Summary
Changes in fat content have been associated with dietary restriction (DR), but whether they play a causal role in mediating various responses to DR remains unknown. We demonstrate that upon DR, Drosophila melanogaster shift their metabolism towards increasing both fatty acid synthesis and breakdown, which is required for various responses to DR. Inhibition of fatty acid synthesis or oxidation genes specifically in the muscle tissue inhibited lifespan extension upon DR. Furthermore, DR enhances spontaneous activity of flies which was found to be dependent on the enhanced fatty acid metabolism. This increase in activity was found to be at least partially required for the lifespan extension upon DR. Over-expression of adipokinetic hormone (dAKH), the functional ortholog of glucagon, enhances fat metabolism, spontaneous activity and lifespan. Together, these results suggest that enhanced fat metabolism in the muscle and physical activity play a key role in the protective effects of DR.
Introduction
Dietary restriction (DR) is defined as the reduction of a particular or total nutrient intake without causing malnutrition. DR slows age-related diseases and extends lifespan in many species, including both vertebrates and invertebrates (Masoro, 2003). In mice and flies, restriction of protein or specific amino acids is sufficient for lifespan extension (De Marte and Enesco, 1986; Min and Tatar, 2006). In Drosophila melanogaster, restriction of yeast, the major source of protein in the fly diet, robustly extends lifespan and is commonly used as a method for DR (Kapahi et al., 2004; Mair et al., 2005). Pathways including the target of rapamycin (TOR) and insulin-like signaling (ILS) have been implicated in the lifespan extension by DR, but the biochemical processes that mediate this lifespan extension are not fully understood (Kapahi et al., 2010).
DR, imposed using yeast restriction in D. melanogaster, enhances resistance to starvation stress and lipid content (Bradley and Simmons, 1997; Chippindale et al., 1993). In flies, nutrient composition has been shown to be critical for fat metabolism, with lower concentrations of yeast and higher concentrations of sugar promoting increased steady state triglyceride levels (Skorupa et al., 2008). Elevated triglyceride content is also observed in TOR and ILS pathway mutants that extend lifespan in D. melanogaster (Bohni et al., 1999; Zhang et al., 2000). While body fat in the mouse decreases under caloric restriction (Harrison et al., 1984), the ability to maintain adipose tissue levels correlates with lifespan extension upon caloric restriction in mice (Liao et al., 2011). Similarly, ob/ob mice, which normally live shorter than controls on an ad libitum (AL) diet, achieve a lifespan similar to control levels under caloric restriction, yet their percentage of body fat is much greater than that of controls (Harrison et al., 1984). Furthermore, mice that are fed on a diet with reduced methionine content are long-lived and show an increase in body fat (Miller et al., 2005). These studies suggest that different dietary and genetic manipulations that extend lifespan do not show a simple correlation with adiposity. Often, steady state levels of triglycerides are reported, but one cannot deduce whether the change in triglycerides is due to alterations in synthesis, storage, or breakdown of triglycerides. In the current study, we characterize the dynamics of fat metabolism upon DR and examine their causal link with extended longevity using D. melanogaster.
Results
DR enhances fat synthesis and utilization in D. melanogaster
We confirmed that flies on DR (0.5 % yeast extract) show an increase in steady state levels of triglyceride content compared to flies on AL diet (5% yeast extract) (Figure 1A). Triglycerides are the major storage form of energy and are mostly stored as fat droplets in the fat bodies in flies. We observed that the flies upon DR show larger fat droplets in the fat body (Figures 1B and S1A). We also observed that fat droplet size is reduced with age in both AL and DR flies, but overall a greater size is maintained upon DR with age (Figure S1A). To examine changes in fat synthesis and breakdown we developed an assay to measure the dynamic changes in triglyceride content upon DR. The assay is based on the incorporation of radioactively labeled glucose into various lipid fractions. Flies were raised on AL or DR diets for 10 days, after which their diets were spiked with 14C labeled glucose for 24 hours. We observed a 2.8 fold increase in the rate of triglyceride synthesis but no change in phospholipid synthesis upon DR (Figure 1C). We ruled out the possibility that upon DR flies may be eating more label by measuring the food intake of the flies (Figure S1B). Next, we measured triglyceride breakdown in vivo by transferring the label fed flies back to the respective diets without label for 60 hours. We observed that upon DR there is an increase in triglyceride breakdown, as there is a 63% reduction in labeled triglyceride levels upon DR. The flies on AL diet however, showed no significant decrease in labeled triglyceride pool (Figure 1D). Next, we confirmed the increase in triglyceride breakdown by measuring the in vitro lipolytic capacity and free fatty acids of corresponding whole fly lysates. Lipolysis was enhanced on DR by 30% (Figure 1E), whereas a 20% increase in free fatty acids levels was observed in vivo upon DR (Figure 1F). Together, these results suggest that flies show both an increase in lipogenesis and lipolysis with DR.
