (1992); 5, Andrade et al. (2007); 6, Arroyo and Cabrera (1977); 7, Baumgratz (1989); 8, Borges (1991); 9, Borges (2000); 10, Dent-Acosta and Breckon (1991); 11, Fracasso and Sazima (2004); 12, Freire-Fierro (2002); 13, Goldenberg (1994); 14, Goldenberg (2000b); 15, Goldenberg and Shepherd (1998); 16, Goldenberg and Varassin (2001); 17, Gross (1993); 18, Guimarães (1997); 19, Guimarães and Ranga (1997); 20, Kral and Bostick (1969); 21, Larson and Barrett (1999); 22, Lumer (1980); 23, Lumer, 1982; 24, Martins (1997); 25, Melo and Machado (1996); 26, Melo and Machado (1998); 27, Melo et al. (1999); 28, Michelangeli (2005); 29, Michelangeli (2010); 30, Pinheiro (1995); 31, Ramirez and Brito, 1990; 32, Renner (1987); 33, Renner (1989a); 34, Renner (1989b); 35, Renner (1990); 36, Renner (1994); 37, Romero (2000); 38, Romero and Goldenberg (1999); 39, Romero and Woodgyer (2010); 40, Santos et al. (2010); 41, Saraiva et al., 1996; 42, Sobrevila and Arroyo, 1982; 43, Souza and Baumgratz (2004); 44, Souza-Silva (2000); 45, Flora Mesoamericana (www.tropicos.org/Project/FM); 46, The International Plant Names Index (www.ipni.org); 47, Plants of the Eastern Caribbean (www.ecflora.cavehill.uwi.edu); 48, www.tropicos.org.
LITERATURE CITED
- Allem AC. Optimization theory in plant evolution: an overview of long-term evolutionary prospects in the angiosperms. Botanical Review. 2003;69:225–251. [Google Scholar]
- Almeda F. Systematics of the Neotropical genus Centradenia (Melastomataceae) Journal of Arnold Arboretum. 1977;85:73–108. [Google Scholar]
- Almeda F. Systematics of the genus Monochaetum (Melastomatacee) in Mexico and Central America. University of California Publications in Botany. 1978;75:1–134. [Google Scholar]
- Almeda F, Chuang TI. Chromosome numbers and their systematic significance in some Mexican Melastomataceae. Systematic Botany. 1992;17:583–593. [Google Scholar]
- Almeida AF, Sarmento FNM. Parque Estadual da Serra de Caldas – Plano de Manejo. Goiânia: CTE – Centro Tecnológico de Engenharia Ltda, FEMAGO – Fundação Estadual do Meio Ambiente; 1998. [Google Scholar]
- Andrade PMA, Forni-Martins RB, Martins FRB. Reproductive system of Eriocnema fulva Naudin (Melastomataceae), an endemic species of Minas Gerais state, SE Brazil. Brazilian Journal of Biology. 2007;67:313–319. doi: 10.1590/s1519-69842007000200017. [DOI] [PubMed] [Google Scholar]
- Arroyo MTK, Cabrera E. Preliminary self-incompatibility test for some tropical cloud forest species in Venezuela. Incompatibility Newsletter. 1977;8:72–76. [Google Scholar]
- Baker HG. Support for Baker′s law – as a rule. Evolution. 1967;21:853–856. doi: 10.1111/j.1558-5646.1967.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Colautti RI, Eckert CG. Plant reproductive systems and evolution during biological invasion. Molecular Ecology. 2008;17:373–383. doi: 10.1111/j.1365-294X.2007.03503.x. [DOI] [PubMed] [Google Scholar]
- Baruch ZR, Robert RP, Goldstein G. Responses to light and water availability of four invasive Melastomataceae in the Hawaiian islands. International Journal of Plant Sciences. 2000;161:107–118. doi: 10.1086/314233. [DOI] [PubMed] [Google Scholar]
- Baumgratz JFA. O gênero Bertolonia Raddi (Melastomataceae): revisão taxonômica e considerações anatômicas. Arquivos do Jardim Botânico do Rio de Janeiro. 1989;30:69–213. [Google Scholar]
- Baumgratz JFA, Silva NMF. Ecologia da polinização e biologia da reprodução de Miconia stenostachya DC. (Melastomataceae) Rodriguésia. 1988;38(64):11–24. [Google Scholar]
- Bernardello G, Anderson GJ, Lopez-S P, Cleland MAX, Stuessy TF, Crawford DJ. Reproductive biology of Lactoris fernandeziana (Lactoridaceae) American Journal of Botany. 1999;86:829–840. [PubMed] [Google Scholar]
