Abstract
Background
Perioperative blood transfusion in pancreatic cancer patients is linked to decreased survival; however, a causal mechanism has not been determined. During processing and storage of packed red blood cells (pRBCs) biologically active molecules accumulate in the acellular plasma fraction; therefore, we hypothesize that the plasma fraction of pRBCs promotes tumor progression.
Methods
Proliferation and migration of murine pancreas cancer and control cells were determined in vitro in response to the plasma fraction from leukocyte and non-leukocyte reduced, fresh versus stored pRBCs. Lastly, an immunocompetent murine model was used to assess the affect of the plasma fraction of pRBCs on pancreas cancer progression.
Results
Incubation of pancreatic cancer cells with the plasma fraction of pRBCs increased proliferation and migration. Intravenous delivery of the acellular plasma fraction to mice with pancreatic cancer significantly increased the tumor weight in both leukocyte reduced and non-leukocyte reduced pRBCs groups (p<0.01) although tumor growth and morbidity was greatest in the non-leukocyte reduced group.
Conclusions
The plasma fraction of stored pRBCs promotes murine pancreatic cancer proliferation and migration in vitro and when administered intravenously significantly augments pancreatic cancer growth in immunocompetent mice.
Keywords: transfusion, pancreas cancer, metastasis, proliferation, erythrocytes, immunomodulation
INTRODUCTION
Transfusion of stored packed red blood cells (pRBCs) often represents a lifesaving clinical intervention designed to rapidly correct deficits of volume and oxygen carrying capacity. Unfortunately, allogeneic blood transfusions expose patients to foreign cells, antigens, and soluble factors that may affect host immune function, a clinical phenomenon described as transfusion related immunomodulation (TRIM). TRIM was first described in 1973 when renal transplant allografts were found to survive longer in patients that had previously received blood transfusions.1 Based upon these observations, Gantt 2 questioned whether this “TRIM” effect might also be associated with an increased risk of cancer recurrence in patients undergoing resection for malignancy. The clinical corollary to this hypothesis proposes that allogeneic blood transfusion modulates the host immune system or directly affects cellular activities, resulting in increased tumor growth and metastasis, hastening cancer-related death.
Since these initial studies, more than 150 clinical studies have examined the association of perioperative allogeneic blood transfusion with cancer recurrence. 3 Most of these are observational cohort studies comparing patients who did or did not receive a transfusion. 4 Notably, several studies showed worsened outcomes in patients that were carefully matched for stage and other pertinent clinicopathologic characteristics such as stage and operative difficulty, that might otherwise be confounding in terms of separating tumor biology from transfusion effect. 5–7 Interestingly, it has been shown in one study that even the recipients of autologous blood transfusions have increased cancer related morbidity and mortality as compared to patients who do not require transfusions.8 These data suggest that something other than the immunogenicity of an allogeneic transfusion is responsible for poorer outcomes, wherein, processing or blood storage may play a role. In a seminal preclinical study by Blajchman et al. it was found that prestorage leukocyte depletion reduced pulmonary metastatic events, as did syngeneic versus allogeneic transfusions.9 Further, at least eight randomized control trials have compared the risk of cancer recurrence between patients receiving pRBCs with and without processing to remove elements believed to be responsible for TRIM,10,11 Likely due to limitations inherent to clinical trials, especially sample size, these studies failed to demonstrate a causal relationship between perioperative allogeneic blood transfusion and cancer recurrence or death due to malignant progression.12,13 Nevertheless, clinical 14–16 and laboratory observations support Gantt’s original hypothesis and have lead to continued investigations. More recently, two relatively large clinical studies have shown that blood transfusion negatively impacts survival in patients with periampullary (pancreas) cancer.17,18
During routine storage, cytokines and other bioactive substances accumulate in the plasma fraction of within pRBCs.19–21 Plasma from stored, but not fresh pRBCs contain lipids, which prime neutrophils (PMNs) cause PMN-mediated cytotoxicity, induce acute lung injury as the second event in a two event model and have been implicated in transfusion-related acute lung injury.21–26 As the ability of the plasma from stored pRBCs has never been tested on tumor growth and metastasis, we hypothesized that the plasma from stored pRBCs may be pro-oncogenic and augment the growth of solid tumors, or affect host immunity thereby allowing more rapid tumor progression.
METHODS
Plasma preparation from stored pRBCs
After obtaining informed consent under a protocol approved by the Colorado Multi-Institutional Review Board, ten healthy donors donated 450 ml of whole blood per American Association of Blood Banks (AABB) criteria. 27 The whole blood was separated into components and 50% (by weight) of the packed red blood cells was pre-storage leukoreduced by filtration using a Pall BPF4™ leukoreduction filter; and the other 50% of the unit was left as an unmodified control. The division of these units was completed without air contamination in a closed system and stored per AABB Guidelines.28 Samples from these pRBCs units, both pre-storage leukoreduced units (LR) and non-leukoreduced units (NLR) on day 1 (D.1) day 28 (D.28) and day 42 (D.42) were obtained via sterile couplers and the plasma was separated by centrifugation.27 Following plasma isolation a second spin (12,500g for 5 min) was performed to remove acellular debris.21 Plasma was aliquotted and stored at −80 °C.
Cell proliferation
Cells were maintained at 37 °C in a mixture of 5% CO2 and 95% air in Dulbecco’s minimal essential medium (DMEM, Gibco; Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, Gemini Bio-Products, Woodland, CA). bEnd.3 (mouse vascular endothelial, ATCC, Manassas, VA), RAW 274.1 (mouse macrophage, ATCC), and Pan02 (mouse pancreatic carcinoma, NCI) cells were cultured and harvested using 1% Trypsin-EDTA. The cells were placed in 96-well dishes (Falcon) in DMEM + 10% FBS for 6 hours to allow the cells to adhere. Media was removed and experimental media was added with varying conditions as follows: DMEM alone (control), DMEM + 10% FBS, DMEM + 10% D.1/D.28/D.42 LR or NLR plasma. To determine Cell proliferation was determined in triplicate at 24 hours using a commercially available MTS assay (Cell Titer 96® Aqueous One Solution Proliferation Assay, Promega Corporation, Madison, WI, Cat. #G3580) according to the manufacturer’s guidelines and absorbance quantified using a FLUOstar OPTIMA plate reader (BMG LabTech, Offenburg, Germany). Data are presented as percent of control (DMEM alone).
Cell Migration
Pan02 cells were harvested into DMEM alone in a modified Boyden chamber with experimental media in the lower chamber containing the following reagents: DMEM alone (control), DMEM + 10% FBS, DMEM + D.1 LR/NLR plasma, or D.42 LR/NLR. Cells were kept at 37 °C for 24 hours. Membranes were then stained, excised and mounted on slides and examined using a Nikon inverted microscope (Eclipse E600) at 200X total magnification. Cells migration across the membrane was quantified in six fields of view per membrane, with 2 membranes per treatment condition. Data were presented as mean number of cells that migrated across the membrane per field (total magnification, 200×).