Figure 1. Dietary restriction enhances triglyceride metabolism in control (w1118) female D. melanogaster.
(A) Comparison of triglyceride content in whole flies under different nutrient conditions, DR diet (black bars), and AL diet (grey bars). (B) Fat bodies of female flies were dissected and stained for the content and number of lipid droplets (Red are triglycerides (Nile red) and blue is F-actin (Phalloidin)). Scale bar is 20 μm.(C) Rate of de novo lipid synthesis measured by incorporation of 14C glucose in triglyceride and phospholipid fraction under DR and AL conditions. (D) Triglyceride breakdown rates were measured in flies on DR and AL diets spiked with 14C labeled glucose for 24 hours (0 hrs fraction) and then flies were transferred to non-labeled food for 60 hours (60 hrs fraction) and incorporation of 14C glucose in triglyceride fraction was measured. (E) Lipolytic activity was measured from whole fly homogenates obtained from AL and DR fed female flies to assess breakdown of 3H labeled triglycerides in vitro. (F) Comparison of free fatty acid levels in whole fly homogenates under different nutrient conditions. The error bars indicate S.E.M. of 4–5 independent preparations. (* indicates p < 0.05). See also Figure S1.
Inhibition of Acetyl CoA Carboxylase (dACC) inhibits DR-dependent responses
To examine whether the increase in lipogenesis plays a role in modulating physiological changes in response to DR, we examined the effect of inhibiting dACC, the fly ortholog of a key enzyme in fat metabolism. Inhibition of dACC was achieved by using the RU486 inducible and ubiquitously expressing Actin 5C-GS Gal4 strain and verified using real time PCR (Figure S2A). As expected, dACC RNAi flies led to a marked inhibition of fatty acid incorporation into triglycerides (Figure S2B). dACC RNAi flies on DR also showed reduced steady state triglyceride content (Figure S2C) and impaired triglyceride remobilization in the fat body (Figure S2D). Combined, the results suggest that dACC plays a key role in mediating the changes in fat metabolism observed upon DR in D. melanogaster.
We examined whether changes in fat metabolism are required for the DR-dependent increase in stress resistance and lifespan. In dACC RNAi flies, starvation resistance was reduced by 24% compared to control flies under DR conditions (p < 0.0001) but no significant change was observed under AL conditions (Figure 2A and Table S1). Similarly, the DR-dependent increase in cold stress resistance was repressed upon dACC inhibition (Figure 2B). As both starvation resistance and cold stress resistance phenotypes could be directly dependent on the reduction of triglyceride levels, we measured the response of ACC RNAi flies to oxidative stress. Upon ACC inhibition, flies showed increased sensitivity to oxidative stress generated by paraquat (Figure S2E), but the survival under hyperoxia was not altered (Figure S2F), suggesting that though fat metabolism may be required for various stress responses it does not appear to be important under hyperoxia. Next, we tested the hypothesis that the switch towards increased fat metabolism upon DR is critical for its lifespan extension effects. Using the RU486 inducible GAL4-UAS system to inhibit dACC, a significant inhibition of DR -dependent increase in lifespan was observed across a range of yeast concentrations in both female (Figures 2C and S2G) and male flies (Figures 2C and S2H). While control female flies showed a 113% increase in lifespan upon DR compared to AL conditions, upon dACC inhibition there was a significant reduction in the lifespan extension with DR (52%). Similarly, control male flies showed a 22% increase in lifespan under DR, while RNAi of dACC resulted in a 5% reduction in lifespan with DR (Figures 2C). Similar effects on female lifespan upon DR were observed using a different RNAi construct for dACC (Figure S2I). We also verified that RU486 by itself does not affect lifespan (Figure S2J) or fat metabolism in flies (Figure S2K). Together, these experiments implicate an important role for dACC in mediating DR-dependent changes to fat metabolism, stress resistance and lifespan.
Figure 2. Fat metabolism in muscle tissue plays a key role in DR-dependent responses in D. melanogaster.