- Bierzychudek P. The evolution of sex and its consequences. Basel: Birkhäuser; 1987. Patterns in plant parthenogenesis; pp. 97–217. [Google Scholar]
- Borges HBN. Biologia reprodutiva de quatro espécies de Melastomataceae. Campinas: Dissertação de Mestrado, Universidade Estadual de Campinas; 1991. [Google Scholar]
- Borges HBN. Biologia reprodutiva e conservação do estrato lenhoso numa comunidade do Cerrado. Campinas: Tese de Doutorado, Universidade Estadual de Campinas; 2000. [Google Scholar]
- Brito VLG. Biologia da polinização, reprodução e genética de duas populações de Tibouchina pulchra Gogn. (Melastomataceae) em gradiente altitudinal no sudeste do Brasil. Campinas.: Dissertação de Mestrado, Universidade Estadual de Campinas; 2010. [Google Scholar]
- Brito VLG, Sazima M. 2012 Tibouchina pulchra (Melastomataceae): reproductive biology of a tree species at two sites of an elevational gradient in the Atlantic Rainforest in Brazil. Plant Systematics and Evolution, in press. http://dx.doi.org/10.1007/s00606-012-0633-5 . [Google Scholar]
- Brown JH, Stevens GC, Kaufman M. The geographic range: size, shape, boundaries, and internal structure. Annual Review of Ecology and Systematics. 1996;27:597–623. [Google Scholar]
- Bullock SH. Breeding systems in the flora of a tropical deciduous forest. Biotropica. 1985;17:287–301. [Google Scholar]
- Carman JG. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biological Journal of the Linnean Society. 1997;61:51–94. [Google Scholar]
- Carvalho DA, Oliveira PE. Biologia reprodutiva e polinização de Senna sylvestris (Vell.) H.S. Irwin & Barneby (Leguminosae, Caesalpinioideae) Revista Brasileira de Botânica. 2003;26:319–328. [Google Scholar]
- Clausing G, Renner SS. Molecular phylogenetics of Melastomataceae and Memecylaceae: implications for character evolution. American Journal of Botany. 2001;88:486–498. [PubMed] [Google Scholar]
- Dent-Acosta SR, Breckon GJ. Reproductive biology of six species of Melastomataceae in western Puerto Rico. Washington DC: First International Melastomataceae Symposium, Smithsonian Institution; 1991. [Google Scholar]
- Eckert CG, Allen M. Cryptic self-incompatibility in tristylous Decodon verticillatus (Lythraceae) American Journal of Botany. 1997;84:1391–1397. [PubMed] [Google Scholar]
- Eckert CG, Samis KE, Dart S. Reproductive assurance and the evolution of uniparental reproduction in flowering plants. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 183–203. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Erbar C. Pollen tube transmitting tissue: place of competition of male gametophytes. International Journal of Plant Sciences. 2003;164:265–277. [Google Scholar]
- Erbar C, Leins P. Secondary pollen presentation and a curious rupture of the style in Spigelia (Spigeliaceae, Gentianales) Plant Biology. 1999;1:389–402. [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. New York, NY: Pergamon Press; 1979. [Google Scholar]
- Faria CA, Romero R, Leoni L. Flora fanerogâmica do Parque Nacional do Caparaó: Melastomataceae. Pabstia. 2006;17:1–31. [Google Scholar]
- Fracasso CM. Biologia da polinização e reprodução de espécies de Melastomataceae do Parque Nacional da Serra da Canastra, MG. Campinas: Tese de Doutorado, Universidade Estadual de Campinas; 2008. [Google Scholar]
- Fracasso CM, Sazima M. Polinização de Cambessedesia hilariana (Kunth) DC. (Melastomataceae): sucesso reprodutivo versus diversidade, comportamento e freqüência de visitas de abelhas. Revista Brasileira de Botânica. 2004;27:797–804. [Google Scholar]
- Freire-Fierro A. Monograph of Aciotis (Melastomataceae) Systematic Botany Monographs. 2002;62:1–99. [Google Scholar]