Orthotopic immunocompetent murine model of pancreatic cancer
Two sets of animal experiments were performed using plasma from different donors. All murine studies were carried out according to the guidelines of the American Association for Accreditation of Laboratory Care and The University of Texas Southwestern Animal Care and Use Committee. Mice underwent orthotopic injection of 3×105 GFP-expressing Pan02 cells as previously described.29 Mice were randomized one week post tumor injection to receive a lateral tail vein (LTV) injection of either saline, D.1 LR or NLR, or D.42 LR or NLR plasma extract while undergoing a second sham surgery. Two non-tumor bearing groups (n=3/group) were given either D.1 LR or NLR plasma or D.42 LR or NLR plasma as a control. Units of pRBCs contain 60–80 ml of acellular plasma, thus if one assumes that the average weight of a healthy human adult is 70 kg; the estimated acellular plasma dose for a patient receiving a single unit of blood would be approximately 1 ml/kg; therefore all animals were given a dose of 1 ml/kg of this acellular plasma diluted into 100 μL of saline. Mice were clinically followed,30 weighed 3 times per week and sacrificed 3 weeks after the orthotopic injection of tumor cells. Non-tumor bearing mice were maintained for 4 weeks after LTV injection of plasma extract to observe for any adverse effects from injection of human plasma.
Necropsy was performed and extent of disease was quantified noting tumor size, visible metastatic disease and tumor sequelae. Necropsy was performed by two individuals blinded to randomization (SEH, CCB). Lesions were examined under blue light (485 nm) to confirm presence of GFP tumor cells. Selected lymph nodes were embedded in paraffin, sectioned and stained with H&E to confirm the presence of metastasis. Jaundice was determined subjectively by direct visual inspection of the abdominal viscera while visceral obstruction was assessed by of dilated viscera proximal to GFP positive implants.
Tumor immunohistochemistry (IHC)
Tumors from control, D.42 NLR and D.42 LR animals were examined for microvessel density, mature macrophage (F4/80) infiltration and activated (Mac-3) macrophage infiltration using fluorescent IHC. Sectioning was performed in 10 μm serial sections of frozen tumors. Sections were air dried for 30 minutes, fixed to slides with acetone for 30 seconds, rehydrated with TBS-0.2% Tween (TBST) and blocked with 20% Aquablock (East Coast Biologics, Cat. #PP82-A3021) and diluted in TBST for two hours. Primary antibody (Rat anti-CD 31 [MEC13.3; BD Biosciences Cat. #550274]) a vascular endothelial cell surface antibody; rat anti-F4/80 (Serotec, Cat # MCA497R), a surface marker for mature macrophages; rat anti-MAC-3 [M3/84; BD Biosciences Cat. #553322], a surface marker for activated macrophages) diluted in DAKO antibody diluent (DAKO Corporation, Carpinteria, CA Code # S3022) at 10 μg/mL was then placed onto separate tissue sections. These were incubated overnight at 4 °C, washed three times with TBST and fluorescently labeled secondary antibody was then placed on the tissue for one hour (goat anti-rat IgG), after washing the slides three times in TBST, the tissue was mounted with ProLong® Gold Antifade reagent (Invitrogen, Carlsbad, CA; Cat # P36930) and fluorescent signal was quantified. Pixel quantification was performed using previously described techniques. 31
Statistical analysis
The data are expressed as mean ± the standard error of the mean. One-way ANOVA testing was performed to determine the significance of observed differences. Fisher’s PLSD was performed for post hoc comparisons. Statistical significance was determined at p<0.05.
RESULTS
Cellular proliferation
Three cell lines were examined, Pan02 (cancer), bEnd.3 (control) and RAW 274.1(control) cells demonstrated similar proliferation effects in response to the acellular plasma from fresh (D.1) and stored (D.42) pRBCs. All data is represented as percentage over control (DMEM alone). The presence of 10% FBS caused significant proliferation in all cell lines tested and was used as a positive control. When specifically examining response to the plasma fraction of pRBCs, RAW cells showed a significant increase in proliferation when comparing plasma from LR and NLR pRBCs whether the units were fresh, D.1, (p<0.05) or stored, D.42 (p<0.01); moreover, increasing storage time of the pRBCs from identical donors also increased RAW cell proliferation wherein plasma from stored pRBCs showed a significant increase over fresh from both LR pRBCs and NLR pRBCs: D.1 vs. D.42 LR (p<0.01) and D.1NLR vs. D.42NLR (p<0.01). With regard to bEnd.3 proliferation, there was no difference in proliferation between plasma from D.1 LR pRBCs vs. D.1 NLR pRBCs (p=0.11). However, there was significant difference when comparing stored D.42 LR vs. D.42 NLR (p<0.01). Storage time did not significantly affect the LR group when comparing plasma from fresh or stored units, although there was a trend that approached statistical significance (p=0.07). However, storage time did have a significant effect on bEnd.3 proliferation when examining NLR plasma comparing fresh to stored plasma from NLR pRBCs (p<0.01). Finally, examining Pan02 proliferation, there was a significant difference between the plasma from LR and NLR pRBCs in both the fresh and stored groups (P=0.04) and (p<0.01), respectively. pRBCs storage time did not significantly affect Pan02 proliferation in the LR group (although the trend was in this direction) conversely it did significantly affect the NLR group such that the plasma from stored NLR pRBCs evidenced greater proliferation as compared to Pan02 cells treated with plasma from fresh NLR-pRBCs (Figure 1).
Figure 1.
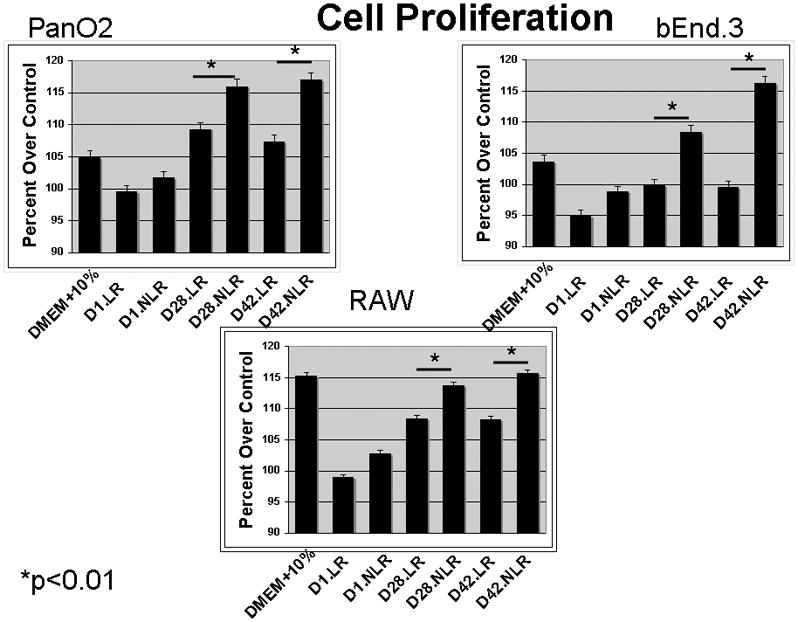
Cell proliferation was significantly increased in Pan02, bEND3 and RAW cells when exposed to NLR plasma as storage time increased.