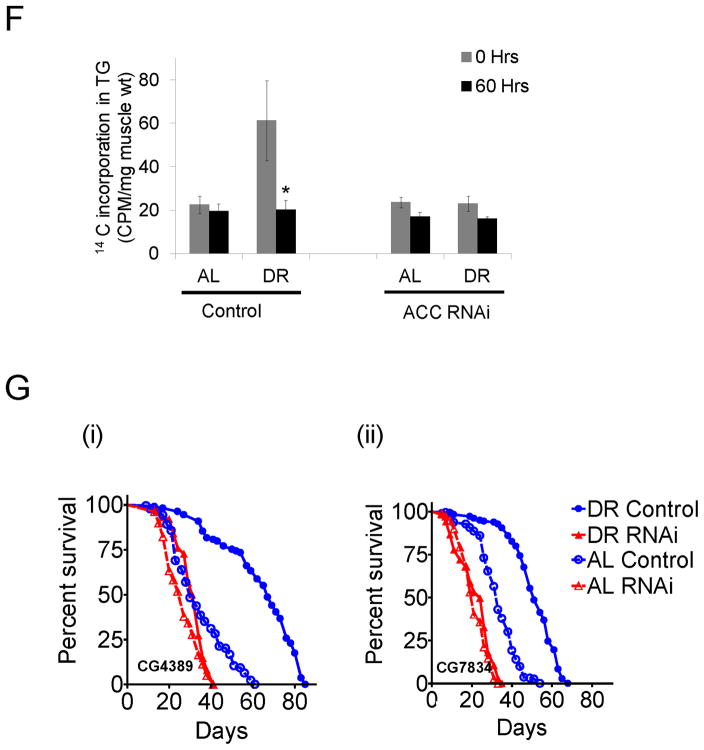
(A) Kaplan Meier survival analysis for survival under starvation in control flies (+/+; Act5c-GS-Gal4/+; UAS-CG11198/+, without RU486, blue) and dACC RNAi flies (with RU486, red) under DR (solid line) and AL (dashed line) conditions. (B) Survival analysis after cold coma recovery for control and dACC RNAi flies under DR or AL conditions. (C) Median lifespan was calculated from Kaplan Meier survival analysis of female and male flies upon dACC RNAi in whole body under different yeast (YE) concentrations. The individual survival curves are shown in Figure S2G (female) and S2H (male). (D) dACC RNAi reverses the effects of DR on transcription of several genes that were specifically up-regulated under DR. The heatmap was generated by selecting the group of genes whose expression was significantly altered between control and dACC RNAi flies between the DR groups but not between AL groups. (E) Effect of tissue specific dACC RNAi on DR-dependent lifespan extension. Kaplan Meier survival analysis of female flies upon dACC RNAi in the fat body ((+/+; +/+; S1106-Gal4/UAS-CG11198); panel i), neurons ((+/+; +/+; Elav-GS-Gal4/UAS-CG11198); panel ii) and muscle ((+/+; Mhc-GS-Gal4/+; UAS-CG11198/+); panel iii) under DR (solid line) and AL (dashed line) conditions; control flies (without RU486, blue) and dACC RNAi flies (with RU486, red). (F) Triglyceride synthesis and breakdown rates were measured in muscle from control and muscle specific dACC RNAi flies on DR and AL diets. Flies were fed DR and AL diets spiked with 14C labeled glucose for 24 hours (0 hrs fraction) and then were transferred to non-labeled food for 60 hours (60 hrs fraction). Thoraces were isolated and incorporation of 14C glucose in triglyceride fraction was measured. The error bars indicate S.E.M of 4–5 independent preparations. (* indicates p < 0.05). (G) Effect of muscle specific RNAi of β-oxidation genes on DR-dependent lifespan extension. Kaplan Meier survival analysis of female flies upon muscle specific RNAi of CG4389 ((+/+; Mhc-GS-Gal4/+; UAS-CG4389/+); panel i) and CG7834 ((+/+; Mhc-GS-Gal4/+; UAS-CG7834/+); panel ii) under DR (solid line) and AL (dashed line) conditions; control flies (without RU486, blue) and RNAi flies (with RU486, red). See also Figure S2 and Table S1–S4.
To better understand how changes in fat metabolism modulate lifespan under DR, we conducted a genome-wide transcriptional analysis. Both control and dACC RNAi flies were fed AL or DR diets for 10 days before assessing transcriptional changes in six independent biological replicate samples. To identify gene functions that mediate lifespan extension upon DR in a dACC dependent manner, expression changes that correlated with lifespan for the four groups were identified. Figure 2D shows a heatmap with cluster analysis of the genes that were changed upon DR (Table S2) but were reversed upon dACC inhibition. GO enrichment analysis on this cluster of genes found a significant enrichment of genes whose products are involved in muscle structure and function (Table S3). Some of the GO categories that changed upon DR, but were not significantly affected by ACC RNAi, were related to proteolysis, detection of visible light, visual perception, monovalent inorganic cation transport and synaptic vesicle exocytosis (Table S4). These global gene expression studies suggest that inhibition of dACC may attenuate the lifespan extension upon DR by reversing gene expression changes upon DR, especially of genes related to muscle function.