- Fritsch PW, Almeda F, Renner SS, Martins AB, Cruz BC. Phylogeny and circumscription of the near-endemic Brazilian tribe Microlicieae (Melastomataceae) American Journal of Botany. 2004;91:1105–1114. doi: 10.3732/ajb.91.7.1105. [DOI] [PubMed] [Google Scholar]
- Giulietti AM, Menezes NL, Pirani JR, Wanderley MGL. Flora da Serra do Cipó, Minas Gerais: caracterização e lista das espécies. Boletim de Botânica da Universidade de São Paulo. 1987;9:1–151. [Google Scholar]
- Goldenberg R. Estudos sobre a biologia reprodutiva de espécies de Melastomataceae de Cerrado em Itirapina, SP. Campinas: Dissertação de Mestrado, Universidade Estadual de Campinas; 1994. [Google Scholar]
- Goldenberg R. Apomixia como alternativa à reprodução sexuada em Melastomataceae. In: Cavalcanti TB, Walter BMT, editors. Tópicos atuais de botânica. Brasília: EMBRAPA – Recursos Genéticos; 2000a. pp. 225–230. [Google Scholar]
- Goldenberg R. 2000b O gênero Miconia Ruiz & Pav. (Melastomataceae). I. Listagens analíticas. II. Revisão taxonômica da seção Hypoxanthus (Rich.ex DC.) Hook F. Tese de Doutorado, Universidade Estadual de Campinas, Campinas. [Google Scholar]
- Goldenberg R, Shepherd GJ. Studies on the reproductive biology of Melastomataceae in ‘cerrado’ vegetation. Plant Systematics and Evolution. 1998;211:13–29. [Google Scholar]
- Goldenberg R, Varassin IG. Sistemas reprodutivos de espécies de Melastomataceae da Serra do Japi. Revista Brasileira de Botânica. 2001;24:283–288. [Google Scholar]
- Goldenberg R, Penneys DS, Almeda F, Judd WS, Michelangeli FA. Phylogeny of Miconia (Melastomataceae): patterns of stamen diversification in a megadiverse Neotropical genus. International Journal of Plant Sciences. 2008;169:963–979. [Google Scholar]
- Gross CL. The breeding system and pollinators of Melastoma affine (Melastomataceae), a pioneer shrub in Tropical Australia. Biotropica. 1993;25:468–473. [Google Scholar]
- Guimarães PJF. 1997 Estudo taxonômicos de Tibouchina sect. Pleroma (D.Don) Cogn. (Melastomataceae). Tese de Doutorado, Universidade Estadual de Campinas, Campinas. [Google Scholar]
- Guimarães PJF, Ranga NT. Sistema de reprodução de Rhynchanthera dichotoma (Lam.) DC. Acta Botanica Brasilica. 1997;11:41–44. [Google Scholar]
- Harder LD, Barrett SCH. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. [Google Scholar]
- Harley RM, Simmons NA. Flórula of Mucugê, Chapada Diamantina, Bahia, Brazil. Kew: Royal Botanical Gardens; 1986. [Google Scholar]
- Hörandl E. The complex causality of geographical parthenogenesis. New Phytologist. 2006;171:525–538. doi: 10.1111/j.1469-8137.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Hörandl E, Paun O. Patterns and sources of genetic diversity in apomictic plants: implications for evolutionary potentials. In: Hörandl E, Grossniklaus U, van Dijk P, Sharbel TF, editors. Apomixis: evolution, mechanisms and perspectives. Liechtenstein: Gantner, Ruggell; 2007. pp. 169–194. [Google Scholar]
- Hörandl E, Cosendai AC, Temsch EM. Understanding the geographic distributions of apomictic plants: a case for a pluralistic approach. Plant Ecology & Diversity. 2008;1:309–320. doi: 10.1080/17550870802351175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye TN. From flowering to dispersal: reproductive ecology of an endemic plant, Astragalus australis var. olympicus (Fabaceae) American Journal of Botany. 1999;86:1248–1256. [PubMed] [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologists. Niwot, CO: Colorado University Press; 1993. [Google Scholar]
- Kral R, Bostick PE. The genus Rhexia (Melastomataceae) Sida. 1969;3:387–440. [Google Scholar]
- Larson BMH, Barrett SCH. The ecology of pollen limitation in buzz-pollinated Rhexia virginica (Melastomataceae) Journal of Ecology. 1999;87:371–381. [Google Scholar]