Cellular Migration
Cellular migration was measured in Pan02 cells. As in the proliferation experiments, differences in leukocyte reduction and storage time were compared; such that storage time was compared within a single group (either LR or NLR). Interestingly, exposure to the acellular plasma fraction from D.1 LR-pRBCs provoked more Pan02 migration than the plasma from D.1 NLR pRBCs (90±6.3 vs. 66±7.8 cells/hpf, NS). However, with storage the plasma from D.42 NLR pRBCs significantly enhanced the Pan02 migration as compared to plasma from D.42 LR pRBCs (D.42 NLR pRBCs: 206±22.9 vs. D.42 LR pRBCs: 161±15.1 cells/hpf, p<0.01). Within groups storage time had a significant effect on migration in that the plasma from D.42 LR pRBCs increased Pan02 migration compared to the plasma from D.1 LR pRBCs (161±15.1 vs. 90±6.3 cells/hpf, p<0.01), and plasma from D.42 NLR pRBCs caused a similar increased migration versus the plasma from these identical units on D.1: (206±22.9 vs. 66±7.8 cells/hpf, p<0.01) (Figure 2).
Figure 2.
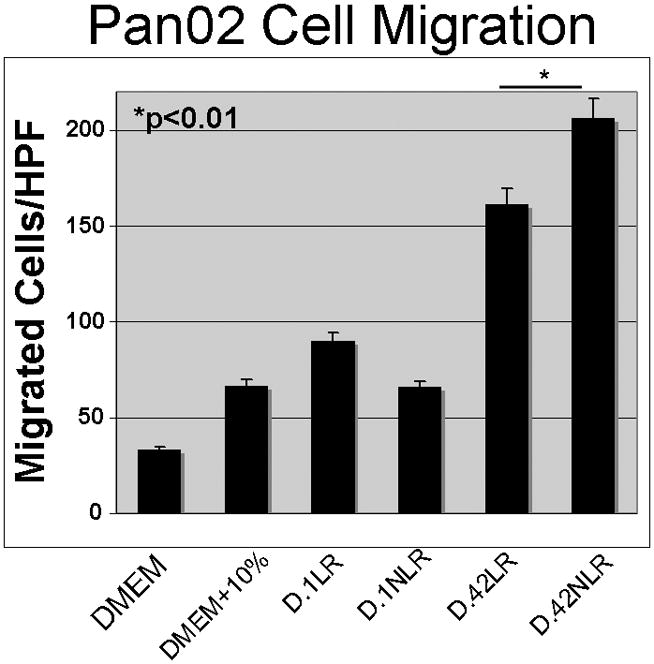
Pan02 cell migration was significantly increased when cells were exposed to NLR plasma from stored pRBCs at “outdate”-day 42 of storage.
Orthotopic Pancreas Cancer Progression
Animals with established tumors received intravenous plasma from fresh (D.1) or stored (D.42) LR or NLR pRBCs, while control animals received intravenous saline. The mean tumor weight in the control and treated animals is as follows: controls: 56.0 ± 11.7 mg, D1 LR pRBCs: 146 ± 7.5 mg, D.1 NLR pRBCs: 200.6 ± 16.8 mg, D.42 LR pRBCs: 150.4 ± 13.3 mg, and D.42 NLR pRBCs: 213 ± 14.1 mg. All mice transfused with pRBCs plasma had statistically larger tumors than the control group (p<0.01). Animals infused with plasma from fresh or stored NLR pRBCs had tumors that were statistically larger from mice infused with plasma from fresh or stored LR pRBCs (p=0.04 and p=0.01, respectively). Storage time did not affect tumor growth in animals transfused with plasma from either the LR or NLR pRBCs (p=0.88 and p=.50, respectively) (Figure 3).
Figure 3.
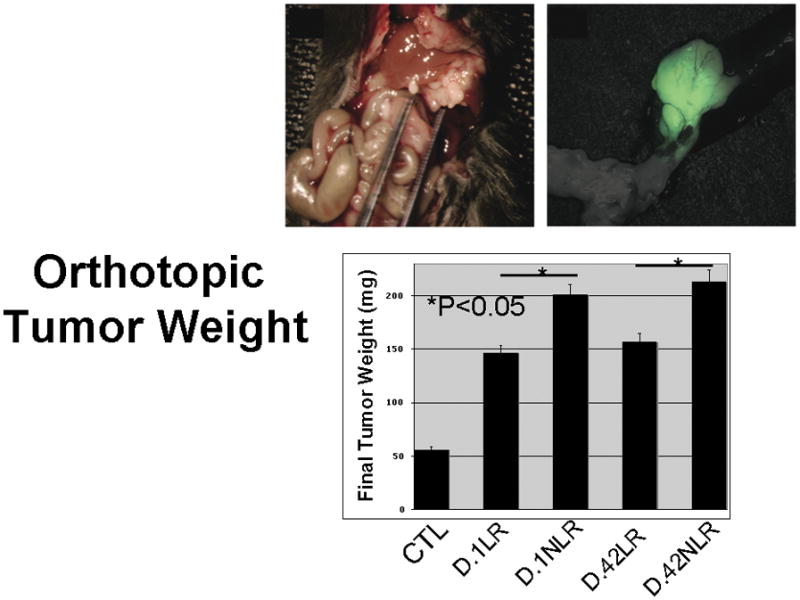
All mice transfused with pRBCs plasma had statistically larger tumors than the control group (p<0.01). Animals infused with plasma from fresh or stored NLR pRBCs had tumors that were statistically larger from mice infused with plasma from fresh or stored LR pRBCs (p=0.04 and p=0.01, respectively). Storage time did not affect tumor growth in animals transfused with plasma from either the LR or NLR pRBCs (p=0.88 and p=.50, respectively).