Fatty acid metabolism in the muscle tissue modulates DR-dependent lifespan extension
To examine the role of various tissues in mediating the effects of dACC on lifespan upon DR, we inhibited dACC in the fat body, neurons and muscle tissues. Interestingly, fat body and neuronal inhibition of dACC using RU486 inducible fat body (S1106-GS-Gal4) or neuronal (Elav-GS-Gal4) enhancer traps did not reduce the lifespan extension upon DR (Figures 2E, panel i and ii). However, muscle specific inhibition of dACC using a RU486 inducible muscle enhancer trap (Mhc-GS-Gal4) reduced the DR-dependent increase in lifespan (Figure 2E, panel iii). While the control flies showed a 47.2 % lifespan extension upon DR, flies with muscle specific inhibition of dACC failed to show any increase upon DR (Figure 2E, panel iii). To gain further insight, we examined changes in fat metabolism in dissected muscle tissue. Inhibition of dACC in the muscle diminished the increase in triglyceride synthesis and breakdown observed under DR conditions (Figure 2F). A significant reduction in lifespan extension, specifically upon DR, was also obtained with a non-inducible muscle driver (Mhc-Gal4) (Figure S2L). To verify the role of fatty acid breakdown in muscle tissue on DR-dependent lifespan extension, we examined two genes that are involved in the breakdown of fatty acids. CG4389 and CG7834 encode for mitochondrial long-chain-3-hydroxyacyl-CoA dehydrogenase and electron transport flavoprotein β subunit proteins respectively. Muscle specific induction of RNAi of CG4389 and CG7834 showed 25–35% reduction in mRNA (Figure S2M). Similar to inhibition of dACC, muscle specific knock down of CG4389 (Figure 2G, panel i) or CG7834 (Figure 2G, panel ii) significantly reduced the DR-dependent lifespan extension. The percentage increase in lifespan upon DR was only 20% in flies with CG4389 RNAi compared to 123% in controls (Figure 2G, panel i). Similarly, CG7834 RNAi flies showed only a 14% increase compared to 55% in the respective control flies (Figure 2G, panel ii). Combined, these experiments demonstrate that DR-dependent increase in muscle fatty acid metabolism is critical for its lifespan extension effects.
DR enhances muscle activity which is required for the maximal lifespan extension upon DR
DR is known to enhance spontaneous movement related activity in a variety of species including flies, rodents and primates (Bross et al., 2005; McCarter et al., 1997; Weed et al., 1997). The increase in movement is likely to be part of an evolutionary adaptation that facilitates foraging behavior under conditions of nutritional scarcity. We examined if changes in fat metabolism are required for the increase in spontaneous activity upon DR. Upon DR, control flies showed significantly higher levels of spontaneous activity compared to AL flies over a 24hr period (Figure S3A). Control flies on DR also showed significantly higher levels of spontaneous activity compared to dACC RNAi inhibited flies at all ages (Figure 3A). We also examined whether dACC would influence the age-related decline in muscle function. Muscle strength and function were assessed using an assay that measures the ability of flies to land on the side of a cylinder when dropped from a height (Palladino et al., 2002). dACC inhibition reduced the protective effects of DR on age-related decline in muscle activity (Figures 3B and S3B). These results suggest that enhanced fat metabolism is critical to preserve age-related decline in muscle function upon DR.
Figure 3. Enhanced movement and muscle function is critical for DR-dependent lifespan extension in female D. melanogaster.
(A) Age dependent measurement of total activity in control flies (+/+; Act5c-GS-Gal4/+; UAS-CG11198/+, without RU486) and dACC RNAi flies (with RU486). Daily activity was measured in the Drosophila activity monitors (statistical analysis by two-way ANOVA; DR control/DR RNAi (F1,3 = 27.9, p = 0.0129); AL control/DR control (F1,3 = 92.9, p = 0.0025; AL control/AL RNAi (F1,3 = 77.8, p = 0.0172). (B) The effects of dACC RNAi on the ability of female flies to maintain flight under different nutrient conditions. Flying ability was measured in young (day 10) and old flies (day 40) (Error bars indicates S.E.M of 4–5 independent observations, * indicates p < 0.001, when compared to young, for complete analysis see Figure S3B). (C) Effect of genetic ablation of wings on DR-dependent increase in lifespan. Kaplan Meier survival analysis of wing ablated (1096-Gal4/+; UAS-rpr/+; +/+) and control (1096-Gal4/+; +/+; +/+) female flies. (D) Effect of mechanically clipping wings of control flies on DR-dependent increase in lifespan. Kaplan Meier survival analysis of control female flies with non-clipped wings (blue) and clipped wings (black) under a DR (solid line) or AL (dashed line) diet. (E) Effect of mechanically clipping wings on DR-dependent lifespan extension in dACC RNAi flies. Kaplan Meier survival analysis of dACC RNAi female flies with non-clipped wings (red) and clipped wings (black) under a DR or AL diet. See also Figure S3 and Table S1.