- Lousada JM, Borba EL, Ribeiro KT, Ribeiro LC, Lovato MB. Genetic structure and variability of the endemic and vulnerable Vellozia gigantea (Velloziaceae) associated with the landscape in the Espinhaco Range, in southeastern Brazil: implications for conservation. Genetica. 2011;139:431–440. doi: 10.1007/s10709-011-9561-5. [DOI] [PubMed] [Google Scholar]
- Lowry E, Lester SE. The biogeography of plant reproduction: potential determinants of species range sizes. Journal of Biogeography. 2006;33:1975–1982. [Google Scholar]
- Lumer C. Rodent pollination of Blakea (Melastomataceae) in a Costa Rican Cloud Forest. Brittonia. 1980;32:512–517. [Google Scholar]
- Lumer C. 1982. The pollination ecology, breeding systems and phenology of Blakea and Topobea (Melastomataceae) in Monteverde, Costa Rica. Doctoral dissertation, City University, New York. [Google Scholar]
- Luo Z, Zhang D, Renner SS. Why two kinds of stamens in buzz-pollinated flowers? Experimental support for Darwin's division-of-labour hypothesis. Functional Ecology. 2008;22:794–800. [Google Scholar]
- Machado IC, Lopes AV, Sazima M. Plant sexual systems and a review of the breeding system studies in the Caatinga, a Brazilian tropical dry forest. Annals of Botany. 2006;97:277–287. doi: 10.1093/aob/mcj029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FN. Staining and observation pollen tubes in the style by means of fluorescence. Stain Technology. 1959;34:125–128. doi: 10.3109/10520295909114663. [DOI] [PubMed] [Google Scholar]
- Martins E. 1997 Revisão taxonômica do gênero Trembleya DC. (Melastomataceae). Tese de Doutorado, Universidade Estadual de Campinas, Campinas. [Google Scholar]
- Martins RL, Oliveira PE. Evidence for apomixis and clonal populations in Eriotheca (Bombacaceae) Plant Biology. 2003;5:338–340. [Google Scholar]
- Melo GFA, Machado ICS. Biologia da reprodução de Henriettea succosa DC. (Melastomataceae) Revista Brasileira de Biologia. 1996;56:383–389. [Google Scholar]
- Melo GFA, Machado ICS. Auto-incompatibilidade em Miconia ciliata (L.C. Rich.) DC. (Miconieae-Melastomataceae) Acta Botanica Brasilica. 1998;12:113–120. [Google Scholar]
- Melo GF, Machado IC, Luceño M. Reprodución de tres especies de Clidemia (Melastomataceae) en Brasil. Revista de Biología Tropical. 1999;47:359–363. [Google Scholar]
- Mendes-Rodrigues C, Oliveira PE. Polyembryony in Melastomataceae from Brazilian Cerrado: multiple embryos in a small world. Plant Biology. 2012 doi: 10.1111/j.1438-8677.2011.00551.x. in press. http://dx.doi.org/10.1111/j.1438-8677.2011.00551.x . [DOI] [PubMed] [Google Scholar]
- Mendes-Rodrigues C, Carmo-Oliveira R, Talavera S, Arista M, Ortiz PL, Oliveira PE. Polyembryony and apomixis in Eriotheca pubescens (Malvaceae – Bombacoideae) Plant Biology. 2005;7:533–540. doi: 10.1055/s-2005-865852. [DOI] [PubMed] [Google Scholar]
- Meyer J. Observations on the reproductive biology of Miconia calvescens DC (Melastomataceae), an alien invasive tree on the island of Tahiti (South Pacific Ocean) Biotropica. 1998;30:609–624. [Google Scholar]
- Michelangeli FA. Tococa (Melastomataceae) Flora Neotropica. 2005;98:1–114. [Google Scholar]
- Michelangeli FA. Neotropical myrmecophilous Melastomataceae – an annotated list and key. Proceedings of the California Academy of Sciences, Series 4. 2010;61:409–440. [Google Scholar]
- Mitchell RJ. Effects of floral traits, pollinator visitation and plant size on Ipomopsis aggregata fruit production. American Naturalist. 1994;143:870–889. [Google Scholar]
- MMA/IBAMA. Plano de manejo – Parque Nacional da Serra da Canastra. Brasília: MMA Ministério do Meio Ambiente/IBAMA – Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis; 2005. [Google Scholar]