Determination of Metastatic Disease
Animals developed metastatic lesions with the same pattern and site-dependent frequency (hepatic, peritoneal, and lymph node) as has been observed in human patients with adenocarcinoma of the pancreas.32 Hepatic Metastases: The difference in number of visible liver metastases did not reach statistical significance between any of the groups; however, three of the mice receiving plasma from NLR pRBCs: one D.1 and two D.42 NLR developed a miliary pattern of liver metastases that made visual inspection and counting difficult. This miliary pattern was not observed in the saline-treated controls or mice infused with plasma from D.1 or D.42 LR pRBCs (Figure 4). Peritoneal Metastases: In comparing groups, leukocyte reduction did not reduce peritoneal metastases in mice treated with plasma from fresh pRBCs, LR pRBCs versus NLR pRBCs (p=0.3) whereas in mice infused with plasma from D.42 NLR pRBCs, there were significantly more metastases as compared to controls (p=0.04). Storage time did not increase the metastatic potential for animals infused with plasma from LR pRBCs, however, for D.42 NLR pRBCs the difference between stored, D.42, and fresh, D.1, approached significance (p=0.08) (Figure 4). Lymph Node Metastases- There was a significant difference in the number of lymph node metastases in mice when comparing the infusion of plasma from NLR pRBCs to LR pRBCs from both fresh (D.1 NLR pRBCs vs. D.1 LR pRBCs, p=0.02) and stored groups (D.42 NLR pRBCs vs. D.42 LR pRBCs, p=0.03). However, storage time did not increase the number of lymph node metastases in the homologous groups, plasma from D.1 vs. D.42 from LR pRBCs or NLR pRBCs (Figure 5).
Figure 4.
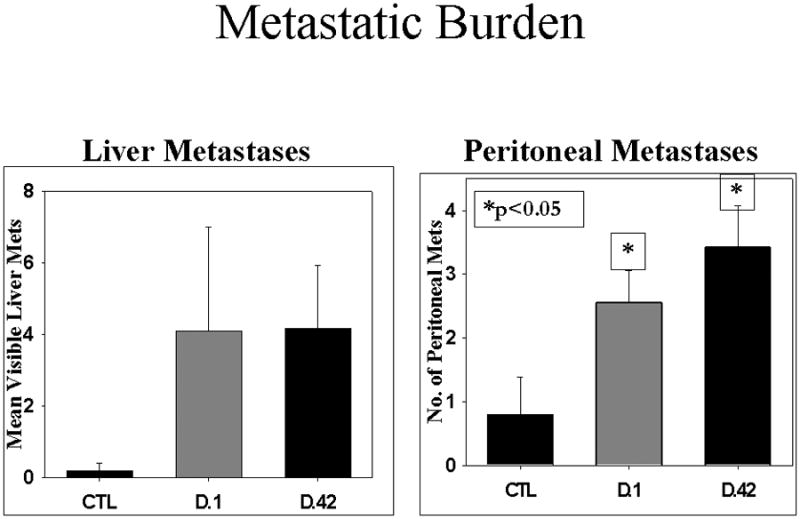
There was no difference in hepatic metastasis when comparing fresh versus stored plasma from NLR pRBCs, however, mice receiving stored plasma had significantly more peritoneal metastases.
Figure 5.
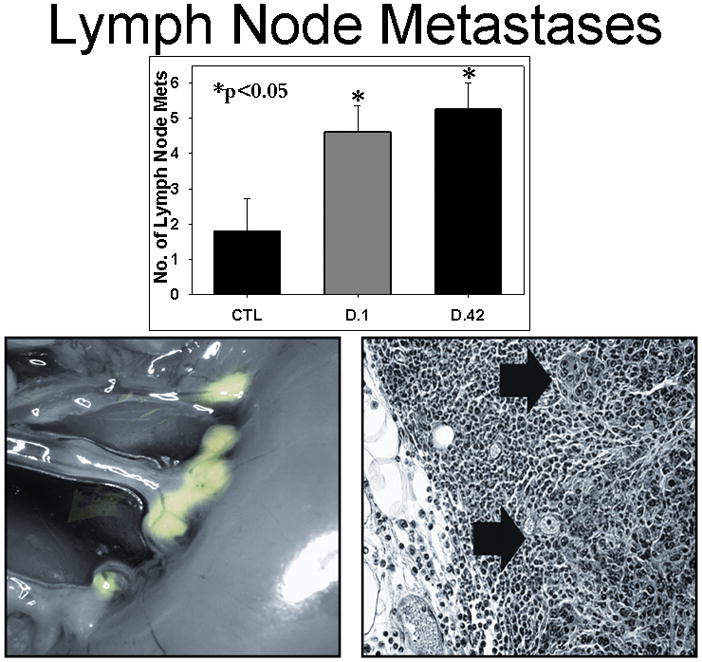
Lymph Node metastases were significantly higher in mice receiving plasma from stored NLR pRBCs versus fresh (data from NLR stored plasma shown). Inset shows GFP expression in lymph node metastases and histology of metastatic adenocarcinoma within a lymph node.
Clinical status of mice
Within two weeks of IV injection of plasma from both fresh and stored NLR pRBCs, the tumor-bearing mice demonstrated roughened haircoat, loss of body condition, and inactivity, 33 while tumor-bearing control animals (saline) appeared normal. Tumor-bearing animals transfused with plasma from LR pRBCs had variable rates of distress, with most animals appearing well (data not shown). The change in animal weight for each group closely paralleled these qualitative observations (data not shown). Mice without cancer, which received plasma from D.42 NLR gained weight appropriately for C57BL/6J mice30 and control, tumor bearing animals gained in parallel until approximately 14 days after tumor cell injection, when weight gain tapered off (presumably due to increasing tumor burden). Mice receiving IV NLR pRBCs plasma, whether fresh or stored, demonstrated poor weight gain compared to controls initially, however mice receiving stored pRBCs plasma gained weight rapidly after one week (Figure 6). Findings at necropsy offer some explanation for the poor clinical course of these mice. Ascites was present in 7/12 (58%) animals receiving plasma from stored NLR pRBCs and only 1/11 of the mice infused with plasma from D.1 NLR pRBCs and none of the control mice had ascites. Interestingly, no animals in the infused with plasma from fresh LR pRBCs, and only one animal (20%) infused with plasma from stored LR pRBCs developed ascites. Clinical jaundice, along with a distended gallbladder and biliary tree, was present in 6 (50%) mice infused with plasma from stored NLR pRBCs versus 2 animals (16%) treated with plasma from fresh NLR pRBCs and none of the control mice or those receiving plasma from D.1 LR-pRBCs. (Figure 7). These differences represent a trend toward more severe disease in the mice receiving plasma from stored pRBCs, with the difference in ascites formation reaching statistical significance (p<0.05), and biliary obstruction/jaundice trending toward a difference (p=0.11). Taken together, these data demonstrate a more virulent pattern of tumor growth in plasma-treated mice over control and mitigation of the “severe clinical effects” by pre-storage leukoreduction.
Figure 6.