Next, we examined whether increased spontaneous activity under DR condition plays a causal role in lifespan extension. We examined this by using flies with reduced movement due to wing defects. Flies with ablated wings caused by over-expressing reaper (UAS-Rpr) with a wing specific Gal4 enhancer trap (1096-Gal4) showed only 14% extension in lifespan compared to control flies which showed a 61% extension upon DR (Figure 3C). We also used partial clipping of the wings, as an alternate method, to reduce physical activity (Figure S3C). Similar to the ablated winged flies, the clipped-wing flies showed a modest 33% increase while the control flies showed a 97% lifespan extension upon DR (Figure 3D). This reduction in lifespan extension upon DR by curtailing movement was specific to control flies as lifespan was not further reduced with dACC inhibition in clipped-wing flies (Figure 3E). Cox regression analysis performed on two independent repeats of this experiment suggests that the impact of clipped wings on the effect of DR on longevity is significantly less in ACC RNAi flies compared to control flies (supplemental experimental procedures). The control and clipped-wing flies showed a similar level of fat synthesis and breakdown in both groups (Figure S3D). The muscle specific RNAi of dACC, CG7834 or CG4389, resulted in a significant reduction in movement of flies upon DR (Figure S3E). Together these observations further support the hypothesis that the enhanced muscle activity due to enhanced fat metabolism plays a critical role in ensuring extended lifespan upon DR.
dAKH over-expression increases fat metabolism, spontaneous activity and lifespan in a nutrient dependent manner
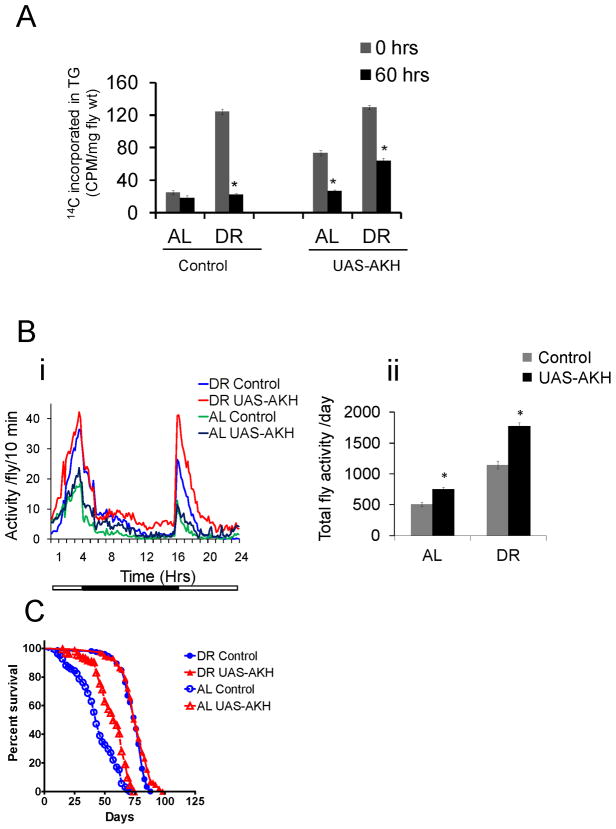
Glucagon, a catabolic hormone, is critical for maintaining glucose and triglyceride homeostasis in both mammals and insects. In flies and other insects, AKH is a circulating peptide that is considered a functional ortholog of glucagon based on its role in glucose and lipid metabolism (Kim and Rulifson, 2004; Lee and Park, 2004). We examined whether increasing AKH levels would improve fat metabolism and extend lifespan. Flies over-expressing dAKH in a ubiquitous manner showed significant increase in triglyceride synthesis and breakdown under AL conditions (Figure 4A). dAKH over-expression was sufficient to increase spontaneous movement under both AL (148%) and DR (154%) conditions (Figure 4B) and lifespan on AL (33%) conditions (Figure 4C). However, despite an increase in movement under DR conditions, lifespan was not increased, suggesting that DR and dAKH over-expression may work through overlapping mechanisms to increase lifespan (Figure 4C). Together, these experiments suggest that AKH modulates changes in fat metabolism, movement and lifespan in response to changes in nutrient status.