- Oliveira AAM, Costa DD, Salgado DP, et al. Plano de gestão das unidades de conservação estaduais em Goiás – Proposta metodológica e executiva. Goiânia: Agência Ambiental de Goiás; 2002. [Google Scholar]
- Oliveira PE, Gibbs PE. Reproductive biology of woody plants in a cerrado community. Flora. 2000;195:311–329. [Google Scholar]
- Oliveira PE, Gibbs PE, Barbosa AA, Talavera S. Contrasting breeding systems in two Eriotheca (Bombacaceae) species of the Brazilian cerrados. Plant Systematics and Evolution. 1992;179:207–219. [Google Scholar]
- Penneys DS, Michelangeli FA, Judd WS, Almeda F. Henrietteeae (Melastomataceae): a new Neotropical berry-fruited tribe. Systematic Botany. 2010;35:783–800. [Google Scholar]
- Pereira AC, Santos JB, Goldenberg R, Melo GAR, Varassin IG. Flower color change accelerated by bee pollination in Tibouchina (Melastomataceae) Flora. 2011;206:491–497. [Google Scholar]
- Peters HA. Clidemia hirta invasion at the Pasoh Forest Reserve: an unexpected plant invasion in an undisturbed tropical forest. Biotropica. 2001;33:60–68. [Google Scholar]
- Pinheiro MCB. Biologia da reprodução de cinco espécies de Melastomataceae da restinga de Maricá-RJ. Campinas: Tese de doutorado, Universidade Estadual de Campinas; 1995. [Google Scholar]
- Pirani JR, Mello-Silva R, Giulietti AM. Flora de Grão Mongol, Minas Gerais, Brasil. Boletim de Botânica da Universidade de São Paulo. 2003;21:1–24. [Google Scholar]
- Prado DE, Gibbs PE. Patterns of species distributions in the dry seasonal forest South America. Annals of the Missouri Botanical Garden. 1993;80:902–927. [Google Scholar]
- Proença C. Buzz pollination – older and more widespread than we think? Journal of Tropical Ecology. 1992;8:115–120. [Google Scholar]
- Ramirez N, Brito Y. Reproductive biology of a tropical palm swamp community in the Venezuelan Llanos. American Journal of Botany. 1990;77:1260–1271. [Google Scholar]
- Ramos ACS, De Lemos JP, Lovato MB. Phylogeographical structure of the Neotropical forest tree Hymenaea courbaril (Leguminosae: Caesalpinioideae) and its relationship with the vicariant Hymenaea stigonocarpa from Cerrado. Journal of Heredity. 2009;100:206–216. doi: 10.1093/jhered/esn092. [DOI] [PubMed] [Google Scholar]
- Renner SS. Sandemania hoehnei (Melastomataceae: Tibouchineae): taxonomy, distribution, and biology. Brittonia. 1987;39:441–446. [Google Scholar]
- Renner SS. A survey of reproductive biology in Neotropical Melastomataceae and Memecylaceae. Annals of the Missouri Botanical Garden. 1989a;76:496–518. [Google Scholar]
- Renner SS. Systematic studies in the Melastomataceae: Bellucia, Loreya, and Macairea. Memoirs of the New York Botanical Garden. 1989b;50:1–112. [Google Scholar]
- Renner SS. A revision of Rhynchanthera (Melastomataceae) Nordic Journal of Botany. 1990;9:601–630. [Google Scholar]
- Renner SS. Phylogeny and classification of the Melastomataceae and Memecylaceae. Nordic Journal of Botany. 1993;13:519–540. [Google Scholar]
- Renner SS. A revision of Pterolepis (Melastomataceae: Melastomeae) Nordic Journal of Botany. 1994;14:73–104. [Google Scholar]
- Ribeiro JF, Walter BMT. As principais fitofisionomias do bioma Cerrado. In: Sano M, Almeida SP, editors. Cerrado: ecologia e flora. Vol. 1. Brasília-DF: Embrapa Informação Tecnológica; 2008. pp. 151–212. [Google Scholar]
- Richards AJ. Plant breeding systems. London: Allen & Unwin; 1986. [Google Scholar]
- Romero R. A Família Melastomataceae no Parque Nacional da Serra da Canastra, Minas Gerais, Brasil. Campinas: Tese de Doutorado, Universidade Estadual de Campinas; 2000. [Google Scholar]
- Romero R, Goldenberg R. A new species of Miconia (Melastomataceae) from Serra da Canastra National Park, Minas Gerais, Brazil. Novon. 1999;9:98–100. [Google Scholar]