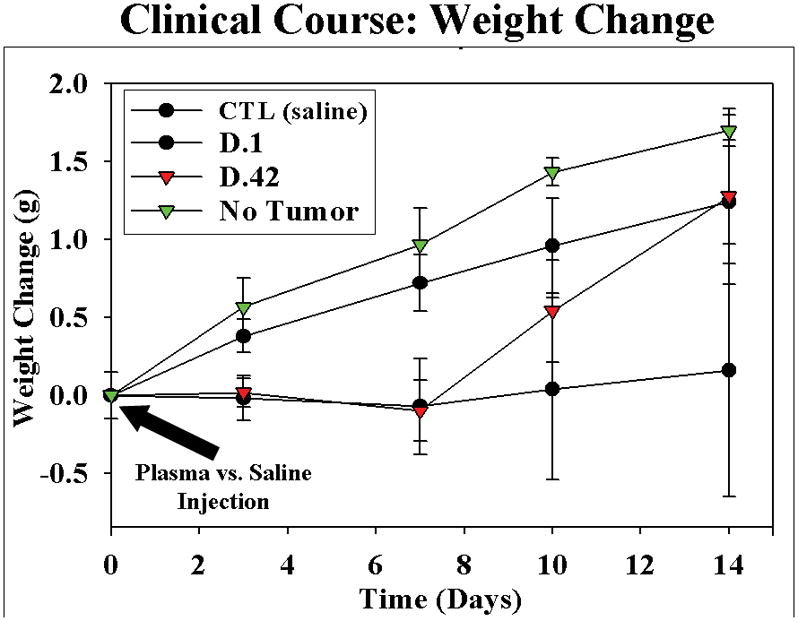
Mice receiving plasma from fresh and stored NLR pRBCs, demonstrated failure to gain compared to controls initially. Interestingly, mice receiving stored plasma developed rapid weight gain after 7–8 days, representing ascites formation in greater than 50%.
Figure 7.
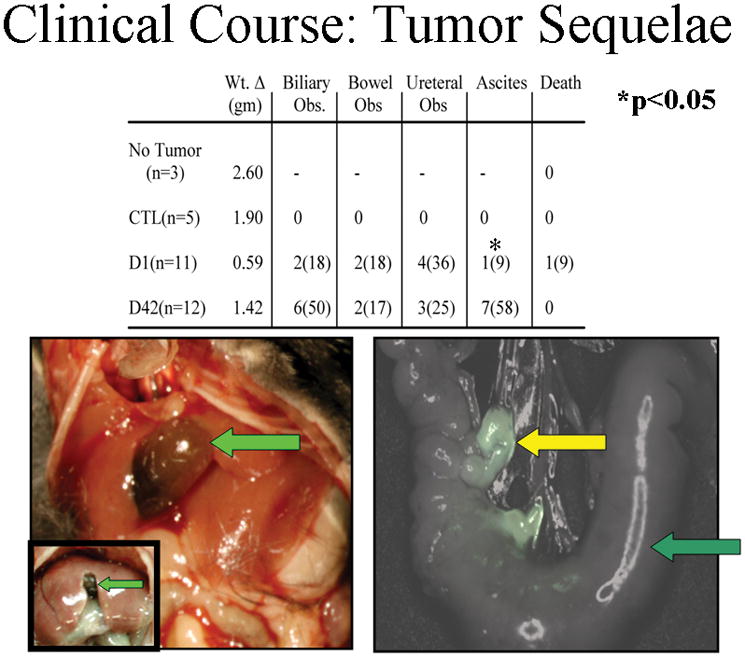
Mice receiving either fresh or stored NLR plasma developed clinical morbidity. Mice receiving plasma from stored NLR pRBCs developed significant ascites (58% versus 9%) compared to fresh. Inset left shows a normal mouse gallbladder compared to an enlarged gallbladder due to biliary obstruction. Inset right shows a bowel obstruction developing due to lymph node metastasis.
Tumor immunohistochemistry
Tumors from animals treated with plasma from both LR and NLR pRBCs had significantly higher microvessel density than tumors in saline-treated animals (p<0.001). Mature macrophage infiltration as determined by F4/80 staining did not differ between groups, but activated macrophage infiltration was higher in mice infused with stored NLR pRBCs or LR pRBCs compared to saline-treated controls treated. Additionally, tumors from animals receiving plasma from stored NLR pRBCs had significantly more activated macrophage infiltration than tumors in animal receiving stored or fresh plasma from LR pRBCs (p<0.001). (Figure 8).
Figure 8.
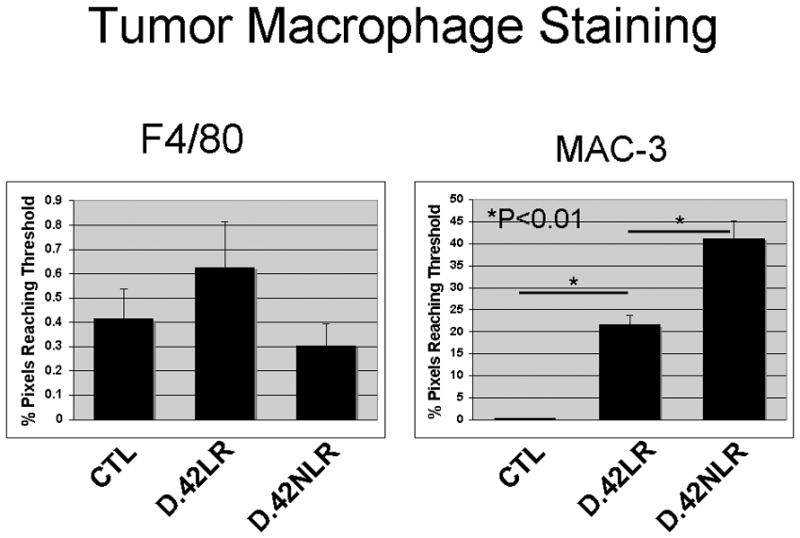
Tumors from mice receiving plasma from stored NLR pRBCs had significantly more activated macrophage infiltration than tumors in animal receiving stored or fresh plasma from LR pRBCs.