Figure 4. dAKH over-expression in flies enhances fat metabolism, spontaneous activity, and lifespan in a nutrient dependent manner in D. melanogaster.
(A) Triglyceride synthesis and breakdown rates were measured in control (+/+; Act5c-GS-GAL4/UAS-AKH; +/+; without RU486) and AKH over-expression (with RU486) flies on DR and AL diets. The error bars indicate S.E.M of 4–5 independent preparations (* indicates p < 0.05). (B) Effect of dAKH over-expression on spontaneous activity. The graph shows averaged activity (four vials per group with 25 flies in each vial) per 10 minutes for control and AKH over-expression flies. The X axis represents time (in hrs) after the flies were moved to the activity monitors. The activity was started at 4:00pm(panel i). The data in the graph is also plotted as bar graphs representing the total activity/fly/day (panel ii). Error bar indicates S.E.M, with n=4 for each group (* indicates p < 0.05). (C) The effects of dAKH over-expression on nutrient dependent changes in lifespan in female flies. Kaplan Meier survival analysis for control and AKH over-expression flies on DR and AL diets. See also Table S1.
Discussion
The relationship between survival, fat metabolism and lifespan remains poorly understood. In D. melanogaster, elevated lipid content has been observed in both long lived mutants in TOR and ILS pathways and the short lived mutants lacking Brummer and AKHR (AKH receptor) lipases (Gronke et al., 2007). These apparently contradictory observations may be reconciled if one takes into account the rate of fat synthesis and breakdown rather than the steady state levels of the measured triglycerides. Our data demonstrates that even though flies under DR show higher steady state triglyceride levels, the rates of synthesis and breakdown were also enhanced in these flies. In contrast, the flies on AL show little or no change in TG breakdown even after 60 hrs of unlabeled food. This was surprising, as this would suggest a continuous synthesis and accumulation of low level of TG in flies on AL diet. As our assay measures TGs synthesized from labeled glucose and not the TG obtained from the AL diet itself, one possibility is that the basal level of TG synthesized de novo from sugars, incorporated and distributed in different tissues, might be getting recycled rather than utilized. Our data from D. melanogaster is consistent with previous data from mice where increased fat synthesis and oxidation is observed during caloric restriction (Bruss et al., 2009). A positive correlation between lifespan extension upon caloric restriction and the ability to maintain fat levels was also observed in a large number of inbred mice strains (Liao et al., 2011). Similarly, by taking advantage of radiolabeled nucleotides from nuclear testing, fat turnover in humans has also been directly measured, showing that low levels of lipid turnover are associated with multiple metabolic diseases (Arner et al., 2011).
Here we demonstrate that the metabolic shift towards enhanced fatty acid metabolism under DR is critical for some of its protective effects. In flies, ACC is coded by a single gene, CG11198, whereas in mammals, two different genes (ACC1 and ACC2) code for separate isoforms of ACC. Mice that are null for ACC1 do not survive, whereas ACC2 null mice are viable. Although it is not known how ACC2 null animals will behave upon caloric restriction, upon regular diet these animals show an increased triglyceride breakdown, leaner phenotype, increased insulin sensitivity and no effect on lifespan (Choi et al., 2007). Interestingly, we also observed some benefits of dACC RNAi that were dependent on diet and tissue specific knockdown. dACC RNAi flies on AL show increased spontaneous activity (Figure 4A) and fat body specific dACC RNAi showed a small but significant increase in lifespan upon AL conditions (Figure 2E, panel i). In some of our experiments, we noted that though the benefits of DR were reduced, the fly strains were short lived on both AL and DR diets. One possible explanation is that our genetic and physical manipulations are very strong and which made the flies sick under standard laboratory conditions. This could not be ruled out as some cellular processes like fat metabolism and movement maybe critical for ensuring a normal lifespan. For this reason, the percentage increase in lifespan upon nutrient limiting diets was deemed critical for their importance in DR. Furthermore, to support our conclusions, we examined the effects of dACC inhibition using multiple tissue specific drivers and identified muscle as the critical tissue for its effects. Similar results using β-oxidation genes (Figure 2G) and AKH (Figure 4) support the role of fat metabolism in lifespan extension upon DR.
Mitochondrial β-oxidation plays a critical role in enhancing fat utilization for energy purposes. Physiological conditions like exercise and fasting, which require increased fatty acid oxidation, show up-regulation of mitochondrial biogenesis and mitochondrial β-oxidation (Hood, 2001) by modulating multiple pathways. We have previously observed that flies upon DR have enhanced mitochondrial function which is mediated by enhanced mRNA translation of nuclear encoded mitochondrial mRNAs in a d4E-BP dependent manner (Zid et al., 2009). Given the role of mitochondria in fatty acid oxidation, it is possible that enhancing mitochondrial function allows for the switch to increased fat utilization and physical activity upon DR.