- Romero R, Martins AB. Melastomataceae do Parque Nacional da Serra da Canastra, Minas Gerais, Brasil. Revista Brasileira de Botânica. 2002;25:19–24. [Google Scholar]
- Romero R, Nakajima JN. Espécies endêmicas do Parque Nacional da Serra da Canastra, Minas Gerais. Revista Brasileira de Botânica. 1999;22(Suppl. 2):259–265. [Google Scholar]
- Romero R, Woodgyer EM. Validation of the name Microlicia petiolulata (Melastomataceae), a new species from the Espinhaço Range, Minas Gerais, Brazil. Kew Bulletin. 2010;65:69–72. [Google Scholar]
- Santos APM, Romero R, Oliveira PE. Biologia reprodutiva de Miconia angelana (Melastomataceae), endêmica da Serra da Canastra, Minas Gerais. Revista Brasileira de Botânica. 2010;33:333–341. [Google Scholar]
- Saraiva LC, Cesar O, Monteiro R. Breeding systems of shrubs and trees of a Brazilian Savanna. Arquivos de Biologia e Tecnologia. 1996;39:751–763. [Google Scholar]
- Simon MF, Grether R, de Queiroz LP, Skema C, Pennington RT, Hughes CE. Recent assembly of the Cerrado, a Neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proceedings of the National Academy of Sciences of the USA. 2009;106:20359–20364. doi: 10.1073/pnas.0903410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrevila C, Arroyo MTK. Breeding systems in a montane tropical cloud forest in Venezuela. Plant Systematics and Evolution. 1982;140:19–37. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York, NY: Freeman; 1981. [Google Scholar]
- Souza MLD, Baumgratz JFA. Notas taxonômicas em Leandra Raddi (Melastomataceae) Insula. 2004;33:89–99. (Florianópolis) [Google Scholar]
- Souza-Silva SCS. Biologia reprodutiva e polinização em Melastomataceae no Parque do Sabiá, Uberlândia, MG. Brasília: Dissertação de mestrado, Universidade de Brasília; 2000. [Google Scholar]
- Stannard BL. Flora of the Pico das Almas, Chapada Diamantina, Bahia, Brazil. Kew: Royal Botanic Gardens; 1995. [Google Scholar]
- Vamosi JC, Knight TM, Steets JA, Mazer SJ, Burd M, Ashman TL. Pollination decays in biodiversity hotspots. Proceedings of the National Academy of Sciences of the USA. 2006;103:956–961. doi: 10.1073/pnas.0507165103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baarlen P, van Dijk P, Hoekstra RF, de Jong JH. Meiotic recombination in sexual diploid and apomictic triploid dandelions (Taraxacum officinale L.) Genome. 2000;43:827–835. doi: 10.1139/g00-047. [DOI] [PubMed] [Google Scholar]
- Vasconcelos MF, Modonado-Coelho M, Buzzetti DRC. Range extensions for the gray-backed tachuri (Polystictus superciliaris) and the pale-throated serra-finch (Embernagra longicauda) with a revision on their geographic distribution. Ornitologia Neotropical. 2003;14:477–489. [Google Scholar]
- Vieira MF, Grabelos R. Sistema reprodutivo de Oxypetalum mexiae Malme (Asclepiadaceae), espécie endêmica de Viçosa, MG, Brasil, em perigo de extinção. Acta Botanica Brasilica. 2003;17:137–145. [Google Scholar]
- Vitta FA. Diversidade e conservação da flora nos campos rupestres da Cadeia do Espinhaço em Minas Gerais. In: Araújo EL, Moura AN, Sampaio EVSB, Gestinari LMS, Carneiro JMT, editors. Biodiversidade, conservação e uso sustentável da Flora do Brasil. Recife: Imprensa Universitária; 2002. pp. 90–94. [Google Scholar]
- Williams CF. Effects of floral display size and biparental inbreeding on outcrossing rates in Delphinium barbeyi (Ranunculaceae) American Journal of Botany. 2007;94:1696–1705. doi: 10.3732/ajb.94.10.1696. [DOI] [PubMed] [Google Scholar]
- Williams CF, Ruvinsky J, Scott PE, Hews DK. Pollination, breeding system, and genetic structure in two sympatric Delphinium (Ranunculaceae) species. American Journal of Botany. 2001;88:1623–1633. [PubMed] [Google Scholar]