DISCUSSION
The role of perioperative blood transfusion in augmenting pancreatic cancer progression remains unclear due to clinical variability amongst patients and individual tumor biology, although large clinical series of patients with pancreatic cancer suggest a negative impact of transfusion on survival.17,18 Similarly, the specific constituent(s) of allogeneic blood that mediate TRIM as it relates to cancer progression are unclear. Plasma, platelets, and leukocytes, which inherently accompany NLR pRBCs, and proteins (cytokines or chemokines) that accumulate during PRBC storage have been implicated in the pathogenesis of TRIM.34–37 Clinical studies in non-cancer patients support the link between TRIM and the length of storage of banked blood.24,38–40 Furthermore, in vitro data have demonstrated that tumor cell growth is enhanced by blood that has been stored for increasing time periods.41 The release of bioactive substances by leukocytes and platelets and the accumulation of these substances during storage is generally accepted, 21,42,43 and animal studies suggest that early removal of leukocytes from banked blood ameliorates the tumor growth promoting effects of blood transfusion.36,37,44 However, five randomized controlled trials designed to evaluate the effect of allogeneic white blood cell transfusion on cancer recurrence failed to yield convincing results with regard to an enhanced TRIM effect from stored versus fresh pRBCs.11
This study has demonstrated that the acellular plasma fraction from stored pRBCs promotes cellular proliferation and Pan02 migration (surrogates for cancer growth and metastasis) and promotes in vivo pancreatic cancer growth. Although previous clinical studies have shown similar conclusions10,17,18,45 these studies are difficult to interpret given the large number of variables inherent to any cancer patient population including tumor biology, tumor stage, and untoward events during surgery as well as innumerable host factors. Use of this well established immunocompetent model of pancreatic cancer29 has allowed us to control for many factors that cause clinical trials to be dismissed. First, a clonal pancreatic cancer cell tumor was surgically introduced into the pancreas itself, which allowed for consistent tumor biology within all test animals. Second the use of an identical tumor inoculum (cell number) in all mice as well as ultrasound examination to confirm tumor presence allows control for “tumor stage” prior to administration of plasma from pRBCs. Third, the use of syngeneic animals controls for “patient variability”, and fourth, the same surgical stress is applied to all animals.
Interestingly, when examining the affect of the plasma from pRBCs on cellular proliferation in vitro, it appears that presence of leukocytes is the predominant factor that promotes proliferation although increasing storage time trended toward increasing proliferation in the Pan02 group as well. Moreover, as opposed to proliferation, storage time appeared to be more important in Pan02 migration even though the presence of leukocytes was clearly important in the stored D.42 blood.
The significant difference in tumor growth and metastasis observed in the animals that received plasma from pRBCs suggests that infusion of bioactive substances within the plasma of pRBCs promotes tumor progression, irrespective of storage time. In addition, acellular plasma harvested from stored D.42 pRBCs did not differentially affect tumor size and metastatic burden when infused into tumor-bearing animals. However, there was a trend toward more severe disease in the D.42 animals, with these animals demonstrating a higher rate of jaundice and ascites formation. Current transfusion literature supports these findings somewhat, suggesting that transfusion of blood with increased storage time augments the detrimental effects of TRIM. 40,41,46,43 Although levels of biologically active cytokines increase during storage of pRBCs, pre-storage leukoreduction decreases the cancer promoting effects of such transfusions;21,48,49 moreover, contrary to the presented data, these studies showed reduction of white blood cells before storage reduced the metastasis formation to control levels. However, until recently, little was known about the effects of pre-storage leukoreduction on platelet-derived growth factors, e.g. VEGF, PDGF or EGF all of which may have a role in tumor growth and may be eliminated from pRBCs by the current generation of filters. Interestingly, the infusion of the plasma fraction from day 1 and day 42 of storage from non-leukoreduced packed pRBCs were not different. These findings suggest that substances with sufficient bioactivity to promote tumor growth are present in the plasma after only one day of storage.
An unexpected finding was the observed rapid decline in the health of the mice harboring pancreatic cancer and receiving plasma versus tumor-bearing control animals. After sham surgery and plasma transfusion, mice with tumors appeared ill within days and failed to gain appropriate weight while tumor bearing control animals gained weight in parallel with non-tumor bearing controls until late in the study. The causes for this “clinical” picture appears to have been identified at necropsy where predominantly animals receiving plasma from D.1 and D.42 NLR pRBCs suffering biliary, gastrointestinal and urinary tract obstruction and a large portion of the mice treated with the plasma from D.42 NLR pRBCs plasma developing ascites within the study period. This difference may be more profound than is initially apparent as human clinical studies have demonstrated that presence of malignant cells in the peritoneum is a strong negative prognostic marker in pancreatic cancer, independent of the size of the primary tumor.50 Thus, there may be a differential effect of stored pRBCs versus fresher erythrocytes. The rapid decline in health in these animals closely parallels cancer-related morbidity witnessed in human patients and suggests that substances within plasma are promoting a virulent cancer progression that is not due to surgical trauma or the presence of tumor alone, but rather a combination of plasma transfusion and surgery in a tumor-bearing host. Although the observed increased morbidity may be related to storage age of the blood, it will require further study for elucidation; furthermore, these findings suggest that transfusion of pRBCs may “tip the balance” of the tumor-host interaction in favor of the tumor.
There are several limitations of this study that warrant discussion: 1) plasma contains many substances that could affect the host response, and the effect of individual agents is difficult to determine. 2) Human plasma was used in this model, and the mouse immune response to these foreign proteins is difficult to predict. Using blood from a syngeneic source would be ideal, but would be logistically difficult considering the need for similar harvesting, processing and storage techniques to make the model clinically relevant. Multiple studies by Silliman, et al 23,51 have demonstrated acute lung injury after transfusion of plasma from stored but not fresh pRBCs that is reproducible using multiple blood donors with no idiosyncratic effects noted in those studies.23,51,52 3) Many of the parameters used to determine disease severity, such as identification of cancerous lesions (lymph node, liver, peritoneal metastases) as well as jaundice and visceral obstruction are subjective. Two blinded observers were employed for confirmation of findings at necropsy to avert bias. Finally, statistical comparisons were necessarily made at high multiplicity comparing tumor growth, which should be viewed with some caution. Despite these issues, this model has clinical relevance and warrants further confirmation with more sophisticated studies in the future.
Blood products are given intravenously to cancer patients in the perioperative period everyday and the effects on cancer-related morbidity and death have yet to be proven. This study has attempted to control for many of the variables present in the clinical setting. In summary, this data suggests that bioactive substances capable of affecting the tumor biology within an organism are present within 24 hours of storage of erythrocytes. The substances and mechanism of action remain to be elucidated; however, these studies offer an avenue for investigation and identification of strategies for intervention.
Acknowledgments
This work supported in part by ACS Grant #MRSG-09-034-01-CCE (CCB) and NHLBI Grant # R01 HL59355, NIGMS # GM49222 (CCS)
Footnotes
The authors declare that they have no competing interests.