Our data suggests that increased spontaneous activity might be a critical component of DR dependent lifespan extension. Both wings ablated and clipped-winged flies showed a reduction in lifespan extension. This result does not agree with a recent report showing that singly housed male flies that were restricted in small activity tubes showed significant increase in lifespan in a sugar restriction based DR paradigm (Linford et al., 2012). However, it is conceivable that restricting space may not limit muscle activity of flies similarly to loss of wings. Furthermore, as the sugar restricted male flies showed an increase neither in triglyceride content nor in total activity in response to diet, it is possible that the mechanism of lifespan extension by the two DR paradigm is different. It is also conceivable that in our experiments, upon wings ablation or clipping, there could be damaging effects on muscle tissue which limit lifespan. Future work exploring the relationship between physical activity and lifespan upon dietary restriction will help resolve these confounding issues.
Enhanced fat metabolism in the muscle has been associated with increased physical activity. Increased storage and utilization of fatty acids in muscle for energy purposes is seen in mice that over-express PEPCK (phosphoenolpyruvate carboxykinase) in the muscle tissue (Hanson and Hakimi, 2008). These mice are long lived, store up to five times more triglyceride in their skeletal muscle, and show increased levels of physical activity compared to control mice (Hanson and Hakimi, 2008). However, increased fat deposition in skeletal muscle is also associated with insulin resistance, as is commonly seen in obesity and type 2 diabetes (Pan et al., 1997). Paradoxically, exercise training that enhances insulin sensitivity also increases muscle fat content which is termed as the “athlete’s paradox” (Goodpaster et al., 2001). One potential explanation is that athletes breakdown their muscle triglycerides much more frequently than type 2 diabetics. Our data demonstrates that diminishing fatty acid oxidation in the muscle limits lifespan extension while enhancing fat mobilization by over-expressing AKH can extend lifespan. Our data also suggests that DR may induce changes in muscle similar to those observed under endurance exercise and that molecule like AKH that enhance fat breakdown could serve as potential DR mimetics. The protective effects of DR may be linked to increased spontaneous activity through enhanced fat metabolism, which helps maintain muscle function with age.
Experimental Procedures
Fly husbandry and lifespan analysis
Flies were developed on standard lab food (Caltech food recipe) and adults were transferred within 2–3 days of eclosion to a yeast extract (YE) diet (variable concentrations of YE) as described previously (Zid et al., 2009). Details of fly stocks, genotype, and crosses are available in the Supplemental Experimental Procedures, statistical analyses of the lifespan, and additional repeats data are provided in Table S1.
Lipid analysis
After 10 days feeding on an AL or DR diet, 240 flies were transferred to an AL or DR diet with 2 μCi of 14C labeled glucose. After 24 hours, half the flies were snap-frozen in liquid Nitrogen (0 hrs sample). The other half was transferred to a fresh non-radioactive AL or DR diet, was kept on this food for the next 60 hours (60 hrs sample), and then immediately frozen. The frozen samples (20 flies/replicate) were homogenized in chloroform and total lipid was fractionated into triglyceride and phospholipid fractions by using DSC-NH2 cartridges and different solvents (Bodennec et al., 2000; Kaluzny et al., 1985). The fractions were dried under nitrogen, re-suspended in the scintillation fluid, and counted. 0 hrs samples indicate the rate of incorporation of glucose in fatty acids and 60 hrs samples indicate the breakdown of the labeled fatty acids. Lipolysis measurements from whole flies were carried out by following a previously described protocol (Gronke et al., 2007). Triglyceride and free fatty acid were measured using commercially available kits (Stanbio labs, Boerne, TX). Fat body staining is described in detail in the supplementary text.
Gene-array expression analysis
Total RNA was extracted from approximately 35 flies per group. Six independent biological replicates were collected and expression array analysis was carried out. Details of RNA extraction, amplification, labeling and hybridization and analyses are provided in the supplementary text. The Gene Expression Omnibus (GEO) accession number for the microarray data reported in this paper is GSE37537.
Spontaneous activity measurements, muscle strength assays, and stress resistance assays
For measurement of spontaneous activity we used Drosophila population activity monitors (Trikinetics Inc., Waltham, MA). Muscle strength, starvation, and cold coma stress assays were performed using standard protocols and are available in detail in the supplemental experimental procedures.
Supplementary Material
Highlights.
DR enhances both fatty acid synthesis and utilization in Drosophila melanogaster
Enhanced fatty acid metabolism in the muscle increases activity and lifespan.
Increased physical activity upon DR is required for lifespan extension.
Over-expression of dAKH enhances fat metabolism, activity, and lifespan.
Acknowledgments
We thank Roger Chang, Akio Nada, Emily Chang (lifespan data collection), Krysta Felkey (microarrays) and Vishal Patel (staining and quantification). We also thank John Tower, Haig Keshishian and Kai Zinn for providing various Gal4 drivers used in the study. We thank Matthew Laye, Gordon Lithgow, Judith Campisi, Martin Brand and members of the Kapahi Lab for helpful discussions and suggestions. This work was funded by grants from the AFAR and the NIH (R01 AG031337-01A1; RO1 AG038688; RL1 AAG032113 and P01 AG025901-S1) (PK), RL9 AG032114 (SDK), a Nathan Shock Award (P30AG025708) (SM) and R01 AR057352 (NP). FD is an EMF/AFAR postdoctoral fellow. NP is an HHMI investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, Buchholz BA, Eriksson M, Arner E, Hauner H, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 2011;478:110–113. doi: 10.1038/nature10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodennec J, Koul O, Aguado I, Brichon G, Zwingelstein G, Portoukalian J. A procedure for fractionation of sphingolipid classes by solid-phase extraction on aminopropyl cartridges. J Lipid Res. 2000;41:1524–1531. [PubMed] [Google Scholar]
- Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell. 1999;97:865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- Bradley TJ, Simmons FH. An analysis of resource allocation in response to dietary yeast in Drosophila melanogaster. J Insect Physiol. 1997;43:779–788. doi: 10.1016/s0022-1910(97)00037-1. [DOI] [PubMed] [Google Scholar]
- Bross TG, Rogina B, Helfand SL. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell. 2005;4:309–317. doi: 10.1111/j.1474-9726.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab. 2009;298:E108–116. doi: 10.1152/ajpendo.00524.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. J Evol Biology. 1993;6:171–193. [Google Scholar]
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, et al. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A. 2007;104:16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marte ML, Enesco HE. Influence of low tryptophan diet on survival and organ growth in mice. Mech Ageing Dev. 1986;36:161–171. doi: 10.1016/0047-6374(86)90017-5. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- Gronke S, Muller G, Hirsch J, Fellert S, Andreou A, Haase T, Jackle H, Kuhnlein RP. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol. 2007;5:e137. doi: 10.1371/journal.pbio.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson RW, Hakimi P. Born to run; the story of the PEPCK-Cmus mouse. Biochimie. 2008;90:838–842. doi: 10.1016/j.biochi.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Archer JR, Astle CM. Effects of food restriction on aging: separation of food intake and adiposity. Proc Natl Acad Sci U S A. 1984;81:1835–1838. doi: 10.1073/pnas.81.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DA. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol. 2001;90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- Kaluzny MA, Duncan LA, Merritt MV, Epps DE. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res. 1985;26:135–140. [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Gelfond JA, Diaz V, Nelson JF. Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell. 2011;10:629–639. doi: 10.1111/j.1474-9726.2011.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linford NJ, Chan TP, Pletcher SD. Re-Patterning Sleep Architecture in Drosophila through Gustatory Perception and Nutritional Quality. PLoS Genet. 2012;8:e1002668. doi: 10.1371/journal.pgen.1002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Subfield history: caloric restriction, slowing aging, and extending life. Sci Aging Knowledge Environ. 2003:RE2. doi: 10.1126/sageke.2003.8.re2. [DOI] [PubMed] [Google Scholar]
- McCarter RJ, Shimokawa I, Ikeno Y, Higami Y, Hubbard GB, Yu BP, McMahan CA. Physical activity as a factor in the action of dietary restriction on aging: effects in Fischer 344 rats. Aging (Milano) 1997;9:73–79. doi: 10.1007/BF03340130. [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech Ageing Dev. 2006;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Palladino MJ, Hadley TJ, Ganetzky B. Temperature-sensitive paralytic mutants are enriched for those causing neurodegeneration in Drosophila. Genetics. 2002;161:1197–1208. doi: 10.1093/genetics/161.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed JL, Lane MA, Roth GS, Speer DL, Ingram DK. Activity measures in rhesus monkeys on long-term calorie restriction. Physiol Behav. 1997;62:97–103. doi: 10.1016/s0031-9384(97)00147-9. [DOI] [PubMed] [Google Scholar]
- Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000;14:2712–2724. doi: 10.1101/gad.835000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.