References
- 1.Opelz G, Teraski P. Improvement of Kidney-Graft Survival with Increased Numbers of Blood Transfusions. N Engl J Med. 1978;299(15):799–803. doi: 10.1056/NEJM197810122991503. [DOI] [PubMed] [Google Scholar]
- 2.Gantt C. Red blood cells for cancer patients. Lancet. 1981;2:363. doi: 10.1016/s0140-6736(81)90673-5. [DOI] [PubMed] [Google Scholar]
- 3.Dionigi G, Rovera F, Boni L, et al. The impact of perioperative blood transfusion on clinical outcomes in colorectal surgery. Surgical Oncology Colorectal Cancer: Biology, Diagnosis and Therapy. 2007;16(Supplement 1):177–182. doi: 10.1016/j.suronc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Vamvakas EC. Perioperative blood transfusion and cancer recurrence: meta-analysis for explanation. Transfusion. 1995;35(9):760–768. doi: 10.1046/j.1537-2995.1995.35996029162.x. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg N, Heal JM, Murphy P, Agarwal MM, Chuang C. Association between transfusion of whole blood and recurrence of cancer. Br Med J. 1986;293:530–533. doi: 10.1136/bmj.293.6546.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wobbes T, Joosen KHG, Kuypers JHC, Beerthuizen GIJM, Theeuwes AGM. The effect of packed cells and whole blood transfusions of survival after curative resection for colorectal carcinoma. Dis Colon Rectum. 1989;32:743–748. doi: 10.1007/BF02562121. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg N, Chuang-Stein C, Heal JM. The relationship of blood transfusion, tumor staging, and cancer recurrence. Transfusion. 1990;30:291–294. doi: 10.1046/j.1537-2995.1990.30490273432.x. [DOI] [PubMed] [Google Scholar]
- 8.Busch O, Hop W, van Papendrecht MH, et al. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328(19):1372–1376. doi: 10.1056/NEJM199305133281902. [DOI] [PubMed] [Google Scholar]
- 9.Bordin JO, Heddle NM, Blajchman MA. Biologic effects of leukocytes present in transfused cellular blood products. Blood. 1994;84:1705–1721. [PubMed] [Google Scholar]
- 10.Busch O, Hop WC, Marquet RL, Jeekel J. Blood transfusions and local tumor recurrence in colorectal cancer. Evidence of a noncausal relationship. Ann Surg Oncol. 1994;220(6):791–797. doi: 10.1097/00000658-199412000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vamvakas EC. Meta-analysis of randomized controlled trials investigating the risk of postoperative infection in association with white blood cell-containing allogeneic blood transfusion: the effects of the type of transfused red blood cell product and surgical setting. Transfus Med Rev. 2002;16(4):304–314. doi: 10.1053/tmrv.2002.35209. [DOI] [PubMed] [Google Scholar]
- 12.Sene AJJ, Robinson C, Walsh S, Kingston RD. Blood transfusion does not have an adverse effect on survival after operation for colorectal cancer. Ann R Coll Surg Eng. 1993;75(4):261–266. [PMC free article] [PubMed] [Google Scholar]
- 13.Francis D. Relationship between blood transfusion and tumour behaviour. Br J Surg. 1991;78(12):1420–1428. doi: 10.1002/bjs.1800781205. [DOI] [PubMed] [Google Scholar]
- 14.Blumberg N, Heal JM. Effects of Transfusion on Immune Function. Cancer Recurrence and Infection. Arch Pathol Lab Med. 1994;118(4):371–379. [PubMed] [Google Scholar]
- 15.Hoh H, Umpleby H, Cooper A, Taylor I. Recurrence of Colorectal Cancer and Perioperative Blood Transfusion. Is Blood Storage Time Important? Dis Colon Rectum. 1990;33(2):127–130. doi: 10.1007/BF02055541. [DOI] [PubMed] [Google Scholar]
- 16.Mynster T, Nielsen HJ Danish RANX05 Colorectal Cancer Study Group. Storage time of transfused blood and disease recurrence after colorectal cancer surgery. Dis Colon Rectum. 2001;44(7):955–964. doi: 10.1007/BF02235483. [DOI] [PubMed] [Google Scholar]
- 17.Yeh JJGM, Tomlinson JS, Idrees K, Brennan MF, Fong Y. Effect of blood transfusion on outcome after pancreaticoduodenectomy for exocrine tumour of the pancreas. British Journal of Surgery. 2007;94(4):466–472. doi: 10.1002/bjs.5488. [DOI] [PubMed] [Google Scholar]
- 18.Park SJKS, Jang JY, Lee KU, Park YH. Intraoperative Transfusion: Is It a Real Prognostic Factor of Periampullary Cancer Following Pancreatoduodenectomy? World Journal of Surgery. 2002;26(4):487–492. doi: 10.1007/s00268-001-0254-6. [DOI] [PubMed] [Google Scholar]
- 19.Shanwell AKM, Remberger M, Ringdén O. Generation of cytokines in red cell concentrates during storage is prevented by prestorage white cell reduction. Transfusion. 1997;37(7):678–84. doi: 10.1046/j.1537-2995.1997.37797369441.x. [DOI] [PubMed] [Google Scholar]
- 20.Kristiansson MSM, Saraste L, Sundqvist KG. Cytokines in stored red blood cell concentrates: promoters of systemic inflammation and simulators of acute transfusion reactions? Acta Anaesthesiol Scand. 1996;40(4):496–501. doi: 10.1111/j.1399-6576.1996.tb04475.x. [DOI] [PubMed] [Google Scholar]
- 21.Silliman CC, Clay KL, Thurman GW, et al. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124(5):684–94. [PMC free article] [PubMed] [Google Scholar]
- 22.Wyman TH, Bjornsen AJ, Elzi DJ, et al. A two-insult in vitro model of PMN-mediated pulmonary endothelial damage: requirements for adherence and chemokine release 10.1152/ajpcell.00540.2001. Am J Physiol Cell Physiol. 2002;283(6):C1592–1603. doi: 10.1152/ajpcell.00540.2001. [DOI] [PubMed] [Google Scholar]
- 23.Silliman CC, Voelkel NF, Allard JD, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101(7):1458–67. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors 10.1182/blood-2002–03–0958. Blood. 2003;101(2):454–462. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 25.Kao K. Mechanisms and New Approaches for the Allogeneic Blood Transfusion-Induced Immunomodulatory Effects. Transfus Med Rev. 2000;14(1):12–22. doi: 10.1016/s0887-7963(00)80112-1. [DOI] [PubMed] [Google Scholar]
- 26.Vamvakas EC, Carven JH. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: effect of the length of storage of transfused red cells. Transfusion. 1999;39(7):701–710. doi: 10.1046/j.1537-2995.1999.39070701.x. [DOI] [PubMed] [Google Scholar]
- 27.Technical Manual. American Association of Blood Banks; Bethesda: 2003. [Google Scholar]
- 28.Technical Manual. 14. American Association of Blood Banks; Bethesda: 2002. [Google Scholar]
- 29.Holloway S, Davis M, Jaber R, Fleming J. A clinically relevant model of human pancreatic adenocarcinoma identifies patterns of metastasis associated with alterations of the TGF-beta/Smad4 signaling pathway. Int J Gastrointest Cancer. 2003;33(1):61–9. doi: 10.1385/IJGC:33:1:61. [DOI] [PubMed] [Google Scholar]
- 30.Animal Resources Body Weight Study of 1993. The Jackson Labs; 1993. [Google Scholar]
- 31.Fleming JB, Shen G-L, Holloway SE, et al. Molecular Consequences of Silencing Mutant K-ras in Pancreatic Cancer Cells: Justification for K-ras-Directed Therapy 10.1158/1541–7786.MCR-04–0206. Mol Cancer Res. 2005;3(7):413–423. doi: 10.1158/1541-7786.MCR-04-0206. [DOI] [PubMed] [Google Scholar]
- 32.Fleming J, Brekken RA. Functional imaging of angiogenesis in an orthotopic model of pancreatic cancer. Journal of Cellular Biochemistry. 2003;90(3):492–501. doi: 10.1002/jcb.10644. [DOI] [PubMed] [Google Scholar]
- 33.Council N. Guide for the Care and Use of Laboratory Animals. National Academy Press; 2004. [Google Scholar]
- 34.Dineen S, Roland CL, Toombs JE, Kelher M, Silliman CC, Brekken RA, Barnett CC. The Acellular Fraction of Stored Platelets Promotes Tumor Cell Invasion. J Surg Res. 2009;153:132–137. doi: 10.1016/j.jss.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Neilsen HJ, Reimert CM, Pedersen AM. Time-dependent, spontaneous release of white cell- and platelet-derived bioactive substances from stored human blood. Transfusion. 1996;36:960–965. doi: 10.1046/j.1537-2995.1996.36111297091738.x. [DOI] [PubMed] [Google Scholar]
- 36.Blajchman MA, Bardossy L, Carmen R, Sastry A, Singal DP. Allogeneic blood transfusion-induced enhancement of tumor growth: two animal models showing amelioration by leukodepletion and passive transfer using spleen cells. Blood. 1993;81:1880–1882. [PubMed] [Google Scholar]
- 37.Dzik S, Mincheff M, Puppo F. Apoptosis, transforming growth factor-beta, and the immunosuppressive effect of transfusion. Transfusion. 2002;42(9):1221–1223. doi: 10.1046/j.1537-2995.2002.00175.x. [DOI] [PubMed] [Google Scholar]
- 38.Biffl WL, Carnaggio R, Moore EE, et al. Clinically relevant hypertonicity prevents stored blood- and lipid-mediated delayed neutrophil apoptosis independent of p38 MAPK or caspase-3 activation. Surgery. 2003;134(1):86–91. doi: 10.1067/msy.2003.178. [DOI] [PubMed] [Google Scholar]
- 39.Biffl WL, Moore EE, Offner PJ, et al. Plasma from aged stored red blood cells delays neutrophil apoptosis and primes for cytotoxicity: abrogation by poststorage washing but not prestorage leukoreduction. J Trauma. 2001;50(3):426–31. doi: 10.1097/00005373-200103000-00005. discussion 432. [DOI] [PubMed] [Google Scholar]
- 40.Offner PJ, Moore EE, Biffl WL, et al. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137(6):711–6. doi: 10.1001/archsurg.137.6.711. discussion 716–7. [DOI] [PubMed] [Google Scholar]
- 41.Bordin JO, Blajchman MA. Blood transfusion and tumor growth in animal models. In: Vamvakas E, Blajchman MA, editors. Immunomodulatory Effects of Blood Transfusion. Bethesda, Maryland: AABB Press; 1997. [Google Scholar]
- 42.Silliman CC, Thurman GW, Ambruso DR. Stored blood components contain agents that prime the neutrophil NADPH oxidase through the platelet-activating-factor receptor. Vox Sang. 1992;63(2):133–136. doi: 10.1111/j.1423-0410.1992.tb02500.x. [DOI] [PubMed] [Google Scholar]
- 43.Silliman CC, Johnson CA, Clay KL, et al. Compounds biologically similar to platelet activating factor are present in stored blood components. Lipids. 1993;28(5):415–418. doi: 10.1007/BF02535939. [DOI] [PubMed] [Google Scholar]
- 44.Vamvakas EC, Blajchman MA. Prestorage versus poststorage white cell reduction for the prevention of the deleterious immunomodulatory effects of allogeneic blood transfusion. Transfus Med Rev. 2000;14(1):23–33. doi: 10.1016/s0887-7963(00)80113-3. [DOI] [PubMed] [Google Scholar]
- 45.Murata N, Idezuki Y, Konishi T, Watanabe H, Ushirokoji Y, Shinohara K, Shibusawa M, Haga S, Hiraishi M, Bandai Y, Yamamura T, Yumoto S, Gunji A, Nishigaki K. Influence of Perioperative Blood Transfusion on the Prognosis of Patients With Gastric Cancer Receiving Anticancer Chemotherapy. Gastric Cancer. 2000:24–27. doi: 10.1007/pl00011685. [DOI] [PubMed] [Google Scholar]
- 46.Adamson JW. New blood, old blood, or no blood? NEJM. 2008;358:1295–1296. doi: 10.1056/NEJMe0800520. [DOI] [PubMed] [Google Scholar]
- 47.Aiboshi J, Moore EE, Ciesla DJ, Silliman CC. Blood transfusion and the two-insult model of post-injury multiple organ failure. Shock. 2001;15(4):302–306. doi: 10.1097/00024382-200115040-00009. [DOI] [PubMed] [Google Scholar]
- 48.Silliman CC, Paterson AJ, Dickey WO, et al. The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion. 1997;37(7):719–726. doi: 10.1046/j.1537-2995.1997.37797369448.x. [DOI] [PubMed] [Google Scholar]
- 49.Bordin JO, Chiba AK, Carvalho KIL, et al. The effect of unmodified or prestorage white cell-reduced allogeneic red cell transfusions on the immune responsiveness in orthopedic surgery patients. Transfusion. 1999;39(7):718–723. doi: 10.1046/j.1537-2995.1999.39070718.x. [DOI] [PubMed] [Google Scholar]
- 50.Nakatsuka AYK, Shimizu S, Yokohata K, Morisaki T, Chijiiwa K, Tanaka M. Positive washing cytology in patients with pancreatic cancer indicates a contraindication of pancreatectomy. Int J Surg Investig. 1999;1(4):311–317. [PubMed] [Google Scholar]
- 51.Silliman CC, Bjornsen AJ, Wyman TH, et al. Plasma and lipids from stored platelets cause acute lung injury in an animal model. Transfusion. 2003;43(5):633–640. doi: 10.1046/j.1537-2995.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 52.Silliman C, Kelher M, Masuno T, Moore EE, Damle S, Cheng A. A two-event in vivo model of transfusion-related acute lung injury. Blood. 2006;108(suppl):10a. [Google Scholar]


